- 1Department of Medical Oncology/Hematology, National Centre for Cancer Care and Research, Hamad Medical Corporation, Doha, Qatar
- 2Department of Hematology, Sultan Qaboos University Hospital, Muscat, Oman
- 3Department of Pharmacy, National Center for Cancer Care and Research, Hamad Medical Corporation, Doha, Qatar
- 4College of Pharmacy, QU Health, Qatar University, Doha, Qatar
- 5Department of Hematology, Kuwait Cancer Control Center, Kuwait City, Kuwait
- 6Translational Research Department, Dasman Diabetes Institute, Kuwait City, Kuwait
- 7Department of Medicine, Division of Hematology, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates
- 8Department of Hematology, Dubai Hospital, Dubai, United Arab Emirates
- 9Department of Hematology, Tawam Hospital, Al Ain, Abu Dhabi, United Arab Emirates
- 10Department of Hematology and Oncology, Faculty of Medicine, Augsburg University Hospital, University of Augsburg, Augsburg, Germany
The treatment landscape for chronic lymphocytic leukemia (CLL) has expanded dramatically over the last decade, with a wide range of effective treatments now available. Clinical management of CLL varies widely depending on patient profile, meaning the optimal treatment in Arab patients, who tend to be young and often present with comorbidities, including diabetes and obesity, requires specific considerations. In the absence of regional guidelines, a group of experts from across the Gulf region and one international expert from Germany convened to discuss and agree upon a position statement for venetoclax-based fixed-duration treatment strategies for Arab patients with CLL. Our position is that ibrutinib-venetoclax should be the first choice as first-line therapy for all fit CLL patients in the region, regardless of age. The advantages of an all-oral, fixed-duration treatment are discussed in the context of a young Arab patient population, including excellent patient and physician convenience, limited accumulative risk of toxicities, uncomplicated logistics, and low burden of healthcare administration costs. Finally, we discuss the management of key safety considerations in Arab populations including ethnic neutropenia, risk of cardiotoxic events, considerations during intermittent fasting, and avoiding adverse drug–drug interactions, e.g., with anti-tuberculosis or anti-obesity medications.
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by a slow but progressive accumulation of clonal, mature, dysfunctional B cells that primarily affect the blood, bone marrow and lymph nodes (1). CLL is the most common leukemia in Western countries, comprising 25–30% of all leukemias, with a considerable burden including an incidence of >100,000 cases and > 40,000 deaths per year globally (2). However, its regional prevalence is highly variable, with a substantially lower incidence observed throughout Asia and Africa, comprising as little as 3% of all leukemia cases in the Middle East (2, 3). As such, data on CLL are sparse in Arab populations (4), and as most clinical trials have enrolled Western patients, differences in demographic and genetic backgrounds indicate that established standard clinical parameters need to be adjusted when considering treatment in Arab populations. Patients from the Middle East region tend to be younger, with a median age at diagnosis of 59 years compared with 70 years in the West, and have different baseline comorbidity and hematological profiles (5–7). Therefore, a different clinical management strategy is required for Arab patients with active disease to that which is used for Western patients. In this article, we discuss the utility of venetoclax-based fixed duration (FD) treatment regimens for previously untreated CLL and review the key clinical data in this regard, with a particular focus on the combination of ibrutinib plus venetoclax and obinutuzumab plus venetoclax, both of which have been approved for use in this setting. We highlight the advantages of a FD treatment strategy in the context of a young Arab patient population, and in the absence of regional treatment guidelines, we discuss what we believe to be the most important considerations when selecting and treating Arab patients with a time-limited treatment strategy.
CLL treatment landscape
The CLL treatment landscape has been revolutionized in recent years, with a wide range of novel treatments available including Bruton tyrosine kinase inhibitors (BTKis), anti-apoptopic protein B-cell lymphoma 2 (BCL-2) inhibitors, phosphoinositide 3-kinase (PI3K) inhibitors, and anti-CD20 monoclonal antibodies (1, 8). The BTKis are highly effective as once- or twice-daily continuous treatments, such that traditional chemoimmunotherapy regimens including rituximab with either fludarabine and cyclophosphamide (FCR) or bendamustine (BR) are no longer widely used (8). Choice of initial therapy depends on a number of factors, including clinical stage, tumor genetic risk stratification [particularly 17p deletion and/or TP53 and immunoglobulin heavy-chain variable region (IGHV) mutational status], patient age, fitness, and presence of comorbidities (1, 8). In particular, cardiac/renal history and concomitant medications are important, as are patient-specific factors such as financial and logistic considerations, as well as individual treatment preferences (9). Single-agent BTKis such as ibrutinib, and more recently acalabrutinib and zanubrutinib, have proven highly effective as once- or twice-daily continuous treatments until disease progression or occurrence of toxicity, and are widely recommended as first-line treatment for CLL (8). While continuous therapies continue to be used with clinical success in CLL, deep remissions are rarely achieved, and potential drawbacks associated with long-term continuous treatment include development of resistance mutations, financial costs, and patient compliance (10). As such, there has been considerable interest in FD treatments, which are able to circumvent some of the problems associated with continuous therapy, and which offer a time-limited treatment option that can limit the development of toxicities that may otherwise accumulate over time. Given CLL is considered an incurable disease, an effective treatment plan is needed that considers the patient’s baseline age and the potential lifelong nature of the disease, while minimizing overall treatment burden on the patient and the impact on their quality of life (11). In this regard, several trials have demonstrated remarkable success of the FD treatment approach in treatment-naïve CLL, particularly combinations of the BCL-2 inhibitor venetoclax alongside ibrutinib (ibrutinib-venetoclax) (12, 13) or the anti-CD20 inhibitor obinutuzumab (obinutuzumab-venetoclax) (14–16).
Fixed duration treatment: ibrutinib-venetoclax
The rationale for combining ibrutinib with venetoclax is based on their complementary mechanisms of actions, whereby BTKis shrink nodal disease by releasing malignant cells out of lymph nodes into peripheral blood, subsequently allowing venetoclax to actively induce apoptosis of these cells and clear them from the peripheral blood and bone marrow (8, 13, 17, 18). Additionally, ibrutinib sensitizes CLL cells to venetoclax-mediated BCL-2 inhibition, thus allowing a further synergy of the treatment combination (19).
CAPTIVATE, a phase 2 trial in patients aged ≤70 years with previously untreated CLL evaluated treatment with ibrutinib-venetoclax (13). The FD cohort received 3 cycles of ibrutinib lead-in (420 mg once daily), followed by venetoclax (400 mg once daily) for 12 cycles, after a standard 5-week ramp-up. Overall, 92% of patients completed the oral FD combination treatment, with a complete response (CR) rate of 56% in patients without del(17p) and 55% in the all-treated population (13). Undetectable minimal residual disease (MRD) rate was achieved by 77%, with 24-month progression-free (PFS) and overall survival (OS) rates of 95 and 98%, respectively (13). A post-hoc exploratory analysis suggested the deep responses were maintained and comparable outcomes were seen in patients with or without high-risk genetic features, including del(17p), TP53 mutated, or unmutated IGHV (20).
The most common grade ≥ 3 adverse event (AE) in CAPTIVATE was neutropenia (33%), followed by hypertension (6%), with one fatal AE of sudden death during the ibrutinib lead-in (13). Cardiovascular events associated with BTKis occurred at a reduced incidence, likely due to shorter treatment duration (13, 21). Any-grade atrial fibrillation occurred in 4%, and any-grade bleeding events were observed in 61% of patients, with grade 3 and 4 events in 0 and 2% of patients, respectively (13). Tumor lysis syndrome (TLS) risk with venetoclax was mitigated by ibrutinib’s 3-cycle lead-in, reducing high-risk TLS classification in 94% of patients and eliminating TLS events per Howard criteria (13, 22). Only 16% of patients required TLS-hospitalization, easing monitoring and prophylaxis burdens (13, 22).
Another finding of note from CAPTIVATE was the absence of typical resistance mutations to BTKi or BCL2-i after FD treatment with ibrutinib-venetoclax. After 38 months of follow up, 15% of patients who completed FD treatment developed progressive disease (PD), with no significant association with baseline genetic risk factors or resistance mutations in BTK, PLCG2, or BCL2 at progression (23). Most patients with PD (19 of 29 patients) were successfully re-treated with either single-agent ibrutinib (n = 16) or ibrutinib-venetoclax (n = 3), supporting the hypothesis that combination regimens may reduce resistance risk and allow effective re-treatment after PD (23).
