- 1Department of General Affairs, Shenzhen Baoan Women’s and Children’s Hospital, Shenzhen, Guangdong, China
- 2Department of Infectious Diseases, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 3Department of Spleen and Gastroenterology, Dongxihu District Hospital of Traditional Chinese Medicine, Wuhan, Hubei, China
- 4School of Traditional Chinese Medicine, Hubei University of Chinese Medicine, Wuhan, Hubei, China
- 5Department of Spleen and Gastroenterology, Hubei Provincial Hospital of Traditional Chinese Medicine, Affiliated Hospital of Hubei University of Chinese Medicine, Wuhan, Hubei, China
- 6Hubei Shizhen Laboratory, Wuhan, Hubei, China
Background: Colorectal adenomas, which are precancerous lesions that can develop into colorectal cancer, present a significant challenge due to the lack of comprehensive early screening and clear identification of risk factors.
Objectives: We conduct a double-blind, prospective cross-sectional analysis to examine the relationship between lifestyle, mental health, and colorectal adenomas.
Methods: Between June 2023 and July 2024, we surveyed 246 participants at Hubei Provincial Hospital of Traditional Chinese Medicine in Wuhan using a self-administered online questionnaire.
Results: The majority of participants were over the age of 50 (49.6%), married or living with a partner (87.08%), and employed as office workers or technicians (44.3%). Among the total population, 435 individuals (53.5%) were diagnosed with colorectal adenomas. A significant positive association was observed between being a manager (OR = 2.340; 95% CI = 1.043–5.248) and the presence of colorectal adenomas, as well as having a BMI over 28 (OR = 6.000; 95% CI = 1.501–23.991). After adjusting for professional role and BMI, no significant associations were found between scores on the HADS-D (AOR = 1.031; 95% CI = 0.967–1.099) or PSS-10 (AOR = 0.971; 95% CI = 0.923–1.022) scales and colorectal adenomas. However, higher scores on the AUDIT (AOR = 1.001–1.144), CDS-12 (AOR = 1.028; 95% CI = 1.003–1.054), PSQI (AOR = 1.079; 95% CI = 1.003–1.161), and HADS-A (AOR = 1.156; 95% CI = 1.059–1.262) scales were significantly associated with an increased likelihood of colorectal adenomas.
Conclusion: The study highlights the significance of addressing alcohol consumption, smoking, sleep quality, and anxiety to reduce the risk of colorectal adenomas. Targeted mental health interventions may play a crucial role in alleviating this health burden and enhancing overall population health.
1 Introduction
Colorectal cancer (CRC) is a significant global health challenge, ranking as the third most common cancer (1) and the second leading cause of cancer-related deaths worldwide (2). In China, the burden of CRC is particularly high, with the country accounting for 29% of all newly diagnosed cases globally, totaling 555,477 cases (2). Colorectal adenomas, which are the primary precursors of CRC, represent approximately 85–90% of sporadic CRC cases and pose a critical public health concern due to their potential to progress into cancer (3). Screening efforts have identified adenomas in up to 50% of asymptomatic individuals undergoing colonoscopy or CT colonography, highlighting the widespread nature of this condition (4). Of these adenomas, 3.4%–7.6% are classified as advanced, and 0.2%–0.6% are malignant (4). Screening tests, including surveillance colonoscopy, circulating plasma microRNAs, and a novel fecal Lachnoclostridium marker, have demonstrated potential in detecting colorectal adenomas (5–8) and early-stage cancer, contributing to reduced mortality rates (9). The increasing incidence of colorectal adenomas in China (10) underscores the need for enhanced screening and the identification of risk factors to mitigate this growing public health issue.
Recent research has underscored the significant impact of lifestyle factors on disease prevention and management (11). Increasing evidence suggests that lifestyle factors, including alcohol consumption (12–14), smoking (13, 15), and sleep quality (16, 17), are associated with an elevated risk of colorectal cancer. Globally, smoking and alcohol use were identified as the leading contributors to CRC disability-adjusted life years in 2019 (18). Additionally, circadian rhythm disruption plays a key role in tumorigenesis (19), and poor sleep quality has been associated with adverse health outcomes, including cardiovascular disease (20), obesity (21), and diabetes (22). Recent studies have also pointed out the significant positive relationship between sleep disturbances and colorectal adenomas (23, 24). These findings suggest that unhealthy lifestyle factors increase the risk of CRC. However, limited research in China has investigated the relationship between lifestyle factors and colorectal adenomas, which are precursors to CRC, particularly for early screening.
In addition to established risk factors like age (25) and family history (26), emerging research suggests that psychological factors may significantly influence the development and progression of colorectal adenomas (17, 27–33). There is a growing concern on the relationship between anxiety (31), depression (32), stress (33), and colorectal cancer. Chronic psychological distress can lead to immune system dysregulation (34) and increased systemic inflammation (35), both of which have been implicated in colorectal cancer pathogenesis (36, 37). Although psychological factors can increase the risk of CRC, few studies in China have specifically examined their association with colorectal adenomas.
To address these gaps, we conducted a prospective cross-sectional survey in Wuhan, China, to examine the association between colorectal adenomas and demographic, lifestyle, and mental health factors among adults (Figure 1). We initially performed univariate logistic regression analysis and included variables with significant differences (p < 0.05) in a multivariate model for further evaluation. Through the use of multiple logistic regression models, our study aims to deepen understanding of the disease’s etiology and identify potential targets for intervention and prevention.
2 Materials and methods
2.1 Study design
Between June 2023 and July 2024, a cross-sectional survey was conducted among adults in Wuhan.
2.2 Sample size
Our sample size (N) was determined utilizing the formula: . Considering a desired confidence level of 95% (Z -score: 1.96), an estimated population proportion of 0.2 (p), and a desired margin of error of 0.05 (d), the minimum sample size (N) required is calculated to be 246 participants. Out of the selected 320 participants, 38 declined to participate in the study, and 36 withdrew from the study. The remaining 246 participants completed all self-administered online questionnaires that took approximately 20 min. The survey achieved a response rate of 76.9%.
