
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 07 February 2025
Sec. Rheumatology
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1469879
 Caiyu Zheng1,2†
Caiyu Zheng1,2† Qingwen Tong1,3†
Qingwen Tong1,3† Zhijun Zhang1
Zhijun Zhang1 Chunmei Lin1
Chunmei Lin1 Zhiyi Wang1
Zhiyi Wang1 Dongshu Kang1
Dongshu Kang1 Yanmei Lin1
Yanmei Lin1 Jianqing Tian1*
Jianqing Tian1*Objective: The study focuses on comparing the efficacy of a low-dose combination of febuxostat and benzbromarone versus each drug used alone for the treatment of gout.
Methods: A prospective, randomized, open-labeled trial of men with gout and renal uric acid underexcretion was conducted. We randomly assigned 100 patients to either low-dose febuxostat or low-dose benzbromarone for initial treatment. 43 patients with complete data were analyzed in this study. The analysis of medication Treatment effects, Period effects and Patient-Within-Sequence effects for different treatment options was performed by cross-test ANOVA.
Result: The cross-trial analysis revealed no significant differences in the magnitude of uric acid decline among the three groups after treatment. Similarly, there were no statistically significant differences in blood lipid levels, CRP, CA724, eGFR, and ALT among the three groups post-treatment. The null model without participant-specific benefits was a linear mixed model for ALT, AST, eGFR, TC, TG, LDL, UA, CRP, CA724, and 24-h uric acid with age, BMI, treatment period and treatment as fixed factors, and the intercept as a random factor by participant. The results indicated no significant differences on relevant indicators among the three treatment regimens.
Conclusion: Our study did not identify a difference in the efficacy of reducing uric acid excretion in gout patients when comparing conventional dose febuxostat and benzbromarone monotherapy versus low-dose combination therapy.
Over the last few decades, gout has become a substantial burden on human health and the global economy. Current evidence suggests it is closely linked to chronic kidney disease (CKD), stroke, and cardiovascular diseases (CVD) (1). Patients with uncontrolled gout experience higher indirect and total costs compared to those with controlled gout (2). The incidence of gout increases with elevated levels of hyperuricemia (3). An increasing body of evidence suggests that the pathophysiology of hyperuricemia is heterogeneous in gout patients (4–7). Renal uric acid underexcretion is the dominant cause of hyperuricemia (70–90% of gout patients) (5). Hence, criteria such as fractional excretion of uric acid (FEUA) less than 5.5% and UUE less than or equal to 600 mg/day/1.73 m2 are employed to define the subset of gout associated with renal uric acid underexcretion (7).
Medications designed for gout function as anti-inflammatory agents to address acute arthritis or as urate-lowering agents to manage hyperuricemia. Urate-lowering therapy (ULT) stands as the central approach for effectively controlling hyperuricemia and gout (8, 9). ULT operates through two mechanisms: promoting urate excretion and inhibiting urate production. Uricosurics contribute to the former, while xanthine oxidase (XO) inhibitors are involved in the latter (10). In China, the most frequently employed urate-lowering drugs include Allopurinol, Febuxostat, and benzbromarone.
Within the Asian study population, the prevalence of HLA-B*5801 is reportedly higher in individuals experiencing Allopurinol hypersensitivity, particularly among the Han and Korean populations (7.4%) (11–14). In Europe, benzbromarone is only recommended as a second-line ULT agent due to potential hepatotoxic effects (15).
It has been suggested that patients taking benzbromarone alone or combined allopurinol and benzbromarone therapy achieve a faster reduction in urate levels compared to those taking allopurinol alone. Some experts argue that the primary focus should be on comparing allopurinol and placebo. Additionally, they highlight allopurinol versus febuxostat and versus benzbromarone as the most clinically relevant active comparisons, and recommend restricting reporting to these comparisons (16).