While the CAPTIVATE trial enrolled a relatively young, fit CLL patient population, the Phase 3 GLOW study investigated ibrutinib-venetoclax in older and/or less fit comorbid patients (≥65 years or 18–64 years with Cumulative Illness Rating Scale (CIRS) ≥6) (12). Patients received either the same 12-cycle ibrutinib-venetoclax FD regimen as in CAPTIVATE (following the 3-cycle ibrutinib lead-in), or six cycles of chlorambucil-obinutuzumab. The FD ibrutinib-venetoclax combination showed significantly improved PFS versus chemoimmunotherapy (hazard ratio 0.216) (12). Greater undetectable MRD was achieved with ibrutinib-venetoclax at 3 months in both bone marrow (51.9 vs. 17.1% with chlorambucil-obinutuzumab) and peripheral blood (54.7 vs. 39.0%) (12). At a 4-year follow-up, PFS (74.6 vs. 24.8%) and OS (87.5 vs. 77.6%) rates favored ibrutinib-venetoclax, suggesting long-term benefit of this combination after the end of the FD treatment period (24).
In the primary safety analysis in GLOW, the ibrutinib-venetoclax combination resulted in slightly more patients with grade ≥3 AEs compared to chlorambucil and obinutuzumab (75.5 vs. 69.5%), with neutropenia being the most common (34.9 vs. 49.5%). The AE profile may have been influenced by the greater number of patients with multiple comorbidities in the ibrutinib plus venetoclax group (12). All-cause mortality was similar between treatment arms, but four on-treatment cardiac/sudden deaths occurred in the ibrutinib-venetoclax group, all patients with a CIRS score >10 and an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of at least 2 (12). These findings alongside the general risk of cardiac events with BTKi warrant investigation into improved predictive biomarkers for cardiac events among elderly and/or comorbid CLL patients receiving BTKi treatment (12). Of note however, at 4-year follow-up, the number of overall deaths were twice higher in patients receiving chemoimmunotherapy than those receiving ibrutinib-venetoclax, resulting in a significant OS advantage of ibrutinib-venetoclax vs. chemoimmunotherapy (HR 0.48) (24).
The results of CAPTIVATE and GLOW expand upon earlier phase 2 studies that investigated two-year time-limited treatment strategies with ibrutinib-venetoclax in previously untreated, older and/or high-risk patients (25, 26), and demonstrated clinically relevant efficacy of the combination with manageable safety, with an all-oral, 15-month FD treatment that had a favorable benefit–risk ratio which was particularly apparent in the fit patient population.
Fixed duration treatment: obinutuzumab-venetoclax
The CLL14 trial compared obinutuzumab-venetoclax to chlorambucil-obinutuzumab as first-line treatment of patients with CLL and existing comorbidities (i.e., CIRS of >6) (14). At 24 weeks, obinutuzumab-venetoclax showed superior PFS (88.2 vs. 64.1%), higher undetectable MRD rates in both peripheral blood (75.5 vs. 35.2%) and bone marrow (56.9 vs. 17.1%), and more patients achieved a CR (49.5 vs. 23.1%) compared to chemoimmunotherapy (14). This trend was observed in patients with/without risk factors such as TP53 mutations or unmutated IGHV, but PFS was considerably shorter in these patients regardless of venetoclax or chlorambucil treatment (14). At long-term follow-up (median 39.6 months), median PFS was not reached for obinutuzumab-venetoclax versus 35.6 months for chemoimmunotherapy (27).
The most common grade ≥3 AEs in the obinutuzumab-venetoclax arm were neutropenia (52.8%), thrombocytopenia (13.7%), and infusion-related reactions (9.0%). TLS was observed in three patients in the venetoclax-obinutuzumab group and five patients in the chemoimmunotherapy group, with none of the events meeting Howard criteria. After treatment completion, there were 11 fatal AEs in the obinutuzumab-venetoclax group compared with 4 in the chemoimmunotherapy group (14).
More recently, the CLL13 study compared obinutuzumab-venetoclax ± ibrutinib with rituximab-venetoclax or chemoimmunotherapy in previously untreated fit patients with CLL (median age 60–62 years, with a median CIRS of 2) (16). At 15 months, undetectable MRD in peripheral blood was 52.0% (chemoimmunotherapy) vs. 57.0% (rituximab-venetoclax) vs. 86.5% (obinutuzumab-venetoclax) vs. 92.2% (obinutuzumab-venetoclax-ibrutinib), with CR rates at 15 months of 31.0, 49.4, 56.8, and 61.9%, respectively (16). PFS was significantly improved with obinutuzumab-venetoclax ± ibrutinib except in IGHV unmutated patients, who achieved very high PFS in all treatment groups. Early treatment discontinuation due to AEs was highest in the chemoimmunotherapy group (15.3%), followed by the triple therapy obinutuzumab-venetoclax-ibrutinib group (12.6%) (16).
Considerations for treatment strategies
With multiple highly effective therapeutic options and treatment strategies now available, the choice of appropriate first-line therapy should be based on several disease-related and patient-related factors. The 2025 NCCN guidelines advocate targeted therapies as the primary treatment for CLL patients with 17p deletion or TP53 mutations, based on evidence of its impact on PFS. The recommended options include BTKis and the BCL2 inhibitor venetoclax combined with obinutuzumab (28). The ELEVATE-TN trial demonstrated that acalabrutinib ± obinutuzumab, resulted in notable PFS improvements in patients with del(17p) or TP53 mutations. The estimated 72-month PFS rate was 56% for both treatment arms. Projected 72-month OS rates were 68% for acalabrutinib alone, 72% for acalabrutinib plus obinutuzumab, and 53% for chemoimmunotherapy (29). The SEQUOIA study, a non-randomized trial, prospectively enrolled 111 CLL patients with del(17p) or TP53 mutations. In this group, zanubrutinib monotherapy outperformed bendamustine plus rituximab, showing higher overall response rate (ORR) and substantial PFS improvements. For patients with high del(17p) levels (≥20%), zanubrutinib achieved a 98% ORR and an 89% 18-month PFS rate. In those with low del(17p) (7–20%), the ORR was 92%, with an 88.9% 18-month PFS (30). In lower-risk patients, venetoclax-based FD regimens are likely to be a good option. Considerations between FD regimens may include risk of TLS and barriers to infusion-based therapy (where ibrutinib-venetoclax may be preferred), or uncontrolled cardiac comorbidities and receiving anticoagulation plus antiplatelet therapy (where obinutuzumab-venetoclax may be preferred). The ongoing phase 3 CLL17 trial (NCT04608318) is investigating non-inferiority of FD ibrutinib-venetoclax or obinutuzumab-venetoclax compared with standard-of-care single-agent continuous BTKi inhibitor in first-line treatment of CLL and is anticipated to provide direct head-to-head comparisons on the relative efficacy and safety of FD treatments compared with continuous BTK inhibitor monotherapy.
Methodology
A group of experts from across the Gulf region of the Middle East and one international expert from Germany convened twice in June 2024, once virtually and once in person in Doha, Qatar. The expert group comprised eight hematologists and one pharmacist who were selected due to their recognized seniority and expertise in the management of CLL. During these meetings the expert group collectively discussed and agreed upon a position statement for venetoclax-based fixed-duration treatment strategies and proposed specific considerations for treatment of Arab patients with CLL. The experts individually took responsibility for the development of specific sections of this article, which were compiled and circulated among all experts for critical review and revisions in a timely manner following the expert meetings.
First-line, fixed-duration, venetoclax-based treatment strategy for CLL patients in the Middle East
To aid treatment decisions for CLL in the Middle East region, we have developed a position statement based on our personal clinical experience. Our preference for initial treatment with ibrutinib-venetoclax is based on its suitability in patients with a low-risk profile who favor a FD therapy, are averse to infusion therapy, and/or where infusion therapy is deemed too risky from the perspective of the treating physician. This regimen is also our first choice for high-risk patients (e.g., patients with 17p deletion or TP53 mutations) who are not suitable for continuous BTK inhibitor treatment or are in favor of FD oral therapy. As ibrutinib-venetoclax is an oral therapy, patients should have a good understanding of their therapeutic regimen and common AEs.
Position statement
Ibrutinib-venetoclax should be the first choice as first-line therapy for all fit CLL patients regardless of age. Ibrutinib-venetoclax should be selected regardless of any cytogenetic high-risk features. Caution should be exercised when considering patients with cardiac comorbidities for treatment with ibrutinib and referral to cardio-oncology or cardiology is advised for risk assessment. Patients with uncontrolled cardiac disease are not candidates for treatment with ibrutinib.
