- 1Graduate School, Beijing University of Chinese Medicine, Beijing, China
- 2Guang’anmen Hospital, China Academy of Traditional Chinese Medicine, Beijing, China
Introduction: Depressive symptoms and the severity of osteoporosis in cancer survivors significantly affect the patient’s quality of life. The correlation between osteoporosis and depressive symptoms in this population has not been examined in prior studies. This cross-sectional study utilized National Health and Nutrition Examination Survey data from 2005 to 2020 to explore the correlation between osteoporosis and depressive symptoms in cancer patients.
Method: We utilized confounder-adjusted multivariate logistic regression models to examine the relationship between osteoporosis and depressive symptoms. Subgroup and interaction analyses were performed according to age and gender to detect potential differences among various demographic groups. Furthermore, smoothing curve fitting and subgroup smoothing curve fitting were employed to evaluate the nonlinear association between bone mineral density and depressive symptom scores.
Results: Two thousand one hundred and fifty-five adult cancer patients satisfied the inclusion and exclusion criteria and were recruited in the research. Statistical analysis demonstrated a significant negative correlation between depressive symptoms and the risk of developing osteoporosis in cancer patients after controlling for multiple variables [OR 0.57, 95% CI (0.46–0.70), p < 0.01]. Subgroup analyses revealed more pronounced associations in women [OR 0.18, 95% CI (0.12–0.27), p < 0.01] and older adults [OR 0.09, 95% CI (0.06–0.13), p < 0.01]. Furthermore, smooth curve fitting results displayed a W-shaped curve between bone mineral density and depressive symptom scores. This W-shaped curve association was especially prominent among older patients in subgroup analyses.
Discussion: This study demonstrates a negative correlation between depressive symptoms and the incidence of osteoporosis in cancer survivors, notably evident among the elderly and female populations. Our research addresses the relationship between depressed symptoms and osteoporosis in cancer survivors, revealing a negative association that may alleviate psychological distress and enhance the quality of life in this population.
Conclusion: A negative correlation between depressive symptoms and osteoporosis in cancer patients, particularly pronounced in the elderly and female survivors. Additionally, a W-shaped relationship was observed between bone mineral density and depression scores, with greater significance in the older group.
1 Introduction
Osteoporosis, abbreviated as OP, is a common disease of the bones primarily characterized by a significant decrease in the mass of the bones throughout the body and irreversible destruction of the bone microstructure. This elevates bone fragility and the likelihood of fractures, even from modest falls or impacts. Individuals with OP are at a high risk of fractures due to this pathological change (1, 2). As the world population ages, osteoporotic fractures are becoming increasingly prevalent and adversely affecting individuals’ quality of life (3). The global incidence of OP is estimated to be around 18.3%. Regarding gender inequalities, the prevalence of OP was 11.7% in males and 23.1% in women. The incidence in undeveloped countries is 22.1%, but in affluent nations, it is 14.5% (4). Considering that OP predominantly impacts older women and healthcare expenses are rising in several low-income nations, research on OP in this demographic possesses heightened therapeutic significance (5). In recent years, due to the ongoing advancement of molecular biology, theories of cellular senescence (6–8), intestinal flora and bone immunity disorder (9, 10), osteoblast energy metabolism, iron homeostasis, and iron death (11) have played a crucial role in the study of its pathogenesis.
Depression is a mental disorder characterized by persistent sadness, loss of interest or pleasure in activities, and impairments in daily functioning that significantly impact a person’s quality of life and career (12). According to a World Health Organization (WHO) survey, around 5% of adults worldwide suffer from depression (13). There is no definite conclusion on the many factors that contribute to the development of depression, such as psychological factors, social pressure, dietary habits, and genetic factors (14). It has been reported that only 3.6% of patients with depression receive specialized antidepressant treatment (15); hence, studying depression can significantly improve people’s quality of life. There is a correlation between depression and the development of OP. Beyond limiting physical activity due to complications such as fractures and chronic pain, OP can also manifest as a psychosomatic illness, affecting patients emotionally. Studies have shown that patients with depression have a heightened chance of acquiring OP, implying that depression may contribute to its etiology (16, 17). A comprehensive and nationally representative study done in the United States identified a link between depression and OP in the older population (18). Furthermore, depression may result in metabolic abnormalities due to neurohormonal imbalances. A stronger correlation has been observed between OP and depressive symptoms in postmenopausal women. The prevalence of concomitant OP is twice as high in those with depression compared to those without depressive symptoms. The correlation between depressed symptoms and OP in postmenopausal women is notably robust (19). The rapid loss of bone mass caused by a decline in estrogen levels during menopause contributes to OP development in women (20, 21). Investigating the connection between depression symptoms and OP is beneficial for both the diagnosis and treatment of OP.
Cancer is a significant public health issue in the twenty-first century, contributing to approximately 1/6 of all fatalities and 3/10 of premature deaths (30–69 years old) worldwide (22, 23). It also has a significant social and financial cost. The idea of cancer-related depression has drawn the attention of some experts and scholars. This is related to the pressures imposed on the patient throughout their illness because of their diagnosis of cancer and the treatment they are undergoing (24). Cancer survivors with depressive symptoms who then experience OP fractures or bone pain are likely to choose suicide (25).
Additionally, medications used to treat cancer, such as letrozole and other aromatase inhibitors, increase the risk of OP in cancer patients (26). Consequently, it is imperative to examine the correlation between depressive symptoms and OP in cancer patients since many of these individuals now contend with both conditions. This study analyzed data from a large, nationally representative U.S. population to investigate the relationship between depressed symptoms and OP in cancer patients, aiming to enhance their quality of survival.
2 Materials and methods
2.1 Study population in NHANES
This study employed data from the National Health and Nutrition Examination Survey (NHANES) administered by the National Center for Health Statistics (NCHS). NHANES data collection is conducted at all the United States locations. NHANES gathers extensive data about the health and nutritional condition of individuals in the United States using questionnaires, laboratory analyses, and physical assessments. All participants granted informed consent, and the NCHS Research Ethics Review Board sanctioned the protocols for the NHANES study. Our study employed cross-sectional data from participant questionnaires, laboratory results, physical exams, and dietary information. For additional details on the NHANES study design and data collection, please refer to the official website: http://www.cdc.gov/nchs/NHANES/. The study was designed following the guidelines of the STROBE Epidemiology Report.
