- 1The Seventh Clinical College of Guangzhou University of Chinese Medicine, Shenzhen Bao’an Traditional Chinese Medicine Hospital, Guangzhou University of Chinese Medicine, Shenzhen, China
- 2Clinical Medical College of Acupuncture, Moxibustion and Rehabilitation, Guangzhou University of Chinese Medicine, Guangzhou, China
Background: Elderly individuals with inadequate vitamin B level are at increased risk of degenerative conditions, notably cardiovascular disorders, cognitive impairments, and osteoporosis. The relationship between niacin (vitamin B3) consumption and osteoporosis risk remains a subject of debate. This study aimed to clarify the relationship between dietary niacin intake and the incidence of osteoporosis in postmenopausal women aged ≥50 years.
Methods: In this study, we gathered details on participants’ bone mineral density, osteoporosis status, dietary niacin intake, and several other critical variables. Multivariate logistic regression models were constructed to determine the association between dietary niacin intake and the incidence of osteoporosis. Restricted cubic splines were employed to further assess the linearity and explore the shape of the dose-response associations. Additionally, we performed stratified and interaction analyses to illustrate the stability of the observed relationships across different subgroups.
Results: After adjusting for all covariates, there was a significant inverse association with osteoporosis (OR = 0.87; 95% CI: 0.77–0.97; p = 0.016). A negative relationship was observed between dietary niacin intake and the risk of osteoporosis (nonlinear: p = 0.672). While stratified analyses revealed some differences in the association between dietary niacin intake and osteoporosis risk, these differences were not statistically significant.
Conclusion: Dietary niacin intake exhibited an inverse correlation with the incidence of osteoporosis. The risk of osteoporosis was significantly reduced by 13% with every 10 mg/day increase in daily dietary niacin consumption among postmenopausal women.
1 Introduction
Osteoporosis, a prevalent and debilitating skeletal disorder, is characterized by a reduction in bone mineral density, destruction of the bone microarchitecture, increased bone fragility and risk of fracture (1). Elderly individuals, particularly postmenopausal women, are at a significantly elevated risk for developing osteoporosis. Globally, the prevalence of osteoporosis among postmenopausal women is significantly high, reaching 27.4% (2). Fracture complications can significantly impair a patient’s ability to perform daily activities, which poses a substantial threat to the economy and healthcare infrastructure worldwide. Considering the severity and widespread nature of postmenopausal osteoporosis, identifying and effectively addressing the contributing risk factors is imperative for disease management.
Niacin, or vitamin B3, is an essential micronutrient predominantly derived from a variety of rich dietary sources, such as dairy, meat, seafood, legumes, cereals, and leafy vegetables. Moreover, humans are capable of synthesizing niacin in vivo through the tryptophan pathway (3, 4). Niacin serves as a dietary precursor essential for the synthesis of the coenzymes nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADPH), which are pivotal for numerous cellular redox reactions (3). Therefore, niacin may exert its potential osteoprotective effects through mitigating inflammation and modulating the expression of the NAD-dependent deacetylase sirtuin 1 (SIRT1) (5, 6).
In recent years, an increasing number of studies (7–9) have linked osteoporosis to nutrients (including niacin). However, the relationship between dietary niacin and osteoporosis risk in postmenopausal women still remains unclear. In this study, our hypothesis was that individuals with osteoporosis have lower dietary niacin intake, based on nutritional patterns observed in this population and findings from preceding related literatures. And we aimed to assess whether dietary niacin is associated with the incidence of osteoporosis in a relatively large and nationally representative population aged 50–80 years in the USA.
2 Materials and methods
2.1 Study population
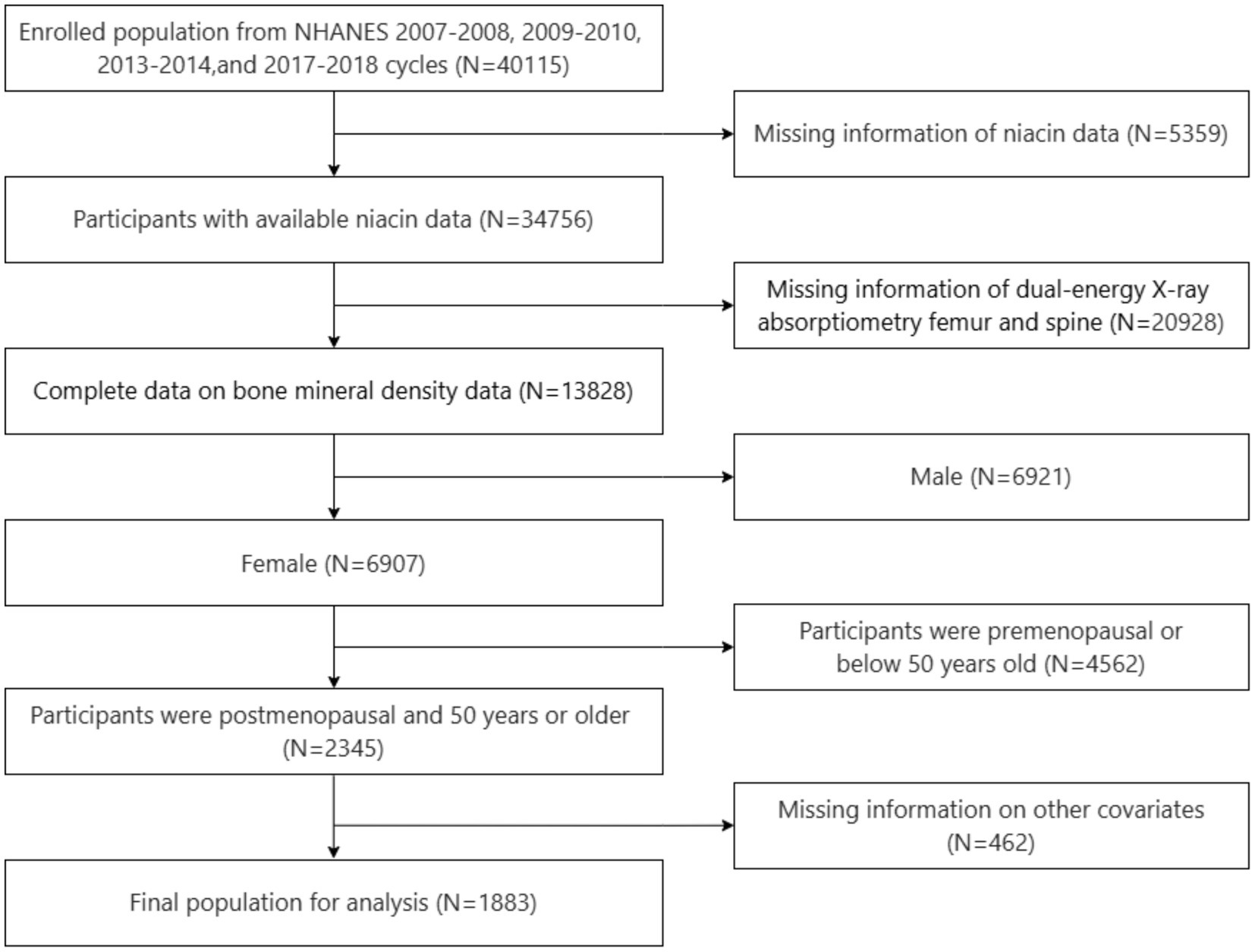
All subject information utilized in this cross-sectional study was extracted from the National Health and Nutrition Examination Survey (NHANES) conducted during the years 2007–2010, 2013–2014, and 2017–2018. The NHANES is a national continuous cross-sectional survey initiated by the National Centre for Health Statistics (NCHS), which aims to assess the health and nutrition of the U.S. civilian population. It collects comprehensive data through a stratified multistage probability sampling approach, with updates every two years (10). The NHANES provides valuable data for secondary analysis, contributing to insights into American Health and Nutrition. NHANES data are available on the NHANES website (http://www.cdc.gov/nchs/nhanes.htm; accessed on June 28, 2024). The NCHS Research Ethics Review Board approved the NHANES study protocol, and written informed consent was obtained from all participants before engaging in the study. The inclusion criteria were: population from NHANES 2007–2008, 2009–2010, 2013–2014, and 2017–2018; participants with available niacin data; participants with complete bone mineral density (BMD) data; postmenopausal women who were ≥50 years old. The exclusion criteria were: participants with missing dual-energy X-ray absorptiometry (DXA) data for the femur and spine; participants with missing data on other covariates. After the inclusion and exclusion criteria were applied, the final study cohort consisted of 1,883 participants (Figure 1).