Obinutuzumab-venetoclax should be selected for patients who cannot use ibrutinib-venetoclax. For these patients, obinutuzumab-venetoclax should only be prescribed if the patient can tolerate the therapy. Obinutuzumab-venetoclax is not a first-choice treatment in patients with high-risk features (including unmutated IGHV, del(17p) or TP53 mutation). Obinutuzumab-venetoclax should not be used in patients with active hepatitis B infection.
Safety considerations in Arab populations
There are several important safety considerations when selecting a FD treatment strategy in Arab patients in the Middle East region. These may differ from treatment strategies in other regions due to unique clinical, socio-economic, and cultural factors in the region, and we have summarized some of the key considerations below.
Ethnic neutropenia
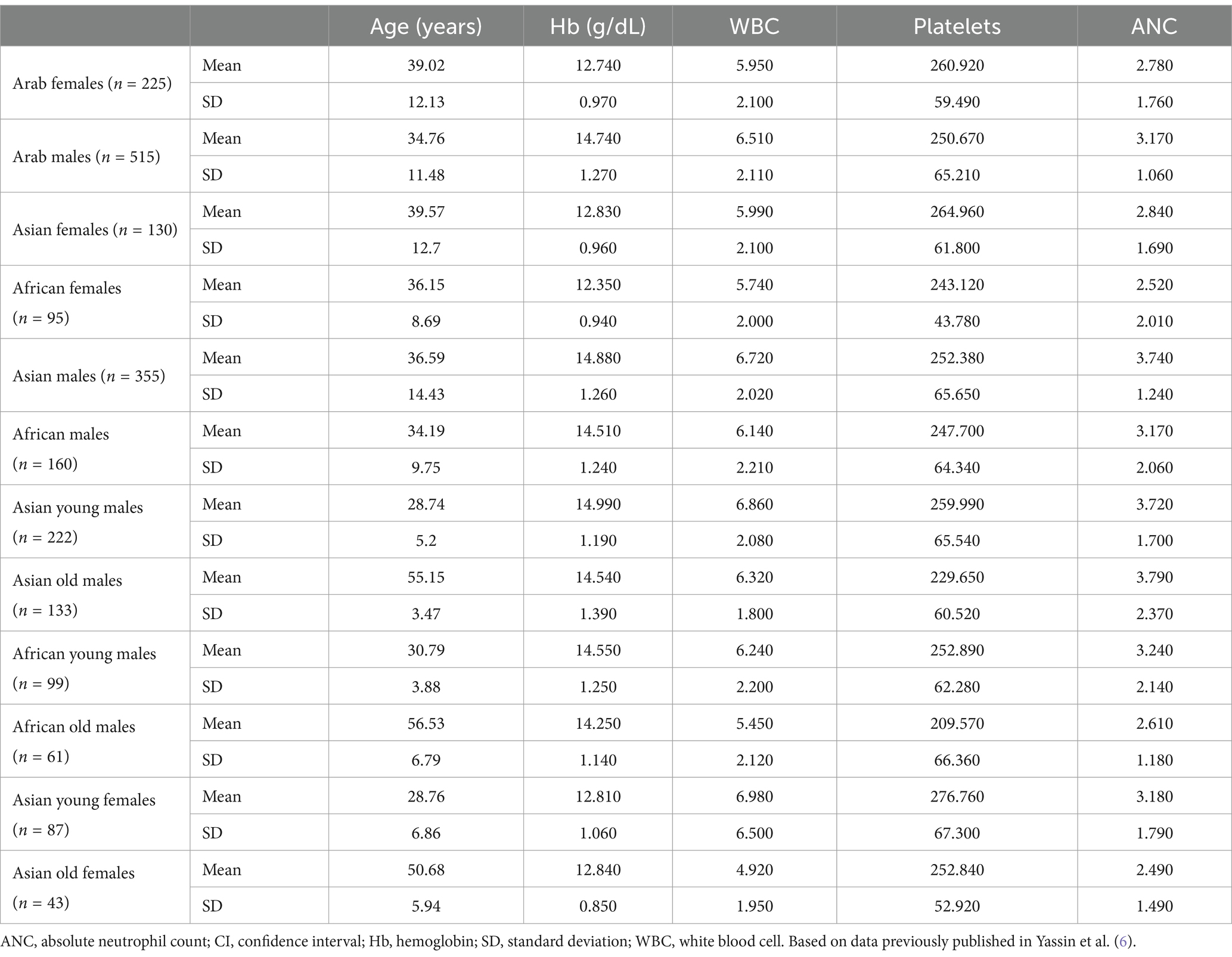
One of the most common AEs in patients receiving venetoclax-based FD treatment regimens is neutropenia, which in clinical trials was grade ≥3 in around a third of patients treated with ibrutinib-venetoclax (12, 13) and in around half of patients treated with obinutuzumab-venetoclax (14, 16). Guidance for the management of neutropenia in the prescribing information is based largely on clinical trial data from Western populations; however, there are key differences in hematological profiles between Arab and Western populations that need to be considered when classifying neutropenia and baseline blood cell counts (6, 7). The prevalence of baseline neutropenia is around 11% in Middle Eastern populations, with a higher prevalence seen in ethnic Arab females versus males (32 vs. 6%) (7). As such, we propose three distinct patient categories to guide neutropenia management in Arab CLL populations. Category A includes those patients without neutropenia at baseline, who should be managed according to the standard label recommendations related to dose adjustments. Category B includes patients who have a complete blood cell (CBC) count prior to CLL diagnosis that is consistent with ethnic neutropenia, and who should be managed according to their baseline level of neutropenia regarding assumptions for recovery after dose adjustment. Lastly, Category C encompasses patients for whom no prior CBC data are available prior to the diagnosis of CLL. In these cases, it is essential to establish a family history of neutropenia and medical professionals should exercise clinical judgment when determining whether dose adjustments or interruptions are necessary to manage neutropenia. The hematological reference ranges for white blood cells, absolute neutrophil count, hemoglobin, and platelet count for ethnic Arab patients are shown in Table 1 (6) and should serve as a useful guide for expected baseline ranges in healthy populations.
Risk of TLS
TLS is a life-threatening risk that can be associated with venetoclax therapy due to reactivation of apoptosis and rapid lysis of CLL lymphocytes, resulting in the release of tumor cell contents that can lead to multiorgan failure (31, 32). Early clinical trials identified TLS as a venetoclax-associated AE that in some cases could be associated with fatality (33), meaning prevention strategies had to be quickly adopted including the introduction of a gradual dose ramp-up strategy over 5 weeks, prophylactic and monitoring protocols, and patient TLS risk stratification (34). Tumor debulking strategies with an ibrutinib lead-in before venetoclax initiation have also proven effective at mitigating the risk of TLS when treating with ibrutinib-venetoclax in both fit and high-risk patients (22). Recent evidence has emerged to suggest that a 5-week ramp-up of venetoclax may not be a necessary strategy (35); however, while we await further studies to strengthen these data, we continue to recommend the ramp-up protocols. While the general management strategies can be used regardless of ethnicity, special considerations are needed for patients undergoing intermittent fasting, for example during the holy month of Ramadan, because aggressive hydration is needed to limit the risks of life-threatening consequences of TLS, and fasting can interfere with the hydration goal (36). In addition, low urinary output as a result of intermittent fasting can limit the kidney’s ability to counteract the metabolic imbalances during tumor lysis, potentially increasing the risk of negative consequences of TLS (36). Therefore, an additional risk assessment is needed in patients who are committed to practicing intermittent fasting and are due to be initiated on venetoclax.
Peptic ulcer disease and risk of gastrointestinal bleeding
BTK inhibitors are also associated with an increased risk of bleeding events due to their potential for on- and off-target inhibition of Tec family kinases in thrombocytes that mediate platelet signaling (37). A small but sizeable proportion (4%) of patients in clinical trials receiving ibrutinib experienced major hemorrhage, including gastrointestinal (GI) bleeding, especially when there is comedication with anticoagulants (36). This rate may be slightly higher with ibrutinib than for other BTK inhibitors, possibly due to the wider spectrum of off-target activity seen with ibrutinib (38). GI bleeding can be caused or exacerbated by peptic ulcer disease (PUD), which is an additional concern in patients from the Midde East region who have a relatively high prevalence of PUD (39). Increases in bleeding and PUD complications due to increased gastric acid levels can also occur during the holy month of Ramadan (36, 40, 41), suggesting that fasting may be associated with increased risk of hemorrhage and should therefore be taken into consideration when designing an appropriate treatment strategy. The rate of PUD in the Middle East also means that patients are often receiving proton-pump inhibitors (PPIs) or histamine H2 receptor antagonists (H2RAs). Drug interactions can lead to lack of efficacy of one or both agents via changes in active plasma concentrations due to alterations in drug absorption, distribution, metabolism, and excretion, or indeed can lead to new or exacerbated adverse reactions, by direct effects on drug metabolism. Additionally, effects can be via direct interference with biological or physiological drug function (42). We have compiled potential drug–drug interactions of PPIs/H2RAs with common CLL treatments, along with the mechanism and recommended action, and summarized these in Table 2.
