
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 29 January 2025
Sec. Intensive Care Medicine and Anesthesiology
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1505408
This article is part of the Research Topic Post-Operative Neuropsychiatric Disorders, volume II View all 3 articles
Background: Emergence delirium(ED) is a common postoperative complication in children undergoing tonsillectomy and adenoidectomy under general anesthesia. There is no high-quality evidence on the relationship between esketamine and ED. The systematic review and meta-analysis was performed to investigate the effect of perioperative esketamine use on ED in children undergoing tonsillectomy and adenoidectomy.
Method: We searched Embase, The Cochrane Library, PubMed, MEDLINE, Web of Science, China National Knowledge Infrastructure (CNKI), WanFang, VIP, and SinoMed from inception to 1 September, 2024. Two evaluators identified randomized controlled trials comparing perioperative use of esketamine with placebo or other drugs in children undergoing tonsillectomy and adenoidectomy. Incidence of ED was the primary outcome of the study. Data synthesis was performed by using Review Manager 5.4 software.
Results: Twenty-three relevant studies involving a total of 1,996 children were identified. Perioperative use of esketamine reduced the incidence of ED in children undergoing tonsillectomy and adenoidectomy (RR = 0.33, 95% CI: [0.25, 0.44], p < 0.00001, I2 = 0%). Scores of ED were lower in the esketamine group than in the control group (SMD = -1.20, 95% CI: [−1.56,-0.84], p < 0.00001, I2 = 88%). Children in the esketamine group have lower postoperative pain scores (SMD = -0.51, 95% CI: [−0.80,-0.39], p < 0.00001, I2 = 74%). Esketamine was also associated with a lower incidence of adverse events (RR = 0.75, 95% CI: [0.57, 0.99], p = 0.04, I2 = 62%). We also found that the use of esketamine reduced the length of stay in the post-anesthetic care unit (PACU) but had no effect on the time to extubation.
Conclusion: Perioperative use of esketamine could significantly reduce the incidence of ED in children undergoing tonsillectomy and adenoidectomy. However, the optimal dose and timing of esketamine administration for preventing ED remains to be explored.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=558560, PROSPERO: CRD42024558560.
Emergence delirium(ED) is a clinically recognized condition that often occurs during the recovery phase of anesthesia and is characterized by agitation, confusion, and restlessness (1). ED is a common perioperative complication in children with a prevalence of approximately 10–80% (2). ED is self-limiting and lasts in typically 15–30 min, but the long-term postoperative cognitive changes of ED in children are unknown (3). Hazards of emergence delirium include wound dehiscence, accidental removal of intravenous infusion tubes or drains, and falling out of bed (4). ED may also cause harm to healthcare workers and family members caring for the child, increase the difficulty of postoperative care, increase the incidence of postoperative complications, and be detrimental to the child’s postoperative recovery (1, 5).
Tonsillectomy and adenoidectomy are the most common surgeries performed on children, with more than 500,000 annually in the United States (6). Pediatric adenoidectomies and tonsillectomies are characterized by short operating times, severe stress response, and high levels of postoperative pain. Currently, general anesthesia with opioids combined with propofol and sevoflurane is widely used for tonsillectomy and adenoidectomy. However, sevoflurane causes a high incidence of delirium during the awakening period, and opioids have the disadvantage of respiratory and circulatory depression (7, 8). After tonsillectomy and adenoidectomy, children often experience complications such as pain, bleeding, nausea, and vomiting. ED can lead to an increased incidence of these complications (9). Therefore, it is necessary to find an appropriate anesthesia plan to reduce the occurrence of delirium during the awakening period and provide comfortable medical treatment for children.
Esketamine is the dextro isomer of ketamine, and its anesthetic and analgesic effects are mainly achieved by non-competitive antagonism of N-methyl-D-aspartic acid (NMDA) receptors (10). Esketamine is an intravenous anesthetic with analgesic properties that can be safely used for the induction and maintenance of general anesthesia and postoperative analgesia. Esketamine has the advantages of good analgesic effect, slight respiratory depression, and inhibition of inflammatory response (11). Subanesthetic doses of esketamine can exert antidepressant effects and improve postoperative cognitive dysfunction (12, 13). Esketamine can also reduce the use of opioid analgesics and even antagonize opioid-induced respiratory depression (14, 15).
Although a number of clinical trials have been conducted to study the relationship between esketamine and ED in pediatrics under general anesthesia, they are all small-sample studies and still lack high-quality evidence. In this study, we investigated the effect of esketamine on delirium during the awakening period after tonsillectomy and adenoidectomy by meta-analysis method to provide a clinical reference.
This meta-analysis was conducted following the recommendations of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement and registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (registration number CRD42024558560).
The literature search was conducted on Embase, The Cochrane Library, PubMed, MEDLINE, Web of Science, CNKI, WanFang, VIP, and SinoMed from inception to 1 September 2024. Four key search terms (‘Emergence Delirium’, ‘Esketamine’, ‘Tonsillectomy or Adenoidectomy’ and ‘Chindren’), with varition, were used and combined using Boolean operators. There were no restrictions on language, gender, sample size, or geographic location during the literature search. Supplementary Table 1 lists the search strategies adapted for each database. Articles that may be eligible by reviewing the reference lists of retrieved studies will be included in this meta-analysis (16, 17).
To assess the eligibility of the acquired studies for the meta-analysis, we adopted the following criteria: (1) Population: children undergoing tonsillectomy and adenoidectomy under general anesthesia, (2) Intervention: perioperative intravenous administration of esketamine, (3) Comparison: placebo or other drugs, (4) Outcomes: development of emergence delirium, (5) Study design: randomized controlled trials, (6) statistical methods used correctly, and (7) complete data.
Exclusion criteria were: (1) duplication of published literature, (2) failure to provide valid data or missing data, (3) non-RCT studies such as reviews and animal experiments, and (4) low quality of literature. Included studies were assessed using the Cochrane risk of bias tool. Studies with high risk of bias for randomization or allocation concealment were judged to be of low quality and excluded (18).
Records from searches were imported into an EndNote library (EndNote 20) and duplicate studies were removed. The remaining records were transferred into an Excel spreadsheet (Microsoft). Articles were screened by 2 independent reviewers who evaluated the article title, abstract, and full text. Studies that did not meet the established inclusion criteria were excluded. Disagreements between two reviewers regarding the inclusion of studies were resolved through discussion or consultation with a third reviewer.
Two reviewers independently assessed the included studies using the Cochrane risk-of-bias tool. The Cochrane tool was used to assess the possibility of different biases across the included randomized controlled trials, including selection bias, implementation bias, measurement bias, follow-up bias, reporting bias, and other biases (18). Each bias of the studies was categorized as low risk, high risk, and unclear risk. Disagreements settled in consultation with a third reviewer.
Two reviewers independently extracted data items from the included studies. Information collected included first author, year of publication, age, gender, sample size, mode of induction of anesthesia, study design, experimental group intervention, control group intervention, incidence of emergence delirium, severity of emergence delirium, level of pain, time to extubation, length of stay in the post-anesthetic care unit (PACU), and incidence of adverse events. Disagreements between the two reviewers regarding the data were resolved by consulting a third reviewer.
In this study, data synthesis was performed by using Review Manager 5.4 software (Cochrane Collaboration; Oxford, UK). Meta-analysis of categorical variables was performed by Mantel–Haenszel (M-H) statistics to calculate the risk ratio (RR) and 95% confidence interval (CI). Meta-analysis of continuous variables was performed by Inverse-Variance (I-V) statistics to calculate the mean difference (MD), standard mean difference (SMD), and 95% confidence interval. When the units and scales of the outcome indicators were the same (such as time to extubation and the length of stay in PACU), MD was used to interpret the results; conversely, SMD was used to interpret the results (such as delirium score and pain score). The results of the 3 studies (19–21) were presented as medians with interquartile ranges. We transformed them into means ± standard deviation (SD) by a reported methodology (22, 23). The I2 statistic was used to assess statistical heterogeneity between pooled data. I2 < 50% indicated that study heterogeneity was relatively small, in which case a fixed effects model (FEM) was used to synthesize the data. In contrast, I2 ≥ 50% indicated that study heterogeneity was relatively large, in which case a random effects model (REM) was used to synthesize the data. All tests were two-tailed test and were defined as statistically significant when p < 0.05.
We performed a subgroup analysis to assess whether the relationship between esketamine application and ED was modified by clinical characteristics. The subgroup plan included (1) dose of esketamine administration: ≥ 0.5 mg/kg or < 0.5 mg/kg; (2) timing of perioperative esketamine administration: before anesthesia, during anesthesia (at the time of induction, induction combined with intraoperative maintenance) and at the end of surgery; and (3) type of drug in the control group: saline or blank control, opioid anesthetic drug control, and other anesthetic drug control.
