
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 26 February 2025
Sec. Ophthalmology
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1513803
This article is part of the Research Topic Enhancing Patient Outcomes After Cataract, Corneal and Refractive Surgery View all 11 articles
Background: To evaluate refractive, visual, and patient-reported outcomes three months after bilateral implantation of a novel bi-aspheric, non-diffractive extended depth of focus (EDOF) intraocular lens (IOL) using PhaseRing technology to achieve good vision across distances with reduced dysphotopsia.
Methods: Twenty-two patients received bilateral Asqelio EDOF IOLs (AST VisionCare Inc.) and were evaluated 3 months post-surgery. The main outcomes assessed were refractive error, monocular and binocular visual acuities at distance, intermediate (67 cm) and near (40 cm), low contrast visual acuity, defocus curves, contrast sensitivity, and patient questionnaires.
Results: The average postoperative spherical equivalent was −0.31 ± 0.30 D. Astigmatism of ≤ 1.00 D was present in all eyes (100%, n = 44), with 75% (n = 33) showing astigmatism of ≤ 0.50 D. Every patient attained a corrected distance visual acuity (CDVA) of 20/25 or better and a distance-corrected intermediate visual acuity (DCIVA) of 20/32 or better. Contrast sensitivity met or exceeded normal levels under both photopic and mesopic conditions, with and without glare, except at 12 cycles per degree under mesopic conditions with glare. Light distortion index was comparable to published reports on monofocal IOLs and other non-diffractive EDOF IOLs, and lower than diffractive multifocal IOLs. Post-surgery, 90.9% (n = 20) of patients reported being satisfied with their vision. No significant visual symptoms were reported.
Conclusion: Asqelio™ EDOF IOL offers an efficient design, providing good clinical outcomes for distance and intermediate vision, while some patients reach functional levels of near vision. Its non-diffractive design minimizes dysphotopsia and reduces light distortion compared to other presbyopia-correcting IOLs.
Cataracts stand as a leading cause of blindness globally, with cataract surgery ranking among the most commonly performed procedures worldwide. After the extraction of the cataract, the implantation of an intraocular lens (IOL) is performed to compensate for the lost power of the extracted lens and at the same time correct the patient’s ametropia, which is done in some cases without waiting for the cataract to develop, in what is known as refractive lensectomy. The use of monofocal IOLs allows for the correction of the patient’s ametropia and can provide excellent vision at a single distance (typically far), but falls show at providing good vision at multiple distances (i.e., far and near). The introduction of bifocal IOLs in the 1990s revolutionized patient care by offering near vision correction alongside ametropia correction. Trifocal IOLs, emerging in the European market in 2012, further enhanced patient outcomes by incorporating an intermediate vision focus, reducing reliance on corrective eyewear across a broader range of distances.
The approval of the first extended depth of focus (EDOF) lens by the FDA in 2016 (1) marked a milestone in vision correction. Subsequently, various EDOF models have entered the international market. Numerous studies have since investigated the visual and refractive efficacy of this type of lenses (2–4). A meta-analysis contrasting trifocal and EDOF IOLs indicates that while trifocal IOLs offer performance in near vision, they tend to induce more photic phenomena (5). Variations in multifocal IOLs stem from their optical principles (6), needing enhanced optical performance in modern IOLs to optimize reading capabilities and enhance patients’ quality of life post-cataract surgery (7, 8).
The present study aims to assess the clinical performance, light distortion and patient reported outcomes three months after bilateral implantation of Asqelio™ EDOF IOL following cataract surgery or refractive lensectomy. This is the first report on the clinical outcomes after implantation with this new non-diffractive EDOF IOL design.
This prospective, single-arm observational post-marketing study was approved by the Ethics Committee of Hospital Clínico San Carlos in Madrid, Spain, and was conducted in accordance with the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all patients before their participation, and the potential consequences of the study were thoroughly explained. Study registration was also carried out at www.clinicaltrials.gov (registration number: NCT06229756).
Inclusion criteria included patients of at least 50 years of age who were submitted to cataract surgery seeking spectacle-independence and bilaterally implanted with Asqelio™ EDOF IOL (model ELIO130C), transparent intraocular media, other than the cataract preoperatively, and a potential visual acuity of 20/25 or better. Exclusion criteria included preoperatory corneal astigmatism exceeding 0.75 D, patients not providing informed consent, patients with concomitant ocular conditions, previous corneal surgery or trauma, extremely shallow anterior chamber, non-age-related cataracts, pregnancy, rubella, and those currently participating in other clinical investigations or expecting to undergo another ocular surgery during the study period.
The Asqelio™ EDOF IOL is manufactured by AST VisionCare, Inc. (previously AST Products, Inc.) (Billerica, MA, USA) via its proprietary Phase-Ring™ technology. It is a one-piece foldable posterior chamber, UV absorbing optical implantable lens with non-diffractive design for the correction of presbyopia. The lens features a bi-aspheric geometry, spherical aberration of −0.27 microns, 360-degree sharp edge and Phase-Ring™-structured design on its posterior surface to extend the depth of focus for intermediate to near distances while maintaining distance vision. It has a total diameter of 13.0 mm with an optical zone of 6.0 mm and is manufactured in a power range from −10.00 to +40.00 D in 0.50 D increments. Crafted from a hydrophobic acrylic soft material, characterized by its glistening-free properties, the lens has a refractive index of 1.5 and an Abbe number of 50.
A limbal incision of 2.2 mm was performed, followed by standard phacoemulsification using the Centurion® Vision System (Alcon Labs Inc., Fort Worth, TX, USA). After removing the cataract and polishing the posterior capsule, the capsular bag was filled with 1.0% sodium hyaluronate (Provisc™, Alcon, Fort Worth, TX, USA) to maintain the capsular space and facilitate IOL implantation. All patients were prescribed moxifloxacin 5 mg/mL (Vigamox™; Alcon), prednisolone 10 mg/mL (Pred-Forte™; Allergan, Inc., Irvine, CA, USA), and diclofenac-Lepori 1 mg/mL, administered in tapering doses over the first four weeks after surgery.
Before surgery, patients underwent comprehensive ophthalmologic examinations, including slit-lamp evaluation, measurements of logMAR uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA), subjective and objective refraction assessments, intraocular pressure measurement, funduscopy, corneal topography, and biometry using the IOLMaster® 700 (Carl Zeiss Meditec AG, Jena, Germany). The Barrett Universal II and Hoffer Q formulas were utilized for intraocular lens (IOL) calculations. The dominant eye was targeted for emmetropia, while slight myopia was aimed for in the non-dominant eye.
Three months post-implantation, patients underwent postoperative evaluations. Standard ophthalmologic assessments, including refraction and slit-lamp biomicroscopy, were conducted. Specifically, monocular and binocular logMAR UDVA, CDVA, uncorrected intermediate visual acuity (UIVA), and distance-corrected intermediate visual acuity (DCIVA) at 60 cm, as well as uncorrected near visual acuity (UNVA) and distance-corrected near visual acuity (DCNVA) at 40 cm, all measured under photopic conditions. DCNVA was also assessed monocularly and binocularly under mesopic conditions (3 cd/m2). Early treatment diabetic retinopathy study (ETDRS) charts were used for the measurements. Monocular and binocular defocus curves were generated with best distance correction using the ETDRS chart located at 4 m under photopic conditions, covering vergences from +2.00 to −5.00 D in 0.50 D increments (including 0.25 D steps between 0 and ± 0.50 D). All data were presented as mean ± standard deviation (SD) and ranges.
Binocular contrast sensitivity was tested with distance correction under photopic conditions (85 cd/m2), both with and without glare, for spatial frequencies of 3, 6, 12, and 18 cycles per degree (cpd), and under mesopic conditions (3 cd/m2) for spatial frequencies of 1.5, 3, 6, and 12 cpd, using the Clinical Trial Suite® (M&S Technologies, Inc., IL, USA). Log absolute contrast threshold values were determined for each patient, spatial frequency, and luminance level combination. Mean values and standard deviations were calculated, and corresponding contrast sensitivity values (log CS) were derived from these thresholds to plot the contrast sensitivity function.
