- 1Department of Pharmacy, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Evidence-Based Pharmacy Center, West China Second University Hospital, Sichuan University, Chengdu, China
- 3Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, Chengdu, China
Background: Patients with duchenne muscular dystrophy (DMD) have an increased risk of complications when they undergo sedation or general anesthesia. However, due to improvements in cardiopulmonary therapies during anesthetic care, patients with DMD are experiencing an unprecedented duration of survival. We performed a systematic analysis to assess the benefits and risks of pharmacological interventions for the management of anesthesia and sedation in DMD patients.
Methods: We included any type of study reporting any drug intervention to manage anesthesia and sedation in participants previously diagnosed with DMD. Our primary outcomes were the onset time, recovery time, and neurodevelopmental disabilities. Seven electronic databases and three clinical trial registry platforms were searched. Data from the eligible studies were combined to calculate pooled risk ratios or standardized mean differences, and some included studies are presented in a narrative synthesis.
Results: Forty studies with 196 DMD participants were included in the analysis. Compared with those of the control group, the sensitivity of patients with DMD to neuromuscular blocking agents (NMBAs) may have resulted in a prolonged onset time [MD = −0.96, 95% CI (0.71, 2.60), I2 = 33%, P < 0.0001] and recovery time [MD = 2.22, 95% CI (1.14, 3.30), I2 = 76%, P < 0.0001] from anesthesia. The neuromuscular blocking effects showed a significant age dependence in DMD patients, and the safe use of 2 mg/kg sugammadex to antagonize deep neuromuscular blockade and rapid recovery has been reported. Furthermore, DMD patients are at risk of developing malignant hyperpyrexia with general/inhaled anesthesia, and dantrolene is often used for effective rescue. In addition, general anesthesia and central neuraxial blockade in patients with severe DMD are unsafe because respiratory depression and myocardial complications may occur after the administration of volatile anesthetics and depolarizing muscle relaxants (succinylcholine) during the induction of anesthesia.
Conclusions: Patients with DMD are more sensitive to NMBAs with delayed onset times and prolonged recovery times. Precautions for DMD patients should include quantitative neuromuscular monitoring, electrocardiographic monitoring and rapid airway protection throughout anesthesia. Compared with general anesthesia, regional anesthesia may be a relatively safe option.
1 Introduction
Duchenne muscular dystrophy (DMD) is a progressive neuromuscular disease transmitted by X-linked inheritance with an incidence of ~1 in 3,500 live male births (1, 2). The onset of clinical symptoms usually occurs during early childhood, and progression of the disease leads to a loss of ambulation in late childhood. At present, no cure exists for DMD, and treatment is aimed at minimizing symptoms (3).
Since patients with DMD present a wide range of symptoms, the associated treatment of health concerns is particularly necessary for each individual patient. Thus, patients with DMD often require anesthetic care during muscle biopsy or correction of progressive orthopedic deformities (4). Depending on the type of surgical procedure and the neurocognitive level of the patient, options include general anesthesia, regional anesthesia or procedural sedation (5, 6). However, the potential impacts of DMD on perioperative morbidity and even mortality cannot be ignored, as the literature has suggested a significantly increased risk during anesthetic care in these patients (8, 9). Earlier reports have outlined the potential for perioperative mortality with cardiac arrest and death in 2 of 25 patients requiring anesthetic care (10).
However, more recent reports have shown that with a better understanding of the pathophysiology of the disease, end-organ involvement and improvements in favorable perioperative care outcomes are possible even in this challenging patient population. In a review of 91 DMD patients who underwent 232 orthopedic surgical procedures, Muenster et al. reported no severe anesthesia-related complications and no cases of unexplained fever or rhabdomyolysis (11). Furthermore, in nearly all patients, neuromuscular blockade agents (NMBAs) were used; therefore, the complete spontaneous recovery of neuromuscular blockade (NMB) in DMD patients remains unclear, and the safest anesthetic technique has yet to be established (12–16).
Given the indispensable nature of sedatives and/or analgesia in DMD surgery/diagnosis and the frequency with which medically compromised DMD patients present for treatment, an increased need exists for reliable data that can inform clinical decision-making. Moreover, the relevant question of whether developments and changes in anesthetic techniques in recent years have improved the safety of anesthesia in this special group of DMD patients has gradually attracted widespread attention, but no studies have been published. Thus, the present review aimed to search for current evidence related to the use of analgosedation in the context of both the drugs used and their side effects and to formulate recommendations in this respect. This review is an up-to-date summary of the medical literature concerning this topic and identifies areas in need of future research.
2 Materials and methods
This systematic review and meta-analysis was performed according to the recommendations in the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) statement and the guidelines described in the Cochrane Handbook (17).
2.1 Search strategy
Our search comprised three English electronic databases (PubMed, Embase, and Cochrane Library) and four Chinese electronic databases (China National Knowledge Infrastructure, Wan Fang Database, Chinese Biomedical Literature Database, and VIP Database for Chinese Technical Periodicals). Three clinical trial registry platforms were used to identify additional studies, including Clinical Trials.gov, the World Health Organization Clinical Trials Registry Platform and the Cochrane Central Registry of Controlled Trials. The search strategy was specific for each database and included a combination of medical subject headings and free text terms (“DMD” or “Duchenne muscular dystrophy”) and (“sedation” or “anesthesia” or “analgosedation”). We looked for additional studies in the reference lists of the selected articles and contacted the authors when the information was unclear. The deadline for the retrieval of all studies was September 2024.
2.2 Inclusion criteria
The following studies were included: (1) studies examining human participants of any age and sex who were previously diagnosed with DMD; (2) any type of drug intervention used for the management of pain and/or sedation; (3) type of study—randomized/non-randomized controlled trials (RCTs), observational studies, case series, and case reports reporting on patients with previously diagnosed DMD; (4) outcomes—the degree and effectiveness of sedation provided by different pharmacological agents, feasibility, and tolerability were assessed, whereas the secondary outcomes included other adverse events. The exclusion criteria were as follows: (1) studies with incomplete or missing information; (2) studies were not published in Chinese or English; (3) abstracts from conferences and unpublished data; (4) no outcomes related to sedative/narcotic drugs or a lack of a specific sedative/narcotic drug detailed regimen.
2.3 Data extraction
Two authors independently extracted the data using a previously designed data extraction table. The data extracted were the authors, year of publication, country, experimental design, sample size, mean age, intervention measure, dose, type of procedure, and any outcome that met the inclusion criteria.
Two independent reviewers screened all the titles and abstracts to identify potentially eligible articles. They independently applied the eligibility criteria to perform the final selection. When discrepancies occurred between the two reviewers regarding the inclusion of the articles, they discussed and identified the reasons to either include or exclude the articles and then made the final decision. If they could not reach an agreement, the final decision was made by a third reviewer.
2.4 Risk of bias assessment
The Interventions' (MINORS) tool was used to assess the risk of bias in non-randomized studies (18). The quality of case report studies was evaluated using the Joanna Briggs Institute of Australia (JBI) quality assessment tool (19).
2.5 Statistical analysis
The meta-analysis was conducted with RevMan 5.3. The data were pooled and reported as relative risks (RR) or Mean Difference (MD) with 95% confidence interval (CI). Heterogeneity was assessed using I-squared (I2) statistics. A fixed effects model was initially constructed. If significant heterogeneity existed among trials (I2 > 50%), potential sources of heterogeneity were considered, and where appropriate, a random effects model was used (20, 21). We planned to report outcome data in tables if a meta-analysis was deemed inappropriate, for example, because of clinical or statistical heterogeneity.
3 Results
3.1 Study search and characteristics
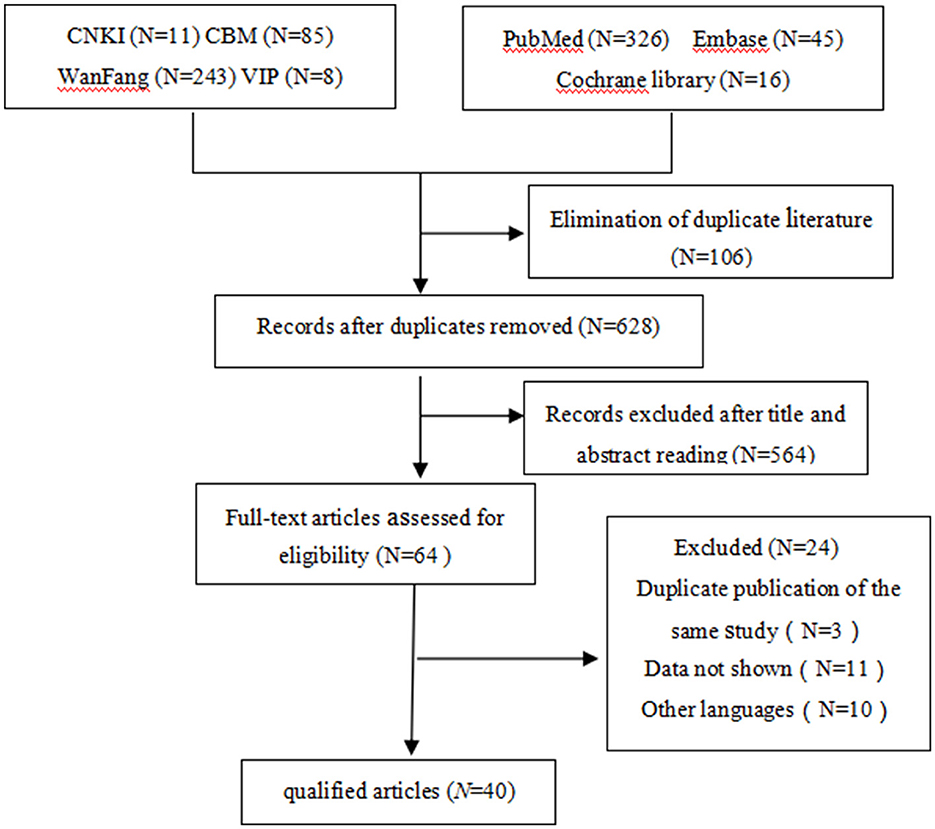
The titles and abstracts of a total of 734 studies were screened, of which 670 were deemed irrelevant (Figure 1). The full texts of the remaining 64 studies were read, and 24 were excluded, leaving 40 studies with 196 patients to be included (35 case reports and five non-randomized controlled trials; Tables 1–3) (4, 7, 12–16, 22–54).
