- 1Department of Clinical Pharmacy, College Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
- 2Department of Social and Administrative Pharmacy, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
Background: During hospitalization, a significant number of patients at risk of thromboembolism do not receive prophylaxis, despite established standards and viable procedures for preventing deep vein thrombosis (DVT). This study aimed to assess the appropriateness of vein thrombosis prophylaxis use among patients admitted to the medical ward of Debre Tabor Comprehensive Specialized Hospital (DTCSH) in Northwest Ethiopia.
Methods: An observational follow-up study was conducted in the medical wards of Debre Tabor Comprehensive Specialized Hospital in Northwest Ethiopia to determine whether thromboprophylaxis was appropriately used, based on the Padua risk assessment tool. To identify factors associated with the occurrence of inappropriate thromboprophylaxis use, a binary logistic regression model was used. Statistical significance was considered when the p-value was <0.05, with a 95% confidence interval.
Results: Among the 365 patients in the study, 21.37% received inappropriate thromboprophylaxis, while 78.63% received it correctly. Patients admitted to the ICU [AOR = 4.276, 95% CI: 1.878–16.134; p = 0.000], those who stayed for more than 6 days [AOR =6.192, 95% CI: 2.085–14.391; p = 0.000], and general practitioners [AOR = 1.816, 95% CI: 1.007–3.207; p = 0.048] were more likely to receive inappropriate thrombophylaxis.
Conclusion: The appropriateness of DVT prophylaxis use was suboptimal, especially among the patients treated by general practitioners, those hospitalized in the intensive care unit, and those who stayed for more than a few days in the ward. Using an integrated risk stratification checklist is an effective way to promote the more rational use of DVT prophylaxis.
Introduction
Deep vein thrombosis (DVT) continues to be a serious condition with a high mortality and morbidity rate (1). Hospitalized patients who are critically ill and have limited movement are at an increased risk of venous thromboembolism (VTE), which can lead to deep vein thrombosis (DVT) and pulmonary embolism (PE) (2). Between 50,000 and 200,000 of the 600,000 hospital admissions associated with DVT result in a pulmonary embolism (3). An estimated 10 million cases of hospital-related venous thromboembolism occur annually, making it a major source of illness and mortality worldwide (4). The occurrence of these complications can be reduced by healthcare personnel implementing effective prevention strategies, such as early mobilization and pharmaceutical prophylaxis. Furthermore, educating patients about symptom recognition and mobility maintenance can improve outcomes and reduce the impact of VTE (5).
In the absence of prevention, a significant number of medical patients experience an increased DVT rate (6). The occurrence of VTE often complicates the treatment plan for hospitalized patients (7). The risk of DVT and its associated consequences in hospitalized patients is considerably decreased when appropriate thromboprophylaxis, such as anticoagulant drugs or mechanical devices, is used (8, 9). However, in hospital settings, thromboprophylaxis is often either underappreciated or overused (10, 11). Negative effects, such as increased morbidity, mortality, and medical expenses, can result from the improper use of DVT prophylaxis—whether through underutilization or overutilization (12).
Inappropriate or non-existent DVT prevention in hospitalized patients can have fatal consequences. Patients with DVT are at a high risk of developing serious complications, including pulmonary embolism, which can lead to significant long-term health issues or even death (13). Furthermore, the development of DVT can lead to long-term complications, such as bleeding, recurrent venous thrombosis, chronic post-thrombotic syndrome (PTS) sequelae, persistent dyspnea following PE, and chronic thromboembolic pulmonary hypertension (14).
In the context of Ethiopia, only a small number of studies have evaluated whether DVT prophylaxis should be used among hospitalized patients in Ethiopia. Research from Jimma University Specialized Hospital revealed that only 37.3% of eligible patients received appropriate thromboprophylaxis (15). Another study conducted at Tikur Anbessa Specialized Hospital at Addis Ababa University revealed that 54.7% of patients received the recommended DVT prophylaxis (16).
The aim of this study was to assess the appropriateness of DVT prophylaxis use among patients admitted to the medical ward of Debre Tabor Comprehensive Specialized Hospital in Northwest Ethiopia. Understanding the fundamental causes of inadequate DVT prophylaxis is essential for formulating interventions that enhance patient outcomes. A detailed assessment of current practices and the identification of opportunities for improvement can help shape the development of targeted interventions to enhance patient safety and quality of care.
Materials and methods
Study setting, period, and design
The study was conducted at Debre Tabor Comprehensive Specialized Hospital (DTCSH), located in the South Gondar zone of the Amhara Region in Northwest Ethiopia, 100 km from Bahirdar and 666 from Addis Ababa. In the catchment area, it serves approximately 3 million people. The hospital has several departments, including internal medicine, pediatrics, obstetrics and gynecology, surgery, dentistry, psychiatry, ophthalmology, hospital pharmacy, dermatology, laboratory services, and an antiretroviral therapy clinic. In the medical wards of the hospital, an observational follow-up study design was used to evaluate the appropriateness of pharmacological prophylaxis against deep vein thrombosis between 18 March and 30 May 2024.
Study participants and sampling technique
The sample size was calculated using the single population formula, . The prevalence of inappropriate DVT prevention was obtained from a study conducted at UoCSH (17). A total sample size of 332 was calculated with a p-value of 31.6% (0.5), a marginal error (d) of 5%, a threshold of significance (α) of 0.05, Zα/2 = 1.96, and q = 1−p, based on prior research of this type. The final sample size was 365 individuals after accounting for a 10% contingency. All patients admitted to the DTCSH medical ward during the study period who met the inclusion criteria were included in the study. Patients who were readmitted during the study period, those with a diagnosis of DVT and taking anticoagulant therapy, those who refused to participate, and those with a length of stay of less than 24 h were excluded from the study. A consecutive sampling technique was used to select study participants.
Operational definition
Appropriate prophylaxis: When pharmacological prophylaxis was administered to the patient when indicated and not contraindicated or when no pharmacological prophylaxis was given to a patient who was either not eligible or had an absolute contraindication.
Inappropriate prophylaxis: When pharmacological prophylaxis was not administered to the patient when indicated and not contraindicated or when pharmacological prophylaxis was given to a patient who was either not eligible or had an absolute contraindication.
DVT prophylaxis indicated: When the Padua risk score was ≥4 and the IMPROVE bleeding risk score was <7.
