
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 17 January 2025
Sec. Pulmonary Medicine
Volume 12 - 2025 | https://doi.org/10.3389/fmed.2025.1388814
The respiratory system is continuously exposed to the outside world, making it vulnerable to airborne particles and harmful pathogens like bacteria and viruses that can enter through breathing. Antigen presenting cells (APCs) have a vital function in the innate immune response as they present antigens to T cells and initiate the response of adaptive immune cells. Professional APCs engulf foreign microorganisms and display their peptides to T lymphocytes using MHC molecules. MHC II on their cell surface and potentially present antigen to CD4+T cells. Furthermore, various other types of cells have similar function that can also serve as APCs by expressing MHC II, thus impacting the progression of lung diseases, such as alveolar epithelial cells (AECs), endothelial cells (ECs), fibroblasts, innate lymphoid cells (ILCs), eosinophils, interstitial cells, mast cells, etc. express MHC II and present antigen. The non-professional APCs type and the extra signals it provides have a direct impact on CD4+T cell programming and downstream effector mechanisms. Here, we summarize the existing research on the expression of MHC II on non-professional APCs in different lung diseases and its influence on CD4+T differentiation types and disease outcomes, in order to further clarify the role of MHC II of different non-professional APCs in lung diseases, such as asthma, chronic obstructive pulmonary disease (COPD), etc.
Professional Antigen presenting cells (APCs) in the lung, such Dendritic cells (DCs), macrophages (MACs), and B cells, come into contact with T cells that are specific to the antigen and have a crucial and distinct function in promoting interaction between the innate and adaptive immune systems, guaranteeing defensive immunity and creating immune memory. When encountering foreign pathogens, APCs activate naïve T cells through antigenic peptides and signals (1). The interaction leads to the stimulation of T cell differentiation and proliferation, resulting in the formation of long-lasting memory T cell populations like central memory T (TCM) cells, effector memory T (TEM) cells, and resident memory T (TRM) cells (2–4). Earlier research has recognized separate groups of APCs in the respiratory system, where the interaction between APCs and T cells heavily relies on major histocompatibility complex (MHC) molecules. T lymphocytes recognize antigens through the presence of MHC molecules on the surface of APCs. MHC molecules responsible for displaying antigens comprise MHC class I, MHC class II, and MHC-like CD1 molecules (5).
CD4+T cells play an important role in lung disease, showing a high degree of heterogeneity and plasticity, and are regulated in response to stimulation with cognate antigens bound to MHC class II molecules. Thus, the antigen-presenting cell type and the additional signals it provides directly affect CD4+T cell programming and its downstream effector mechanisms. The expression of MHC II is mainly limited to professional APCs, but epithelial cells and fibroblasts can also express MHC II. However, the functional consequences of CD4+T cell interactions with these “non-professional” APCs are unknown.
Additionally, it has been proposed that various cell types in the lung, including alveolar epithelial cells (AECs), endothelial cells (ECs), innate Lymphoid Cells (ILCs) and eosinophils in the lung, may have the ability to present exogenous antigens to T cells (6, 7). The cell categories are commonly known as non-professional APCs. APCs load foreign antigen-derived peptides onto MHC II, which then present peptide–MHC II complexes to CD4+T cells (8–11). It is interesting to note that approximately half of the cells expressing MHC II in the lung are CD45-nonhematopoietic cells (12).
Mounting evidence suggests that non-hematopoietic cell populations residing in tissues play a crucial role in controlling immune responses specific to organs (13). During perinatal development, AECs progenitors undergo a process of differentiation into mature alveolar type 1 (AEC I) and type 2 (AEC II) cells. According to recent findings, it has been indicated that additional cell types in the respiratory system, like AEC II, possess the ability to present antigens (14–16).
The literature on immune signaling and immune cellular changes in lung inflammatory diseases has experienced significant growth in recent years. Currently, there have been studies on the impact of non-immune cells presenting antigens on the onset and progression of various illnesses. Non-professional APCs have gradually become the focus of research. In the realm of cardiovascular and other disease research, the discovery of the involvement of non-professional APCs in disease is an ongoing process. A recent report in Nature revealed that cardiac fibroblasts play a role in promoting cardiac fibrosis and dysfunction through antigen presentation (17).