2.2 Menopausal status definition
Menopausal status was ascertained through a self-administered reproductive health questionnaire in the NHANES survey. Women were classified as postmenopausal if they responded “no” to the question “have you had at least one menstrual period in the past 12 months? (Please do not include bleeding caused by medical conditions, hormone therapy, or surgeries.),” and selected either “hysterectomy” or “menopause/change of life” as the reason for amenorrhea. Further detailed information regarding the questionnaire is accessible in the NHANES database, which can be accessed through their official website (11).
2.3 Dietary niacin intake
The NHANES collects dietary intake data via the Computer-Assisted Dietary Interview (CADI) system, which is administered by bilingual (Spanish and English), trained interviewers. This comprehensive, computerized system employs a multiple-pass recall method to record the types and amounts of food and beverages consumed within a 24-h period prior to the interview. Each MEC dietary interview room is equipped with a standard set of measurement guidelines, developed with expert consensus to ensure accurate reporting of food volume and size. The NHANES Dietary Interviewer Procedure Manual provides a complete overview of these dietary survey methods (12). Niacin intake in this study refers exclusively to dietary sources and does not include supplemental niacin.
2.4 Determination of BMD and the diagnosis of osteoporosis
In the NHANES, BMD was assessed across various regions, including the total femur, femur neck, and total spine, utilizing DXA scans with Hologic QDR-4500A fan-beam densitometers (Hologic, Inc., Bedford, Massachusetts). The left hip was typically scanned, with exceptions made for subjects with a history of fracture, pin, or replacement on the left side, necessitating examination of the right hip. Participants were excluded from the DXA examination if they were pregnant; had a history of radiographic contrast material, bilateral hip fractures or replacements, or pins; or weighed over 450 lbs. Osteoporosis was diagnosed in accordance with the classification criteria established by the World Health Organization. When the BMD T-score in any region of the femur is ≤ −2.5, a value greater than 2.5 standard deviations below the reference mean is indicated for a young adult population (13, 14). Patients with osteopenia and those with normal BMD were considered as non-osteoporosis. Therefore, participants were also regarded as having osteoporosis if they answered “yes” to the question “Has a doctor ever told you that you had osteoporosis, sometimes called thin or brittle bones?” In cases where there is a discrepancy between the patient’s self-reported information and the data obtained from DXA scans, the diagnosis of osteoporosis should be based on the DXA scan data.
2.5 Covariates
On the basis of the NHANES website, we gathered covariate data through questionnaires, physical exams and laboratory testing. In this study, the following covariates were taken into account: age (year), race/ethnicity, education level (year), marital status, family poverty income ratio (PIR), smoking status, alcohol consumption status, body mass index (BMI) (kg/m2), physical activity, hypertension, diabetes, coronary heart disease (CHD), history of previous fractures, history of prednisone or cortisone, dietary supplements taken, blood calcium (mg/dL), and serum 25(OH)D (nmol/L) (15, 16). Race/ethnicity was classified as non-Hispanic White, non-Hispanic Black, Mexican American, or others. Education level was divided into three groups: less than 9 years, 9–12 years, and over 12 years. Marital status was categorized as living alone, married or living with a partner. Household income was stratified into low (PIR ≤1.3), middle (PIR 1.3–3.5), and high (PIR >3.5). Smoking status was defined as never (fewer than 100 cigarettes), current, or former (quit smoking after smoking 100+ cigarettes). Drinkers were defined as individuals who consume at least 12 alcoholic beverages per year. BMI was calculated using a standardized method based on weight and height measurements. Physical activity was labeled as sedentary, moderate (≥10 min of movement in the past 30 days causing only mild sweating or a slight to moderate increase in breathing or heart rate), or vigorous (≥10 min of activity in the past 30 days causing heavy sweating or a significant increase in breathing or heart rate). Previous diseases like hypertension, diabetes, and CHD were determined by questionnaire responses regarding physician diagnoses. The history of previous fractures, the use of prednisone or cortisone, and the intake of dietary supplements were classified as “yes” or “no” based on the participants’ reports. Blood calcium and serum 25(OH)D concentrations were determined via laboratory analysis of participants’ blood samples (12, 17).