Light distortion was determined using the Light Distortion Analyzer (LDA) system, first under monocular conditions and then binocularly. In this assessment, patients responded to small peripheral light stimuli presented around a central light source, providing feedback to the system. Based on these responses, the LDA calculated several indices that quantify the size and regularity of distortion surrounding the central light source—specifically, the distortion index, the radius of the best-fit circle, and the irregularity of the best-fit circle (9).
Patient-reported outcomes were gathered through questionnaires. The Catquest-9SF (10), a widely recognized 9-item questionnaire, was used to determine limitations in daily activities due to poor vision, selected for its documented responsiveness in cataract surgery. This questionnaire consists of nine items with four response options, ranging from 1 (“no difficulty/very satisfied”) to 4 (“very great difficulty/very dissatisfied”), along with an additional “cannot decide” option treated as missing data. Items labeled A and C1 to C7 focus on difficulty levels, while item B addresses patient satisfaction.
Subjective visual symptoms were assessed using a questionnaire based on a validated quality-of-vision instrument (11). This questionnaire explores the frequency, intensity, and bothersomeness of ten common visual symptoms: glare, halos, starbursts, foggy vision, blurred vision, distortion, double vision, fluctuations in vision, difficulty focusing, and difficulty judging distances or depth. To aid in understanding, patients were shown simulated images depicting each symptom. They were then asked to rate each symptom on frequency (from 1 “Never” to 4 “Very often”), intensity (from 1 “None” to 4 “Severe”), and bothersomeness (from 1 “None” to 4 “A lot”).
Adverse events were recorded based on both solicited inquiries and spontaneous patient comments, as well as from observations made by the investigator.
The study’s estimated sample size was determined using the highest standard deviation observed in the defocus curve of typical monocular visual acuity (12). Specifically, a standard deviation of 0.24 logMAR at +2.00 diopters blur, a 95% confidence interval, and a maximum allowable margin of error of 0.10 logMAR were applied, resulting in a minimum required sample size of 22 patients.
Categorical variables were presented as frequencies and percentages, while continuous variables were summarized using means and standard deviations. Cumulative histograms of postoperative refractive error and refractive cylinder were constructed to evaluate refractive accuracy. Additionally, a cumulative histogram of postoperative visual performance was generated to assess the efficacy of refractive correction.
A total of 22 consecutive patients were enrolled in the study, with a mean age of 67.95 ± 8.40 years (ranging from 55 to 83 years); half of them were female (n = 11, 50%). The preoperative demographic characteristics are summarized in Table 1. Standardized graphs depicting refractive and visual acuity outcomes at the three-month follow-up were constructed in line with established reporting guidelines (13).
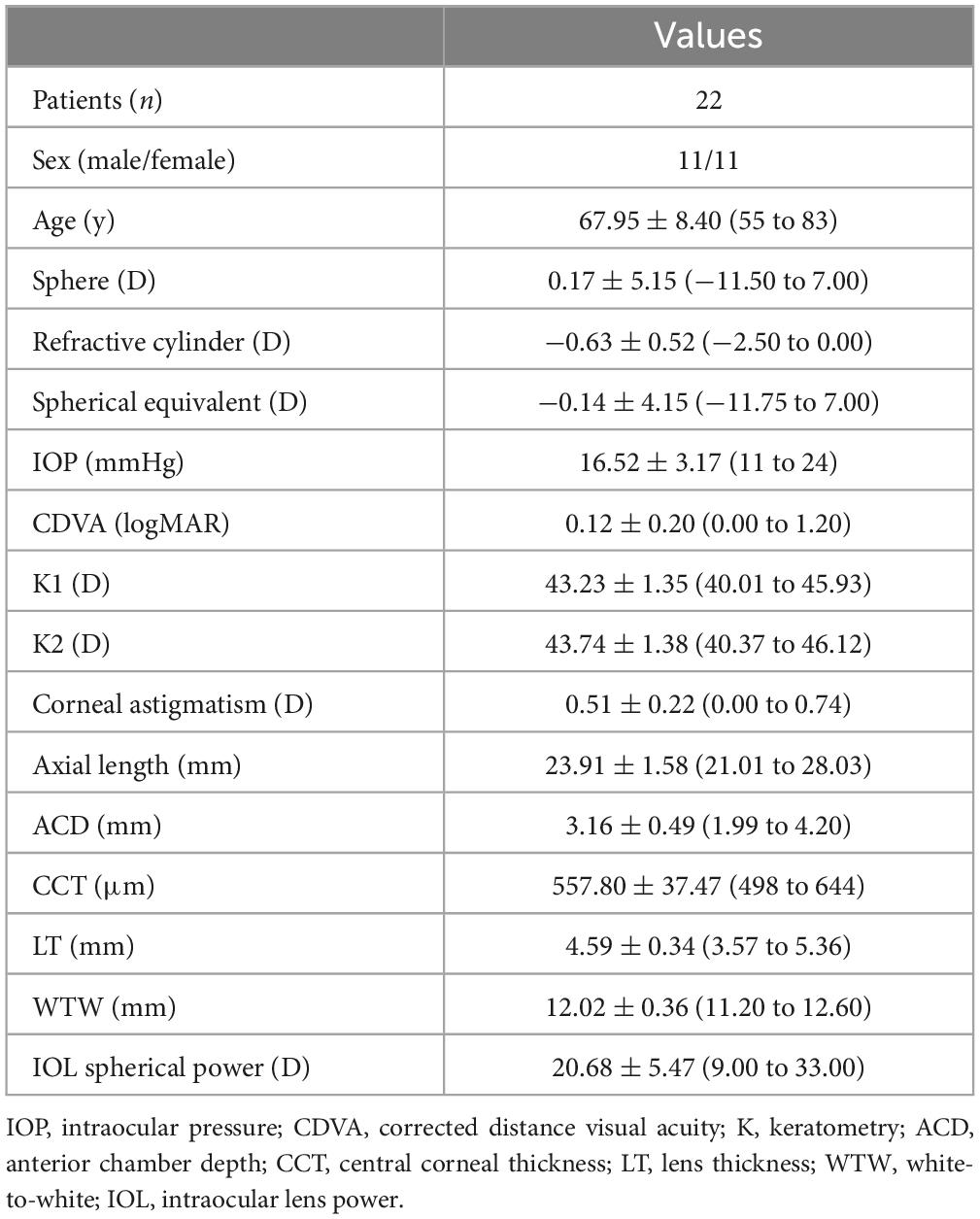
Table 1. Demographic characteristics of participants shown as means, standard deviations (SD) and ranges.
For the assessment of predictability, Figure 1 presents a histogram of postoperative spherical equivalent refraction relative to the intended target, while Figure 2 illustrates the postoperative refractive astigmatism. Concerning the spherical equivalent, the largest proportion of eyes, 43.18% (n = 19), fell within the range of −0.50 to −0.14 diopters (D), followed by 29.55% (n = 13) within the ± 0.13 D range, highlighting a high refractive accuracy, with the vast majority of patients achieving results close to the planned refraction. Overall, 97.73% (n = 43) of eyes were within ± 1.00 D of the target refraction, and 91% (n = 40) were within ± 0.50 D. The mean postoperative spherical equivalent was −0.31 ± 0.30 D, ranging from −1.50 D to +0.25 D.

Figure 1. Histogram of postoperative spherical equivalent refractive accuracy 3 months after surgery relative to the intended target refraction.