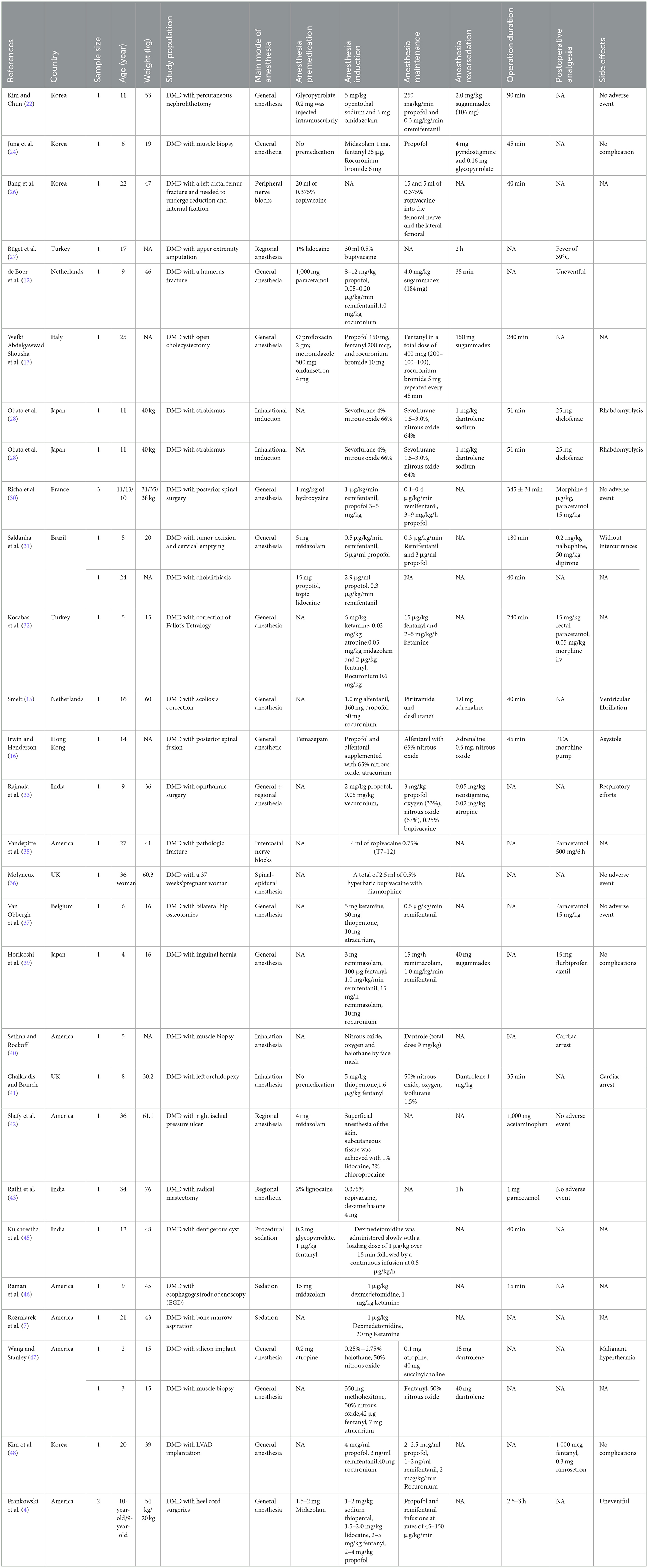
All patients needed sedation or anesthesia before surgery or diagnostic procedures, had an American Society of Anesthesiologists (ASA) grade of I–III, and had no history of allergies. Most of the patients were male, whereas three were female and underwent radical mastectomy, cesarean section, and laparoscopic hysterectomy (25, 36, 43). The age ranged from 5 to 58 years. The sample sizes of the included studies varied between 1 and 29. The studies were conducted in Korea (n = 4), Iran (n = 2), China (n = 3), Germany (n = 2), America (n = 9), Turkey (n = 2), the UK (n = 3), the Netherlands (n = 2), Italy (n = 1), Japan (n = 5), France (n = 1), Brazil (n = 1), Belgium (n = 1), and India (n = 4) from 1995–2019. Some patients with DMD underwent general anesthesia for percutaneous nephrolithotomy, muscle biopsy, corrective orthopedic surgery, laparoscopic cholecystectomy, tumor excision, cholelithiasis, cholecystectomy, etc. Some other patients with DMD underwent local anesthesia for reduction internal fixation, upper extremity amputation, fistulectomy and intercostal nerve blocks. In addition, some patients with DMD underwent general anesthesia supplemented with regional anesthesia for laparoscopic cholecystectomy and traumatic cataract surgery. The duration of most anesthesia operations arranged from 30 min to 2 h.
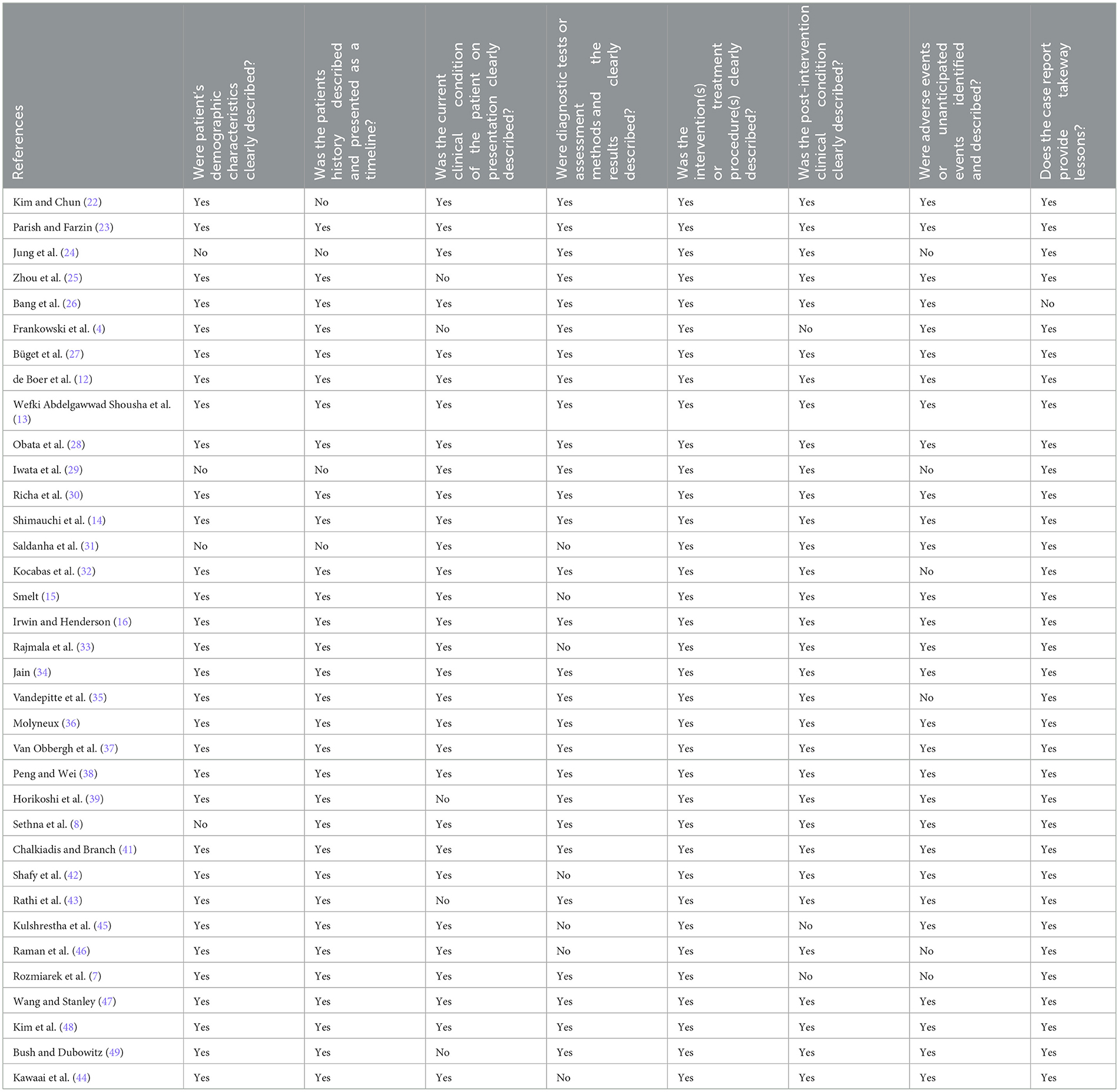
3.2 Quality assessment (risk of bias assessment)
The quality of case report studies was evaluated using the JBI quality assessment tool. Thirty-five case report studies were included (4, 7, 22–54). The results of the quality evaluation revealed that 82.86% (29/35) of the studies described adverse events and unexpected events, 88.57% (31/35) of the studies clearly described patients' history and the researched intervention and/or treatment measures, 88.57% (31/35) of the studies clearly described patients' demographic characteristics. A total of 91.43% (32/35) of the studies clearly described the health status of the patients after the intervention, and 61.5% (8/13) of the studies described the implications of the study. A total of 85.71% (30/35) of the studies clearly presented the current clinical health problems of the patients. Eighty percent (28/35) of the studies clearly described the diagnosis, assessment method, and outcomes, indicating that the overall quality of case reports was high. The results of quality evaluation of the case reports are shown in Table 4.