This study aimed to refine the classification of hyperuricemia causes and select appropriate urate-lowering drugs based on the underlying pathogenesis. This approach seeks to improve patient compliance with uric acid management and gout treatment by utilizing smaller or conventional drug doses. While benzbromarone and Febuxostat have been employed in gout treatment, comparative studies assessing their urate-lowering effects have yielded conflicting results. Therefore, this study focuses on comparing the efficacy of a low-dose combination of febuxostat and benzbromarone versus each drug used alone for the treatment of gout.
A total of 100 gout patients exhibiting reduced uric acid excretion and receiving treatment at the Outpatient Department of Endocrinology, Fujian Medical University Xiamen Humanity Hospital, Xiamen, Fujian Province, from October 2019 to June 2023, were included in the present study. Our study included patients who were primarily middle-aged and young men with mild gout conditions, and there was no significant tophi formation or other severe gout manifestations in our subjects. These patients had mild gout attacks and had not been regularly treated to manage uric acid levels. Inclusion criteria comprised individuals aged 18 to 60, male gender, primary gout with serum uric acid levels ranging from 480 μmol/L to 600 μmol/L, uric acid excretion fraction <5.5%, 24-h uric acid ≤600 mg/d, and morning urine pH < 6.0. Additionally, participants should not have experienced an acute gouty arthritis attack in the 2 weeks preceding inclusion and during the 2-week washout period. Volunteers were required to sign an informed consent form. Exclusion criteria included severe diseases affecting blood, immune system, central nervous system, etc.; malignant tumors; acute gouty arthritis caused by chemoradiotherapy, drugs, or other factors; severe inflammatory reactions in other body parts; and the use of drugs affecting serum uric acid metabolism during treatment. The participants were classified using a random number method, and all participants provided written informed consent. The study was approved by Medical Ethics Committee of Xiamen Humanity Hospital (Approval NO. HAXM-MEC-20200608-006-01).
In this study, the randomization principle was employed to assign patients meeting the enrollment criteria to either treatment A or treatment B. Treatment A involved a low-dose regimen of febuxostat (20 mg febuxostat once daily), while treatment B commenced with a low dose of benzbromarone, specifically 25 mg of benzbromarone once daily. Treatment A + B involved a combination of low-dose benzbromarone and low-dose febuxostat, i.e., 20 mg of febuxostat once daily combined with 25 mg of benzbromarone once daily to reduce uric acid. All participants underwent a 14-day washout period during which they refrained from taking uric acid-lowering medications, other medications, and adhered to a low-purine diet. Throughout the study, the use of other uric acid-lowering drugs or medications known to impact serum uric acid levels was prohibited. Participants were required to orally consume sodium bicarbonate daily to alkalize their urine. Exclusion criteria included patients who did not take medicine for three consecutive days or did not attend regular reviews. The study spanned 26 weeks, during which patients underwent eight examinations and follow-up visits at the end of the 2nd, 4th, 8th, 13th, 17th, 20th, 22nd, and 26th weeks after treatment at the gout specialized clinic. Anthropometric and biochemical measurements, including age, body weight, height, uric acid, alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), cholesterol (TC), CRP (C-reactive protein), and eGFR (Estimating the glomerular filtration rate), were collected at each follow-up (Figure 1). In the present study, no patient discontinued treatment due to drug intolerance or other adverse reactions. Finally, 43 patients completed the follow-up.
A modified cross-design method was utilized, and mixed-effect models were employed to account for confounding factors. IBM SPSS Statistics 21.0 was used for data analysis. Continuous variables were expressed as mean ± standard deviation. Drug main effects, phase effects, and sequential effects were analyzed using a multifactor mixed-effect model, with treatment, age, and BMI as fixed factors and the intercept as random factors for participants. Models were fitted using the maximum-likelihood method, and p-values were obtained through parameter bootstrap with 10,000 iterations.