2.3 Participants and data collection
Participants in this study were adults aged eighteen and over who underwent lower gastrointestinal endoscopy at Hubei Provincial Hospital of Traditional Chinese Medicine, including both inpatients and outpatients. The research team used a volunteer sampling approach to recruit participants. A questionnaire, developed by a panel of four gastroenterology clinicians, two epidemiologists, and one psychologist, was used to gather data. This questionnaire was created using Wenjuanxing and was initially evaluated with fifteen randomly selected participants. Based on their feedback, the panel made necessary adjustments to finalize the questionnaire. The questionnaire is available in the Supplementary material.
Before undergoing colonoscopy, all eligible participants completed the questionnaire without any knowledge of the microscopic findings and pathological results. Participants were invited to a dedicated consulting room where well-trained workers provided detailed information about the study and confirmed their eligibility. Participants were assured of their anonymity and informed of their right to withdraw from the study at any time without any consequences. Written informed consent was obtained from all participants prior to their inclusion in the study. Upon completing the survey, participants received a ¥20 (US$2.81) cash coupon as compensation for their time. There was no missing data in this study. All data and codes used in this study were available at https://github.com/BennyZhu2025/Cross_sectional_studies.git.
2.4 Ethics approval
The study received ethical approval from the Ethics Committee of Hubei Provincial Hospital of Traditional Chinese Medicine (Approval Number: HBZY2024-C29-02).
2.5 Measurements
2.5.1 Background characteristics of the participants
Participants provided sociodemographic details encompassing age, gender, marital status, highest educational attainment, monthly income, occupation, and BMI.
2.5.2 Dependent variables
Participants diagnosed with colorectal adenomas had initially been found to have polyps during their first lower gastrointestinal endoscopy, with postoperative pathology confirming the presence of adenomas. In contrast, both the endoscopic evaluations and postoperative pathology of patients without colorectal adenomas showed normal results. The diagnosis of colorectal adenomas was confirmed through pathological examination, considered the gold standard, while polyps were identified through endoscopic evaluation.
2.5.3 Lifestyle variables
The Alcohol Use Disorder Identification and Testing Instrument, commonly referred to as AUDIT (38), contains 10 items designed to measure various aspects of alcohol consumption such as frequency and quantity as well as episodes of binge drinking (three items), symptoms associated with dependence (three), as well as its damaging social and health repercussions associated with its use (four). The overall Cronbach’s α (39) of AUDIT was 0.864 and the McDonald’s omega (40) was 0.897.
The Cigarette Dependence Scale (CDS-12) (41) is a meticulously designed 12-item questionnaire intended to assess specific dimensions of dependence. Notably, it examines indicators encompassing smoking compulsion, withdrawal symptoms, loss of control over time or activities that one used to enjoy and the persistence in smoking even when there is potential harm involved. Furthermore, CDS-12 includes items which capture self-perceptions of addiction or smoking rates with responses on a five-point Likert scale for each question. Cronbach’s alpha coefficients (39) were 0.983 for CDS-12 and the McDonald’s omega (40) was 0.985.
The Pittsburgh Sleep Quality Index (PSQI) (42), developed in 1989, is a validated questionnaire employed to assess sleep-related difficulties. The PSQI is administered as a self-report questionnaire that requires individuals to answer nineteen questions regarding their sleep patterns during the last month, plus five optional ones from cohabitants who share rooms or beds with them. This questionnaire aims to provide comprehensive insights into various aspects of sleep quality. Cronbach’s alpha coefficients (39) were 0.763 for PSQI and the McDonald’s omega (40) for this scale was 0.772.
2.5.4 Mental health variables
The Hospital Anxiety and Depression Scale (HADS) (43) is a validated instrument comprising 14 items, specifically developed to evaluate symptoms of anxiety and depression among clinical patients. Notably, the scale aims to minimize the influence of physical illness on the overall score. The items pertaining to depression primarily target anhedonia symptoms commonly associated with depression. Participants rate each item on a 4-point severity scale, allowing for a comprehensive assessment of symptom severity. The overall Cronbach’s α (39) of HADS was 0.842 and the McDonald’s omega (40) for this scale was 0.848.
The Perceived Stress Scale-10 (PSS-10), originally developed by Cohen et al. (44), is an widely utilized 10-item questionnaire used to assess stress levels among individuals aged 12 years or over - this measure includes both children and adults alike. Response options are recorded using a five-point Likert scale from 0 to 4, where 4 corresponds with never; three with rarely; 2 with sometimes; 1 with frequently and 0 with always. Additionally, items 4, 5, 7, and 8 are positively scored. A total score of 13 indicates a normal stress level, while scores of 20 or higher denote elevated stress levels warranting therapeutic intervention (45). The Cronbach’s α (39) and McDonald’s omega (40) were 0.749 and 0.760, respectively.
2.6 Statistical analysis
The figure illustrating the schematic overview of the study design was created using Figdraw1. Statistical analyses were conducted using R (version 4.4.2) and Python (version 3.12.3) in the Microsoft Visual Studio Code environment. Categorical variables were encoded into numeric types using Scikit-learn’s LabelEncoder. Categorical variables were analyzed at each level using descriptive statistics, including frequencies and percentages. For continuous variables, means and standard deviations were reported. Univariate logistic regression was performed for all variables, followed by stepwise multivariate logistic regression for variables with significant differences in the univariate analysis. Multicollinearity among independent variables in each logistic regression model was assessed using the variance inflation factor (46). The linearity of the logit was assessed using the Box-Tidwell Test. Outliers were identified using Casewise Diagnostics, applying a 3-standard deviation default threshold. The adequacy of the multivariable models was evaluated using the Hosmer and Lemeshow goodness-of-fit tests (47). Overdispersion was examined using studentized permutations. A two-sided p < 0.05 was considered statistically significant. The Python environment was equipped with some packages, including pandas (version 2.1.4), numpy (version 1.24.3), scikit-learn (version 1.3.0), statsmodels (version 0.14.2) and scipy (version 1.11.4). Similarly, the R environment included necessary packages such as MASS (version 7.3-61), ltm (version 1.2-0), dplyr (version 1.1.4), tableone (version 0.13.2), openxlsx (version 4.2.7.1), and broom (version 1.0.7).
