
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 07 March 2025
Sec. Hepatobiliary Diseases
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1423780
This article is part of the Research TopicInnovative Multidisciplinary Insights into the Gut-Liver Axis and CancerView all 5 articles
Transjugular intrahepatic portosystemic shunt (TIPS) placement alleviates portal hypertension symptoms. Hepatic encephalopathy (HE) is a common complication of TIPS, impacting patient quality of life and the healthcare burden. Post-TIPS HE is associated with portosystemic shunting, elevated blood ammonia levels, and inflammation. Increasing attention has been given to the liver and intestinal circulation in recent years. An imbalance in intestinal microecology plays a role in the occurrence of HE and may be a new target for treatment. This review discusses the causes, diagnosis, and treatment strategies for post-TIPS HE and focuses on exploring treatment strategies and their relationships with the gut microbiota, suggesting an innovative approach to address this complication.
Transjugular intrahepatic portal system shunt (TIPS) is one of the main methods used to reduce portal vein pressure and works by establishing a new channel between the portal vein and hepatic vein, which can quickly reduce portal vein pressure, achieve hemostasis, and relieve ascites (1–4). Compared with abdominal paracentesis, TIPS significantly enhances transplant-free survival in cirrhosis patients suffering from refractory ascites. It also diminishes the likelihood of recurrent ascites and hepatorenal syndrome. However, TIPS is associated with an increased risk of developing hepatic encephalopathy (HE) (5). The gut–liver axis has emerged as a focal point in chronic liver disorders, prompting more research into the role of the gut microbiota in liver cirrhosis (6). In individuals with liver cirrhosis, changes in the structure and function of the gut microbiota are closely tied to clinical prognosis (7–9). The gut microbiota is closely related to the occurrence of HE (10–13). At the same time, changes in portal pressure can also affect the gut microbiota (14–17). Intestinal congestion in patients with portal hypertension not only affects the absorption of nutrients but also leads to local inflammatory reactions, damages the intestinal barrier function, affects the intestinal microenvironment, and thus affects the composition of the intestinal microbiota. TIPS can improve the intestinal microenvironment to a certain extent by reducing portal pressure, reducing intestinal congestion, alleviating local inflammation, and repairing the intestinal barrier. Studies have shown that the better the intestinal microbiota recovers after TIPS, the lower the incidence of HE (18).
HE is a common complication after TIPS that affects the quality of life of patients and their families. Therefore, this review aims to summarize the relevant risk factors for HE occurrence after TIPS, the role of the gut microbiota in diagnosing HE and predicting patient prognosis, and prevention and treatment strategies.
HE is a complex neuropsychiatric syndrome caused by acute and chronic liver dysfunction or portal-systemic shunt abnormalities characterized by metabolic disorders with varying degrees of severity (19). In general, HE can be divided into three types depending on the type of liver disease: type A (caused by acute liver failure), type B (associated with portocaval shunt), and type C (associated with chronic liver injury such as cirrhosis with the presence of portosystemic shunts) (19). The West Haven HE classification standard (0–4 levels) (20) has been widely utilized. This classification system includes non-HE, minimal hepatic encephalopathy (MHE), HE Level 1, and HE Levels 2–4. The first three grades are collectively referred to as covert hepatic encephalopathy (CHE). Patients with CHE have only mild cognitive difficulties such as decreased attention, memory, and delayed responses. HE Levels 2–4 are collectively referred to as overt hepatic encephalopathy (OHE). Patients with OHE may experience personality changes, comas, or other neurological abnormalities. The diagnosis of HE relies mainly on symptoms and neuropsychological tests, while excluding altered mental status caused by other reasons. MHE can affect the clinical prognosis and may progress to OHE (21). MHE may persist even after recovery (22). In addition to increasing falls, fractures, and traffic accidents, HE can also increase the length of hospital stays, rate of rehospitalization (21), and mortality (23, 24), affecting patient prognosis (25) and the quality of life of patients’ families (26) and leading to an increasing healthcare burden (27).
Transjugular intrahepatic portosystemic shunt (TIPS) placement is one of the main methods used to treat portal hypertension. However, HE is one of the most frequent postoperative complications after TIPS (28). The incidence of HE after TIPS varies due to factors such as previous history of HE, puncture site, and follow-up time with an average incidence of 20–50% (29–31). In a retrospective study of 75 patients with no previous episodes of HE, the incidence of HE at 6 months after TIPS was 36% (30), whereas it was 27% at 12 months. A study involving 82 patients with liver cirrhosis with previous HE reported that the incidence of OHE after TIPS at 6 months was 43% (29). Among patients who underwent right portal TIPS within 1 year, 46.7% had HE, whereas 26.6% of patients who underwent left portal TIPS had HE (32). HE after TIPS is related to the establishment of a shunt channel between the portal and central veins during surgery, which limits the ability of the liver to detoxify intestinal toxins. When the toxin crosses the blood–brain barrier, they lead to impaired brain function, resulting in new or exacerbations of existing HE. Many related factors may affect the occurrence of post-TIPS HE (Figure 1).
Age (sHR 1.05, CI 1.02–1.08, p = 0.002), Child-Pugh score (sHR 1.29, CI 1.06–1.56, p = 0.01), and CHE (sHR 3.16, CI 1.43–6.99, p = 0.004) have been reported to be associated with post-TIPS HE (29). OHE after TIPS developed significantly more frequently in patients with a history of OHE or MHE (33). A meta-analysis (34) also revealed that patients with HE before TIPS or a higher Child-Pugh class or score had an increased risk of post-TIPS HE. A recent study revealed (35) that liver function deteriorated initially after TIPS, followed by recovery. Recovery of liver function at 3 months was associated with reduced OHE. Nutritional status is related to liver function, and sarcopenia can directly reflect nutritional status, while muscles participate in ammonia detoxification. Sarcopenia is a common complication of liver cirrhosis, is related to the severity of liver disease, and increases the incidence of other liver disease complications, including HE (36). In a prospective study (37) of 46 patients with cirrhosis, sarcopenia (sHR 31.3, CI 4.5–218.07, p < 0.001) was independently associated with the development of HE after TIPS. The mechanism underlying the relationship between sarcopenia and HE may be related to decreased ammonia detoxification secondary to sarcopenia. Because muscles are another important site for ammonia metabolism, creating glutamine through glutamine synthase, muscle loss may lead to a decrease in the ability to clear ammonia in the body, thereby increasing the risk of HE (38). There is a complex interaction between sarcopenia and HE (39), so it is not clear which is the cause and which is the effect of sarcopenia and HE. Previous studies have suggested that sustained high blood ammonia levels may increase the levels of muscle growth inhibitors while stimulating autophagy, leading to muscle protein synthesis disruption and muscle atrophy (40, 41). However, a recent meta-analysis (36) revealed via multivariate analysis that HE did not increase the risk of sarcopenia (2.14, 95% CI 0.56–8.16) (I2 = 62%, p = 0.07).
Dilutional hyponatremia commonly develops in patients with advanced cirrhosis and portal hypertension when patients have insufficient oral sodium intake and excessive use of diuretics, causing brain edema and a series of neurological manifestations. One study aimed to assess the effect of hyponatremia on the development of OHE within 1 week of TIPS and reported that the odds ratio for developing HE with hyponatremia (pre-TIPS Na <135 mEq/L) was 8.6 (42). Proton pump inhibitors (PPIs) are commonly used to alleviate gastric acid discomfort in patients and reduce the risk of bleeding. However, PPI lowers the pH of the gastrointestinal tract by inhibiting gastric acid secretion. While this may lead to more ammonia being absorbed into the bloodstream through the intestine, it weakens the gastric acid barrier, causing bacterial translocation and affecting the composition of the gut microbiota. This is beneficial for the growth of urease-producing bacteria, thus increasing the risk of developing HE (43, 44). Research has shown that patients who receive PPI treatment during TIPS exhibit a significantly greater incidence of post-TIPS HE than patients without PPI treatment do (30.4% vs. 11.7%, p < 0.001). The incidence of HE after TIPS increased in a dose-dependently manner with the use of PPI. Patients administered 40 mg PPIS daily presented a notably greater incidence of post-TIPS HE than those on a 20 mg PPI daily dose (30.9% vs. 10.0%, p = 0.028) (45).
With respect to stent diameter, a randomized controlled trial (46) revealed that the incidence of HE was similar in groups with an 8 mm and 10 mm stent diameter. However, a meta-analysis (47) aimed at exploring the optimal diameter of TIPS suggested that for Asians, the use of a stent with a diameter of 8 mm was beneficial for reducing the incidence of postoperative HE (OR = 0.49, CI 0.27–0.87, p = 0.02). Another meta-analysis revealed that (48) the rate of postoperative HE was significantly lower in the group with the puncture site in the left portal vein group than in the group with the site in the right portal vein (5.7% vs. 18.1%, OR 0.19, p < 0.00001). The blood flow inside the stent corresponds to the degree of decrease in the portosystemic gradient (PSG), and the greater the decrease in the PSG before and after TIPS, the greater the degree of diversion into the body is. The greater the venous blood flow is, the greater the likelihood of developing HE (49). According to reports, TIPS reducing the hepatic venous pressure gradient by >9–10 mmHg or > 60% increases the risk of HE after TIPS. A study conducted in 1280 patients who underwent TIPS due to refractory ascites or variceal bleeding revealed that a one-third reduction in PSG can reduce the risk of HE and liver function damage and achieve good clinical results (50).