In addition, we assessed publication bias by funnel plots when at least 10 studies reported on the primary outcome measure (24). Sensitivity analysis was performed to explore the impact of study quality on the overall results using successive exclusion of included individual studies.
A total of 108 potentially relevant articles were initially identified from the 9 databases, 52 articles were removed due to duplication, and the remaining 56 studies were screened. We excluded 29 articles due to insufficient relevance based on the title and abstract. The characteristics of the excluded studies are shown in the PRISMA diagram (Figure 1). 27 studies were included in the systematic review, 23 of which were further included in the meta-analysis.
In the 23 studies included, esketamine was administered intravenously. The dose range was 0.15 mg/kg to 1 mg/kg, and the time of administration included before anesthesia, during anesthesia (maintained at induction or in combination with induction), and at the end of surgery (dosage and time of administration in specific studies were recorded in detail in Table 1).
The risks of bias in individual studies were presented in Figure 2. As for the domain of randomization, 5 RCTs (17, 25–28) were lack of information about randomized methods and 19 RCTs (17, 21, 25–41) were lack of information about allocation concealment. In the domain of intended intervention, 9 RCTs (16, 20, 25, 27, 28, 30, 34, 35, 37) were rated high risk due to specialized intervention methods and 9 RCTs (26, 29, 31–33, 36, 38, 40, 41) were rated unclear risk due to a lack of information on blinding. One RCT (16) was rated high risk in the domain of measurement bias because the researchers know the subgroups. All RCTs were rated low risk in follow-up bias and reporting bias. Regarding other biases, one RCT (30) was rated unclear risk because control group information was not detailed (Figure 3).
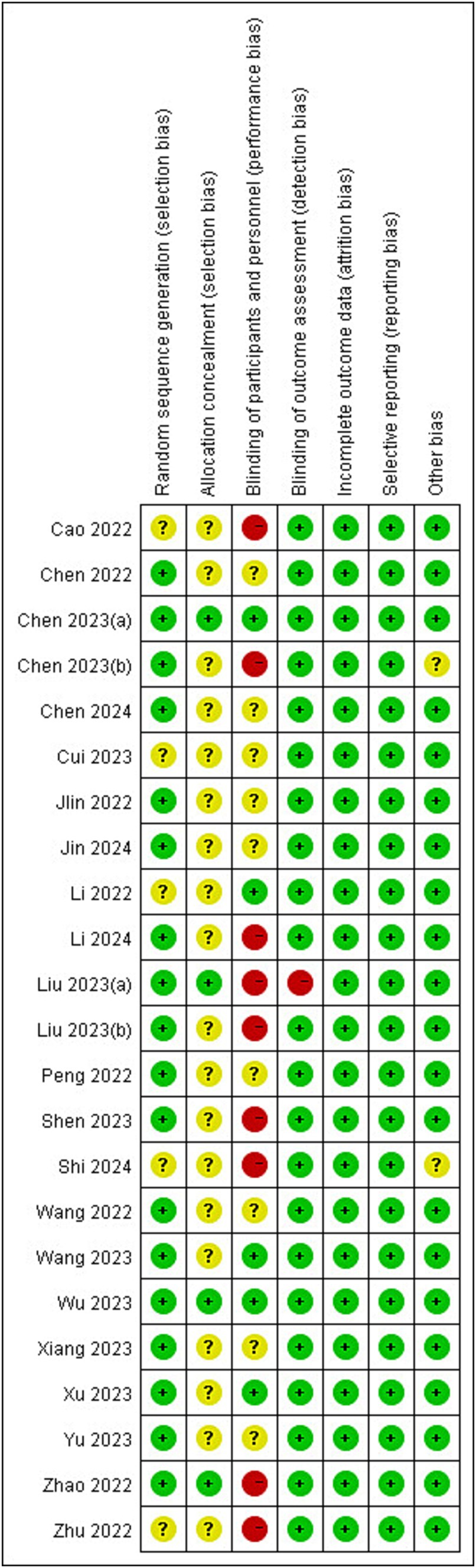
Figure 2. Risks of bias of the included studies. Risk of bias. Green: low risk; yellow: some concern; red: high risk.
The main indicator of this study is the incidence of emergence delirium. According to different control groups, different doses of esketamine, and different administration times, the corresponding subgroup analysis was carried out. The results of the subgroup analysis were presented in Table 2.
Of 23 included studies, 13 reported the incidence of emergence delirium (Figure 4). The incidence of ED in pediatrics treated with esketamine perioperatively was significantly lower than that in the control group (RR = 0.33, 95% CI: [0.25, 0.44], p < 0.00001, I2 = 0%, 13 trials, 1,113 participants).
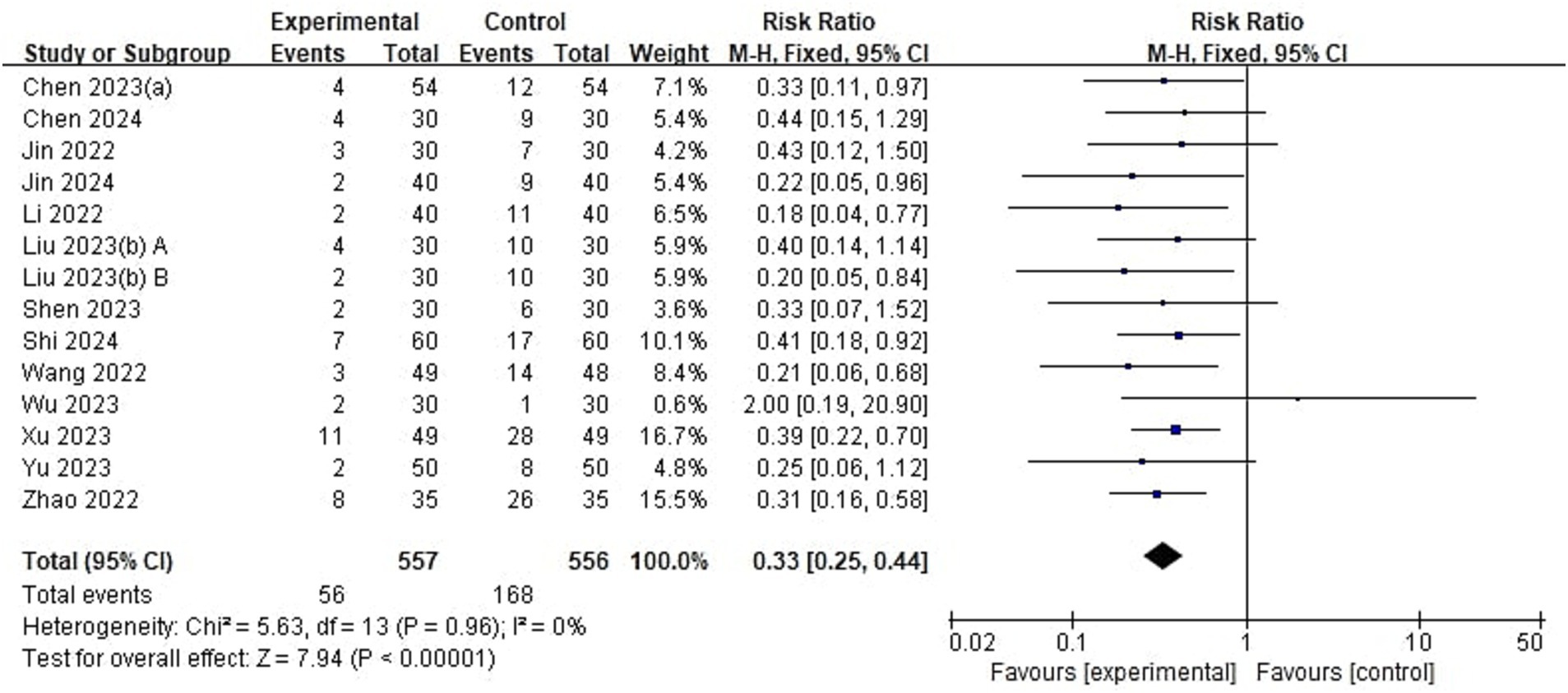
Figure 4. Forest plot comparing the risk of emergence delirium between esketamine and control groups. M-H, Mantel–Haenszel; CI, confidence interval.
The control group in 7 studies (17, 19, 21, 27, 35, 37, 38) was saline or blank. These studies showed the incidence of ED in pediatrics treated with esketamine perioperatively was significantly lower than that in pediatrics treated with saline or the blank control (RR = 0.35, 95% CI: [0.25, 0.49], p < 0.00001, I2 = 0%, 7 trials, 663 participants). 3 studies (31–33) indicated pediatrics using esketamine have a lower incidence of emergence delirium compared with opioids (RR = 0.28, 95% CI: [0.13, 0.58], p = 0.0006, I2 = 0%, 3 trials, 220 participants). In the other 3 studies (20, 41, 42), the control groups were other anesthetic drugs including remimazolam, midazolam, and dexmedetomidine. Pediatrics using esketamine also have a lower incidence of emergence delirium compared with other anesthetic drugs (RR = 0.34, 95% CI: [0.20, 0.60], p = 0.0002, I2 = 18%, 3 trials, 230 participants). These results suggest that esketamine could reduce the incidence of ED compared with different control groups, and there were no significant differences between subgroups (p = 0.86, Supplementary Figure 1).