2.6 Statistical analysis
Continuous variables with a normal distribution were presented as mean ± SD and contrasted through a one-way analysis of variance. Categorical variables were expressed as absolute values (percentages) and contrasted using Chi-square test. Multivariate logistic regression analysis was used to analyse the association between dietary niacin intake and osteoporosis while controlling for other covariates. The odds ratio (OR) and 95% confidence interval (CI) were used to reflect the direct relationship between dietary niacin intake and osteoporosis. Model 1 was adjusted for sociodemographic variables (age, marital status, race/ethnicity, education level, and PIR). Model 2 was further adjusted for smoking status, alcohol consumption status, BMI, physical activity, hypertension, diabetes, CHD, history of previous fractures, history of prednisone or cortisone, and dietary supplements taken. Model 3 was fully adjusted, similar to model 2, and additional adjustments for blood calcium and serum 25(OH)D. The restricted cubic spline (RCS) model was used to further assess the linearity and explore the shape of the dose-response relationship between dietary niacin intake and osteoporosis incidence. The smooth curve fitting graph was established and adjusted on the basis of the covariables contained in model 3. Moreover, logistic regression models were used to conduct interaction and subgroup analyses based on all covariates.
Given that the sample size was determined solely on the basis of the data provided, no a priori estimate of statistical power was made. All analyses were performed using the statistical software packages R 4.3.3 and Free Statistics software version 1.9.2 (18). A descriptive study was conducted on all the participants. A p-value of <0.05 indicated significance by a two-tailed testing.
3 Results
3.1 Baseline characteristics of the study population
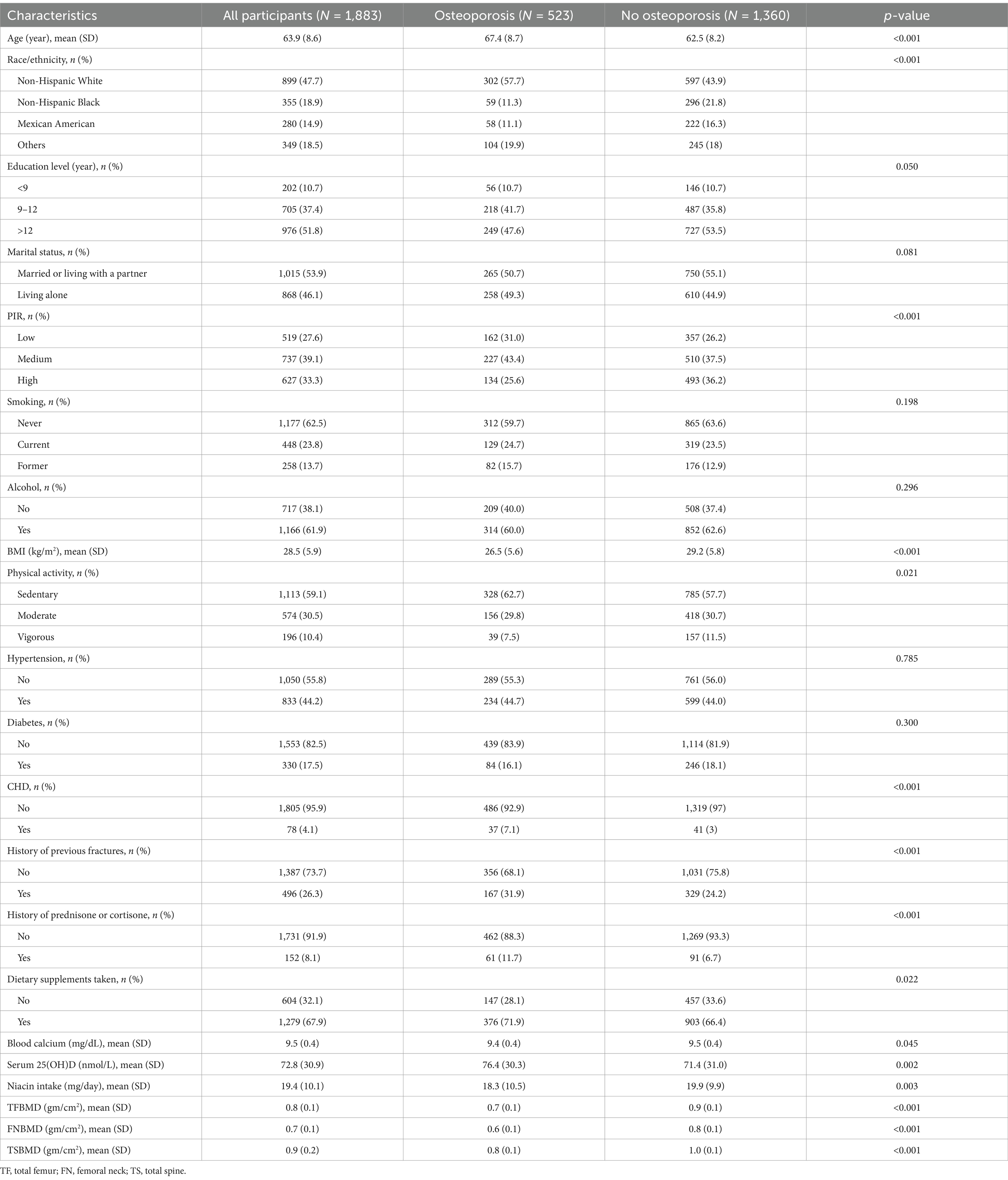
A total of 1,883 qualified participants were included in this study. The prevalence of osteoporosis was 27.8% (523/1,883). Table 1 presents the characteristics of all the subjects with and without osteoporosis. The average age of the study population was 63.9 ± 8.6 years. Compared with participants without osteoporosis, osteoporosis patients often tended to be thinner and older, with a low or medium family income, coronary heart disease, a sedentary lifestyle, previous fractures, a history of prednisone or cortisone, higher consumption of dietary supplements, higher serum 25(OH)D level, and more likely to be non-Hispanic white. Notably, the non-osteoporosis population had a greater amount of dietary niacin.
