
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 28 January 2025
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1446835
 Wei Wang1,2
Wei Wang1,2 Xiaomeng Wang1,2
Xiaomeng Wang1,2 Songhua Chen1
Songhua Chen1 Jun Li3
Jun Li3 Qinglin Cheng4
Qinglin Cheng4 Yu Zhang1
Yu Zhang1 Qian Wu1,2
Qian Wu1,2 Kui Liu1,2
Kui Liu1,2 Xuli Jiang5
Xuli Jiang5 Bin Chen1,2*
Bin Chen1,2*Objective: To identify the composition of comorbidities among patients with newly diagnosed pulmonary tuberculosis and assess the impact of comorbidities on the clinical characteristics of patients.
Methods: This study was conducted in 13 hospitals across 13 counties in Zhejiang province, China. Patient data collected in this study included demographic characteristics, chest radiography results, etiological results, and comorbidities. Descriptive statistics were conducted to describe the composition of comorbidities of all participants. Univariate and multivariate logistic regression analyzes were performed to identify the effects of comorbidities on the clinical features of the participants.
Results: Of the 8,421 total participants, 27.6% reported cavities in the chest radiography results, 41.9% were Mycobacterium tuberculosis-positive in the etiology test results, and 38.7% (3,258/8,421) had at least one type of comorbidity. The most predominant comorbidity was pleuritis (1,833, 21.8%), followed by diabetes mellitus (763, 9.1%), other extrapulmonary tuberculosis (421, 5%), tracheobronchial tuberculosis (275, 3.3%), and silicosis (160, 1.9%). Participants with diabetes mellitus had the highest rate of chest cavities on X-ray (54.8%), followed by those with silicosis (33.1%). In addition, a higher percentage of the M. tuberculosis-positive etiology (45%) was observed in participants without comorbidities than in participants with comorbidities (37.1%). Compared to patients without comorbidities, patients with diabetes mellitus (adjusted odds ratio [AOR]: 2.88, 95% confidence interval [CI]: 2.42–3.43) were more likely to show cavities in chest X-ray, while patients with pleuritis (AOR: 0.27, 95% CI: 0.23–0.32), other extrapulmonary tuberculosis (AOR: 0.48, 95% CI: 0.36–0.64), and tracheobronchial tuberculosis (AOR: 0.40–0.79) were less likely to show chest cavities in X-ray. In addition, patients with diabetes mellitus (AOR: 2.05, 95% CI: 1.72–2.45), tracheobronchial tuberculosis (AOR: 3.22, 95% CI: 2.4–4.32) were more likely to show Mycobacterium tuberculosis-positive in the etiology, and patients with pleuritis (AOR: 0.25, 95% CI: 0.22–0.29), other extrapulmonary tuberculosis (AOR: 0.61, 95% CI: 0.48–0.76) were less likely to show Mycobacterium tuberculosis-positive in the etiology.
Conclusion: The prevalence of comorbidities was high in patients newly diagnosed with pulmonary tuberculosis. Thus, integration of screening and personalized management is needed for the control of tuberculosis and its comorbidities.
In 2022, an estimated total of 10.6 million people worldwide were ill with tuberculosis (TB), with 1.13 million TB-associated deaths (1, 2). It has been estimated that a quarter of the world’s population has been infected with TB (3), and approximately 5–15% of these infections will progress to active TB during the patient’s lifetime (2). TB is a serious public health threat. To effectively address this problem through a multisectoral response, the End TB Strategy set the target of a 90% reduction in TB incidence and a 95% reduction in TB mortality between 2015 and 2035 (1, 4), as proposed by the World Health Organization (WHO) and approved by the 67th World Health Assembly.
Over the past several decades, tremendous progress has been achieved in TB control after the implementation of a series of measures based on different TB control strategies, including directly observed treatment, short-course (DOTS) strategies, the Stop TB Strategy, and the Global Plan to Stop TB (4, 5). In the past, TB services were limited due to inadequate healthcare capacity and low economic levels in geographic regions of focus, and these efforts were typically focused on single conditions while neglecting to identify and manage other coexisting conditions (6). TB is a chronic inflammatory disease that may increase a person’s susceptibility to other non-communicable and communicable diseases, such as diabetes mellitus (DM), depression, malaria, and human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) (7, 8). Other conditions that commonly co-occur with TB may worsen its clinical course, affect the efficacy of TB treatment, and increase the risk of relapse.
The proportion of people with coexisting medical problems is increasing as the global population is aging (9), which not only increases the challenges of practicing clinicians but also places significant health and financial burdens on patients with TB and health services (10, 11). Accordingly, TB comorbidities have received increasing attention in recent years (6). An exploratory survey of 27 high-TB burden countries identified HIV, DM, depression, and tobacco and alcohol use disorders as the most common comorbid conditions in TB (12). Several studies have been conducted to estimate the prevalence of different comorbidities in patients with TB. The global prevalence of DM in TB patients is approximately 15% (13). The prevalence of TB and noncommunicable disease comorbidity was 26.9% among public primary care patients in South Africa (14). A World Health Survey conducted in 48 low- and middle-income countries showed that the prevalence of one or more comorbid noncommunicable diseases was 68.8% in individuals with TB (8). The coexistence of silicosis and TB was also observed in many studies, where the incidence of TB in patients with silicosis is higher than the incidence of TB in the general population (15, 16).