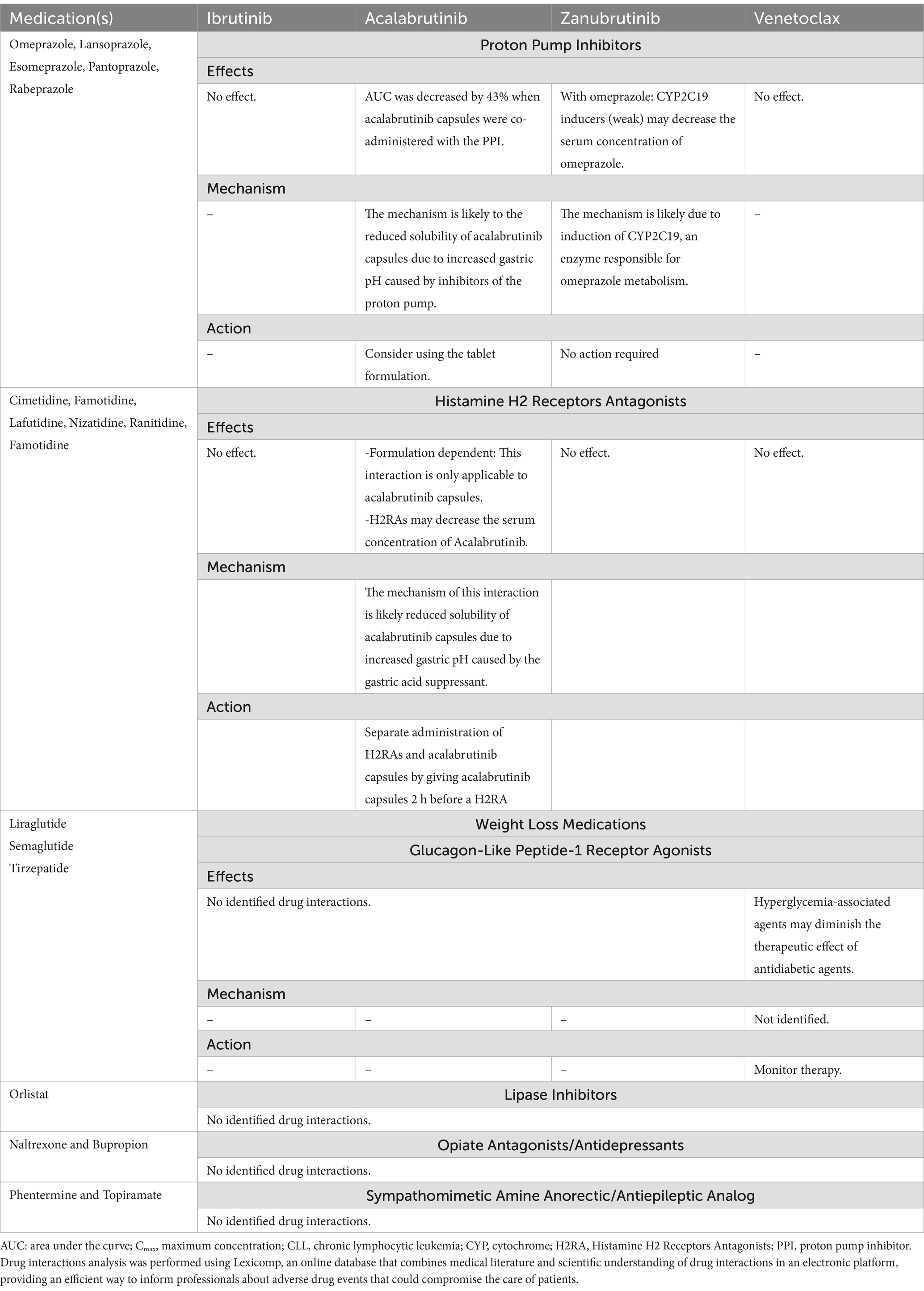
Table 2. Summary of potential drug–drug interactions of CLL treatments with PPIs, H2RAs, and anti-obesity.
Tuberculosis and concomitant use of anti-tuberculosis agents
CLL increases patient susceptibility to infections, with factors such as immune dysregulation, IGHV status, and treatment, contributing to higher infection rates (43). Tuberculosis (TB) is also endemic in the Middle East and Arab nations, with a reported incidence of 26.79 per 100,000 across the Middle East and North Africa in 2019 (44). Commonly used anti-tuberculosis medications can alter pharmacokinetic parameters of co-medications by direct effects on liver metabolism (45), therefore devising a treatment strategy for CLL patients taking anti-TB medications has to take this into consideration. To help physicians in the region circumvent such drug–drug interactions, we have checked for potential interactions between first- and second-line anti-TB medications and summarized these, along with our recommendations, in Table 2.
Risk of adverse cardiotoxic events
Cardiac AEs are a class effect that can occur with all available BTKis (46–48), and are thought to be mediated by co-inhibition of BTK and homologous non-receptor tyrosine kinases within cardiomyocytes (48). Physicians should exercise caution in concluding that second-generation BTKis are safer than ibrutinib in this regard (46). While the majority of such cardiac AEs are low grade, high-grade, life-threatening events do occur (47). Trials with stringent cardiac observations of patients have resulted in low rates of such BTKi-related toxicity (49). This emphasizes the need to address this issue in all patients undergoing such treatment, particularly in patients with cardiac comorbidities who have a 3–4-fold higher risk of developing cardiac AEs upon BTKi treatment (50). This is particularly relevant for Middle Eastern populations, who have a substantial burden of cardiac comorbidities, including high levels of diabetes (51), obesity (52), hyperlipidemia (53), and uncontrolled hypertension (54). It is therefore essential to identify patients with such baseline risk, optimize cardiac disorder-related medication before BTKi initiation, and monitor at-risk patients accordingly while on treatment. This includes regular clinical examination and ECG evaluation every 3 months or upon any signs and symptoms suggestive of heart-related origin, and patients should be advised accordingly. Furthermore, we recommend regular cardiology visits to monitor current cardiac status and related medication. Individuals with poorly controlled heart disease should not receive BTKis as first-line CLL therapy, neither continuous nor FD, and patients on BTKis who experience cardiovascular or other bothersome AEs should undergo dose modification. This approach frequently resolves and reduces reoccurrence of the event and enables patients to remain on drug for a longer period of time, and several prospective and retrospective studies suggest that such dose reductions for AE management do not impair the efficacy of treatment (55–59). It is important to consider potential drug interactions of CLL treatments with anti-obesity agents, due to the high prevalence of obese and overweight individuals (21.17 and 33.14, respectively) across the Middle East region (60). We have summarized potential drug–drug interactions of CLL treatments with anti-obesity agents, along with mechanisms and recommendations to avoid interactions in Table 3.