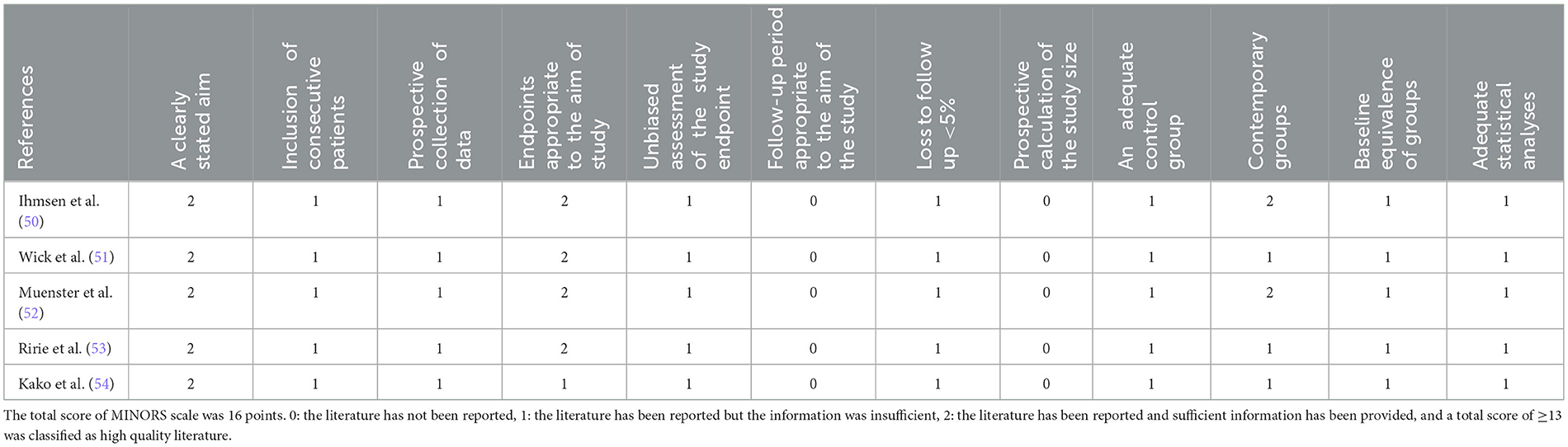
A methodological appraisal of the selected non-randomized studies using the MINORS tool is presented in Table 5 (50–54). The assessment scores ranged from 11 to 13, with a maximum global score of 16. Three studies clearly stated the aim of the investigation, reported the prospective collection of data, and properly described the main outcomes. Additionally, no loss of treated subjects during the follow-up period was reported. However, limitations were found in the description of the inclusion of consecutive patients and in the prospective calculation of the study size (50–54). Thus, three studies were classified as “moderate quality” (51, 53, 54) and two studies as “high quality” (50, 52).
3.3 Preoperative evaluation
The cardiac status needs to be carefully considered in the preoperative evaluation of DMD patients. Most of the included studies performed electrocardiography or pulmonary auscultation before surgery and reported the results. The American Society of Anesthesiologists' physical status of the patients was reported to be I–III (13, 14, 31, 51). The preoperative evaluation should focus on the end-organ involvement of DMD, its evaluation, and the development of an anesthetic drug plan based on these findings. In addition to cardiac involvement, as noted above, respiratory involvement is universally present in patients with DMD. For a full discussion regarding the respiratory concerns of patients with DMD, the reader is referred to the review in the journal written by the pulmonologists who participated in the development of the consensus statement from the American College of Chest Physicians (55).
3.4 Pharmacological interventions
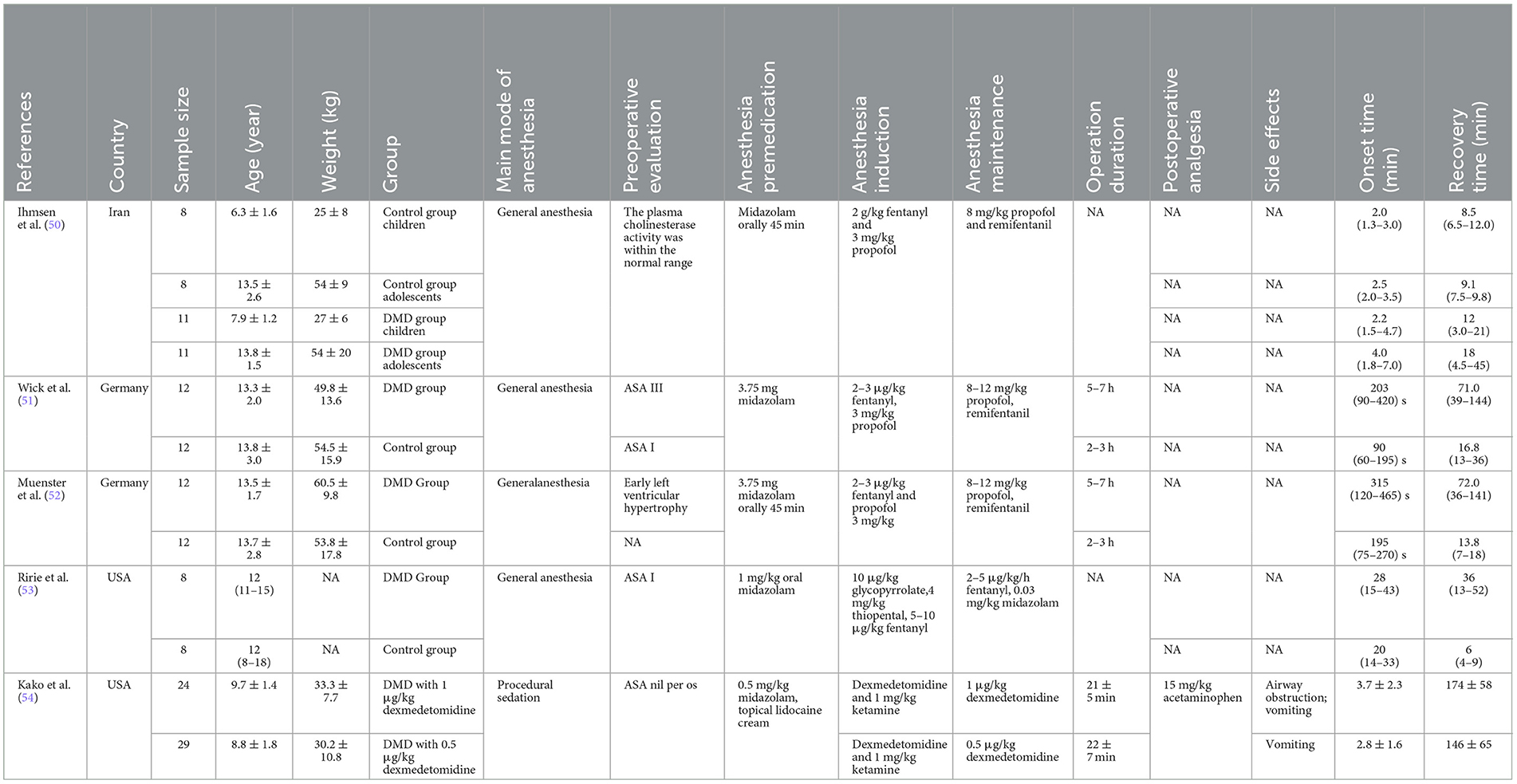
Tables 1, 2 present the drug management strategies used for DMD patients during the induction, maintenance, and recovery periods from anesthesia. Five non-randomized controlled trials compared the DMD group with the control group. Wick et al. (51) determined the onset time and complete spontaneous recovery from neuromuscular blockade after the administration of a standard dose of 0.6 mg/kg rocuronium in patients with advanced DMD compared with controls. Ihmsen et al. (50) compared children with DMD with normal patients to investigate the effects of mivacurium on neuromuscular blockade. Tino Muenster et al. investigated the onset time, peak effect and complete spontaneous recovery from neuromuscular blockade after the administration of a single dose of 0.3 mg/kg rocuronium in DMD patients and compared the data with those of controls (52). Ririe et al. (53) used vecuronium to characterize the neuromuscular blockade of patients with DMD and the response to that of the controls. In addition, Kako et al. (54) evaluated a combination of ketamine with two different doses of dexmedetomidine for sedation during muscle biopsy in patients with DMD.