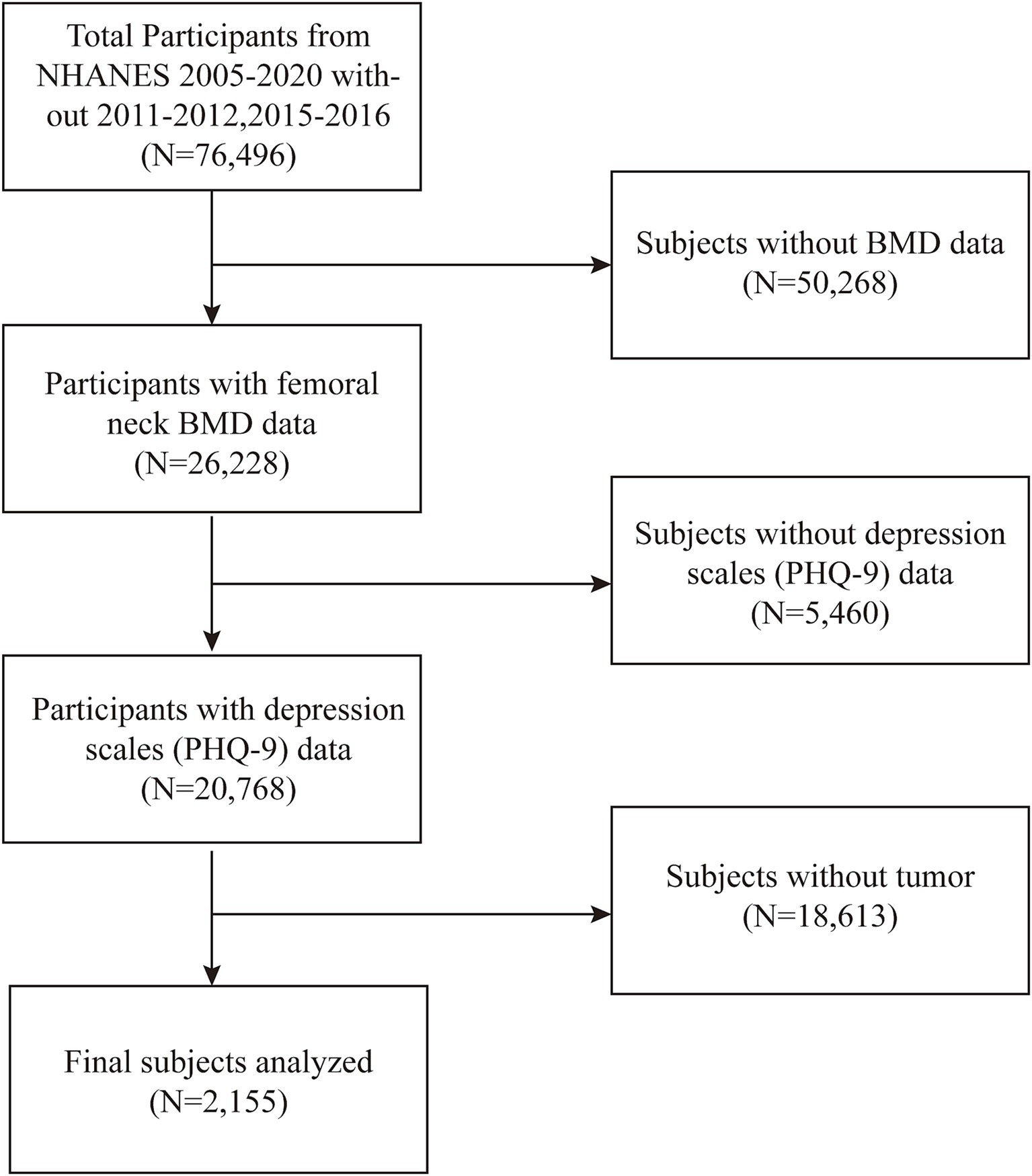
We analyzed participant data collected from 2005 to 2020, with the 2017–2020 data representing pre-pandemic data gathered from 2017 to March 2020. Data from 2011–2012 and 2015–2016 were excluded due to incomplete femoral neck bone mineral density (BMD) measurements. Bone density data were indispensable as an outcome variable in our study. OP diagnosis was determined by dual-energy X-ray absorptiometry (DXA)-measured femoral neck bone mineral density (BMD). In this study, femoral neck BMD was the primary outcome variable, with BMD values below 0.556 g/cm2 classified as OP (27). Depression was assessed as the exposure variable, with a total PHQ-9 score ≥ 5 indicating depressive symptoms. The study population for this study was cancer survivors. Participants who answered “yes” to the MCQ220 questionnaire section on whether they had ever been told by a doctor that they had cancer were included in the study. In MCQ230a, participants answered specific types of cancer; therefore, the NHANES study covered the full range of cancer types. We removed all incorrect values and missing values. In the initial sample of 76,496 participants, 50,268 were excluded due to missing BMD data, 5,460 due to missing depression scale scores, and 18,613 due to the absence of a cancer diagnosis. Ultimately, the final analysis comprised 2,155 individuals (Figure 1).
2.2 Measures
The World Health Organization defines OP using the T-score of BMD calculated as (BMD-reference BMD)/reference standard deviation (SD). This research categorized individuals with a femoral neck BMD below 0.556 g/cm2 as having OP. Depressive symptoms were evaluated with the Patient Health Questionnaire-9 (PHQ-9), a nine-item measure for assessing depression. A score of ≥5 on the PHQ-9 was utilized to signify the existence of depressive symptoms, with elevated scores corresponding with a heightened probability of depression. Factors including low level of education, poor marital status, and low income may all contribute to depressed mood or reduced nutritional supplementation in patients, thereby affecting the relationship between depressive symptoms and OP. Based on the experience of previous studies, the occurrence of depression and OP may be associated with body mass index (BMI) (28, 29), smoking status (30, 31), age (32, 33), gender (34, 35), race (34, 36), education (36, 37), marital status (38), household income to poverty ratio (PIR) (39), and diabetes status (40, 41). The study encompassed the following covariates: BMI, smoking status, age, gender, race, educational attainment, marital status, PIR, and diabetes status. We used multivariate logistic regression to adjust for covariates to reduce the effect of confounders on the results. Comprehensive measurement methods for these variables are available at http://www.cdc.gov/nchs/nhanes/.