Most patients undergoing TIPS treatment have a foundation for liver disease, such as cirrhosis. On the one hand, the efficiency of ammonia metabolism by damaged liver cells is reduced; on the other hand, due to the placement of the stent, the amount of ammonia entering the brain tissue further increases, saturating the glutamine metabolism pathway (51–53). Excessive glutamine can damage the morphology and function of astrocytes, thereby affecting the integrity of the blood–brain barrier. Modified astrocytic function ultimately results in disrupted neuroglial interactions and an imbalance within the neurotransmitter system. This disruption affects synaptic plasticity and the functioning of cerebral oscillatory networks, creating a pathological setting that is indicative of HE (54, 55). The possible mechanism is that excessive glutamine is transported to the mitochondria and metabolized by glutaminase into glutamate and ammonia. Excess ammonia interferes with normal mitochondrial function, producing excessive reactive oxygen species and reactive nitrogen and inducing mitochondrial permeability transition (56, 57).
The ammonia toxicity theory is one of the main mechanisms of HE, and the ammonia produced by the intestinal flora is a key driving factor for HE (58). With the development of technologies such as metagenomics and a deeper understanding of the intestinal microbiota, increasing attention has been given to the role of the microbiota, inflammation, and metabolic pathways in the pathogenesis of HE, providing a new perspective for treatment (11, 59).
The intestinal microecology is an ecosystem formed by the interaction between intestinal microorganisms and the human body. The balance of the intestinal microecology depends on the stable intestinal flora, normal intestinal mucosal barrier, and normal operation of related lymphatic tissues. The intestinal microbiota comprises bacteria, fungi, viruses, and protozoa. The intestine and liver are interconnected and interact through the biliary tract, portal vein, and systemic circulation, which is known as the intestine–liver axis (60). On the one hand, when liver function is impaired, bile acid synthesis is reduced, and the intestinal tract is more susceptible to competitive colonization by bacteria. Reduced albumin synthesis and portal hypertension lead to intestinal edema, resulting in excessive bacterial growth and damage to the intestinal mucosal barrier. On the other hand, when the intestinal microbiota is dysfunctional, the immune system can affect the degree of liver steatosis, inflammation, and fibrosis. As observed in non-alcoholic fatty liver disease, the abundance of bacteria in feces is independently associated with non-alcoholic steatohepatitis, and the abundance of Ruminococcus is independently associated with significant liver fibrosis (F ≥ 2) (61). The intestinal microbiota is closely linked to liver diseases (62). An increasing number of studies have reported different microbiota phenotypes in various liver diseases (60, 63, 64). Understanding the intestinal liver axis has led to the development of new diagnostic, prognostic, and therapeutic methods for liver diseases.
The occurrence of HE is closely related to disturbance of intestinal microbiota (10). Bajaj et al. (65) found that patients with HE had lower Roseburia and higher Enterococcus, Veillonella, Megasphaera, and Burkholderia abundances in the mucosal microbiome than non-HE patients did. The abundance of butyrate-producing bacteria increased in the intestinal mucosa of patients with cirrhosis without HE. Moreover, autochthonous genera (Blautia, Faecalibacterium, Roseburia, and Dorea) were associated with good cognition and decreased inflammation in patients both with and without HE, whereas Enterococcus, Megasphaera, and Burkholderia were associated with poor cognition and inflammation (65). However, there was no significant difference in the fecal microbiota between patients with cirrhosis with and without HE, suggesting that the fecal microbiota may have a smaller impact on immunity and overall health than the intestinal mucosal microbiota does. Another study (66) comparing the biological differences in stool microflora between patients with cirrhosis with and without MHE reported that the abundance of salivary streptococci in patients with MHE was significantly greater than that in patients without MHE (p = 0.030), and the change in the number of these bacteria was positively correlated with ammonia accumulation (R = 0.58, p = 0.003). Some studies found that Saboo et al. (67) have shown that the function and composition of the intestinal microbiota in cirrhosis patients with HE are related to sex. As the disease progresses in male patients with liver cirrhosis, the flora related to hormone metabolism changes, and the microbial composition is similar to that in female patients. In general (68), patients with liver cirrhosis have an imbalance in the intestinal flora, which is manifested mainly by an increase in bacteria that promote inflammation and ammonia production. As our understanding of the liver gut–brain axis increased, an increasing number of microbiota have been identified as contributing to HE.
The establishment of portal-systemic shunt channels reduces the detoxification effect of the liver against intestinal endotoxins, which may increase the incidence of HE after TIPS. It also improves portal hypertension, which can affect the composition of the intestinal microbiota to a certain extent and improve the intestinal microecology (14). The specific mechanism of post-TIPS HE, as well as whether the change in the intestinal flora is a concomitant phenomenon of HE or is involved in the occurrence of HE, remains to be further explored. There is an interaction between portal hypertension and the intestinal flora. On the one hand, portal hypertension can cause intestinal congestion, damage the intestinal barrier, and lead to intestinal flora translocation. On the other hand, flora translocation can also increase liver inflammation, damage liver function, and further increase portal vein pressure (14, 16), which can increase the occurrence of adverse events such as HE.
One study (69) divided patients into three groups (non-HE, MHE, and OHE) according to prognosis after TIPS to investigate the changes in the gut microbiota after TIPS in patients with MHE. After TIPS, the non-HE group presented significant increases in the native flora Dialister, Coprococcus, Ruminococcaceae_uncultured, Flavonifractor, and Clostridium_sensu_stricto_1, whereas the MHE group presented significant reductions in the abundance of the harmful flora Granulicatella, Enterococcus, Streptococcus, and Rothia and significant increases in the abundance of Veillonella and Megasphaera after TIPS, whereas the OHE group presented a significant increase in the abundance of Veillonella only after surgery. There was a significant difference in the changes in the gut microbiota after TIPS between patients with different prognoses. The increase in the abundance of native flora may influence the remission of MHE. Another study (18) aimed to evaluate alterations in the microbiota after TIPS and the relationship between these changes and HE. After TIPS, the autochthonous taxa increased, whereas the potential pathogenic taxa decreased in the non-HE group, and the autochthonous taxon Lachnospiraceae decreased in the HE group. The variations in five autochthonous taxa, namely, Coprococcus, Ruminococcus, Blautia, Ruminococcaceae_uncultured, and Roseburia, were negatively correlated with the severity of HE. The gut microbiota could be a promising potential biological target for screening suitable patients receiving TIPS and prevention and for the treatment of post-TIPS HE. Hong-Wei Zhao (70) reported that the abundance of gut microbiota at the phylum level did not differ between the HE group and the non-HE group after TIPS. However, the abundances of Haemophilus and Eggerthella increased, whereas those of Anaerostipes, Dialister, Butyricicoccus, and Oscillospira decreased in the HE group. The abundances of Eggerthella, Streptococcus, and Bilophila increased, whereas those of Roseburia and Ruminococcus decreased in the non-HE group, and the pathogenic genus Morganella appeared in the HE group but not in the non-HE group.
To date, few studies have focused on metabolic changes after TIPS. A recent Italian study (71) evaluated whether TIPS placement modified the gut microbiota composition and metabolic function. The results revealed that (71) abundance of Flavonifractor spp. increased (p = 0.049) after TIPS and the abundance of Clostridiaceae decreased (p = 0.024). No differences were detected in the short-chain fatty acid signature, whereas analysis of medium-chain fatty acid (MCFA) profiles revealed a decreased abundance of proinflammatory isohexanoic (p < 0.01), 2-ethylhexanoic (p < 0.01), and octanoic (p < 0.01) acids after TIPS. Correction of portal hypertension following TIPS resulted in modifications of the gut microbiota composition, which could be beneficial and reduce the levels of fecal proinflammatory MCFA. A non-targeted metabolomics study conducted in 22 patients with cirrhosis who underwent TIPS revealed that the placement of TIPS stents affects metabolomic changes, indicating that low levels of bile acids in peripheral blood after TIPS are associated with an increase in the severity of HE (72). Another study revealed that peptides, amino acids, and lipid metabolites significantly increased among the early postoperative metabolites of TIPS, mainly enriched in the pathway of amino acid metabolism. The most significant metabolite consumed was the lipid metabolite. Moreover, it was found that 9 portal vein metabolites had good predictive value in predicting liver function decline after TIPS, and 12 portal vein metabolites had moderate classification performance in predicting HE grade (73).
Changes in the intestinal microecology after TIPS may be related to decreased portal vein pressure and reduced intestinal congestion. Therefore, the intestinal flora before TIPS may be related to patient survival, and the degree of recovery of the intestinal flora after TIPS may be related to the incidence and severity of HE. Previous studies have shown that (74) the fecal flora is associated with the onset of HE in patients with liver cirrhosis and that the relative abundance of individual flora is associated with HE recurrence and overall survival during follow-up. Therefore, the intestinal microbiota may become a new marker for predicting HE after TIPS, but there is still little research on this topic. The role of the gut microbiota in the diagnosis and prediction of HE is shown in Table 1.
