
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 19 February 2025
Sec. Gastroenterology
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1460975
Introduction: The objective of our study was to externally validate the value of the fibrinogen-to-albumin ratio (FAR), a new biomarker used to identify active inflammatory bowel disease (IBD).
Materials and methods: A total of 245 ulcerative colitis (UC) and 543 Crohn’s disease (CD) patients were included in our study. Multivariate logistic regression analysis was used to investigate the independent association between FAR and disease activity in patients with UC or CD. The area under the receiver operating characteristic curve was used to assess the prediction accuracy of biomarkers in distinguishing disease states.
Results: Multivariate logistic regression analysis identified the FAR as the strongest predictor to discriminate disease activity of UC (odds ratio: 24.871, 95% confidence interval: 9.831–38.912, p < 0.001) and CD (odds ratio: 28.966, 95% confidence interval: 21.009–37.250, p < 0.001). The FAR gave the highest area under the curve in identifying both active UC (0.870, 95% confidence interval: 0.824–0.916) and CD (0.925, 95% confidence interval: 0.904–0.946). The probability of both UC and CD patients being in the active stage significantly increased when the FAR was more than or equal to the optimal cutoff values.
Conclusion: The FAR, a simple prognostic indicator, performs well in identifying active IBD.
Inflammatory bowel disease (IBD) is a chronic and recurrent immunological disorder with an increasing incidence and prevalence over time (1–3). The main types of IBD, Crohn’s disease (CD) and ulcerative colitis (UC), are believed to be caused by the dysfunction of the immune system (4). Early detection of IBD activity is crucial for timely treatment and effective prevention of complications, ultimately improving patients’ quality of life and prognosis (5–7). Endoscopic biopsy remains the gold standard for assessing and monitoring inflammatory activity in IBD, while it is an invasive procedure that poses challenges in specimen collection and can be costly.
The neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) obtained from the complete blood count (CBC) have been demonstrated as effective markers for predicting IBD activity and severity (8–11). Red blood cell distribution width (RDW) has shown good ability in evaluating disease activity, specifically in CD (12). C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) have been demonstrated as significant biomarkers for early diagnosis of IBD and accurate monitoring of IBD activity (13–15). Inflammation and coagulation are interdependent processes, each activating and propagating the other, which is crucial for the progression of IBD (16, 17). Distinct pro-inflammatory stimuli activate the clotting cascade, which subsequently propagates the inflammatory state by activating signaling pathways or recruiting more inflammatory cells to inflamed tissues (18). Fibrinogen can modify various aspects of inflammatory cell function by engaging leukocytes through different cellular receptors and mechanisms (19). In our previous study, we externally validated for the first time fibrinogen’s diagnostic ability to identify patients with IBD in the active stage and demonstrated that fibrinogen had a high discriminative capacity for active IBD (20). Albumin is a negative acute-phase protein, and its synthetic rate has a direct correlation with the severity of acute inflammation (21). Additionally, albumin was deemed to possess a strong discriminatory ability in identifying children with CD (22). Thus, we proposed a novel biomarker, the fibrinogen-to-albumin ratio (FAR), for identifying active IBD. This study aims to assess the association between FAR and IBD activity and to evaluate the diagnostic ability of FAR in detecting active IBD.
The research was a retrospective observational study. We included IBD patients admitted to the First Affiliated Hospital of Wenzhou Medical University from September 2011 to September 2019. The diagnosis of IBD was based on clinical evidence such as diarrhea, abnormal laboratory findings, imaging findings, endoscopic findings, and histopathology. Patients meeting the following criteria were excluded: other immune-related diseases, infectious disease, carcinoma, liver cirrhosis, renal or heart or respiratory failure, and loss of fibrinogen or albumin data on admission. We excluded patients with clinical, laboratory, and histological evidence of infectious colitis, radiation colitis, indeterminate colitis, microscopic colitis, or with a doubtful diagnosis of IBD. The clinical data we collected included demographic data, laboratory parameters, and endoscopic findings. The FAR was calculated by dividing fibrinogen by albumin. Disease activity of UC and CD was assessed by the Total Mayo Score and Crohn’s Disease Activity Index (CDAI), respectively. Total Mayo Score was a comprehensive scoring system that contained stool frequency, rectal bleeding, endoscopic findings, and the physician’s global assessment (23, 24). The Total Mayo Score can simply and effectively classify patients with UC, and a score of more than 2 indicated that patients with UC were in the active stage (23, 24). The CDAI criteria included body weight, general health condition, hematocrit, abdominal pain severity, daily blood stool count, and complications (25). The CDAI of more than 150 indicated that patients with CD were in the active stage (25). The ethics committee of the First Affiliated Hospital of Wenzhou Medical University approved this research.
The Mann–Whitney U-test was used to compare quantitative variables, which were presented as median [interquartile range (IQR)]. The chi-square test or Fisher’s exact test was used to compare categorical variables, which were presented as absolute numbers (frequencies). Multivariate logistic regression analysis was used to investigate the independent association between biomarkers and disease activity in patients with UC or CD to calculate the odds ratio with a confidence interval of 95%. The relationship between FAR and IBD activity was determined by the Spearman’s correlation analysis. The area under the receiver operating characteristic curve (AUROC) was used to assess the prediction accuracy of biomarkers in distinguishing disease states. The AUROCs of various biomarkers were compared using the DeLong test (26). We compared sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio at an optimum cutoff value according to the curve. We divided patients in UC and CD cohorts, respectively, into two groups based on the optimum cutoff values for the FAR. All tests were two-sided, and a p-value less than 0.05 was considered statistically significant. All statistical procedures were performed with STATA (version 14.0; StataCorp, State of Texas, USA).