2.3 Statistical analysis
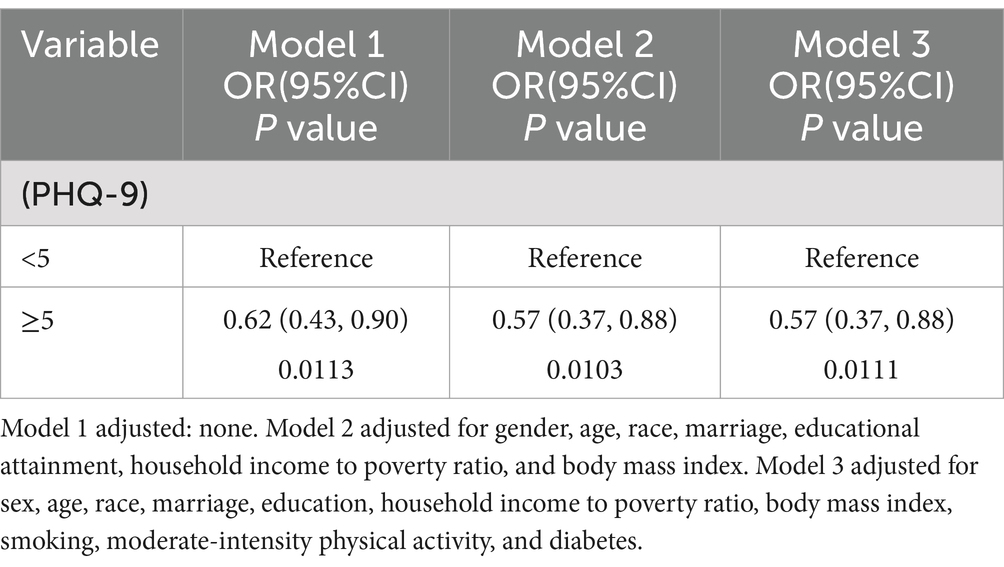
In this cross-sectional research, we employed t-tests and chi-square tests to examine the baseline characteristics of patients with and without OP. The correlation between OP risk and depressive symptoms was assessed by three multivariate logistic regression models, with outcomes reported as odds ratios (OR) and 95% confidence intervals (CI). Model 1 did not incorporate any modifications for variables. Model 2 incorporated modifications for age, gender, race, educational attainment, marital status, family income-to-poverty ratio, and BMI. Model 3 was also adjusted for diabetes, smoking status, and moderate-intensity physical activity alongside the variables from Model 2. We conducted a subgroup stratified analysis of the relationship between depressive symptoms and OP in a population of cancer survivors based on age, equally divided into 4 groups (Q1–Q4) and gender (male and female). A smoothed curve fitting technique was employed to analyze the linear correlation between PHQ-9 scores and BMD. Statistical analyses were conducted utilizing R1 and EmpowerStats.2
3 Results
3.1 Baseline characteristics
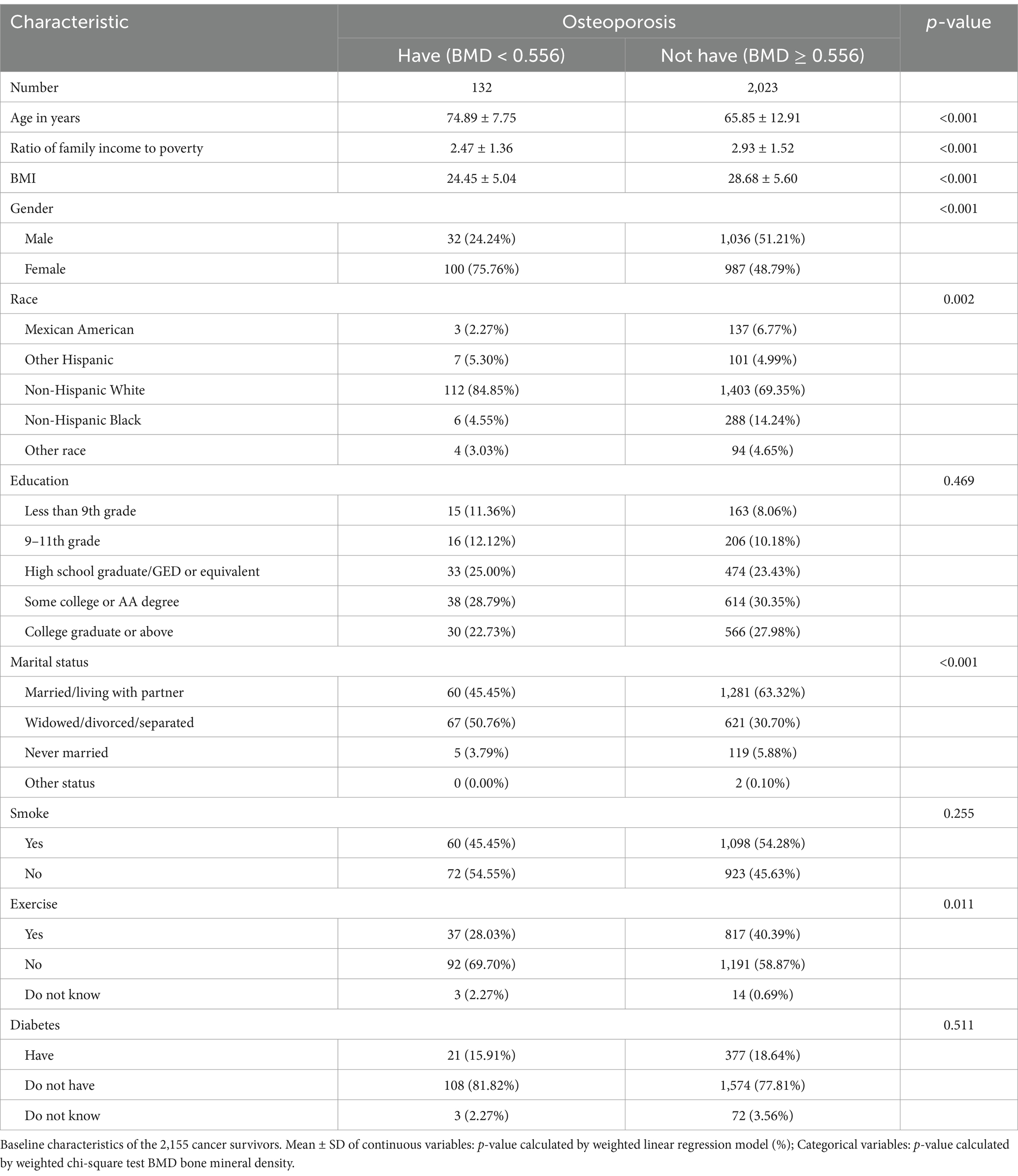
This investigation involved 2,155 subjects in total, 132 of whom had an OP diagnosis and 2,023 of whom did not (132/2,023). The age distribution of the patients with and without OP was 74.89 ± 7.75 years and 65.85 ± 12.91 years, respectively. There were no significant differences in the components of the variables of physical activity, education, smoking status and prevalence of diabetes (p > 0.05). In contrast, age, gender, income, race, and marital status were significantly different between those who had OP and those without OP (p < 0.05) (Table 1).
3.2 Association between depression and OP in the cancer survivor population
We investigated the relationship between OP and depression in cancer patients utilizing three multivariate logistic regression models (Table 2). The findings showed that the risk of OP was reduced by 38% [OR 0.62, 95% CI (0.43, 0.90), p = 0.01] in depressed patients in model 1; in model 2, the risk was reduced to 48% [OR 0.60, 95% CI (0.47, 0.76), p < 0.01]; and in model 3, the risk was 57% [OR 0.57, 95% CI (0.46, 0.70), p < 0.01]. These findings reveal a negative correlation between depressive symptoms and OP in cancer patients, with the odds of getting OP among cancer patients being 57% lower in those with depression than in those without. Nevertheless, no causal relationship between the two diseases can be inferred.
3.3 Subgroup analyses
We conducted logistic regression for additional covariates and grouped the continuous variables into four quartiles (Q1-Q4) to analyze the association between depressive symptoms and OP in cancer patients (Table 3). Our findings indicated that the negative association was more significant in women [OR 0.18, 95% CI (0.12, 0.27) p < 0.01] and older adults [OR 0.09, 95% CI (0.06, 0.13) p < 0.01]. In this investigation of cancer patients, smoothed curve fitting (Figure 2) showed a W-shaped relationship between depression score (PHQ-9) and BMD. Based on subgroup analyses, we stratified the cancer survivor population by age. We performed smoothed curve fitting for each subgroup (Figure 3) and found that the W-shaped relationship was more significant in the older survivor groups (Q3 and Q4).