DVT prophylaxis not indicated: When the Padua risk score was <4, regardless of the IMPROVE bleeding risk score.
DVT prophylaxis contraindicated: When the IMPROVE bleeding risk score was ≥7.
Data collection tools, procedures, and quality control
Data extraction tools were developed following a review of the literature (13, 17–21), with modifications made based on the type and context of patient medical data. These tools included participant sociodemographic details, pertinent laboratory findings, coagulation profile, length of hospital stay, diagnosis, number of diseases, the Padua assessment tool, and the IMPROVE bleeding risk score criteria. All of the information was obtained from the patients’ medical records. The format for data abstraction was pretested on 5% of the sample population, and any necessary modifications were made before the actual data collection period. Two pharmacists, who had received training on the study’s goals and fundamental data-gathering techniques, collected the data. Every day, the primary investigator checked the consistency, accuracy, and completeness of the data that had been gathered.
Outcome measurements
Each patient’s risk of thromboembolism was assessed using the modified Padua risk assessment model. Each criterion was assigned a risk value between 1 and 4 based on the extent of its impact on the development of thromboembolism. The patient’s total risk score was determined by summing the points assigned to each Padua risk assessment parameter. Using the IMPROVE bleeding risk score criteria, the contraindications of DVT prophylaxis were evaluated. A total score of 7 or more was considered to indicate an absolute contraindication to prophylaxis. When a patient is hospitalized in a medical ward, stays for more than 24 h, and has no contraindications, a total score of 4 or more indicates a high risk of thromboembolism and qualifies them for pharmacologic prophylaxis (21, 22).
Data processing and analysis
After the data collection, the data were entered into EpiData version 4.6, cleansed, and analyzed using STATA version 17. The results of the descriptive statistics were summarized using tables and figures. A Q–Q plot and a histogram were used to assess the normal distribution of the data. Depending on the distribution of the data, continuous variables were presented using the mean (standard deviation) and median (interquartile range), while categorical variables were presented using frequency and percent. After performing a Hosmer–Lemeshow goodness-of-fit test, a logistic regression model was used. A binary logistic regression analysis was conducted to identify the independent factors associated with the inappropriateness of DVT prophylaxis. Independent variables from the bivariate logistic regression analysis with a p-value of less than 0.2 were included in the multivariable logistic regression analysis to account for potential confounding. A p-value less than 0.05 was considered statistically significant.
Research approval and the participants’ consent
The study was approved by the Debre Tabor University Institutional Research Ethics Review Committee (approval number DTU/Re/305/2016). The hospital’s medical director provided a letter of authorization, which was received by the medical ward director. Written informed consent was obtained from each respondent after they were informed of the study’s goals and purpose. Participants were informed that their participation in the study was voluntary and that their information would remain private and confidential. The names and addresses of the participants were excluded from the data abstraction format to protect their confidentiality. The study adhered to the Helsinki Declaration, ensuring that it was conducted in an anonymous and confidential manner.
Results
Sociodemographic characteristics of the participants
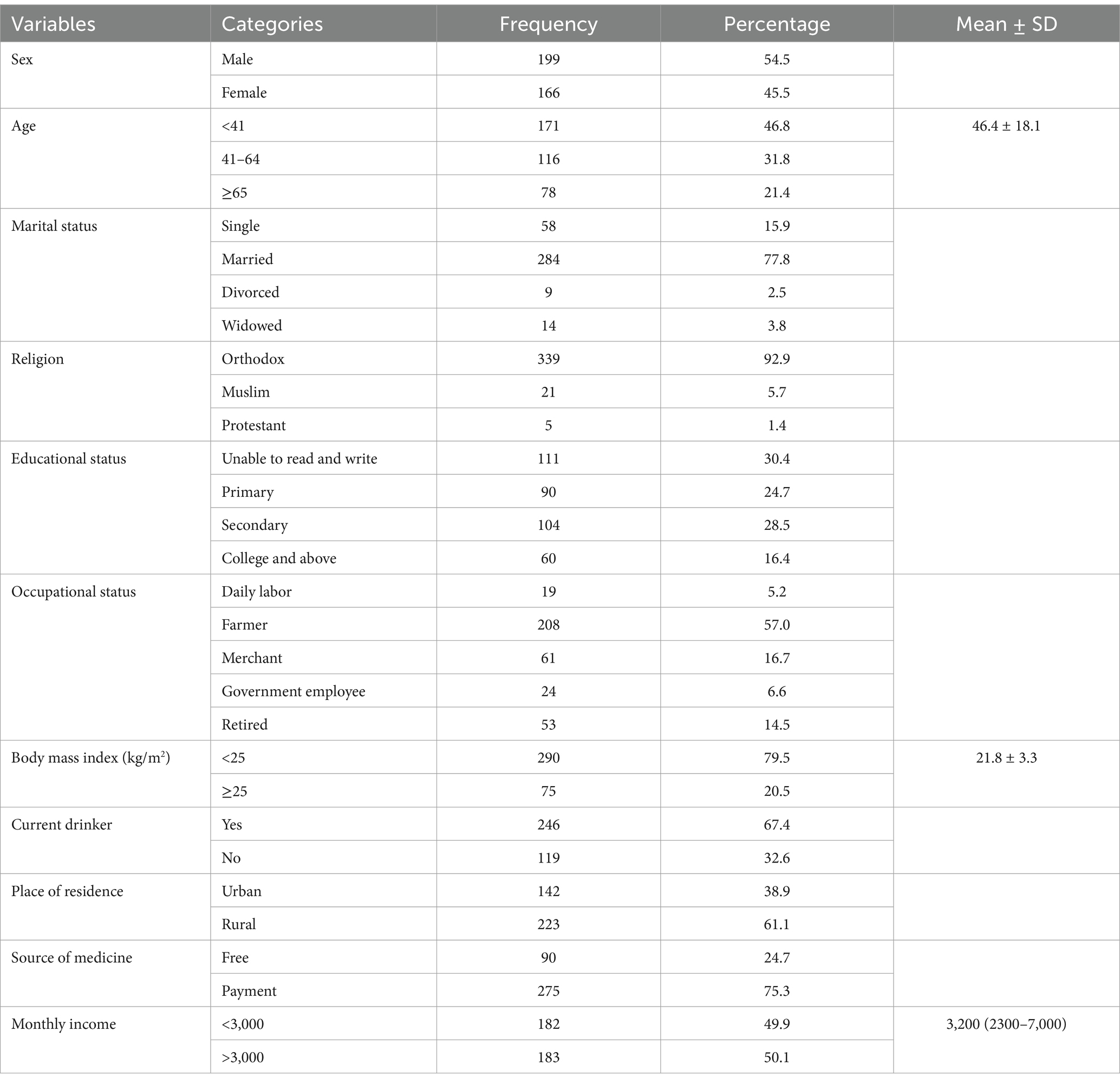
A total of 365 individuals participated in the study during the study period. The majority of the patients (54.5%) were male, and their mean (±SD) age was 46.4 ± 18.1 years. Payment for medical expenses was utilized by the great majority of the study participants (75.3%). Approximately one-fifth of the study participants were overweight (Table 1).