Our study included 788 IBD patients, of whom 245 (31.1%) had UC and 543 (68.9%) had CD. Overall, patients in the UC group were older than those in the CD group. In addition, the CD group had a higher proportion of male patients than the UC group. More details about the characteristics of the IBD cohort are listed in Table 1. The FAR, CRP, ESR, PLR, and NLR levels in both active UC and CD patients scored significantly higher compared with those in the remission stage, whereas the LMR levels scored significantly lower (all p < 0.001), as shown in Tables 2, 3. Multivariate logistic regression analysis identified that FAR and CRP were independent predictors for identifying both active UC and CD. Table 4 lists the multivariate logistic regression analysis results. Table 5 presents significantly positive correlations of both Total Mayo Score and CDAI with FAR, NLR, PLR, RDW, CRP, and ESR, and negative correlations with LMR (all p < 0.001). Table 5 indicates correlations among biomarkers and disease activity of IBD. FAR, RDW, ESR, CRP, NLR, and PLR had significant positive correlations with the Total Mayo Score of UC, whereas LMR was negatively related to the Total Mayo Score of UC (all p < 0.001). In addition, the correlation analysis showed significant positive correlations of CDAI with the FAR, RDW, ESR, CRP, NLR, and PLR and negative correlations with LMR (all p < 0.001). Figures 1, 2 show that the FAR gave the highest area under the curve in identifying both active UC and CD compared with NLR, LMR, PLR, RDW, ESR, and CRP (all p < 0.05). Referring to the area under the curve (AUC) of these markers, we found that, while the