3.4.1 Main mode of anesthesia
Twenty-six of the included studies involved general anesthesia (4, 12–16, 22–24, 28, 30–32, 37, 39–41, 44, 47–54). Standard intraoperative monitoring, including electrocardiography, automatic blood pressure, and pulse oximetry, was used in the studies. General anesthesia was mostly induced with propofol, fentanyl, pentothal sodium, and midazolam (22–26, 50–52). Rocuronium was administered as a muscle relaxant (14, 15, 24, 32, 39, 48). Anesthesia was mostly maintained with propofol and remifentanil with an oxygen–air mixture (13, 14, 22–25, 28–31). Moreover, a few studies used inhalation induction for general anesthesia with sevoflurane and nitrous oxide (28, 40, 41).
Six studies included regional anesthesia (26, 27, 35, 36, 42, 43). These studies reported local nerve blockade with lidocaine, ropivacaine and other drugs. These studies suggested that general anesthesia or central neuraxial blockade in patients with severe DMD is an unsafe approach to anesthesia because of hemodynamic instability and respiratory depression. Peripheral nerve block is the best way to reduce the risk of critical complications and is a safe and feasible approach to anesthesia in patients with severe DMD.
Four of the included studies involved general anesthesia supplemented with regional anesthesia (29, 33, 34, 38). Anesthesia was induced and maintained with propofol, remifentanil, and fentanyl; local nerve block with ropivacaine and lidocaine was performed.
Moreover, four of the included studies involved the use of procedural sedation (7, 45, 46, 54). Procedural sedation was induced and maintained with dexmedetomidine, ketamine and midazolam (7, 45, 46, 54).
3.4.2 Anesthesia premedication
Nineteen studies involved the use of anesthesia drugs as a premedication (4, 12, 13, 16, 22, 23, 30, 31, 42, 44–47, 49–54). The drugs used included midazolam, acetaminophen, morphine, ondansetron, and hydroxyzine. Kim and Chun (22) reported that 0.2 mg of glycopyrrolate was injected intramuscularly for anesthesia premedication. Parish and Farzin (23) reported that a total of 200 mg of hydrocortisone was injected as the stress dose. Some studies have used midazolam as an anesthesia premedication at doses ranging from 1 to 5 mg (4, 50–54).
3.4.3 Anesthesia induction
Twenty-three studies involved the use of anesthesia induction drugs (4, 13–16, 22–25, 28–33, 37–39, 41, 44, 47–49). The drugs used included midazolam, pentothal sodium, rocuronium bromide, sodium thiopental, propofol, and fentanyl. In patients in whom a muscle relaxant is used, monitoring of muscle relaxation was performed via acceleromyography. The agents used for anesthetic induction should be based on the patient's comorbid cardiac condition. Although the effect of etomidate on adrenal function has led to a re-evaluation of its use during endotracheal intubation in critically ill ICU patients, it may still be an appropriate choice for anesthetic induction in patients with diminished myocardial function (56). The depolarizing agent succinylcholine is absolutely contraindicated and should not even be drawn into a syringe. Rocuronium bromide, with its usually short onset time, could be a suitable alternative to succinylcholine in DMD patients when the clinical conditions require rapid muscle relaxation for airway protection. When motor-evoked potentials are used to monitor spinal cord function, a single dose of a non-depolarizing NMBA can be used to facilitate endotracheal intubation. However, in patients with myopathic conditions such as DMD, the duration of blockade is prolonged (57).
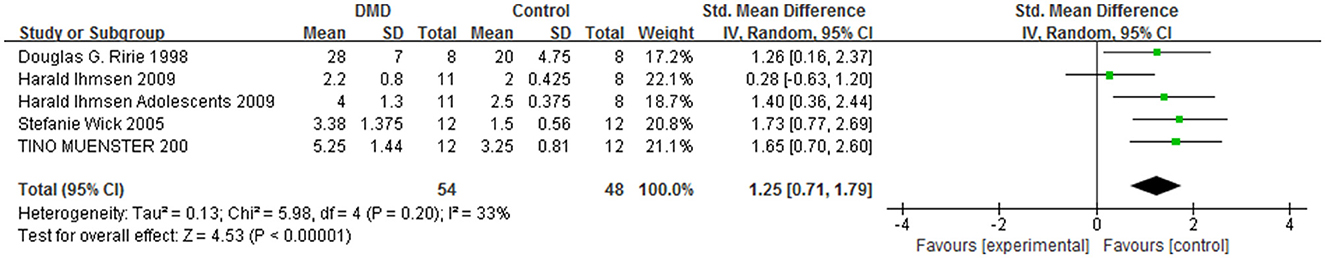
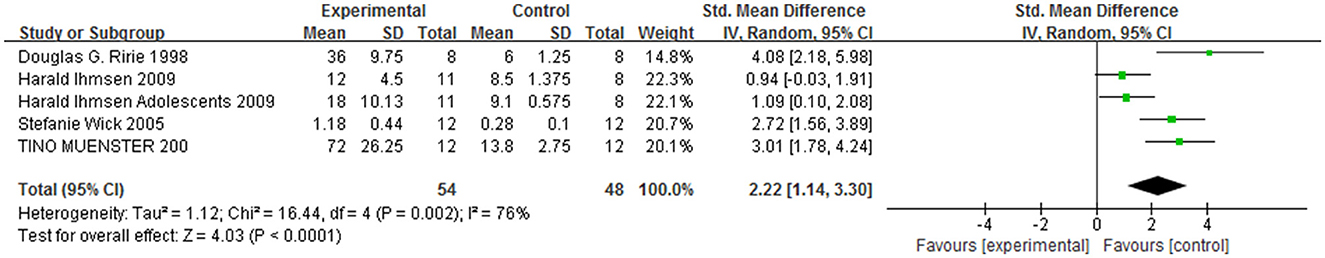
Moreover, five non-randomized controlled trials, including a total of 155 patients, compared the DMD group with the control group to determine the onset time and recovery time after the administration of a standard dose of NMBAs in patients (50–54). Compared with the control group, the sensitivity of patients with DMD to NMBAs may result in a prolonged onset time [MD = −0.96, 95% CI (0.71, 2.60), I2 = 33%, P < 0.0001; Figure 2] and recovery time [MD = 2.22, 95% CI (1.14, 3.30), I2 = 76%, P < 0.0001; Figure 3] from anesthesia. Therefore, the anesthetic management of these patients is challenging and may cause serious problems for anesthesiologists. A sensitivity analysis of each comparison revealed no robust changes in significance.
Similarly, Jung et al. (24) documented that the responsiveness of DMD patients administered a standard dosage of non-depolarizing NMBAs differs from that of normal patients. The delayed onset of blockade in DMD patients following the administration of standard-dose rocuronium and prolonged recovery from rocuronium-induced blockade necessitate the need for a careful assessment of neuromuscular function. If rocuronium is administered to patients with DMD, an quantitative assessment of complete neuromuscular recovery, such as acceleromyography, is mandatory.
3.4.4 Anesthesia maintenance
Thirty-two of the included studies involved anesthesia maintenance (4, 7, 12–16, 22–25, 28–34, 37, 39–41, 44–47, 49–54). Intravenous anesthetics such as propofol, fentanyl, remifentanil, ketamine, dexmedetomidine, and rocuronium bromide are reasonable alternatives and are commonly used at variable doses (Table 1). In addition, some studies used inhalation anesthesia with nitrous oxide, oxygen, sevoflurane, isoflurane and halothane to maintain anesthesia (40, 41, 44).
Richa et al. (30) recommended remifentanil for children with DMD. They reported that the combination of propofol and remifentanil infusions with nitrous oxide in oxygen was successful for patients with DMD undergoing spinal surgery. Exaggerated reactions to drugs were not observed. The patient's intraoperative blood pressure and heart rate were stable, and the wake-up test was successful. Alternatively, endotracheal intubation can be accomplished with a combination of propofol and remifentanil to avoid the need for a neuromuscular blocking agent (41).
Moreover, the multidisciplinary panel suggested the use of total intravenous anesthesia (TIVA) to induce and maintain general anesthesia (e.g., propofol and short-acting opioids) (55). Maintenance anesthesia during surgery for scoliosis generally includes TIVA not only due to the abovementioned concerns of rhabdomyolysis related to volatile anesthetic agents but also to facilitate neurophysiological monitoring using motor and somatosensory evoked potentials. Despite the popularity and clinical experience of the use of propofol for TIVA in these patients, recent concerns have been expressed regarding the effect of propofol on mitochondrial oxidative function (10). These concerns have been raised because rhabdomyolysis, which is thought to be secondary to the disruption of mitochondrial fatty acid oxidation, can occur with prolonged propofol infusion in the pediatric ICU setting, and a defect in mitochondrial oxidative capacity is known to occur in patients with muscular dystrophies (58–61). Despite such concerns, TIVA with propofol and a synthetic opioid remains the most commonly chosen anesthetic regimen (11). However, dexmedetomidine may be added to decrease the propofol dose (62, 63). Kako et al. (54) reported that the use of dexmedetomidine (0.5 μg/kg) and ketamine (1 mg/kg) as loading doses followed by continuous infusion of 0.5 kg/kg/h dexmedetomidine achieved the appropriate sedation level with a shorter total recovery time than the higher-dose dexmedetomidine regimen. Therefore, the combination of dexmedetomidine and ketamine is safe and effective for moderately painful procedures with limited respiratory and cardiovascular effects on high-risk patients.