Although there have been some research reports on TB comorbidity in China (11, 17, 18), most of these studies focused on TB and DM comorbidity only (19–22), and further evidence is needed to guide the optimization of TB prevention and control policies. The objective of this study was to identify and describe the composition of comorbidities among newly diagnosed patients with TB, and to assess the impact of comorbidities on the clinical characteristics of TB in real-world settings as based on patients from 13 designated TB hospitals across Zhejiang Province, China.
The study was conducted from January 1, 2017 to February 28, 2019 in 13 hospitals across 13 counties in Zhejiang province, eastern China. Patients with pulmonary TB (PTB) were newly diagnosed as outpatients, and drug-susceptible and HIV-negative patients were included in the study. Patients with retreated, drug-resistant, or HIV-positive TB were excluded. With informed consent from all eligible primary TB patients, investigations were performed by trained medical personnel.
The data collected in this study included basic demographic characteristics (gender, age, ethnicity, occupation, household registration), chest radiography examination results, and etiological examination results of the participants. In addition, we collected information on whether the participants had any of the following diseases: Pleuritis, tracheal bronchial TB, DM, silicosis, or other extrapulmonary TB, which clinicians believe may affect the course or effectiveness of TB treatment.
Patients with confirmed PTB based on the National Diagnostic Criteria for TB (WS 288–2017) and the Classification of TB criteria (WS196–2017) were included in the study. Laboratory diagnosis was based on the results of detectable acid-fast bacilli obtained from sputum smears and/or sputum cultures, or a positive result from molecular diagnosis. The clinical diagnosis was based on chest radiographs, epidemiological surveys, clinical symptoms, and other relevant tests. Since pleurisy and tracheobronchial TB typically occur outside the lungs and the treatment regimen is different from that of PTB, these two diseases were classified as concurrent diseases of PTB in this study. The comorbidities investigated in this study included pleuritis, tracheobronchial TB, DM, silicosis, and other extrapulmonary TB, which were diagnosed by physicians based on national diagnostic criteria, or as reported by participants whether they had been diagnosed with one or more diseases in another healthcare facility prior to the present TB visit.
Descriptive statistics were used to demonstrate participant characteristics. UpSet plots were generated using the R package UpSetR to describe the comorbidities of all participants (23). Chi-square test was used to explore the correlation between comorbidity and demographic characteristics. The results of chest radiography and etiology were used to represent the clinical characteristics of PTB in this study. If cavity was reported in chest X-ray examination results or positive was reported in Mycobacterium tuberculosis in etiology test, which were usually considered to be serious clinical manifestations of TB that require the focus of clinicians. In this study, the cavity in chest X-ray examination results, positive in Mycobacterium tuberculosis etiology test were taken as dependent variables, and comorbidities were taken as independent variables to identify the effects of comorbidities on the participants’ clinical features by multivariate logistic regression analyses. In the multivariate logistic regression analysis, the effects of sex, age, ethnicity, occupation, and household registration were adjusted. The estimation of their odds ratios (ORs) and 95% confidence intervals (CIs) were determined. Statistical significance was set at p < 0.05.
A total of 8,421 participants participated in the study, the characteristics of which are presented in Table 1. Of this total, 68.1% were female and 67.0% were from local provincial residences. Farmers accounted for the largest proportion (39.1%) of participants, followed by workers (18.9%), the unemployed (11.1%), migrant workers (9.3%), and business/service workers (5.8%). The predominant age group was ages 25–35 years (21.4%), followed by age 15–25 years (18.4%), age 45–55 years (15.0%), and age 35–45 years (13.9%). Ethnic Han participants were the most common ethnicity (97.9%). Of all the participants, 27.6% showed cavities in the chest X-ray examination results, and 41.9% were Mycobacterium tuberculosis-positive in the etiology test results.
Of all the newly diagnosed PTB patients included in our study, 38.7% (3,258/8,421) had at least one comorbidity. Approximately 40% of male participants and 35.9% of female participants reported having investigated comorbidities. The proportion of patients with comorbidities in the overall population of PTB patients increased with age. The highest proportion of comorbidities was observed in participants aged 75 years and older (54.6%), followed by the 65–75 years age group (53.2%), 55–65 years age group (49.8%), 45–55 years age group (45.8%), 35–45 years age group (33.6%), and 15–25 years age group (28.8%). The proportion of comorbidities in local provincial residences was 43.2 and 29.6% in participants with household registration outside the province. The proportions of comorbidities in retirees, farmers, workers, business and service workers were 56.1, 46.8, 28.4, 25.7, and 27.2%, respectively.
The predominant type of comorbidity was pleuritis (1,833, 21.8%), followed by DM (763, 9.1%), other extrapulmonary TB (421, 5%), tracheobronchial TB (275, 3.3%), and silicosis (160, 1.9%), which are shown in Figure 1. Among the 3,069 participants with only one type of comorbidity, 1,696 had pleuritis, 652 had DM, 351 had other extrapulmonary TB, 240 had tracheobronchial TB, and 130 had silicosis. Among the 189 participants with two or more comorbidities, 72 had both pleuritis and DM, 38 had both pleuritis and other extrapulmonary TB, 14 had both DM and other extrapulmonary TB, and 5 had three or more comorbidities.