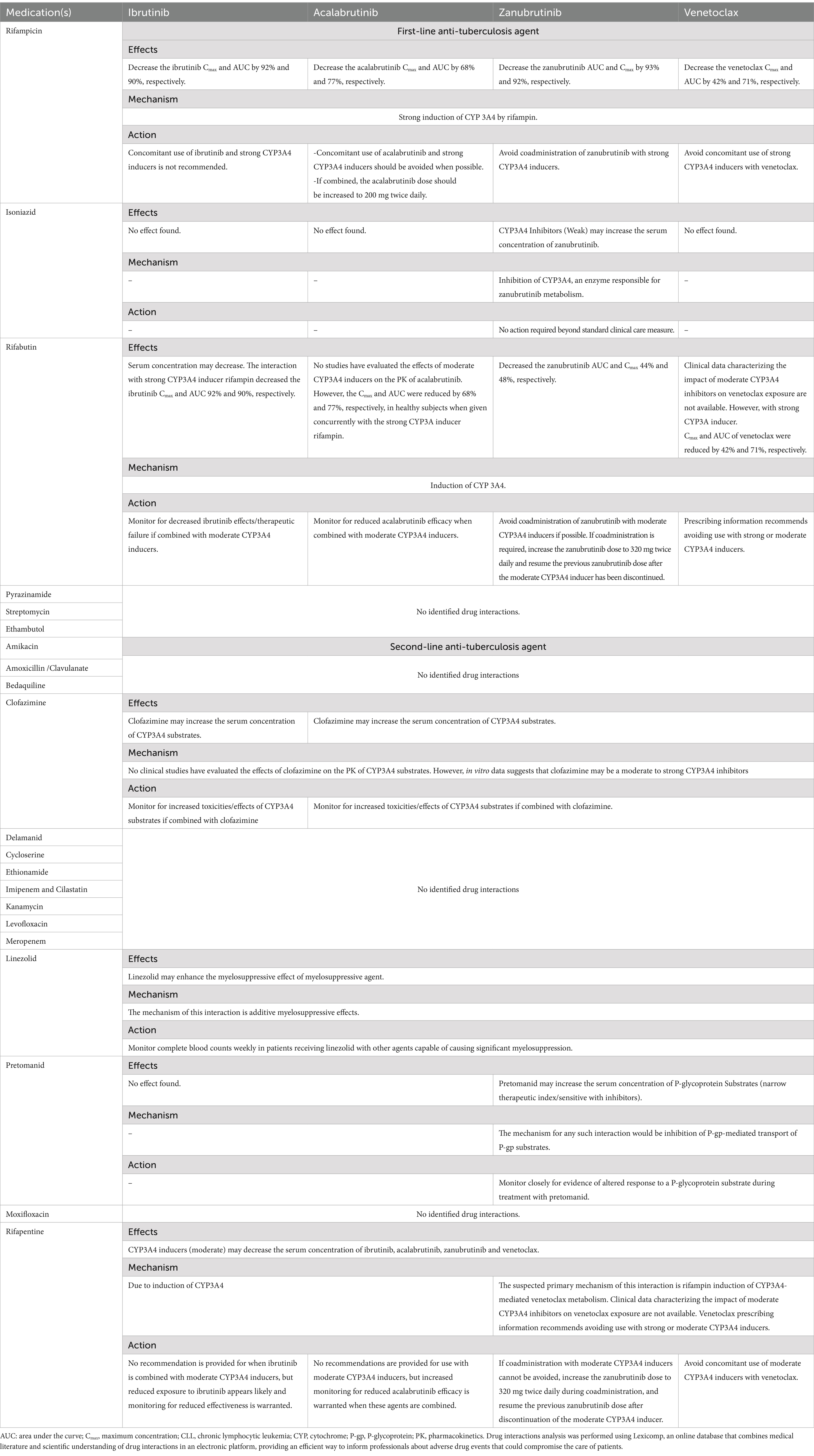
Table 3. Summary of potential drug–drug interactions of CLL treatments with anti-tuberculosis agents.
Renal impairment
Renal insufficiency is an important factor in treatment decisions and clinical management. The incidence of chronic kidney disease (CKD), the risk of which is increased in the presence of other comorbidities including diabetes, obesity and hypertension, is increasingly common in the Middle East (61). The presence of CKD requires certain dose adjustments, with consideration of tumor lysis risk, that varies by disease stage, and should be closely monitored for the potential impact on drug–drug interactions.
Fertility and pregnancy
Pregnancy and fertility-related concerns are important in the treatment planning and monitoring of patients with CLL in the Middle East region, given the younger age of patients at diagnosis, compared to Western patients (5). Unlike the well-documented effects of traditional chemo-immunotherapies, very little is known about the impact of BTKis and BCL2 antagonists on reproductive health, and there are no data on the effect of these agents on fertility or to guide the use of these agents during pregnancy. Female patients of reproductive age as well as male patients should all be counseled on the potential risk of infertility and advised about methods of fertility preservation prior to the initiation of therapy (32, 62, 63). All female patients with reproductive potential should be tested for pregnancy prior to initiation of therapy, and given the potential for fetal harm, the use of effective contraception is recommended in these patients while receiving therapy. Similarly, in the absence of data on the presence of these agents or their metabolites in breast milk, lactating women are advised not to breastfeed during treatment to avoid potential AEs in the breastfed child. Female patients are advised to avoid becoming pregnant for at least 30 days from last dose of these agents, and male patients are advised not to father a child while receiving treatment or for at least 10–30 days from last dose (62, 63).
Discussion
FD treatment strategies have the potential to offer several advantages over continuous therapy for patients from Arab nations. Firstly, Arab patients tend to be approximately 10 years younger than Western patients at diagnosis (5), meaning effective alternatives to potentially lifelong continuous therapies are even more important from both patient and clinical perspectives. Time-limited treatments in a younger population can effectively limit the cumulative risk of toxicity associated with continuous treatment, particularly with respect to potential cardiac toxicities that are known to be associated with long-term BTKi use. This is particularly advantageous in Middle Eastern populations that are susceptible to cardiovascular diseases due to relatively high baseline levels of obesity, diabetes, hypercholesterolemia, and hypertension (51–54). Our position statement for Arab patients is that ibrutinib-venetoclax should be the first choice as first-line therapy for all fit CLL patients regardless of age. It is of note that combination therapies like ibrutinib-venetoclax can produce overlapping toxicities such as TLS, cardiac issues, and cytopenia which necessitate close patient monitoring, and tailored prophylaxis. Figure 1 summarizes what we believe to be the key safety considerations in this population based on our clinical experience.
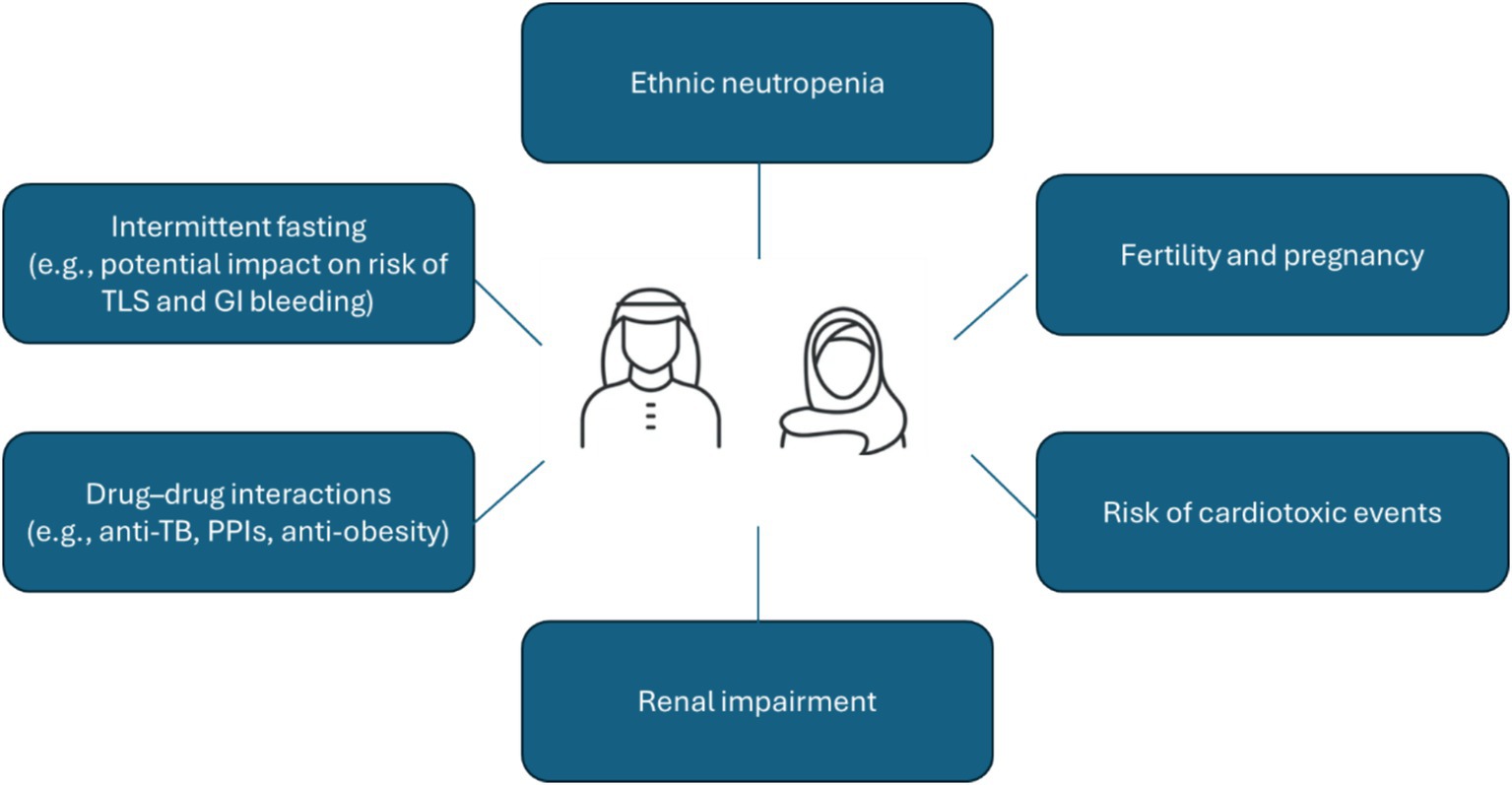
Figure 1. Key safety considerations for CLL patients in the Middle East region. CLL, chronic lymphocytic leukemia; GI, gastrointestinal; PPI, proton pump inhibitors; TB, tuberculosis; TLS, tumor lysis syndrome.