FAR is better than CRP in identifying active CD, the difference is not significant. Tables 6, 7 show more details about the diagnostic performance of biomarkers. According to the FAR classification of UC patients (Group A: <0.076 and Group B: ≥0.076), the proportion of active UC patients in Groups A and B was 30.8% (24/78) and 88.6% (148/167), respectively, and the difference was statistically significant (p < 0.001). According to the FAR classification of CD patients (Group C: <0.107 and group D: ≥0.107), the incidence of active CD in Groups C and D was 18.2% (50/275) and 88.1% (236/268), respectively (p < 0.001). It can be seen that the probability of IBD patients being active increased significantly when the FAR was more than or equal to the optimal threshold. Based on the FAR classification for UC patients (Group A: <0.076 and Group B: ≥0.076), the active UC patients’ rates among Groups A and B were 30.8% (24/78) and 88.6% (148/167), respectively (p < 0.001). Based on the FAR classification for CD patients (Group C: <0.107 and Group D: ≥0.107), the active CD patients’ rates among Groups C and D were 18.2% (50/275) and 88.1% (236/268), respectively (p < 0.001). It is clear that the probability of IBD patients in the active stage significantly increased when the FAR was more than or equal to the optimal cutoff values.
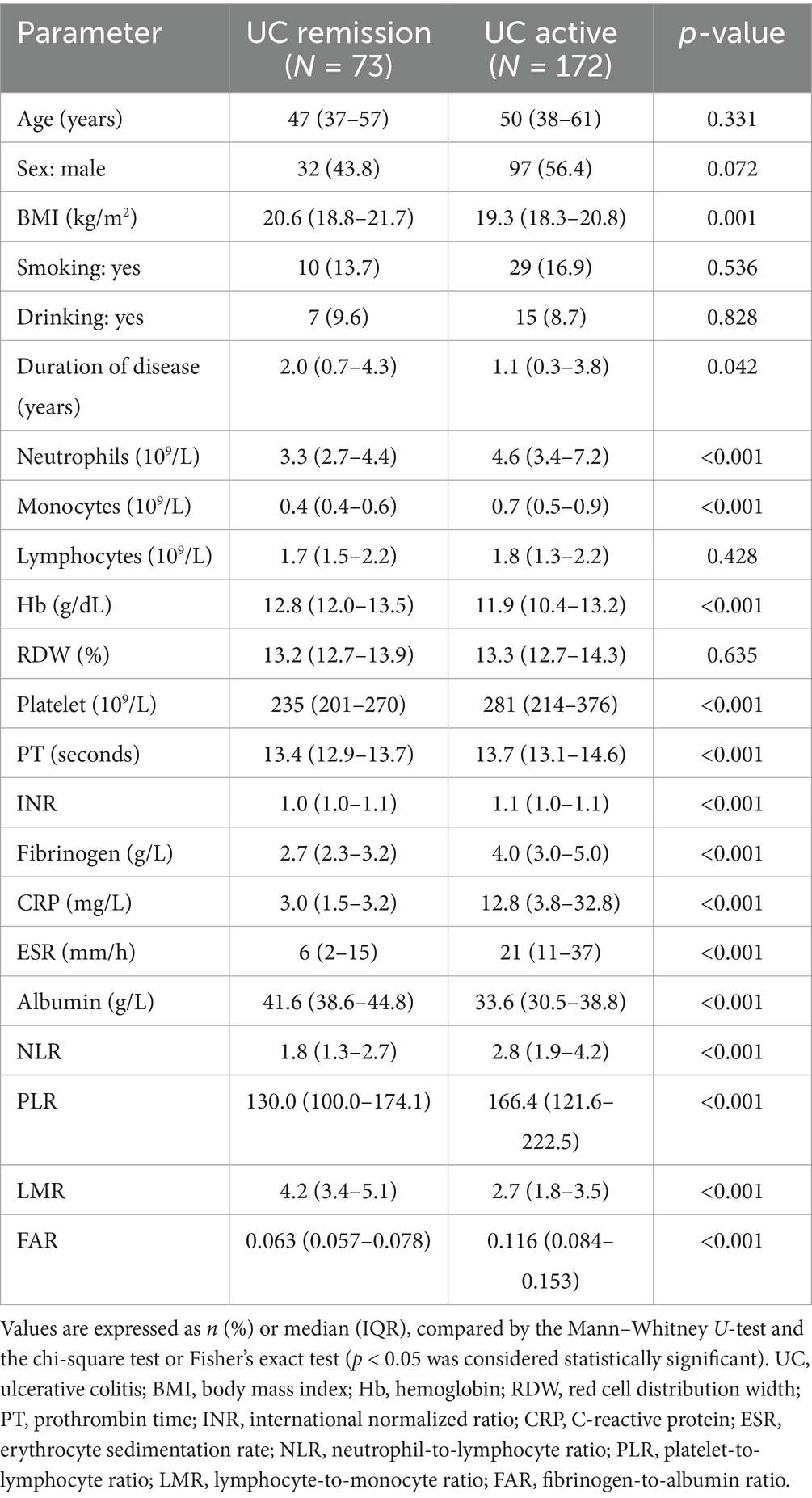
Table 2. Epidemiology and laboratory parameters of ulcerative colitis patients, stratified by disease activity.
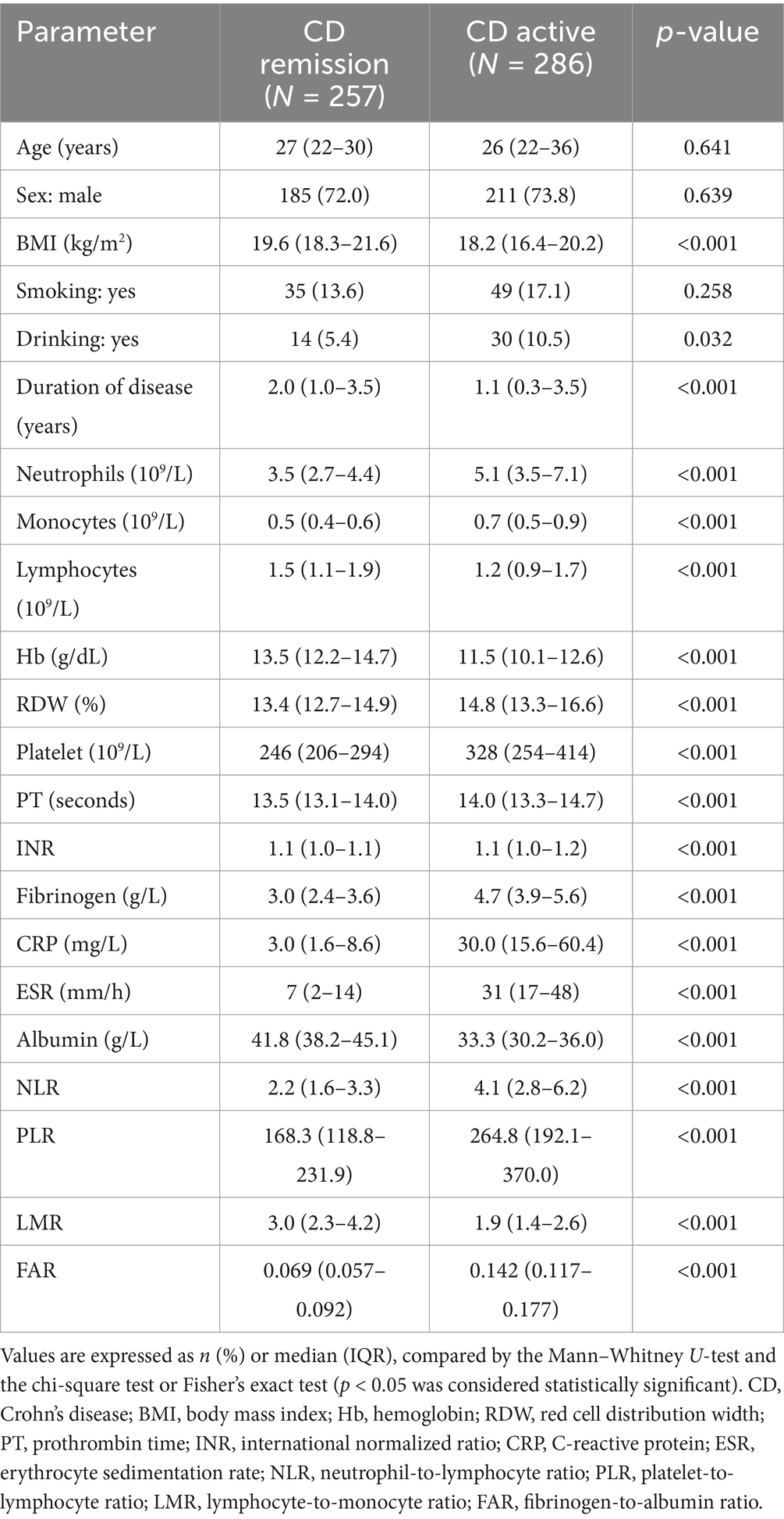
Table 3. Epidemiology and laboratory parameters of Crohn’s disease patients, stratified by disease activity.