While professional APCs in the lung are well understood in their role of activating T cells and generating immune memory, the functional implications of interactions between non-professional APCs and CD4+T cells remain unclear. CD4+T cells are activated by other lung cells such as AECs, ECs, fibroblasts, innate lymphoid cells (ILCs), eosinophils, interstitial cells, mast cells, etc. through antigen presentation. Specifically, the ability of these non-professional APCs to influence T cell differentiation, programming, and effector functions, as well as their contribution to immune responses in lung diseases, is not fully elucidated. The main emphasis of this review focuses on the expression of MHC II on non-professional APCs cell surface and potentially present antigen to CD4+T cells in lung development and different lung diseases. In detail, we summarize the expression of MHC II on non-professional APCs and CD4+T cells formation by non-professional APCs during lung diseases, such as asthma, chronic obstructive pulmonary disease (COPD), etc. This analysis will help to understand the significance of MHC II mediate multiple effects of crosstalk with CD4+T cells in lung diseases.
In order to comprehend the reason behind the ability of specific non-immune cells to function as APCs, as well as the correlation between their function and lung diseases, it is imperative to initially grasp the attributes of APCs. To start with, it is necessary to possess the capacity to modify the proteasome, such as through the activation of the pertinent genes that stimulate the proteasome, as these immunoproteasomes play a role in the processing of antigenic peptide (18). Additionally, in order to present antigens to CD8+T or CD4+T cells, APCs must express either MHC I or MHC II, respectively. It is necessary for cells to have the capability of increasing the expression of co-stimulatory proteins like B7, which interacts with CD28 found on the outer layer of T cells (19). Successful activation of T cells necessitates this interaction. Activation of antigen-dependent T cells necessitates the presence of these three essential functions in APCs. While these genes can be expressed by various cell types and their expression can increase in response to disease or stimulation, the upregulation of these genes alone is not enough to ascribe APC function. Additionally, it must be demonstrated that APCs are effective and capable of inducing T cell activation. In short, the most direct way to identify a cell as an APC is to test whether it expresses MHC I and MHC II molecules. The expression of costimulatory molecules (such as CD80, CD86, etc.) was examined. To verify whether it has the ability to uptake and process antigens. The effective activation of specific T cells was verified by co-culture assay or T cell proliferation/activation assay. With these methods, can effectively determine whether a cell has antigen-presenting function.
The cellular contribution of the lung to the adaptive immune response is emerging but not fully understood. New findings indicates that there may be significant functional connections between T cells within the lungs and non-professional APCs, which could potentially contribute to development of diseases and lung-related issues. The results reveal that cells like the AECs function as the primary APCs in the lung and carry significant implications for disease comprehension.
Pulmonary immunity is significantly influenced by CD4+T cells. Furthermore, their traditional role in aiding the initiation of CD4+T cell activation in lymph nodes is also noteworthy. In the lung, CD4+T cells are also responsible for regulating both proinflammatory and anti-inflammatory immune responses. In addition, CD4+T cells have the ability to express cytotoxic programs that are comparable to those observed in CD8+T cells. CD4+T cells display a significant amount of diversity and flexibility and are controlled when exposed to MHC II-bound specific antigens. Hence, the specific cell category displaying the antigen and the supplementary cues it offers have a direct impact on the programming of CD4+T cells and subsequent effector mechanisms. While MHC II expression is primarily limited to APCs, there are certain situations where MHC II can also be expressed. Nevertheless, the functional implications of intrapulmonary interactions between CD4+T cells and this particular non-professional APCs (AECs, ECs, fibroblasts, ILCs, eosinophils, interstitial cells, and mast cells) are currently being examined.