In total, 43 patients were included in the current study, and Table 1 presents their baseline characteristics. The mean age in this small cohort was 33.33 ± 8.33 years. Additionally, the mean BMI, mean eGFR, and mean uric acid level for these 43 patients were 27.80 ± 3.26 kg/m2, 99.54 ± 12.34 mL/min/1.73m2, and 563.97 ± 92.40 μmol/L, respectively.
Table 2 displays the results of the cross-test analysis, with randomization used for grouping. Cross-trial ANOVA for different treatment options. Group A received doses of febuxostat, group A + B received a combination of low-dose febuxostat and low-dose benzbromarone, and group B underwent benzbromarone therapy. The cross-trial analysis revealed no significant differences in the magnitude of uric acid decline among the three groups after treatment. Similarly, there were no statistically significant differences in blood lipid levels, CRP, CA724, eGFR, and ALT among the three groups post-treatment.
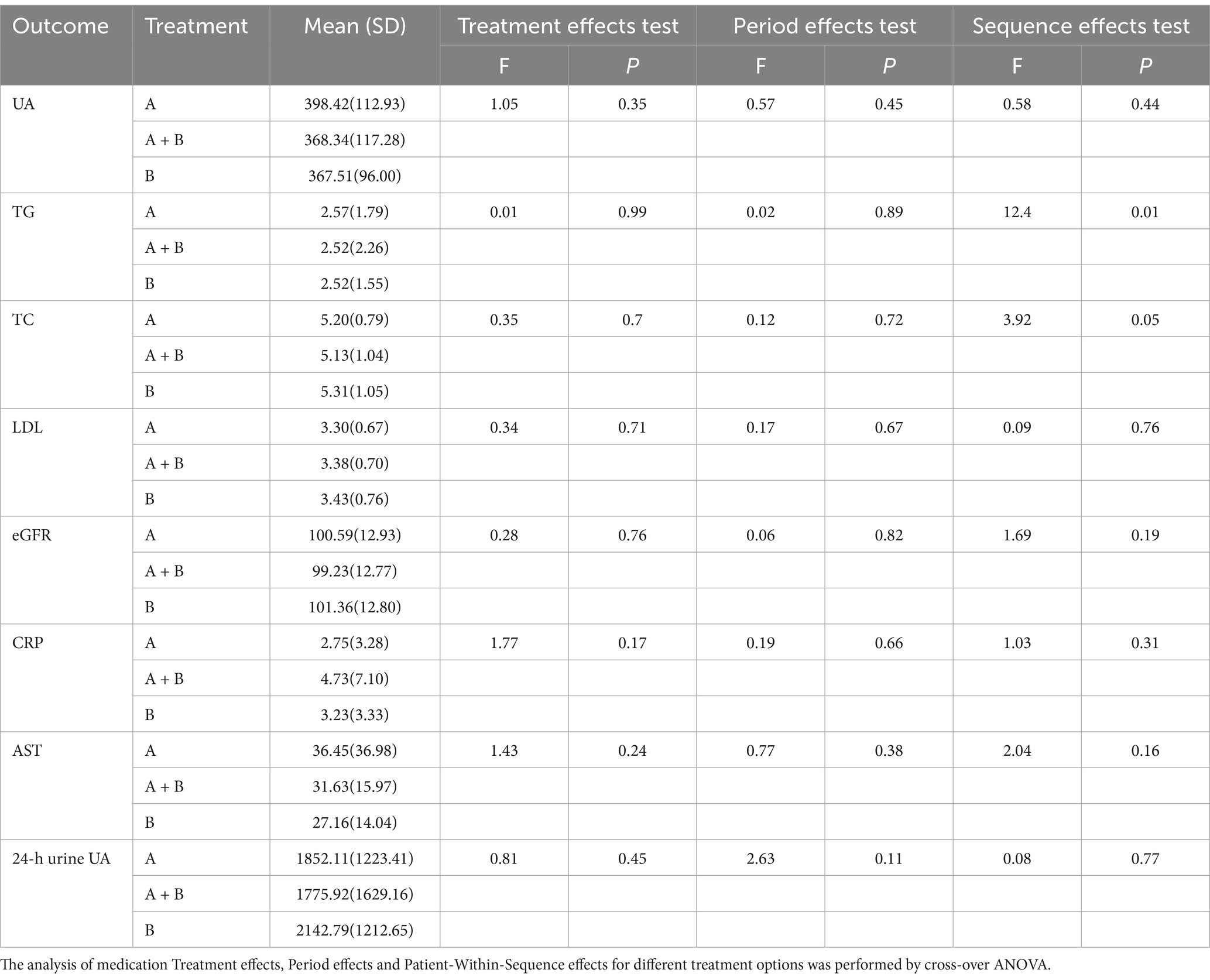
Table 2. p Values of main effects and interaction effects between treatment and stage on outcome measures.