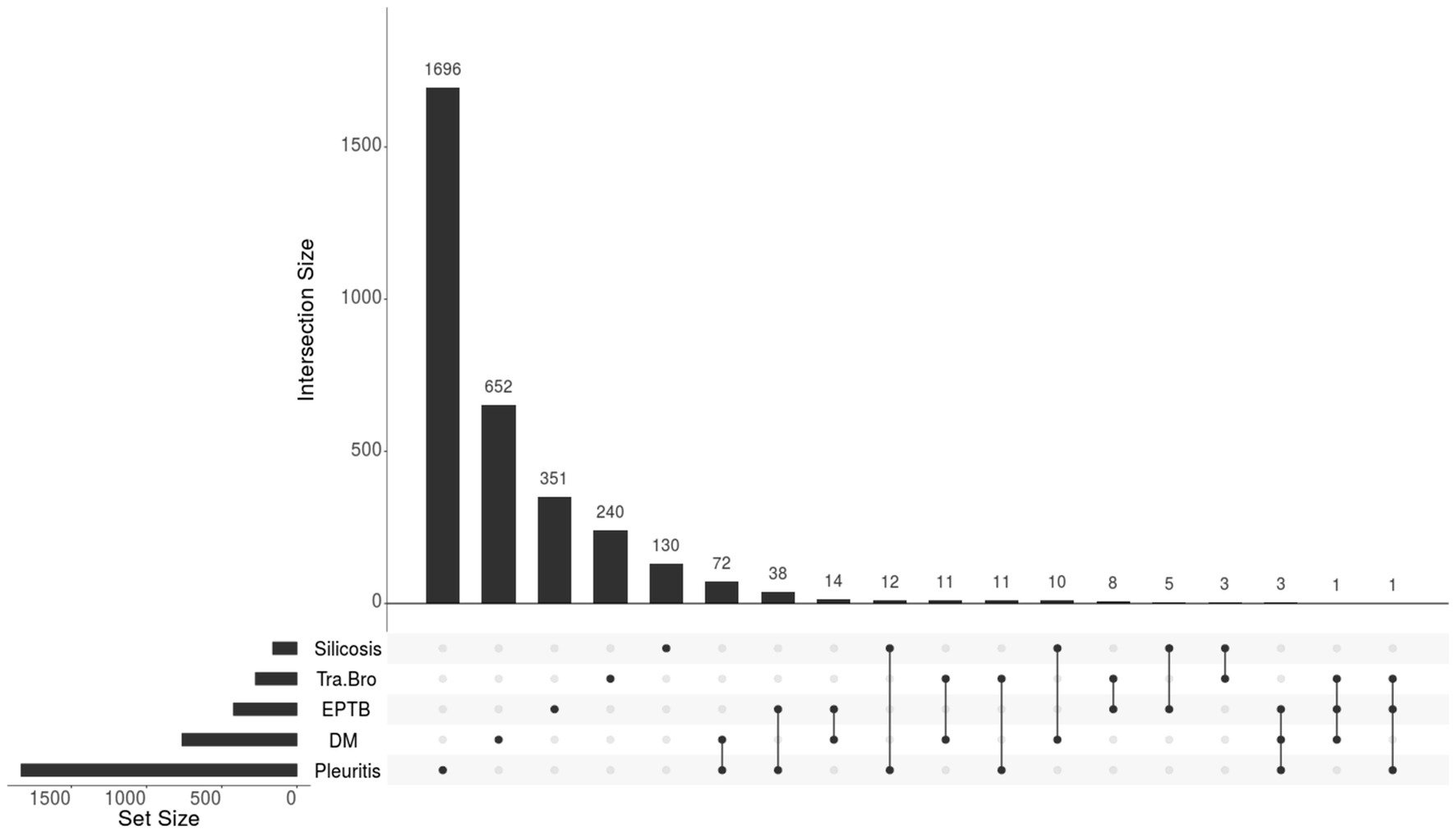
Figure 1. The composition of comorbidities in newly pulmonary tuberculosis patients (N = 3,258). Tra-Bro, Tracheobronchial tuberculosis; EPTB, Other Extrapulmonary Tuberculosis; DM, Diabetes Mellitus.
The clinical profiles of comorbidities in patients with newly diagnosed PTB are shown in Table 2. Of the 5,163 newly diagnosed TB patients without comorbidities in this study, 30.6% showed cavities in the chest X-ray examination results, which was higher than that of participants in the comorbidities group (22.7%). Participants with DM had the highest rate of chest cavities on X-ray (54.8%), followed by those with silicosis only (33.1%). In addition, a higher percentage of M. tuberculosis-positive etiology (45%) was observed in participants without comorbidities than in participants with comorbidities (37.1%). Among the 3,069 participants who had only one type of comorbidity, the proportion of M. tuberculosis-positive etiology was highest in participants with tracheobronchial TB (72.5%), followed by participants with DM (67.8%).