Clinical and laboratory profiles of the participants
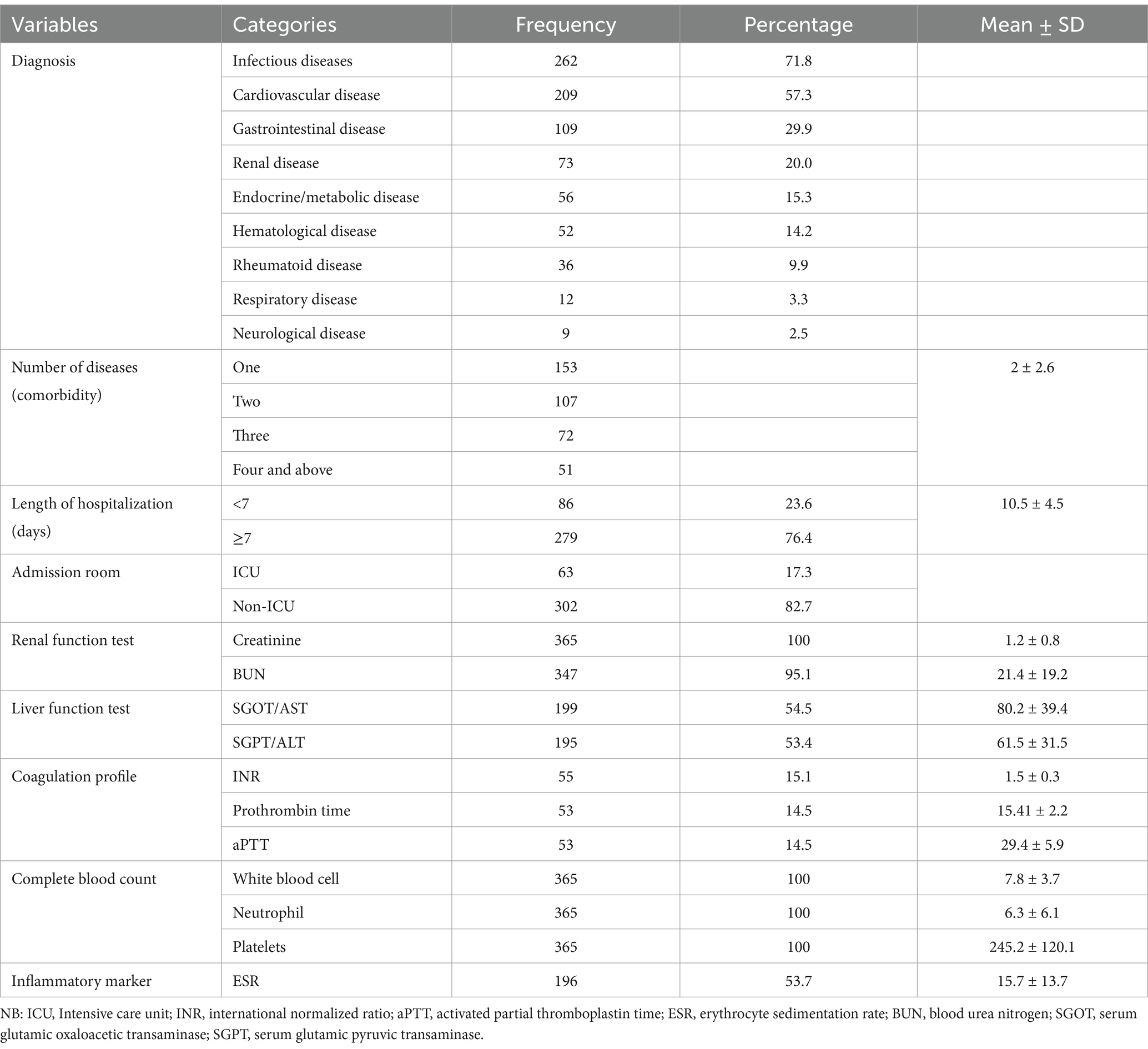
According to the study, patients were most frequently diagnosed with infectious diseases (71.8%), followed by cardiovascular (57.3%) and gastrointestinal disorders (29.9%). The average length of hospital stay was 10.5 ± 4.5 days. In addition, 17.3% of patients were admitted to the intensive care unit (Table 2).
Risk factors for thromboembolisms
The common risk factors for thromboembolism included restricted mobility (40.0%), heart or respiratory failure (29.3%), acute infection and rheumatologic illness (86.5%), and other conditions (Table 3).

Table 3. Risk factors for thromboembolism based on the Padua assessment tool in the hospitalized patients.
DVT risk stratification
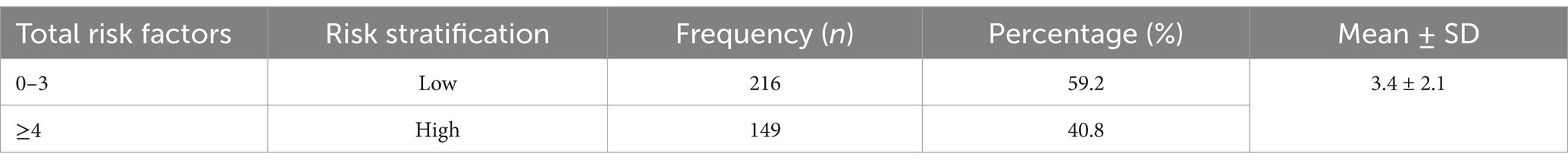
The Padua assessment tool for thromboembolism was used in this study to determine the risk of DVT and design appropriate thromboprophylaxis. As a result, the classification of DVT risk was based on the sum of each specific risk factor. Consequently, 59.2% of the patients were classified as low risk. In terms of overall risk, the mean Padua score for the participants was 3.4 ± 2.1 (Table 4).