3.4.5 Adverse events of anesthesia
The sensitivity of patients with DMD to sedative, anesthetic and neuromuscular blocking agents may result in intraoperative and early postoperative cardiovascular and respiratory complications, as well as prolonged recovery from anesthesia. When sedation/anesthetic was excessive, sedation/anesthesia reversal was particularly necessary. Sixteen of the included studies involved the use of anesthesia reversal (12–16, 22, 24, 28, 33, 34, 38–41, 47, 49). Sugammadex, neostigmine and atropine were administered at different doses to reverse cisatracurium besylate-induced neuromuscular blockade (Table 1).
These studies described the efficacy of sugammadex for reversing a prolonged blockade in this setting, but no adverse events were observed (12–14, 22). Jung et al. (24) reported the use of 4 mg of pyridostigmine and 0.16 mg of glycopyrrolate to reverse deep NMB in a child with DMD. Rajmala et al. (33) used 0.05 mg/kg neostigmine and 0.02 mg/kg atropine after the appearance of respiratory efforts, and the postoperative course was uneventful. Similarly, Peng and Wei (38) used 0.2 mg of neostigmine and 0.1 mg of atropine to reverse deep NMB. Treatment with inotropic agents such as milrinone or dobutamine may be necessary to support myocardial function. Close monitoring of cardiac rhythm should be standard, and rhythm abnormalities should be promptly treated (57). Pyridostigmine has been shown to be an effective reversal agent in patients with DMD (24).
Notably, many reports of fatal hyperkalemic cardiac arrest associated with the use of succinylcholine in patients with DMD have raised anesthetists' awareness of this potential complication (16, 28, 40, 41, 47, 49). Therefore, the anesthesia community now commonly accepts that this drug should be strictly avoided in patients with DMD (11). However, rhabdomyolysis may occur in the absence of succinylcholine intraoperatively and during postoperative cardiac arrest as a result of hyperkalemia in patients with DMD (16, 27, 28, 40, 41, 47, 49, 64). The eventual contribution of general anesthetic agents to the cause of the event cannot be ascertained because events occurred during IV and inhaled anesthetic exposure without succinylcholine (16, 28). Moreover, we identified seven cases of rhabdomyolysis and intraoperative cardiac arrest secondary to hyperkalemia during the use of the inhaled anesthetics isoflurane, halothane, and sevoflurane (16, 28, 40, 41, 47, 49). In these patients, a clear precipitant rhythm or event was difficult to discern. Resuscitations persisted in excess of 60 min, with full recoveries obtained in six patients. However, one patient was discharged home with no subjective changes in cerebral function. However, he was paraplegic (sensory level T) (41). Dantrolene is often used empirically after documented concomitant metabolic and respiratory acidosis, with or without modest temperature increases. These cases suggest a predisposition to rhabdomyolysis upon exposure to volatile anesthetics, regardless of surgical stress. The disease is not known to be associated with MH; the components of effective resuscitation are difficult to discern, but a reduction in serum potassium levels is crucial (28, 40, 41, 47, 49).
3.4.6 Postoperative pain control
Appropriate analgesics should be encouraged to provide postoperative analgesia without affecting the patient's normal respiratory function. As in other patients, the analgesic drugs of choice for patients with DMD are opioids. Depending on the duration of the surgical intervention, the choice of opioid should be based on the pharmacological effect and pharmacokinetics. We have administered nearly all clinically used opioids in our series (Tables 1, 2). Six of the included studies investigated postoperative pain control (14, 16, 25, 26, 28–32, 35, 37, 39, 42, 43, 48, 54). Postoperative interventions, such as paracetamol, PCA, morphine pumps, pethidine, nalbuphine, dipirone, flurbiprofen axetil, diclofenac, sufentanil, ketorolac and atropine, were administered at different doses to provide postoperative analgesia (Table 1).
Given the severity of the surgical procedure, several options exist for the provision of postoperative analgesia. In patients undergoing spinal fusion surgery, neuraxial techniques have been used to achieve analgesia through the intermittent or continuous infusion of opioids and/or local anesthetics via epidural catheters, with minimal respiratory side effects (65).
In addition, given their effects on the central control of ventilation and cough effort, options that limit the use of opioids, including adjunct agents or regional anesthesia, should be considered. Preliminary data from the adult population have shown the potential role of the preoperative administration of pregabalin or gabapentin (66). Additionally, postoperative administration of the a2-adrenergic agonist dexmedetomidine and intravenous acetaminophen may play a role. Moreover, caution has been suggested with the use of non-steroidal anti-inflammatory agents given their anecdotal and temporal association with rhabdomyolysis (67, 68).
4 Discussion
Patients with DMD are uniquely vulnerable to the adverse physiological effects of general anesthesia and procedural sedation (55). Tachycardia, ventricular fibrillation and cardiac arrest have been reported during the induction of anesthesia (40, 41, 69–71). In almost all patients assessed in these case reports, DMD was not suspected until a further investigation was prompted by the occurrence of cardiac manifestations. In addition, ventricular fibrillation or cardiac arrest has also been described in patients who are known to have DMD following a return of consciousness while the patient is still in the recovery room (72). Therefore, patients with DMD should receive a detailed preoperative assessment, thoughtful disease-specific intraoperative management and aggressive postoperative monitoring if they are to avoid anesthesia- and surgery-related morbidity and mortality. Moreover, all children presenting for the administration of general anesthesia or sedation should be screened for motor milestones. The inability to walk at an age >18 months or other signs of motor loss or elevated levels of CPK should prompt a suspicion of subclinical myopathy and should warrant a neurological evaluation and genetic testing before elective surgery. Most cases of DMD are detected via genetic testing (55). In addition, timing disease-related major surgical procedures, such as scoliosis surgery, early in a child's life prior to the onset of significant myocardial dysfunction is recommended to minimize the cardiovascular risk. Finally, surgery for DMD patients should be performed in a hospital equipped to address the unique issues faced by patients with neuromuscular disorders (57).
In addition, because postoperative pulmonary complications might be one of the causes of postoperative complications in DMD patients, before general anesthesia or procedural sedation, the following lung function parameters should be measured to assess the patients' risk of respiratory complications and the need for perioperative and postoperative assisted ventilation or cough. The application of non-invasive ventilation modalities in the preoperative and postoperative setting to limit pulmonary postoperative complications using personalized non-invasive respiratory support is important (55). Options for respiratory support include manual ventilation using a flow-inflated manual resuscitation bag (standard “anesthesia bag”) with a full face or nasal mask interface and mechanical support using a conventional or non-invasive positive pressure ventilator via a full face or nasal mask.
Furthermore, these included case studies also revealed that general anesthesia and central neuraxial blockade in patients with severe DMD are unsafe approaches to anesthesia. Peripheral nerve blocks are the best way to reduce the risk of critical complications and are a safe and feasible approach to anesthesia in patients with severe DMD (26, 27, 42, 43). Notably, the general anesthetics that resulted in cardiac complications at induction were succinylcholine and volatile anesthetics. Therefore, the anesthesia community now commonly accepts that anesthetic machines free of volatile agents (including a new disposable breathing circuit) should be used and that succinylcholine is avoided. Monitoring should include a temperature probe, an ECG, and a nerve stimulator (73).
In addition, although general anesthesia may be required for specific procedures, moderately painful procedures such as bone marrow aspiration and biopsy can be performed with procedural sedation and the maintenance of spontaneous ventilation. Given these issues, a need remains for a better agent or agents for procedural sedation. The current evidence suggests the use of a total intravenous anesthesia (TIVA) technique to induce and maintain general anesthesia (e.g., propofol and short-acting opioids) and is advised rather than the use of depolarizing muscle relaxants (48). The authors reported their experience with a combination of ketamine and dexmedetomidine for sedation during bone aspiration and biopsy in an adolescent with DMD (7), which revealed that the application of dexmedetomidine in patients with DMD has the potential to be a promising treatment option in the future.
Moreover, the sensitivity of patients with DMD to NMBAs may result in prolonged onset and recovery times from anesthesia. Muenster et al. speculated that one reason for the prolonged duration of NMB in these patients could be the known degradation of muscle fibers and their replacement by fatty and fibrous tissue with the progression of the disorder. These structural changes are obviously accompanied by a decrease in the total number of neuromuscular junctions and receptors. Consistently, in an experimental study in mdx mice, accelerated degradation of adult nicotinic acetylcholine receptors was observed (74). Such a situation with a reduced number of receptors strongly influences the dose–response relationship of administered non-depolarizing NMBA. Therefore, the wide interpatient variability in the recovery time after the administration of a reduced dose does not allow an estimation of the time needed for complete recovery in a single patient (52). In particular, regarding the prolonged onset time, special attention must be paid to the effect of the relaxant agent used. The use of muscle relaxants is a major concern when performing anesthesia in DMD patients (52). Several prospective investigations have shown that nearly all commonly used non-depolarizing NMBAs can be used in patients with DMD (11). This situation is especially true for rocuronium and mivacurium (11, 52). These reports also revealed that the response to non-depolarizing NMBAs is altered in patients with DMD. The most striking difference is the delayed onset of blockade in DMD patients compared with normal patients. This effect should be considered in situations where rapid airway protection is necessary. Another significant difference is the prolonged duration of recovery from NMB in DMD following standard doses of non-depolarizing NMBAs. Notably, depending on the time of reversal, the duration of residual block after rocuronium may exceed the duration of antagonism by the reversal agent. Therefore, using reversal agents in this situation involves the risk of possible “recurarization” (51). Therefore, even after the administration of a reversal agent, monitoring of muscle strength in the recovery room either quantitatively or clinically should be performed (51). Furthermore, these effects depend on the stage of the disease, with more pronounced effects observed with ongoing progression. This altered response to non-depolarizing NMBAs in patients with DMD makes a quantitative assessment of complete neuromuscular recovery, such as acceleromyography, necessary (11).