The lung contains several non-professional APCs that also have the ability to present antigens. These cells induce the expression of MHC II molecules and possess a limited capacity for antigen presentation. The non-professional APCs found in the lung include AECs, ECs, fibroblasts, ILCs, Eosinophils, Interstitial cells, and Mast Cells.
In the upcoming parts of this analysis, we will gather the newly gathered compelling proof that certain non-immune cells in the respiratory organ can carry out numerous of these tasks and can thus can function as APCs in particular situations or contribute to the disease procession.
AECs consist of epithelial cells, namely AEC I and AEC II (20). AEC I have the primary responsible of facilitating gas exchange and also play a crucial role in the regulation and defense mechanisms (21). As progenitor cells, AEC II have the ability to either self-renew or differentiate into AEC I cells, primarily generating surfactant (21, 22). Simultaneously, they have the ability to generate cytokines and chemokines when faced with different types of lung damage caused by bacteria, viruses, or mechanical ventilation, and also play a role in immune reactions (23). AECs have direct interactions with the surrounding environment, making them crucial elements in the regulation of barrier immunity (13). AEC II has a barrier function to prevent external pathogens and harmful substances from entering the lung tissue. In the case of infection or injury, it secrete cytokines, activates the immune system, attracts and recruits immune cells to the inflammatory site, and also provides antigen information to T cells through the expression of MHC molecules to play a local antigen presentation role. When injury, occurs AEC II differentiates to AEC I to ensure renewal and homeostasis. Here, we summarize the functional role of AEC II in pulmonary immunity in Figure 1.
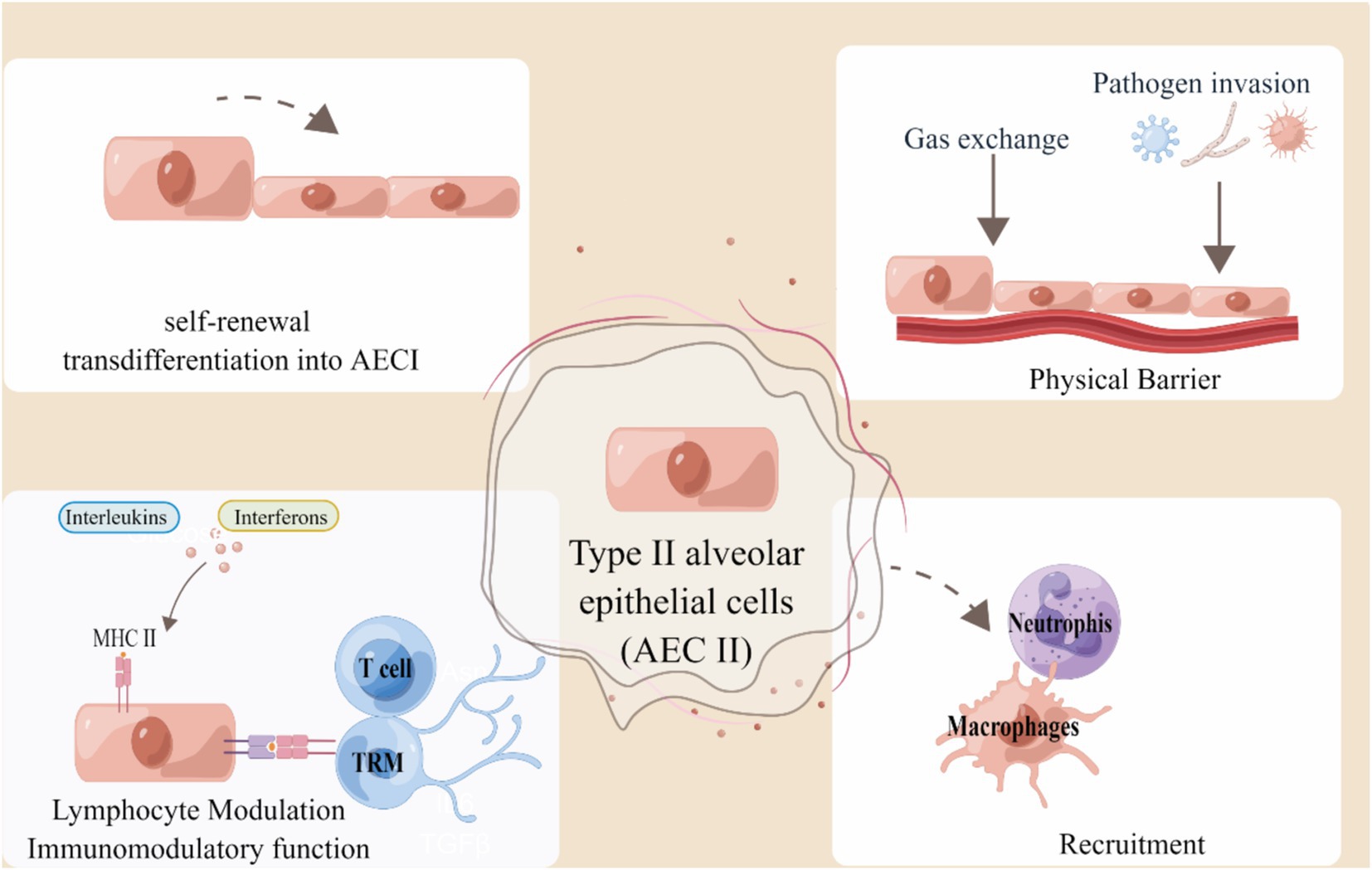
Figure 1. Alveolar type II epithelial cells in lung immunity. AEC II serves as an external obstacle and activates the innate immune response to engage in immune modulation. Type II epithelial cells can act as non-professional APCs by presenting antigen to T cells and TRM cells in a manner restricted by MHC II, thereby indicating their involvement in the regulation of immune function. The figure was created using Figdraw (www.figdraw.com).
Decades ago, it was demonstrated that AECs express MHC II. However, the precise role of this expression is still not understood. Currently, there has been extensive research on AECs, which are increasingly acknowledged for their role in maintaining immune balance. Increasing evidence suggests that alveolar epithelial cells play a role in the lung’s adaptive immune response (21). AECs have the ability to express MHC II protein and associated processing molecules, potentially serving as non-professional APCs (24).