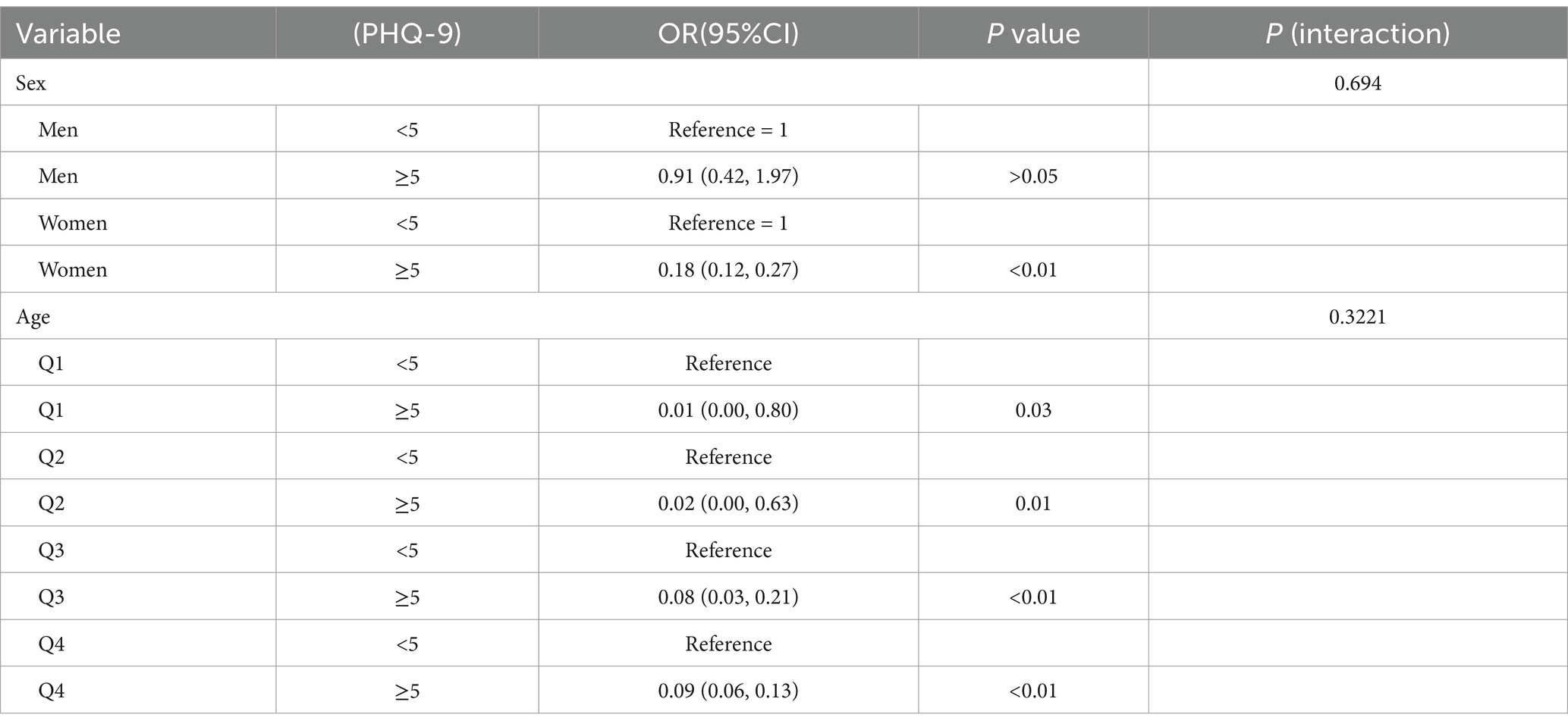
Table 3. Subgroup analysis of the relationship between depression and osteoporosis in cancer survivors.
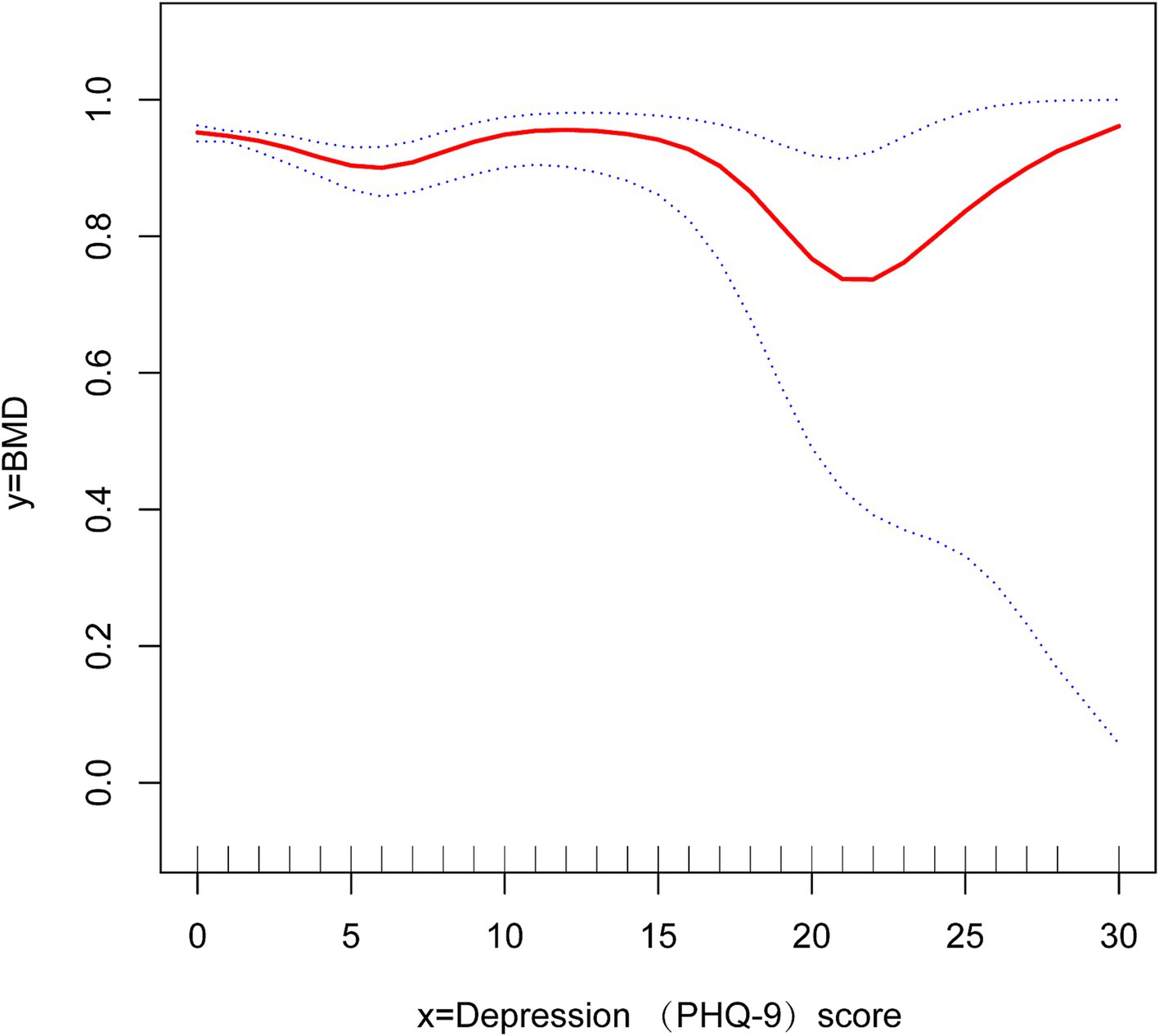
Figure 2. Smooth curve fitting reveals a w-shaped relationship between depression scores and bone density in cancer survivors.