The dose of esketamine ≥0.5 mg/kg was the anesthetic dose group and the dose of esketamine <0.5 mg/kg was the subanaesthetic dose group. Anesthetic doses of esketamine were given in 7 studies (27, 31, 32, 35, 37, 41, 42). The incidence of ED was significantly lower in the anesthetic dose group compared with the control group (RR = 0.38, 95% CI: [0.24, 0.60], p < 0.0001, I2 = 0%, 7 trials, 520 participants). Subanaesthetic doses of esketamine were given in 7 studies (17, 19–21, 33, 35, 38). The incidence of emergence delirium was also significantly lower in the low-dose esketamine group compared with the control group (RR = 0.31, 95% CI: [0.22, 0.43], p < 0.00001, I2 = 0%, 7 trials, 593 participants). These results suggest that different doses of esketamine could reduce the incidence of ED, and there were no significant differences between subgroups (p = 0.48, Supplementary Figure 2).
Depending on the time of administration of esketamine, these studies were classified as administered before anesthesia, during anesthesia, and at the end of surgery. Esketamine given before anesthesia reduced the incidence of ED compared with the control group (RR = 0.31, 95% CI: [0.15, 0.63], p = 0.001, I2 = 0%, 4 trials, 317 participants). Esketamine administrated during anesthesia reduced the occurrence of emergence delirium compared with the control group (RR = 0.36, 95% CI: [0.25, 0.52], p < 0.00001, I2 = 0%, 5 trials, 478 participants). Pediatrics who were given esketamine at the end of surgery had a lower incidence of emergence delirium compared with the control group (RR = 0.31, 95% CI: [0.19, 0.49], p < 0.00001, I2 = 0%, 4 trials, 318 participants). These results suggest that esketamine administration at different times could reduce the incidence of ED, and there were no significant differences between subgroups (p = 0.87, Supplementary Figure 3).
Secondary outcomes of this study included delirium scores, pain scores, time to extubation, length of stay in the PACU, and incidence of adverse events (Table 3).
Of the 23 included studies, 14 provided details regarding delirium scores (Figure 5). Analysis results showed that the delirium score in the esketamine group was lower than that in the control group (SMD = −1.20, 95% CI: [−1.56,-0.84], p < 0.00001, I2 = 88%, 14 trials, 1,319 participants), indicating that esketamine was beneficial in reducing the severity of postoperative delirium in children.
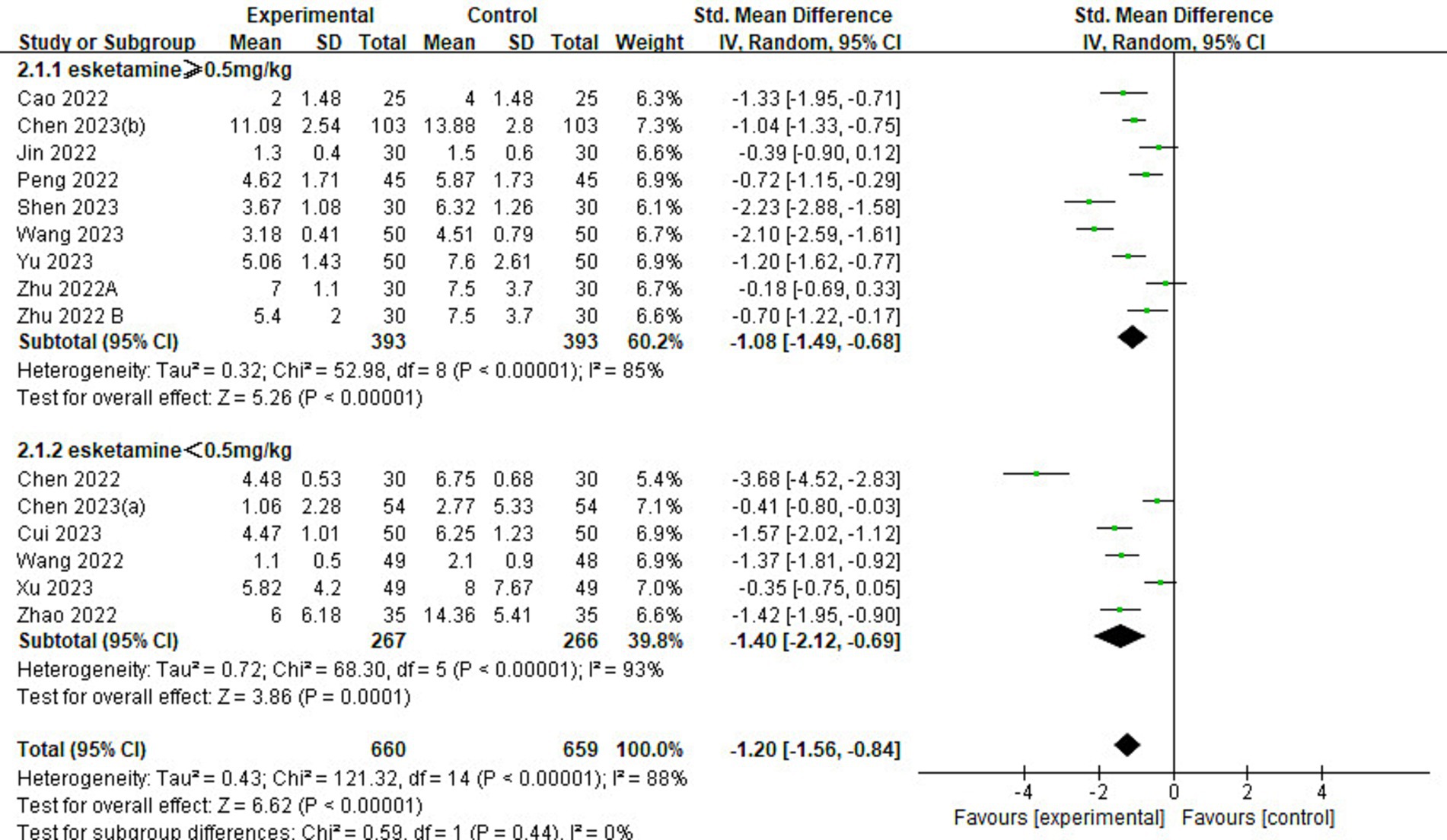
Figure 5. Forest plot comparing the delirium scores between esketamine and control groups. IV, Inverse Variance; CI, confidence interval.
Of the 23 included studies, 16 reported information on pain scores (Figure 6). Pain scores in the esketamine group were lower than those in the control group (SMD = −0.51, 95% CI: [−0.80,-0.39], p < 0.00001, I2 = 74%, 16 trials, 1,563 participants), indicating that esketamine had a positive effect on postoperative pain reduction relief in children.