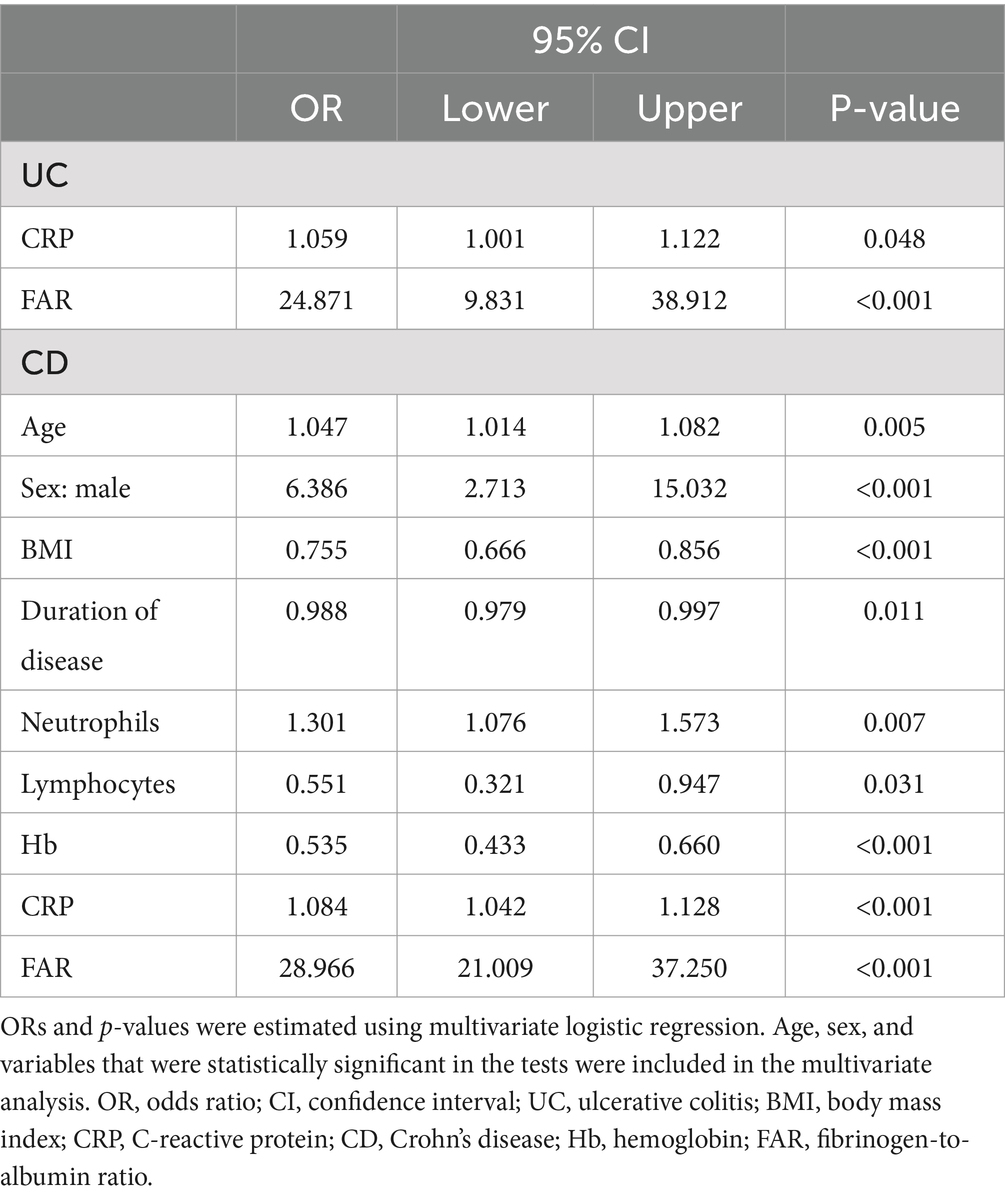
Table 4. Multivariate logistic regression analysis results of disease activity for patients with inflammatory bowel disease.