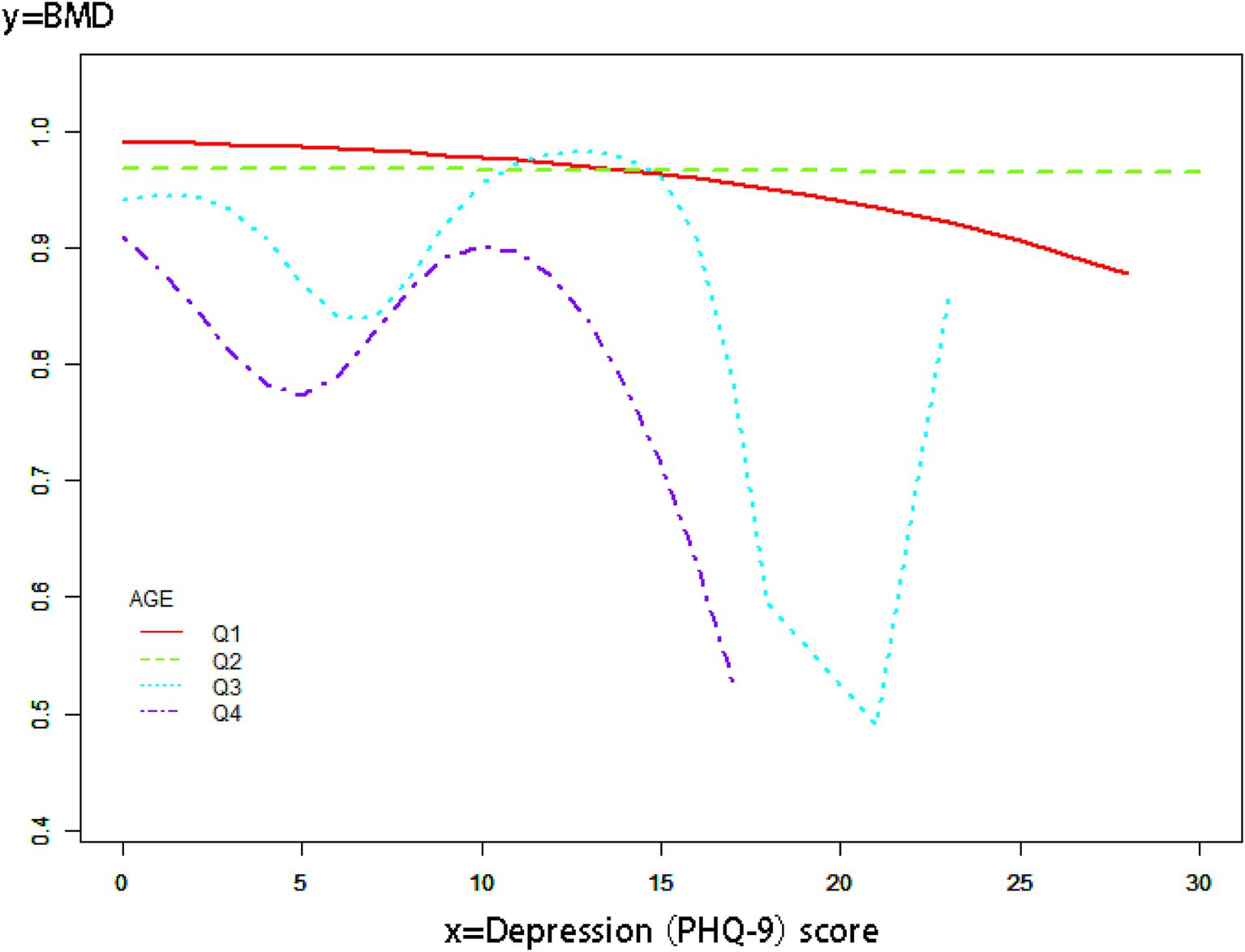
Figure 3. Smooth curve fitting of subgroup analyses describes the relationship between depression scores and BMD in cancer survivors of different age groups.
4 Discussion
Depressive symptoms in cancer survivors may lead to suicide when coupled with bone pain or fractures due to OP. The association between OP and depressed symptoms in cancer survivors has not been investigated before. Many contemporary epidemiological studies examining the relationship between depressed symptoms and OP have concentrated on the general and elderly populations. Prior research has indicated a potential bidirectional causal link between depressed symptoms and OP. A cross-sectional NHANES study identified a favorable correlation between depressed symptoms and OP (18). Numerous expert consensus studies, systematic reviews, and meta-analyses have established a strong positive link between depressed symptoms and OP throughout the whole population, identifying depression as a definitive risk factor for OP (42, 43). A research examining adverse reactions to alendronate within the Formulated Adverse Event Reporting System (FAERS) revealed that the prevalent OP medication alendronate was linked to heightened incidences of depression and anxiety (44). In the elderly population, depressive symptoms impair physical activity, exacerbating OP in older individuals with the condition (45). Female patients aged over 50 may constitute a distinct demographic. A clinical investigation has demonstrated a negative correlation between depressed symptoms and OP in women over the age of 50 (46).
Cancer survivor populations exhibit a higher prevalence of depressive symptoms compared to the general population, attributable to low survival rates, and are at an increased risk for suicide owing to physical distress (47). A significant consensus exists about the association between cancer and OP. Patients with breast and prostate malignancies are more predisposed to developing OP as a result of hormonal imbalances (48). OP-related bone pain in cancer survivors typically results in significant discomfort (49). OP may elevate the chance of pathological fractures, particularly in the hip, perhaps resulting in extended bed rest and the gradual deterioration of the patient. Extended bed rest progressively undermines the patient’s mental health and induces depressed symptoms (50). The co-morbidity of depression and OP in cancer survivors can be profoundly detrimental, perhaps resulting in severe repercussions that may drive the individual to contemplate suicide (51). Consequently, it is essential to examine the correlation between depressed symptoms and OP in cancer survivors.
