
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med., 17 March 2025
Sec. Hematology
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1515002
 Yaqing Feng1*†
Yaqing Feng1*† Hongjin Wang2†
Hongjin Wang2† Lidong Zhang1
Lidong Zhang1 Jinying Gong3,4
Jinying Gong3,4 Xi Liu1
Xi Liu1 Caiqin Mu1
Caiqin Mu1 Jun Qiao1
Jun Qiao1 Haitao Meng1
Haitao Meng1 Yanfang Zhang1
Yanfang Zhang1Objective: Chronic myeloid leukemia (CML) is a malignancy driven by the BCR::ABL1fusion gene, with the e19a2 transcript being a rare variant, accounting for 0.4% of CML cases. Patients with the e19a2 transcript often show poor response to first-line treatment with imatinib, and no standard therapy has been established for this subtype.
Methods: We report a case of a 28-year-old female with e19a2-positive CML. The patient exhibited poor response and tolerance to dasatinib. After 6 months, she achieved partial cytogenetic response (PCyR) but developed grade 3 pleural effusion. Following treatment discontinuation and prednisone therapy, the patient continued dasatinib (80 mg/d). At 12 months, the patient achieved complete cytogenetic response (CCyR), but BCR::ABL1 levels remained suboptimal, with recurrent pleural effusion. The patient was then switched to flumatinib (600 mg/d), achieving major molecular response (MMR) at 6 months and deep complete molecular response (MR4.5) at 24 months, with good tolerance.
Conclusion: Flumatinib demonstrated excellent deep molecular response and good tolerability in e19a2-positive CML patients, suggesting that it may be one of the preferred treatment options for such patients.
The BCR::ABL1 fusion gene is the primary molecular marker of chronic myeloid leukemia (CML) (1). The typical BCR::ABL1 transcripts are e13a2 and e14a2, which encode the p210 BCR::ABL1 protein. However, e19a2 is a rare variant that encodes the p230 BCR::ABL1 protein. The breakpoint cluster region (BCR) is located between exons 17 and 20 in the μ region, producing a fusion protein with a molecular weight of 230 kDa (2). Since Saglio et al. first reported the e19a2 variant in 1990, the number of related cases has gradually increased (3). Before the advent of tyrosine kinase inhibitors (TKIs), the treatment of CML mainly relied on allogeneic stem cell transplantation, chemotherapy, and interferon. However, chemotherapy and interferon treatment could only achieve limited hematologic responses, while allogeneic stem cell transplantation was limited by the availability of matched donors and treatment-related complications.
The introduction of the first-generation TKI, imatinib, in 2001 significantly improved the prognosis of CML patients. However, approximately 20% of patients develop resistance, leading to the emergence of second-generation TKIs (4). Although second-generation TKIs often provide deeper molecular responses (MR), they are also associated with adverse events such as pleural effusion, cytopenias, and vascular spasms or occlusive events, as well as pulmonary arterial hypertension (1). This case report discusses an e19a2-positive CML patient who showed poor response and tolerability to dasatinib but achieved a favorable molecular response following treatment with flumatinib.
A 28-year-old female patient, during the Chinese New Year in 2020, experienced a gradual decrease in appetite while staying at home due to the COVID-19 pandemic, resulting in a weight loss of approximately 10 kg by July 2020. Subsequently, the patient developed worsening fatigue and nausea, accompanied by vomiting after eating, and eventually sought medical attention. On examination: anemia-like appearance, spleen tip 10 cm below the rib margin. No enlarged superficial lymph nodes were palpated, and no suspicious masses were detected. Laboratory tests showed: white blood cell count of 314.45 × 109 g/L, with eosinophils at 1%, basophils at 11%, hemoglobin at 86 g/L, and platelet count at 524 × 109 g/L. Peripheral blood analysis showed 5% blast cells. Ultrasound revealed splenomegaly (thickness 6.5 cm, length 22.8 cm), with Sokal risk score indicating intermediate risk (1.18), EUTOS score indicating high risk (117.0), and ELTS score indicating intermediate risk (1.76) (see Table 1). Ultrasound of the spleen revealed a thickness of 6.5 cm and length of 22.8 cm. Bone marrow aspiration showed hypercellularity with 7% blast cells (see Figure 1A). Neutrophil alkaline phosphatase (N-ALP) positivity rate was 2.00% (see Figure 1B). Fluorescence in situ hybridization (FISH) showed BCR::ABL1 fusion signals in 40% of cells (see Figures 1G,H). Cytogenetic analysis revealed a karyotype of 46, XX, t(9; 22)(q34; q11.2)/47, XX, t(9; 22), +der (22)t(9; 22) (see Figure 1I).