3 Results
3.1 Characteristics of participants
The characteristics of the participants are summarized in Table 1. Half of the participants were over 50 years old (49.6%), and the majority were either married or cohabiting (87.8%). Office workers and technicians made up the largest professional group (44.3%), and most participants had a BMI between 18 and 24 (54.9%). Additionally, 31.7% of the participants held a university degree or higher. Female participants represented 54.9% of the total sample, while 34.6% had a monthly income between 700 and 1,400. The study found that 107 participants (43.5%) were diagnosed with colorectal adenomas, while 139 (56.5%) were not.
3.2 Scale scores of lifestyle-level and mental health-level variables
The lifestyle-related scales showed an average score of 2.10 (SD 4.58) on the AUDIT scale, 16.42 (SD 11.00) on the CDS-12 scale, and 6.78 (SD 3.77) on the PSQI scale. Regarding mental health, the HADS-A subscale had a mean score of 7.09 (SD 4.21), the HADS-D sub-scale averaged 6.94 (SD 3.31), and the PSS-10 scale recorded a mean score of 16.15 (SD 3.94). These results are detailed in Figure 2.
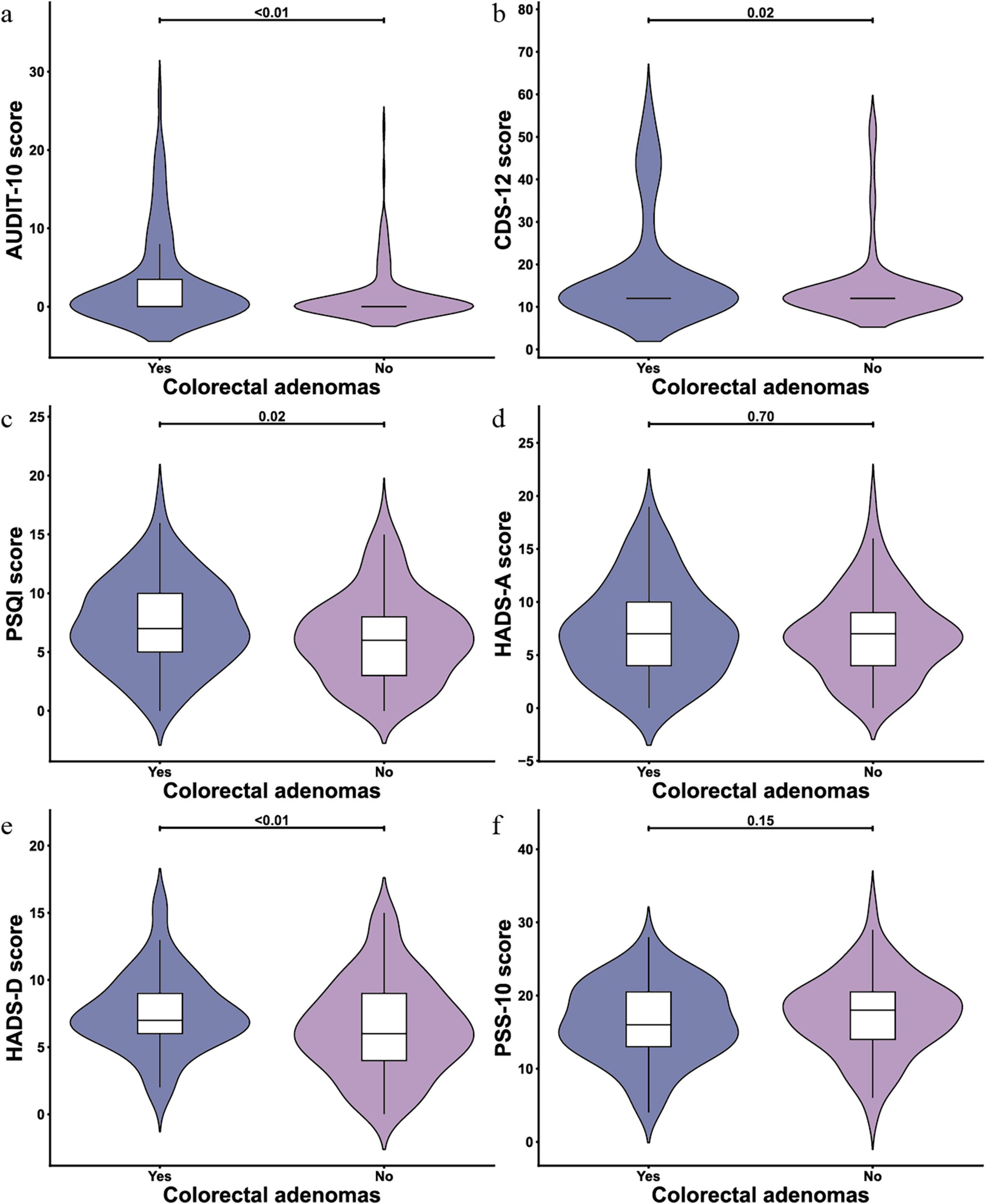
Figure 2. Violin plots of scale scores for lifestyle and mental health variables (N = 246). (a) AUDIT-10 scale; (b) Cigarette Dependence Scale (CDS)-12 scale; (c) Pittsburgh Sleep Quality Index (PSQI) scale; (d) Hospital Anxiety and Depression Scale (HADS)-A scale; (e) HADS-D scale; (f) Perceived Stress Scale-10 (PSS-10) scale.