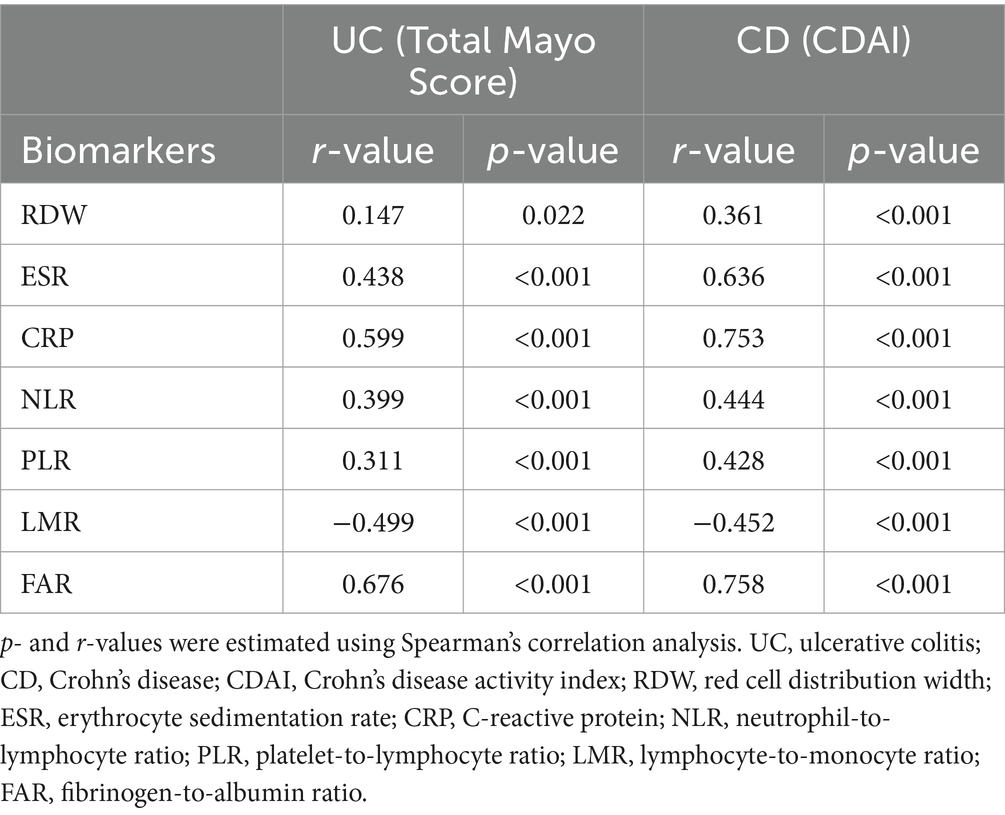
Table 5. Spearman’s correlation coefficients between biomarkers and disease activity of patients with inflammatory bowel disease.
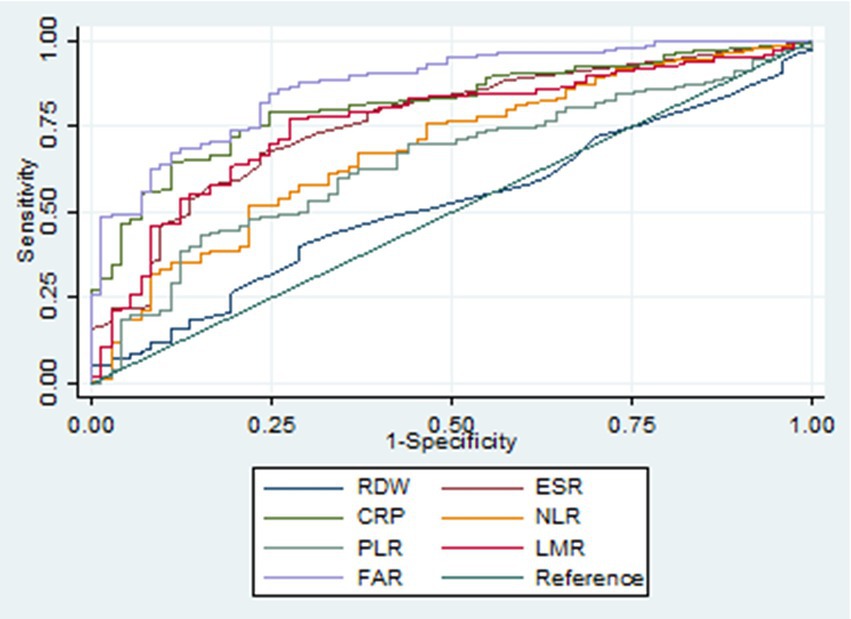
Figure 1. Receiver operating characteristic curves of various biomarkers in identifying active ulcerative colitis. RDW, red cell distribution width; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; FAR, fibrinogen-to-albumin ratio.
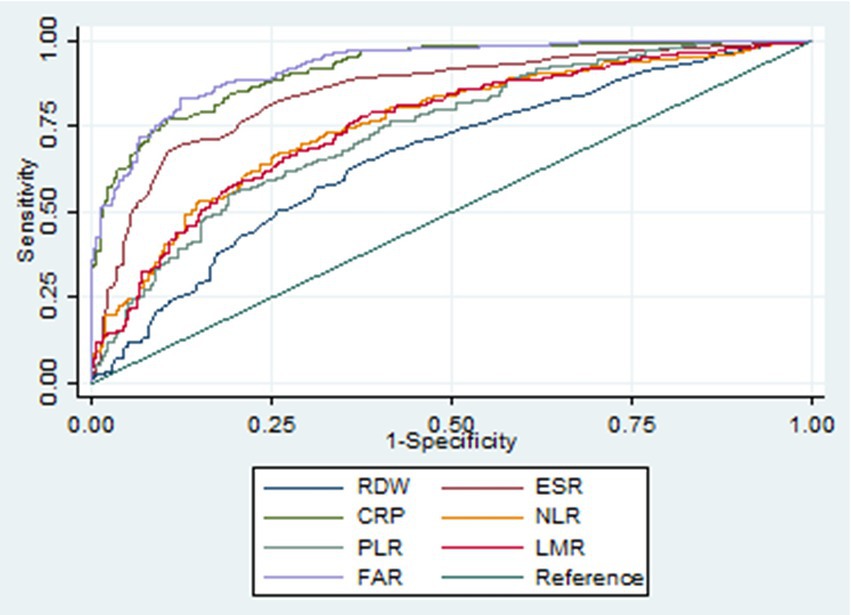
Figure 2. Receiver operating characteristic curves of various biomarkers in identifying active Crohn’s disease. RDW, red cell distribution width; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; FAR, fibrinogen-to-albumin ratio.