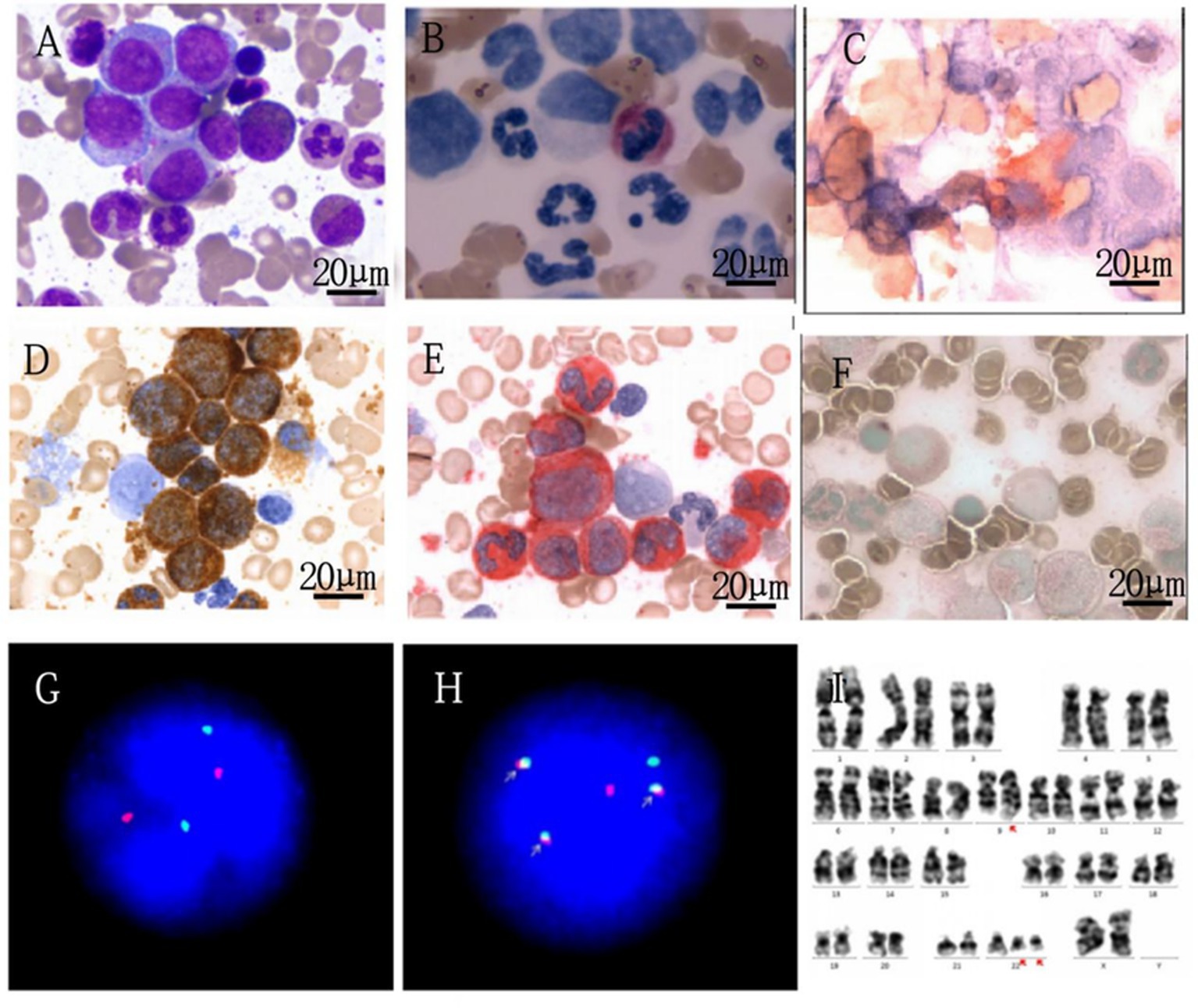
Figure 1. Laboratory examination of the patient. (A) Patient's bone marrow cell morphological analysis (1000x). (B) Patient's cytochemical staining (1000x). (C) CD41 (1000x). (D) Peroxidase staining (1000x). (E) Specific esterase staining (1000x). (F) Nonspecific esterase staining (1000x). (G,H) FISH analysis–normal control and patient’s image, respectively.