3.3 Background characteristics associated with colorectal adenomas
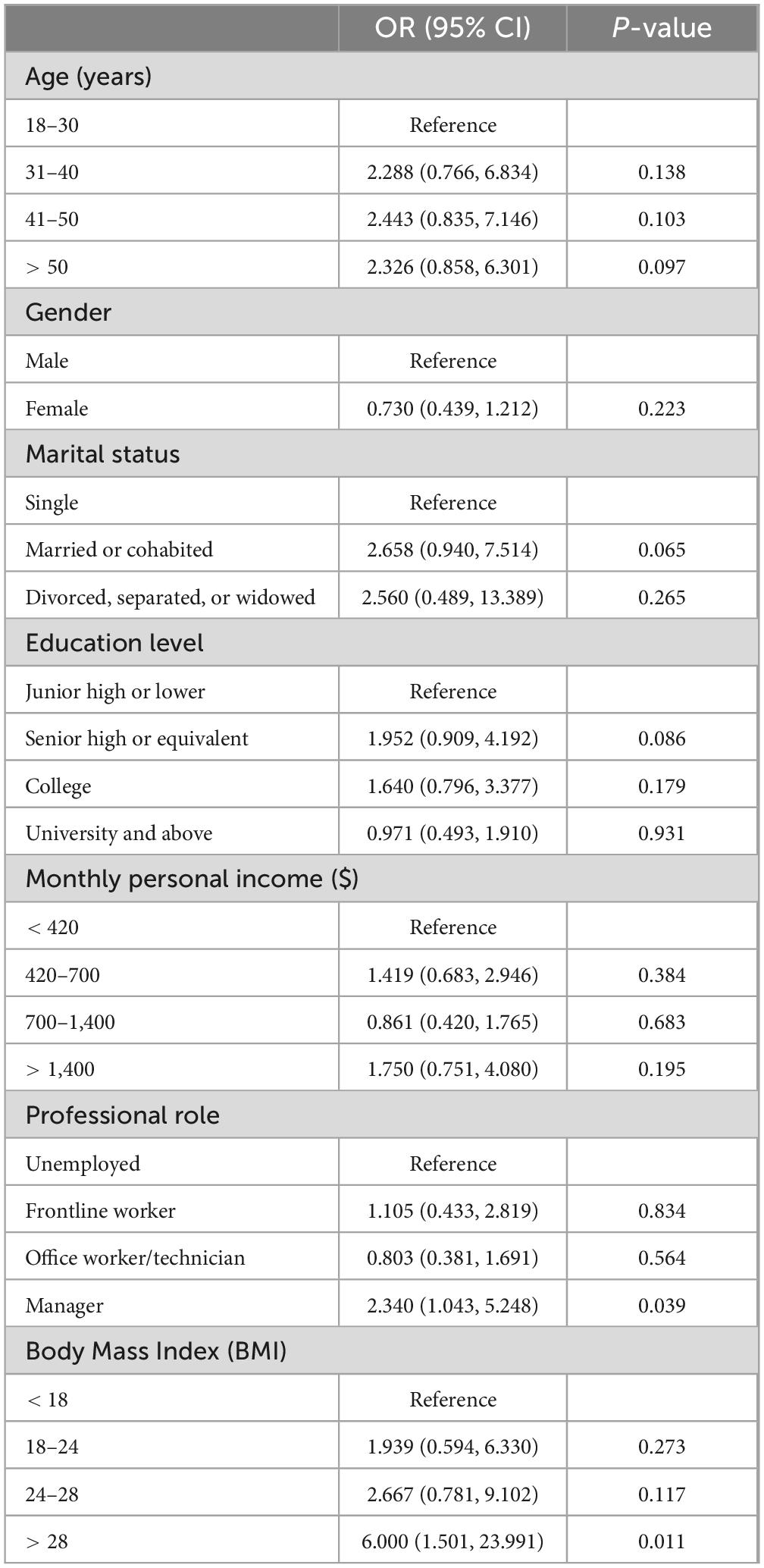
Table 2 shows that there are significant positive associations between being a manager (OR = 2.340; 95% CI = 1.043–5.248) and colorectal adenomas, as well as having a BMI over 28 (OR = 6.000; 95% CI = 1.501–23.991) and colorectal adenomas. However, no significant correlations were found between colorectal adenomas and factors such as age, gender, marital status, education level, or monthly income.
3.4 Lifestyle-level and mental health-level scales associated with colorectal adenomas
Before adjusting for demographic variables, significant associations with colorectal adenomas were identified across various measurement scales. For lifestyle factors, AUDIT showed a significant relationship (OR = 1.097, 95% CI: 1.030–1.168), as did CDS-12 (OR = 1.030, 95% CI: 1.005–1.055) and PSQI (OR = 1.083, 95% CI: 1.012–1.160). Among mental health assessments, HADS-A was notably correlated with colorectal adenomas (OR = 1.108, 95% CI: 1.024–1.199), while HADS-D did not show a significant connection (OR = 1.017, 95% CI: 0.958–1.080). Additionally, no significant association was observed between PSS-10 (OR = 0.966, 95% CI: 0.921–1.013) and colorectal adenomas.
After controlling for professional role and BMI, higher AUDIT scores remained significantly associated with colorectal adenomas (AOR = 1.070; 95% CI: 1.001–1.144). Significant associations were also found between CDS-12 scores (AOR = 1.028; 95% CI: 1.003–1.054) and PSQI scores (AOR = 1.079; 95% CI = 1.003–1.161) with colorectal adenomas. The HADS-A subscale continued to show a significant association (AOR = 1.156; 95% CI: 1.059–1.262), while the HADS-D subscale did not (AOR = 1.031; 95% CI: 0.967–1.099). Furthermore, no significant association was found between PSS-10 scores (AOR = 0.971; 95% CI: 0.923–1.022) and colorectal adenomas. These findings are detailed in Table 3.