Disturbances in the gut microbiota, excessive bacterial growth in the small intestine, and changes in the intestinal barrier can lead to bacterial translocation and further systemic inflammation (75), which is also a major driver of liver cirrhosis-related immune dysfunction (76). Inflammatory reactions play an important role in HE development. Previous studies revealed that the level of endotoxin in the portal vein was the highest in patients with liver disease (77, 78), which indicated that the main source was the intestine and that changes in intestinal bacteria and intestinal permeability can affect the level of endotoxin (79). The reticuloendothelial system (RES) (79, 80) is the main defense system against bacteremia and other infections acquired through blood-borne pathways, with Kupffer cells as the main component. The RES is damaged during liver cirrhosis, and blood bypasses the RES during the portosystemic shunt. Therefore, endotoxemia may worsen after TIPS, which may increase the incidence of HE. One study (81) tested the hypothesis that TIPS exacerbates endotoxemia and reported that 1 h after TIPS, the level of endotoxin in peripheral blood and the brain flux of ammonia increased, but there was no significant change in arterial blood ammonia. Another study (82) also indicated that TIPS can weaken the clearance of endotoxins in the liver after TIPS. However, one study (83) reported that the level of endotoxin in peripheral blood did not increase after TIPS, and it was considered that endotoxemia was due to decreased liver cell function rather than blood shunting. Another study (84) found that patients with alcoholic liver cirrhosis who were on TIPS had similar endotoxins in the portal and hepatic veins during TIPS and decreased portal pressure in 2 weeks after TIPS. No change in endotoxin levels in the portal and hepatic veins indicates that the liver itself may have only cleared a small amount of endotoxin. In addition, one early study reported that (85), after TIPS, the levels of lipopolysaccharide and endothelin in the portal vein are significantly reduced, which can reduce the occurrence of complications, such as endotoxemia caused by intestinal bacterial ectopia.
The differences in the results of the above studies may be due to differences in measurement methods, study time, and patient disease severity, but they also suggests that HE after TIPS may be related not only to portosystemic shunt after stent implantation but also to the intestinal microbiota. Interestingly, hepatic coma and hyperammonemia occur in germ-free animals (86). It was found (87) that hyperammonemia after TIPS may be caused to a large extent by the metabolism of small intestinal cells and has little relationship with intestinal bacteria. Compared with parenteral infusion, enteral infusion had a greater portal ammonia load (29 (21–36) vs. 14 (8–21) mmol/L/240 min) and a higher degree of systemic hyperammonemia (14 (11–17) vs. 9 (6–12) mmol/L/240 min). The small intestinal mucosa extracts glutamine from arterial blood for metabolism by enterocytes and releases considerable quantities of ammonia into the portal vein. Following TIPS, small intestinal ammonia production may lead to a higher degree of systemic hyperammonemia when nutrition is provided via the enteral route rather than the parenteral route. Hence, parenteral nutrition may be superior to enteral nutrition in patients after TIPS because of lower ammonia levels.
Inflammatory changes can not only manifest as endotoxemia but also activate neuroglial cells, induce neuroinflammation, lead to the activation of microglia and a subsequent neuroinflammatory response (88), and cause parenchymal changes in the brain and neurological dysfunction (89). Studies in mice (90) revealed that administration of azoxymethane in mice can reduce the level of IL-6 in plasma and brain tissue, the activation of microglia, and peripheral and cerebral inflammation. Therefore, neuroinflammatory changes may be involved in the occurrence of HE.
In addition to the correlations among the intestinal microbiota, inflammation, and HE, metabolites of the intestinal microbiota such as short-chain fatty acids (91) (mainly butyrate, propionate, and acetate), ethanol, bile acids, and choline also affect the metabolism of fat and glucose to varying degrees, affecting the pathophysiology of liver diseases (92) and may also participate in the occurrence of HE (13). For example, bile acids can induce antimicrobial peptides by binding to farnesol X receptors, thereby inhibiting excessive growth of the intestinal microbiota (93, 94). Circulating levels of butyrate were inversely related to portal hypertension, endotoxemia, and systemic inflammation in patients with cirrhosis (95). The relationship between the intestinal microbiota and post-TIPS HE is shown in Figure 2.
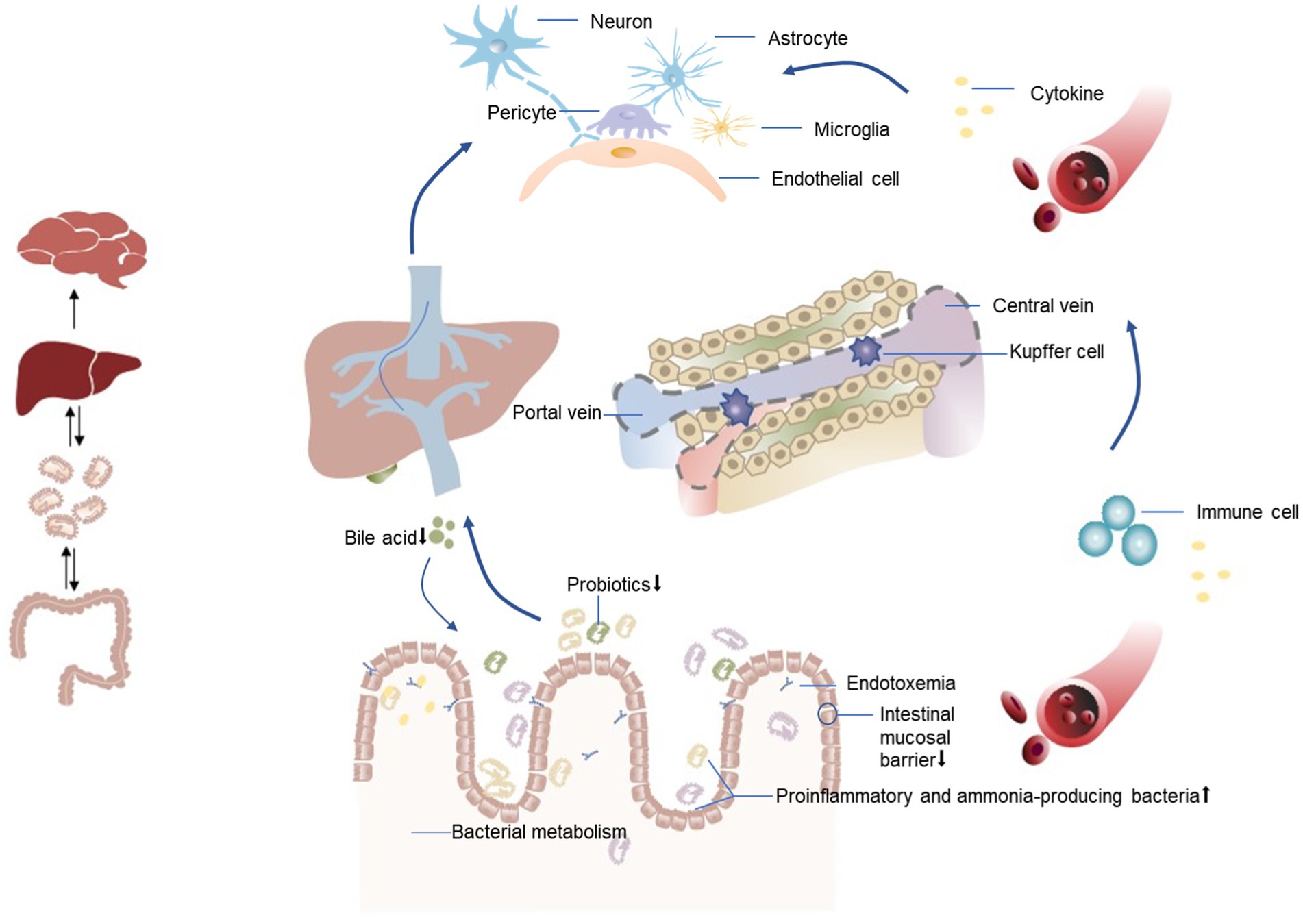
Figure 2. Relationship between the intestinal microbiota and HE after TIPS. An altered gut microbiota in patients with liver disease manifests as a decrease in beneficial bacteria and an increase in harmful bacteria (proinflammatory and ammonia-producing bacteria). This dysbiosis is associated with compromised integrity of the intestinal mucosal barrier, leading to bacterial translocation. Consequently, inflammatory cytokines and toxic substances, including bacterial endotoxins and ammonia, are transported from the intestines to the liver via the portal vein. When liver function is impaired, the ability of Kupffer cells to clear endotoxins is reduced, leading to the entry of endotoxins into the systemic circulation through shunts. Simultaneously, decreased bile acid synthesis promotes bacterial overgrowth, resulting in a systemic inflammatory state. This inflammation may directly trigger neuroinflammation, leading to neurological dysfunction, or facilitate the recruitment of immune cells to brain tissues, ultimately altering brain function.
The development of HE after TIPS affected the survival rate in a previous study (32). However, a recent observational study revealed that unlike patients with cirrhosis who did not receive TIPS, episodic OHE after TIPS did not increase the risk of death. However, in patients who underwent TIPS and subsequently died, the incidence of persistent OHE after TIPS was higher (8% vs. 3%) (96). Postoperative HE after TIPS has adverse effects on survival outcomes, and drug strategies for preventing OHE after TIPS remain an unmet need due to the limited evidence of effective preventive treatment.
Current treatment strategies for HE focus primarily on reducing ammonia production and accumulation, inhibiting inflammation, and regulating the intestinal flora (97). The treatment strategy for HE after TIPS is approximately the same as that for common HE (98). MHE and HE Level 1 are generally treated with symptomatic treatment, dietary modification, and medication to prevent further progression of the disease. It is necessary to identify the cause, remove the cause, and strengthen drug treatment for level 2–4 HE. With respect to protein intake (99), in patients with HE Levels 1–2, protein should be limited to 20 g/d in the first few days. With the improvement of symptoms, 10–20 g protein can be added every 2–3 days. Patients with HE Level 3–4 should be prohibited from supplementing protein from the intestine. Plant proteins are superior to animal proteins. Patients who fail to respond to treatment can be treated with stent flow limitation or temporary closure of shunts, artificial extracorporeal liver support can be performed if necessary, and liver transplantation is the ultimate treatment option (99).