3.2 Relationship between dietary niacin intake and osteoporosis
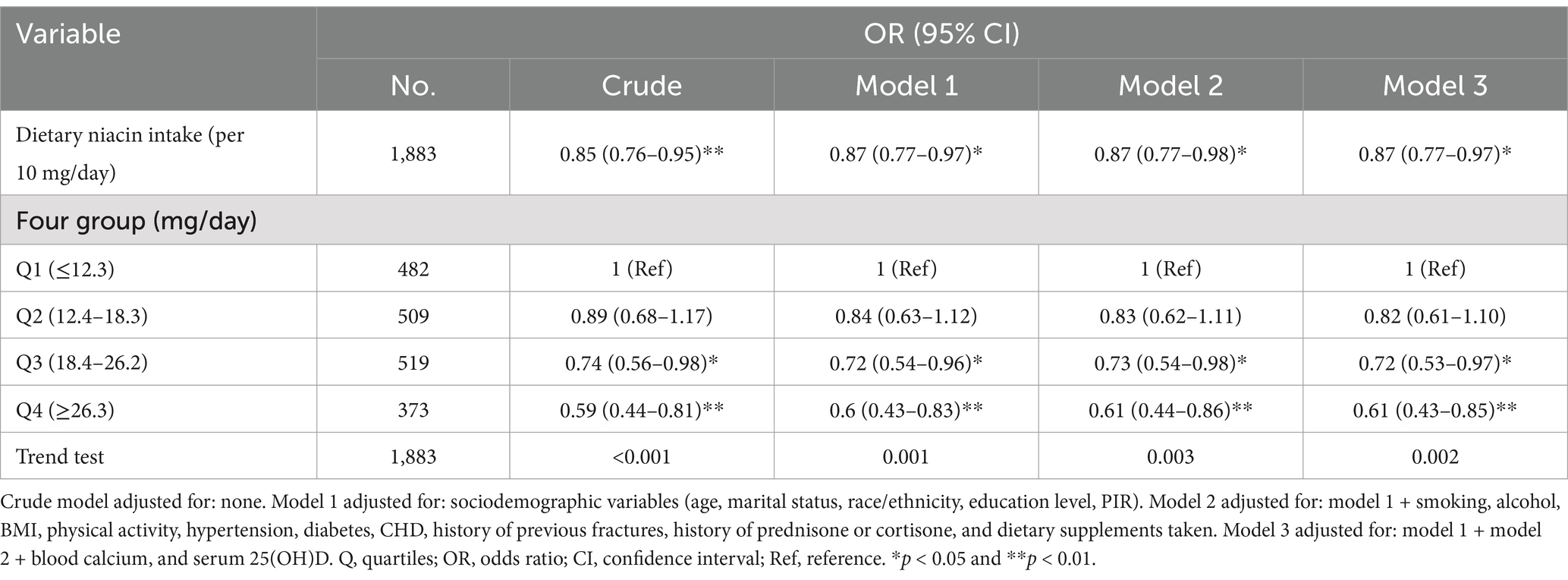
Univariate regression analysis revealed that age, race and ethnicity, PIR, BMI, physical activity, CHD, history of previous fractures, history of prednisone or cortisone, dietary supplements taken, blood calcium, serum 25(OH)D, dietary niacin intake were significantly associated to osteoporosis (p < 0.05) (Supplementary Table). Besides, the relationship between dietary niacin intake and the risk of osteoporosis was also analysed by conducting multivariate logistic regression (Table 2). When niacin was analysed as a continuous variable, a significant independent negative association was discovered between dietary niacin intake and the risk of osteoporosis in the non-adjusted crude model (OR: 0.85, 95% CI: 0.76–0.95; p = 0.003); moreover, further adjustment did not significantly affect the results. Consistent findings were also obtained when niacin was analysed as a categorical variable. Compared with individuals with lower niacin consumption Q1 (<12.3 mg/day), the fully adjusted OR values for dietary niacin intake and osteoporosis in Q2 (12.4–18.3 mg/day), Q3 (18.4–26.2 mg/day), and Q4 (≥26.3 mg/day) were 0.82 (95% CI: 0.61–1.10, p = 0.191), 0.72 (95% CI: 0.53–0.97, p = 0.030), and 0.61 (95% CI: 0.43–0.85, p = 0.004), respectively. Accordingly, the RCS of the relationship between dietary niacin intake and osteoporosis was negative when all potential confounders were taken into account (nonlinearity: p = 0.672) (Figure 2).
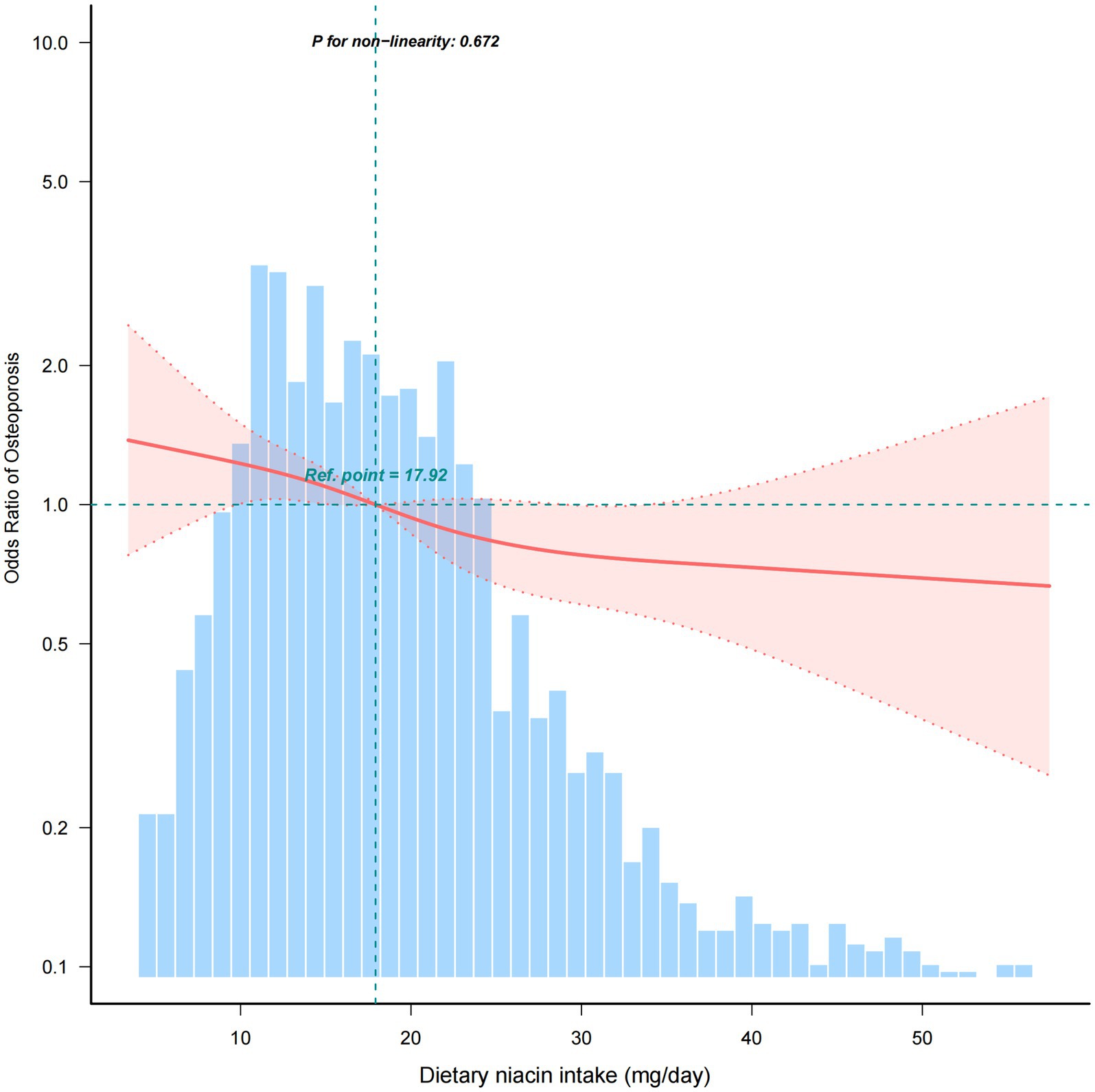
Figure 2. Association between dietary niacin intake and the osteoporosis odds ratio. The solid and dashed lines represent the predicted value and 95% confidence intervals, respectively. They were adjusted for age, marital status, race/ethnicity, education level, PIR, smoking, alcohol, BMI, physical activity, hypertension, diabetes, CHD, history of previous fractures, history of prednisone or cortisone, dietary supplements taken, blood calcium, and serum 25(OH)D. Only 99% of the data is shown.
3.3 Subgroup analyses
Stratified and interaction analysis was performed to assess whether the association between dietary niacin intake and osteoporosis was consistent across several subgroups. However, there was no statistically significant interactions in any subgroups after stratifying by age, race/ethnicity, education level, marital status, PIR, or BMI (Figure 3).

Figure 3. The relationship between dietary niacin intake (per 10 mg/day) and osteoporosis according to basic features. Each stratification factor was adjusted for all other variables (age, marital status, race/ethnicity, education level, PIR, smoking status, alcohol, BMI, physical activity, hypertension, diabetes, CHD, history of previous fractures, history of prednisone or cortisone, dietary supplements taken, blood calcium, and serum 25(OH)D).
4 Discussion