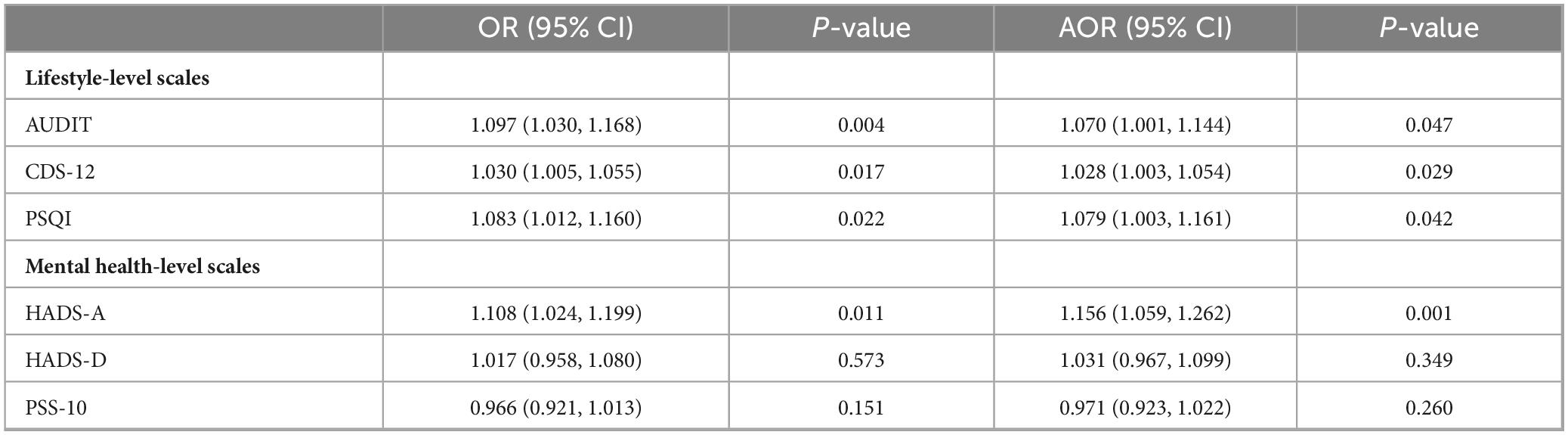
Table 3. Lifestyle-level and mental health-level scales associated with colorectal adenomas (N = 246).
4 Discussion
By integrating pathological examinations, endoscopic evaluations, and validated questionnaire data, our study provides a comprehensive understanding of the factors contributing to colorectal adenomas. This multi-faceted approach enhances the reliability of our findings by allowing for cross-validation and reducing biases inherent in single-method studies. The use of validated scales to assess lifestyle and mental health factors further strengthens our results, enabling the identification of significant associations with colorectal adenoma risk, such as alcohol consumption, smoking, sleep quality, and anxiety. These findings underscore the importance of incorporating both lifestyle and mental health considerations into routine screening and prevention strategies, offering a robust foundation for future research and the development of more effective interventions to reduce the burden of colorectal cancer.
The survey was conducted in Wuhan to investigate the prevalence and risk factors associated with colorectal adenomas among adults. The study population demonstrated a colorectal adenoma prevalence rate of 43.5% in Wuhan, China. Interestingly, consistent with previous research work (48, 49), no significant associations were observed between age, marital status, gender, education level, and monthly personal income, and colorectal adenomas. However, study findings revealed the professional roles played by managers as an influential modifiable risk factor for colon adenomas development. Furthermore, our investigation revealed a statistically significant association between a BMI exceeding 28 and colorectal adenomas, suggesting potential variations in the exposure to risk factors.
The present study has revealed significant positive associations between lifestyle factors, alcohol consumption, smoking, and colorectal adenomas. While previous studies have focused on lifestyle factors such as alcohol abuse and smoking (50–52), there remains limited research exploring the relationship between sleep quality and colorectal adenomas. lifestyle factors may disrupt various biological processes, including immune function, DNA repair mechanisms, and gut microbiota composition, all of which could contribute to the development and progression of colorectal adenomas (53). Consequently, further comprehensive investigations, including basic research and well-designed cohort studies, are indispensable for a more thorough understanding of this subject matter.
Our study makes a groundbreaking investigation into the influence of mental health on the screening risk factors associated with colorectal adenomas. While prior research conducted by Aceto et al. has shed some light on the potential microenvironmental alterations resulting from poor mental health, which may promote colorectal adenomas (54), the precise relationship between mental health and colorectal adenomas remains elusive. In our study, we provide compelling and interesting evidence that underscores the significant role of anxiety in the likelihood of developing colonic adenomatous polyps. Notably, emerging research indicates that anxiety exerts distinct effects on the patient’s immune system, metabolism, and physiological functions (55). Therefore, placing paramount importance on fostering a positive attitude becomes crucial in reducing the incidence of colorectal adenomatous polyps. Furthermore, disseminating the concept that an optimistic mood can enhance intestinal function holds immense promise as a potentially efficacious strategy. By elucidating the intricate association between mental health and colorectal adenomas, our findings contribute to a deeper comprehension of the multifaceted factors underpinning the etiology of this condition.
Our work offers a meaningful contribution toward reducing the medical burden associated with colorectal adenomas and minimizing the need for lower gastrointestinal endoscopy. By recognizing the association between mental health and colorectal adenomas, healthcare providers can adopt more comprehensive and tailored approaches to prevention and management. However, further research is necessary to elucidate the underlying mechanisms through which mental health influences colorectal adenomas. This would facilitate the development of targeted interventions that promote both mental well-being and gastrointestinal health. Furthermore, we advocate for the integration of lifestyle and mental health considerations into colorectal adenoma screening protocols, allowing for more comprehensive and tailored approaches to prevention and management. Through these efforts, we can address the multifaceted factors contributing to colorectal adenomas, reducing the burden of this condition and improving population health outcomes.