However, there is no consensus on whether and which drugs should be used to prevent the occurrence of HE after TIPS. The American and European Clinical Guidelines on HE in Chronic Liver Diseases (100) recommend the use of lactulose to prevent recurrence after the initial onset of HE. The Chinese HE Guidelines (99) mention that lactulose can be used as a preventive drug. According to the European Association for the Study of the Liver (101), lactulose can be used as a secondary preventive measure for HE. Pharmacological prophylaxis is not recommended in North America recommendation for patients who undergo TIPS without a history of HE (102). The BAVENO VII Portal Hypertension Consensus (103) noted that rifaximin can be used for secondary prevention of HE. Currently, there are differences in the effectiveness of drugs in preventing HE after TIPS. In an early study (104) of 75 patients with cirrhosis after TIPS, there was no significant difference in the incidence of HE 1 month after TIPS between the two groups receiving rifaximin or lactulose preventive treatment and without intervention. However, in a recent study (31) involving 197 patients with alcoholic cirrhosis after TIPS, rifaximin prophylaxis reduced the incidence of HE at 6 months after TIPS compared with the non-prevention group (34% vs. 53%; OR 0.48, CI 0.27–0.87). A network meta-analysis (105) aimed to investigate the efficacy of multiple pharmacological regimens for the prevention of post-TIPS HE and revealed that rifaximin alone, lactulose alone, and rifaximin plus lactulose did not significantly reduce the incidence of post-TIPS HE. However, the combination of rifaximin plus lactulose showed the most promising trend toward preventing post-TIPS HE.
Rifaximin is a selective intestinal antibiotic (106). Compared with placebo/no intervention, rifaximin may improve health-related quality of life in patients with MHE. Compared with non-absorbable disaccharides, rifaximin may not have an overall impact on mortality, serious adverse events, health-related quality of life, or HE. However, when used in combination with non-absorbable disaccharides, it may reduce the overall risk of death and the risk of serious adverse events, improve HE, shorten the hospital stay, and prevent the occurrence/recurrence of HE. Therefore, the mechanism by which rifaximin improves cognition may be related to changes in metabolic functions related to the microbiota (107). A study (108) conducted in 20 patients with liver cirrhosis and MHE revealed no significant changes in the fecal microbiota at baseline or 8 weeks after rifaximin 550 mg BID, except for a moderate decrease in Veillonellaceae and an increase in Eubacteraceae. Another (109) comparison of the feces of patients with liver cirrhosis at baseline and after 4 weeks of rifaximin 400 mg TID also revealed no significant changes in the intestinal composition but a decrease in the relative abundances of the Veillonella and Streptococcus genera.
Lactulose is a disaccharide composed of galactose and fructose that can be selectively utilized by host microorganisms and is therefore a prebiotic. A study (110) reported that lactulose affects human metabolism and the gut microbiota in a dose-dependent manner. Another study (111) targeting healthy adults revealed that taking 10 g of lactulose daily for 1 month significantly increased the absolute count of the Bifidobacterium genus but did not increase the count of other bacteria. After 1 month of treatment with a daily dose of 20 g of lactulose, the counts of cultivable Bifidobacterium, Lactobacillus, and Streptococcus increased, whereas the counts of Bacteroides, Clostridium, Escherichia coli, and Eubacterium decreased (112). Interestingly, unlike healthy individuals, after intervention with lactulose, the gut microbiota of patients with liver cirrhosis did not change, indicating that the impact of lactulose on HE may not be related to changes in the gut microbiota (113). However, there is still a lack of research regarding the efficacy of intestinal microecological agents in the prevention or treatment of HE following TIPS. A summary of the literature on drug prevention of post-TIPS HE is provided in Table 2.
Intestinal microecological therapy is a method of treating diseases by restoring normal flora, inhibiting pathogenic bacteria, and promoting microecological balance and includes mainly microecological agents (probiotics, etc.) and fecal flora transplantation therapy (114). A meta-analysis based on multiple randomized controlled trials (115, 116) revealed that the use of probiotics in patients with HE could reduce hospitalization rates, improve CHE, and prevent progression to OHE. A randomized trial conducted in patients with recurrent HE (117) revealed that compared with standard treatment, fecal flora transplantation could reduce readmission rates, improve patient cognition, and increase flora diversity. Research on the prevention or treatment of HE after TIPS is limited, and interventions are mostly lactulose, rifaximin, or ornithine aspartate (118, 119). There are limited studies and inconsistencies in the findings. It is unclear whether intestinal microecological intervention after TIPS affects changes in the intestinal flora and the relationship between such changes and postoperative HE. In a case report on the application of fecal microbial transplantation in patients with HE after TIPS (120), beneficial bacteria such as Ruminococcus decreased in patients with HE after TIPS, whereas harmful bacteria and opportunistic pathogenic bacteria such as Veillonella increased. After fecal microbial transplantation, changes in the intestinal flora of patients were significant. There were no further hospital admissions for HE during the 1-year follow-up period. Therefore, intestinal microecological therapy may be a new treatment for preventing HE after TIPS.
Clostridium butyricum (121) is an inherently beneficial bacterium that has been proven to have potential protective or beneficial effects on the body in patients with inflammatory bowel diseases, neurodegenerative diseases, metabolic diseases, and colorectal cancer. Previous studies have shown that beneficial bacteria such as Clostridium butyricum are significantly reduced in patients with liver cirrhosis (122). Clostridium butyricum, also known as Clostridium butyricum, can inhibit pathogenic bacteria and promote the growth of beneficial bacteria in the intestine. It can convert lactic acid to butyric acid, accelerate mucin production, reduce propionic acid and acetic acid levels, and prevent the destruction of epithelial mucin, thereby maintaining a healthy intestinal tract and enhancing its barrier effect. It has been found that (123) Clostridium butyricum can reduce the expression of Toll-like receptor 4 in colon epithelial cells, and inhibiting the activation of NF-κB signaling pathway can reduce the expression of lipopolysaccharide-induced proinflammatory cytokines, alleviate intestinal mucosal damage, and thereby reduce the level of endotoxin in the body, which can delay the progression of liver fibrosis. There is still a lack of literature on whether it can reduce the incidence of HE after TIPS. Research on the prevention or treatment of HE with probiotics is shown in Table 3. In recent years, studies have also begun to explore the efficacy of fecal microbiota transplantation in the treatment of recurrent HE, but this research is still in clinical trials. Research has shown that FMT has good safety and tolerability, but the efficacy of treating HE varies from person to person. We have also added this information to the literature after (Table 3).
HE after TIPS affects the quality of life of patients and their families, and there are currently no effective preventive measures. The intestinal microbiota has been shown to be associated with the occurrence and prognosis of various diseases. The intestinal microbiota has become increasingly widely used in clinical practice, and restoring intestinal homeostasis is one of the goals of liver disease treatment. The predictive value of the intestinal microbiota in post-TIPS HE and the significance of intestinal microecological agents in the prevention and treatment of post-TIPS HE require further study. In summary, the intestinal flora has a promising future in the occurrence and treatment of HE after TIPS.
XX: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. TZ: Conceptualization, Writing – original draft, Writing – review & editing. CJ: Supervision, Writing – original draft, Writing – review & editing. MJ: Writing – original draft, Methodology, Visualization. YF: Writing – original draft, Resources. FX: Supervision, Writing – original draft, Writing – review & editing. QM: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing. JL: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Scientific Research Project of Beijing Youan Hospital, CCMU, 2022 (No. BJYAYY-YN2022-14). The project leader is Jianjun Li.