Using a dual color and dual fusion BCR-ABL1 probe, the BCR (22q11) gene is marked with green, the ABL1 (9q34) gene is marked with red, and the BCR::ABL1 fusion gene is represented by a yellow or red, green superimposed signal. The negative signal is a 2 red and 2 green signal modes. The ISCN result of this patient is nuc ish (ABL1, BCR) × 3 (ABL1 con BCR × 2) [221/500]/(ABL1, BCR) × 4 (ABL1 con BCR × 3) [2000/500], indicating a positive detection rate of 84.2% for the BCR-ABL1 fusion gene. Among them, 40% of positive cell fusion signals increased with the addition of a fusion copy. ABL1 (9q34), Abelson murine leukemia viral oncogene homolog 1 is located on chromosome; 9q34BCR (22q11), breakpoint cluster region gene is located on chromosome 22q11; FISH, fluorescence in situ hybridization.
I Chromosome Analysis Karyotype Description 46,XX,t(9;22)(q34;q11.2) (5)/47,XX,t(9;22),+der(22)t(9;22) (6).
Bone marrow biopsy indicated active marrow proliferation with fibrosis, and reticulin staining was graded as MF-2 (see Figure 2). The BCR/ABL P210 fusion gene was negative, the BCR/ABL P190 fusion gene was negative, and the BCR/ABL P230 fusion gene was positive, confirming the diagnosis of e19a2-positive CML.
In July 2020, the patient began treatment with dasatinib at a dose of 100 mg/d. After 3 months of treatment, the patient achieved complete hematological response (CHR), but no cytogenetic response (CyR) was observed, with BCR::ABL1 > 10%. After 6 months, the patient achieved partial cytogenetic response (PCyR) with BCR::ABL1 < 10%, but grade 3 pleural effusion developed. The spleen thickness reduced to 4.5 cm and the length reduced to 16 cm. The tuberculin test was negative, and no acid-fast bacilli or fungi were detected. Liquid-based cytology showed proliferation of mesothelial cells and lymphocytes, but no malignant changes were observed. The pleural effusion was attributed to a side effect of dasatinib. The patient discontinued dasatinib treatment and was given diuretics and prednisone (40 mg/d). According to the 2020 European LeukemiaNet (ELN) guidelines, the treatment evaluation was in the warning zone, and a change in therapy was recommended. However, the patient chose to continue dasatinib treatment with a dose reduction to 80 mg/d. After 12 months, the patient achieved complete cytogenetic response (CCyR) with BCR::ABL1 reduced to 0.17%, but did not reach the optimal response. The spleen thickness further reduced to 3.8 cm and the length reduced to 11 cm. The patient developed grade 3 pleural effusion again, along with skin itching, cytopenia, and iron-deficiency anemia (possibly due to excessive menstrual bleeding). She resumed prednisone therapy, underwent pleural drainage, and received iron supplementation. The pleural effusion resolved after discontinuing dasatinib.