Our research establishes a scientific basis for enhancing colorectal adenoma screening, prevention strategies, and targeted interventions by incorporating lifestyle and mental health considerations into clinical practice. Recent studies support the integration of these factors, indicating that lifestyle choices such as diet and physical activity significantly impact adenoma risk, thereby suggesting that personalized interventions could improve screening outcomes (56). Additionally, mental health factors, including stress and anxiety, have been linked to health behaviors that influence colorectal cancer risk, underscoring the importance of a holistic approach in clinical settings (57, 58). Community-based interventions that address both lifestyle and mental health components have also demonstrated potential in increasing screening rates and patient engagement (59). Despite these advances, challenges remain in effectively implementing these strategies across diverse populations, highlighting the need for further research to optimize and tailor interventions (60). Therefore, our research contributes to the ongoing effort to refine and improve colorectal adenoma prevention and care.
This study has some limitations. Firstly, the study did not fully account for confounders like comorbidities, diet, and genetics. Conditions such as diabetes or inflammatory bowel disease, along with dietary factors like fiber intake, could independently affect both psychological states and colorectal adenoma development, potentially skewing results. Similarly, genetic predispositions could influence both psychological resilience and adenoma growth. Future research should gather detailed clinical, dietary, and genetic data, using standardized tools and advanced statistical methods to adjust for these factors. Collaboration with multidisciplinary teams would help clarify whether observed relationships are causal or arise from shared underlying mechanisms. Secondly, our research did not collect data from participants who declined to take part. Thirdly, no statistical analyses were performed regarding specific polyp locations, sizes or postoperative pathological classification for patients diagnosed with colorectal polyps. Fourthly, even though the study was anonymous, patients’ self-report might cause evaluation bias, which can be reduced by computer assistants. Moreover, we confined our sample to patients undergoing their first colonoscopy. As the perfection of examination and follow-up treatment intervention would have led to changes in patients’ mood and lifestyle, causing errors. Ideally, our data analysis should have focused on stratifying patients according to whether they had previously undergone colonoscopy and completed subsequent treatment after examination. Furthermore, since our study used cross-sectional design with no longitudinal data collection we cannot draw definitive conclusions regarding causal links among depression, individual press, sleep quality and colorectal adenomas.
5 Conclusion
This study identified significant correlations between alcohol consumption, smoking, sleep quality, anxiety, and the occurrence of colorectal adenomas among adults in China. These findings highlight the importance of targeted mental health interventions, which could help reduce the incidence of colorectal adenomas and improve overall public health outcomes.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Hubei Provincial Hospital of Traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MY: Conceptualization, Investigation, Writing – original draft. SZ: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review and editing. XT: Data curation, Formal Analysis, Investigation, Resources, Software, Validation, Visualization, Writing – original draft. CY: Formal Analysis, Investigation, Resources, Visualization, Writing – original draft. HH: Resources, Writing – original draft. YL: Conceptualization, Investigation, Project administration, Supervision, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to express their gratitude to all participants for their engagement in this study. The figure illustrating the schematic overview of the study design was created using Figdraw (https://www.figdraw.com).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1475987/full#supplementary-material
Footnotes
References
1. Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag C, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut. (2023) 72:338–44. doi: 10.1136/gutjnl-2022-327736
2. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. (2019) 16:713–32. doi: 10.1038/s41575-019-0189-8
5. Meester R, Lansdorp-Vogelaar I, Winawer S, Zauber A, Knudsen A, Ladabaum U. Intensity of surveillance for patients with colorectal adenomas. Ann Internal Med. (2020) 172:441–2. doi: 10.7326/l19-0828
6. Verma A, Patel M, Aslam M, Jameson J, Pringle J, Wurm P, et al. Circulating plasma microRNAs as a screening method for detection of colorectal adenomas. Lancet. (2015) 385:S100. doi: 10.1016/S0140-6736(15)60415-9
7. Liang J, Li T, Nakatsu G, Chen Y, Yau T, Chu E, et al. A novel faecal Lachnoclostridium marker for the non-invasive diagnosis of colorectal adenoma and cancer. Gut. (2020) 69:1248–57. doi: 10.1136/gutjnl-2019-318532
8. Senore C, Inadomi J, Segnan N, Bellisario C, Hassan C. Optimising colorectal cancer screening acceptance: A review. Gut. (2015) 64:1158–77. doi: 10.1136/gutjnl-2014-308081
9. Waldmann E, Kammerlander A, Gessl I, Penz D, Majcher B, Hinterberger A, et al. Association of adenoma detection rate and adenoma characteristics with colorectal cancer mortality after screening colonoscopy. Clin Gastroenterol Hepatol. (2021) 19:1890–8. doi: 10.1016/j.cgh.2021.04.023
10. Hong W, Dong L, Stock S, Basharat Z, Zippi M, Zhou M. Prevalence and characteristics of colonic adenoma in mainland China. Cancer Manag Res. (2018) 10:2743–55. doi: 10.2147/cmar.S166186