In addition, the existing evidence implicates calcium dysregulation as an underlying crucial event in the pathophysiology of DMD (73). In malignant hyperthermia, defective influx and efflux of Ca from the sarcoplasmic reticulum has been observed in mouse models. Since a malignant hyperthermia-like syndrome may occur in DMD patients during anesthesia, maneuvers capable of reducing Ca influx into cells have beneficial effects on these patients DMD; thus, the possibility that a reduction in Ca influx from the sarcoplasmic reticulum by a Ca antagonist, such as dantrolene, may result in additional benefits for patients with DMD (74).
Several limitations of the study should be acknowledged. The strengths of this systematic review include the broad and complete search strategy, the publication of a protocol a priori, and the validated methodology used to assess the included studies, e.g., Cochrane's “Risk of Bias” 2.0 tool and ROBINS-I. We adhered to the protocol to minimize intellectual bias in conducting and reporting the findings. Two authors independently screened studies for inclusion and performed the risk-of-bias assessment. Potential limitations include our broad approach, i.e., for example, we included all studies regardless of the type of drugs, which may have contributed to the high degree of clinical heterogeneity of the included studies. Furthermore, our choice of the definition of outcomes could be discussed. We chose analgesia, sedation and mortality at discharge as the primary outcomes. Unfortunately, a possibility of poorly documented minor complications or minor adverse events caused by anesthetic medication always exists. Finally, we did not explore the significance of a diagnosis of respiratory or cardiac involvement in the prediction of perioperative complications in DMD patients, and we were not able to perform a retrospective evaluation. No cases of patients requiring postoperative ventilatory support were documented, regardless of whether NMBAs had been used. Follow-up examinations after adverse reactions to general anesthesia are often incomplete, and some patients receive the same type of anesthesia again (75). Whether the negative responses in DMD patients originate during multiple exposures to anesthesia or sedation is unknown, and additional high-quality research is needed to provide more comprehensive information.
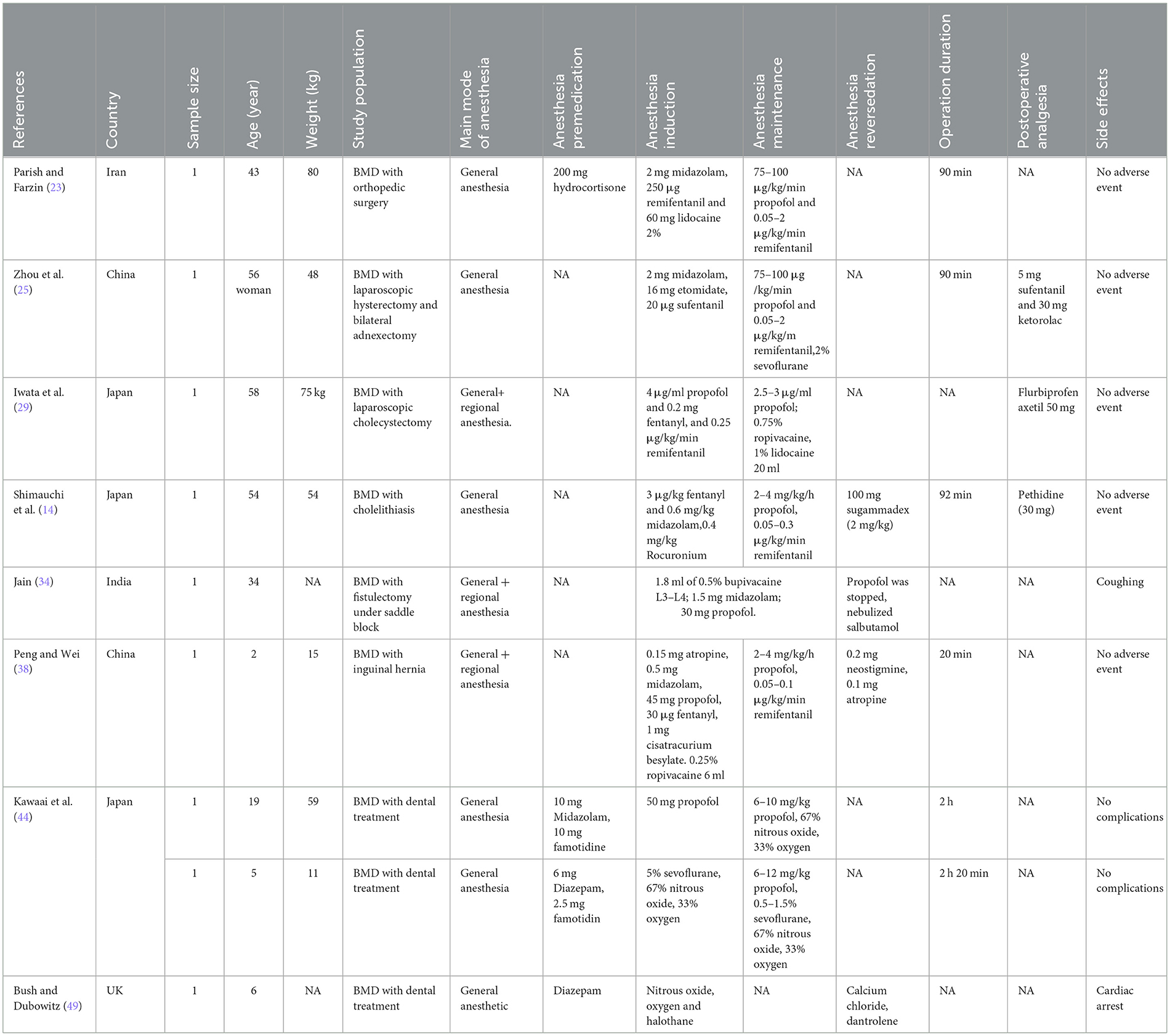
Finally, the primary difference between DMD and BMD is the quantity of dystrophin present in skeletal and cardiac muscle. In patients with DMD, dystrophin is almost always absent, whereas partially functional dystrophin is present in patients with BMD and results in a milder form of the disorder and longer survival, which was consistent with the data in Tables 2, 3. However, 2 patients with BMD who were very young were described (23, 49). In addition, compared with patients with BMD, patients with DMD had more comorbid conditions and higher rates of cardiomyopathy and severe restrictive lung disease. However, patients with BMD are present with serious postoperative adverse reactions (49). Postoperatively, DMD and BMD patients must be monitored until cardiorespiratory function returns to the baseline. The current case reports are insufficient for generating definitive conclusions regarding the significant differences between patients with BMD and DMD. Overall, the anesthesia technique must be customized and adjusted for each patient.