Contraindications to thromboprophylaxis
There is a contraindication: patients with a total risk score of 4 or higher should receive thromboprophylaxis. The highest risk of bleeding was observed among individuals aged 65 years or older (21.4%) and those of the male sex (54.5%). A mean bleeding score of 8.5 ± 3.5 was obtained (Table 5).

Table 5. Contraindications for thromboprophylaxis according to the IMPROVE bleeding risk score criteria.
Appropriateness of thromboprophylaxis
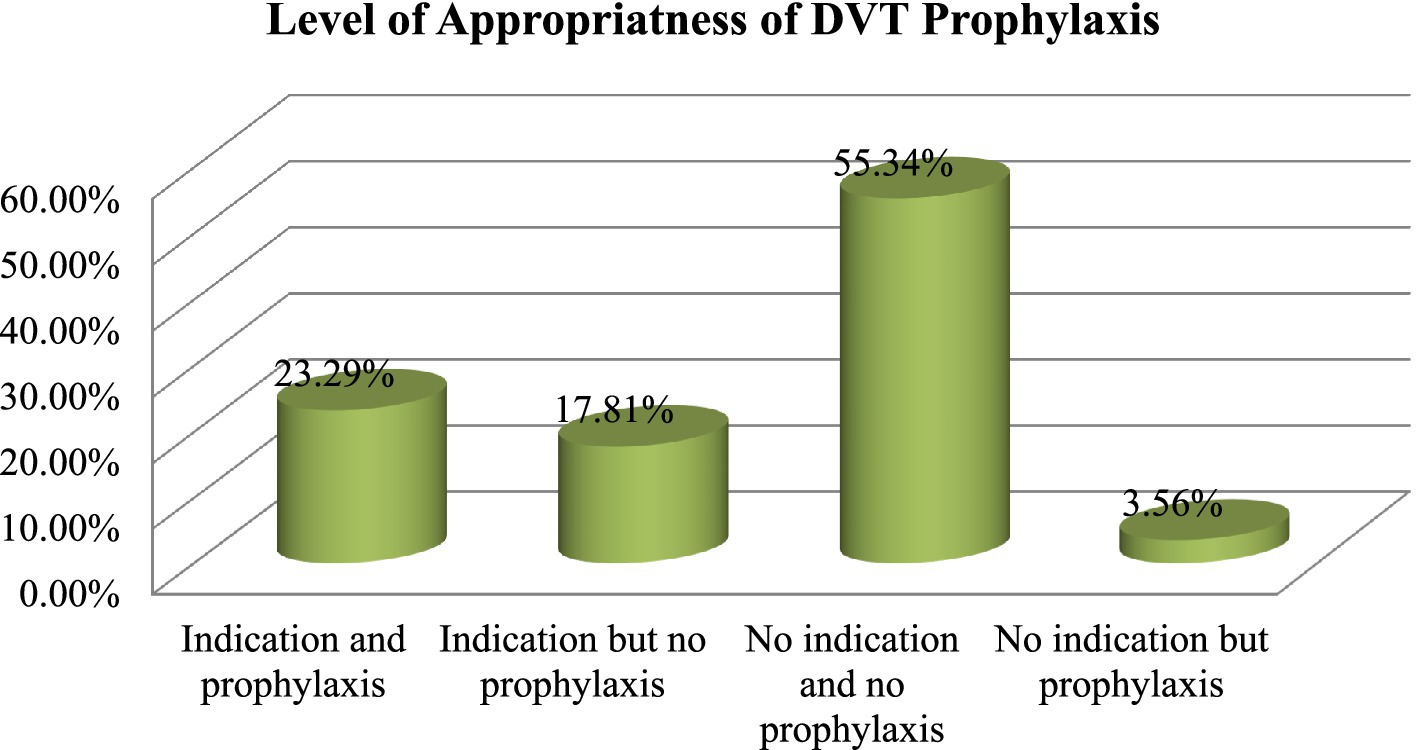
Among the patients in our study, 21.37% received inappropriate thromboprophylaxis. Approximately 55.34% of the patients were those who were not eligible for thromboprophylaxis and who did not receive prophylaxis, while 23.29% were those who were eligible and received prophylaxis. The total of these two factors indicated the appropriateness of thromboprophylaxis (Figure 1).
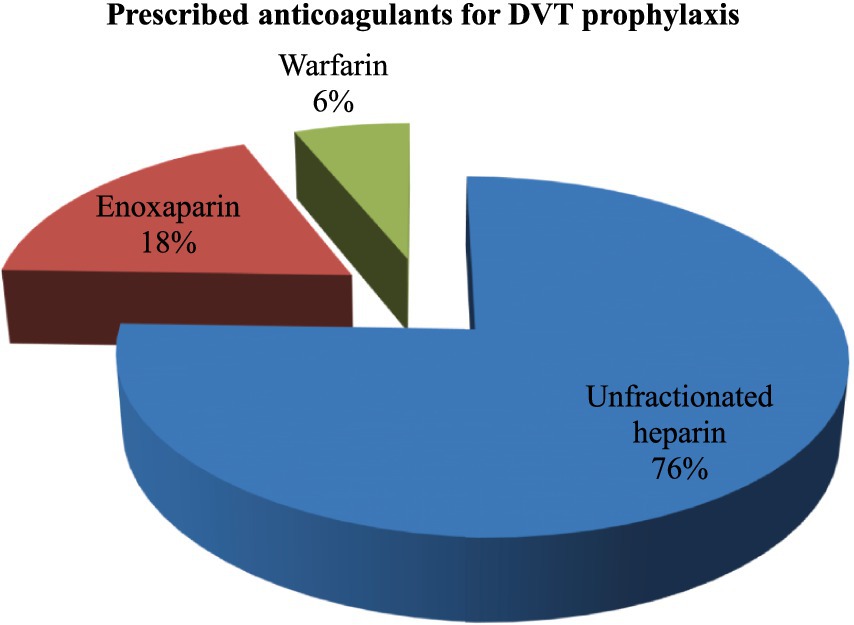
Prescribed anticoagulants for DVT prophylaxis
The most frequently prescribed anticoagulants were unfractionated heparin (76%), a low molecular weight anticoagulant (enoxaparin) (18%), and warfarin (6%) (Figure 2).