This nationally cross-sectional study of 1,883 American postmenopausal women from the NHANES (2007–2008, 2009–2010, 2013–2014, and 2017–2018) evaluated the association between dietary niacin intake and the incidence of osteoporosis. To our knowledge, this is the first study to explore the potential relationship between dietary niacin intake and osteoporosis risk in American postmenopausal women. After adjustment for potential confounders, dietary niacin was negatively associated with the incidence of osteoporosis. Moreover, when niacin was converted from a continuous variable to a categorical variable, the higher niacin quartile group (third quartile and fourth quartile) presented a lower osteoporosis risk than did the lowest niacin quartile group. Subgroup analysis further confirmed the stability of this association. Our findings highlight the importance of maintaining a niacin-rich diet in the prevention of osteoporosis among postmenopausal women.
Oxidative stress (OS) and inflammation are crucial pathogenic mechanisms underlying postmenopausal osteoporosis. Extensive animal researches have yielded robust evidence implicating reactive oxygen species (ROS) in the etiology of age-associated bone loss. Researchers have reported that ovariectomy-induced osteoporosis and osteoclast activity were markedly attenuated in mice deficient in key coenzymes necessary for ROS generation, such as nicotinamide adenine dinucleotide phosphate oxidase, or in those lacking antioxidants such as glutathione (19–21). After menopause, the body experiences an increase in the production of oxidizing substances, including ROS, malondialdehyde, and hydrogen peroxide (22–24). Concurrently, the activities of antioxidants, such as certain vitamins and folic acid, as well as those of antioxidant enzymes like superoxide dismutase, are significantly reduced (25). This leads to a disruption in the redox equilibrium of cells. The excessive accumulation of ROS can induce damage to lipids, proteins, and DNA in the cell membrane and nucleus. The OS state disrupts bone homeostasis by enhancing osteoclastogenesis, promoting apoptosis in osteoblasts and osteocytes, suppressing osteoblast activity, and inhibiting osteoprogenitor differentiation into the osteoblast cell lineage (26). OS enhances osteoclastogenesis through the upregulation of receptor activator of NF-kB ligand (RANKL) and the downregulation of osteoprotegerin (OPG), both of which are modulated via the Wnt/β-catenin pathway and facilitated by the activation of protein kinases (ERK1/2 and JNK) (27). SIRT1, a distinctive NAD+-dependent deacetylase, is among the most conserved members of the seven mammalian sirtuin family (28). It utilizes NAD+ to remove acetyl groups from proteins, particularly histone and non-histone acetylations, thereby regulating gene expression and protein function. As mentioned previously, niacin serves as a precursor of NAD and NADP and thus can elevate the level of NAD+ in the body, subsequently activating SIRT1. SIRT1 subsequently activates the Forkhead box O (FoxO)1/β-catenin signaling pathway through deacetylation, thereby mitigating oxidative stress-induced cellular damage from hydrogen peroxide (H2O2) and curbing apoptosis in osteoblasts (29, 30). This mechanism ultimately contributes to the therapeutic potential of SIRT1 in preventing osteoporosis.