This study discovered a substantial negative connection between depressed symptoms and OP among cancer survivors. The negative link was more evident in female patients, aligning with other research that indicated a negative correlation between depressed symptoms and OP in women over 50 years old. Cancer survivors exhibiting depressed symptoms had a markedly reduced incidence of OP compared to those without depressive symptoms [OR 0.57, 95% CI (0.46, 0.70), p < 0.01]. The precise pathways between depression and OP among cancer survivors remain unidentified. In cancer patients, particularly those with breast and prostate malignancies, hormonal imbalances are linked to the onset of OP (52). As cancer severity increases, the probability of depression rises, leading to more systematic treatment by an oncologist in a hospital setting, therefore establishing a negative link between depressive symptoms and OP in cancer survivors. Cancer survivors experiencing depressive symptoms frequently have less tolerance for external stimuli and are prone to exaggerating somatic feelings, resulting in heightened alertness about physical changes, which may lead patients to pursue BMD screening or anti-OP therapy (53). In late-stage cancer survivors, malignant circumstances influence BMD, leading to the emergence of depression symptoms. Consequently, physicians often prescribe anti-osteoporotic drugs, establishing a negative association between depressive symptoms and OP. Cancer survivors in the advanced stages of malignancy will experience a reduction in their BMD; patients often exhibit depressive symptoms, prompting physicians to recommend anti-OP pharmacotherapy, thereby establishing a negative correlation between depressive symptoms and OP (54). The negative link may be particularly evident in women owing to the substantial hormonal fluctuations during menopause.
A W-shaped correlation exists between BMD and depression ratings in populations of cancer survivors. The W-shaped association may result from a mix of hormonal and neurotransmitter influences. Chronic stress in mild to severe depression elevates hormone levels, which suppresses bone production and results in decreased BMD (55). In those with moderate to severe depression, tailored therapy, together with enhanced outdoor exercise and dietary intake, leads to a temporary improvement in BMD (56). Patients with severe depression may endure extended durations of bed rest and malnutrition resulting from eating problems, which can precipitate a further loss in BMD (57). The results we obtained indicate that the type W relationship is more prominent in the older demographic, maybe due to their greater availability to seek hospital care for antidepressants or anti-osteoporotic medications compared to younger individuals.
The benefits and contributions of this work are as follows: This study represents the first investigation into the correlation between depressed symptoms and OP among cancer survivors, enhancing OP studies within this demographic. Compared with other studies, this study has a large sample size and high reliability of results. Furthermore, to enhance the trustworthiness of the findings, this study was comprehensively adjusted for confounding variables that might influence the results. Subgroup analyses were performed to clarify the relationship between depressed symptoms and OP in cancer patients across various ages and genders. This study identified a W-shaped correlation between BMD and depression scores among cancer survivors through smoothed curve fitting. The relationship was particularly pronounced in the elderly demographic.
This study’s limitations encompass the challenge of establishing a direct causal association between depressed symptoms and OP in cancer patients due to its cross-sectional methodology. We intend to pursue case–control studies in the future to investigate the causal link between these two factors. The impact of various malignancies on the association between OP and depressed symptoms necessitates more comprehensive data about distinct cancer kinds; the relatively uniform NHANES cancer-related data fails to provide specific information on cancer severity and stage. Cancer treatments may influence the correlation between depressed symptoms and OP in cancer survivors, and the NHANES database lacks medication data for cancer survivors, so we cannot dismiss the potential impact of anticancer drugs on the findings. Our team aims to gather more comprehensive patient data in the future to further explore the correlation between OP and depressed symptoms in cancer survivors with varying severity and drug regimes. A comprehensive cohort research will be undertaken to examine the correlation between variations in depression scale scores and OP in cancer survivors, therefore establishing a causal link between depressed symptoms and OP in this population. Despite the study’s consideration of many features and adjustments for confounders, the influence of all confounding variables could not be entirely eradicated.
Depressive symptoms exhibited a negative correlation with OP in cancer survivors. Communicating this finding to patients may alleviate the stress associated with concurrent illnesses and indirectly offer psychological solace to cancer survivors. The negative correlation between depressive symptoms and OP in cancer survivors was more pronounced in female patients, whereas it was not significant in male patients. Therefore, greater attention should be directed toward male cancer survivors in efforts to prevent the co-morbidity of depressive symptoms and OP.
5 Conclusion
This study found that depressive symptoms were negatively correlated with OP in cancer patients, which may be related to the population of cancer patients, and this effect was more significant in the elderly population and the female population. There was a W-shaped relationship between BMD and depressive scores, and the W-shaped relationship was more significant in the elderly population.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SH: Data curation, Methodology, Writing – original draft, Writing – review & editing. FX: Supervision, Writing – review & editing. GZ: Conceptualization, Writing – review & editing. YZ: Validation, Writing – review & editing. LS: Methodology, Project administration, Validation, Writing – review & editing. LZ: Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study is funded by the Scientific and Technological Innovation project of the China Academy of Chinese Medical Sciences (CI2021A03101) and High Level Chinese Medical Hospital Promotion Project (No. HLCMHPP2023046).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Compston, JE, McClung, MR, and Leslie, WD. Osteoporosis. Lancet (Lond Engl). (2019) 393:364–76. doi: 10.1016/S0140-6736(18)32112-3
2. Anam, AK, and Insogna, K. Update on osteoporosis screening and management. Med Clin North Am. (2021) 105:1117–34. doi: 10.1016/j.mcna.2021.05.016
3. Salari, N, Ghasemi, H, Mohammadi, L, Behzadi, MH, Rabieenia, E, Shohaimi, S, et al. The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg Res. (2021) 16:609. doi: 10.1186/s13018-021-02772-0
4. Xiao, PL, Cui, AY, Hsu, CJ, Peng, R, Jiang, N, Xu, XH, et al. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: a systematic review and meta-analysis. Osteoporos Int. (2022) 33:2137–53. doi: 10.1007/s00198-022-06454-3
5. Shen, L, Gao, C, Hu, S, Kang, D, Zhang, Z, Xia, D, et al. Using artificial intelligence to diagnose osteoporotic vertebral fractures on plain radiographs. J Bone Miner Res. (2023) 38:1278–87. doi: 10.1002/jbmr.4879
6. Farr, JN, Rowsey, JL, Eckhardt, BA, Thicke, BS, Fraser, DG, Tchkonia, T, et al. Independent roles of estrogen deficiency and cellular senescence in the pathogenesis of osteoporosis: evidence in young adult mice and older humans. J Bone Miner Res Off J Am Soc Bone Miner Res. (2019) 34:1407–18. doi: 10.1002/jbmr.3729
7. Xiao, Y-Z, Yang, M, Xiao, Y, Guo, Q, Huang, Y, Li, C-J, et al. Reducing hypothalamic stem cell senescence protects against aging-associated physiological decline. Cell Metab. (2020) 31:534–548.e5. doi: 10.1016/j.cmet.2020.01.002
8. Farr, JN, and Khosla, S. Cellular senescence in bone. Bone. (2019) 121:121–33. doi: 10.1016/j.bone.2019.01.015
9. Lyu, Z, Hu, Y, Guo, Y, and Liu, D. Modulation of bone remodeling by the gut microbiota: a new therapy for osteoporosis. Bone Res. (2023) 11:31. doi: 10.1038/s41413-023-00264-x
10. Li, C-J, Xiao, Y, Sun, Y-C, He, W-Z, Liu, L, Huang, M, et al. Senescent immune cells release grancalcin to promote skeletal aging. Cell Metab. (2021) 33:1957–1973.e6. doi: 10.1016/j.cmet.2021.08.009
11. Yang, Y-Y, Zhou, Y-M, Xu, J-Z, Sun, L-H, Tao, B, Wang, W-Q, et al. Lgr4 promotes aerobic glycolysis and differentiation in osteoblasts via the canonical Wnt/β-catenin pathway. J Bone Miner Res Off J Am Soc Bone Miner Res. (2021) 36:1605–20. doi: 10.1002/jbmr.4321
12. McCarron, RM, Shapiro, B, Rawles, J, and Luo, J. Depression. Ann Intern Med. (2021) 174:ITC65–ITC80. doi: 10.7326/AITC202105180
13. World Health Organization. (2023). Depression (depressive disorder) [Fact sheet]. Available online at: https://www.who.int/news-room/fact-sheets/detail/depression.