To further account for confounding factors, a mixed-effects model analysis was conducted. The effects of different treatment options on relevant indicators were analyzed using a multifactor mixed effect model. The null model without participant-specific benefits was a linear mixed model for ALT, AST, eGFR, TC, TG, LDL, UA, CRP, CA724, and 24-h uric acid with age, BMI, treatment period and treatment as fixed factors, and the intercept as a random factor by participant. Treatment, age, and BMI were considered fixed factors, while the intercept served as the random factor for participants. The results indicated no significant differences on relevant indicators among the three treatment regimens (Table 3).
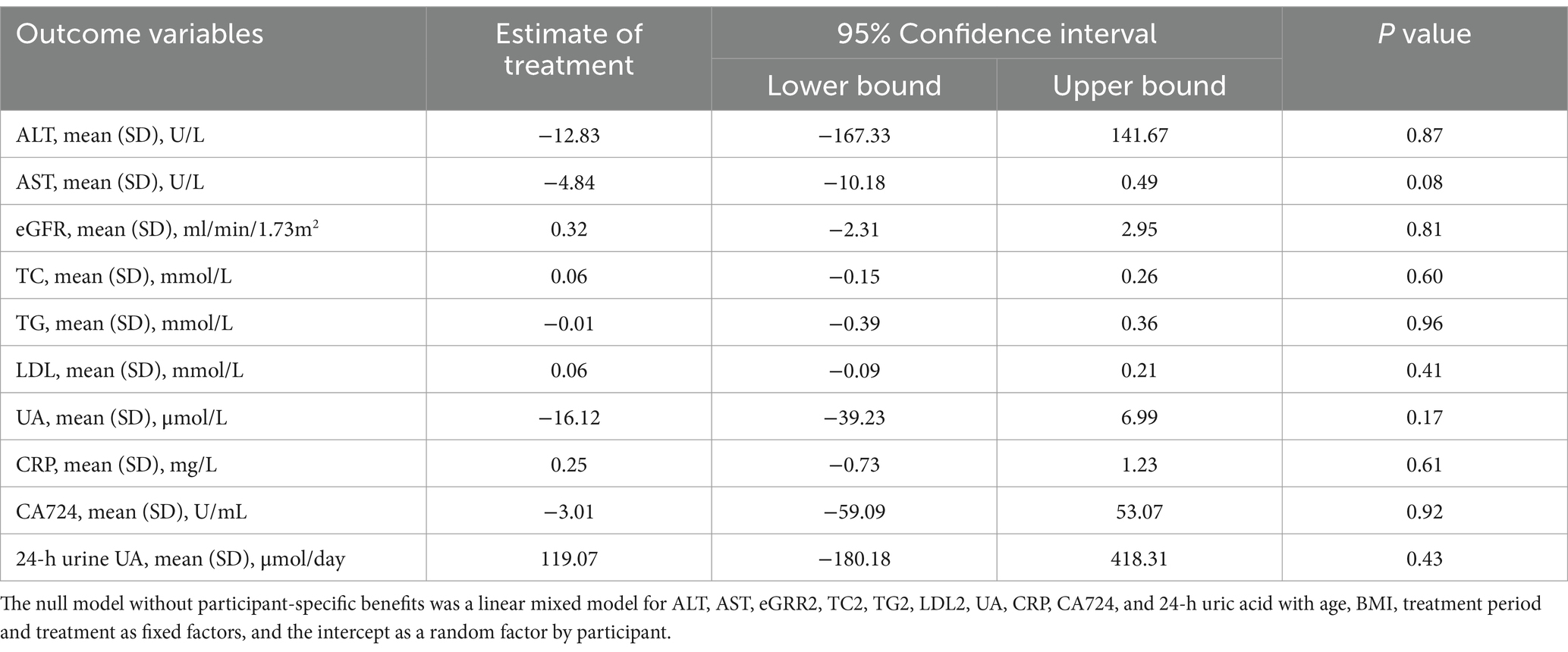
Table 3. Results of the linear mixed-effect model analysis for assessing treatment on outcome variables.