Historically, DCs, MACs, and B cells have been categorized as professional APCs and would normally express MHC II. Nevertheless, numerous researches have detected the presence of MHC II expression in lung epithelial cells under normal conditions and in reaction to viral damage or cancer (25, 26). It is worth noting that the presence of invariant chain expression has been observed on fetal alveolar epithelium in humans at 12–14 weeks gestational age, even in the absence of MHC II co-expression (27). During gestation, it seems that MHC II is not expressed on the surfaces of AECs in fetal lung tissue, unless there is active inflammation (28). Initially, it was demonstrated that adult AECs consistently express MHC II on the bronchial and alveolar epithelium, particularly on AEC II and ciliated ECs (29).
There is limited research on the role of AEC II expressing MHC II during lung development. Studies have shown that immune control in the lung is mediated through the expression of MHC II by pulmonary epithelial cells. These cells act as sentinels with anti-infection capabilities. In cell culture, pulmonary epithelial cells use these molecules to instruct T cells on their actions. This allows them to respond appropriately to infectious microorganisms, suggesting that AEC II may play a role in infection monitoring during lung development through antigen presentation.
There is increasing evidence that AEC II in the lung contribute to adaptive immune responses in the lungs. AEC II cells express MHC II and present antigen. In lung infectious diseases, Toulmin et al. showed that AEC II had antigen presentation ability, but it was lower than DCs (25). In vitro antigen-presenting experiments AEC II with antigen specific hybridoma, they revealed that while AEC II can present antigens to CD4+T cells and induce interferon-γ (IFN-γ) production, the efficiency is lower than DCs, especially in the context of influenza A virus (IAV) infection. The absence of MHC II on AEC II cells led to a slight deterioration in respiratory viral illness after being infected with influenza and Sendai virus (25, 30). The presence of IFN-γ in the lung microenvironment has the ability to control the presentation of different antigens and the subsequent interactions between T cells and non-professional APCs (31). The presentation of antigens by AEC II cells could play a crucial role in viral infections by enabling a proficient group of APCs to quickly obtain antigens from infected epithelial cells. For instance, immune cells can receive MHC II from epithelial cells (32).