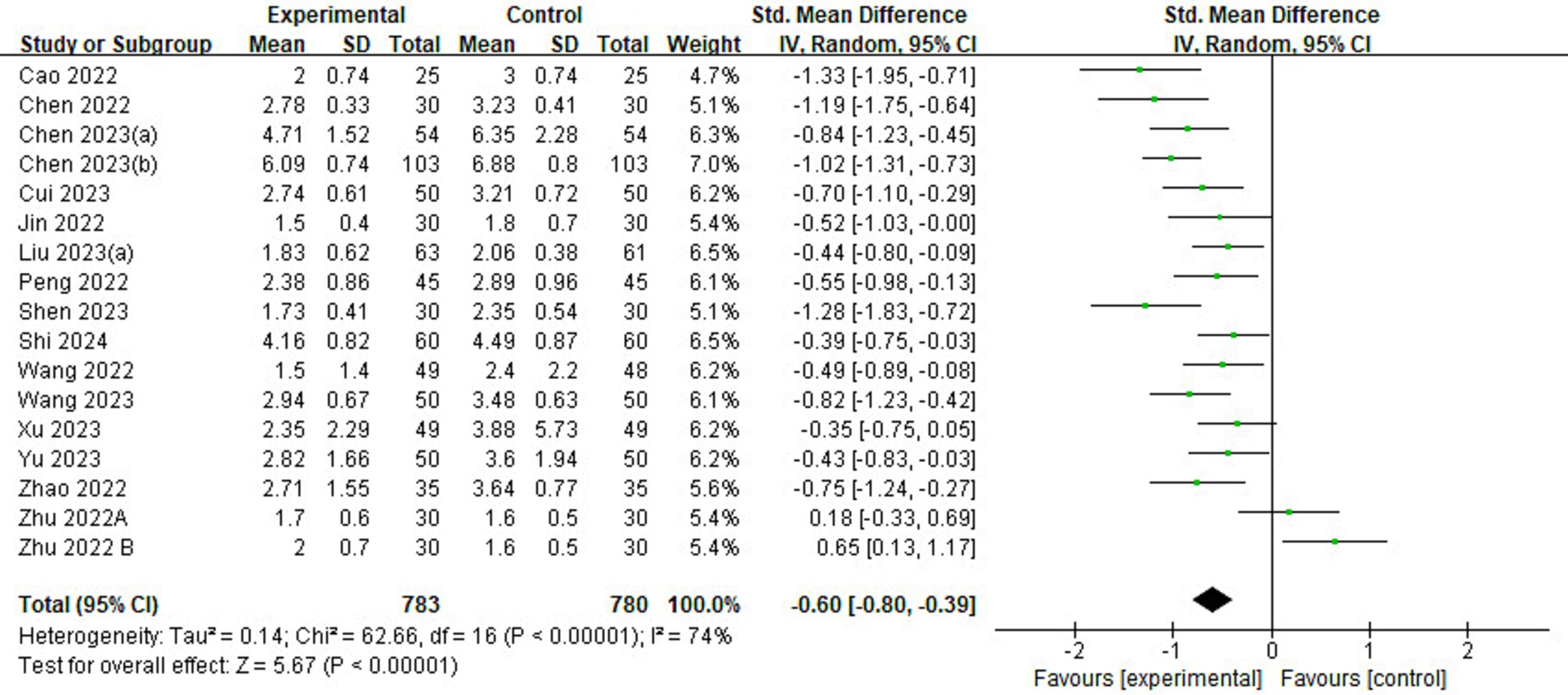
Figure 6. Forest plot comparing the pain scores between esketamine and control groups. IV, inverse variance; CI, confidence interval.
Of the 23 included studies, 11 provided data on time to extubation (Figure 7). The results showed no significant difference in extubation time between esketamine and control groups (MD = 0.01, 95% CI: [−1.44, 1.46], p = 0.99, I2 = 95%, 11 trials, 1,072 participants), implying that esketamine had little effect on postoperative extubation time in children.
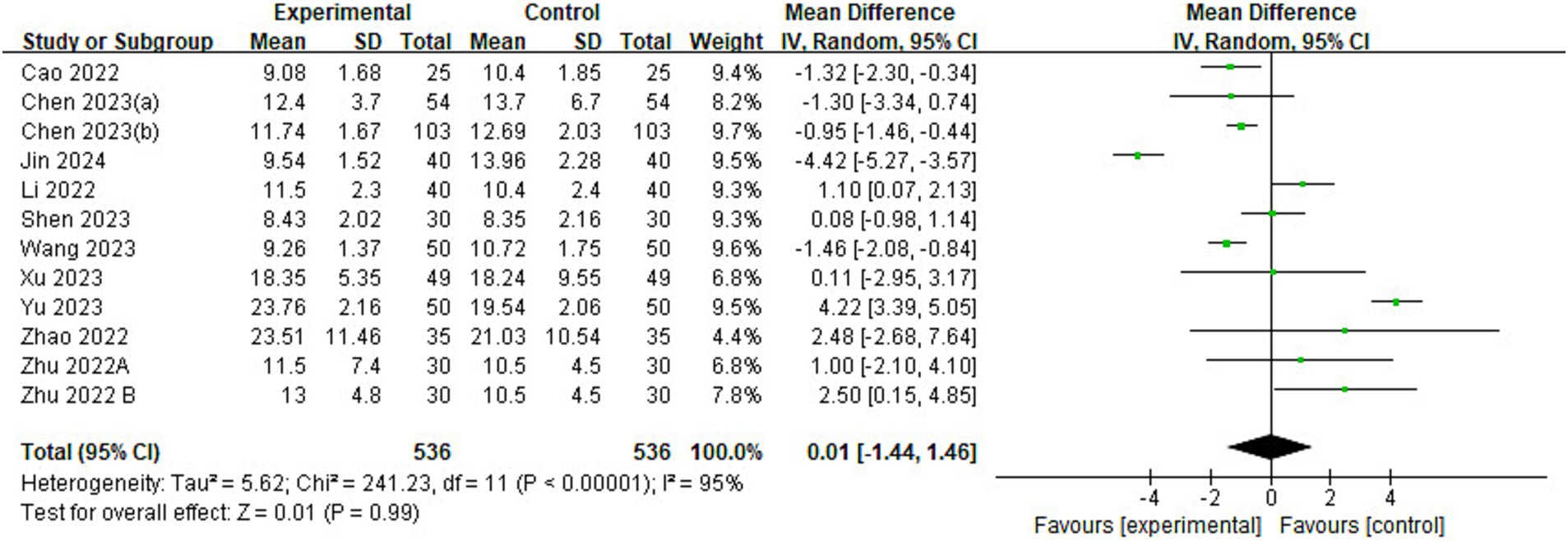
Figure 7. Forest plot comparing the time to extubation between esketamine and control groups. IV, Inverse Variance; CI, confidence interval.
Of the 23 included studies, 12 reported information on length of stay in the PACU (Figure 8). The analysis found that children in the esketamine group had a shorter stay in the PACU than the control group (MD = −2.03, 95% CI: [−4.20, 0.14], p = 0.07, I2 = 95%, 12 trials, 1,025 participants), and although the p value was close to the significance level, it still showed a trend that esketamine may shorten the length of stay in the PACU.
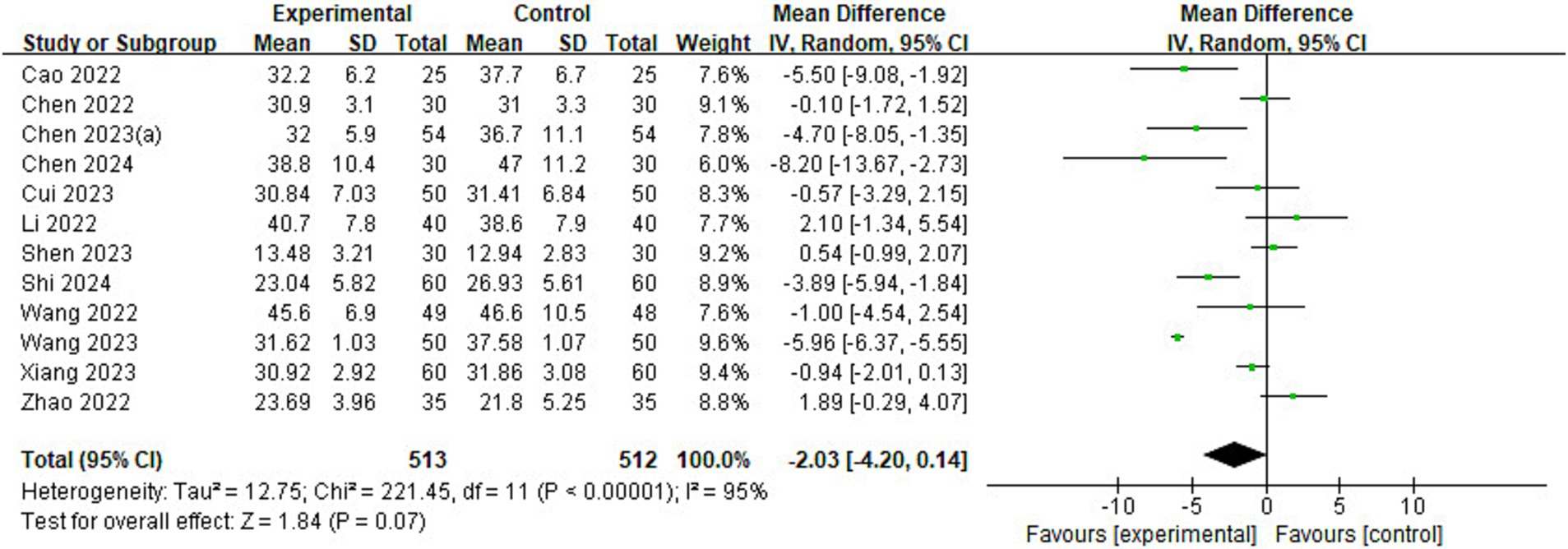
Figure 8. Forest plot comparing the length of stay in the PACU between esketamine and control groups. IV, Inverse Variance; CI, confidence interval; PACU, post-anesthetic care unit.
Of the 23 included studies, and 22 reported details on the incidence of adverse events (Figure 9). The risk of adverse events was lower in the esketamine group than in the control group (RR = 0.75, 95% CI: [0.57–0.99], p = 0.04, I2 = 62%, 22 trials, 2,142 participants), suggesting that esketamine has some advantages in reducing the incidence of postoperative adverse events in children.