11. Martinez M, Mcpherson R, Levin B, Glober GA. A case-control study of dietary intake and other lifestyle risk factors for hyperplastic polyps. Gastroenterology. (1997) 113:423–9. doi: 10.1053/gast.1997.v113.pm9247459
12. Zhou X, Wang L, Xiao J, Sun J, Yu L, Zhang H, et al. Alcohol consumption, DNA methylation and colorectal cancer risk: Results from pooled cohort studies and Mendelian randomization analysis. Int J Cancer. (2022) 151:83–94. doi: 10.1002/ijc.33945
13. Sandler R, Lyles C, Mcauliffe C, Woosley J, Kupper L. Cigarette smoking, alcohol, and the risk of colorectal adenomas. Gastroenterology. (1993) 104:1445–51. doi: 10.1016/0016-5085(93)90354-F
14. Kilian C, Rovira P, Neufeld M, Ferreira-Borges C, Rumgay H, Soerjomataram I, et al. Modelling the impact of increased alcohol taxation on alcohol-attributable cancers in the WHO European Region. Lancet Regional Health Eur. (2021) 11:100225. doi: 10.1016/j.lanepe.2021.100225
15. Botteri E, Borroni E, Sloan E, Bagnardi V, Bosetti C, Peveri G, et al. Smoking and colorectal cancer risk, overall and by molecular subtypes: A meta-analysis. Am J Gastroenterol. (2020) 115:1940–9. doi: 10.14309/ajg.0000000000000803
16. Ton M, Watson N, Sillah A, Malen R, Labadie J, Reedy A, et al. Colorectal cancer anatomical site and sleep quality. Cancers (Basel). (2021) 13:2578. doi: 10.3390/cancers13112578
17. Jiao L, Duan Z, Sangi-Haghpeykar H, Hale L, White D, El-Serag H. Sleep duration and incidence of colorectal cancer in postmenopausal women. Br J Cancer. (2013) 108:213–21. doi: 10.1038/bjc.2012.561
18. GBD 2019 Colorectal Cancer Collaborators. Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. (2022) 7:627–47. doi: 10.1016/s2468-1253(22)00044-9
19. Miro C, Docimo A, Barrea L, Verde L, Cernea S, Sojat A, et al. “Time” for obesity-related cancer: The role of the circadian rhythm in cancer pathogenesis and treatment. Semin Cancer Biol. (2023) 91:99–109. doi: 10.1016/j.semcancer.2023.03.003
20. Jackson C, Redline S, Emmons K. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health. (2015) 36:417–40. doi: 10.1146/annurev-publhealth-031914-122838
21. Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. (2009) 5:253–61. doi: 10.1038/nrendo.2009.23
22. Song Y, Chang Z, Song C, Cui K, Yuan S, Qiao Z, et al. Association of sleep quality, its change and sleep duration with the risk of type 2 diabetes mellitus: Findings from the English longitudinal study of ageing. Diabetes/Metab Res Rev. (2023) 39:e3669. doi: 10.1002/dmrr.3669
23. Tran C, Paragomi P, Tran M, Nguyen M, Tuong T, Tran Q, et al. Association between sleep duration and colorectal adenomas: Findings from a case–control study in vietnam. Cancer Epidemiol Biomark Prevent. (2023) 32:1160–8. doi: 10.1158/1055-9965.Epi-23-0056
24. Thompson C, Larkin E, Patel S, Berger N, Redline S, Li L. Short duration of sleep increases risk of colorectal adenoma. Cancer. (2011) 117:841–7. doi: 10.1002/cncr.25507
25. Chen H, Li N, Ren J, Feng X, Lyu Z, Wei L, et al. Participation and yield of a population-based colorectal cancer screening programme in China. Gut. (2019) 68:1450–7. doi: 10.1136/gutjnl-2018-317124
26. Regge D, Laudi C, Galatola G, Della Monica P, Bonelli L, Angelelli G, et al. Diagnostic accuracy of computed tomographic colonography for the detection of advanced neoplasia in individuals at increased risk of colorectal cancer. JAMA. (2009) 301:2453–61. doi: 10.1001/jama.2009.832
27. Thomsen M, Jørgensen M, Pedersen L, Erichsen R, Sørensen H, Mikkelsen E. Mental disorders, participation, and trajectories in the Danish colorectal cancer screening programme: A population-based cohort study. Lancet Psychiatry. (2023) 10:518–27. doi: 10.1016/S2215-0366(23)00179-7
28. Protani M, Alotiby M, Seth R, Lawrence D, Jordan S, Logan H, et al. Colorectal cancer treatment in people with severe mental illness: A systematic review and meta-analysis. Epidemiol Psychiatric Sci. (2022) 31:e82. doi: 10.1017/S2045796022000634
29. Qaderi S, Van Der Heijden J, Verhoeven R, De Wilt J, Custers J, Beets G, et al. Trajectories of health-related quality of life and psychological distress in patients with colorectal cancer: A population-based study. Eur J Cancer. (2021) 158:144–55. doi: 10.1016/j.ejca.2021.08.050
30. Jiang Y, Hu Y, Yang Y, Yan R, Zheng L, Fu X, et al. Tong-Xie-Yao-Fang promotes dendritic cells maturation and retards tumor growth in colorectal cancer mice with chronic restraint stress. J Ethnopharmacol. (2023) 319:117069. doi: 10.1016/j.jep.2023.117069
31. Kroenke C, Bennett G, Fuchs C, Giovannucci E, Kawachi I, Schernhammer E, et al. Depressive symptoms and prospective incidence of colorectal cancer in women. Am J Epidemiol. (2005) 162:839–48. doi: 10.1093/aje/kwi302
32. Chambers S, Lynch B, Aitken J, Baade P. Relationship over time between psychological distress and physical activity in colorectal cancer survivors. J Clin Oncol. (2009) 27:1600–6. doi: 10.1200/jco.2008.18.5157
33. Nielsen N, Kristensen T, Strandberg-Larsen K, Zhang Z, Schnohr P, Grønbæk M. Perceived stress and risk of colorectal cancer in men and women: A prospective cohort study. J Internal Med. (2008) 263:192–202. doi: 10.1111/j.1365-2796.2007.01826.x
34. Cui B, Peng F, Lu J, He B, Su Q, Luo H, et al. Cancer and stress: NextGen strategies. Brain Behav Immun. (2021) 93:368–83. doi: 10.1016/j.bbi.2020.11.005
35. Christian L, Franco A, Glaser R, Iams J. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav Immun. (2009) 23:750–4. doi: 10.1016/j.bbi.2009.02.012
36. Zhang L, Pan J, Chen W, Jiang J, Huang J. Chronic stress-induced immune dysregulation in cancer: Implications for initiation, progression, metastasis, and treatment. Am J Cancer Res. (2020) 10:1294–307.
37. Borowczak J, Szczerbowski K, Maniewski M, Kowalewski A, Janiczek-Polewska M, Szylberg A, et al. The role of inflammatory cytokines in the pathogenesis of colorectal carcinoma-recent findings and review. Biomedicines. (2022) 10:1670. doi: 10.3390/biomedicines10071670
38. Babor T, Higgins-Biddle J, Saunders J, Monteiro M. The Alcohol Use Disorders Identification Test. Geneva;: World Health Organization (2001).