SPClowMHC IIhigh AEC II cells act as APCs to generate CD4+TRM cells (33). In vitro, AEC II cells that present antigens stimulate naïve CD4+T cells and promote the development of regulatory T cells (34). Barrier epithelial cells not only activate CD4+T cells but also attract and retain CD8+TRM cells in close proximity to the sites of antigen exposure and reactivate them through local antigen presentation (2, 35). Recent studies have provided fresh insights into the interactions between epithelial cells and CD4+TRM cells (33).
Research conducted on mice with a targeted elimination of MHC II in AEC II has shown the significance of MHC II in relation to these cells. The absence of MHC II on AEC II cells resulted in a decrease in the quantity of T cells in the lungs and the percentage of CD4+T cells that exhibited PD-1 (35). In a more striking manner, animals that do not have MHC II in their epithelial cells experience a mortality rate that is twice as high as that of control animals after IAV infection (25). This emphasizes the benefit of survival provided by MHC II+ AEC II cells. Interestingly, Shenoy and colleagues discovered that the targeted removal of MHC II in lung epithelial cells led to a decrease in PD-L1 expression by AECs, both in normal conditions and in pneumonia caused by bacteria (33). After being re-infected, AEC II cells that present antigens may reactivate effector T cells or start the recruitment of regulatory T cells in order to support the regeneration of epithelial cells (36). CD4 +TRM cell maintenance around airways was facilitated by airway epithelial cells in a model of S. pneumoniae infection (33). The suppression of CD4+T cells may occur due to the inhibition of overly exuberant and potentially harmful responses, which is caused by the expression of PD-L1 in epithelial cells (37).
AECs from individuals with allergy or autoimmunity, such as chronic bronchitis, asthma, idiopathic pulmonary fibrosis (IPF), or lung transplant rejection, exhibit increased MHC II expression in lung tissue (30, 38–40). Increased MHC II expression on AEC II is a phenomenon of interest in these lung diseases. The increase in MHC II may be related to bystander effect during any chronic inflammatory condition, but the current understanding of its specific mechanisms and effects is still incomplete. This effect may modulate the local immune environment or promote inflammatory responses. However, there is no direct evidence that upregulation of MHC II plays a direct antigen presentation role in these diseases. Although the increase in MHC II on AEC II suggested that these cells may have potential function for antigen presentation, no study has clearly demonstrated efficient antigen presentation by MHC II on AEC II in these lung diseases. In IPF and lung transplant rejection, the increased expression of MHC II on AEC II may be related to the fibrotic process of the disease and immune rejection, but its specific role in antigen presentation needs to be further explored. A summary of the expression of these MHC II-expressing non-professional APCs and their detection methods is important for further investigation of the function of these non-professional APCs. Based on this, we summarize the current relevant in vitro and in vivo studies on the expression and outcome of MHC II molecules on AECs in lung disease shown in Table 1.
The lung has a high amount of blood vessels due to a unique arrangement of endothelial cells, primarily made up of microvascular endothelial cells. Capillaries, which are small blood vessels, closely interact with the alveoli to enable the exchange of gasses between the apical circulation and the air in the basal alveoli (41). The pulmonary ECs are the cells that line the inner surface of these capillaries, Immune-cell recruitment primarily takes place in the lung’s capillaries, which are usually just a few micrometers wide. Due to the narrowing of capillaries, recruited immune cells tend to slow down as they traverse these vessels, resulting in a longer contact time between immune cells and ECs to potentially modulate the immune response. T cells must pass through these cells, which compose the inner lining of blood vessels, in order to reach their target, thus establishing a direct barrier between blood and tissue (42). Hence, ECs have the ability to effectively display antigens derived from blood and neighboring tissues to circulating T cells. Krupnick AS et al. showed that the activation and induction of CD4+Foxp3+ regulatory T cells occur when antigens are presented to CD4+T cells through the vascular endothelium (43). Infection of lung ECs, such as capillary ECs, by IAV, could potentially trigger the presentation of viral antigens and aid in the prompt activation of memory T cells located within blood vessels or surrounding tissues (44).