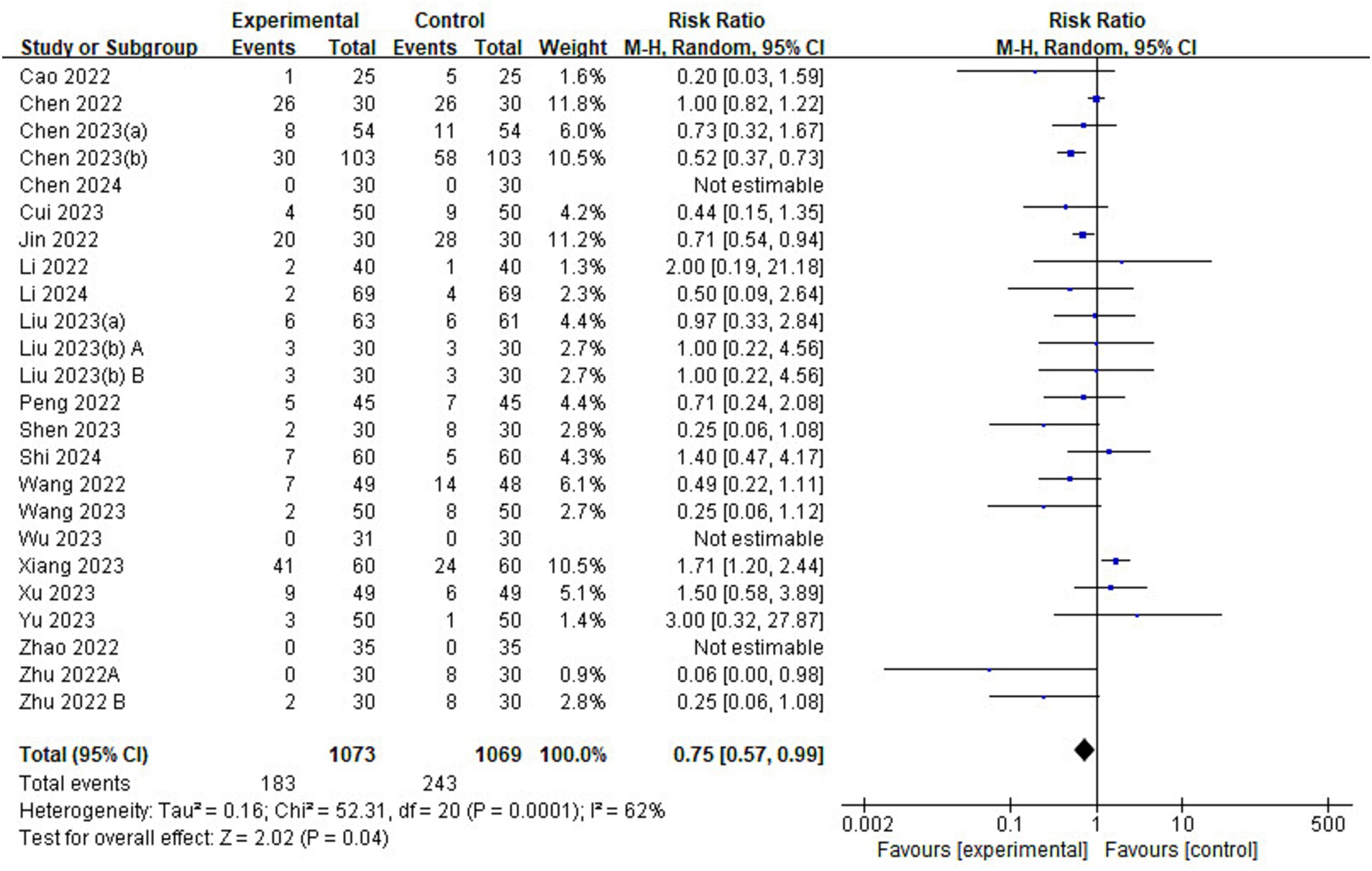
Figure 9. Forest plot comparing the risk of adverse events between esketamine and control groups. M-H, Mantel–Haenszel; CI, confidence interval.
In the analysis of the incidence of ED, one study (42) was found to be at high risk of publication bias by plotting a funnel plot (Figure 10). After excluding the study with high risk of publication bias, the results showed that the funnel plot was symmetrical and suggested that publication bias was small (Figure 11).
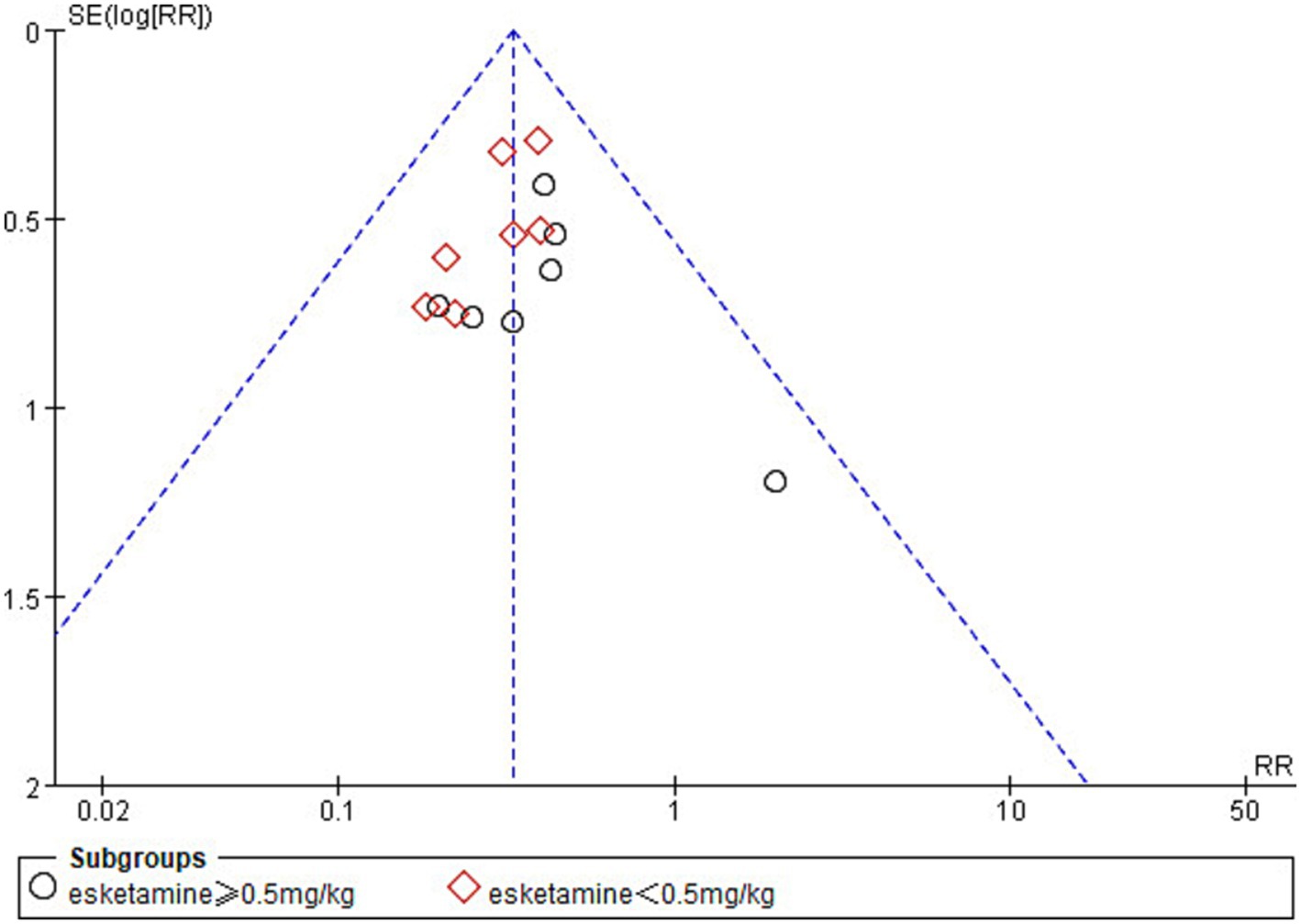
Figure 10. Funnel plot of the incidence of emergence delirium between esketamine and control groups.