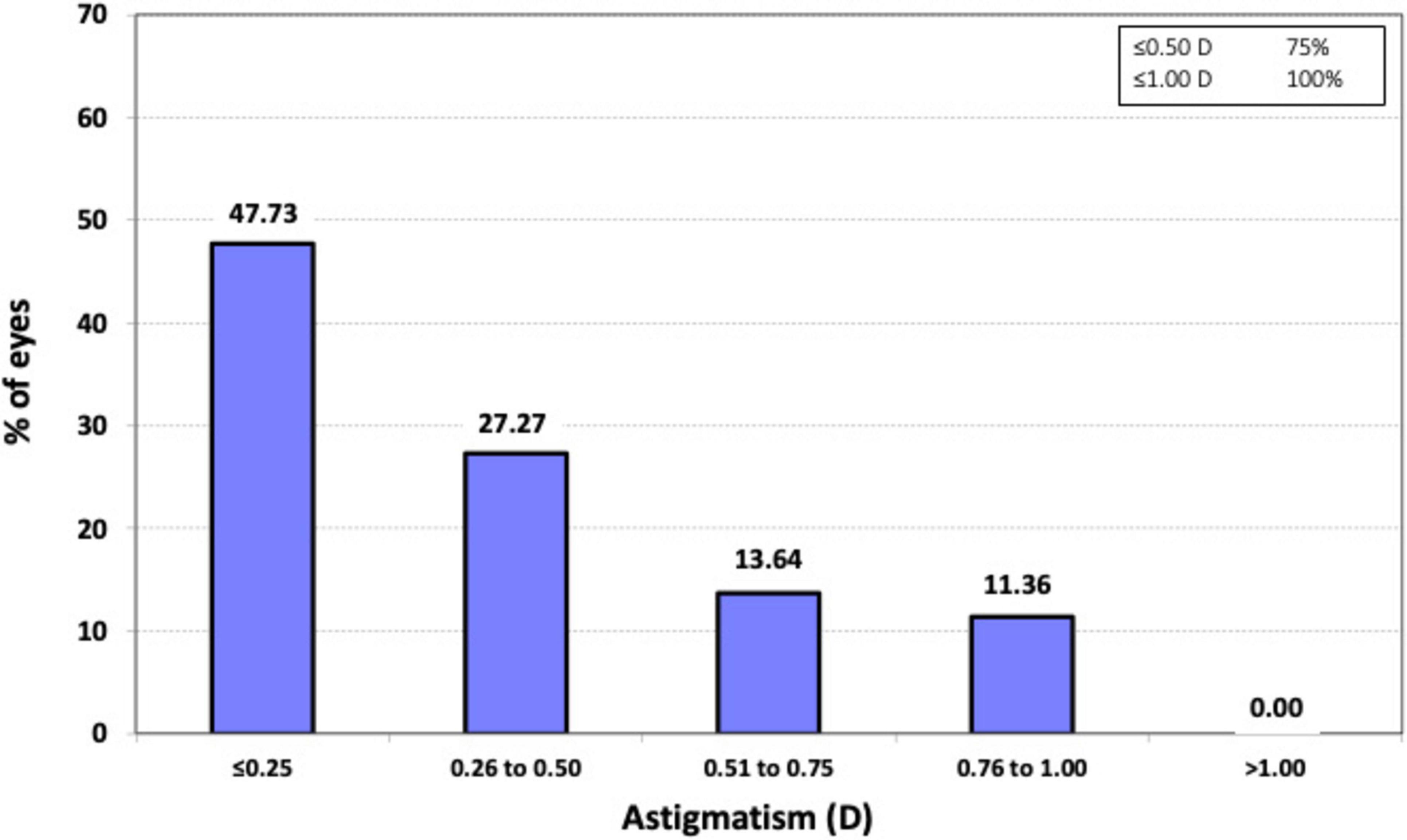
Figure 2. Histogram of the prevalence of postoperative refractive astigmatism at 3 months after surgery.
Regarding astigmatism, all eyes (100%, n = 44) exhibited postoperative refractive cylinder values of ≤ 1.00 D, and 75% (n = 33) had values of ≤ 0.50 D. The mean postoperative refractive cylinder was −0.41 ± 0.33 D, with a range from 0 to −1.0 D.
To evaluate the efficacy of the procedure, Figure 3 presents the cumulative postoperative binocular logMAR UDVA and CDVA (A), UIVA and DCIVA (B), and UNVA and DCNVA (C), respectively. All patients (100%) showed cumulative CDVA of 20/25 or better, and DCIVA of 20/32 or better. Specifically, 90.91% (n = 20) of patients showed an UDVA of 20/25 or better compared to 100% (n = 44) for CDVA, 72.73% (n = 16) of patients showed an UIVA of 20/25 or better compared to 90.91% (n = 20) for DCIVA, and 18.18% (n = 4) of patients showed an UNVA of 20/25 or better compared to 4.55% (n = 1) for DCNVA. Table 2 presents detailed measurements of visual acuity at different distances under both photopic and mesopic conditions. The monocular and binocular defocus curves with best correction for distance (Figure 4) show that visual acuity remained relatively stable across a wide range of defocus levels, indicating a smooth and extended depth of focus. The best performance was observed at 0.00 D (distance vision), with a gradual decline as defocus increased. The results show binocular vision maintained better performance than monocular vision at all vergences. A binocular visual acuity of 0.2 logMAR (20/32) or better was maintained up to −2.00 D of defocus, which corresponds to approximately 50 cm, demonstrating the lens’s ability to provide functional intermediate vision, and supporting the effectiveness of the lens in providing extended vision while maintaining good distance acuity.
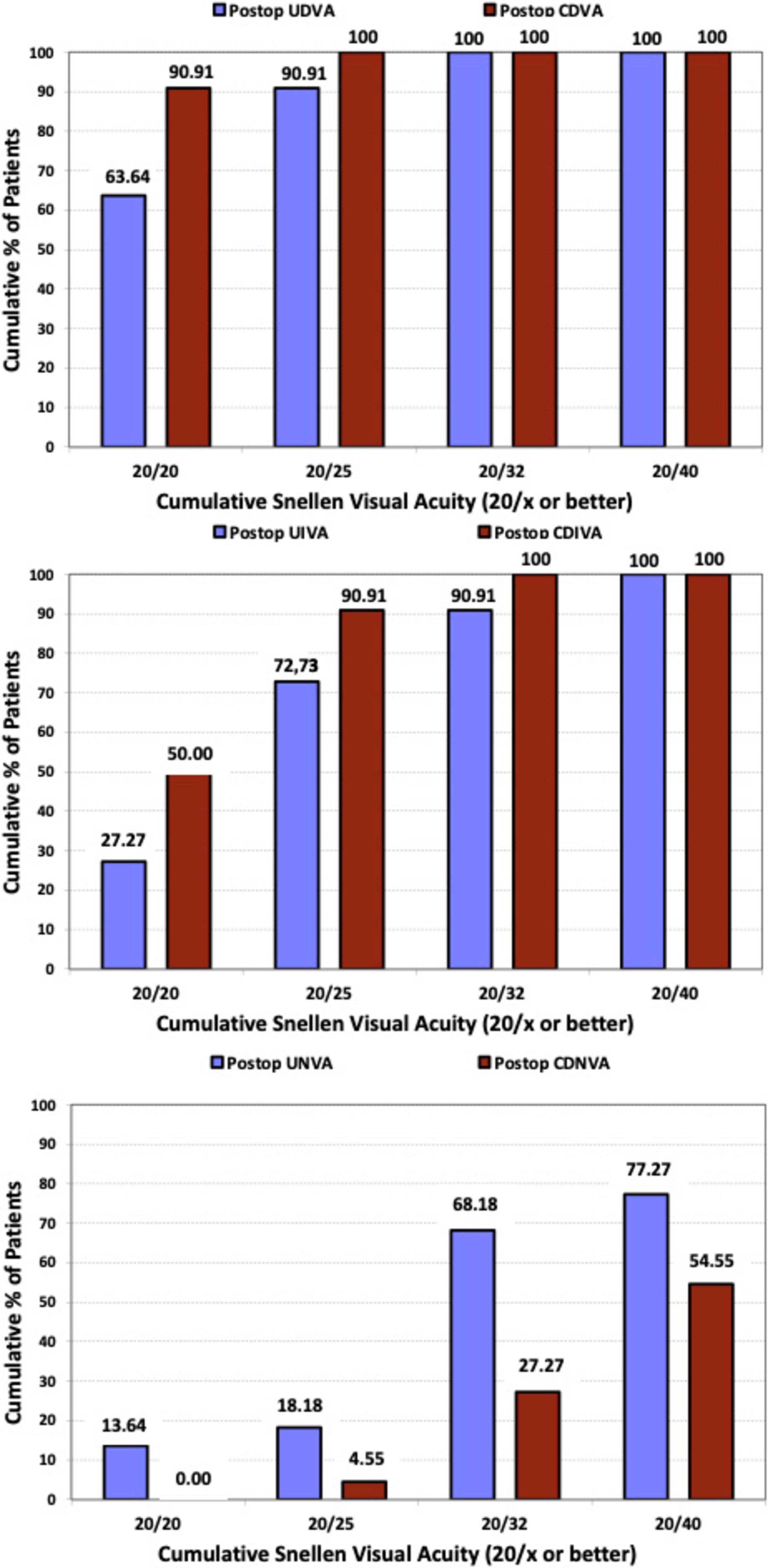
Figure 3. Cumulative proportion of patients having a given photopic binocular: uncorrected distance visual acuity (UDVA) and best-corrected distance visual acuity (CDVA) values (upper); binocular uncorrected intermediate visual acuity (UIVA) and distance-corrected intermediate visual acuity (DCIVA) values (middle); uncorrected near visual acuity (UNVA) and distance-corrected near visual acuity (DCNVA) values (lower), at 3 months after surgery.