40. Revelle W, Zinbarg R. Coefficients alpha, beta, omega, and the glb: Comments on Sijtsma. Psychometrika. (2009) 74:145–54.
41. Etter J, Le Houezec J, Perneger TV. A self-administered questionnaire to measure dependence on cigarettes: The cigarette dependence scale. Neuropsychopharmacology. (2003) 28:359–70.
42. Buysse D, Reynolds Iii C, Monk T, Berman S, Kupfer D. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213.
43. Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatrica Scand. (1983) 67:361–70.
44. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. (1983) 24:385–96.
45. Cohen S. Perceived stress in a probability sample of the United States. In: S Spacapan, S Oskamp editors. The Social Psychology of Health. Thousand Oaks, CA: Sage Publications, Inc (1988). p. 31–67.
46. Patel M, Niemann C, Sally M, De La Cruz S, Zatarain J, Ewing T, et al. The impact of hydroxyethyl starch use in deceased organ donors on the development of delayed graft function in kidney transplant recipients: A propensity-adjusted analysis. Am J Transpl. (2015) 15:2152–8.
47. Vittinghoff E, Glidden D, Shiboski S, Mcculloch C. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. Berlin: Springer (2006).
48. Kang H, Kim D, Kim H, Kim C, Kim Y, Park M, et al. Visceral obesity and insulin resistance as risk factors for colorectal adenoma: A cross-sectional, case-control study. Am J Gastroenterol. (2010) 105:178–87. doi: 10.1038/ajg.2009.541
49. Jacobs E, Giuliano A, Roe D, Guillén-Rodríguez J, Alberts D, Martínez M. Baseline dietary fiber intake and colorectal adenoma recurrence in the wheat bran fiber randomized trial. J Natl Cancer Inst. (2002) 94:1620–5. doi: 10.1093/jnci/94.21.1620
50. Sninsky J, Shore B, Lupu G, Crockett S. Risk factors for colorectal polyps and cancer. Gastrointestinal Endoscopy Clin North Am. (2022) 32:195–213.
51. Erhardt J, Kreichgauer H, Meisner C, Bode J, Bode C. Alcohol, cigarette smoking, dietary factors and the risk of colorectal adenomas and hyperplastic polyps–A case control study. Eur J Nutr. (2002) 41:35–43.
52. Ji B, Weissfeld J, Chow W, Huang W, Schoen R, Hayes R, et al. Tobacco smoking and colorectal hyperplastic and adenomatous polyps. Cancer Epidemiol Biomark Prevent. (2006) 15:897–901.
53. Wong S, Yu J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. (2019) 16:690–704.
54. Aceto G, Catalano T, Curia M. Molecular aspects of colorectal adenomas: The interplay among microenvironment, oxidative stress, and predisposition. BioMed Res Int. (2020) 2020:1726309. doi: 10.1155/2020/1726309
55. Lücke J, Sabihi M, Zhang T, Bauditz L, Shiri A, Giannou A, et al. The good and the bad about separation anxiety: Roles of IL-22 and IL-22BP in liver pathologies. Semin Immunopathol. (2021) 43:591–607.
56. Li J, Li Z, Ruan W, Wang W. Colorectal cancer screening: The value of early detection and modern challenges. World J Gastroenterol. (2024) 30:2726–30. doi: 10.3748/wjg.v30.i20.2726
57. Shokar N, Dwivedi A, Molokwu J. Psychosocial risk profiles and colorectal cancer screening: A latent profile analysis in a colorectal cancer screening intervention setting. Cancer Prev Res (Phila). (2023) 16:571–9. doi: 10.1158/1940-6207.Capr-23-0114
58. Telles V, Rodriguez S, Torres M, Schneider J, Haughton J, Maldonado M, et al. Barriers and facilitators to implementing a multilevel, multicomponent intervention promoting colorectal cancer screening in health centers: A qualitative study of key informant perspectives. BMC Health Serv Res. (2024) 24:404. doi: 10.1186/s12913-024-10749-y
59. Yakoubovitch S, Zaki T, Anand S, Pecoriello J, Liang P. Effect of behavioral interventions on the uptake of colonoscopy for colorectal cancer screening: A systematic review and meta-analysis. Am J Gastroenterol. (2023) 118:1829–40. doi: 10.14309/ajg.0000000000002478
60. Castañeda S, Gupta S, Nodora J, Largaespada V, Roesch S, Rabin B, et al. Hub-and-Spoke centralized intervention to optimize colorectal cancer screening and follow-up: A pragmatic, cluster-randomized controlled trial protocol. Contemp Clin Trials. (2023) 134:107353. doi: 10.1016/j.cct.2023.107353
Keywords: colorectal adenomas, cross-sectional study, lifestyle, mental health, Wuhan
Citation: Ye M, Zhu S, Tan X, Yu C, Huang H and Liu Y (2025) Impact of lifestyle and mental health on colorectal adenomas in China: a prospective cross-sectional survey. Front. Med. 12:1475987. doi: 10.3389/fmed.2025.1475987
Received: 04 August 2024; Accepted: 20 February 2025;
Published: 03 March 2025.
Edited by:
Dorota Formanowicz, Poznan University of Medical Sciences, PolandReviewed by:
Babak Pakbin, Texas A&M University, United StatesYudong Li, Capital Medical University, China
Vincent Wing-Hei Wong, The Chinese University of Hong Kong, China
Copyright © 2025 Ye, Zhu, Tan, Yu, Huang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Liu, bGl1eWFuZ18wOTMzQDEyNi5jb20=
†These authors have contributed equally to this work
 Min Ye
Min Ye Shiben Zhu
Shiben Zhu Xinyi Tan3,4†
Xinyi Tan3,4† Chenxi Yu
Chenxi Yu Yang Liu
Yang Liu