All participants in this study are gratefully acknowledged by the authors. We are grateful for the funding from the Scientific Research Project of Beijing Youan Hospital, CCMU.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tripathi, D, Stanley, AJ, Hayes, PC, Travis, S, Armstrong, MJ, Tsochatzis, EA, et al. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut. (2020) 69:1173–92. doi: 10.1136/gutjnl-2019-320221
2. Rajesh, S, George, T, Philips, CA, Ahamed, R, Kumbar, S, Mohan, N, et al. Transjugular intrahepatic portosystemic shunt in cirrhosis: an exhaustive critical update. World J Gastroenterol. (2020) 26:5561–96. doi: 10.3748/wjg.v26.i37.5561
3. Deltenre, P, Zanetto, A, Saltini, D, Moreno, C, and Schepis, F. The role of transjugular intrahepatic portosystemic shunt in patients with cirrhosis and ascites: recent evolution and open questions. Hepatology. (2023) 77:640–58. doi: 10.1002/hep.32596
4. Rodrigues, SG, Sixt, S, Abraldes, JG, De Gottardi, A, Klinger, C, Bosch, J, et al. Systematic review with meta-analysis: portal vein recanalisation and transjugular intrahepatic portosystemic shunt for portal vein thrombosis. Aliment Pharmacol Ther. (2019) 49:20–30. doi: 10.1111/apt.15044
5. Bai, M, Qi, XS, Yang, ZP, Yang, M, Fan, DM, and Han, GH. TIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World J Gastroenterol. (2014) 20:2704–14. doi: 10.3748/wjg.v20.i10.2704
6. Zhu, X, Zhou, Z, and Pan, X. Research reviews and prospects of gut microbiota in liver cirrhosis: a bibliometric analysis (2001-2023). Front Microbiol. (2024) 15:1342356. doi: 10.3389/fmicb.2024.1342356
7. Bajaj, JS, Heuman, DM, Hylemon, PB, Sanyal, AJ, White, MB, Monteith, P, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. (2014) 60:940–7. doi: 10.1016/j.jhep.2013.12.019
8. Yamamoto, K, Honda, T, Inukai, Y, Yokoyama, S, Ito, T, Imai, N, et al. Identification of the microbiome associated with prognosis in patients with chronic liver disease. Microorganisms. (2024) 12:610. doi: 10.3390/microorganisms12030610
9. Wu, Z, Zhou, H, Liu, D, and Deng, F. Alterations in the gut microbiota and the efficacy of adjuvant probiotic therapy in liver cirrhosis. Front Cell Infect Microbiol. (2023) 13:1218552. doi: 10.3389/fcimb.2023.1218552
10. Bajaj, JS. The role of microbiota in hepatic encephalopathy. Gut Microbes. (2014) 5:397–403. doi: 10.4161/gmic.28684
11. Won, SM, Oh, KK, Gupta, H, Ganesan, R, Sharma, SP, Jeong, JJ, et al. The link between gut microbiota and hepatic encephalopathy. Int J Mol Sci. (2022) 23:8999. doi: 10.3390/ijms23168999
12. Zhu, R, Liu, L, Zhang, G, Dong, J, Ren, Z, and Li, Z. The pathogenesis of gut microbiota in hepatic encephalopathy by the gut-liver-brain axis. Biosci Rep. (2023) 43:BSR20222524. doi: 10.1042/bsr20222524
13. Chen, Z, Ruan, J, Li, D, Wang, M, Han, Z, Qiu, W, et al. The role of intestinal bacteria and gut-brain axis in hepatic encephalopathy. Front Cell Infect Microbiol. (2020) 10:595759. doi: 10.3389/fcimb.2020.595759
14. Lombardi, M, Troisi, J, Motta, BM, Torre, P, Masarone, M, and Persico, M. Gut-liver axis dysregulation in portal hypertension: emerging frontiers. Nutrients. (2024) 16:1025. doi: 10.3390/nu16071025
15. Simbrunner, B, Mandorfer, M, Trauner, M, and Reiberger, T. Gut-liver axis signaling in portal hypertension. World J Gastroenterol. (2019) 25:5897–917. doi: 10.3748/wjg.v25.i39.5897
16. Arab, JP, Martin-Mateos, RM, and Shah, VH. Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol Int. (2018) 12:24–33. doi: 10.1007/s12072-017-9798-x
17. Baffy, G. Potential mechanisms linking gut microbiota and portal hypertension. Liver Int. (2019) 39:598–609. doi: 10.1111/liv.13986
18. Li, M, Li, K, Tang, S, Lv, Y, Wang, Q, Wang, Z, et al. Restoration of the gut microbiota is associated with a decreased risk of hepatic encephalopathy after TIPS. JHEP Rep. (2022) 4:100448. doi: 10.1016/j.jhepr.2022.100448
19. Association, H.B.o.t.C.M. Guidelines for the diagnosis and treatment of liver cirrhosis and hepatic encephalopathy (Beijing, 2018). In: H.B.o.t.C.M. Association In:. Chin J Gastrointestinal Endoscopy, Chinese Medical Association Hepatology Branch. (2018). 97–113.
20. Blei, AT, and Córdoba, J. Hepatic encephalopathy. Am J Gastroenterol. (2001) 96:1968–76. doi: 10.1111/j.1572-0241.2001.03964.x
21. Patidar, KR, Thacker, LR, Wade, JB, Sterling, RK, Sanyal, AJ, Siddiqui, MS, et al. Covert hepatic encephalopathy is independently associated with poor survival and increased risk of hospitalization. Am J Gastroenterol. (2014) 109:1757–63. doi: 10.1038/ajg.2014.264
22. Wang, AJ, Peng, AP, Li, BM, Gan, N, Pei, L, Zheng, XL, et al. Natural history of covert hepatic encephalopathy: an observational study of 366 cirrhotic patients. World J Gastroenterol. (2017) 23:6321–9. doi: 10.3748/wjg.v23.i34.6321
23. Krishnarao, A, and Gordon, FD. Prognosis of hepatic encephalopathy. Clin Liver Dis. (2020) 24:219–29. doi: 10.1016/j.cld.2020.01.004
24. Bajaj, JS, O'Leary, JG, Tandon, P, Wong, F, Garcia-Tsao, G, Kamath, PS, et al. Hepatic encephalopathy is associated with mortality in patients with cirrhosis independent of other extrahepatic organ failures. Clin Gastroenterol Hepatol. (2017) 15:565–574.e4. doi: 10.1016/j.cgh.2016.09.157
25. Rose, CF, Amodio, P, Bajaj, JS, Dhiman, RK, Montagnese, S, Taylor-Robinson, SD, et al. Hepatic encephalopathy: novel insights into classification, pathophysiology and therapy. J Hepatol. (2020) 73:1526–47. doi: 10.1016/j.jhep.2020.07.013
26. Montagnese, S, and Bajaj, JS. Impact of hepatic encephalopathy in cirrhosis on quality-of-life issues. Drugs. (2019) 79:11–6. doi: 10.1007/s40265-018-1019-y
27. Hirode, G, Vittinghoff, E, and Wong, RJ. Increasing burden of hepatic encephalopathy among hospitalized adults: an analysis of the 2010-2014 National Inpatient Sample. Dig Dis Sci. (2019) 64:1448–57. doi: 10.1007/s10620-019-05576-9
28. Loffroy, R, Favelier, S, Pottecher, P, Estivalet, L, Genson, PY, Gehin, S, et al. Transjugular intrahepatic portosystemic shunt for acute variceal gastrointestinal bleeding: indications, techniques and outcomes. Diagn Interv Imaging. (2015) 96:745–55. doi: 10.1016/j.diii.2015.05.005
29. Nardelli, S, Gioia, S, Pasquale, C, Pentassuglio, I, Farcomeni, A, Merli, M, et al. Cognitive impairment predicts the occurrence of hepatic encephalopathy after Transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. (2016) 111:523–8. doi: 10.1038/ajg.2016.29
30. Fonio, P, Discalzi, A, Calandri, M, Doriguzzi Breatta, A, Bergamasco, L, Martini, S, et al. Incidence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS) according to its severity and temporal grading classification. Radiol Med. (2017) 122:713–21. doi: 10.1007/s11547-017-0770-6
31. Bureau, C, Thabut, D, Jezequel, C, Archambeaud, I, D'Alteroche, L, Dharancy, S, et al. The use of Rifaximin in the prevention of overt hepatic encephalopathy after Transjugular intrahepatic portosystemic shunt: a randomized controlled trial. Ann Intern Med. (2021) 174:633–40. doi: 10.7326/m20-0202
32. Bai, M, He, CY, Qi, XS, Yin, ZX, Wang, JH, Guo, WG, et al. Shunting branch of portal vein and stent position predict survival after transjugular intrahepatic portosystemic shunt. World J Gastroenterol. (2014) 20:774–85. doi: 10.3748/wjg.v20.i3.774
33. Berlioux, P, Robic, MA, Poirson, H, Métivier, S, Otal, P, Barret, C, et al. Pre-transjugular intrahepatic portosystemic shunts (TIPS) prediction of post-TIPS overt hepatic encephalopathy: the critical flicker frequency is more accurate than psychometric tests. Hepatology. (2014) 59:622–9. doi: 10.1002/hep.26684
34. Bai, M, Qi, X, Yang, Z, Yin, Z, Nie, Y, Yuan, S, et al. Predictors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients: a systematic review. J Gastroenterol Hepatol. (2011) 26:943–51. doi: 10.1111/j.1440-1746.2011.06663.x
35. Wang, C, Yao, J, Niu, H, Yang, C, Liu, J, Bai, Y, et al. Dynamic changes in liver function after transjugular intrahepatic portosystemic shunt in patients with cirrhosis. J Int Med. (2022) 5:207–12. doi: 10.1016/j.jimed.2022.09.001
36. Tuo, S, Yeo, YH, Chang, R, Wen, Z, Ran, Q, Yang, L, et al. Prevalence of and associated factors for sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. Clin Nutr. (2024) 43:84–94. doi: 10.1016/j.clnu.2023.11.008
37. Nardelli, S, Lattanzi, B, Torrisi, S, Greco, F, Farcomeni, A, Gioia, S, et al. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clin Gastroenterol Hepatol. (2017) 15:934–6. doi: 10.1016/j.cgh.2016.10.028