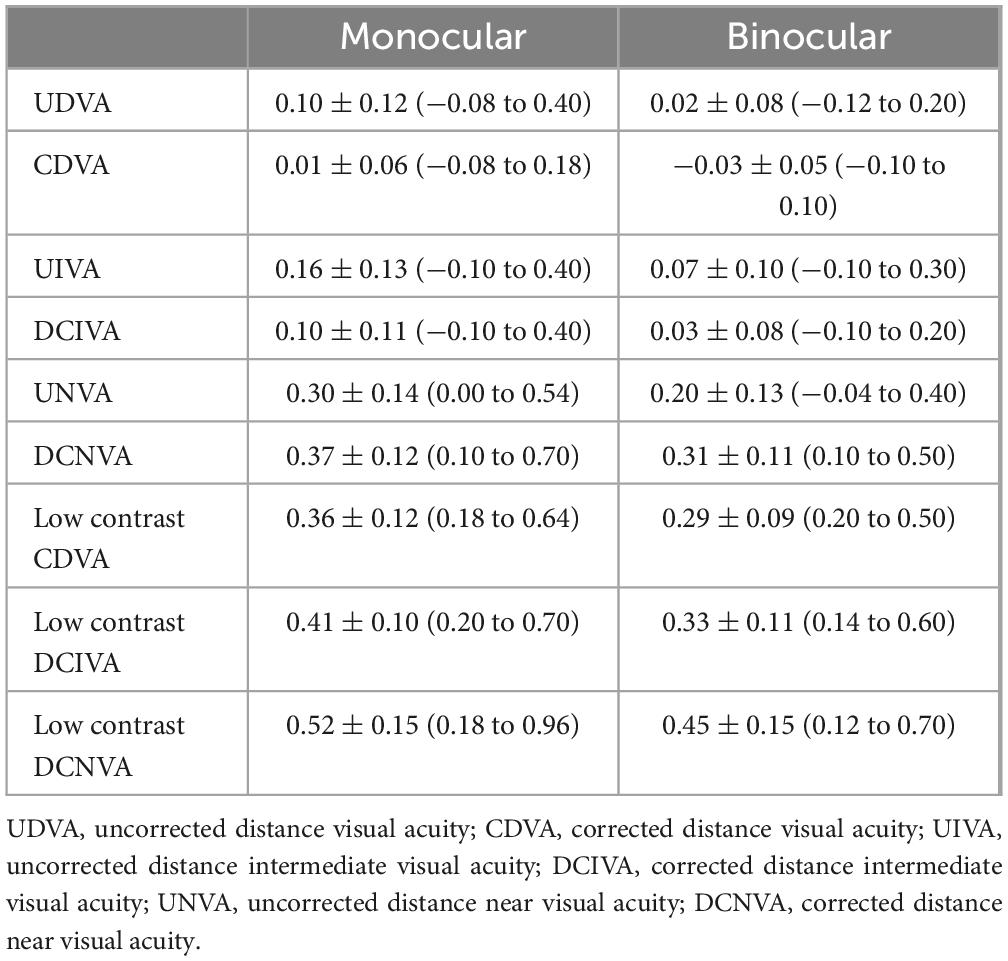
Table 2. LogMAR visual acuity outcomes of patients implanted with the Asqelio EDOF intraocular lens shown as means, standard deviations (SD) and ranges.
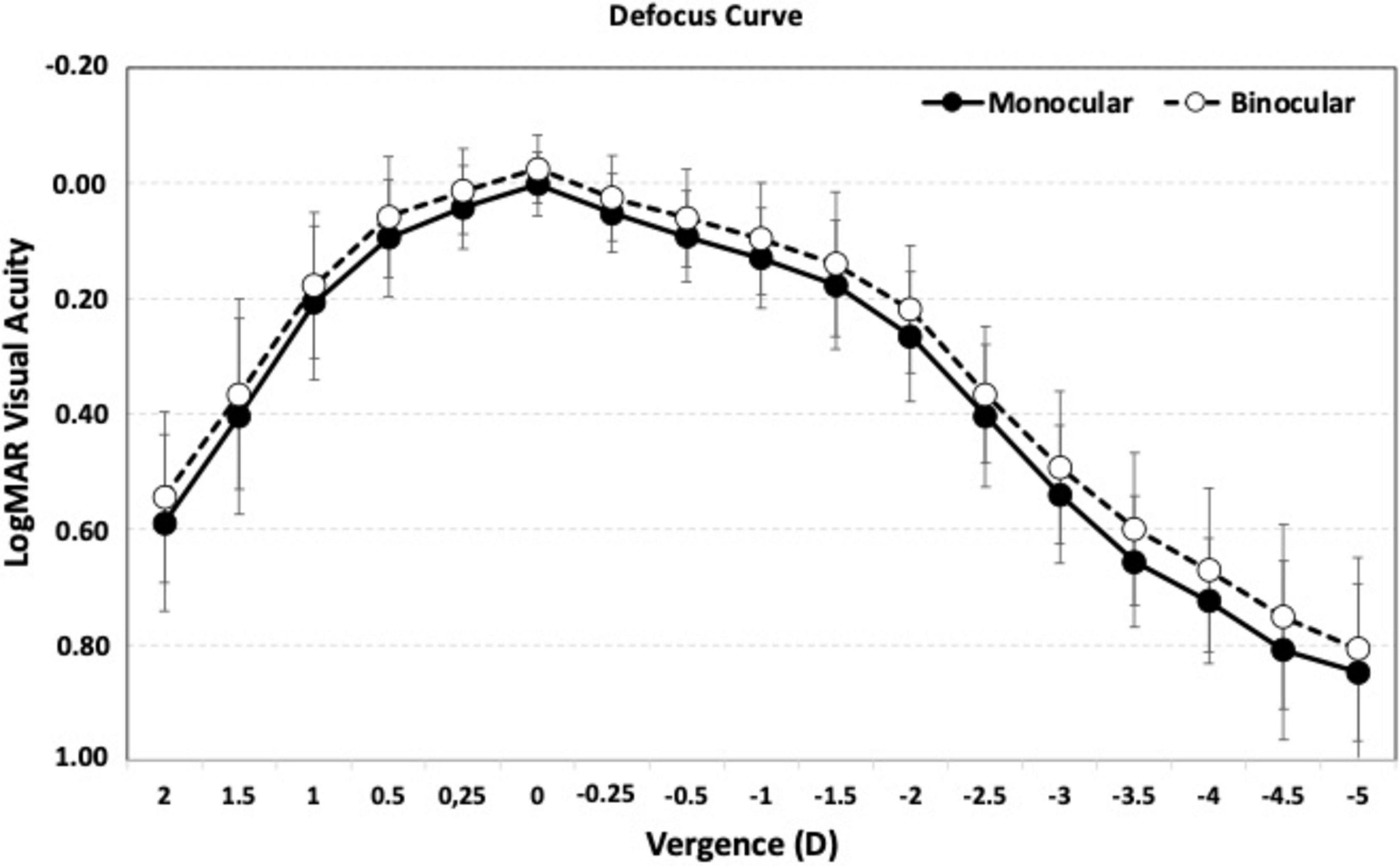
Figure 4. Mean, high-contrast, photopic, monocular and binocular logMAR visual acuity with best correction for distance, as a function of the chart vergence. Error bars represent standard deviation.