14. LeMoult, J, Humphreys, KL, Tracy, A, Hoffmeister, J-A, Ip, E, and Gotlib, IH. Meta-analysis: exposure to early life stress and risk for depression in childhood and adolescence. J Am Acad Child Adolesc Psychiatry. (2020) 59:842–55. doi: 10.1016/j.jaac.2019.10.011
15. Lu, J, Xu, X, Huang, Y, Li, T, Ma, C, Xu, G, et al. Prevalence of depressive disorders and treatment in China: a cross-sectional epidemiological study. Lancet Psychiatry. (2021) 8:981–90. doi: 10.1016/S2215-0366(21)00251-0
16. Miziak, B, Chrościńska-Krawczyk, M, and Czuczwar, SJ. An update on the problem of osteoporosis in people with epilepsy taking antiepileptic drugs. Expert Opin Drug Saf. (2019) 18:679–89. doi: 10.1080/14740338.2019.1625887
17. Ma, M, Liu, X, Jia, G, Liu, Z, Zhang, K, He, L, et al. The association between depression and bone metabolism: a US nationally representative cross-sectional study. Arch Osteoporos. (2022) 17:113. doi: 10.1007/s11657-022-01154-1
18. Chen, K, Wang, T, Tong, X, Song, Y, Hong, J, Sun, Y, et al. Osteoporosis is associated with depression among older adults: a nationwide population-based study in the USA from 2005 to 2020. Public Health. (2024) 226:27–31. doi: 10.1016/j.puhe.2023.10.022
19. Bener, A, Saleh, NM, and Bhugra, D. Depressive symptoms and bone mineral density in menopause and postmenopausal women: a still increasing and neglected problem. J Fam Med Prim Care. (2016) 5:143–9. doi: 10.4103/2249-4863.184640
20. Pineda-Moncusí, M, Servitja, S, Casamayor, G, Cos, ML, Rial, A, Rodriguez-Morera, J, et al. Bone health evaluation one year after aromatase inhibitors completion. Bone. (2018) 117:54–9. doi: 10.1016/j.bone.2018.09.010
21. Hung, S-C, Liao, K-F, Hung, H-C, Lin, C-L, Lee, P-C, Hung, S-J, et al. Tamoxifen use correlates with increased risk of hip fractures in older women with breast cancer: a case-control study in Taiwan. Geriatr Gerontol Int. (2019) 19:56–60. doi: 10.1111/ggi.13568
22. Bray, F, Laversanne, M, Weiderpass, E, and Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. (2021) 127:3029–30. doi: 10.1002/cncr.33587
23. Chen, S, Cao, Z, Prettner, K, Kuhn, M, Yang, J, Jiao, L, et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. (2023) 9:465–72. doi: 10.1001/jamaoncol.2022.7826
24. Yuan, T, Zhou, Y, Wang, T, Li, Y, and Wang, Y. Impact research of pain nursing combined with hospice care on quality of life for patients with advanced lung cancer. Medicine. (2024) 103:e37687. doi: 10.1097/MD.0000000000037687
25. Kane, CM, Hoskin, P, and Bennett, MI. Cancer induced bone pain. BMJ (Clin Res Ed). (2015) 350:h315. doi: 10.1136/bmj.h315
26. Lee, YK, Lee, EG, Kim, HY, Lee, Y, Lee, SM, Suh, DC, et al. Osteoporotic fractures of the spine, hip, and other locations after adjuvant endocrine therapy with aromatase inhibitors in breast cancer patients: a meta-analysis. J Korean Med Sci. (2020) 35:e403. doi: 10.3346/jkms.2020.35.e403
27. Kanis, JA, McCloskey, EV, Johansson, H, Oden, A, Melton, LJ, and Khaltaev, N. A reference standard for the description of osteoporosis. Bone. (2008) 42:467–75. doi: 10.1016/j.bone.2007.11.001
28. Malmir, H, Mirzababaei, A, Moradi, S, Rezaei, S, Mirzaei, K, and Dadfarma, A. Metabolically healthy status and BMI in relation to depression: a systematic review of observational studies. Diabetes Metab Syndr. (2019) 13:1099–103. doi: 10.1016/j.dsx.2019.01.027
29. Tang, G, Feng, L, Pei, Y, Gu, Z, Chen, T, and Feng, Z. Low BMI, blood calcium and vitamin D, kyphosis time, and outdoor activity time are independent risk factors for osteoporosis in postmenopausal women. Front Endocrinol. (2023) 14:1154927. doi: 10.3389/fendo.2023.1154927
30. Wu, Z, Yue, Q, Zhao, Z, Wen, J, Tang, L, Zhong, Z, et al. A cross-sectional study of smoking and depression among US adults: NHANES (2005-2018). Front Public Health. (2023) 11:1081706. doi: 10.3389/fpubh.2023.1081706
31. Jing, Z, Li, Y, Zhang, H, Chen, T, Yu, J, Xu, X, et al. Tobacco toxins induce osteoporosis through ferroptosis. Redox Biol. (2023) 67:102922. doi: 10.1016/j.redox.2023.102922
32. Gopinath, V. Osteoporosis. Med Clin North Am. (2023) 107:213–25. doi: 10.1016/j.mcna.2022.10.013
33. Liu, J, Liu, Y, Ma, W, Tong, Y, and Zheng, J. Temporal and spatial trend analysis of all-cause depression burden based on global burden of disease (GBD) 2019 study. Sci Rep. (2024) 14:12346. doi: 10.1038/s41598-024-62381-9
34. Onizuka, N, and Onizuka, T. Disparities in osteoporosis prevention and care: understanding gender, racial, and ethnic dynamics. Curr Rev Musculoskelet Med. (2024) 17:365–72. doi: 10.1007/s12178-024-09909-8
35. Guo, X, She, Y, Liu, Q, Qin, J, Wang, L, Xu, A, et al. Osteoporosis and depression in perimenopausal women: from clinical association to genetic causality. J Affect Disord. (2024) 356:371–8. doi: 10.1016/j.jad.2024.04.019
36. Oh, H, Pickering, TA, Martz, C, Lincoln, KD, Breslau, J, and Chae, D. Ethno-racial differences in anxiety and depression impairment among emerging adults in higher education. SSM Popul Health. (2024) 26:101678. doi: 10.1016/j.ssmph.2024.101678
37. Kjeldgaard, HK, Omsland, TK, Skirbekk, VF, O’flaherty, M, Meyer, HE, and Holvik, K. Forecasting the burden of hip fracture in Norway towards 2050 by educational level: the norwegian epidemiologic osteoporosis studies (NOREPOS). Scand J Public Health. (2025):14034948251325760. doi: 10.1177/14034948251325760