38. Nardelli, S, Lattanzi, B, Merli, M, Farcomeni, A, Gioia, S, Ridola, L, et al. Muscle alterations are associated with minimal and overt hepatic encephalopathy in patients with liver cirrhosis. Hepatology. (2019) 70:1704–13. doi: 10.1002/hep.30692
39. Jindal, A, and Jagdish, RK. Sarcopenia: ammonia metabolism and hepatic encephalopathy. Clin Mol Hepatol. (2019) 25:270–9. doi: 10.3350/cmh.2019.0015
40. Qiu, J, Tsien, C, Thapalaya, S, Narayanan, A, Weihl, CC, Ching, JK, et al. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab. (2012) 303:E983–93. doi: 10.1152/ajpendo.00183.2012
41. Kumar, A, Davuluri, G, Silva, RNE, Engelen, M, Ten Have, GAM, Prayson, R, et al. Ammonia lowering reverses sarcopenia of cirrhosis by restoring skeletal muscle proteostasis. Hepatology. (2017) 65:2045–58. doi: 10.1002/hep.29107
42. Merola, J, Chaudhary, N, Qian, M, Jow, A, Barboza, K, Charles, H, et al. Hyponatremia: a risk factor for early overt encephalopathy after Transjugular intrahepatic portosystemic shunt creation. J Clin Med. (2014) 3:359–72. doi: 10.3390/jcm3020359
43. Imhann, F, Bonder, MJ, Vich Vila, A, Fu, J, Mujagic, Z, Vork, L, et al. Proton pump inhibitors affect the gut microbiome. Gut. (2016) 65:740–8. doi: 10.1136/gutjnl-2015-310376
44. Macke, L, Schulz, C, Koletzko, L, and Malfertheiner, P. Systematic review: the effects of proton pump inhibitors on the microbiome of the digestive tract-evidence from next-generation sequencing studies. Aliment Pharmacol Ther. (2020) 51:505–26. doi: 10.1111/apt.15604
45. Sturm, L, Bettinger, D, Giesler, M, Boettler, T, Schmidt, A, Buettner, N, et al. Treatment with proton pump inhibitors increases the risk for development of hepatic encephalopathy after implantation of transjugular intrahepatic portosystemic shunt (TIPS). United European Gastroenterol J. (2018) 6:1380–90. doi: 10.1177/2050640618795928
46. Riggio, O, Ridola, L, Angeloni, S, Cerini, F, Pasquale, C, Attili, AF, et al. Clinical efficacy of transjugular intrahepatic portosystemic shunt created with covered stents with different diameters: results of a randomized controlled trial. J Hepatol. (2010) 53:267–72. doi: 10.1016/j.jhep.2010.02.033
47. Huang, PC, Zhao, M, Wei, YY, Xia, FF, and Li, H. Transjugular intrahepatic portosystemic shunt: a meta-analysis of 8 mm versus 10 mm stents. Wideochir Inne Tech Maloinwazyjne. (2021) 16:623–32. doi: 10.5114/wiitm.2021.104198
48. Zuo, K, Wang, C, Wang, J, Xia, FF, and Song, T. Transjugular intrahepatic portosystemic shunt through left branch versus right branch of portal vein: a meta-analysis. Abdominal Radiol. (2021) 46:1718–25. doi: 10.1007/s00261-020-02789-9
49. Li, W, Duan, Y, Liu, Z, Lu, X, She, J, Qing, J, et al. Clinical value of hemodynamic changes in diagnosis of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Scand J Gastroenterol. (2022) 57:713–7186. doi: 10.1080/00365521.2022.2029938
50. Luo, SH, Zhou, MM, Cai, MJ, Han, SL, Zhang, XQ, and Chu, JG. Reduction of portosystemic gradient during transjugular intrahepatic portosystemic shunt achieves good outcome and reduces complications. World J Gastroenterol. (2023) 29:2336–48. doi: 10.3748/wjg.v29.i15.2336
51. Trivedi, S, Lam, K, Ganesh, A, Hasnain, Y, Hassan, W, Herren, J, et al. Hepatic encephalopathy after Transjugular intrahepatic portosystemic shunt creation. Semin Interv Radiol. (2023) 40:9–14. doi: 10.1055/s-0043-1764282
52. Schindler, P, Heinzow, H, Trebicka, J, and Wildgruber, M. Shunt-induced hepatic encephalopathy in TIPS: current approaches and clinical challenges. J Clin Med. (2020) 9:3784. doi: 10.3390/jcm9113784
53. Butterworth, RF. Hepatic encephalopathy in cirrhosis: pathology and pathophysiology. Drugs. (2019) 79:17–21. doi: 10.1007/s40265-018-1017-0
54. Cudalbu, C, and Taylor-Robinson, SD. Brain edema in chronic hepatic encephalopathy. J Clin Exp Hepatol. (2019) 9:362–82. doi: 10.1016/j.jceh.2019.02.003
55. Häussinger, D. Low grade cerebral edema and the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology. (2006) 43:1187–90. doi: 10.1002/hep.21235
56. Brusilow, SW, Koehler, RC, Traystman, RJ, and Cooper, AJ. Astrocyte glutamine synthetase: importance in hyperammonemic syndromes and potential target for therapy. Neurotherapeutics. (2010) 7:452–70. doi: 10.1016/j.nurt.2010.05.015
57. Weiss, N, Miller, F, Cazaubon, S, and Couraud, PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. (2009) 1788:842–57. doi: 10.1016/j.bbamem.2008.10.022
58. Hassouneh, R, and Bajaj, JS. Gut microbiota modulation and fecal transplantation: an overview on innovative strategies for hepatic encephalopathy treatment. J Clin Med. (2021) 10:330. doi: 10.3390/jcm10020330
59. Liu, J, Xu, Y, and Jiang, B. Novel insights into pathogenesis and therapeutic strategies of hepatic encephalopathy, from the gut microbiota perspective. Front Cell Infect Microbiol. (2021) 11:586427. doi: 10.3389/fcimb.2021.586427
60. Wang, R, Tang, R, Li, B, Ma, X, Schnabl, B, and Tilg, H. Gut microbiome, liver immunology, and liver diseases. Cell Mol Immunol. (2021) 18:4–17. doi: 10.1038/s41423-020-00592-6
61. Boursier, J, Mueller, O, Barret, M, Machado, M, Fizanne, L, Araujo-Perez, F, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. (2016) 63:764–75. doi: 10.1002/hep.28356
62. Tilg, H, Cani, PD, and Mayer, EA. Gut microbiome and liver diseases. Gut. (2016) 65:2035–44. doi: 10.1136/gutjnl-2016-312729
63. Chopyk, DM, and Grakoui, A. Contribution of the intestinal microbiome and gut barrier to hepatic disorders. Gastroenterology. (2020) 159:849–63. doi: 10.1053/j.gastro.2020.04.077
64. Milosevic, I, Vujovic, A, Barac, A, Djelic, M, Korac, M, Radovanovic Spurnic, A, et al. Gut-liver axis, gut microbiota, and its modulation in the management of liver diseases: a review of the literature. Int J Mol Sci. (2019) 20:395. doi: 10.3390/ijms20020395
65. Bajaj, JS, Hylemon, PB, Ridlon, JM, Heuman, DM, Daita, K, White, MB, et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. (2012) 303:G675–85. doi: 10.1152/ajpgi.00152.2012
66. Zhang, Z, Zhai, H, Geng, J, Yu, R, Ren, H, Fan, H, et al. Large-scale survey of gut microbiota associated with MHE via 16S rRNA-based pyrosequencing. Am J Gastroenterol. (2013) 108:1601–11. doi: 10.1038/ajg.2013.221
67. Saboo, K, Shamsaddini, A, Iyer, MV, Hu, C, Fagan, A, Gavis, EA, et al. Sex is associated with differences in gut microbial composition and function in hepatic encephalopathy. J Hepatol. (2021) 74:80–8. doi: 10.1016/j.jhep.2020.06.046
68. Bajaj, JS, Ridlon, JM, Hylemon, PB, Thacker, LR, Heuman, DM, Smith, S, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. (2012) 302:G168–75. doi: 10.1152/ajpgi.00190.2011
69. Li, MH, Li, K, Tang, SH, Wang, ZY, Guo, WG, Yin, ZX, et al. Changes in gut microbiota after transjugular intrahepatic portosystemic shunt in cirrhotic patients with mild hepatic encephalophy in different prognosis groups. J Clin Hepatol. (2021) 37:326–30. doi: 10.3969/j.issn.1001-5256.2021.02.016
70. Zhao, HW, Zhang, JL, Liu, FQ, Yue, ZD, Wang, L, Zhang, Y, et al. Alterations in the gut microbiome after transjugular intrahepatic portosystemic shunt in patients with hepatitis B virus-related portal hypertension. World J Gastroenterol. (2024) 30:3668–79. doi: 10.3748/wjg.v30.i31.3668
71. Gitto, S, Vizzutti, F, Baldi, S, Campani, C, Navari, N, Falcini, M, et al. Transjugular intrahepatic Porto-systemic shunt positively influences the composition and metabolic functions of the gut microbiota in cirrhotic patients. Dig Liver Dis. (2022) 55:622–8. doi: 10.1016/j.dld.2022.11.017
72. Dantas Machado, AC, Ramos, SF, Gauglitz, JM, Fassler, AM, Petras, D, Aksenov, AA, et al. Portosystemic shunt placement reveals blood signatures for the development of hepatic encephalopathy through mass spectrometry. Nat Commun. (2023) 14:5303. doi: 10.1038/s41467-023-40741-9
73. Chen, Q, Bao, L, Yue, Z, Wang, L, Fan, Z, and Liu, F. Adverse events after the transjugular intrahepatic portal shunt are linked to serum metabolomic changes following the procedure. Front Mol Biosci. (2023) 10:1168782. doi: 10.3389/fmolb.2023.1168782
74. Sung, CM, Lin, YF, Chen, KF, Ke, HM, Huang, HY, Gong, YN, et al. Predicting clinical outcomes of cirrhosis patients with hepatic encephalopathy from the fecal microbiome. Cell Mol Gastroenterol Hepatol. (2019) 8:301–318.e2. doi: 10.1016/j.jcmgh.2019.04.008