In our study, no significant difference in efficacy was observed among patients with gout and reduced uric acid excretion when comparing conventional-dose monotherapy with febuxostat and benzbromarone versus low-dose combination therapy.
During clinical practice, a significant portion of patients with hyperuricemia or gout, currently receiving urico-synthesis inhibitors, often do not undergo comprehensive testing, such as 24-h urinary uric acid excretion and uric acid excretion fraction. In both domestic and foreign studies on hyperuricemia or gout treatment, there has been a lack of classification based on the causes of hyperuricemia, leading to a limited understanding of the condition. Existing research suggests that although approximately 70% of gout patients in China have received treatment, the compliance rate for uric acid management is only around 10%. Moreover, only 20% of individuals treated for more than 6 months exhibit regular adherence, indicating a significant challenge in maintaining a satisfactory compliance rate (17).
Earlier studies, including the work of scholars like Perez-Ruiz et al. (18), have suggested that in gout patients with low uric acid excretion, the uric acid excretion drug benzbromarone can achieve lower uric acid levels than allopurinol, a uric acid synthesis inhibitor. Notably, the efficacy of febuxostat 40 mg is comparable to that of allopurinol (11). Research by Ohta Yuko et al. (19) and Kojima et al. (20) suggested that combining febuxostat, a low-dose uric acid synthesis inhibitor, with benzbromarone, a uricoproexcretion drug, yields a significantly greater effect on lowering blood uric acid than conventional doses of febuxostat or benzbromarone alone. Other investigations have indicated that patients using febuxostat exhibit higher estimated glomerular filtration rates, a reduced risk of kidney disease progression, and lower serum uric acid levels compared to those using allopurinol, suggesting potential renal protective benefits of febuxostat. In China, studies have shown that febuxostat is more effective in treating hyperuricemia than benzbromarone, effectively reducing uric acid levels and demonstrating a higher safety profile (21, 22). Additionally, previous findings suggest that lower doses of benzbromarone have a greater impact on lowering serum uric acid compared to lower doses of febuxostat (23).
However, the present study did not identify a difference in uric acid reduction between febuxostat and benzbromarone, whether used as conventional dose monotherapy or in low-dose combination therapy. Notably, we found that both monotherapy and low-dose treatments exhibited equal effectiveness. These findings align with a recent study that demonstrated comparable efficacy between benzbromarone (25 mg/d) and febuxostat (20 mg/d) in reducing serum urate levels (24). In addition, Nan et al. emphasized the significance of considering uric acid levels and renal function when recommending low doses of febuxostat and benzbromarone to patients with gout. Their research indicates that the efficacy of both medications is influenced by renal function.