Interventions for inappropriate thromboprophylaxis
Following the discovery of the inappropriate use of thromboprophylaxis on the medical ward, interventions were implemented. Of the interventions provided, 55% were initiated for treatment, and 27% involved informing the prescribers only (Figure 3).

Figure 3. Interventions for the inappropriate use of DVT among patients admitted to the medical ward.
Factors associated with inappropriate thromboprophylaxis use among the participants
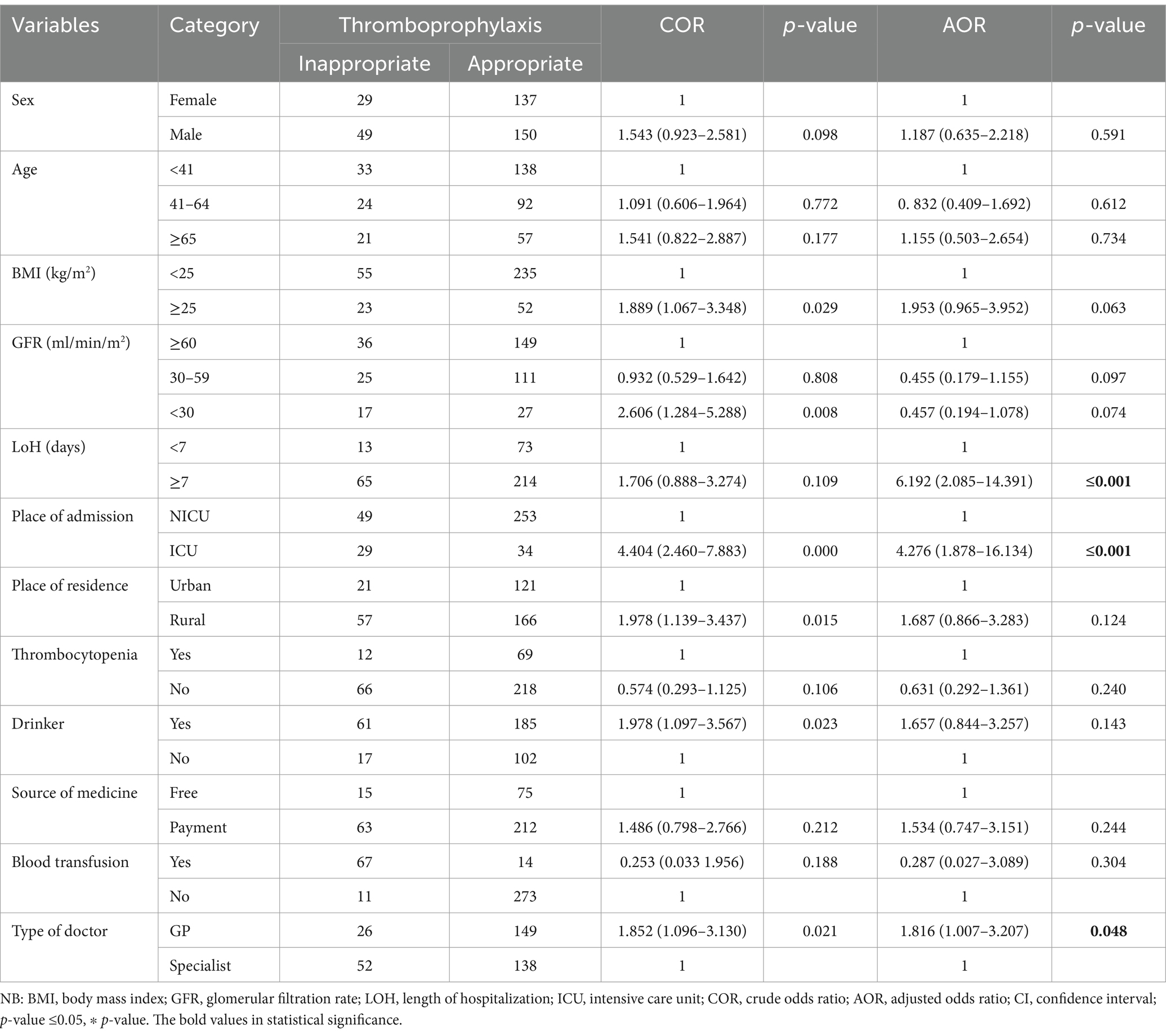
According to the multivariate logistic regression analysis, the patients’ place of admission, length of stay, and type of doctor were significantly associated with the occurrence of inappropriate thromboprophylaxis use.
Thus, assuming all other variables remained constant, compared to the NICU patients, the patients admitted to the ICU were more likely to receive inappropriate thromboprophylaxis [AOR = 4.276, 95% CI: 1.878–16.134; p = ≤0.001]. Compared to the patients who stayed for less than 7 days, the patients who stayed for more than 6 days had a higher likelihood of receiving inappropriate thromboprophylaxis [AOR = 6.192, 95% CI: 2.085–14.391; p = ≤0.001]. In addition, it was observed that the patients receiving treatment from GPs were more likely to receive inappropriate thromboprophylaxis than those receiving care from specialists [AOR = 1.816, 95% CI: 1.007–3.207; p = 0.048] (Table 6).
Discussion
Prophylactic treatment of deep vein thrombosis can save lives and prevent non-fatal symptomatic thromboembolism. It can also help avoid post-thrombotic syndrome, which is estimated to affect 15–40% of those who have had DVT in the past (23). These outcomes are highly beneficial in terms of human resources. Therefore, patients in our hospitals should receive careful evaluation regarding the prophylaxis of deep vein thrombosis. Many patients who are at significant risk of complications do not receive thromboprophylaxis, despite its potential benefits. If thromboprophylaxis is not contraindicated, patients with a total risk score higher than 4 should be studied. In our findings, the most common absolute contraindications that excluded the patients from receiving thromboprophylaxis included being older than 65 years, ICU admission, and a GFR of less than 30 mL/min/a. Nonetheless, a study conducted by the American University of Beirut Medical Center found that the most common contraindication was renal impairment (24).