An additional benefit of FD treatment is the potential for a reduction in treatment costs, where lifetime savings versus continuous treatment would be far greater in Arab patients given they are typically diagnosed with CLL approximately 10 years younger than their Western counterparts (5). This necessitates a particular emphasis on limiting the cumulative risk of toxicity associated with lifelong therapies, especially the potential cardiac toxicity linked to BTKis (64). This consideration is particularly relevant for Middle Eastern populations, who exhibit a higher susceptibility to cardiovascular diseases due to the relatively high prevalence of baseline obesity, diabetes, and hypertension (65).
From a health economics perspective, fixed-duration ibrutinib-venetoclax as an all-oral regimen should be the treatment of choice for indicated CLL patients in the front-line setting. Ongoing work demonstrates the all-oral regimen leads to cost savings including drug costs, direct medical costs, direct non-medical costs, and indirect costs. This comprises lower utilization of healthcare resources including daycare unit visits, nursing time, pharmacy time, intravenous fluids, medical consumables, transportation time, and home carers, in addition to reductions in absenteeism and presenteeism (66). Furthermore, the majority of CLL patients in the Gulf region are expatriates whose families depend on their incomes (66). This underscores the necessity for well-tolerated therapies that not only improve overall quality of life but also minimize work absences, thereby supporting both the patient and their economic stability.
A further benefit of oral therapy over infusion therapy is the lack of infusion-related reactions. These reactions can range from mild to severe and often require close monitoring and supportive care during and after administration, which can be logistically challenging in healthcare settings with limited infusion resources or where access to emergency medical intervention might be delayed. Moreover, in some healthcare systems within the Middle East, logistical barriers such as the availability of infusion centers, patient preference for convenience, and challenges in travel or access to specialized care facilities can make infusion therapy less favorable. These practical considerations often influence the risk–benefit assessment and the preference for an oral FD regimen, which offers efficacy comparable to infusion-based regimens without the associated infusion-related risks. Additionally, being an FD treatment, ibrutinib-venetoclax would have lower cumulative drug costs compared to other available continuous treatment oral options. FD therapy could also positively impact quality of life as patients will have the opportunity to be treatment-free after only 15 months.
When using a FD treatment strategy in the Middle East, it is important to note that most centers rely on clinical assessment to evaluate treatment efficacy, while MRD is still largely viewed as an unvalidated, experimental assessment that is not routinely used. This needs to be addressed across the region, given that MRD is the most effective way to assess the depth of remission and determine the likelihood of patient relapse (67). Flow cytometry is widely available throughout the region, meaning most centers have the capability to directly assess MRD, while next-generation sequencing facilities are also available, albeit in a limited number of specialized facilities. Despite these capabilities, MRD assessment has not been widely adopted in the region due to lack of expertise and validation in Arab patient populations, alongside the lack of standardized local protocols to support its widespread use. At present there is a lack of data on long-term outcomes and sustainability of FD treatment strategies; however, the European research initiative in CLL (ERIC) is initiating a multicenter project to further examine the ibrutinib-venetoclax FD protocol and work is being conducted in several centers across the Gulf region to generate real-world data on the use of this treatment strategy. A focus of future CLL clinical management initiatives in Gulf countries should include education and uptake on the use of MRD, which is an accessible prognostic technique that can be used as an effective tool to guide treatment decisions (67).
Conclusion
In the absence of large clinical datasets in Arab patients, we have drawn on our collective experience to outline key considerations for clinicians in the Middle East region who are managing CLL patients using a FD treatment strategy. The selection of the optimal treatment strategy depends on a number of factors, most importantly patient demographics, baseline comorbidities, and preferences/logistics. Our position statement is that ibrutinib-venetoclax should be the first choice as first-line therapy for all fit CLL patients in the region, irrespective of their age. In addition, the option of an all-oral treatment with ibrutinib-venetoclax presents several advantages in terms of patient convenience, logistics, and reducing the burden on healthcare administration costs compared with monoclonal antibody-based treatments.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MY: Conceptualization, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. KF: Conceptualization, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. AH: Conceptualization, Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing. RG: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. AA: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. KM: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. HY: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. HO: Data curation, Formal analysis, Investigation, Validation, Writing – original draft, Writing – review & editing. MT: Data curation, Formal analysis, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors are grateful to the Emirates Society of Haematology (ESH) and Meeting Minds Experts for their support and endorsement of this manuscript. The authors would like to thank the Qatar International Hematology Conference, organized by the National Center for Cancer Care & Research, Doha, Qatar, for their generous collaboration in hosting the expert panel meeting. The authors acknowledge John Bett and Laura D’Castro from Astraeus Medical for editorial support in the preparation of this manuscript. All authors met the International Committee of Medical Journal Editors authorship criteria.
Conflict of interest
MY, AH, and RG are employed by the Hamad Medical Corporation. MT is an employee at Augsburg University Hospital. He received travel grants and speakers honoraria by Johnson & Johnson.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mukkamalla, SKR, Taneja, A, Malipeddi, D, and Master, SR. Chronic lymphocytic leukemia. Treasure Island, FL: StatPearls Publishing (2024).
2. Yao, Y, Lin, X, Li, F, Jin, J, and Wang, H. The global burden and attributable risk factors of chronic lymphocytic leukemia in 204 countries and territories from 1990 to 2019: analysis based on the global burden of disease study 2019. Biomed Eng Online. (2022) 21:4. doi: 10.1186/s12938-021-00973-6
3. Alam, A, Al Qawasmeh, K, Alam, N, and McCarthy, PL. Patterns of disease, risk factors and treatment in chronic lymphocytic leukemia (CLL). A retrospective report of real world patient cohort from United Arab Emirates. Blood. (2023) 142:6558–8. doi: 10.1182/blood-2023-182574
4. Alshemmari, SH, Hamdah, A, Pandita, R, and Kunhikrishnan, A. Chronic lymphocytic leukemia in a young population. Leuk Res. (2021) 110:106668. doi: 10.1016/j.leukres.2021.106668
5. Alshemmari, SH, AlSarraf, A, Kaempf, A, and Danilov, AV. Prognostic impact of chronic lymphocytic leukemia comorbidity index in a young population: a real-world evidence study of a national gulf region cohort. BMC Cancer. (2024) 24:584. doi: 10.1186/s12885-024-12343-1
6. Yassin, MA, Soliman, AT, Nashwan, AJ, Alamami, AA, Abdulla, MAJ, Hmissi, SM, et al. Hematological indices reference intervals for a healthy Arab population in Qatar: effect of age, gender, and geographic location. Medicine. (2022) 101:e29271. doi: 10.1097/MD.0000000000029271