75. Maslennikov, R, Ivashkin, V, Efremova, I, Poluektova, E, and Shirokova, E. Gut-liver axis in cirrhosis: are hemodynamic changes a missing link? World J Clin Cases. (2021) 9:9320–32. doi: 10.12998/wjcc.v9.i31.9320
76. Albillos, A, Lario, M, and Álvarez-Mon, M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. (2014) 61:1385–96. doi: 10.1016/j.jhep.2014.08.010
77. Prytz, H, Holst-Christensen, J, Korner, B, and Liehr, H. Portal venous and systemic endotoxaemia in patients without liver disease and systemic endotoxaemia in patients with cirrhosis. Scand J Gastroenterol. (1976) 11:857–63. doi: 10.1080/00365521.1976.12097199
78. Lumsden, AB, Henderson, JM, and Kutner, MH. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology. (1988) 8:232–6. doi: 10.1002/hep.1840080207
79. Garcia-Tsao, G, and Wiest, R. Gut microflora in the pathogenesis of the complications of cirrhosis. Best Pract Res Clin Gastroenterol. (2004) 18:353–72. doi: 10.1016/j.bpg.2003.10.005
80. Rimola, A, Soto, R, Bory, F, Arroyo, V, Piera, C, and Rodes, J. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology. (1984) 4:53–8. doi: 10.1002/hep.1840040109
81. Jalan, R, Olde Damink, SW, Ter Steege, JC, Redhead, DN, Lee, A, Hayes, PC, et al. Acute endotoxemia following transjugular intrahepatic stent-shunt insertion is associated with systemic and cerebral vasodilatation with increased whole body nitric oxide production in critically ill cirrhotic patients. J Hepatol. (2011) 54:265–71. doi: 10.1016/j.jhep.2010.06.042
82. Benten, D, Schulzezur Wiesch, J, Sydow, K, Koops, A, Buggisch, P, Böger, RH, et al. The transhepatic endotoxin gradient is present despite liver cirrhosis and is attenuated after transjugular portosystemic shunt (TIPS). BMC Gastroenterol. (2011) 11:107. doi: 10.1186/1471-230x-11-107
83. Kaser, A, Ludwiczek, O, Waldenberger, P, Jaschke, W, Vogel, W, and Tilg, H. Endotoxin and its binding proteins in chronic liver disease: the effect of transjugular intrahepatic portosystemic shunting. Liver. (2002) 22:380–7. doi: 10.1034/j.1600-0676.2002.01666.x
84. Trebicka, J, Krag, A, Gansweid, S, Appenrodt, B, Schiedermaier, P, Sauerbruch, T, et al. Endotoxin and tumor necrosis factor-receptor levels in portal and hepatic vein of patients with alcoholic liver cirrhosis receiving elective transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol. (2011) 23:1218–25. doi: 10.1097/MEG.0b013e32834a75dc
85. Meng, J, Wang, Q, Liu, K, Yang, S, Fan, X, Liu, B, et al. Systemic and splanchnic lipopolysaccharide and Endothelin-1 plasma levels in liver cirrhosis before and after transjugular intrahepatic portosystemic shunt. Gastroenterol Res Pract. (2016) 2016:8341030. doi: 10.1155/2016/8341030
86. Schalm, SW, and van der Mey, T. Hyperammonemic coma after hepatectomy in germ-free rats. Gastroenterology. (1979) 77:231–4. doi: 10.1016/0016-5085(79)90270-1
87. Plauth, M, Roske, AE, Romaniuk, P, Roth, E, Ziebig, R, and Lochs, H. Post-feeding hyperammonaemia in patients with transjugular intrahepatic portosystemic shunt and liver cirrhosis: role of small intestinal ammonia release and route of nutrient administration. Gut. (2000) 46:849–55. doi: 10.1136/gut.46.6.849
88. Jaeger, V, DeMorrow, S, and McMillin, M. The direct contribution of astrocytes and microglia to the pathogenesis of hepatic encephalopathy. J Clin Transl Hepatol. (2019) 7:352–61. doi: 10.14218/jcth.2019.00025
89. Butterworth, RF. Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology. (2011) 53:1372–6. doi: 10.1002/hep.24228
90. Chastre, A, Bélanger, M, Beauchesne, E, Nguyen, BN, Desjardins, P, and Butterworth, RF. Inflammatory cascades driven by tumor necrosis factor-alpha play a major role in the progression of acute liver failure and its neurological complications. PLoS One. (2012) 7:e49670. doi: 10.1371/journal.pone.0049670
91. He, J, Zhang, P, Shen, L, Niu, L, Tan, Y, Chen, L, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. (2020) 21:6356. doi: 10.3390/ijms21176356
92. Anand, S, and Mande, SS. Host-microbiome interactions: gut-liver axis and its connection with other organs. NPJ Biofilms Microbiomes. (2022) 8:89. doi: 10.1038/s41522-022-00352-6
93. Giannelli, V, Di Gregorio, V, Iebba, V, Giusto, M, Schippa, S, Merli, M, et al. Microbiota and the gut-liver axis: bacterial translocation, inflammation and infection in cirrhosis. World J Gastroenterol. (2014) 20:16795–810. doi: 10.3748/wjg.v20.i45.16795
94. Xiang, J, Zhang, Z, Xie, H, Zhang, C, Bai, Y, Cao, H, et al. Effect of different bile acids on the intestine through enterohepatic circulation based on FXR. Gut Microbes. (2021) 13:1949095. doi: 10.1080/19490976.2021.1949095
95. Juanola, O, Ferrusquía-Acosta, J, García-Villalba, R, Zapater, P, Magaz, M, Marín, A, et al. Circulating levels of butyrate are inversely related to portal hypertension, endotoxemia, and systemic inflammation in patients with cirrhosis. FASEB J. (2019) 33:11595–605. doi: 10.1096/fj.201901327R
96. Nardelli, S, Riggio, O, Marra, F, Gioia, S, Saltini, D, Bellafante, D, et al. Episodic overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt does not increase mortality in patients with cirrhosis. J Hepatol. (2024) 80:596–602. doi: 10.1016/j.jhep.2023.11.033
97. Singh, J, Ibrahim, B, and Han, SH. Nontraditional treatment of hepatic encephalopathy. Clin Liver Dis. (2024) 28:297–315. doi: 10.1016/j.cld.2024.01.007
98. Wang, LJ, Yao, X, Qi, Q, and Qin, JP. Prevention and treatment of hepatic encephalopathy during the perioperative period of transjugular intrahepatic portosystemic shunt. World J Gastrointest Surg. (2023) 15:1564–73. doi: 10.4240/wjgs.v15.i8.1564
99. Association, I.M.B.o.C.M. CCI clinical practice guidelines: management of TIPS for portal hypertension (2019 edition) In: I.M.B.o.C.M. Association In:. Chinese journal of liver diseases Chinese Medical Association Hepatology Branch. (2019). 582–93.
100. Vilstrup, H, Amodio, P, Bajaj, J, Cordoba, J, Ferenci, P, Mullen, KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the liver. Hepatology. (2014) 60:715–35. doi: 10.1002/hep.27210
101. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of hepatic encephalopathy. J Hepatol. (2022) 77:807–24. doi: 10.1016/j.jhep.2022.06.001
102. Boike, JR, Thornburg, BG, Asrani, SK, Fallon, MB, Fortune, BE, Izzy, MJ, et al. North American practice-based recommendations for transjugular intrahepatic portosystemic shunts in portal hypertension. Clin Gastroenterol Hepatol. (2022) 20:1636–1662.e36. doi: 10.1016/j.cgh.2021.07.018
103. Li, XM, Luo, BH, Yuan, J, and Han, GH. Baveno VII - renewing consensus in portal hypertension: personalized care for portal hypertension. Chinese J Liver Dis. (2022) 30:21–9. doi: 10.3760/cma.j.cn501113-20220109-00010
104. Riggio, O, Masini, A, Efrati, C, Nicolao, F, Angeloni, S, Salvatori, FM, et al. Pharmacological prophylaxis of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt: a randomized controlled study. J Hepatol. (2005) 42:674–9. doi: 10.1016/j.jhep.2004.12.028
105. Ahmed, Z, Hassan, M, Arif, SF, Aziz, M, Iqbal, U, Nawaz, A, et al. Comparative efficacy of treatment options for the prevention of post-TIPS hepatic encephalopathy: a systematic review and network meta-analysis. J Gastrointestin Liver Dis. (2023) 32:70–6. doi: 10.15403/jgld-4508
106. Zacharias, HD, Kamel, F, Tan, J, Kimer, N, Gluud, LL, and Morgan, MY. Rifaximin for prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. (2023) 7:Cd011585. doi: 10.1002/14651858.CD011585.pub2
107. Bajaj, JS. Review article: potential mechanisms of action of rifaximin in the management of hepatic encephalopathy and other complications of cirrhosis. Aliment Pharmacol Ther. (2016) 43:11–26. doi: 10.1111/apt.13435
108. Bajaj, JS, Heuman, DM, Sanyal, AJ, Hylemon, PB, Sterling, RK, Stravitz, RT, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. (2013) 8:e60042. doi: 10.1371/journal.pone.0060042
109. Kaji, K, Takaya, H, Saikawa, S, Furukawa, M, Sato, S, Kawaratani, H, et al. Rifaximin ameliorates hepatic encephalopathy and endotoxemia without affecting the gut microbiome diversity. World J Gastroenterol. (2017) 23:8355–66. doi: 10.3748/wjg.v23.i47.8355
110. Ruszkowski, J, and Witkowski, JM. Lactulose: Patient- and dose-dependent prebiotic properties in humans. Anaerobe. (2019) 59:100–6. doi: 10.1016/j.anaerobe.2019.06.002
111. Morales, P, Fujio, S, Navarrete, P, Ugalde, JA, Magne, F, Carrasco-Pozo, C, et al. Impact of dietary lipids on colonic function and microbiota: an experimental approach involving orlistat-induced fat malabsorption in human volunteers. Clin Transl Gastroenterol. (2016) 7:e161. doi: 10.1038/ctg.2016.20