In July 2021, the patient switched to treatment with flumatinib at a dose of 600 mg/d. After 6 months, the patient achieved major molecular response (MMR), with BCR::ABL1 at 0.1%. After 18 months, the patient reached MR4.0, and at 24 months, BCR::ABL1 was undetectable, indicating achievement of MR4.5. During the early phase of flumatinib treatment, the patient experienced mild gastrointestinal side effects, which resolved quickly, and she tolerated the treatment well without recurrence of pleural effusion. The molecular response remained stable. At the same time, she resumed her daily activities, and her quality of life significantly improved.
The patient expressed satisfaction with the treatment outcome.
CML is a malignancy caused by clonal mutations of hematopoietic stem cells, characterized by the BCR::ABL1 fusion gene. CML is usually detected during physical exams or blood tests, and the presence of Ph chromosome abnormalities is confirmed by routine cytogenetic analysis, fluorescence in situ hybridization (FISH), or molecular studies (1). The typical BCR::ABL1 transcripts are e13a2 and e14a2, which encode the p210 fusion protein. Rare transcripts such as e19a2, e14a3, e1a2, and e13a3 account for 0.4, 0.3, 0.9, and 0.1% of CML cases, respectively. Other rare transcripts, including e1a3, e6a2, and e2a2, have also been reported (7). The e19a2 transcript encodes the p230 fusion protein, which differs clinically from the more common p210 isoform. Initially, the e19a2 subtype was often observed in neutrophilic CML, presenting with a benign clinical course. However, it has later been found predominantly in typical CML patients, some of whom exhibit more aggressive clinical manifestations. Patients with the p210 transcript tend to reach treatment goals more rapidly and have a lower treatment failure rate. In contrast, patients with the p230 transcript generally take longer to respond and have a higher risk of treatment failure. However, these patients show better responses to second-generation TKIs (2GTKIs) (2).
In the early stages, CML patients with the e19a2 transcript were treated with interferon and hydroxyurea, but these treatments only induced hematological responses, and most patients died due to disease progression. These patients also showed poor responses to the first-generation TKI imatinib, with most failing to achieve or maintain deep MR, leading to continued disease progression (2). Studies comparing patients with the p230 transcript to those with the p210 transcript revealed significant differences in treatment outcomes. The 1-year CCyR rate for p230 was 44.4%, while for p210 it was 87.9%; the 1-year MMR rate for p230 was 48.6%, compared to 6.3% for p210; and the 2-year event-free survival (EFS) rate for p230 was 94.4%, compared to 69.1% for p210 (8). These findings are consistent with another study’s results (5).
Due to the lower efficacy of imatinib in achieving MMR, 2GTKIs, such as nilotinib and dasatinib, have become the preferred first-line treatment options. Currently, the guidelines recommend the use of imatinib, dasatinib, bosutinib, and nilotinib as first-line TKIs for CML (1). Although the number of reported cases is limited, some studies show that second-generation TKIs can induce deeper molecular responses more quickly. In one study, 10 patients receiving nilotinib (300 mg daily, divided into two doses) as first-line treatment showed good therapeutic effects: one patient achieved MR4.5 within 3 months and maintained it for 31 months, while the other patients achieved MMR within 2 to 6 months. Similarly, two patients treated with dasatinib (100 mg daily) reached MMR within 6 months, with the duration of MMR ranging from 3 months to 43 months (9, 10).
Our patient initially received dasatinib treatment and achieved CCyR at 12 months with BCR::ABL1 < 1%. However, due to suboptimal response, recurrent pleural effusion, rash, and poor tolerance, the treatment was switched to flumatinib. After 6 months of flumatinib treatment, the patient achieved MMR and reached MR4.5 at 24 months, with only mild side effects. This response is consistent with the ELN guidelines for CML treatment (11).The patient’s laboratory test results and treatment response (see Table 1 and Figure 3).