Other factors that may influence the results include: First, we provided simple lifestyle intervention guidance to each study subject, but the degree of adherence to lifestyle management may vary among individuals, which could affect the measurement of uric acid levels. Second, the follow-up period for this study was relatively short, and the dosages were not further increased to full dose across groups. Further exploration of the results after increasing the dosage is warranted. Our study findings align with existing guidelines. Despite the various types of hyperuricemia, both domestic and international guidelines currently do not recommend tailoring uric acid-lowering therapy based on specific types. Due to reports of hepatocellular necrosis associated with benzbromarone in Caucasians, it is often recommended as a second-line drug in European guidelines. The American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) recommend benzbromarone at 50 mg once daily, gradually increasing to 100 mg daily and the 2012 ACR recommends that clinicians consider the cause of hyperuricemia for gout patients (evidence level C) (25). However, the ACR’s latest updated gout management guidelines conditionally advise against checking urine for drug selection accuracy and uric acid adjuvant therapy strategy (26). The 2016 European Congress of Rheumatology (EULAR) Gout Management Guidelines and the 2020 ACR guidelines recommend allopurinol as a first-line treatment. While the 2016 EULAR guidelines support uric acid excretion as a second-line treatment for gout, the 2020 ACR guidelines conditionally recommend probenecid as a second-line agent only after allopurinol has failed, and benzbromarone is not part of this clinical guideline due to its unavailability in the United States (27, 28).
Our research distinguishes itself by focusing on a specific advantage: while numerous clinical trials and studies globally intervene directly or analyze the causes of hyperuricemia without categorizing patients into types, our study uniquely targets gout patients with low uric acid excretion. Through distinct group trials, we administered two conventional doses of uric acid-lowering drugs with varying mechanisms of action as standalone treatments for individuals with low uric acid excretion. Additionally, the treatment group explored the effectiveness of different treatment regimens by incorporating a combination of low doses of two uric acid-lowering drugs with distinct mechanisms of action. It is essential to acknowledge some limitations, such as the study being exclusively conducted in males and our study primarily focused on comparing the efficacy of drugs between different groups and did not include analyses of the differences in the timing and duration of gout attacks between groups. We did not further increase the drug dosage in each group to explore the differences in efficacy following dose escalation. Future research with a larger sample size is needed to validate and further confirm our findings.
In conclusion, our study did not identify a difference in the efficacy of reducing uric acid excretion in gout patients when comparing conventional dose febuxostat and benzbromarone monotherapy versus low-dose combination therapy.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the study was approved by Medical Ethics Committee of Xiamen Humanity Hospital (Approval NO. HAXM-MEC-20200608-006-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
CZ: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. QT: Data curation, Methodology, Writing – original draft. ZZ: Investigation, Methodology, Data curation, Writing – review & editing. CL: Data curation, Investigation, Supervision, Writing – review & editing. ZW: Data curation, Writing – review & editing. DK: Data curation, Writing – review & editing. YL: Data curation, Writing – review & editing. JT: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by Xiamen City Medical and health Guidance project. (No. 3502Z20199158).