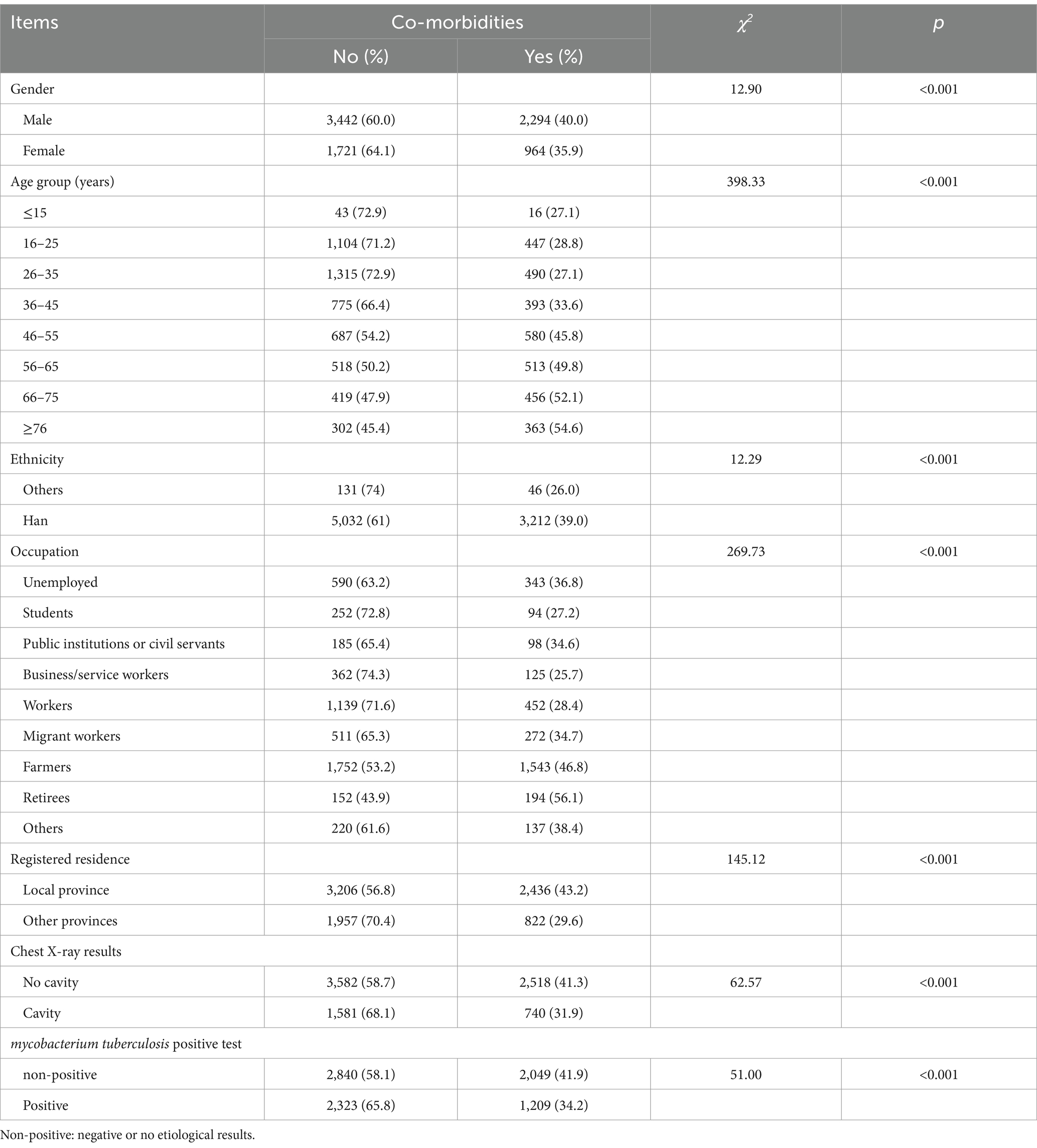
Table 2. The correlation between comorbidity and demographic characteristics of newly pulmonary tuberculosis patients.
The results of chi-square analysis showed that significant statistical difference of comorbidities was observed in different gender, age, ethnicity, occupation, household registration (Table 2), respectively. In multivariate logistic analysis, when compared to patients without comorbidities (Table 3), patients with DM (AOR: 2.88, 95% CI: 2.42–3.43) were at a higher risk for observed cavities in chest X-ray, while patients with pleuritis (AOR: 0.27, 95% CI: 0.23–0.32), other extrapulmonary TB (AOR: 0.48, 95% CI: 0.36–0.64), and tracheobronchial TB (AOR:0.56, 95% CI: 0.40–0.79) were at a lower risk for observed cavities in chest X-ray. In addition, with respect to the proportion of patients with M. tuberculosis-positive etiology, a higher risk was observed in patients with DM (AOR: 2.05, 95% CI: 1.72–2.45) and tracheobronchial TB (AOR: 3.22, 95%: 2.40–4.32), and a lower risk in patients with pleuritis (AOR: 0.25, 95% CI: 0.22–0.29) and extrapulmonary TB (AOR: 0.61, 95%: 0.48–0.76).