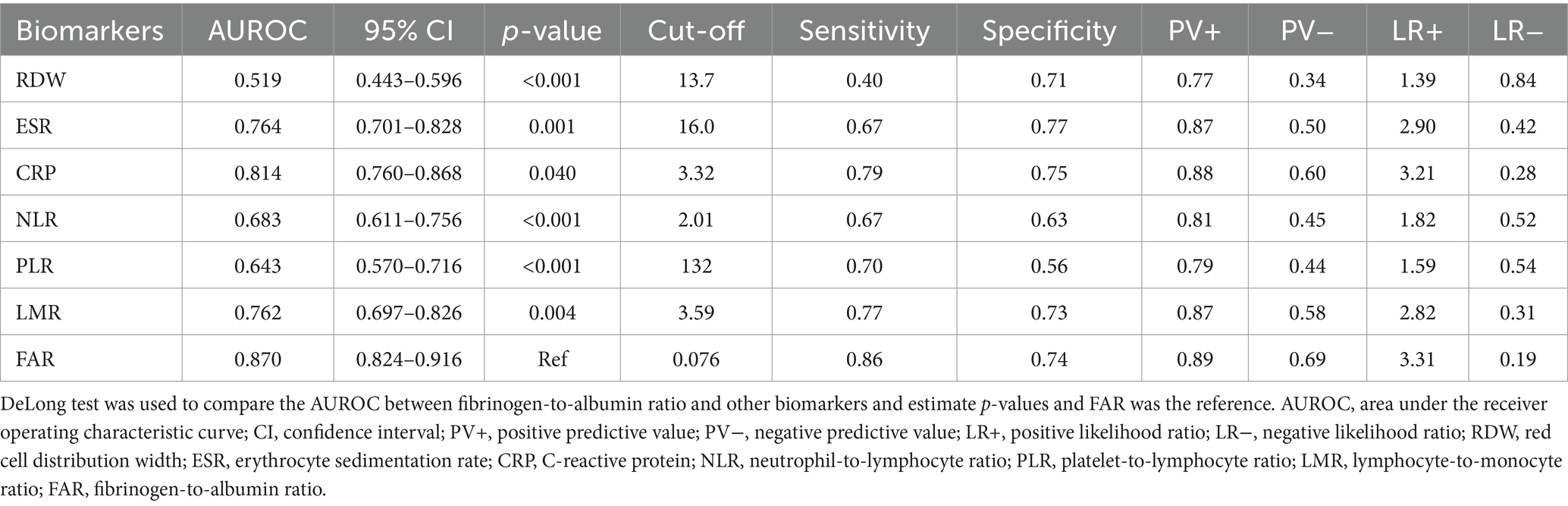
Table 6. Diagnostic accuracy of various biomarkers in identifying ulcerative colitis in the active stage.
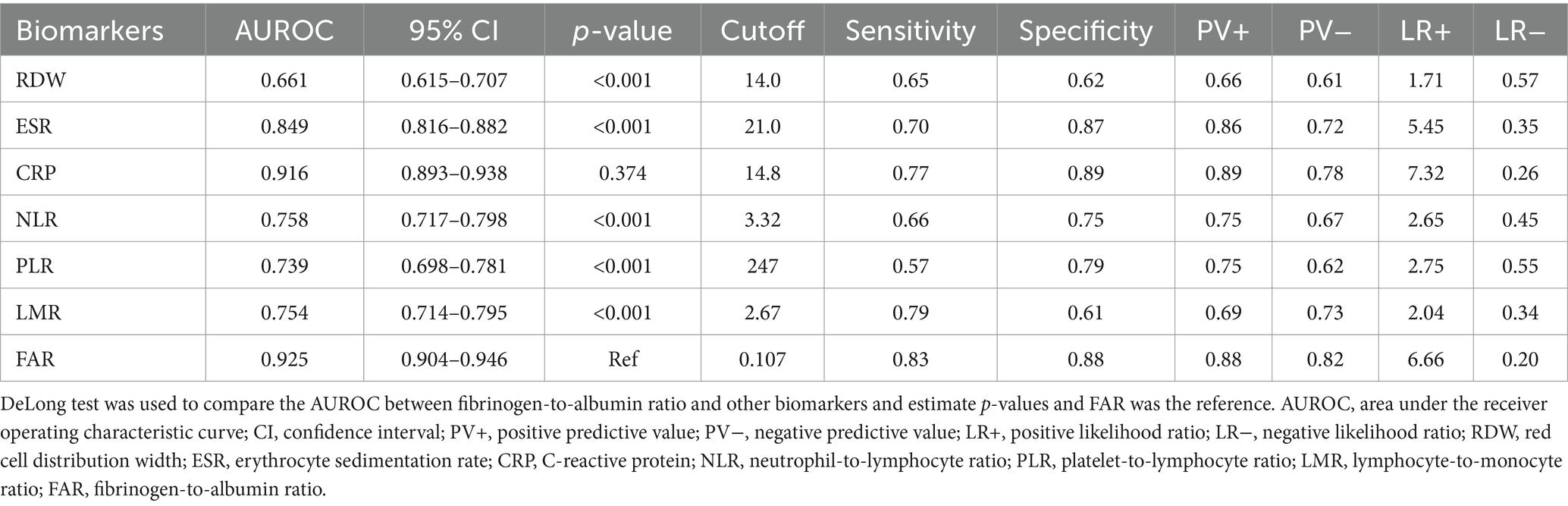
Table 7. Diagnostic accuracy of various biomarkers in identifying Crohn’s disease in the active stage.