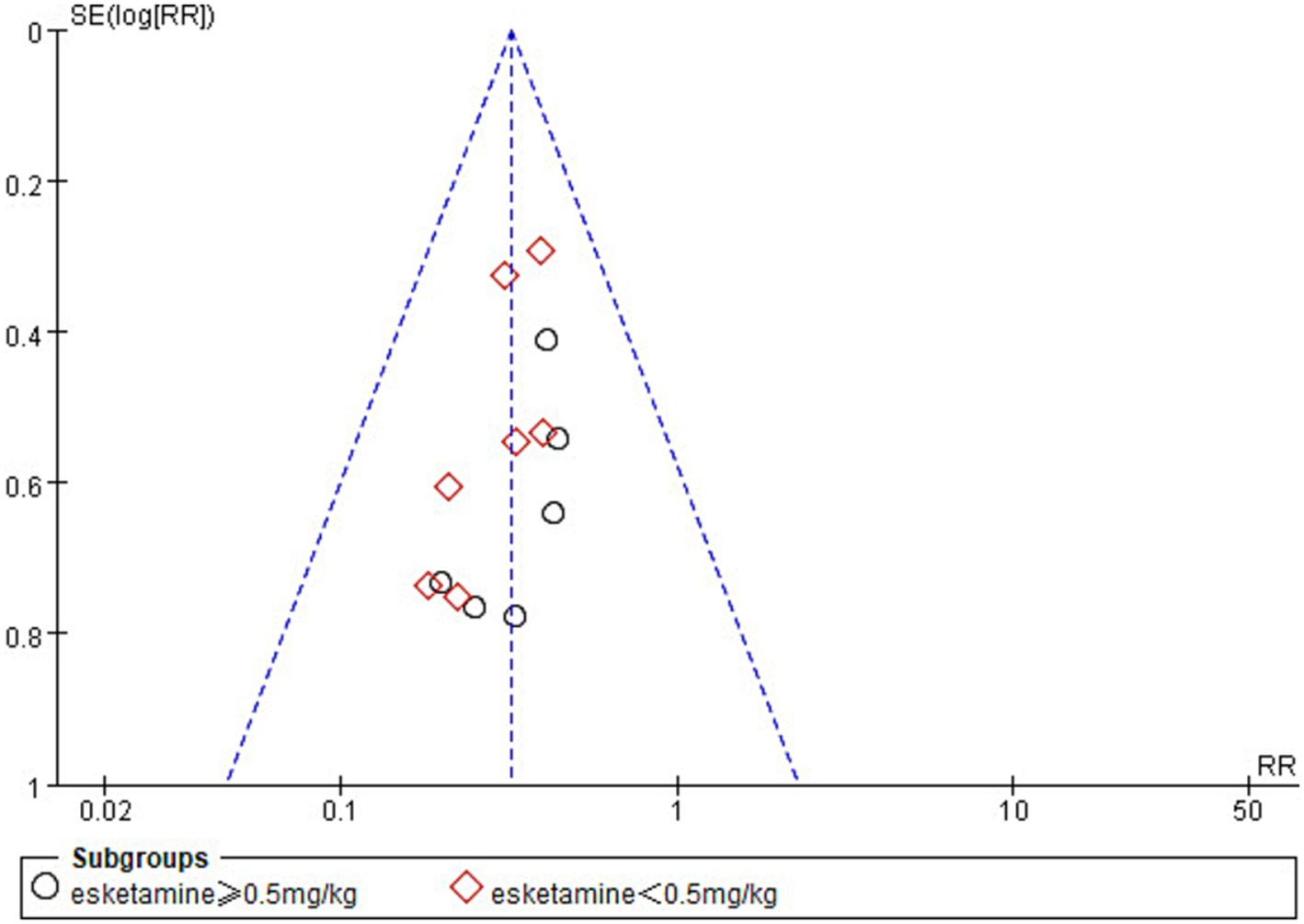
Figure 11. Funnel plot comparing the incidence of emergence delirium between esketamine and control groups. The funnel plot removes one study with high bias.
After excluding each study, the effect of esketamine on the incidence of ED remained significant (RR = 0.32–0.34, 95% CI: [0.24–0.46]). The results showed studies with a high risk of bias did not unduly affect the pooled results (Table 4). This further strengthened the reliability of our conclusion that perioperative use of esketamine could reduce the incidence of ED in children undergoing tonsillectomy and adenoidectomy.
Emergence delirium (ED) is an early complication of general anesthesia in pediatrics, presenting with perceptual deficits and psychomotor agitation (2). ED may cause postoperative complications, prolong hospital stay, and increase medical costs. The mechanism of ED in children remains unclear, and the risk factors of ED may include preschool age, ophthalmological and otorhinolaryngological procedures, inhalational anesthetics with low blood gas partition coefficients, prolonged duration of surgery, preoperative anxiety, and postoperative pain (43). The incidence of ED was significantly higher in pediatrics undergoing otorhinolaryngology procedures than in other pediatrics (44). The effect of different perioperative anesthetic drug use on emergence delirium also varies. However, perioperative use of ketamine may reduce the risk of ED (45, 46). Esketamine is the dextro isomer of ketamine and its pharmacological characteristics are similar to those of ketamine. However, compared to ketamine, esketamine has a stronger receptor affinity, stronger analgesic effect, faster metabolism, and fewer and milder adverse effects (11). Therefore, esketamine is widely used for the induction and maintenance of general anesthesia, ambulatory surgery, pediatric surgery, and postoperative analgesia.
In this systematic review and meta-analysis, we identified 23 randomized controlled trials that examined the effect of perioperative esketamine use on the incidence of delirium, delirium scores, pain scores, time to extubation, length of stay in the PACU, and incidence of adverse events in children undergoing tonsillectomy and adenoidectomy. Our results suggested that perioperative use of esketamine could reduce the risk of ED after general anesthesia when compared with a blank control or saline group, opioids, or other anesthetic drugs. Meanwhile, esketamine administrations before anesthesia, during anesthesia, or at the end of surgery could significantly reduce the incidence of ED. In addition, perioperative use of both anesthetic and subanaesthetic doses of esketamine significantly reduced the incidence of ED. Our study also found that perioperative use of esketamine reduced delirium and pain scores, shortened the length of stay in PACU, and reduced the risk of adverse events, but had little effect on the time to extubation.
ED is a common postoperative adverse event and prevention of ED is necessary (47). Our findings support the notion that perioperative use of esketamine could prevent ED. The ability of esketamine to reduce the risk of pediatric ED may be related to its unique pharmacological properties. Esketamine produces anesthesia and analgesia mainly through the inhibition of NMDA receptors and also produces analgesia through the inhibition of opioid receptors via G-protein coupling (48). While, one of the included studies had different results. The study of Wu et al. (42) reported there was no difference in the incidence of ED between the esketamine group and control group, which may be due to the administration of remimazolam in the the control group. Remazolam is a new ultra-short-acting benzodiazepine that can relieve preoperative anxiety. In addition, Yang et al. (49) found that administration of remimazolam at the end of the surgery could reduce the incidence of ED in children following tonsillectomy and adenoidectomy under sevoflurane anesthesia.
Three of the included studies were esketamine versus other anesthetic drugs (20, 41, 42). The control groups in these three studies were administrated remimazolam, midazolam, and dexmedetomidine, respectively. Although we found that esketamine reduced the risk of ED compared with midazolam and dexmedetomidine, there was only one study with a small sample size to support this result, respectively. Further multi-center, large sample-size clinical trials are needed to determine the effect of esketamine on ED compared to other anesthetics. Compared with the blank control or saline group, esketamine significantly reduced the incidence of ED. This suggests that adding esketamine to the standard anesthetic regimen may help prevent ED. However, Chen et al.’s findings were contrary, showing that a single dose of near-anesthetic for anesthesia induction may increase the risk of ED in preschool children after surgery (50). This may be due to a higher proportion of children in the esketamine group who were treated with sevofluorine for maintenance of anesthesia. In order to determine the effect of perioperative esketamine use on ED, more standardized anesthetic regimens are necessary in the future. In summary, based on our findings, it can be inferred that perioperative esketamine use could prevent ED, but further evidence from higher-quality studies is needed.
Pain is an important risk factor for ED in children. Esketamine could provide effective analgesia, reduce postoperative pain scores, decrease opioid consumption, and improve the quality of perioperative recovery (51, 52). This meta-analysis showed that esketamine can reduce postoperative pain levels, which is consistent with the findings of Qian et al. (53). Five of the included studies showed that perioperative use of esketamine reduced postoperative pain in children undergoing tonsillectomy and adenoidectomy compared with perioperative use of opioids and one of the included studies showed that esketamine was comparable to opioids for analgesia (28). These results suggest that esketamine is effective in improving postoperative pain in children undergoing tonsillectomy and adenoidectomy. Heavy opioid use can cause pain hypersensitivity, which is a state of hypersensitivity to painful stimuli (54). However, esketamine could relieve opioid-induced pain hypersensitivity and enhance opioid analgesia (55).
It is important to note that doses of perioperative esketamine use vary (from 0.15 mg/kg to 1 mg/kg). Meanwhile, times of esketamine administration are also different (including before anesthesia, during anesthesia, and at the end of surgery). Although this meta-analysis showed that different times of administration of esketamine and different doses of esketamine both could reduce the incidence of ED, we are unable to simultaneously determine an optimal timing and dosage of administration to prevent ED. The pharmacological effect of esketamine at different doses and times may be different in extent and duration, which may have different effects on results. The difference in the dose and time of esketamine administration between studies may also introduce confounding factors and bias the results.