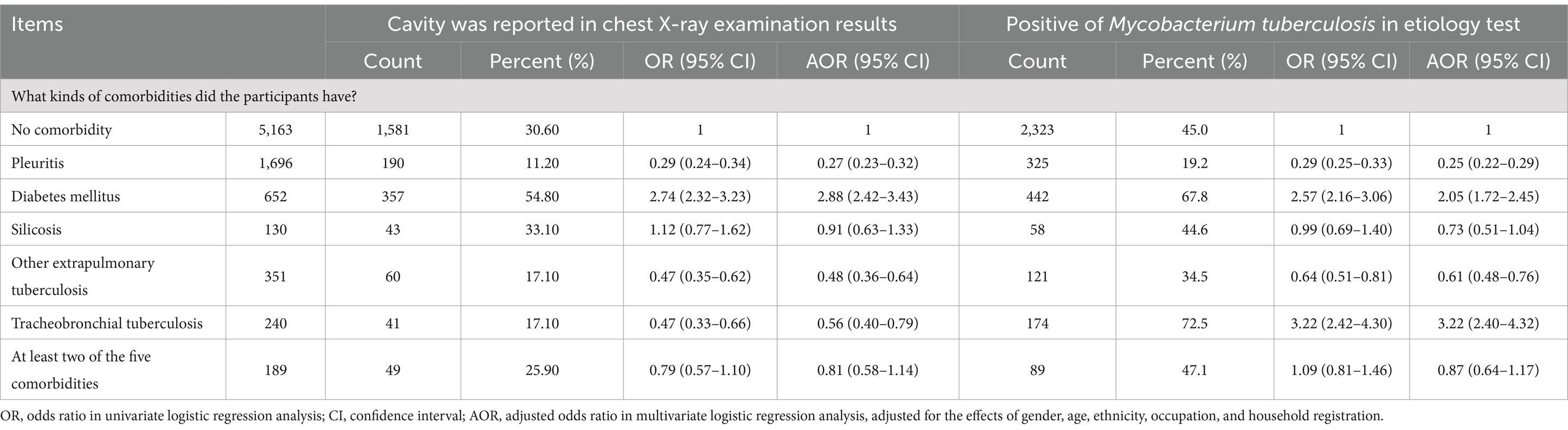
Table 3. The analysis of the influence of different co-morbidities on the clinical features of newly pulmonary tuberculosis.
This study investigated the prevalence of five comorbidities that may affect the treatment course of PTB. We found that the prevalence of the five comorbidities investigated was as high as 38.7% in patients with newly diagnosed PTB. Pleurisy and DM were the most common comorbidities. Compared to PTB patients without any comorbidities, the risk of pulmonary cavity in X-ray and positive in Mycobacterium tuberculosis etiology test was higher in PTB patients complicated with DM and lower in those complicated with pleurisy or other extrapulmonary TB.
PTB comorbidities are commonly reported in low- and middle-income countries, and can include non-communicable diseases (NCD), chronic communicable diseases, and mental disorders (24). These comorbid conditions pose a great challenge for both individual health and healthcare systems. In this study, comorbidities were observed in nearly 40% of all participants. Another study in China reported common comorbidities such as DM and hypertension among patients with PTB (17). In India, more than half of PTB patients reported multimorbidity, with depression, DM, acid peptic disease, and hypertension being the most common comorbidities (25). Among the five investigated comorbidities in our study, the predominant form of comorbidity was pleuritis, followed by DM, extrapulmonary TB, tracheobronchial TB, and silicosis. Additionally, pleuritis, and DM, pleuritis and other extrapulmonary TB, DM and other extrapulmonary TB were the most commonly clustering comorbidities. Multimorbidity was also observed in our study. The clustering of different comorbidities greatly increases the complexity of treatment and management and adversely affects health, economic, and mortality outcomes (6), threatening the capacity to intervene in a global TB epidemic. Evidence-based frameworks for integration and person-centered care, screening approaches, and effective interventions have been demonstrated to help control for TB comorbidities (26). Thus, integrating screening and management of these comorbidities within TB programs and addressing TB multimorbidity in policy and practice are essential to meet the End TB targets (12).
This study demonstrated that the proportion of patients with PTB comorbidities increased with age and was higher in male patients than in female patients. A study conducted in South Africa on NCD multimorbidity among TB patients in public primary care clinics also reported that the likelihood of multimorbidity was higher among older patients (14). In India and 48 other low- and middle-income countries, increasing age was also significantly associated with multimorbidity among patients with TB (8, 25). Studies have shown that older people are more susceptible to infections and other chronic diseases (27), which may be related to a decline in the immune system (28). A study in South Korea revealed an increasing prevalence of comorbidities among female TB patients with increasing age (29). Differences in diseases between male and female patients may be influenced by differences in sex hormones, sex-related genetic backgrounds, genetic regulation and metabolism, and differing living habits (30). This study also found that retirees and farmers have relatively high comorbidity rates. Generally, retirees are older, so similar results as those of older TB patients are understandable. Farmers are more likely to face socioeconomic deprivation (31), such as a lack of education, low income, overcrowding, and poor nutrition, which may increase the risk of disease. These findings acknowledge the need for the personalized management of TB, and evaluation of the risks for comorbidities should consider the sex, age, and occupation of the patient (6, 29).