The research externally validated for the first time a new and non-invasive biomarker, the FAR’s diagnostic ability to identify active IBD patients.
Timely diagnosis of the disease activity of IBD can help us choose the best treatment plan and improve the prognosis of patients. Endoscopic biopsy remains the gold standard for assessing and monitoring inflammatory activity in IBD. Fecal calprotectin was a good biological index to evaluate IBD activity (27–29). However, this index had a high cost, long time, and inconvenient sample collection and processing. Thus, there is an urgent need for a simple, readily obtainable, low-cost, and efficient biomarker to optimally manage IBD patients.
Fibrinogen deposits are a near-universal feature of tissue injury, including injury driven by immunological derangements (19). Low albumin levels often indicate malnutrition, while patients’ nutriture is associated with disease activity, and the nutriture of active IBD patients is worse. In addition, the inflammatory response can influence albumin synthesis (30). Thus, we proposed the FAR to identify active IBD patients. The FAR has its unique advantages. First, the FAR can be rapidly calculated using two parameters and an easy formula without the need for a tedious calculation process. Second, fibrinogen and albumin levels can be easily determined using an inexpensive and non-invasive blood test. The FAR is a biomarker of high prediction accuracy and practicality and is objectively assessed. Third, the FAR is a potential biomarker that may have high accuracy and practicality. According to our findings, a FAR greater than 0.076 in UC and 0.107 in CD may indicate the active phase of the disease. Of course, the value of FAR requires further verification by a prospective multi-center large-sample study. Finally, the FAR may have potential clinical applications in the evaluation of the therapeutic effect and prediction of the complications of IBD. These need further study.
However, the research has its limitations. First, this study was retrospective single-center research. We will conduct a prospective multi-center large-sample study in the future. In addition, we did not take into account several factors, including immunosuppressant and corticosteroid use, which may have an impact on inflammatory biomarker levels. In the following research, we will distinguish between new cases and those that are under treatment. Finally, we included some commonly used inflammatory biomarkers but others were excluded. Problems will be addressed in the following studies.
In conclusion, the laboratory-based FAR is a simple and practical biomarker to identify active IBD patients. Further study is needed to explore and validate other potential clinical application values of FAR.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the ethics committee of First Affiliated Hospital of Wenzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
X-FC: Writing – original draft. Z-MH: Writing – review & editing. X-LH: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Molodecky, NA, Soon, IS, Rabi, DM, Ghali, WA, Ferris, M, Chernoff, G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. (2012) 142:46–54.e42. doi: 10.1053/j.gastro.2011.10.001
2. Xavier, RJ, and Podolsky, DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. (2007) 448:427–34. doi: 10.1038/nature06005
3. Jeong, DY, Kim, S, Son, MJ, Son, CY, Kim, JY, Kronbichler, A, et al. Induction and maintenance treatment of inflammatory bowel disease: a comprehensive review. Autoimmun Rev. (2019) 18:439–54. doi: 10.1016/j.autrev.2019.03.002
4. Park, JH, Peyrin-Biroulet, L, Eisenhut, M, and Shin, JI. IBD immunopathogenesis: a comprehensive review of inflammatory molecules. Autoimmun Rev. (2017) 16:416–26. doi: 10.1016/j.autrev.2017.02.013
5. Magro, F, Gionchetti, P, Eliakim, R, Ardizzone, S, Armuzzi, A, Barreiro-de Acosta, M, et al. Third European evidence-based consensus on diagnosis and Management of Ulcerative Colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, Cancer surveillance, surgery, and Ileo-anal pouch disorders. J Crohns Colitis. (2017) 11:649–70. doi: 10.1093/ecco-jcc/jjx0008
6. Gomollón, F, Dignass, A, Annese, V, Tilg, H, van, G, Lindsay, JO, et al. 3rd European evidence-based consensus on the diagnosis and Management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. (2017) 11:3–25. doi: 10.1093/ecco-jcc/jjw168
7. Walsh, AJ, Bryant, RV, and Travis, SP. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol. (2016) 13:567–79. doi: 10.1038/nrgastro.2016.128
8. Torun, S, Tunc, BD, Suvak, B, Yildiz, H, Tas, A, Sayilir, A, et al. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: a promising marker in predicting disease severity. Clin Res Hepatol Gastroenterol. (2012) 36:491–7. doi: 10.1016/j.clinre.2012.06.004
9. Gao, SQ, Huang, LD, Dai, RJ, Chen, DD, Hu, WJ, and Shan, YF. Neutrophil-lymphocyte ratio: a controversial marker in predicting Crohn’s disease severity. Int J Clin Exp Pathol. (2015) 8:14779–85.