Figure 5 illustrates the mean contrast sensitivity function measured under photopic conditions (85 cd/m2) with glare (A) and without glare (B), and under mesopic conditions (3 cd/m2) with glare (C) and without glare (D). Since the CTS system does not provide reference ranges for normal contrast sensitivity in healthy subjects under these conditions, for this analysis the normal ranges for non-operated eyes over 60 years old reported by Escaf et al. (14) using the Functional Acuity Contrast Test (FACT) were used. The results indicate that contrast sensitivity was within or above normal levels under both photopic and mesopic conditions, regardless of the presence of glare. The only exception was mesopic contrast sensitivity at 12 cycles per degree (cpd) with glare, where the mean value fell slightly below the normal range.
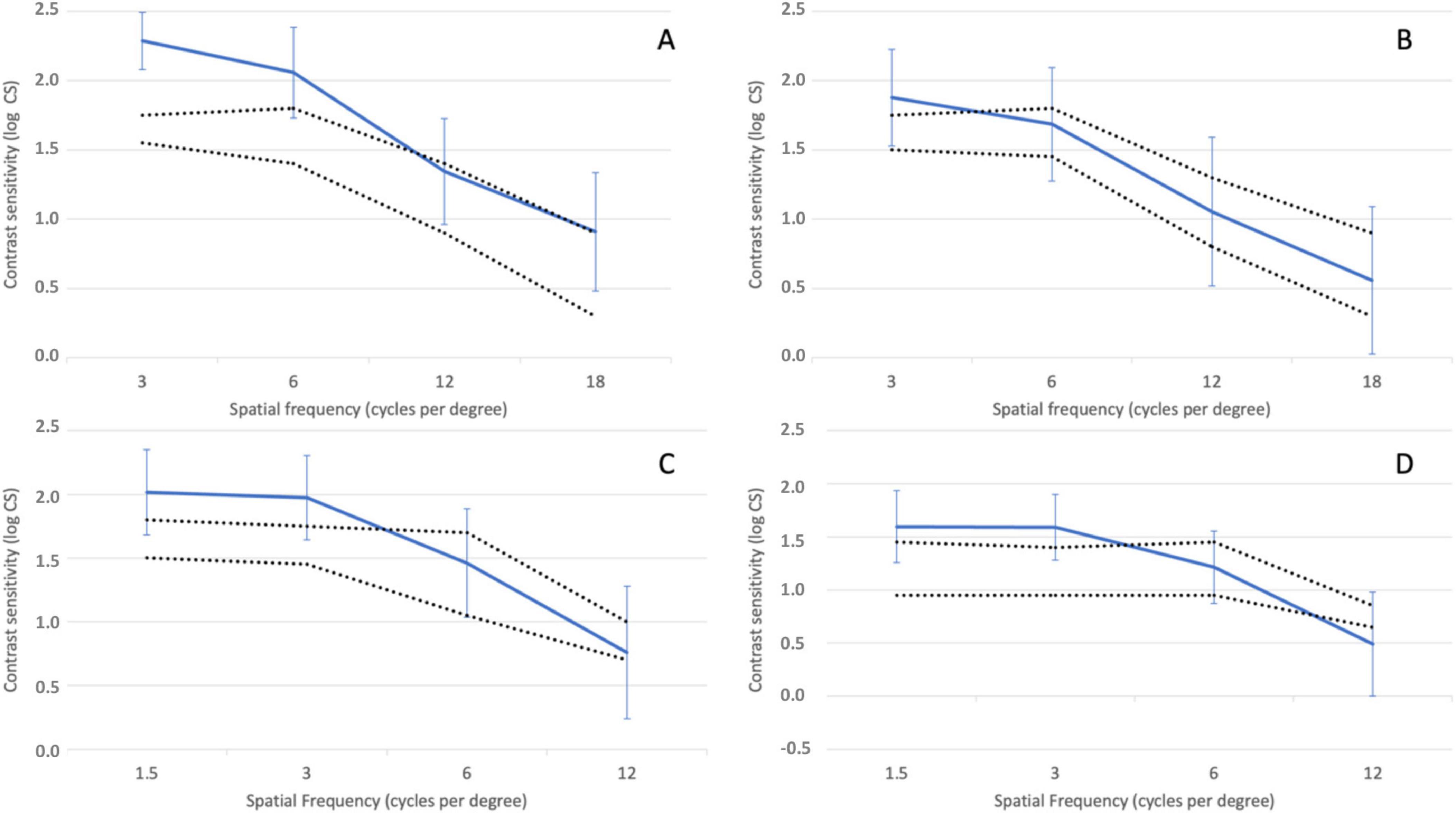
Figure 5. Contrast sensitivity function determined under photopic conditions (85 cd/m2) without (A) and with induced glare (B) and under mesopic conditions (3 cd/m2) without (C) and with induced glare (D) Dotted lines delimit the normal range for non-operated eyes above 60 years of age using the Functional Acuity Contrast Test (FACT) (11). Error bars represent the 95% confidence intervals.
Regarding the questionnaires, the Catquest-9SF results (Table 3) showed that 90.9% of patients were either satisfied (13 out of 22) or very satisfied (7 out of 22) with their vision after surgery, with none reporting being very unsatisfied. This table presents the average scores and frequency of responses to questions related to difficulties in performing daily activities, as assessed by the Catquest-9SF. In most cases, the results indicate higher percentages for no difficulty (R4) in performing any of those activities [ranging from 63.6% (n = 14) to 90.9% (n = 20)], except for reading the newspapers, where half of the patients reported some difficulty and 40.91% (n = 9) reported no difficulty. Table 4 summarizes the results of the visual symptom questionnaire. No significant visual symptoms were reported in terms of frequency, intensity, or bothersomeness following the implantation of the Asqelio EDOF IOL. Halo was the only relevant visual symptom, with only 18.19% (n = 4) of patients reporting their presence quite often or very often, but none experiencing severe bothersome.
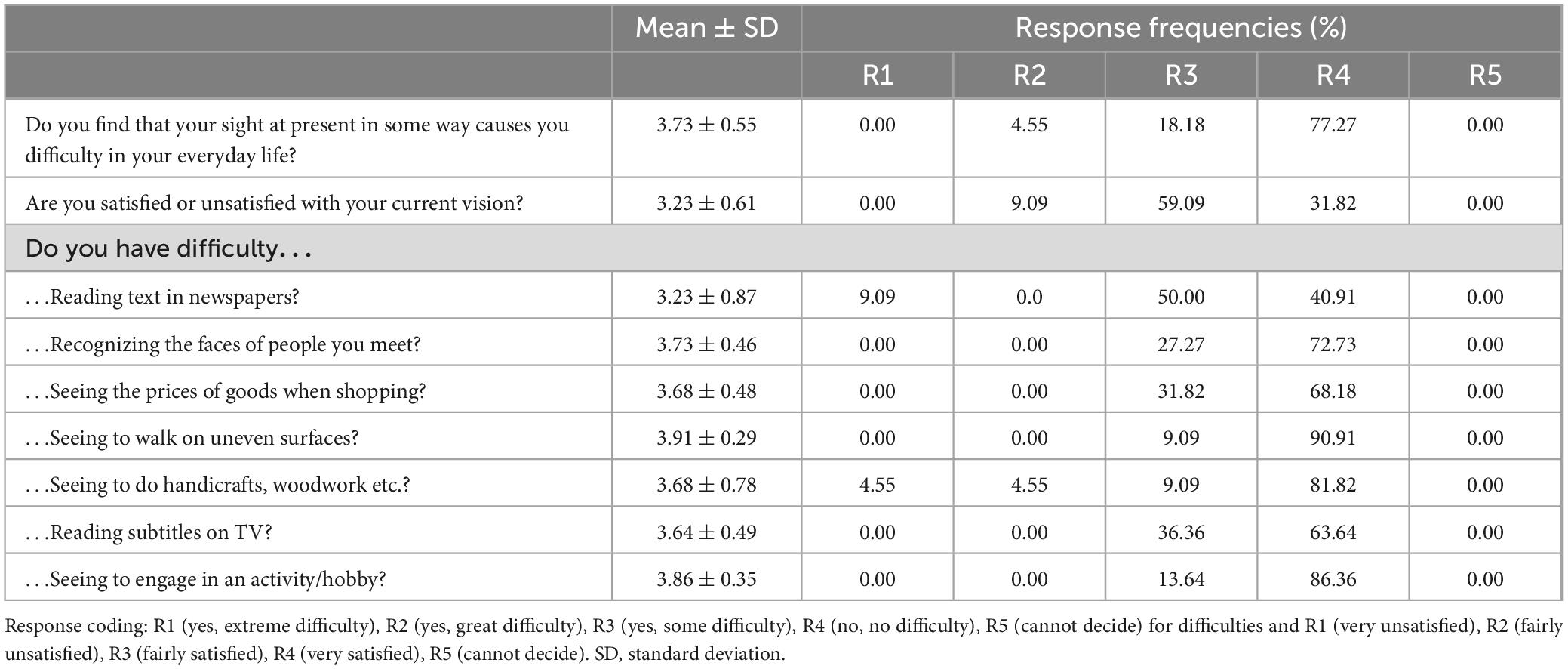
Table 3. Summary of patient-reported difficulties and satisfaction with their vision as per Catquest-9SF.