TB pleurisy is a common form of extrapulmonary TB (32, 33) with exceptionally low sensitivity to M. tuberculosis. This study found that the risks of pulmonary cavity and etiological positivity were lower in participants with TB pleurisy or other types of extrapulmonary TB. A previous study showed that TB pleural effusion is mostly a hypersensitivity reaction (34) and is rarely positive for acid-fast bacilli staining. In addition, obtaining high-quality sputum samples from patients with TB pleurisy is difficult. Similar to the findings of this study, most studies have reported no specific lung or pleural radiological findings that can confirm TB pleurisy (34–36). Multiple factors make the timely diagnosis of TB pleurisy challenging. Therefore, more in-depth scientific research is required for the early detection of extrapulmonary TB, such as TB pleurisy.
DM has been considered one of the two most common NCDs to coexist with TB (6, 13). The prevalence of DM among TB patients in various low- and middle-income countries varies from 1.8 to 45% (37). In a systematic review and meta-analysis of the compiled data from 2.3 million people suffering from active TB, the global prevalence of DM among patients with TB was estimated to be 15% (13), which was slightly higher than the 9% found in this study. Heterogeneity may be the result of disparities in age, gender, region, level of income, and development between the study regions. The results of this study showed that the risk of pulmonary cavity disease and etiological positivity were higher in patients with pulmonary TB complicated by DM, which is consistent with previous studies in China (21, 22), Saudi Arabia (38), and Qatar (39). DM can lead to a greater risk of poor TB outcomes by weakening the immune system, resulting in worse clinical presentation, more symptoms, and death (37, 40). Similarly, better DM control in patients with TB could improve TB treatment success and reduce TB transmission, averting millions of TB cases and deaths (41). Accordingly, in 2011, the WHO declared a framework for the care and control of TB and DM (42), which recommends the collaborative care of TB-related DM.
This study has some limitations. First, only five diseases were investigated in this study at the time of the initial patient visit, which may have introduced differences in the number and type of comorbidities compared to those of other studies. Second, some of the participants’ comorbidities were self-reported based on their historical diagnosis, which may have been biased by recall. Thirdly, the results of this study may be partially overestimated due to the limitations of cross-sectional studies. Despite these limitations, this was a multicenter observational study covering 13 designated hospitals from 13 counties across Zhejiang province, China, with results that could reflect comorbidities among newly diagnosed PTB patients in eastern coastal China and provide valuable scientific insight to policy makers to optimize the prevention and control of TB comorbidities in the future.
The prevalence of the investigated comorbidities was high in patients with newly diagnosed PTB. Among the five comorbidities investigated, pleurisy and DM were the most common comorbidity. The risk of pulmonary cavity and etiological positivity was higher in patients with PTB and DM. Thus, an integration of screening and personalized management is needed in the control of TB and its comorbidities.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of the Zhejiang Provincial Center for Disease Control and Prevention. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
WW: Conceptualization, Writing – review & editing, Funding acquisition, Methodology, Writing – original draft, Data curation, Formal analysis, Investigation, Project administration. XW: Methodology, Writing – review & editing, Investigation, Project administration, Supervision. SC: Investigation, Project administration, Supervision, Writing – review & editing, Methodology. JL: Investigation, Project administration, Supervision, Writing – review & editing, Data curation, Methodology. QC: Investigation, Supervision, Writing – review & editing, Data curation, Methodology, Project administration. YZ: Investigation, Supervision, Data curation, Writing – review & editing, Methodology, Project administration. QW: Data curation, Investigation, Supervision, Writing – review & editing, Formal analysis, Methodology. KL: Investigation, Supervision, Data curation, Writing – review & editing, Formal analysis, Methodology, Software. XJ: Data curation, Investigation, Supervision, Writing – review & editing, Formal analysis, Methodology. BC: Conceptualization, Writing – review & editing, Funding acquisition, Data curation, Resources.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National-Zhejiang Health Commission Major S&T Project (Grant No. WKJZJ-2118), Zhejiang Provincial Medical and Health Project (2024KY886).
The authors would like to thank all the participants involved in the study and all the staff in 13 study sites.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lonnroth, K, and Raviglione, M. The WHO's new end TB strategy in the post-2015 era of the sustainable development goals. Trans R Soc Trop Med Hyg. (2016) 110:148–50. doi: 10.1093/trstmh/trv108
2. World Health Organization. Global Tuberculosis Report 2023. Geneva: World Health Organization (2023).
3. Houben, RM, and Dodd, PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. (2016) 13:e1002152. doi: 10.1371/journal.pmed.1002152
4. Uplekar, M, Weil, D, Lonnroth, K, Jaramillo, E, Lienhardt, C, Dias, HM, et al. WHO's new end TB strategy. Lancet. (2015) 385:1799–801. doi: 10.1016/S0140-6736(15)60570-0
5. Board, E. Global strategy and targets for tuberculosis prevention, care and control after 2015: report by the secretariat. Geneva: World Health Organization (2014).