The current study found that 17.81% of eligible patients did not receive thromboprophylaxis, which is consistent with an Iranian study reporting 18.3% (7). However, compared to a study conducted in Australia, only 23% of patients in the medium-risk group and 5% in the high-risk group received the recommended preventive treatment (23). The inability to accurately classify patients into the appropriate risk group and the difficulty in selecting appropriate prophylaxis for a specific risk group are key factors contributing to inappropriate thromboprophylaxis. Another reason thromboprophylaxis is not administered is that patients with a high risk of DVT may have multiple diagnoses, with the primary and major diagnoses taking priority over the risk of DVT in each individual patient. Clinicians’ lack of awareness of DVT prophylactic protocols and DVT risk stratification may also contribute to the underuse of thromboprophylaxis (23, 25).
According to the results of the current investigation, thromboprophylaxis regimens were administered improperly to approximately 21.37% of patients after 365 patients were assessed using the Padua assessment tool. Similar low rates were also noted in research conducted in other Asian countries (26, 27) and Iran (7). However, the rate in our study was lower than those of previous studies conducted in California (28), Brazilian Society (29), and sub-Saharan Africa (30). Some studies have also indicated that venous thromboembolism remains the primary cause of unexpected mortality, despite patients having received appropriate prophylaxis (19). This discrepancy might result from the fact that our study exclusively focused on pharmacologic prophylaxis, while the prior study evaluated the use of both pharmaceutical and mechanical prophylaxis. Furthermore, although our study used the Padua assessment tool, other studies also used alternative assessment techniques.
Our investigation revealed that the place of the patient’s admission, the duration of their hospital stay, and the types of physicians they saw were the main contributors to the improper use of thromboprophylaxis. As a result, individuals who spent 7 days or more in the hospital had a 6-fold increased risk of inappropriate thromboprophylaxis use compared to those who stayed for fewer days. These findings are in line with those of previous studies (31, 32). As a result, sitting still may make it difficult to move the legs and may even cause compression, reducing blood flow to the legs. Remarkably, the group classified as having sedentary professions had a higher risk of DVT, which medical professionals may overlook or fail to notice if patients remain in the hospital for an extended period. Due to bed shortages, extended stays in acute hospitals hinder patient flow and access to care while also increasing the risk of hospital-acquired illnesses. The shortage of hospital beds raises concerns about patient safety and the adequacy of the healthcare system’s infrastructure (33).
The hospital’s place of admission was another factor contributing to the incidence of improper thromboprophylaxis usage. Consequently, patients admitted to the ICU had approximately a 4-fold higher risk of inappropriate DVT prophylaxis use compared to those admitted to non-ICUs. Inappropriate use of DVT prophylaxis in the ICU has also been reported in previous studies (34–36). Since DVT is often clinically silent in the ICU, especially in patients who are sedated and on mechanical ventilation, the majority of patients admitted there do not receive the recommended thromboprophylaxis. As ICU-acquired thromboembolic events may resemble a variety of other illnesses, they are challenging to detect (37).
In our study, the individuals treated by specialists received appropriate DVT prophylaxis at a rate approximately double that of those treated by general practitioners. This result is consistent with research showing that specialists, compared to general practitioners, typically exhibit better adherence to guidelines, ensuring the proper use of prophylactics (38). One possible explanation for GPs’ improper use of thromboprophylaxis is a lack of awareness and familiarity with evidence-based practices. A problem frequently noted in the sub-Saharan African context is the severe shortage of experts in healthcare systems in low-resource environments, such as Ethiopia, which forces general practitioners to handle complex situations without the necessary resources or experience (39). General practitioners may be encouraged to use prophylaxis in low- to high-risk patients, for whom the risks may outweigh the benefits, using performance indicators that promote DVT prophylaxis for all medical patients. However, specialists are better able to identify people who will actually benefit from it (40). Since similar approaches have significantly enhanced DVT prophylaxis in low-resource healthcare systems, it is recommended that GPs participate in ongoing professional development programs and systematically incorporate thromboprophylaxis guidelines into their daily practices to close these gaps (41).
Our investigation revealed that the supply of interventions resolved 95% of the problems associated with inappropriate deep vein thromboprophylaxis use, a finding supported by previous studies (7, 42), which demonstrated that clinical pharmacy interventions are statistically significant in preventing the improper use of DVT prophylaxis. Another study conducted in Belgium (43) reported that pharmacist-driven interventions increased the percentage of critically ill medical patients receiving VTE prophylaxis, with benefits that persisted over time. Compared to education-based approaches, there was a notable improvement during the pharmacist-intervention phase. It is more effective to use clinical pharmacy interventions, particularly when it comes to maintaining optimum thromboprophylaxis (44, 45). Clinical pharmacists can help healthcare professionals apply antithrombotic prophylaxis and medication use rationally in hospitals by using various risk assessment tools and providing support.
Strengths and limitations
One potential strength of this study is its observational follow-up study design. However, when interpreting the study findings, the following limitations should be considered. The results of this study are limited to one location and cannot be generalized to all hospitals in Ethiopia. In addition, it omitted information regarding the appropriateness of prophylaxis in terms of dosage and treatment duration. Furthermore, based on the sample size estimate, we did not include enough patients to achieve the desired statistical power.