5 Conclusions
The results of the included studies confirmed that patients with DMD are more sensitive to NMBAs, which may result in a delayed onset time and prolonged recovery time from anesthesia, and these effects depend on the stage of the disease, with more pronounced effects observed with ongoing progression. Precautions for DMD patients should include quantitative neuromuscular and electrocardiographic monitoring and rapid airway protection throughout anesthesia. The strict avoidance of succinylcholine and volatile anesthetics during anesthesia in patients with DMD can prevent known anesthetic hazards such as rhabdomyolysis or hypercalcemia. Compared with general anesthesia, regional anesthesia can be a relatively safe option (if the surgical site is appropriate for the technique). Dantrolene should be available in the theater and be readily used if events consistent with a malignant hyperpyrexial response to anesthesia occur. However, further prospective clinical trials are needed to determine the most effective interventions for patients with DMD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XL: Writing – original draft, Data curation, Formal analysis. YJ: Data curation, Formal analysis, Methodology, Writing – original draft. TL: Data curation, Formal analysis, Methodology, Writing – original draft. YG: Data curation, Methodology, Writing – original draft. YL: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Chengdu Pharmaceutical Society pharmaceutical research project (cdyxky5039).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kunkel LM, Monaco AP, Middlesworth W, Ochs HD, Latt SA. Specific cloning of DNA fragments absent from the DNA of a male patient with an X chromosome deletion. Proc Natl Acad Sci USA. (1985) 82:4778–82. doi: 10.1073/pnas.82.14.4778
2. Ray PN, Belfall B, Duff C, Logan C, Kean V, Thompson MW, et al. Cloning of the breakpoint of an X; 21 translocation associated with Duchenne muscular dystrophy. Nature. (1985) 318:672–5. doi: 10.1038/318672a0
3. Mah JK. Current and emerging treatment strategies for Duchenne muscular dystrophy. Neuropsychiatr Dis Treat. (2016) 12:1795–807. doi: 10.2147/NDT.S93873
4. Frankowski GA, Johnson JO, Tobias JD. Rapacuronium administration to two children with Duchenne's muscular dystrophy. Anesth Analg. (2000) 91:27–8. doi: 10.1213/00000539-200007000-00005
5. Caliskan E, Sener M, Kocum A, Aribogan A. Duchenne muscular dystrophy: how I do it? Regional or general anesthesia? Paediatr Anaesth. (2010) 19:624–5. doi: 10.1111/j.1460-9592.2009.03020.x
6. Murat I, Esteve C, Montay G, Delleur MM, Gaudiche O, Saint-Maurice C, et al. Pharmacokinetics and cardiovascular effects of bupivacaine during epidural anesthesia in children with Duchenne muscular dystrophy. Anesthesiology. (1987) 7:249–52. doi: 10.1097/00000542-198708000-00017
7. Rozmiarek A, Corridore M, Tobias JD. Dexmedetomidine-ketamine sedation during bone marrow aspirate and biopsy in a patient with duchenne muscular dystrophy. Saudi J Anaesth. (2011) 5:219–22. doi: 10.4103/1658-354X.82810
8. Sethna NF, Rockoff MA, Worthen HM, Rosnow JM. Anesthesia-related complications in children with Duchenne muscular dystrophy. Anesthesiology. (1988) 68:462–5. doi: 10.1097/00000542-198803000-00028
9. Hayes J, Veyckemans F, Bissonnette B. Duchenne muscular dystrophy: an old anesthesia problem revisited. Paediatr Anaesth. (2008) 18:100–6. doi: 10.1111/j.1460-9592.2007.02302.x
10. Hopkins PM. Anaesthesia and the sex-linked dystrophies: between a rock and a hard place. Br J Anaesth. (2010) 104:397–400. doi: 10.1093/bja/aeq036
11. Muenster T, Mueller C, Forst J, Huber H, Schmitt HJ. Anaesthetic management in patients with Duchenne muscular dystrophy undergoing orthopaedic surgery: a review of 232 cases. Eur J Anaesthesiol. (2012) 29:489–94. doi: 10.1097/EJA.0b013e3283566789
12. de Boer HD, van Esmond J, Booij LH, Driessen JJ. Reversal of rocuronium-induced profound neuromuscular block by sugammadex in Duchenne muscular dystrophy. Paediatr Anaesth. (2009) 19:1226–8. doi: 10.1111/j.1460-9592.2009.03178.x
13. Wefki Abdelgawwad Shousha AA, Sanfilippo M, Sabba A, Pinchera P. Sugammadex and reversal of neuromuscular block in adult patient with duchenne muscular dystrophy. Case Rep Anesthesiol. (2014) 2014:680568. doi: 10.1155/2014/680568
14. Shimauchi T, Yamaura K, Sugibe S, Hoka S. Usefulness of sugammadex in a patient with Becker muscular dystrophy and dilated cardiomyopathy. Acta Anaesthesiol Taiwan. (2014) 52:146–8. doi: 10.1016/j.aat.2014.02.005
15. Smelt WL. Cardiac arrest during desflurane anaesthesia in a patient with Duchenne's muscular dystrophy. Acta Anaesthesiol Scand. (2005) 49:267–9. doi: 10.1111/j.1399-6576.2004.00596.x
16. Irwin MG, Henderson M. Cardiac arrest during major spinal scoliosis surgery in a patient with Duchenne's muscular dystrophy undergoing intravenous anaesthesia. Anaesth Intensive Care. (1995) 23:626–9. doi: 10.1177/0310057X9502300521
17. Higgins J, Thompson SG, Deeks JJ. Cochrane handbook for systematic reviews of interventions version 5.1.0. the cochrane collaboration. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. (2008) 5:S38.
18. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
19. The Joanna Briggs Instatute. Joanna Briggs Instatute Reviewers' Manual: 2016 Edition. Adelaide, SA: The Joanna Bbrigga Institute (2016).
20. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. (2022). Available at: www.training.cochrane.org/handbook
21. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
22. Kim JE, Chun HR. Rocuronium-induced neuromuscular block and sugammadex in pediatric patient with duchenne muscular dystrophy: a case report. Medicine. (2017) 96:e6456. doi: 10.1097/MD.0000000000006456
23. Parish M, Farzin H. Adult patient with Becker dystrophy undergoing orthopedic surgery: an anesthesia challenge. Int Med Case Rep J. (2018) 11:33–6. doi: 10.2147/IMCRJ.S150037
24. Jung HJ, Kim JB, Im KS, Lee JH, Kim DJ, Cho SA, et al. How should we monitor pediatric patients with Duchenne muscular dystrophy? A case report. Korean J Anesthesiol. (2011) 61:159–61. doi: 10.4097/kjae.2011.61.2.159
25. Zhou SY, Wang D, Liu C, Zhang S, Shan BL, Ma HC, et al. Laparoscopic gynecological surgery in an adult woman with Becker muscular dystrophy performed with sevoflurane with cisatracurium anesthesia: a case report. Medicine. (2020) 99:e19733. doi: 10.1097/MD.0000000000019733
26. Bang SU, Kim YS, Kwon WJ, Lee SM, Kim SH. Peripheral nerve blocks as the sole anesthetic technique in a patient with severe Duchenne muscular dystrophy. J Anesth. (2016) 30:320–3. doi: 10.1007/s00540-015-2127-4
27. Büget MI, Eren I, Küçükay S. Regional anaesthesia in a Duchenne muscular dystrophy patient for upper extremity amputation. Agri. (2014) 26:191–5. doi: 10.5505/agri.2014.34713
28. Obata R, Yasumi Y, Suzuki A, Nakajima Y, Sato S. Rhabdomyolysis in association with Duchenne's muscular dystrophy. Can J Anaesth. (1999) 46:564–6. doi: 10.1007/BF03013547
29. Iwata M, Kuzumoto N, Akasaki Y, Morioka M, Nakayama K, Matsuzawa N, et al. The ultrasound-guided nerve blocks of abdominal wall contributed to anesthetic management of cholecystectomy in a patient with Becker muscular dystrophy without using muscle relaxants. JA clinical reports. (2017) 3:64. doi: 10.1186/s40981-017-0134-1
30. Richa F, Yazigi A, Yazbeck P. Use of remifentanil and propofol without muscle relaxant combined with intrathecal morphine in children with Duchenne's muscular dystrophy undergoing spinal surgery. J Med Liban. (2008) 56:181–4.
31. Saldanha RM, Gasparini JR, Silva LS, de Carli RR, de Castilhos VU, das Neves MM, et al. Anesthesia for Duchenne muscular dystrophy patients: case reports. Rev Bras Anestesiol. (2005) 55:445–9. doi: 10.1590/S0034-70942005000400009
32. Kocabas S, Yedicocuklu D, Askar F, Atay Y. Anesthetic management of a child with Duchenne muscular dystrophy undergoing correction of Fallot's tetralogy. Paediatr Anaesth. (2008) 18:448–50. doi: 10.1111/j.1460-9592.2008.02456.x
33. Rajmala X, Savita S, Kirti K, Nandini X. Intravenous anaesthesia combined with peribulbar block in a child with suspected Duchenne muscular dystrophy. Acta Anaesthesiol Scand. (2004) 48:1341. doi: 10.1111/j.1399-6576.2004.00501.x
34. Jain A. Propofol-induced violent coughing in a patient with Becker's muscular dystrophy. Indian J Pharmacol. (2011) 43:476–7. doi: 10.4103/0253-7613.83134
35. Vandepitte C, Gautier P, Bellen P, Murata H, Salviz EA, Hadzic A, et al. Use of ultrasound-guided intercostal nerve block as a sole anaesthetic technique in a high-risk patient with Duchenne muscular dystrophy. Acta Anaesthesiol Belg. (2013) 64:91−4.