6. Siddiqi, K, Stubbs, B, Lin, Y, Elsey, H, and Siddiqi, N. TB multimorbidity: a global health challenge demanding urgent attention. Int J Tuberc Lung Dis. (2021) 25:87–90. doi: 10.5588/ijtld.20.0751
7. Bates, M, Marais, BJ, and Zumla, A. Tuberculosis comorbidity with communicable and noncommunicable diseases. Cold Spring Harb Perspect Med. (2015) 5:a017889. doi: 10.1101/cshperspect.a017889
8. Stubbs, B, Siddiqi, K, Elsey, H, Siddiqi, N, Ma, R, Romano, E, et al. Tuberculosis and non-communicable disease multimorbidity: an analysis of the world health survey in 48 low- and middle-income countries. Int J Environ Res Public Health. (2021) 18:2439. doi: 10.3390/ijerph18052439
9. Salisbury, C. Multimorbidity: redesigning health care for people who use it. Lancet. (2012) 380:7–9. doi: 10.1016/S0140-6736(12)60482-6
10. Fortin, M, Lapointe, L, Hudon, C, Vanasse, A, Ntetu, AL, and Maltais, D. Multimorbidity and quality of life in primary care: a systematic review. Health Qual Life Outcomes. (2004) 2:51. doi: 10.1186/1477-7525-2-51
11. Chen, Q, Che, Y, Xiao, Y, Jiang, F, Chen, Y, Zhou, J, et al. Impact of multimorbidity subgroups on the health care use and clinical outcomes of patients with tuberculosis: a population-based cohort analysis. Front Public Health. (2021) 9:756717. doi: 10.3389/fpubh.2021.756717
12. Jarde, A, Siqueira, N, Afaq, S, Naz, F, Irfan, M, Tufail, P, et al. Addressing TB multimorbidity in policy and practice: an exploratory survey of TB providers in 27 high-TB burden countries. PLOS Glob Public Health. (2022) 2:e0001205. doi: 10.1371/journal.pgph.0001205
13. Noubiap, JJ, Nansseu, JR, Nyaga, UF, Nkeck, JR, Endomba, FT, Kaze, AD, et al. Global prevalence of diabetes in active tuberculosis: a systematic review and meta-analysis of data from 2·3 million patients with tuberculosis. Lancet Glob Health. (2019) 7:e448–60. doi: 10.1016/S2214-109X(18)30487-X
14. Peltzer, K. Tuberculosis non-communicable disease comorbidity and multimorbidity in public primary care patients in South Africa. Afr J Prim Health Care Fam Med. (2018) 10:e1–6. doi: 10.4102/phcfm.v10i1.1651
15. Jamshidi, P, Danaei, B, Arbabi, M, Mohammadzadeh, B, Khelghati, F, Akbari Aghababa, A, et al. Silicosis and tuberculosis: a systematic review and meta-analysis. Pulmonology. (2023) 31:2416791–2. doi: 10.1016/j.pulmoe.2023.05.001
16. Farazi, A, and Jabbariasl, M. Silico-tuberculosis and associated risk factors in central province of Iran. Pan Afr Med J. (2015) 20:333. doi: 10.11604/pamj.2015.20.333.4993
17. Liu, Y, Lin, Y, Sun, Y, Thekkur, P, Cheng, C, Li, Y, et al. Managing comorbidities, determinants and disability at start and end of TB treatment under routine program conditions in China. Trop Med Infect Dis. (2023) 8:341. doi: 10.3390/tropicalmed8070341
18. Yang, Q, Lin, M, He, Z, Liu, X, Xu, Y, Wu, J, et al. Mycobacterium tuberculosis infection among 1,659 silicosis patients in Zhejiang Province, China. Microbiol Spectr. (2022) 10:e01451–22. doi: 10.1128/spectrum.01451-22
19. Zhang, S, Tong, X, Wang, L, Zhang, T, Huang, J, Wang, D, et al. Clinical characteristics and prognostic analysis of patients with pulmonary tuberculosis and type 2 diabetes comorbidity in China: a retrospective analysis. Front Public Health. (2021) 9:710981. doi: 10.3389/fpubh.2021.710981
20. Ling, Y, Chen, X, Zhou, M, Zhang, M, Luo, D, Wang, W, et al. The effect of diabetes mellitus on tuberculosis in eastern China: a decision-tree analysis based on a real-world study. J Diabetes. (2023) 15:920–30. doi: 10.1111/1753-0407.13444
21. Wu, Z, Guo, J, Huang, Y, Cai, E, Zhang, X, Pan, Q, et al. Diabetes mellitus in patients with pulmonary tuberculosis in an aging population in Shanghai, China: prevalence, clinical characteristics and outcomes. J Diabetes Complicat. (2016) 30:237–41. doi: 10.1016/j.jdiacomp.2015.11.014
22. Leung, CC, Yew, WW, Mok, TYW, Lau, KS, Wong, CF, Chau, CH, et al. Effects of diabetes mellitus on the clinical presentation and treatment response in tuberculosis. Respirology. (2017) 22:1225–32. doi: 10.1111/resp.13017
23. Conway, JR, Lex, A, and Gehlenborg, N. UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics. (2017) 33:2938–40. doi: 10.1093/bioinformatics/btx364
24. Jarde, A, Romano, E, Afaq, S, Elsony, A, Lin, Y, Huque, R, et al. Prevalence and risks of tuberculosis multimorbidity in low-income and middle-income countries: a meta-review. BMJ Open. (2022) 12:e060906. doi: 10.1136/bmjopen-2022-060906