Conclusion
In our study, the appropriateness of DVT prophylaxis use was suboptimal, especially among the patients treated by general practitioners, those hospitalized in the intensive care unit, and those confined to the ward for longer than a few days. Using an integrated risk stratification checklist is an effective way to globally increase the use of DVT prophylaxis. Staff members working in medical wards should follow protocols and possess an understanding of thromboprophylaxis and DVT risk factors.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Debre Tabor University Institutional Research Ethical Review Committee gave the study ethical approval with approval number (DTU/Re/305/2016). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Writing – original draft, Writing – review & editing. TM: Data curation, Methodology, Project administration, Supervision, Writing – review & editing. FD: Data curation, Supervision, Validation, Writing – review & editing. AA: Formal analysis, Project administration, Visualization, Writing – review & editing. SA: Formal analysis, Validation, Visualization, Writing – review & editing. AG: Formal analysis, Validation, Visualization, Writing – original draft. GA: Data curation, Formal analysis, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors greatly appreciate the cooperation of the hospital administration and study participants throughout the study period.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stone, J, Hangge, P, Albadawi, H, Wallace, A, Shamoun, F, Knuttien, MG, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. (2017) 7:S276–84. doi: 10.21037/cdt.2017.09.01
2. Geerts, WH, Bergqvist, D, Pineo, GF, Heit, JA, Samama, CM, Lassen, MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2008) 133:381S–453S. doi: 10.1378/chest.08-0656
3. Van Wicklin, SA, Ward, KS, and Cantrell, SW. Implementing a research utilization plan for prevention of deep vein thrombosis. AORN J. (2006) 83:1351–62. doi: 10.1016/S0001-2092(06)60149-X
4. Giri, S, Singh, A, Varghese, J, Ingawale, S, and Roy, A. Outcome of pharmacological thromboprophylaxis in hospitalized patients with cirrhosis–a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. (2023) 35:674–81. doi: 10.1097/MEG.0000000000002564
5. Onwuzo, C, Olukorode, J, Sange, W, Tanna, SJ, Osaghae, OW, Hassan, A, et al. A review of the preventive strategies for venous thromboembolism in hospitalized patients. Cureus. (2023) 15:e48421. doi: 10.7759/cureus.48421
6. Samama, MM, Cohen, AT, Darmon, JY, Desjardins, L, Eldor, A, Janbon, C, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. N Engl J Med. (1999) 341:793–800. doi: 10.1056/NEJM199909093411103
7. Khalili, H, Dashti-Khavidaki, S, Talasaz, AH, Mahmoudi, L, Eslami, K, and Tabeefar, H. Is deep vein thrombosis prophylaxis appropriate in the medical wards? A clinical pharmacists' intervention study. Pharm World Sci. (2010) 32:594–600. doi: 10.1007/s11096-010-9412-y
8. Kahn, SR, Lim, W, Dunn, AS, Cushman, M, Dentali, F, Akl, EA, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (2012) 141:e195S–226S. doi: 10.1378/chest.11-2296
9. Guyatt, GH, Akl, EA, Crowther, M, Gutterman, DD, and Schuünemann, HJ. Executive summary: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2012) 141:7S–47S. doi: 10.1378/chest.1412S3
10. Kahn, SR, Panju, A, Geerts, W, Pineo, GF, Desjardins, L, Turpie, AG, et al. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res. (2007) 119:145–55. doi: 10.1016/j.thromres.2006.01.011
11. Cohen, AT, Tapson, VF, Bergmann, JF, Goldhaber, SZ, Kakkar, AK, Deslandes, B, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. (2008) 371:387–94. doi: 10.1016/S0140-6736(08)60202-0
12. Greene, MT, Spyropoulos, AC, Chopra, V, Grant, PJ, Kaatz, S, Bernstein, SJ, et al. Validation of risk assessment models of venous thromboembolism in hospitalized medical patients. Am J Med. (2016) 129:1001.e9. e18. doi: 10.1016/j.amjmed.2016.03.031
13. Badireddy, M., and Mudipalli, V.R., Deep venous thrombosis prophylaxis. In: StatPearls. Treasure Island (FL): StatPearls Publishing. (2023).
14. Ghanima, W, Wik, HS, Tavoly, M, Enden, T, and Jelsness-Jørgensen, LP. Late consequences of venous thromboembolism: measuring quality of life after deep vein thrombosis and pulmonary embolism. Thromb Res. (2018) 164:170–6. doi: 10.1016/j.thromres.2017.07.025
15. Dominguez-Vicent, A, Brautaset, R, and Venkataraman, AP. Repeatability of quantitative measurements of retinal layers with SD-OCT and agreement between vertical and horizontal scan protocols in healthy eyes. PLoS One. (2019) 14:e0221466. doi: 10.1371/journal.pone.0221466
16. Masters, R, and Powers, D. Clarifying assumptions in age-period-cohort analyses and validating results. PLoS One. (2020) 15:e0238871. doi: 10.1371/journal.pone.0238871
17. Ayalew, MB, Horsa, BA, and Zeleke, MT. Appropriateness of pharmacologic prophylaxis against deep vein thrombosis in medical wards of an Ethiopian referral hospital. J Vasc Med. (2018) 2018:1–7. doi: 10.1155/2018/8176898
18. Mohammed, AS, Taha, NM, and Abdel-Aziz, EM. Nurses' performance regarding venous thromboembolism prophylaxis at intensive care unit. Zagazig Nurs J. (2018) 14:1–17. doi: 10.21608/znj.2018.37454
19. Shah, SS, Abdi, A, Özcem, B, and Basgut, B. The rational use of thromboprophylaxis therapy in hospitalized patients and the perspectives of health care providers in northern Cyprus. PLoS One. (2020) 15:e0235495. doi: 10.1371/journal.pone.0235495
20. Yap, DFS, Ng, ZY, Wong, CY, Saifuzzaman, MM, and Yang, L. Appropriateness of deep vein thrombosis (DVT) prophylaxis use among medical inpatients: a DVT risk alert tool (DRAT) study. Med J Malaysia. (2019) 74:45.
21. Rosenberg, DJ, Press, A, Fishbein, J, Lesser, M, McCullagh, L, McGinn, T, et al. External validation of the IMPROVE bleeding risk assessment model in medical patients. Thromb Haemost. (2016) 116:530–6. doi: 10.1160/TH16-01-0003
22. Lai, J. Venous thromboembolism prophylaxis adult inpatient/ambulatory Clinical Practice Guideline (2014). Available at: https://acforum-excellence.org/Resource-Center/resource_files/1585-2020-07-01-062736.pdf
23. Ahmad, HA, Geissler, A, and MacLellan, DG. Deep venous thrombosis prophylaxis: are guidelines being followed? ANZ J Surg. (2002) 72:331–4. doi: 10.1046/j.1445-2197.2002.02402.x
24. Masroujeh, R, Shamseddeen, W, Isma’eel, H, Otrock, ZK, Khalil, IM, and Taher, A. Underutilization of venous thromboemoblism prophylaxis in medical patients in a tertiary care center. J Thromb Thrombolysis. (2008) 26:138–41. doi: 10.1007/s11239-007-0084-y
25. Rahim, SA, Panju, A, Pai, M, and Ginsberg, J. Venous thromboembolism prophylaxis in medical inpatients: a retrospective chart review. Thromb Res. (2003) 111:215–9. doi: 10.1016/j.thromres.2003.09.010
26. Pinjala, R. Venous thromboembolism risk & prophylaxis in the acute hospital care setting (ENDORSE), a multinational cross-sectional study: results from the Indian subset data. Indian J Med Res. (2012) 136:60–7.