7. Yassin, MA, Soliman, AT, Hmissi, SM, Abdulla, MAJ, Itani, M, Alamami, AA, et al. Prevalence of neutropenia among adult Arabs in Qatar: relation to other hematological parameters and anthropometric data. Medicine. (2022) 101:e30431. doi: 10.1097/MD.0000000000030431
8. Bewarder, M, Stilgenbauer, S, Thurner, L, and Kaddu-Mulindwa, D. Current treatment options in CLL. Cancers. (2021) 13:2468. doi: 10.3390/cancers13102468
9. Wierda, WG, and Tambaro, FP. How I manage CLL with venetoclax-based treatments. Blood. (2020) 135:1421–7. doi: 10.1182/blood.2019002841
10. Stumpf, J, and Al-Sawaf, O. Chronic lymphocytic leukemia: time-limited therapy in the first-line setting and role of minimal residual disease. Curr Oncol Rep. (2024) 26:136–46. doi: 10.1007/s11912-023-01482-6
11. Wartmann, H, Kabilka, A, Deiters, B, Schmitz, N, and Volmer, T. A decade of chronic lymphocytic leukaemia therapy in Germany: real-world treatment patterns and outcomes (2010-2022). EJHaem. (2024) 5:346–52. doi: 10.1002/jha2.888
12. Kater, AP, Owen, C, Moreno, C, Follows, G, Munir, T, Levin, M-D, et al. Fixed-duration Ibrutinib-Venetoclax in patients with chronic lymphocytic leukemia and comorbidities. NEJM Evid. (2022) 1:2006. doi: 10.1056/EVIDoa2200006
13. Tam, CS, Allan, JN, Siddiqi, T, Kipps, TJ, Jacobs, R, Opat, S, et al. Fixed-duration ibrutinib plus venetoclax for first-line treatment of CLL: primary analysis of the CAPTIVATE FD cohort. Blood. (2022) 139:3278–89. doi: 10.1182/blood.2021014488
14. Fischer, K, Al-Sawaf, O, Bahlo, J, Fink, A-M, Tandon, M, Dixon, M, et al. Venetoclax and Obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. (2019) 380:2225–36. doi: 10.1056/NEJMoa1815281
15. Molica, S, and Allsup, D. Fixed-duration therapy comes of age in CLL: long-term results of MURANO and CLL14 trials. Expert Rev Anticancer Ther. (2024) 24:101–6. doi: 10.1080/14737140.2023.2288899
16. Eichhorst, B, Niemann, CU, Kater, AP, Fürstenau, M, Von Tresckow, J, Zhang, C, et al. First-line Venetoclax combinations in chronic lymphocytic leukemia. N Engl J Med. (2023) 388:1739–54. doi: 10.1056/NEJMoa2213093
17. Cervantes-Gomez, F, Lamothe, B, Woyach, JA, Wierda, WG, Keating, MJ, Balakrishnan, K, et al. Pharmacological and protein profiling suggests Venetoclax (ABT-199) as optimal partner with Ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res. (2015) 21:3705–15. doi: 10.1158/1078-0432.CCR-14-2809
18. Kater, AP, Slinger, E, Cretenet, G, Martens, AW, Balasubramanian, S, Leverson, JD, et al. Combined ibrutinib and venetoclax treatment vs single agents in the TCL1 mouse model of chronic lymphocytic leukemia. Blood Adv. (2021) 5:5410–4. doi: 10.1182/bloodadvances.2021004861
19. Deng, J, Isik, E, Fernandes, SM, Brown, JR, Letai, A, and Davids, MS. Bruton’s tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia. Leukemia. (2017) 31:2075–84. doi: 10.1038/leu.2017.32
20. Allan, JN, Flinn, IW, Siddiqi, T, Ghia, P, Tam, CS, Kipps, TJ, et al. Outcomes in patients with high-risk features after fixed-duration Ibrutinib plus Venetoclax: phase II CAPTIVATE study in first-line chronic lymphocytic leukemia. Clin Cancer Res. (2023) 29:2593–601. doi: 10.1158/1078-0432.CCR-22-2779
21. Rogers, KA, and Woyach, JA. A CAPTIVATE-ing new regimen for CLL. Blood. (2022) 139:3229–30. doi: 10.1182/blood.2022015963
22. Barr, PM, Tedeschi, A, Wierda, WG, Allan, JN, Ghia, P, Vallisa, D, et al. Effective tumor Debulking with Ibrutinib before initiation of Venetoclax: results from the CAPTIVATE minimal residual disease and fixed-duration cohorts. Clin Cancer Res. (2022) 28:4385–91. doi: 10.1158/1078-0432.CCR-22-0504
23. Jain, N, Croner, LJ, Allan, JN, Siddiqi, T, Tedeschi, A, Badoux, XC, et al. Absence of BTK, BCL2, and PLCG2 mutations in chronic lymphocytic leukemia relapsing after first-line treatment with fixed-duration Ibrutinib plus Venetoclax. Clin Cancer Res. (2024) 30:498–505. doi: 10.1158/1078-0432.CCR-22-3934
24. Niemann, CU, Munir, T, Moreno, C, Owen, C, Follows, GA, Benjamini, O, et al. Fixed-duration ibrutinib-venetoclax versus chlorambucil-obinutuzumab in previously untreated chronic lymphocytic leukaemia (GLOW): 4-year follow-up from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. (2023) 24:1423–33. doi: 10.1016/S1470-2045(23)00452-7
25. Jain, N, Keating, M, Thompson, P, Ferrajoli, A, Burger, JA, Borthakur, G, et al. Ibrutinib plus Venetoclax for first-line treatment of chronic lymphocytic leukemia: a nonrandomized phase 2 trial. JAMA Oncol. (2021) 7:1213–9. doi: 10.1001/jamaoncol.2021.1649
26. Jain, N, Keating, M, Thompson, P, Ferrajoli, A, Burger, J, Borthakur, G, et al. Ibrutinib and Venetoclax for first-line treatment of CLL. N Engl J Med. (2019) 380:2095–103. doi: 10.1056/NEJMoa1900574
27. Al-Sawaf, O, Zhang, C, Tandon, M, Sinha, A, Fink, A-M, Robrecht, S, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. (2020) 21:1188–200. doi: 10.1016/S1470-2045(20)30443-5
28. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology chronic lymphocytic leukemia/small lymphocytic lymphoma version 1.2025. Washington, DC: National Comprehensive Cancer Network (2024).
29. Sharman, JP, Egyed, M, Jurczak, W, Skarbnik, A, Pagel, JM, Flinn, IW, et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia. (2022) 36:1171–5. doi: 10.1038/s41375-021-01485-x
30. Tam, CS, Brown, JR, Kahl, BS, Ghia, P, Giannopoulos, K, Jurczak, W, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. (2022) 23:1031–43. doi: 10.1016/S1470-2045(22)00293-5
31. Howard, SC, Jones, DP, and Pui, C-H. The tumor lysis syndrome. N Engl J Med. (2011) 364:1844–54. doi: 10.1056/NEJMra0904569
33. Fischer, K, Al-Sawaf, O, and Hallek, M. Preventing and monitoring for tumor lysis syndrome and other toxicities of venetoclax during treatment of chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. (2020) 2020:357–62. doi: 10.1182/hematology.2020000120
34. Roeker, LE, Fox, CP, Eyre, TA, Brander, DM, Allan, JN, Schuster, SJ, et al. Tumor lysis, adverse events, and dose adjustments in 297 Venetoclax-treated CLL patients in routine clinical practice. Clin Cancer Res. (2019) 25:4264–70. doi: 10.1158/1078-0432.CCR-19-0361
35. Ghosh, N, Matusz-Fisher, A, Bose, R, Boselli, D, Magee, G, Chen, T, et al. Evaluation of the impact of monitoring for tumor lysis during Venetoclax ramp-up in chronic lymphocytic leukemia in routine clinical practice. JCO Oncol. Pract. (2024) 22:OP2400417. doi: 10.1200/OP.24.00417
36. Benkhadra, M, Fituri, N, Aboukhalaf, S, Ghasoub, R, Mattar, M, Alfarsi, K, et al. The safety of novel therapies in chronic lymphocytic leukemia in the era of intermittent fasting: a pharmacology-based review. Cancers. (2024) 16:2079. doi: 10.3390/cancers16112079
37. Von Hundelshausen, P, and Siess, W. Bleeding by Bruton tyrosine kinase-inhibitors: dependency on drug type and disease. Cancers. (2021) 13:1103. doi: 10.3390/cancers13051103
38. Kaptein, A, De Bruin, G, Emmelot-van Hoek, M, Van De Kar, B, De Jong, A, Gulrajani, M, et al. Potency and selectivity of BTK inhibitors in clinical development for B-cell malignancies. Blood. (2018) 132:1871–1. doi: 10.1182/blood-2018-99-109973
39. Albaqawi, ASB, El-Fetoh, NMA, Alanazi, RFA, Alanazi, NSF, Alrayya, SE, Alanazi, ANM, et al. Profile of peptic ulcer disease and its risk factors in Arar. Northern Saudi Arabia Electr Physician. (2017) 9:5740–5. doi: 10.19082/5740
40. Iraki, L, Abkari, A, Vallot, T, Amrani, N, Khlifa, RH, Jellouli, K, et al. Effect of Ramadan fasting on intragastric pH recorded during 24 hours in healthy subjects. Gastroenterol Clin Biol. (1997) 21:813–9.
41. Ozkan, S, Durukan, P, Akdur, O, Vardar, A, Torun, E, and Ikizceli, I. Does Ramadan fasting increase acute upper gastrointestinal haemorrhage? J Int Med Res. (2009) 37:1988–93. doi: 10.1177/147323000903700637
42. Palleria, C, Di Paolo, A, Giofrè, C, Caglioti, C, Leuzzi, G, Siniscalchi, A, et al. Pharmacokinetic drug-drug interaction and their implication in clinical management. J Res Med Sci. (2013) 18:601–10.