We are grateful to all the subjects for their participation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Li, Q, Li, R, Zhang, S, Zhang, Y, Liu, M, Song, Y, et al. Relation of BMI and waist circumference with the risk of new-onset hyperuricemia in hypertensive patients. QJM. (2022) 115:271–8. doi: 10.1093/qjmed/hcaa346
2. Flores, NM, Nuevo, J, Klein, AB, Baumgartner, S, and Morlock, R. The economic burden of uncontrolled gout: how controlling gout reduces cost. J Med Econ. (2018) 22:1–6. doi: 10.1080/13696998.2018.1532904
3. Maekawa, M, Tomida, H, Aoki, T, Hishida, M, Morinaga, T, and Tamai, H. Successful treatment of refractory gout using combined therapy consisting of Febuxostat and allopurinol in a patient with chronic renal failure. Intern Med. (2014) 53:609–12. doi: 10.2169/internalmedicine.53.0698
4. Mandal, AK, and Mount, DB. The molecular physiology of uric acid homeostasis. Annu Rev Physiol. (2015) 77:323–45. doi: 10.1146/annurev-physiol-021113-170343
5. Vitart, V, Rudan, I, Hayward, C, Gray, NK, Floyd, J, Palmer, CNA, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. (2008) 40:437–42. doi: 10.1038/ng.106
6. Dalbeth, N, and Merriman, T. Crystal ball gazing: new therapeutic targets for hyperuricaemia and gout. Rheumatology. (2008) 48:222–6. doi: 10.1093/rheumatology/ken460
7. Ichida, K, Matsuo, H, Takada, T, Nakayama, A, Murakami, K, Shimizu, T, et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nature. Communications. (2012) 3:756. doi: 10.1038/ncomms1756
8. Liu, X, Zhai, T, Ma, R, Luo, C, Wang, H, and Liu, L. Effects of uric acid-lowering therapy on the progression of chronic kidney disease: a systematic review and meta-analysis. Ren Fail. (2018) 40:289–97. doi: 10.1080/0886022X.2018.1456463
9. Shoji, A, Yamanaka, H, and Kamatani, N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Care Res. (2004) 51:321–5. doi: 10.1002/art.20405
10. Lin, H-C, Daimon, M, Wang, C-H, Ho, Y, Uang, Y-S, Chiang, S-J, et al. Allopurinol, benzbromarone and risk of coronary heart disease in gout patients: a population-based study. Int J Cardiol. (2017) 233:85–90. doi: 10.1016/j.ijcard.2017.02.013
11. Liang, N, Sun, M, Sun, R, Xu, T, Cui, L, Wang, C, et al. Baseline urate level and renal function predict outcomes of urate-lowering therapy using low doses of febuxostat and benzbromarone: a prospective, randomized controlled study in a Chinese primary gout cohort. Arthritis Res Ther. (2019) 21:200. doi: 10.1186/s13075-019-1976-x
12. Xue, X, Liu, Z, Li, X, Lu, J, Wang, C, Wang, X, et al. The efficacy and safety of citrate mixture vs sodium bicarbonate on urine alkalization in Chinese primary gout patients with benzbromarone: a prospective, randomized controlled study. Rheumatology. (2021) 60:2661–71. doi: 10.1093/rheumatology/keaa668
13. Grusch, B, Rintelen, B, and Leeb, BF. Evidenzbasierte Empfehlungen der European League Against Rheumatism“zur Diagnostik und Therapie der Gicht. Z Rheumatol. (2007) 66:568–72. doi: 10.1007/s00393-007-0209-x
14. Yamanaka, H, Tamaki, S, Ide, Y, Kim, H, Inoue, K, Sugimoto, M, et al. Stepwise dose increase of febuxostat is comparable with colchicine prophylaxis for the prevention of gout flares during the initial phase of urate-lowering therapy: results from FORTUNE-1, a prospective, multicentre randomised study. Ann Rheum Dis. (2018) 77:270–6. doi: 10.1136/annrheumdis-2017-211574
15. Naoyuki, K, Shin, F, Toshikazu, H, Tatsuo, H, Kenjiro, K, Toshitaka, N, et al. An allopurinol-controlled, Multicenter, randomized, open-label, parallel between-group, comparative study of Febuxostat (TMX-67), a non-purine-selective inhibitor of xanthine oxidase, in patients with hyperuricemia including those with gout in Japan. JCR. (2011) 17:S44–9. doi: 10.1097/RHU.0b013e31821d352f