25. Chauhan, A, Parmar, M, Rajesham, JD, Shukla, S, Sahoo, KC, Chauhan, S, et al. Landscaping tuberculosis multimorbidity: findings from a cross-sectional study in India. BMC Public Health. (2024) 24:453. doi: 10.1186/s12889-024-17828-z
26. Byrne, AL, Marais, BJ, Mitnick, CD, Garden, FL, Lecca, L, Contreras, C, et al. Feasibility and yield of screening for non-communicable diseases among treated tuberculosis patients in Peru. Int J Tuberc Lung Dis. (2018) 22:86–92. doi: 10.5588/ijtld.17.0381
27. Gavazzi, G, Herrmann, F, and Krause, K-H. Aging and infectious diseases in the developing world. Clin Infect Dis. (2004) 39:83–91. doi: 10.1086/421559
28. Castelo-Branco, C, and Soveral, I. The immune system and aging: a review. Gynecol Endocrinol. (2014) 30:16–22. doi: 10.3109/09513590.2013.852531
29. Moon, D, Jeong, D, Kang, YA, and Choi, H. Gender differences in tuberculosis patients with comorbidity: a cross-sectional study using national surveillance data and national health insurance claims data in South Korea. PLoS One. (2023) 18:e0280678. doi: 10.1371/journal.pone.0280678
31. Duarte, R, Lönnroth, K, Carvalho, C, Lima, F, Carvalho, ACC, Muñoz-Torrico, M, et al. Tuberculosis, social determinants and co-morbidities (including HIV). Pulmonology. (2018) 24:115–9. doi: 10.1016/j.rppnen.2017.11.003
32. Shaw, JA, Diacon, AH, and Koegelenberg, CFN. Tuberculous pleural effusion. Respirology. (2019) 24:962–71. doi: 10.1111/resp.13673
33. McNally, E, Ross, C, and Gleeson, LE. The tuberculous pleural effusion. Breathe. (2023) 19:230143. doi: 10.1183/20734735.0143-2023
34. Cascio, CML, Kaul, V, Dhooria, S, Agrawal, A, and Chaddha, U. Diagnosis of tuberculous pleural effusions: a review. Respir Med. (2021) 188:106607. doi: 10.1016/j.rmed.2021.106607
35. Andreu, J, Cáceres, J, Pallisa, E, and Martinez-Rodriguez, M. Radiological manifestations of pulmonary tuberculosis. Eur J Radiol. (2004) 51:139–49. doi: 10.1016/j.ejrad.2004.03.009
36. Valdés, L, Alvarez, D, San José, E, Penela, P, Valle, JM, García-Pazos, JM, et al. Tuberculous pleurisy: a study of 254 patients. Arch Intern Med. (1998) 158:2017–21. doi: 10.1001/archinte.158.18.2017
37. McMurry, HS, Mendenhall, E, Rajendrakumar, A, Nambiar, L, Satyanarayana, S, and Shivashankar, R. Coprevalence of type 2 diabetes mellitus and tuberculosis in low-income and middle-income countries: a systematic review. Diabetes Metab Res Rev. (2019) 35:e3066. doi: 10.1002/dmrr.3066
38. Abd El-Hamid El-Kady, R, and Abdulrahman, TS. The footprint of diabetes mellitus on the characteristics and response to anti-tuberculous therapy in patients with pulmonary tuberculosis from Saudi Arabia. Infect Drug Resist. (2021) 14:5303–12. doi: 10.2147/IDR.S344703
39. Dousa, KM, Hamad, A, Albirair, M, Al Soub, H, Elzouki, A-N, Alwakeel, MI, et al. Impact of diabetes mellitus on the presentation and response to treatment of adults with pulmonary tuberculosis in Qatar. Open Forum Infect Dis. (2018) 6:ofy335. doi: 10.1093/ofid/ofy335
40. Restrepo, BI, and Schlesinger, LS. Impact of diabetes on the natural history of tuberculosis. Diabetes Res Clin Pract. (2014) 106:191–9. doi: 10.1016/j.diabres.2014.06.011
41. Pan, S-C, Ku, C-C, Kao, D, Ezzati, M, Fang, C-T, and Lin, H-H. Effect of diabetes on tuberculosis control in 13 countries with high tuberculosis: a modelling study. Lancet Diabetes Endocrinol. (2015) 3:323–30. doi: 10.1016/S2213-8587(15)00042-X
Keywords: pulmonary tuberculosis, comorbidity, prevalence, tuberculosis, observational study
Citation: Wang W, Wang X, Chen S, Li J, Cheng Q, Zhang Y, Wu Q, Liu K, Jiang X and Chen B (2025) Prevalence and clinical profile of comorbidity among newly diagnosed pulmonary tuberculosis patients: a multi-center observational study in eastern China. Front. Med. 12:1446835. doi: 10.3389/fmed.2025.1446835
Received: 12 June 2024; Accepted: 02 January 2025;
Published: 28 January 2025.
Edited by:
Felix Khuluza, Kamuzu University of Health Sciences (Formerly College of Medicine-University of Malawi), MalawiReviewed by:
George Jó Bezerra Sousa, Ministry of Health, BrazilCopyright © 2025 Wang, Wang, Chen, Li, Cheng, Zhang, Wu, Liu, Jiang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Chen, YmNoZW5AY2RjLnpqLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.