43. Murru, R, Galitzia, A, Barabino, L, Presicci, R, La Nasa, G, and Caocci, G. Prediction of severe infections in chronic lymphocytic leukemia: a simple risk score to stratify patients at diagnosis. Ann Hematol. (2024) 103:1655–64. doi: 10.1007/s00277-024-05625-y
44. Moradinazar, M, Afshar, ZM, Ramazani, U, Shakiba, M, Shirvani, M, and Darvishi, S. Epidemiological features of tuberculosis in the Middle East and North Africa from 1990 to 2019: results from the global burden of disease study 2019. Afr Health Sci. (2023) 23:366–75. doi: 10.4314/ahs.v23i3.43
45. Iqbal, P, Soliman, A, De Sanctis, V, and Yassin, MA. Association of tuberculosis in patients with chronic myeloid leukemia: a treatment proposal based on literature review. Expert Rev Hematol. (2021) 14:211–7. doi: 10.1080/17474086.2021.1875818
46. Lyon, AR, López-Fernández, T, Couch, LS, Asteggiano, R, Aznar, MC, Bergler-Klein, J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the international cardio-oncology society (IC-OS). Eur Heart J. (2022) 43:4229–361. doi: 10.1093/eurheartj/ehac244
47. Boriani, G, Menna, P, Morgagni, R, Minotti, G, and Vitolo, M. Ibrutinib and Bruton’s tyrosine kinase inhibitors in chronic lymphocytic leukemia: focus on atrial fibrillation and ventricular Tachyarrhythmias/sudden cardiac death. Chemotherapy. (2023) 68:61–72. doi: 10.1159/000528019
48. Tang, CPS, McMullen, J, and Tam, C. Cardiac side effects of bruton tyrosine kinase (BTK) inhibitors. Leuk Lymphoma. (2018) 59:1554–64. doi: 10.1080/10428194.2017.1375110
49. Munir, T, Cairns, DA, Bloor, A, Allsup, D, Cwynarski, K, Pettitt, A, et al. Chronic lymphocytic leukemia therapy guided by measurable residual disease. N Engl J Med. (2024) 390:326–37. doi: 10.1056/NEJMoa2310063
50. Fernandez Turizo, MJ, Kim, E, Asnani, A, Yankama, T, Von Keudell, G, and Mejías-De, JC. Pre-existing cardiovascular disease increases the risk of cardiovascular adverse events during Bruton tyrosine kinase inhibitor therapy. Blood. (2023) 142:496–6. doi: 10.1182/blood-2023-174560
51. Khan, R, Siddiqui, AA, Alshammary, F, Shaikh, S, Amin, J, and Rathore, HA. Diabetes in the Arab world In: I Laher, editor. Handbook of healthcare in the Arab world. Cham: Springer International Publishing (2021). 1–24.
52. Mamdouh, H, Hussain, HY, Ibrahim, GM, Alawadi, F, Hassanein, M, Zarooni, AA, et al. Prevalence and associated risk factors of overweight and obesity among adult population in Dubai: a population-based cross-sectional survey in Dubai, the United Arab Emirates. BMJ Open. (2023) 13:e062053. doi: 10.1136/bmjopen-2022-062053
53. Al Mahmeed, W, Bakir, S, Beshyah, SA, Morcos, B, Wajih, S, Horack, M, et al. Prevalence of lipid abnormalities and cholesterol target value attainment in patients with stable and acute coronary heart disease in the United Arab Emirates. Heart Views. (2019) 20:37–46. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_32_18
54. Akl, C, Akik, C, Ghattas, H, and Obermeyer, CM. The cascade of care in managing hypertension in the Arab world: a systematic assessment of the evidence on awareness, treatment and control. BMC Public Health. (2020) 20:835. doi: 10.1186/s12889-020-08678-6
55. Rogers, KA, Lu, X, Emond, B, Ding, Z, Lefebvre, P, Lafeuille, M-H, et al. Real-world (RW) dosing patterns and outcomes among chronic lymphocytic leukemia (CLL) patients (pts) with or without an ibrutinib (IBR) dose adjustment (DA) in first-line (1L). JCO. (2023) 41:7537–7. doi: 10.1200/JCO.2023.41.16_suppl.7537
56. Ghosh, N, Wang, R, Ding, Z, He, J, Bokun, A, Mavani, H, et al. Comparative effectiveness of Ibrutinib flexible dosing treatment strategies on time to next treatment in a largely community-based claims database: a target trial emulation study. Blood. (2023) 142:270–14. doi: 10.1182/blood-2023-187743
57. Shadman, M, Karve, S, Patel, S, Rava, A, Sun, H, Howarth, A, et al. Impact of Ibrutinib dose reduction on duration of therapy in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Blood. (2023) 142:269–9. doi: 10.1182/blood-2023-181774
58. Stephens, DM, Brown, JR, Ma, S, Wang, M, Moreno, CD, Robak, T, et al. Ibrutinib dose modifications for management of cardiac adverse events in patients with B-cell malignancies: pooled analysis of 10 clinical trials. JCO. (2023) 41:7538–8. doi: 10.1200/JCO.2023.41.16_suppl.7538
59. Shadman, M, Srivastava, B, Patel, S, Saifan, C, Salkar, M, Emond, B, et al. P 621: real-world dosing patterns and time to next treatment for previously untreated patients with Cll/Sll with or without Ibrutinib dose reduction following an adverse event. Hema Sphere. (2023) 7:e24922b0. doi: 10.1097/01.HS9.0000969388.24922.b0
60. Okati-Aliabad, H, Ansari-Moghaddam, A, Kargar, S, and Jabbari, N. Prevalence of obesity and overweight among adults in the Middle East countries from 2000 to 2020: a systematic review and Meta-analysis. J Obes. (2022) 2022:8074837. doi: 10.1155/2022/8074837
61. Al-Ghamdi, S, Abu-Alfa, A, Alotaibi, T, AlSaaidi, A, AlSuwaida, A, Arici, M, et al. Chronic kidney disease Management in the Middle East and Africa: concerns, challenges, and novel approaches. Int J Nephrol Renovasc Dis. (2023) 16:103–12. doi: 10.2147/IJNRD.S363133
64. Quartermaine, C, Ghazi, SM, Yasin, A, Awan, FT, Fradley, M, Wiczer, T, et al. Cardiovascular toxicities of BTK inhibitors in chronic lymphocytic leukemia: JACC: cardio oncology state-of-the-art review. JACC Cardio Oncol. (2023) 5:570–90. doi: 10.1016/j.jaccao.2023.09.002
65. Bhagavathula, AS, Shehab, A, Ullah, A, and Rahmani, J. The burden of cardiovascular disease risk factors in the Middle East: a systematic review and Meta-analysis focusing on primary prevention. Curr Vasc Pharmacol. (2021) 19:379–89. doi: 10.2174/1573406416666200611104143
66. Aboelkhir, H, Alaoui, YE, Padmanabhan, R, Elomri, A, Omri, HE, and Oomri, AE. Unpacking the clinical burden of leukemia in GCC: Implications for patient care. Scitepress. (2024) 2:444–9. doi: 10.5220/0012373700003657
Keywords: chronic lymphocytic leukemia, fixed-duration treatment, ibrutinib, venetoclax, obinutuzumab, Arab, Middle East
Citation: Yassin MA, Al Farsi K, Hamad A, Ghasoub R, Alhuraiji A, Mheidly K, Yaseen HA, Osman H and Trepel M (2025) Upfront fixed-duration treatment strategies for chronic lymphocytic leukemia in Arab populations: a position statement from the Gulf region. Front. Med. 12:1509074. doi: 10.3389/fmed.2025.1509074
Edited by:
Andrea Visentin, University of Padua, ItalyReviewed by:
Clement Chung, Houston Methodist Hospital, United StatesCopyright © 2025 Yassin, Al Farsi, Hamad, Ghasoub, Alhuraiji, Mheidly, Yaseen, Osman and Trepel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed A. Yassin, eWFzc2luQGhhbWFkLnFh
†ORCID: Anas Hamad, https://orcid.org/0000-0001-8606-8521
Rola Ghasoub, https://orcid.org/0000-0002-1392-8831
 Mohamed A. Yassin
Mohamed A. Yassin Khalil Al Farsi2
Khalil Al Farsi2 Anas Hamad
Anas Hamad Rola Ghasoub
Rola Ghasoub Martin Trepel
Martin Trepel