10. Akpinar, MY, Ozin, YO, Kaplan, M, Ates, I, Kalkan, IH, Kilic, ZMY, et al. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict mucosal disease severity in ulcerative colitis. J Med Biochem. (2018) 37:155–62. doi: 10.1515/jomb-2017-0050
11. Cherfane, CE, Gessel, L, Cirillo, D, Zimmerman, MB, and Polyak, S. Monocytosis and a low lymphocyte to monocyte ratio are effective biomarkers of ulcerative colitis disease activity. Inflamm Bowel Dis. (2015) 21:1769–75. doi: 10.1097/MIB.0000000000000427
12. Yeşil, A, Senateş, E, Bayoğlu, IV, Erdem, ED, Demirtunç, R, and Kurdaş Övünç, AO. Red cell distribution width: a novel marker of activity in inflammatory bowel disease. Gut Liver. (2011) 5:460–7. doi: 10.5009/gnl.2011.5.4.460
13. Henriksen, M, Jahnsen, J, Lygren, I, Stray, N, Sauar, J, Vatn, MH, et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. (2008) 57:1518–23. doi: 10.1136/gut.2007.146357
14. Lewis, JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. (2011) 140:1817–1826.e2. doi: 10.1053/j.gastro.2010.11.058
15. Yoon, JY, Park, SJ, Hong, SP, Kim, TI, Kim, WH, and Cheon, JH. Correlations of C-reactive protein levels and erythrocyte sedimentation rates with endoscopic activity indices in patients with ulcerative colitis. Dig Dis Sci. (2014) 59:829–37. doi: 10.1007/s10620-013-2907-3
16. Danese, S, Papa, A, Saibeni, S, Repici, A, Malesci, A, and Vecchi, M. Inflammation and coagulation in inflammatory bowel disease: the clot thickens. Am J Gastroenterol. (2007) 102:174–86. doi: 10.1111/j.1572-0241.2006.00943.x
17. Owczarek, D, Cibor, D, Głowacki, MK, Rodacki, T, and Mach, T. Inflammatory bowel disease: epidemiology, pathology and risk factors for hypercoagulability. World J Gastroenterol. (2014) 20:53–63. doi: 10.3748/wjg.v20.i1.53
18. Yoshida, H, and Granger, DN. Inflammatory bowel disease: a paradigm for the link between coagulation and inflammation. Inflamm Bowel Dis. (2009) 15:1245–55. doi: 10.1002/ibd.20896
19. Luyendyk, JP, Schoenecker, JG, and Flick, MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. (2019) 133:511–20. doi: 10.1182/blood-2018-07-818211
20. Chen, XF, Zhao, Y, Guo, Y, Huang, ZM, and Huang, XL. Predictive value of fibrinogen in identifying inflammatory bowel disease in active stage. BMC Gastroenterol. (2021) 21:472. doi: 10.1186/s12876-021-02040-9
21. Don, BR, and Kaysen, G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. (2004) 17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x
22. Daniluk, U, Daniluk, J, Krasnodebska, M, Lotowska, JM, Sobaniec-Lotowska, ME, and Lebensztejn, DM. The combination of fecal calprotectin with ESR, CRP and albumin discriminates more accurately children with Crohn’s disease. Adv Med Sci. (2019) 64:9–14. doi: 10.1016/j.advms.2018.08.001
23. D’Haens, G, Sandborn, WJ, Feagan, BG, Geboes, K, Hanauer, SB, Irvine, EJ, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. (2007) 132:763–86. doi: 10.1053/j.gastro.2006.12.038
24. Schroeder, KW, Tremaine, WJ, and Ilstrup, DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. (1987) 317:1625–9. doi: 10.1056/NEJM198712243172603
25. Best, WR, Becktel, JM, Singleton, JW, and Kern, F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. (1976) 70:439–44. doi: 10.1016/S0016-5085(76)80163-1
26. DeLong, ER, DeLong, DM, and Clarke-Pearson, DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. (1988) 44:837–45. doi: 10.2307/2531595
27. Mosli, MH, Zou, G, Garg, SK, Feagan, SG, MacDonald, JK, Chande, N, et al. C-reactive protein, fecal calprotectin, and stool Lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and Meta-analysis. Am J Gastroenterol. (2015) 110:802–19. doi: 10.1038/ajg.2015.120
28. Cerrillo, E, Moret, I, Iborra, M, Pamies, J, Hervás, D, Tortosa, L, et al. A nomogram combining fecal calprotectin levels and plasma cytokine profiles for individual prediction of postoperative Crohn’s disease recurrence. Inflamm Bowel Dis. (2019) 25:1681–91. doi: 10.1093/ibd/izz053
29. Manceau, H, Chicha-Cattoir, V, Puy, H, and Peoc’h, K. Fecal calprotectin in inflammatory bowel diseases: update and perspectives. Clin Chem Lab Med. (2017) 55:474–83. doi: 10.1515/cclm-2016-0522
Keywords: albumin, biomarker, fibrinogen, inflammatory bowel disease, activity
Citation: Chen X-F, Huang Z-M and Huang X-L (2025) Fibrinogen-to-albumin ratio: a new biomarker to identify inflammatory bowel disease in active stage. Front. Med. 12:1460975. doi: 10.3389/fmed.2025.1460975
Received: 07 July 2024; Accepted: 04 February 2025;
Published: 19 February 2025.
Edited by:
Jacopo Troisi, University of Salerno, ItalyReviewed by:
Tsvetelina Velikova, Sofia University, BulgariaCopyright © 2025 Chen, Huang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Ming Huang, d3lodWFuZ3poaW1pbmdAMTI2LmNvbQ==; Xie-Lin Huang, d3lodWFuZ3hpZWxpbkAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.