This systematic review and meta-analysis still has several potential limitations. Firstly, most of the studies included were small studies with a sample size of each group less than 100, which may lead to small effect study bias (56). Second, ED is delirium that occurs in the operating room or PACU after anesthesia has ended. However, the time point of assessing ED was inconsistent among the included studies which may lead to inconsistent measurement results. Third, the usage and dosage of esketamine varied among the included studies, and we are unable to provide valuable recommendations for the use of esketamine in the perioperative period. Fourth, The measurement tools were different in included studies. As a result, there will be some differences in the results. Therefore, further more standardized perioperative esketamine protocols and unified assessment tools for ED should be developed for prevention of ED in children undergoing tonsillectomy and adenoidectomy. Fifth, there are data source limitations in this study, and all included studies were conducted in China. This may affect the external validity of the results due to differences in medical practice, patient characteristics, anesthesia management practices, and so on in different countries. More international studies are needed in the future, including groups of children in different regions, to validate our findings and improve the broad applicability of the results.
In conclusion, this systematic review and meta-analysis suggests that the perioperative use of esketamine could significantly reduce the incidence of ED in children undergoing tonsillectomy and adenoidectomy. In addition, perioperative administration of esketamine reduces the risk of postoperative adverse events. However, the optimal dose and timing of esketamine administration for preventing ED remains to be explored.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
JuL: Conceptualization, Writing – original draft. JiL: Methodology, Writing – review & editing. HS: Visualization, Writing – review & editing. XC: Investigation, Writing – review & editing. CW: Resources, Writing – review & editing. DL: Formal analysis, Writing – review & editing. CH: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1505408/full#supplementary-material
SUPPLEMENTARY FIGURE 1 | Forest plot comparing the risk of emergence delirium between esketamine and different control groups. M-H, Mantel-Haenszel; CI, confidence interval.
SUPPLEMENTARY FIGURE 2 | Forest plot comparing the risk of emergence delirium between different doses of esketamine and control groups. M-H, Mantel-Haenszel; CI, confidence interval.
SUPPLEMENTARY FIGURE 3 | Forest plot comparing the risk of emergence delirium between esketamine administrated at different time and control groups. M-H, Mantel-Haenszel; CI, confidence interval.
1. Somaini, M, Sahillioglu, E, Marzorati, C, Lovisari, F, Engelhardt, T, and Ingelmo, PM. Emergence delirium, pain or both? A challenge for clinicians. Paediatr Anaesth. (2015) 25:524–9. doi: 10.1111/pan.12580
2. Moore, AD, and Anghelescu, DL. Emergence delirium in pediatric anesthesia. Paediatr Drugs. (2017) 19:11–20. doi: 10.1007/s40272-016-0201-5
3. Aldecoa, C, Bettelli, G, Bilotta, F, Sanders, RD, Audisio, R, Borozdina, A, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. (2017) 34:192–214. doi: 10.1097/EJA.0000000000000594
4. Hilly, J, Horlin, AL, Kinderf, J, Ghez, C, Menrath, S, Delivet, H, et al. Preoperative preparation workshop reduces postoperative maladaptive behavior in children. Paediatr Anaesth. (2015) 25:990–8. doi: 10.1111/pan.12701
5. Hudek, K. Emergence delirium: a nursing perspective. AORN J. (2009) 89:509–16. doi: 10.1016/j.aorn.2008.12.026
6. Nguyen, BK, and Quraishi, HA. Tonsillectomy and adenoidectomy - pediatric clinics of North America. Pediatr Clin N Am. (2022) 69:247–59. doi: 10.1016/j.pcl.2021.12.008
7. Veyckemans, F. Excitation and delirium during sevoflurane anesthesia in pediatric patients. Minerva Anestesiol. (2002) 68:402–5.
8. Baldo, BA. Toxicities of opioid analgesics: respiratory depression, histamine release, hemodynamic changes, hypersensitivity, serotonin toxicity. Arch Toxicol. (2021) 95:2627–42. doi: 10.1007/s00204-021-03068-2
9. Johnson, LB, Elluru, RG, and Myer, CM 3rd. Complications of adenotonsillectomy. Laryngoscope. (2002) 112:35–6. doi: 10.1002/lary.5541121413
10. Molero, P, Ramos-Quiroga, JA, Martin-Santos, R, Calvo-Sanchez, E, Gutierrez-Rojas, L, and Meana, JJ. Antidepressant efficacy and tolerability of ketamine and Esketamine: a critical review. CNS Drugs. (2018) 32:411–20. doi: 10.1007/s40263-018-0519-3
11. Zhang, XX, Zhang, NX, Liu, DX, Ding, J, Zhang, YN, and Zhu, ZQ. Research advances in the clinical application of esketamine. Ibrain. (2022) 8:55–67. doi: 10.1002/ibra.12019
12. Daly, EJ, Trivedi, MH, Janik, A, Li, H, Zhang, Y, Li, X, et al. Efficacy of Esketamine nasal spray plus Oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. (2019) 76:893–903. doi: 10.1001/jamapsychiatry.2019.1189
13. Ma, J, Wang, F, Wang, J, Wang, P, Dou, X, Yao, S, et al. The effect of low-dose Esketamine on postoperative neurocognitive dysfunction in elderly patients undergoing general anesthesia for gastrointestinal tumors: a randomized controlled trial. Drug Des Devel Ther. (2023) 17:1945–57. doi: 10.2147/DDDT.S406568
14. Zhu, T, Zhao, X, Sun, M, An, Y, Kong, W, Ji, F, et al. Opioid-reduced anesthesia based on esketamine in gynecological day surgery: a randomized double-blind controlled study. BMC Anesthesiol. (2022) 22:354. doi: 10.1186/s12871-022-01889-x
15. Jonkman, K, van Rijnsoever, E, Olofsen, E, Aarts, L, Sarton, E, van Velzen, M, et al. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. (2018) 120:1117–27. doi: 10.1016/j.bja.2018.02.021
16. Liu, F, Kong, F, Zhong, L, Wang, Y, Xia, Z-f, and Wu, J. Preoperative Esketamine alleviates postoperative pain after endoscopic plasma Adenotonsillectomy in children. Clin Med Res. (2023) 21:79–86. doi: 10.3121/cmr.2023.1818
17. Li, Q, Fan, J, and Zhang, W. Low-dose esketamine for the prevention of emergency agitation in children after tonsillectomy: a randomized controlled study. Front Pharmacol. (2022) 13:991581. doi: 10.3389/fphar.2022.991581
18. Higgins, JP, Altman, DG, Gøtzsche, PC, Jüni, P, Moher, D, Oxman, AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
19. Chen, Y, Ru, F, Ye, Q, Wu, X, Hu, X, Zhang, Y, et al. Effect of S-ketamine administered at the end of anesthesia on emergence delirium in preschool children undergoing tonsillectomy and/or adenoidectomy. Front Pharmacol. (2023) 14:1044558. doi: 10.3389/fphar.2023.1044558
20. Zhao, Z. (2022). Comparison of the effect of esketamine and dexmedetomidine in the prevention of postoperative agitation in children following tonsillectomy and adenoidectomy. Available at: https://link.cnki.net/doi/10.27652/d.cnki.gzyku.2022.001146 (Accessed January 16, 2023).
21. Xu, J, Gao, Z, Wang, F, Zhang, J, Mao, Z, and Zhang, X. Observation of the effect of low-dose esketamine on emergence delirium in children undergoing tonsillectomy. Beijing Med J. (2023) 45:65. doi: 10.15932/j.0253-9713.2023.01.017
22. Wan, X, Wang, W, Liu, J, and Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
23. Luo, D, Wan, X, Liu, J, and Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
24. Sterne, JA, Sutton, AJ, Ioannidis, JP, Terrin, N, Jones, DR, Lau, J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. doi: 10.1136/bmj.d4002
25. Cao, Y, Jia, L, Sheng, M, Yuan, S, Yu, W, and Zhang, Y. Observations on the use of esketamine intravenous injection anesthesia in pediatric tonsillectomy and adenoidectomy. Shandong Med J. (2022) 62:864. doi: 10.3969/j.issn.1002-266X.2022.24.011
26. Cui, X. Analysis of the value of prophylactic application of esketamine in children undergoing tonsillectomy and adenoidectomy. Pract Clin J Integr Tradit Chin Western Med. (2023) 23:156. doi: 10.13638/j.issn.1671-4040.2023.02.028
27. Shi, S, Bai, Y, Zheng, Y, and Yan, H. Effectiveness of esketamine combined with conventional sedative drugs in pediatric adenoidectomy. China Pharm. (2024) 33:92–6. doi: 10.3969/j.issn.1006-4931.2024.07.020
28. Zhu, N. (2022). Effect of esketamine on the quality of the awakening in children undergoing tonsillectomy and adenoidectomy. Available at: https://link.cnki.net/doi/10.27374/d.cnki.gwnyy.2022.000308 (Accessed January 16, 2023).