27. Nekoonam, B, Eshraghi, A, Hajiesmaeili, M, and Sahraei, Z. Deep vein thrombosis prophylaxis evaluation in intensive care unit. Arch Crit Care Med. (2016) 1:e8497. doi: 10.17795/accm-8497
28. White, RH. The epidemiology of venous thromboembolism. Circulation. (2003) 107:I-4–8. doi: 10.1161/01.CIR.0000078468.11849.66
29. Pereira, CA, Brito, SS, Martins, AS, and Almeida, CM. Deep venous thrombosis prophylaxis: practical application and theoretical knowledge in a general hospital. J Vasc Bras. (2008) 7:18–27. doi: 10.1590/S1677-54492008000100005
30. Kingue, S, Bakilo, L, Ze Minkande, J, Fifen, I, Gureja, YP, Razafimahandry, HJC, et al. Epidemiological African day for evaluation of patients at risk of venous thrombosis in acute hospital care settings: cardiovascular topic. Cardiovasc J Afr. (2014) 25:159–64. doi: 10.5830/CVJA-2014-025
31. Mahan, CE, Fisher, MD, Mills, RM, Fields, LE, Stephenson, JJ, Fu, AC, et al. Thromboprophylaxis patterns, risk factors, and outcomes of care in the medically ill patient population. Thromb Res. (2013) 132:520–6. doi: 10.1016/j.thromres.2013.08.013
32. de Bastos, M, Barreto, SM, Caiafa, JS, Bogutchi, T, and Rezende, SM. Assessment of characteristics associated with pharmacologic thromboprophylaxis use in hospitalized patients: a cohort study of 10 016 patients. Blood Coagul Fibrinolysis. (2013) 24:691–7. doi: 10.1097/MBC.0b013e328360a52c
33. Maguire, PA, Taylor, IC, and Stout, RW. Elderly patients in acute medical wards: factors predicting length of stay in hospital. Br Med J (Clin Res Ed). (1986) 292:1251–3. doi: 10.1136/bmj.292.6530.1251
34. Permpikul, C, Chaiyasoot, W, and Panitchote, A. Incidence of proximal deep vein thrombosis in medical critical care patients. Thromb J. (2022) 20:5. doi: 10.1186/s12959-022-00363-5
35. Malato, A, Dentali, F, Siragusa, S, Fabbiano, F, Kagoma, Y, Boddi, M, et al. The impact of deep vein thrombosis in critically ill patients: a meta-analysis of major clinical outcomes. Blood Transfus. (2015) 13:559–68. doi: 10.2450/2015.0277-14
36. Khouli, H, Shapiro, J, Pham, VP, Arfaei, A, Esan, O, Jean, R, et al. Efficacy of deep venous thrombosis prophylaxis in the medical intensive care unit. J Intensive Care Med. (2006) 21:352–8. doi: 10.1177/0885066606292880
37. Minet, C, Potton, L, Bonadona, A, Hamidfar-Roy, R, Somohano, CA, Lugosi, M, et al. Venous thromboembolism in the ICU: main characteristics, diagnosis and thromboprophylaxis. Crit Care. (2015) 19:1–9. doi: 10.1186/s13054-015-1003-9
38. Vallano, A, Arnau, JM, Miralda, GP, and Pérez-Bartolí, J. Use of venous thromboprophylaxis and adherence to guideline recommendations: a cross-sectional study. Thromb J. (2004) 2:3. doi: 10.1186/1477-9560-2-3
39. Naicker, S, Eastwood, JB, Plange-Rhule, J, and Tutt, RC. Shortage of healthcare workers in sub-Saharan Africa: a nephrological perspective. Clin Nephrol. (2010) 74:S129–33. doi: 10.5414/CNP74S129
40. Berhe, DF, Taxis, K, Haaijer-Ruskamp, FM, and Mol, PGM. Healthcare professionals' level of medication knowledge in Africa: a systematic review. Br J Clin Pharmacol. (2018) 84:2729–46. doi: 10.1111/bcp.13746
41. Mokadem, NE, and El-Sayed, S, Effect of educational intervention on critical care Nurses' adherence to the clinical practice guidelines for preventing venous thromboembolism in critically ill patients. American Journal of Nursing. (2019). 7:974–982. doi: 10.12691/ajnr-7-6-10
42. Cohn, SL, Adekile, A, and Mahabir, V. Improved use of thromboprophylaxis for deep vein thrombosis following an educational intervention. J Hosp Med. (2006) 1:331–8. doi: 10.1002/jhm.137
43. Vervacke, A, Lorent, S, and Motte, S. Improved venous thromboembolism prophylaxis by pharmacist-driven interventions in acutely ill medical patients in Belgium. Int J Clin Pharm. (2014) 36:1007–13. doi: 10.1007/s11096-014-9988-8
44. Kiracı, ZK, Yalçın, N, Cennet, Ö, Demirkan, K, and Yorgancı, K. Education and clinical pharmacist-led management strategies for the risk and prophylaxis of venous thromboembolism in general surgery. Thromb J. (2023) 21:86. doi: 10.1186/s12959-023-00530-2
Keywords: deep vein thrombosis, appropriateness of thromboprophylaxis, medical ward, Comprehensive Specialized Hospitals, Northwest Ethiopia
Citation: Dagnew SB, Moges TA, Dagnew FN, Assefa AN, Anberbr SS, Geremew AT and Addis GT (2025) Evaluation of deep vein thrombosis prophylaxis use in a Northwest Ethiopian medical ward: an observational follow-up study. Front. Med. 12:1468190. doi: 10.3389/fmed.2025.1468190
Edited by:
Carmine Siniscalchi, University of Parma, ItalyReviewed by:
Pierpaolo Di Micco, Ospedale Santa Maria delle Grazie, ItalyBudi Setiawan, Diponegoro University, Indonesia
Copyright © 2025 Dagnew, Moges, Dagnew, Assefa, Anberbr, Geremew and Addis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samuel Berihun Dagnew, c2FtdWVsYmVyaWh1bjEyQGdtYWlsLmNvbQ==; c2FtdWJlckBkdHUuZWR1LmV0
 Samuel Berihun Dagnew
Samuel Berihun Dagnew Tilaye Arega Moges
Tilaye Arega Moges Fisseha Nigussie Dagnew
Fisseha Nigussie Dagnew Abraham Nigussie Assefa
Abraham Nigussie Assefa Sisay Sitotaw Anberbr
Sisay Sitotaw Anberbr Adane Tsegaw Geremew1
Adane Tsegaw Geremew1 Getu Tesfaw Addis
Getu Tesfaw Addis