112. Ballongue, J, Schumann, C, and Quignon, P. Effects of lactulose and lactitol on colonic microflora and enzymatic activity. Scand J Gastroenterol Suppl. (1997) 222:41–4. doi: 10.1080/00365521.1997.11720716
113. Sarangi, AN, Goel, A, Singh, A, Sasi, A, and Aggarwal, R. Faecal bacterial microbiota in patients with cirrhosis and the effect of lactulose administration. BMC Gastroenterol. (2017) 17:125. doi: 10.1186/s12876-017-0683-9
114. Muñoz, L, Caparrós, E, Albillos, A, and Francés, R. The shaping of gut immunity in cirrhosis. Front Immunol. (2023) 14:1139554. doi: 10.3389/fimmu.2023.1139554
115. Cao, Q, Yu, CB, Yang, SG, Cao, HC, Chen, P, Deng, M, et al. Effect of probiotic treatment on cirrhotic patients with minimal hepatic encephalopathy: a meta-analysis. Hepatobiliary Pancreatic Dis Int. (2018) 17:9–16. doi: 10.1016/j.hbpd.2018.01.005
116. Saab, S, Suraweera, D, Au, J, Saab, EG, Alper, TS, and Tong, MJ. Probiotics are helpful in hepatic encephalopathy: a meta-analysis of randomized trials. Liver Int. (2016) 36:986–93. doi: 10.1111/liv.13005
117. Bajaj, JS, Kassam, Z, Fagan, A, Gavis, EA, Liu, E, Cox, IJ, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology. (2017) 66:1727–38. doi: 10.1002/hep.29306
118. Seifert, LL, Schindler, P, Schoster, M, Weller, JF, Wilms, C, Schmidt, HH, et al. Recurrence of hepatic encephalopathy after TIPS: effective prophylaxis with combination of lactulose and rifaximin. J Clin Med. (2021) 10:4763. doi: 10.3390/jcm10204763
119. de Wit, K, Schaapman, JJ, Nevens, F, Verbeek, J, Coenen, S, Cuperus, FJC, et al. Prevention of hepatic encephalopathy by administration of rifaximin and lactulose in patients with liver cirrhosis undergoing placement of a transjugular intrahepatic portosystemic shunt (TIPS): a multicentre randomised, double blind, placebo controlled trial (PEARL trial). BMJ Open Gastroenterol. (2020) 7:e000531. doi: 10.1136/bmjgast-2020-000531
120. Li, J, Wang, D, and Sun, J. Application of fecal microbial transplantation in hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Medicine. (2022) 101:e28584. doi: 10.1097/md.0000000000028584
121. Stoeva, MK, Garcia-So, J, Justice, N, Myers, J, Tyagi, S, Nemchek, M, et al. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes. (2021) 13:1–28. doi: 10.1080/19490976.2021.1907272
122. Qin, N, Yang, F, Li, A, Prifti, E, Chen, Y, Shao, L, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. (2014) 513:59–64. doi: 10.1038/nature13568
123. Isono, A, Katsuno, T, Sato, T, Nakagawa, T, Kato, Y, Sato, N, et al. Clostridium butyricum TO-A culture supernatant downregulates TLR4 in human colonic epithelial cells. Dig Dis Sci. (2007) 52:2963–71. doi: 10.1007/s10620-006-9593-3
124. Ahluwalia, V, Betrapally, NS, Hylemon, PB, White, MB, Gillevet, PM, Unser, AB, et al. Impaired gut-liver-brain axis in patients with cirrhosis. Sci Rep. (2016) 6:26800. doi: 10.1038/srep26800
125. Iebba, V, Guerrieri, F, Di Gregorio, V, Levrero, M, Gagliardi, A, Santangelo, F, et al. Combining amplicon sequencing and metabolomics in cirrhotic patients highlights distinctive microbiota features involved in bacterial translocation, systemic inflammation and hepatic encephalopathy. Sci Rep. (2018) 8:8210. doi: 10.1038/s41598-018-26509-y
126. Bajaj, JS, Torre, A, Rojas, ML, Fagan, A, Nandez, IE, Gavis, EA, et al. Cognition and hospitalizations are linked with salivary and faecal microbiota in cirrhosis cohorts from the USA and Mexico. Liver Int. (2020) 40:1395–407. doi: 10.1111/liv.14437
127. Bloom, PP, Luevano, JM Jr, Miller, KJ, and Chung, RT. Deep stool microbiome analysis in cirrhosis reveals an association between short-chain fatty acids and hepatic encephalopathy. Ann Hepatol. (2021) 25:100333. doi: 10.1016/j.aohep.2021.100333
128. Bajaj, JS, Fagan, A, McGeorge, S, Sterling, RK, Rogal, S, Sikaroodi, M, et al. Area deprivation index and gut-brain axis in cirrhosis. Clin Transl Gastroenterol. (2022) 13:e00495. doi: 10.14309/ctg.0000000000000495
129. Wang, Q, Chen, C, Zuo, S, Cao, K, and Li, H. Integrative analysis of the gut microbiota and faecal and serum short-chain fatty acids and tryptophan metabolites in patients with cirrhosis and hepatic encephalopathy. J Transl Med. (2023) 21:395. doi: 10.1186/s12967-023-04262-9
130. Bai, M, He, C, Yin, Z, Niu, J, Wang, Z, Qi, X, et al. Randomised clinical trial: L-ornithine-L-aspartate reduces significantly the increase of venous ammonia concentration after TIPSS. Aliment Pharmacol Ther. (2014) 40:63–71. doi: 10.1111/apt.12795
131. Riggio, O, Nardelli, S, Pasquale, C, Pentassuglio, I, Gioia, S, Onori, E, et al. No effect of albumin infusion on the prevention of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Metab Brain Dis. (2016) 31:1275–81. doi: 10.1007/s11011-015-9713-x
132. Subramanian, SK, Abraham, F, Dutra, BE, Patil, P, Cohen, AM, Purnak, T, et al. Tu1716 survival benefit of the use of lactulose and rifaximin as prophylaxis for portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt. Gastroenterology. (2020) 158:S-1467–8. doi: 10.1016/s0016-5085(20)34328-6
133. Sharma, P, Sharma, BC, Puri, V, and Sarin, SK. An open-label randomized controlled trial of lactulose and probiotics in the treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol. (2008) 20:506–11. doi: 10.1097/MEG.0b013e3282f3e6f5
134. Mittal, VV, Sharma, BC, Sharma, P, and Sarin, SK. A randomized controlled trial comparing lactulose, probiotics, and L-ornithine L-aspartate in treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol. (2011) 23:725–32. doi: 10.1097/MEG.0b013e32834696f5
135. Saji, S, Kumar, S, and Thomas, V. A randomized double blind placebo controlled trial of probiotics in minimal hepatic encephalopathy. Trop Gastroenterol. (2011) 32:128–32.
136. Lunia, MK, Sharma, BC, Sharma, P, Sachdeva, S, and Srivastava, S. Probiotics prevent hepatic encephalopathy in patients with cirrhosis: a randomized controlled trial. Clin Gastroenterol Hepatol. (2014) 12:1003–8.e1. doi: 10.1016/j.cgh.2013.11.006
137. Ziada, DH, Soliman, HH, El Yamany, SA, Hamisa, MF, and Hasan, AM. Can Lactobacillus acidophilus improve minimal hepatic encephalopathy? A neurometabolite study using magnetic resonance spectroscopy. Arab J Gastroenterol. (2013) 14:116–22. doi: 10.1016/j.ajg.2013.08.002
138. Sharma, K, Pant, S, Misra, S, Dwivedi, M, Misra, A, Narang, S, et al. Effect of rifaximin, probiotics, and l-ornithine l-aspartate on minimal hepatic encephalopathy: a randomized controlled trial. Saudi J Gastroenterol. (2014) 20:225–32. doi: 10.4103/1319-3767.136975
139. Pratap Mouli, V, Benjamin, J, Bhushan Singh, M, Mani, K, Garg, SK, Saraya, A, et al. Effect of probiotic VSL#3 in the treatment of minimal hepatic encephalopathy: a non-inferiority randomized controlled trial. Hepatol Res. (2015) 45:880–9. doi: 10.1111/hepr.12429
140. Bajaj, JS, Fagan, A, Gavis, EA, Kassam, Z, Sikaroodi, M, and Gillevet, PM. Long-term outcomes of fecal microbiota transplantation in patients with cirrhosis. Gastroenterology. (2019) 156:1921–1923.e3. doi: 10.1053/j.gastro.2019.01.033
141. Bajaj, JS, Salzman, NH, Acharya, C, Sterling, RK, White, MB, Gavis, EA, et al. Fecal microbial transplant capsules are safe in hepatic encephalopathy: a phase 1, randomized, placebo-controlled trial. Hepatology. (2019) 70:1690–703. doi: 10.1002/hep.30690
142. Bloom, PP, Donlan, J, Torres Soto, M, Daidone, M, Hohmann, E, and Chung, RT. Fecal microbiota transplant improves cognition in hepatic encephalopathy and its effect varies by donor and recipient. Hepatology Commun. (2022) 6:2079–89. doi: 10.1002/hep4.1950
Keywords: transjugular intrahepatic portosystemic shunt, hepatic encephalopathy, gut microbiota, probiotics, treatment, prevention
Citation: Xu X, Zhu T, Jing C, Jiang M, Fu Y, Xie F, Meng Q and Li J (2025) Hepatic encephalopathy treatment after transjugular intrahepatic portosystemic shunt: a new perspective on the gut microbiota. Front. Med. 12:1423780. doi: 10.3389/fmed.2025.1423780
Received: 23 November 2024; Accepted: 24 February 2025;
Published: 07 March 2025.
Edited by:
Rohit Gundamaraju, University of Tasmania, AustraliaReviewed by:
Yu-Chen Fan, Shandong University, ChinaCopyright © 2025 Xu, Zhu, Jing, Jiang, Fu, Xie, Meng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianjun Li, bGpqaXJAY2NtdS5lZHUuY24=; Qinghua Meng, bWVuZ19xaDA4MDVAY2NtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.