29. Chen, J. Effect of Esketamine on agitation and pain during awakening after pediatric tonsillectomy and adenoidectomy. Health Guide. (2022) 9:77–80.
30. Chen, Y, Jia, Y, and Zhou, R. Effect of preventive use of Esketamine on the quality of the awakening period in children with tonsil adenoidectomy. J Henan Med Coll Staff Workers. (2023) 35:864. doi: 10.3969/j.issn.1008-9276.2023.06.002
31. Chen, K, Xie, Y, Xue, Q, and Shen, X. Effect of esketamine on awakening in children after endoscopic adenoidectomy. Fudan Univ J Med Sci. (2024) 51:76–80. doi: 10.3969/j.issn.1672-8467.2024.01.011
32. Jin, B, Jiang, Z, and Guo, C. Use of Esketamine in pediatric adenoidectomy. Chin J Rural Med Pharm. (2022) 29:33–5. doi: 10.19542/j.cnki.1006-5180.006295
33. Jin, M. Effect of prophylactic application of a small dose of esketamine on emergence agitation after general anesthesia in children undergoing tonsillectomy and adenoidectomy. Tianjin Pharm. (2024) 36:47–50. doi: 10.3969/j.issn.1006-5687.2024.02.013
34. Li, L, Ma, W, Gao, Y, and Zhang, Y. Effects of esketamine versus dexmedetomidine adjuvant general anesthesia on PAED scores, quality of awakening, and adverse events in pediatrics following tonsillectomy and adenoidectomy. Clin Misdiagn Mistherapy. (2024) 37:53–7. doi: 10.3969/issn.1002-3429.2024.06.011
35. Liu, W, Ma, B, and Zhang, L. Efficacy of esketamine in preventing restlessness in anesthesia recovery period in children undergoing low-temperature plasma knife tonsil adenoidectomy. Jiangsu Pharm. (2023) 49:35. doi: 10.19460/j.cnki.0253-3685.2023.09.021
36. Peng, Y, and Du, Y. Study of the application of esketamine in pediatric tonsillectomy and adenoidectomy. Gansu Pharm. (2022) 41:78. doi: 10.15975/j.cnki.gsyy.2022.12.014
37. Shen, M, and Liu, D. Effect of low-dose Esketamine hydrochloride on Restlessness, Pain, and hemodynamics during recovery period after tonsillectomy and adenoidectomy in children. Chin Foreign Med Res. (2023) 21:65. doi: 10.14033/j.cnki.cfmr.2023.10.003
38. Wang, X, Yuan, J, Xing, F, Zhu, P, Guo, Y, Ding, X, et al. Effect of small dose of esmketamine on emergence agitation in children undergoing tonsillectomy. J Clin Anesthes. (2022) 38:98. doi: 10.12089/jca.2022.02.009
39. Wang, Y, Wang, Y, Qiu, Y, and Jia, Y. Application of Esketamine intravenous injection anesthesia in tonsillectomy and adenoidectomy in children. Clin Res. (2023) 31:128. doi: 10.12385/j.issn.2096-1278(2023)05-0058-04
40. Xiang, SQ, Zeng, P, Wang, ZP, Wu, SX, and Li, CJ. Clinical anesthetic effect of esketamine on children undergoing tonsillectomy. Mol Cell Toxicol. (2023) 20:573–7. doi: 10.1007/s13273-023-00366-x
41. Yu, G, Cai, Y, Chen, Y, and Xue, R. Effect evaluation of esketamine on tonsillectomy and adenoidectomy in children. J Clin Med Pract. (2023) 27:457. doi: 10.7619/jcmp.20230084
42. Wu, M, Yang, F, Ma, X, and Cai, N. Comparison of clinical effects and safety of remidazolam and esketamine for preoperative sedation in children. Nan fang yi ke da xue xue bao =. J South Med Univ. (2023) 43:2126–31. doi: 10.12122/j.issn.1673-4254.2023.12.18
43. Lee, SJ, and Sung, TY. Emergence agitation: current knowledge and unresolved questions. Korean J Anesthesiol. (2020) 73:471–85. doi: 10.4097/kja.20097
44. Voepel-Lewis, T, Malviya, S, and Tait, AR. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth Analg. (2003) 96:1625–30. doi: 10.1213/01.ANE.0000062522.21048.61
45. Lee, YS, Kim, WY, Choi, JH, Son, JH, Kim, JH, and Park, YC. The effect of ketamine on the incidence of emergence agitation in children undergoing tonsillectomy and adenoidectomy under sevoflurane general anesthesia. Korean J Anesthesiol. (2010) 58:440–5. doi: 10.4097/kjae.2010.58.5.440
46. Abu-Shahwan, I, and Chowdary, K. Ketamine is effective in decreasing the incidence of emergence agitation in children undergoing dental repair under sevoflurane general anesthesia. Paediatr Anaesth. (2007) 17:846–50. doi: 10.1111/j.1460-9592.2007.02298.x
47. Quintão, VC, Sales, CKO, Herrera, EM, Ellerkmann, RK, Rosen, HD, and Carmona, MJC. Emergence delirium in children: a Brazilian survey. Braz J Anesthesiol. (2022) 72:207–12. doi: 10.1016/j.bjane.2020.12.029
48. Zhou, JS, Peng, GF, Liang, WD, Chen, Z, Liu, YY, Wang, BY, et al. Recent advances in the study of anesthesia-and analgesia-related mechanisms of S-ketamine. Front Pharmacol. (2023) 14:1228895. doi: 10.3389/fphar.2023.1228895
49. Yang, X, Lin, C, Chen, S, Huang, Y, Cheng, Q, and Yao, Y. Remimazolam for the prevention of emergence delirium in children following tonsillectomy and adenoidectomy under Sevoflurane anesthesia: a randomized controlled study. Drug Des Devel Ther. (2022) 16:3413–20. doi: 10.2147/DDDT.S381611
50. Chen, S, Yang, JJ, Zhang, Y, Lei, L, Qiu, D, Lv, HM, et al. Risk of esketamine anesthesia on the emergence delirium in preschool children after minor surgery: a prospective observational clinical study. Eur Arch Psychiatry Clin Neurosci. (2024) 274:767–75. doi: 10.1007/s00406-023-01611-z
51. Brinck, ECV, Virtanen, T, Mäkelä, S, Soini, V, Hynninen, VV, Mulo, J, et al. S-ketamine in patient-controlled analgesia reduces opioid consumption in a dose-dependent manner after major lumbar fusion surgery: a randomized, double-blind, placebo-controlled clinical trial. PLoS One. (2021) 16:e0252626. doi: 10.1371/journal.pone.0252626
52. Shen, J, Song, C, Lu, X, Wen, Y, Song, S, Yu, J, et al. The effect of low-dose esketamine on pain and post-partum depression after cesarean section: a prospective, randomized, double-blind clinical trial. Front Psych. (2022) 13:1038379. doi: 10.3389/fpsyt.2022.1038379
53. Qian, Q, Liu, HX, and Li, YQ. Effect of esketamine nasal drops on pain in children after tonsillectomy using low temperature plasma ablation. Front Pediatr. (2023) 11:1110632. doi: 10.3389/fped.2023.1110632
54. Mercadante, S, Arcuri, E, and Santoni, A. Opioid-induced tolerance and hyperalgesia. CNS Drugs. (2019) 33:943–55. doi: 10.1007/s40263-019-00660-0
55. Wang, J, Feng, Y, Qi, Z, Li, J, Chen, Z, Zhang, J, et al. The role and mechanism of esketamine in preventing and treating remifentanil-induced hyperalgesia based on the NMDA receptor-CaMKII pathway. Open Life Sci. (2024) 19:20220816. doi: 10.1515/biol-2022-0816
Keywords: emergence delirium, esketamine, meta-analysis, perioperative medicine, systematic review
Citation: Liu J, Liu J, Sun H, Cheng X, Wang C, Lei D and Han C (2025) Effect of perioperative esketamine use on emergency delirium in children undergoing tonsillectomy and adenoidectomy: a systematic review and meta-analysis of randomized controlled trials. Front. Med. 12:1505408. doi: 10.3389/fmed.2025.1505408
Received: 02 October 2024; Accepted: 14 January 2025;
Published: 29 January 2025.
Edited by:
Yiying Zhang, Harvard Medical School, United StatesReviewed by:
Ying Meng, Chongqing Medical University, ChinaCopyright © 2025 Liu, Liu, Sun, Cheng, Wang, Lei and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Han, c3RhZmY5NDBAeXhwaC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.