In recent years, iron overload has been recognized as a new risk factor for postmenopausal osteoporosis (31, 32). Many researches have indicated that iron overload is prevalent among elderly individuals, particularly in postmenopausal women. Subsequent studies have shown that iron overload can lead to elevated levels of OS and ROS, which in turn compromises osteoblastogenesis and enhances osteoclastogenesis. This disruption in bone cell dynamics contributes to decreased bone mass and even osteoporosis (33, 34). Ma et al. (31) found that the primary cause of bone loss in Hepcidin−/− mice was osteocyte apoptosis, which was attributed to elevated levels of ROS and subsequent alteration in sclerostin and RANKL/OPG expression. Moreover, the findings of Tao et al. (35) suggested that niacin, a potent antioxidant and anti-inflammatory agent, reduced oxidative stress and reversed bone loss caused by iron overload and estrogen deficiency through activating the SIRT1 signaling pathway. In their MC3T3-E1 cell experiment, niacin significantly reversed the adverse effects of iron overload on osteoblast activity and differentiation, as well as the protection of mitochondrial function and reduction in oxidative damage through the upregulation of SIRT1 and SOD2 expression. Additionally, niacin exerted a protective effect on bone health by inhibiting ferric citrate-induced osteoclast differentiation in RAW264.7 cells, further protecting bone health. From the preceding discourse, it is apparent that niacin may contribute to anti-osteoporosis by ameliorating oxidative stress, inflammation, and aging in the body, suggesting that dietary niacin could serve as a potential protective factor against osteoporosis. A modest-sized observational study (36) involving a cohort of 100 Polish women aged 51–70 years also revealed that osteoporosis patients had 16% lower niacin intake than non-osteoporosis individuals did. It is worth mentioning that although there was a negative linear relationship between dietary niacin intake and the risk of osteoporosis in this study, it should not be assumed that higher levels of dietary niacin supplementation are necessarily beneficial to human health. Although the essential requisites for NAD+ biosynthesis can be adequately met by consuming less than 20 mg of niacin daily, there is growing evidence indicating that significantly increasing the rate of NAD+ synthesis may be beneficial for certain degenerative diseases (37). Nevertheless, a nutritional review suggested that the upper limit of tolerable dietary niacin intake for adults is 35 mg/day (38). In addition, experimental studies in chicks have demonstrated that feeding high levels of supplemental niacin was found to be associated with adverse effects on bone strength, dimensions, and susceptibility to fracture (39, 40). And Table 1 indicates that the use of dietary supplements is positively correlated with the incidence of osteoporosis, suggesting the importance of taking dietary supplements in a reasonable and moderate manner. A meta-analysis suggested that the optimal intake levels may vary between healthy individuals and those with specific disease conditions. It prompted a re-evaluation of the dosage thresholds for nicotinic acid supplements (41). Nevertheless, our findings indicate that, within reasonable limits, increasing dietary niacin intake may exert a protective impact on the prevention of osteoporosis. Future prospective researches are warranted to confirm the potential preventative role of niacin in osteoporosis and its underlying mechanisms.
Our study has several noteworthy strengths. First, to our knowledge, this is the largest investigation to date exploring the relationship between dietary niacin intake and the risk of osteoporosis in postmenopausal women, thereby comprising participants from various ethnic backgrounds. Second, the adoption of a large-scale sample design from a nationally representative cohort of U.S. adults, ensured a robust population size. Third, by adjusting for potential confounding factors, including a history of prednisone and dietary supplements, our conclusions are more plausible and persuasive. Notwithstanding the advantages, there are still some limitations that require attention. First, the cross-sectional nature of our study prevents us from drawing conclusions regarding the causal relationship between dietary niacin intake and postmenopausal osteoporosis. Second, our study population may not be fully representative of all demographic groups, which could affect the generalizability of our findings. Third, despite our efforts to control for confounding factors, residual confounding cannot be entirely ruled out. Fourth, the dietary intake assessment in the NHANES, which relies on 24-h recall questionnaires, may be subject to inevitable recall bias. Nonetheless, the food frequency questionnaire, while a valuable tool, offers less comprehensive information regarding food types and quantities than does 24-h dietary recall (42, 43). Fifth, we have included data on dietary niacin intake only and have not incorporated data on supplemental niacin. Hence, it is imperative for future multicentre, large-sample cohort studies to validate these findings by incorporating more potential confounders and employing standardized measures.
In conclusion, dietary niacin intake was negatively associated with the risk of osteoporosis in postmenopausal women. Dietary niacin intake and the incidence of osteoporosis were negatively associated; the risk of osteoporosis was significantly reduced by 13% with every 10 mg/day increase in daily dietary niacin consumption.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Ethics statement
The studies involving humans were approved by the NCHS Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LL: Writing – original draft, Data curation. WL: Data curation, Writing – review & editing. BD: Data curation, Writing – review & editing. YJ: Data curation, Writing – review & editing. XH: Software, Writing – review & editing. YZ: Software, Writing – review & editing. TL: Software, Writing – review & editing. LW: Software, Writing – review & editing. YX: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. GC: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of Guangdong Province (Nos. 2024A1515011874 and 2023A1515011123), the Shenzhen Science and Technology Innovation Committee (No. JCYJ20210324124613037), the Shenzhen Bao’an Traditional Chinese Medicine Development Foundation (No. 2022KJCX-ZJZL-10), and Bao’an District Shenzhen Traditional Chinese Medicine Association (No. 2023ZYYLCZX-20).
Acknowledgments
The authors gratefully thank Dr. Huanxian Liu (Department of Neurology, Chinese PLA General Hospital), Dr. Haibo Li (Fujian Maternity and Child Health Hospital, Fujian, China), and Dr. Jie Liu (Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital) for their guidance and valuable assistance in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1504892/full#supplementary-material
Abbreviations
BMD, Bone mineral density; BMI, Body mass index; CADI, Computer-Assisted Dietary Interview; CHD, Coronary heart disease; CI, Confidence interval; DXA, Dual-energy X-ray absorptiometry; FoxO, Forkhead box O; H2O2, Hydrogen peroxide; NAD, Nicotinamide adenine dinucleotide; NADPH, Nicotinamide adenine dinucleotide phosphate; NCHS, National Centre for Health Statistics; NHANES, National Health and Nutrition Examination Survey; OPG, Osteoprotegerin; OR, Odds ratio; OS, Oxidative stress; PIR, Poverty income ratio; RANKL, Receptor activator of NF-kB ligand; RCS, Restricted cubic spline; ROS, Reactive oxygen species; SIRT1, Sirtuin 1.