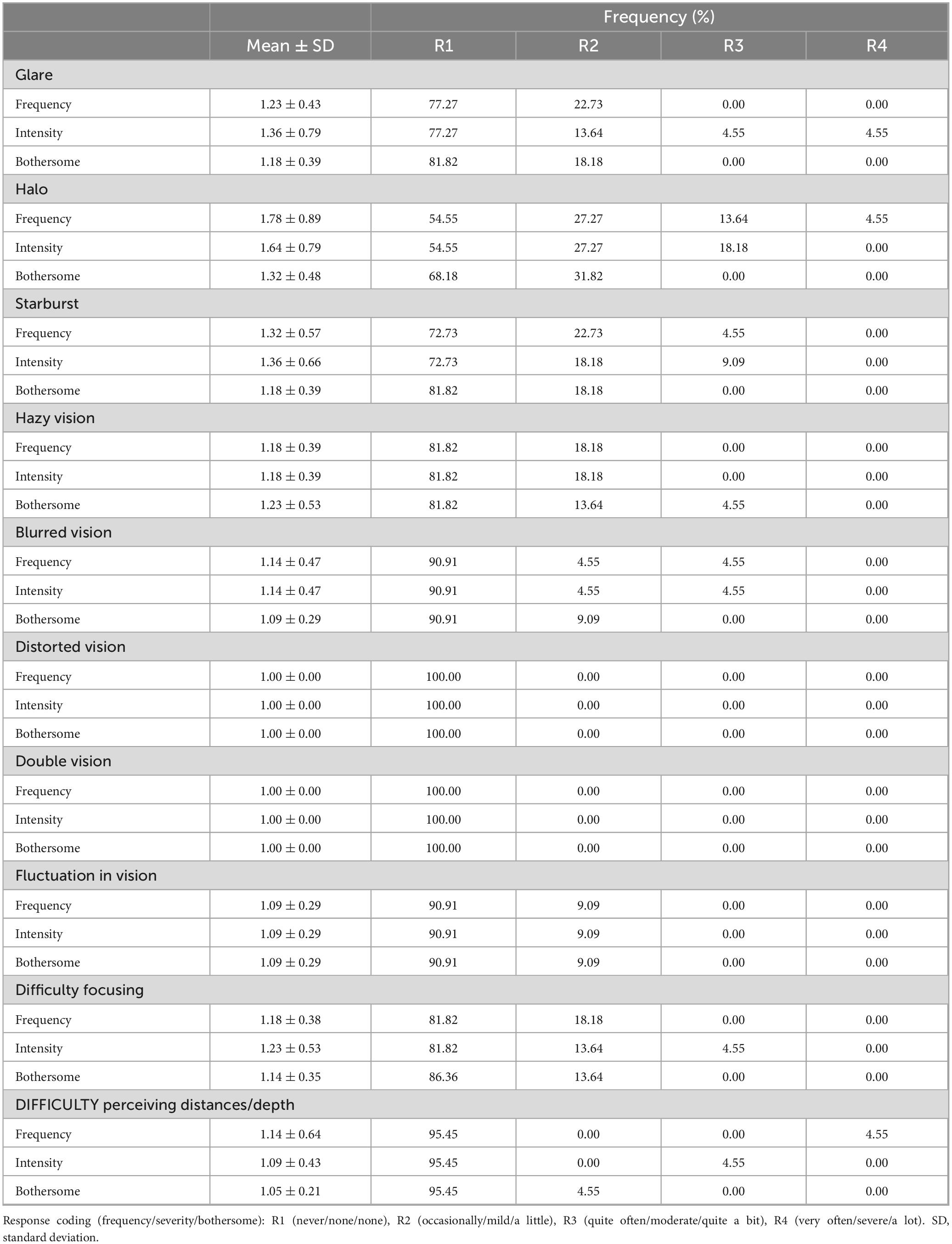
Table 4. Summary of patient reported visual symptoms (mean score, type of symptom and frequency of responses) as per visual quality questionnaire.
Light distortion parameters obtained under monocular and binocular conditions are displayed in Table 5.
No adverse events were reported for any subject enrolled in the present study.
EDOF IOLs are increasingly being implanted alongside trifocal lenses. They provide a continuous extended range of vision without generating specific focal points for particular distances, offering good VA results for distance and intermediate ranges comparable to multifocal IOLs, though with somewhat poorer near VA (15). The technologies used to create the extended range in these IOLs vary considerably among different platforms (3, 16). Due to this variability, the American Academy of Ophthalmology reached a consensus on the criteria for defining and evaluating the performance of EDOF IOLs (17). Based on these recommendations, the US Food and Drug Administration established several clinical criteria for EDOF IOLs in the American National Standards Institute (ANSI)/AAO Standard Z80.35–2018 (18).
According to these criteria, EDOF IOLs should provide a monocular depth of focus at 0.2 logMAR that is at least 0.50 diopters (D) greater than that of a monofocal control. Additionally, the monocular photopic DCIVA at 66 cm should exceed that of a monofocal control lens, with at least 50% of eyes achieving a VA of 0.2 logMAR or better. Finally, the mean monocular photopic CDVA should be non-inferior to that of a monofocal control IOL. Assessments of mesopic contrast sensitivity and visual symptom questionnaires are also required, although there are no specific criteria regarding visual quality or disturbances.
At the present moment, three IOLs in the EDOF IOL category may be considered as non-diffractive EDOF IOLs that do not use spherical aberration as source of EDOF but refractive elements to reshape the wavefront: Acrysof® IQ Vivity® (Alcon Inc., USA) (now also a version with the new Clareon material is available), Lucidis® (SAV-IOL SA, Switzerland), and Asqelio™ EDOF (AST VisionCare, Inc., USA). The present study constitutes the first report on the clinical outcomes with the Asqelio™ EDOF IOL.
Analyzing the visual performance of Asqelio™ EDOF obtained in the present study, all patients (100%) showed cumulative CDVA of 0.1 LogMAR or better, and DCIVA of 0.2 LogMAR or better. Specifically, 90.91% of patients showed an UDVA of 0.1 LogMAR or better compared to 100% for CDVA, 72.73% of patients showed an UIVA of 0.1 LogMAR or better compared to 90.91% for DCIVA, and 18.18% of patients showed an UNVA of 0.1 LogMAR or better compared to 4.55% for DCNVA. The difference observed between uncorrected and corrected visual performance, particularly for near vergences, results from attempting a slight residual myopia in the non-dominant eye, known as mini-monovision, which has been reported to give good clinical outcomes with similar types of IOLs (19) and is common practice in the study center, aiming to expand the range of binocular clear vision. Considering this, the visual performance with the IOL is good under both monocular and binocular conditions.