16. Kydd, AS, Buchbinder, R, Bombardier, C, and Edwards, CJ. Allopurinol for chronic gout (review). Cochrane Database Syst. Rev. (2013) 4:CD006077DOI. doi: 10.1002/14651858.CD006077.pub2
17. Liu, R, Han, C, Wu, D, Xia, X, Gu, J, Guan, H, et al. Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: a systematic review and meta-analysis. Biomed Res Int. (2015) 2015:1–12. doi: 10.1155/2015/762820
18. Perez-Ruiz, F, Alonso-Ruiz, A, Calabozo, M, Herrero-Beites, A, García-Erauskin, G, and Ruiz-Lucea, E. Efficacy of allopurinol and benzbromarone for the control of hyperuricaemia. A pathogenic approach to the treatment of primary chronic gout. Ann Rheum Dis. (1998) 57:545–9. doi: 10.1136/ard.57.9.545
19. Ohta, Y, Ishizuka, A, Arima, H, Hayashi, S, Iwashima, Y, Kishida, M, et al. Effective uric acid-lowering treatment for hypertensive patients with hyperuricemia. Hypertens Res. (2016) 40:259–63. doi: 10.1038/hr.2016.139
20. Kojima, S, Kojima, S, Hifumi, A, Soejima, H, and Ogawa, H. Therapeutic strategy for efficient reduction of serum uric acid levels with allopurinol versus benzbromarone in hyperuricemic patients with essential hypertension — a randomized crossover study (terao study). Int J Cardiol. (2016) 224:437–9. doi: 10.1016/j.ijcard.2016.09.073
21. Hu, AM, and Brown, JN. Comparative effect of allopurinol and febuxostat on long-term renal outcomes in patients with hyperuricemia and chronic kidney disease: a systematic review. Clin Rheumatol. (2020) 39:3287–94. doi: 10.1007/s10067-020-05079-3
23. Yan, F, Xue, X, Lu, J, Dalbeth, N, Qi, H, Yu, Q, et al. Superiority of low-dose Benzbromarone to low-dose Febuxostat in a prospective, randomized comparative effectiveness trial in gout patients with renal uric acid Underexcretion. Arthritis Rheumatol. (2022) 74:2015–23. doi: 10.1002/art.42266
24. Liu, D, Zhou, B, Li, Z, Zhang, Z, Dai, X, Ji, Z, et al. Effectiveness of benzbromarone versus febuxostat in gouty patients: a retrospective study. Clin Rheumatol. (2022) 41:2121–8. doi: 10.1007/s10067-022-06110-5
25. Khanna, D, Fitzgerald, JD, Khanna, PP, Bae, S, Singh, MK, Neogi, T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. (2012) 64:1431–46. doi: 10.1002/acr.21772
26. Jansen, TL. Rational pharmacotherapy (RPT) in goutology: define the serum uric acid target & treat-to-target patient cohort and review on urate lowering therapy (ULT) applying synthetic drugs. Joint Bone Spine. (2015) 82:225–9. doi: 10.1016/j.jbspin.2014.02.015
27. Singh, JA, Neogi, T, and FitzGerald, JD. Patient perspectives on gout and gout treatments: a patient panel discussion that informed the 2020 American College of Rheumatology Treatment Guideline. ACR Open Rheumatol. (2020) 2:725–33. doi: 10.1002/acr2.11199
Keywords: febuxostat, benzbromarone, gout, 24-h uric acid, uric acid
Citation: Zheng C, Tong Q, Zhang Z, Lin C, Wang Z, Kang D, Lin Y and Tian J (2025) The efficacy of a low-dose combination of febuxostat and benzbromarone versus each drug used alone for the treatment of gout. Front. Med. 12:1469879. doi: 10.3389/fmed.2025.1469879
Received: 24 July 2024; Accepted: 20 January 2025;
Published: 07 February 2025.
Edited by:
Mario Salazar-Paramo, University of Guadalajara, MexicoReviewed by:
Carlos Abud-Mendoza, Autonomous University of San Luis Potosi, MexicoCopyright © 2025 Zheng, Tong, Zhang, Lin, Wang, Kang, Lin and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianqing Tian, NDQ5MjU2MTE5QHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.