References
1. Compston, JE, McClung, MR, and Leslie, WD. Osteoporosis. Lancet. (2019) 393:364–76. doi: 10.1016/S0140-6736(18)32112-3
2. Xiao, PL, Cui, AY, Hsu, CJ, Peng, R, Jiang, N, Xu, XH, et al. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: a systematic review and meta-analysis. Osteoporos Int. (2022) 33:2137–53. doi: 10.1007/s00198-022-06454-3
3. Wuerch, E, Urgoiti, GR, and Yong, VW. The promise of niacin in neurology. Neurotherapeutics. (2023) 20:1037–54. doi: 10.1007/s13311-023-01376-2
4. Carbone, LD, Bůžková, P, Fink, HA, Raiford, M, Le, B, Isales, CM, et al. Association of dietary niacin intake with incident hip fracture, BMD, and body composition: the cardiovascular health study. J Bone Miner Res. (2019) 34:643–52. doi: 10.1002/jbmr.3639
5. Ballesteros, J, Rivas, D, and Duque, G. The role of the kynurenine pathway in the pathophysiology of frailty, sarcopenia, and osteoporosis. Nutrients. (2023) 15:3132. doi: 10.3390/nu15143132
6. Koh, JM, Khang, YH, Jung, CH, Bae, S, Kim, DJ, Chung, YE, et al. Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporos Int. (2005) 16:1263–71. doi: 10.1007/s00198-005-1840-5
7. Ilesanmi-Oyelere, BL, Brough, L, Coad, J, Roy, N, and Kruger, MC. The relationship between nutrient patterns and bone mineral density in postmenopausal women. Nutrients. (2019) 11:1262. doi: 10.3390/nu11061262
8. Lee, H, Kim, J, and Lim, H. Coexistence of metabolic syndrome and osteopenia associated with social inequalities and unhealthy lifestyle among postmenopausal women in South Korea: the 2008 to 2011 Korea National Health and Nutritional Examination Survey (KNHANES). Menopause. (2020) 27:668–78. doi: 10.1097/GME.0000000000001518
9. Park, HM, Heo, J, and Park, Y. Calcium from plant sources is beneficial to lowering the risk of osteoporosis in postmenopausal Korean women. Nutr Res. (2011) 31:27–32. doi: 10.1016/j.nutres.2010.12.005
10. Zipf, G, Chiappa, M, Porter, KS, Ostchega, Y, Lewis, BG, and Dostal, J. (2013). National Health and Nutrition Examination Survey: plan and operations, 1999–2010. National Center for Health Statistics, Hyattsville, MD. 1–37.
11. NHANES. (2024). NHANES 2017–2018 questionnaire instruments. Available at: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Questionnaires.aspx?BeginYear=2017. (Accessed August 1, 2024)
12. Liu, H, Wang, L, Chen, C, Dong, Z, and Yu, S. Association between dietary niacin intake and migraine among American adults: National Health and Nutrition Examination Survey. Nutrients. (2022) 14:3052. doi: 10.3390/nu14153052
13. Looker, AC, Orwoll, ES, Johnston, CC, Lindsay, RL, Wahner, HW, Dunn, WL, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. (1997) 12:1761–8. doi: 10.1359/jbmr.1997.12.11.1761
14. Xue, S, Zhang, Y, Qiao, W, Zhao, Q, Guo, D, Li, B, et al. An updated reference for calculating bone mineral density T-scores. J Clin Endocrinol Metab. (2021) 106:e2613–21. doi: 10.1210/clinem/dgab180
15. Chen, R, Gong, K, Chen, W, Chen, Z, Hua, X, Tan, J, et al. Association of serum alkaline phosphatase levels with bone mineral density, osteoporosis prevalence, and mortality in US adults with osteoporosis: evidence from NHANES 2005–2018. Osteoporos Int. (2024) 29. doi: 10.1007/s00198-024-07324-w
16. Zhuang, R, Hou, W, Zhang, T, and Wang, T. Association between dietary vitamin E and osteoporosis in older adults in the United States. Front Endocrinol. (2024) 15:1410581. doi: 10.3389/fendo.2024.1410581
17. Liu, H, Zhang, S, Gong, Z, Zhao, W, Lin, X, Liu, Y, et al. Association between migraine and cardiovascular disease mortality: a prospective population-based cohort study. Headache. (2023) 63:1109–18. doi: 10.1111/head.14616
18. Ruan, Z, Lu, T, Chen, Y, Yuan, M, Yu, H, Liu, R, et al. Association between psoriasis and nonalcoholic fatty liver disease among outpatient US adults. JAMA Dermatol. (2022) 158:745–53. doi: 10.1001/jamadermatol.2022.1609
19. Goettsch, C, Babelova, A, Trummer, O, Erben, RG, Rauner, M, Rammelt, S, et al. NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J Clin Invest. (2013) 123:4731–8. doi: 10.1172/JCI67603
20. Zhuang, J, Chen, X, Cai, G, Wu, D, Tu, C, Zhu, S, et al. Age-related accumulation of advanced oxidation protein products promotes osteoclastogenesis through disruption of redox homeostasis. Cell Death Dis. (2021) 12:1160. doi: 10.1038/s41419-021-04441-w
21. Almeida, M, Han, L, Martin-Millan, M, Plotkin, LI, Stewart, SA, Roberson, PK, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. (2007) 282:27285–97. doi: 10.1074/jbc.M702810200
22. Bourgonje, AR, Abdulle, AE, Al-Rawas, AM, Al-Maqbali, M, Al-Saleh, M, Enriquez, MB, et al. Systemic oxidative stress is increased in postmenopausal women and independently associates with homocysteine levels. Int J Mol Sci. (2020) 21:314. doi: 10.3390/ijms21010314
23. Zhao, F, Guo, L, Wang, X, and Zhang, Y. Correlation of oxidative stress-related biomarkers with postmenopausal osteoporosis: a systematic review and meta-analysis. Arch Osteoporos. (2021) 16:4. doi: 10.1007/s11657-020-00854-w