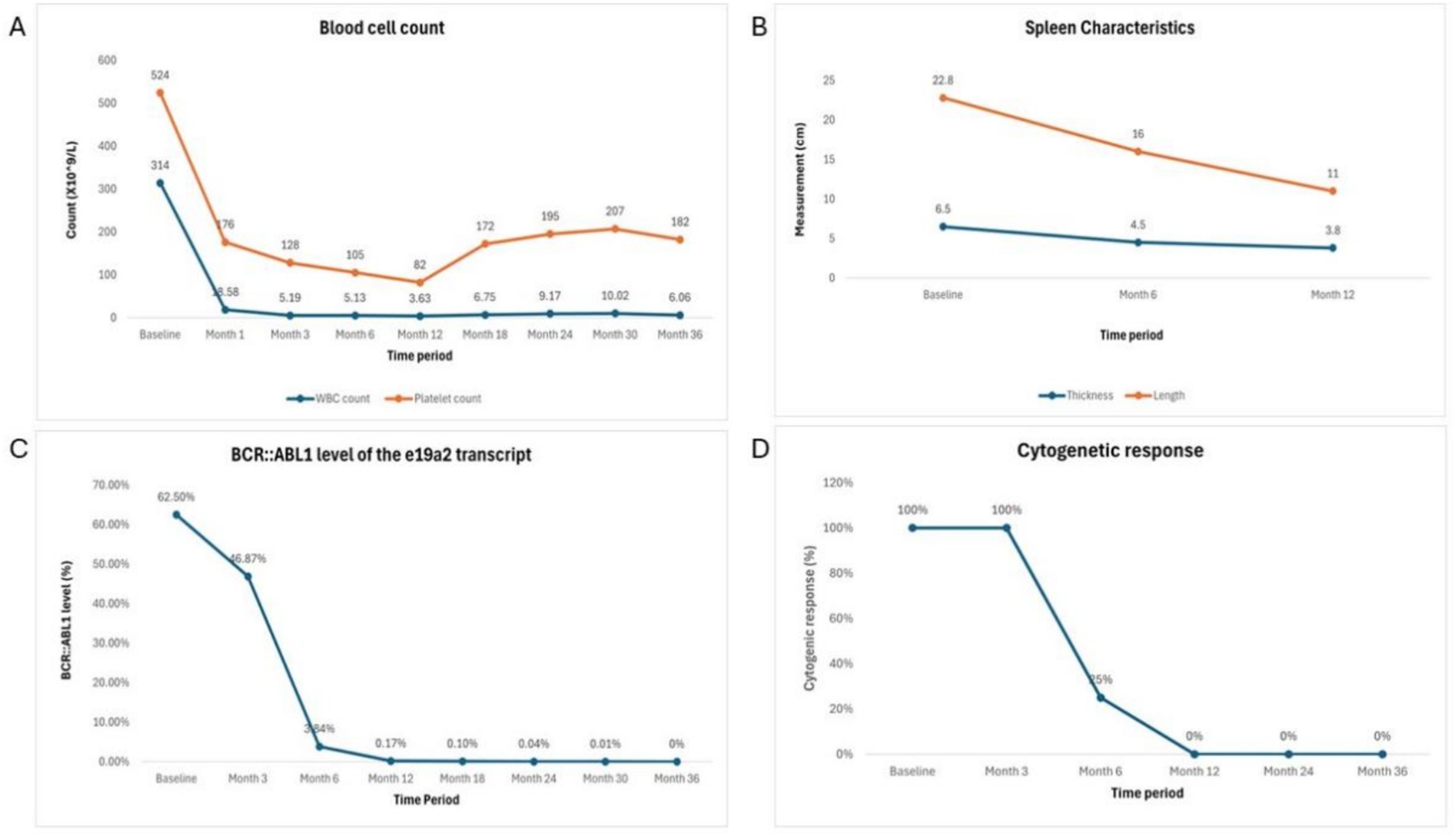
Figure 3. Laboratory test results and treatment response over treatment period. (A) Blood cell counts. (B) Spleen characteristics. (C) BCR::ABL1 level of e19a2 transcript. (D) Cytogenic response.