In a sample of 20 patients implanted with the Acrysof® IQ Vivity® IOL, Sabur and Unsal (20) reported a mean monocular CDVA of 0.02 ± 0.04 LogMAR, DCIVA of 0.18 ± 0.09 and DCNVA of 0.30 ± 0.11LogMAR. These outcomes are comparable to those found in the present study, with mean values of 0.01 ± 0.06, 0.10 ± 0.11, and 0.37 ± 0.12 for monocular CDVA, DCIVA and DCNVA, respectively. With regards to binocular VA values, Sabur and Unsal (20) reported mean values of 0.01 ± 0.03, 0.13 ± 0.07 and 0.24 ± 0.10. Binocular VA values found in the present study were slightly better, −0.03 ± 0.05, 0.03 ± 0.08 and 0.20 ± 0.13 LogMAR for CDVA, DCIVA and DCNVA, respectively. In their study, they compared the outcomes of the non-diffractive EDOF IOL against the TECNIS Eyhance® (Johnson & Johnson Surgical Vision, Inc, USA), categorized as an enhanced monofocal IOL. They concluded that visual performance for distance and intermediate vision was similar between both IOLs, with near vision being significantly better for the EDOF IOL.
van Amelsfort et al. (21) found that adjusting for emmetropia in the dominant eye and inducing slight myopia in the nondominant eye (-0.25 D to -0.50 D) led to improved near visual acuity, greater patient satisfaction, and higher levels of spectacle independence. Similarly, Newsom and Potvin (22) demonstrated that targeting -0.75 D of myopia in the nondominant eye resulted in an improvement of more than one line in near visual acuity compared to other studies focusing on bilateral emmetropia.
In a randomized double-blind comparison study between Acrysof® IQ Vivity® and TECNIS Symfony® (Johnson & Johnson Surgical Vision, Inc, USA), a diffractive EDOF IOL, Scheepers and Hall (23) found binocular VA values of −0.03 ± 0.05, 0.04 ± 0.08 and 0.22 ± 0.12 LogMAR for CDVA, DCIVA and DCNVA, respectively, in patients implanted with the Vivity refractive EDOF 3 months after surgery, very much in agreement with those found in the present study with Asqelio™ EDOF IOL. These were not significantly different from those obtained in patients implanted with the Symfony IOL, which were, respectively, of −0.03 ± 0.05, 0.03 ± 0.10 and 0.26 ± 0.11 LogMAR.
Sabur and Unsal (20) reported binocular defocus curve outcomes with bilateral implantation of the non-diffractive Acrysof Vivity EDOF IOL. According to their mean binocular defocus curve, CDVA peaked at 0 D, with a progressive decline that reached 0.1 LogMAR around −1.25 D vergence and 0.2 LogMAR around −2.0 D. Similar profile was reported by Pantanelli et al. (24) comparing Acryso IQ Vivity to a monofocal control. They also showed two more lines of vision at intermediate and near vergences compared to the control IOL. In the present study, Asqelio™ EDOF IOL showed a similar behavior, with a mean binocular defocus curve that peaked at 0 D and had a similar progressive decline, reaching 0.2 LogMAR at −2.0 D.
According to the literature, eyes implanted with an EDOF IOL should experience fewer dysphotopsia and less loss of contrast sensitivity compared with those fitted with a conventional multifocal IOL (25–28). Sabur and Unsal (20) obtained similar contrast sensitivity outcomes in Acrysof® IQ Vivity® patients and Eyhance® enhanced monofocal IOL, with differences not being statistically significant at any spatial frequency or light condition. In the present study, contrast sensitivity values obtained with Asqelio™ EDOF showed either within or above normal range under both photopic and mesopic conditions, both with and without glare being induced. Given that the CTS system does not provide with a reference range of normality for healthy subjects under photopic and mesopic conditions with and without glare, it must be noted that the normal ranges for non-operated eyes above 60 years of age used by Escaf et al. (14). using the Functional Acuity Contrast Test (FACT) were used as a reference here.
With regards to light distortion, Guarro et al. (29) concluded that diffractive EDOF IOL models induced similar visual disturbances that were worse than those produced by the non-diffractive EDOF model. In their study they found glare, halos, and starbursts to be similar between the non-diffractive EDOF IOL studied, AcrySof® IQ Vivity®, and a monofocal control IOL. The mean LDI values they obtained for the non-diffractive EDOF IOL were 14.36 ± 10.25 (range 4.46 to 42.62) monocularly, and 8.24 ± 3.86 (3.82, 16.31) binocularly. These results are in agreement with those found in the present study in patients implanted with Asqelio™ EDOF, showing that the light distortion of Asqelio™ EDOF and Acrysof® Vivity® is very similar both monocularly and binocularly. These outcomes are considerably better than those reported in the literature using the same method with other presbyopia-correcting IOLs (30, 31).
Patient-reported outcomes show 90.9% of patients as satisfied or very satisfied with their vision after bilateral implantation of Asqelio™ EDOF, while none of the patients’ reports being very unsatisfied. The Catquest-9SF outcomes show no difficulty for performing most of the daily activities, except for reading the newspapers, where half of the patients reported some difficulty, as expected given the close distance range needed. Regarding visual disturbance symptoms, literature shows that diffractive EDOF IOLs perform worse than non-diffractive EDOF IOLs (19), and no significant differences in visual disturbances were found compared to a monofocal lens (29). In the study by Guarro et al. (29), the most frequent visual symptom reported by patients implanted with Acrysof® IQ Vivity® was starbursts, with 4.5% of patients reporting it as very frequent and 13.6% as moderately severe and quite bothersome. These were slightly different in the study by Pantanelli et al. (24), who found glare as the most frequent visual symptom, with 4.2% of patients reporting very frequent and very bothersome. In the study by Newsom and Potvin (22), halos and starbursts had increased frequency (18 and 39%, respectively), severity (33 and 55%, respectively), and bothersome (21 and 18%, respectively). In the present study, patient reported outcomes with Asqelio™ EDOF show halo as the most frequent visual symptom, with 4.5% of patients reporting as very frequent, but none of them considered it as bothersome.
To conclude, the present study supports that the Asqelio™ EDOF IOL is an efficient IOL design providing good clinical outcomes at distance and intermediate distances, while some patients may also reach functional near vision. Its non-diffractive design also benefits the lack of relevant dysphotopsia and reduced light distortion compared to other presbyopia-correcting IOL designs. The high level of patient satisfaction reported after implantation makes it a valuable option for patients seeking spectacle independence with no visual disturbance, and very good visual outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of the Hospital Clínico San Carlos (Madrid, Spain). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
ST-S: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review and editing. PT-S: Formal analysis, Methodology, Writing – review and editing. BE-G: Data curation, Formal analysis, Methodology, Writing – review and editing. PO-V: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review and editing. PT-R: Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review and editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by AST Products, Inc. (Billerica, MA, USA).
PO-V declares grant, collaboration, research support and/or consultant relationships from Alcon Laboratories, AST Products Inc., BVI, Carl Zeiss Meditec, Hoya Surgical, HumanOptics AG, Johnson & Johnson, Ocumension Therapeutics, and Vialase Inc. PT-R declares grant, collaboration, research support and/or consultant relationships from Alcon Laboratories, AST Products Inc., BVI, Carl Zeiss Meditec, Hoya Surgical AG, HumanOptics AG, Johnson & Johnson, and Vialase Inc.
The authors declare that this study received funding from AST Products, Inc. The funder initiated the study and was involved in defining its objectives.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. FDA. FDA News Release. (2016). Available online at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-intraocular-lens-extended-range-vision-cataract-patients (accessed July, 15 2016).
2. Breyer D, Kaymak H, Ax T, Kretz F, Auffarth G, Hagen P. Multifocal intraocular lenses and extended depth of focus intraocular lenses. Asia Pac J Ophthalmol (Phila). (2017) 6:339–49.
3. Kohnen T, Suryakumar R. Extended depth-of-focus technology in intraocular lenses. J Cataract Refract Surg. (2020) 46:298–304.