24. Liang, G, Kow, ASF, Yusof, R, Tham, CL, Ho, YC, and Lee, MT. Menopause-associated depression: impact of oxidative stress and neuroinflammation on the central nervous system-a review. Biomedicines. (2024) 12:184. doi: 10.3390/biomedicines12010184
25. Leanza, G, Conte, C, Cannata, F, Isgrò, C, Piccoli, A, Strollo, R, et al. Oxidative stress in postmenopausal women with or without obesity. Cells. (2023) 12:1137. doi: 10.3390/cells12081137
26. Kimball, JS, Johnson, JP, and Carlson, DA. Oxidative stress and osteoporosis. J Bone Joint Surg Am. (2021) 103:1451–61. doi: 10.2106/JBJS.20.00989
27. Fontani, F, Marcucci, G, Iantomasi, T, Brandi, ML, and Vincenzini, MT. Glutathione, N-acetylcysteine and lipoic acid down-regulate starvation-induced apoptosis, RANKL/OPG ratio and sclerostin in osteocytes: involvement of JNK and ERK1/2 signaling. Calcif Tissue Int. (2015) 96:335–46. doi: 10.1007/s00223-015-9961-0
28. Jin, X, Sun, X, Ma, X, Qin, Z, Gao, X, Kang, X, et al. SIRT1 maintains bone homeostasis by regulating osteoblast glycolysis through GOT1. Cell Mol Life Sci. (2024) 81:204. doi: 10.1007/s00018-023-05043-9
29. Iyer, S, Han, L, Bartell, SM, Kim, HN, Gubrij, I, de Cabo, R, et al. Sirtuin1 (Sirt1) promotes cortical bone formation by preventing β-catenin sequestration by FoxO transcription factors in osteoblast progenitors. J Biol Chem. (2014) 289:24069–78. doi: 10.1074/jbc.M114.561803
30. Yao, H, Yao, Z, Zhang, S, Zhang, W, and Zhou, W. Upregulation of SIRT1 inhibits H2O2-induced osteoblast apoptosis via FoxO1/β-catenin pathway. Mol Med Rep. (2018) 17:6681–90. doi: 10.3892/mmr.2018.8657
31. Ma, J, Wang, A, Zhang, H, Liu, B, Geng, Y, Xu, Y, et al. Iron overload induced osteocytes apoptosis and led to bone loss in Hepcidin−/− mice through increasing sclerostin and RANKL/OPG. Bone. (2022) 164:116511. doi: 10.1016/j.bone.2022.116511
32. Cheng, Q, Zhang, X, Jiang, J, Zhao, G, Wang, Y, Xu, Y, et al. Postmenopausal iron overload exacerbated bone loss by promoting the degradation of type I collagen. Biomed Res Int. (2017) 2017:1345193. doi: 10.1155/2017/1345193
33. Xiao, W, Beibei, F, Guangsi, S, Yu, J, Wen, Z, Xi, H, et al. Iron overload increases osteoclastogenesis and aggravates the effects of ovariectomy on bone mass. J Endocrinol. (2015) 226:121–34. doi: 10.1530/JOE-14-0657
34. Jiang, Z, Wang, H, Qi, G, Jiang, C, Chen, K, and Yan, Z. Iron overload-induced ferroptosis of osteoblasts inhibits osteogenesis and promotes osteoporosis: an in vitro and in vivo study. IUBMB Life. (2022) 74:1052–69. doi: 10.1002/iub.2656
35. Tao, Z, Tao, M, Zhou, M, and Wu, XJ. Niacin treatment prevents bone loss in iron overload osteoporotic rats via activation of SIRT1 signaling pathway. Chem Biol Interact. (2024) 388:110827. doi: 10.1016/j.cbi.2023.110827
36. Wawrzyniak, A, Klimczyk, P, Woźniak, A, Anyżewska, A, and Leonkiewicz, M. Assessment of differences in nutrients consumption in women diagnosed with osteoporosis as compared to a healthy control group. Rocz Panstw Zakl Hig. (2017) 68:143–9.
37. Bogan, KL, and Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. (2008) 28:115–30. doi: 10.1146/annurev.nutr.28.061807.155443
39. Johnson, NE, Qiu, XL, Gautz, LD, and Ross, E. Changes in dimensions and mechanical properties of bone in chicks fed high levels of niacin. Food Chem Toxicol. (1995) 33:265–71. doi: 10.1016/0278-6915(94)00143-c
40. Johnson, NE, Harland, BF, Ross, E, Gautz, L, and Dunn, MA. Effects of dietary aluminum and niacin on chick tibiae. Poult Sci. (1992) 71:1188–95. doi: 10.3382/ps.0711188
41. Minto, C, Vecchio, MG, Lamprecht, M, and Gregori, D. Definition of a tolerable upper intake level of niacin: a systematic review and meta-analysis of the dose-dependent effects of nicotinamide and nicotinic acid supplementation. Nutr Rev. (2017) 75:471–90. doi: 10.1093/nutrit/nux011
42. Cui, Q, Xia, Y, Wu, Q, Chang, Q, Niu, K, and Zhao, Y. Validity of the food frequency questionnaire for adults in nutritional epidemiological studies: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. (2023) 63:1670–88. doi: 10.1080/10408398.2021.1966737
Keywords: niacin intake, osteoporosis, bone health, postmenopausal women, NHANES
Citation: Li L, Liang W, Deng B, Jiang Y, Huang X, Zhang Y, Lu T, Wang L, Xu Y and Chen G (2025) Association of dietary niacin intake with osteoporosis in the postmenopausal women in the US: NHANES 2007–2018. Front. Med. 12:1504892. doi: 10.3389/fmed.2025.1504892
Edited by:
Grazia Daniela Femminella, University of Naples Federico II, ItalyReviewed by:
Majid Hajifaraji, National Nutrition and Food Technology Research Institute, IranCarol Johnston, Arizona State University, United States
Copyright © 2025 Li, Liang, Deng, Jiang, Huang, Zhang, Lu, Wang, Xu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunxiang Xu, eHV5eDE5NjhAMTYzLmNvbQ==; Guizhen Chen, Y2d6aGVuMjAwMEAxNjMuY29t
 Li Li
Li Li Wankun Liang
Wankun Liang Bing Deng
Bing Deng Yue Jiang
Yue Jiang Xiaomin Huang
Xiaomin Huang Yanlin Zhang
Yanlin Zhang Tianrui Lu
Tianrui Lu Lu Wang
Lu Wang Yunxiang Xu
Yunxiang Xu Guizhen Chen
Guizhen Chen