Due to its trifluoromethyl and imidazole ring structure, nilotinib demonstrates better lipophilicity and ATP-binding affinity, but its long-term use is associated with cardiovascular risks (12). Dasatinib, as a dual inhibitor of ABL and SRC kinases, has a lower specificity for the shape and charge of the binding sites, which makes it more likely to induce pleural effusion with lymphocytic infiltration. These immune-mediated effects are thought to be caused by SRC inhibition (13). The advent of the third-generation TKI, ponatinib, provides hope for overcoming the T315I mutation, but the second-phase PACE trial reported a cumulative incidence of arterial occlusive events of 31% in chronic-phase CML patients (6). Therefore, although ponatinib remains effective, its use requires careful risk assessment.
Flumatinib, optimized from imatinib, binds more strongly to the hydrophobic pocket of the tyrosine kinase domain, enhancing its stability and efficacy (14). In vitro studies have shown that flumatinib exhibits excellent inhibitory activity against BCR::ABL1 kinase point mutations (15). In a phase III clinical trial (FESTnd) evaluating newly diagnosed CML patients, flumatinib was mainly associated with mild adverse events (AEs) such as diarrhea, which typically lasted only 1–2 days. The incidence of grade 3 or higher adverse events was low, including grade 3 rash, diarrhea, and thrombocytopenia (all <1.5%). Rare cardiovascular events, such as atrial fibrillation or stroke (in individual cases) (16, 17), were also reported. In our case, the patient responded rapidly and completely to flumatinib, with no severe side effects. Compared to other second-generation TKIs, flumatinib provides excellent efficacy, overall good tolerability, and fewer serious adverse events (16, 17), making it a feasible treatment option for patients with underlying cardiovascular disease or poor physical condition. A summary of related literature is provided in Table 2.
Since this is the first detailed case report describing the use of flumatinib in treating CML patients with the rare e19a2 transcript, further research is required in larger sample cohorts to validate its efficacy and safety, addressing the limitations of single-case studies.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by The Third People’s Hospital of Datong. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YF: Writing – original draft. LZ: Data curation, Writing – review & editing. JG: Data curation, Writing – review & editing. XL: Data curation, Writing – review & editing. CM: Data curation, Writing – review & editing. JQ: Data curation, Writing – review & editing. HM: Data curation, Writing – review & editing. YZ: Supervision, Writing – review & editing. HW: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
JG was employed by Tianjin Union Precision Medical Diagnostics Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Jabbour, E, and Kantarjian, H. Chronic myeloid leukemia: 2025 update on diagnosis, therapy, and monitoring. Am J Hematol. (2024) 99:2191–212. doi: 10.1002/ajh.27443
2. Molica, M, Abruzzese, E, and Breccia, M. Prognostic significance of transcript-type BCR - ABL1 in chronic myeloid leukemia. Mediterr J Hematol Infect Dis. (2020) 12:e2020062. doi: 10.4084/mjhid.2020.062
3. Saglio, G, Guerrasio, A, Rosso, C, Zaccaria, A, Tassinari, A, Serra, A, et al. New type of Bcr/Abl junction in Philadelphia chromosome-positive chronic myelogenous leukemia. Blood. (1990) 76:1819–24. doi: 10.1182/blood.V76.9.1819.1819
4. Huang, X, Jiang, Q, Hu, J, Li, J, Jin, J, Meng, F, et al. Four-year follow-up of patients with imatinib-resistant or intolerant chronic myeloid leukemia receiving dasatinib: efficacy and safety. Front Med. (2019) 13:344–53. doi: 10.1007/s11684-018-0639-7