36. Molyneux MK. Anaesthetic management during labour of a manifesting carrier of Duchenne muscular dystrophy. Int J Obstet Anesth14. (2005) 58–61. doi: 10.1016/j.ijoa.2004.05.009
37. Van Obbergh LJ, Corteel J, Papadopoulos J, Aunac S. Anesthesia for a child suffering from a deletion in the Xp21 loci resulting in Duchenne disease, glycerol kinase deficiency, and congenital adrenal hypoplasia. Paediatr Anaesth. (2011) 21:1085–7. doi: 10.1111/j.1460-9592.2011.03634.x
38. Peng L, Wei W. Anesthesia management in a pediatric patient with Becker muscular dystrophy undergoing laparoscopic surgery: a case report. World J Clin Cases. (2021) 9:8852–7. doi: 10.12998/wjcc.v9.i29.8852
39. Horikoshi Y, Kuratani N, Tateno K, Hoshijima H, Nakamura T, Mieda T, et al. Anesthetic management with remimazolam for a pediatric patient with Duchenne muscular dystrophy. Medicine. (2021) 100:e28209. doi: 10.1097/MD.0000000000028209
40. Sethna NF, Rockoff MA. Cardiac arrest following inhalation induction of anaesthesia in a child with Duchenne's muscular dystrophy. Can Anaesth Soc J. (1986) 33:799–802. doi: 10.1007/BF03027134
41. Chalkiadis GA, Branch KG. Cardiac arrest after isoflurane anaesthesia in a patient with Duchenne's muscular dystrophy. Anaesthesia. (1990) 45:22–5. doi: 10.1111/j.1365-2044.1990.tb14497.x
42. Shafy SZ, Hakim M, Villalobos MA, Pearson GD, Veneziano G, Tobias JD, et al. Caudal epidural block instead of general anesthesia in an adult with Duchenne muscular dystrophy. Local Reg Anesth. (2018) 11:75–80. doi: 10.2147/LRA.S180867
43. Rathi R, Ramekar A, D'souza N. Combination of regional anaesthetic techniques in a Duchenne Muscular Dystrophy carrier undergoing mastectomy. Indian J Anaesth. (2021) 65:260–1. doi: 10.4103/ija.IJA_913_20
44. Kawaai H, Tanaka K, Yamazaki S. Continuous infusion propofol general anesthesia for dental treatment in patients with progressive muscular dystrophy. Anesth Prog. (2005) 52:12–6. doi: 10.2344/0003-3006(2005)52[12:CIPGAF]2.0.CO;2
45. Kulshrestha A, Bajwa SJ, Singh A, Kapoor V. Dexmedetomidine and fentanyl combination for procedural sedation in a case of Duchenne muscular dystrophy. Anesth Essays Res. (2011) 5:224–6. doi: 10.4103/0259-1162.94788
46. Raman V, Yacob D, Tobias JD. Dexmedetomidine-ketamine sedation during upper gastrointestinal endoscopy and biopsy in a patient with Duchenne muscular dystrophy and egg allergy. Int J Crit Illn Inj Sci. (2012) 2:40–3. doi: 10.4103/2229-5151.94899
47. Wang JM, Stanley TH. Duchenne muscular dystrophy and malignant hyperthermia–two case reports. Can Anaesth Soc J. (1986) 33:492–7. doi: 10.1007/BF03010977
48. Kim HJ, Kim SY, Ju MH, Lee SY, Byeon GJ, Kim HY, et al. Early extubation after left ventricular assist device implantation in a patient with Duchenne muscular dystrophy: a case report. J Anesth. (2021) 35:455–8. doi: 10.1007/s00540-021-02925-9
49. Bush A, Dubowitz V. Fatal rhabdomyolysis complicating general anaesthesia in a child with Becker muscular dystrophy. Neuromuscul Disord. (1991) 1:201–4. doi: 10.1016/0960-8966(91)90025-N
50. Ihmsen H, Schmidt J, Schwilden H, Schmitt HJ, Muenster T. Influence of disease progression on the neuromuscular blocking effect of mivacurium in children and adolescents with Duchenne muscular dystrophy. Anesthesiology. (2009) 110:1016–9. doi: 10.1097/ALN.0b013e31819daf31
51. Wick S, Muenster T, Schmidt J, Forst J, Schmitt HJ. Onset and duration of rocuronium-induced neuromuscular blockade in patients with Duchenne muscular dystrophy. Anesthesiology. (2005) 102:915–9. doi: 10.1097/00000542-200505000-00009
52. Muenster T, Schmidt J, Wick S, Forst J, Schmitt HJ. Rocuronium 0.3 mg x kg-1 (ED95) induces a normal peak effect but an altered time course of neuromuscular block in patients with Duchenne's muscular dystrophy. Paediatr Anaesth. (2006) 16:840–5. doi: 10.1111/j.1460-9592.2006.01870.x
53. Ririe DG, Shapiro F, Sethna NF. The response of patients with Duchenne's muscular dystrophy to neuromuscular blockade with vecuronium. Anesthesiology. (1998) 88:351–4. doi: 10.1097/00000542-199802000-00013
54. Kako H, Corridore M, Kean J, Mendell JR, Flanigan KM, Tobias JD, et al. Dexmedetomidine and ketamine sedation for muscle biopsies in patients with Duchenne muscular dystrophy. Paediatr Anaesth. (2014) 24:851–6. doi: 10.1111/pan.12387
55. Birnkrant DJ, Panitch HB, Benditt JO. American College of Chest Physicians consensus statement on the respiratory and related management of patients with Duchenne muscular dystrophy undergoing anesthesia or sedation. Chest. (2007) 132:1977–86. doi: 10.1378/chest.07-0458
56. Scherzer D, Leder M, Tobias JD. Pro-con debate: etomidate or ketamine for rapid sequence intubation in pediatric patients. J Pediatr Pharmacol Ther. (2012) 17:142–9. doi: 10.5863/1551-6776-17.2.142
57. Cripe LH, Tobias JD. Cardiac considerations in the operative management of the patient with Duchenne or Becker muscular dystrophy. Paediatr Anaesth. (2013) 23:777–84. doi: 10.1111/pan.12229
58. Vasile B, Rasulo F, Candiani A. The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome. Intensive Care Med. (2003) 29:1417–25. doi: 10.1007/s00134-003-1905-x
59. Kuznetsov AV, Winkler K, Wiedemann FR. Impaired mitochondrial oxidative phosphorylation in skeletal muscle of the dystrophin-deficient mdx mouse. Mol Cell Biochem. (1998) 183:87–96.
60. Ginns EI, Barranger JA, McClean SW. A juvenile form of glycerol kinase deficiency with episodic vomiting, acidemia, and stupor. J Pediatr. (1984) 104:736–9. doi: 10.1016/S0022-3476(84)80956-7
61. Mak TWL, Wong LM, Wong SN. Glycerol kinase deficiency presenting with hypodipsia, osmotic diuresis and severe hy-hypernatremia. J Inherit Metab Dis. (2005) 28:1159–61. doi: 10.1007/s10545-005-0101-2
62. Tobias JD, Goble TJ, Bates G. Effects of dexmedetomidine on intraoperative motor and somatosensory evoked potential monitoring during spinal surgery in adolescents. Pediatr Anesth. (2008) 18:1082–8. doi: 10.1111/j.1460-9592.2008.02733.x
63. Bala E, Sessler DI, Nair DR. Motor and somatosensory evoked potentials are well maintained in patients given dexmedetomidine during spine surgery. Anesthesiology. (2008) 109:417–25. doi: 10.1097/ALN.0b013e318182a467
64. Gurnaney H, Brown A, Litman RS. Malignant hyperthermia and muscular dystrophies. Anesth Analg. (2009) 109:1043–8. doi: 10.1213/ane.0b013e3181aa5cf6
65. Tobias JD. A review of intrathecal and epidural analgesia after spinal surgery in children. Anesth Analg. (2004) 98:956–65. doi: 10.1213/01.ANE.0000107938.80562.75
66. Rusy LM, Hainsworth KR, Nelson TJ. Gabapentin use in pediatric spinal fusion patients: a randomized, double-blind, controlled controlled trial. Anesth Analg. (2010) 110:1393–8. doi: 10.1213/ANE.0b013e3181d41dc2
67. Poole TC, Lim TY, Buck J. Perioperative cardiac arrest in a patient with previously undiagnosed Becker's muscular dystrophy after isoflurane anaesthesia for elective surgery. Br J Anaesth. (2010) 104:487–9. doi: 10.1093/bja/aeq035
68. Burke SM, Shorten GD. Perioperative pregabalin improves pain and functional outcomes 3 months after lumbar discectomy. Anesth Analg. (2010) 110:1180–5. doi: 10.1213/ANE.0b013e3181cf949a
69. Miller Jr ED, Sanders DB, Rowlingson JC, Berry Jr FA, Sussman MD, Epstein RM, et al. Anesthesia induced rhabdomyolysis in a patient with Duchenne'smuscular dystrophy. Anesthesiology. (1978) 48:146–8. doi: 10.1097/00000542-197802000-00012
70. Brownell AKW, Paasuke RT, Elash A. Malignant hypothermia in Duchenne muscular dystrophy. Anesthesiology. (1983) 58:180. doi: 10.1097/00000542-198302000-00013
71. Linter SP, Thomas PR, Withington PS, Hall MG. Suxamethonium associated hypertonicity and cardiac arrest in unsuspected pseudohypertrophic muscular dystrophy. Br J Anaesth. (1982) 54:1331–2. doi: 10.1093/bja/54.12.1331
72. Boba A. Fatal postanesthetic complications in two muscular dystrophic patients. J Pediatr Surg. (1970) 5:71–5. doi: 10.1016/0022-3468(70)90523-3
73. Vallejo-Illarramendi A, Toral-Ojeda I, Aldanondo G, López de Munain A. Dysregulation of calcium homeostasis in muscular dystrophies. Expert Rev Mol Med. (2014) 16:e16. doi: 10.1017/erm.2014.17
74. Bertorini TE, Palmieri GM, Griffin J, Igarashi M, Hinton A, Karas JG, et al. Effect of dantrolene in Duchenne muscular dystrophy. Muscle Nerve. (1991) 14:503–7. doi: 10.1002/mus.880140603
Keywords: Duchenne muscular dystrophy, sedative, anesthesia, pharmacological interventions, systematic review, meta-analysis
Citation: Lian X, Jing Y, Luo T, Guo Y and Lin Y (2025) Pharmacological interventions for the management of anesthesia and sedation in patients with Duchenne muscular dystrophy: a systematic review and meta-analysis. Front. Med. 12:1497538. doi: 10.3389/fmed.2025.1497538
Received: 19 September 2024; Accepted: 06 January 2025;
Published: 27 January 2025.
Edited by:
Zhongheng Zhang, Sir Run Run Shaw Hospital, ChinaReviewed by:
Giovanni Misseri, Institute Foundation G.Giglio, ItalyTanya Cully, University of Otago, New Zealand
Copyright © 2025 Lian, Jing, Luo, Guo and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunzhu Lin, bGlueXVuemh1OTlAc2N1LmVkdS5jbg==
 Xianghong Lian
Xianghong Lian Yang Jing1,2,3
Yang Jing1,2,3 Yunzhu Lin
Yunzhu Lin