4. Kanclerz P, Toto F, Grzybowski A, Alio J. Extended depth-of-field intraocular lenses: An update. Asia Pac J Ophthalmol (Phila). (2020) 9:194–202. doi: 10.1097/APO.0000000000000296
5. Zhong Y, Wang K, Yu X, Liu X, Yao K. Comparison of trifocal or hybrid multifocal-extended depth of focus intraocular lenses: A systematic review and meta-analysis. Sci Rep. (2021) 11:6699.
6. Fernández-Vega L, Alfonso J, Baamonde B, Madrid-Costa D, Montés-Micó R, Lozano J. Visual and refractive outcomes in hyperopic pseudophakic patients implanted with the Acri.LISA 366D multifocal intraocular lens. Am J Ophthalmol. (2009) 148, 214–220.e1. doi: 10.1016/j.ajo.2009.02.036
7. Ortiz D, Alió J, Bernabéu G, Pongo V. Optical performance of monofocal and multifocal intraocular lenses in the human eye. J Cataract Refract Surg. (2008) 34:755–62.
8. Alió J, Plaza-Puche A, Piñero D, Amparo F, Jiménez R, Rodríguez-Prats J, et al. Optical analysis, reading performance, and quality-of-life evaluation after implantation of a diffractive multifocal intraocular lens. J Cataract Refract Surg. (2011) 37:27–37. doi: 10.1016/j.jcrs.2010.07.035
9. Ferreira-Neves H, Macedo-de-Araújo R, Rico-Del-Viejo L, da-Silva A, Queirós A, González-Méijome J. Validation of a method to measure light distortion surrounding a source of glare. J Biomed Opt. (2015) 20:75002. doi: 10.1117/1.JBO.20.7.075002
10. Lundström M, Llovet F, Llovet A, Martinez Del Pozo M, Mompean B, González JV, et al. Validation of the Spanish Catquest-9SF in patients with a monofocal or trifocal intraocular lens. J Cataract Refract Surg. (2016) 42:1791–6.
11. McAlinden C, Pesudovs K, Moore J. The development of an instrument to measure quality of vision: The Quality of Vision (QoV) questionnaire. Invest Ophthalmol Vis Sci. (2010) 51:5537–45.
12. Sullivan L. Power and Sample Size Determination. Boston, MA: Boston University School of Public Health (2023)
13. Dupps W Jr., Kohnen T, Mamalis N, Rosen E, Koch D, Obstbaum S, et al. Standardized graphs and terms for refractive surgery results [editorial]. J Cataract Refract Surg. (2011) 37:1–3.
14. Escaf L, Escaf L, Polo S, Rodríguez-Vallejo M, Fernández J. Standard results and contrast sensitivity reestablishment after implantation of a trifocal intraocular lens. Curr Eye Res. (2021) 46:672–7. doi: 10.1080/02713683.2020.1828486
15. Bohm M, Petermann K, Hemkeppler E, Kohnen T. Defocus curves of 4 presbyopia-correcting IOL designs: Diffractive panfocal, diffractive trifocal, segmental refractive, and extended-depth-of-focus. J Cataract Refract Surg. (2019) 45:1625–36. doi: 10.1016/j.jcrs.2019.07.014
16. Rocha K. Extended depth of focus IOLs: The next chapter in refractive technology? J Refract Surg. (2017) 33:146–9. doi: 10.3928/1081597X-20170217-01
17. MacRae S, Holladay J, Glasser A, Calogero D, Hilmantel G, Masket S, et al. Special report: American academy of ophthalmology task force consensus statement for extended depth of focus intraocular lenses. Ophthalmology. (2017) 124:139–41. doi: 10.1016/j.ophtha.2016.09.039
18. American National Standard Institute. American National Standard for Ophthalmics - Extended Depth of Focus Intraocular Lenses. Washington, DC: American National Standard Institute (2018).
19. Fernandes M, Nunomura C, Messias A. Visual performance and photic disturbances with diffractive and nondiffractive EDOF intraocular lenses using mini-monovision: Randomized trial. J Cataract Refract Surg. (2024) 50:153–9. doi: 10.1097/j.jcrs.0000000000001330
20. Sabur H, Unsal U. Visual outcomes of non-diffractive extended-depth-of-focus and enhanced monofocal intraocular lenses: A case-control study. Eur J Ophthalmol. (2023) 33:262–8.
21. van Amelsfort T, Webers V, Bauer N, Clement LHH, van den Biggelaar FJHM, Nuijts RMMA. Visual outcomes of a new nondiffractive extended depth-of-focus intraocular lens targeted for minimonovision: 3-month results of a prospective cohort study. J Cataract Refract Surg. (2022) 48:151–6. doi: 10.1097/j.jcrs.0000000000000825
22. Newsom T, Potvin R. Evaluation of quality of vision and visual outcomes with bilateral implantation of a non-diffractive extended vision intraocular lens with a target of slight myopia in the non-dominant eye. Clin Ophthalmol. (2022) 16:183–90. doi: 10.2147/OPTH.S350850
23. Scheepers M, Hall B. Randomized and double-blind comparison of clinical visual outcomes of 2 EDOF intraocular lenses. J Cataract Refract Surg. (2023) 49:354–9. doi: 10.1097/j.jcrs.0000000000001113
24. Pantanelli S, O’Rourke T, Bolognia O, Scruggs K, Longenecker A, Lehman E. Vision and patient-reported outcomes with nondiffractive EDOF or neutral aspheric monofocal intraocular lenses. J Cataract Refract Surg. (2023) 49:360–6.
25. Giers B, Khoramnia R, Varadi D, Wallek H, Son H, Attia M, et al. Functional results and photic phenomena with new extended-depth-of-focus intraocular lens. BMC Ophthalmol. (2019) 19:197. doi: 10.1186/s12886-019-1201-3
26. Bilbao-Calabuig R, Gonzalez-Lopez F, Llovet-Rausell A, Ortega-Usobiaga J, Tejerina Fernandez V, Llovet-Osuna F. Lens-based surgical correction of presbyopia. Where are we in 2020? Arch Soc Esp Oftalmol (Engl Ed). (2021) 96:74–88. doi: 10.1016/j.oftal.2020.07.012
27. Rodov L, Reitblat O, Levy A, Assia E, Kleinmann G. Visual outcomes and patient satisfaction for trifocal, extended depth of focus and monofocal intraocular lenses. J Refract Surg. (2019) 35:434–40.
28. Sachdev G, Ramamurthy S, Sharma U, Dandapani R. Visual outcomes of patients bilaterally implanted with the extended range of vision intraocular lens: A prospective study. Indian J Ophthalmol. (2018) 66:407–10.
29. Guarro M, Sararols L, Londoño G, Goñi I, Vázquez M, Ruiz S, et al. Visual disturbances produced after the implantation of 3 EDOF intraocular lenses vs 1 monofocal intraocular lens. J Cataract Refract Surg. (2022) 48:1354–9. doi: 10.1097/j.jcrs.0000000000000988
30. Escandón-García S, Ribeiro F, McAlinden C, Queirós A, González-Méijome J. Through-focus vision performance and light disturbances of 3 new intraocular lenses for presbyopia correction. J Ophthalmol. (2018) 2018:6165493. doi: 10.1155/2018/6165493
Keywords: extended depth of focus, non-diffractive, intraocular lens, phacoemulsification, cataract
Citation: Tañá-Sanz S, Tañá-Sanz P, Elvira-Giner B, Orts-Vila P and Tañá-Rivero P (2025) Visual performance, light distortion and patient reported outcomes with a new bi-aspheric non-diffractive extended depth of focus intraocular lens. Front. Med. 12:1513803. doi: 10.3389/fmed.2025.1513803
Received: 19 October 2024; Accepted: 06 February 2025;
Published: 26 February 2025.
Edited by:
Mayank Nanavaty, Brighton and Sussex University Hospitals NHS Trust, United KingdomReviewed by:
Neetu Kushwah, Boston Children’s Hospital and Harvard Medical School, United StatesCopyright © 2025 Tañá-Sanz, Tañá-Sanz, Elvira-Giner, Orts-Vila and Tañá-Rivero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pedro Tañá-Rivero, cmRpQG9mdGFsdmlzdC5lcw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.