38. Azizi, H, Esmaeili, ED, Bastani, P, Vaezi, M, Sarbazi, E, and Khodamoradi, F. Prevalence of and risk factors for early marriage and the correlations with depressive symptoms in northwest of Iran. Soc Psychiatry Psychiatr Epidemiol. (2025). doi: 10.1007/s00127-025-02840-4
39. Sabel, CE, Pedersen, CB, Antonsen, S, Webb, RT, and Horsdal, HT. Changing neighborhood income deprivation over time, moving in childhood, and adult risk of depression. JAMA Psychiatry. (2024) 81:919–27. doi: 10.1001/jamapsychiatry.2024.1382
40. Liu, Y, Huang, S-Y, Liu, D-L, Zeng, X-X, Pan, X-R, and Peng, J. Bidirectional relationship between diabetes mellitus and depression: mechanisms and epidemiology. World J Psychiatry. (2024) 14:1429–36. doi: 10.5498/wjp.v14.i10.1429
41. Chen, F, Wang, P, Dai, F, Zhang, Q, Ying, R, Ai, L, et al. Correlation between blood glucose fluctuations and osteoporosis in type 2 diabetes mellitus. Int J Endocrinol. (2025) 2025:8889420. doi: 10.1155/ije/8889420
42. Ji, W, Pan, B, Chen, X, Lao, Z, Yang, W, and Qian, Y. Mendelian randomization studies of risk and protective factors for osteoporosis: a systematic review and meta-analysis. Front Endocrinol. (2024) 15:1486188. doi: 10.3389/fendo.2024.1486188
43. Cizza, G, Primma, S, and Csako, G. Depression as a risk factor for osteoporosis. Trends Endocrinol Metab. (2009) 20:367–73. doi: 10.1016/j.tem.2009.05.003
44. Keshishi, D, Makunts, T, and Abagyan, R. Common osteoporosis drug associated with increased rates of depression and anxiety. Sci Rep. (2021) 11:23956. doi: 10.1038/s41598-021-03214-x
45. Shi, L, Zhou, X, Gao, Y, Li, X, Fang, R, and Deng, X. Evaluation of the correlation between depression and physical activity among older persons with osteoporosis: a cross-sectional study. Front Psych. (2023) 14:1193072. doi: 10.3389/fpsyt.2023.1193072
46. Shin, MK, Kim, H, Choi, S-H, Kim, B-J, and Kwon, O. Sex-specific bi-directional association between osteoporosis and depression from the national representative data of South Korea. Sci Rep. (2022) 12:9500. doi: 10.1038/s41598-022-13401-z
47. Mwobobia, J, White, MC, Osazuwa-Peters, OL, Adjei Boakye, E, Abouelella, DK, Barnes, JM, et al. Depression, non-medical pain prescriptions, and suicidal behavior in cancer survivors. J Cancer Surviv. (2025). doi: 10.1007/s11764-024-01740-x
48. VanderWalde, A, and Hurria, A. Aging and osteoporosis in breast and prostate cancer. CA Cancer J Clin. (2011) 61:139–56. doi: 10.3322/caac.20103
49. Iaconisi, GN, Mancini, R, Ricci, V, Donati, D, Sconza, C, Marvulli, R, et al. Biochemical mechanisms and rehabilitation strategies in osteoporosis-related pain: a systematic review. Clin Pract. (2024) 14:2737–58. doi: 10.3390/clinpract14060216
50. Remelli, F, Ferrara, MC, Triolo, F, Belvederi Murri, M, Caruso, G, Bellelli, G, et al. Depression and functional recovery after hip fracture in community-dwelling older adults. J Frailty Aging. (2024) 13:470–3. doi: 10.14283/jfa.2024.67
51. Liu, Q, Wang, X, Kong, X, Wang, Z, Zhu, M, Ren, Y, et al. Subsequent risk of suicide among 9,300,812 cancer survivors in US: a population-based cohort study covering 40 years of data. Eclinicalmedicine. (2022) 44:101295. doi: 10.1016/j.eclinm.2022.101295
52. Liang, H, Zhao, J, and Tian, T. Pharmacological interventions for glucocorticoid-induced osteoporosis: an umbrella review. Horm Metab Res. (2023) 55:511–9. doi: 10.1055/a-2112-1596
53. Tavares, DF, Suen, P, Moreno, DH, Vieta, E, Moreno, RA, and Brunoni, AR. Distractibility, anxiety, irritability, and agitation symptoms are associated with the severity of depressive and manic symptoms in mixed depression. Rev Bras Psiquiatr. (2022) 44:576–83. doi: 10.47626/1516-4446-2022-2606
54. Holt, A, Khan, MA, Gujja, S, and Govindarajan, R. Utilization of bone densitometry for prediction and administration of bisphosphonates to prevent osteoporosis in patients with prostate cancer without bone metastases receiving antiandrogen therapy. Cancer Manag Res. (2015) 7:13–8. doi: 10.2147/CMAR.S74116
55. Kozopas, V, Humeniuk, V, Semenova, Y, Peana, M, Butnariu, M, Lysiuk, R, et al. Osteoporosis: relevance of biomolecules for diagnosis and treatment. Curr Med Chem. (2025). doi: 10.2174/0109298673358991250210111956
56. Geng, D, Li, X, and Shi, Y. Effect of exercise intervention on health-related quality of life in middle-aged and older people with osteoporosis: a systematic review and meta-analysis. PeerJ. (2025) 13:e18889. doi: 10.7717/peerj.18889
Keywords: cancer survivor, depression, bone density, osteoporosis, cross-sectional study
Citation: Hou S, Xu F, Zong G, Zheng Y, Shi L and Zhai L (2025) Association between depression and osteoporosis in a population of cancer survivors: results from the NHANES 2005–2020. Front. Med. 12:1515435. doi: 10.3389/fmed.2025.1515435
Edited by:
Morteza Taheri, University of Tehran, IranReviewed by:
Maedeh Ahmadpour, University of Tehran, IranRoya Khorrami Estakhri, Guilan University of Medical Sciences, Iran
Copyright © 2025 Hou, Xu, Zong, Zheng, Shi and Zhai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengquan Xu, eHVmZW5ncXVhbkBnYW15eS5jbg==
†These authors have contributed equally to this work and share first authorship
 Shuchen Hou
Shuchen Hou Fengquan Xu
Fengquan Xu Guanhua Zong
Guanhua Zong Yu Zheng2
Yu Zheng2