5. Qin, YZ, Jiang, Q, Jiang, H, Lai, YY, Shi, HX, Chen, WM, et al. Prevalence and outcomes of uncommon BCR-ABL1 fusion transcripts in patients with chronic myeloid leukaemia: data from a single Centre. Br J Haematol. (2018) 182:693–700. doi: 10.1111/bjh.15453
6. Cortes, JE, Kim, DW, Pinilla-Ibarz, J, le Coutre, PD, Paquette, R, Chuah, C, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. (2018) 132:393–404. doi: 10.1182/blood-2016-09-739086
7. Xue, M, Wang, Q, Huo, L, Wen, L, Yang, X, Wu, Q, et al. Clinical characteristics and prognostic significance of chronic myeloid leukemia with rare BCR-ABL1 transcripts. Leuk Lymphoma. (2019) 60:3051–7. doi: 10.1080/10428194.2019.1607329
8. Sazawal, S, Chikkara, S, Singh, K, Chaubey, R, Chandra, D, Mishra, P, et al. Chronic myeloid leukemia with a rare fusion transcript, e19a2 BCR-ABL1: a report of three cases from India. Ann Diagn Pathol. (2017) 27:24–7. doi: 10.1016/j.anndiagpath.2016.12.001
9. Greco, M, Caocci, G, and La Nasa, G. Early complete molecular response to first-line Nilotinib in two patients with chronic myeloid leukemia carrying the p230 transcript. Case Rep Hematol. (2013) 2013:871476. doi: 10.1155/2013/871476
10. Jiang, YQ, Xu, N, Liu, XL, Wang, JS, Yuan, Z, Huang, JX, et al. Clinical effect of tyrosine kinase inhibitors in the treatment of P230 chronic myeloid leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. (2021) 29:1752–6. doi: 10.19746/j.cnki.issn.1009-2137.2021.06.010
11. Hochhaus, A, Baccarani, M, Silver, RT, Schiffer, C, Apperley, JF, Cervantes, F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. (2020) 34:966–84. doi: 10.1038/s41375-020-0776-2
12. Kantarjian, HM, Hughes, TP, Larson, RA, Kim, DW, Issaragrisil, S, le Coutre, P, et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. (2021) 35:440–53. doi: 10.1038/s41375-020-01111-2
13. Hughes, TP, Laneuville, P, Rousselot, P, Snyder, DS, Rea, D, Shah, NP, et al. Incidence, outcomes, and risk factors of pleural effusion in patients receiving dasatinib therapy for Philadelphia chromosome-positive leukemia. Haematologica. (2019) 104:93–101. doi: 10.3324/haematol.2018.188987
14. Yang, M, Xi, Q, Jia, W, and Wang, X. Structure-based analysis and biological characterization of imatinib derivatives reveal insights towards the inhibition of wild-type BCR-ABL and its mutants. Bioorg Med Chem Lett. (2019) 29:126758. doi: 10.1016/j.bmcl.2019.126758
15. Yang, Y, Liu, Y, Sun, H, Meng, L, Lin, H, Chen, C, et al. Safety and efficacy of flumatinib as later-line therapy in patients with chronic myeloid leukemia. Haematologica. (2024) 109:3965–74. doi: 10.3324/haematol.2023.284892
16. Zhang, X, Xu, N, Yang, Y, Lin, H, Liu, B, du, X, et al. Comparison of the efficacy among Nilotinib, Dasatinib, Flumatinib and Imatinib in newly diagnosed chronic-phase chronic myeloid leukemia patients: a real-world multi-center retrospective study. Clin Lymphoma Myeloma Leuk. (2024) 24:e257–66. doi: 10.1016/j.clml.2024.02.008
Keywords: chronic myeloid leukemia, e19a2 transcript, second-generation tyrosine kinase inhibitor, flumatinib, molecular response
Citation: Feng Y, Wang H, Zhang L, Gong J, Liu X, Mu C, Qiao J, Meng H and Zhang Y (2025) Molecular response of a patient with e19a2-positive chronic myeloid leukemia to flumatinib: a case report and literature review. Front. Med. 12:1515002. doi: 10.3389/fmed.2025.1515002
Received: 22 October 2024; Accepted: 11 February 2025;
Published: 17 March 2025.
Edited by:
Eleni Gavriilaki, Aristotle University of Thessaloniki, GreeceReviewed by:
Oscar Gonzalez Ramella, Civil Hospital of Guadalajara, MexicoCopyright © 2025 Feng, Wang, Zhang, Gong, Liu, Mu, Qiao, Meng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaqing Feng, MzQ0MjE4NzE2NkBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.