Fibroblasts frequently reside near lung epithelial cells and have demonstrated an ability to present antigens (17, 45). Several experiments conducted in a laboratory setting using mice have shown that fibroblasts can effectively display antigens to CD4+T cells (17, 46). Ngwenyama et al. studied the interactions between fibroblasts and CD4+T cells in a heart failure mouse model. They found that cardiac fibroblasts present antigens to CD4+T cells through MHC II, induced by IFN-γ. In the lung, recent single-cell RNA sequencing studies have further shed light on fibroblasts, previously thought to be uniformly supportive, for example also having specific pathogenic properties such as antigen presentation and vascular support. Antigen presentation by lung MHC II+fibroblasts is a crucial driver of anti-tumor immunity. The MHC+fibroblasts, known as antigen-presenting cancer-associated fibroblasts (apCAFs), present cancer-specific MHC II peptides to nearby CD4+T cells. In a recent study, Kerdidani and colleagues discovered MHC II+ lung fibroblasts in both normal conditions and in mouse models of lung cancer, which were found adjacent to regions with a high density of CD4+T cells (26). They demonstrated that cancer-specific CD4+T cells exhibited increased expression of effector cytokines when stimulated by apCAFs. This interaction enhances the effector function of cancer-specific CD4+T cells and contributes to the tumor-suppressive effect of apCAFs. Lung antigen-presenting fibroblasts may be unique in their ability to activate effector T cells. Denton et al. discovered lung fibroblasts in the lung infected with IAV that facilitated the development of lung germinal centers by encouraging the recruitment of B cells (47). The fibroblasts in the naïve lung were not detected and expressed MHC II. The findings indicate that MHC II+ lung fibroblasts could potentially interact with lung T cells that have encountered antigens, thereby enhancing immune responses that provide protection (48).
HLA-DR and costimulatory molecules OX-40 L and CD70 are expressed by human lung fibroblasts, enabling the activation and maintenance of CD4+ memory T cells (49). Overall, MHC II expression in lung fibroblasts may play an important role in regulating local immune responses, disease progression, and immune balance.
ILC2s, also known as Type 2 innate lymphocytes, play a crucial role in initiating early immune responses associated with type 2 immunity (50). Previous research has indicated that ILC2s are capable of expressing MHC II molecules, thus functioning as antigen-presenting cells (APCs) (51). ILC2s strongly express OX40L, ICOS, ICOSL, CD80, and CD86, which have the ability to control the differentiation of CD4+T cells (52).
ILC2s isolated from the peripheral blood of patients experiencing acute exacerbation of chronic obstructive pulmonary disease (AECOPD) were co-cultured with CD4+T cells obtained from the peripheral blood of healthy individuals. This co-culture aimed to investigate the potential role of ILC2s as APCs by upregulating MHC II and influencing the shift toward Th2-type responses in AECOPD (53). In their study, Kang and colleagues showed that pulmonary ILC2s have the ability to function as APCs, triggering CD4+T cell activation through the MHC II pathway in the context of respiratory syncytial virus (RSV) infection (54). The presence of RSV infection enhances the presentation of MHC II molecules on the outer layer of pulmonary ILC2s. ILC2s have the ability to trigger and facilitate the growth and specialization of CD4+T cells infected with respiratory syncytial virus. Significantly decreasing the expansion of CD4+T cells was achieved by blocking the interaction between CD4+T cells and ILC2s using a monoclonal antibody against MHC II.
Furthermore, ILC3s have the ability to directly control CD4+T cells by presenting antigens to MHC II (55–57). The study by Teng et al. found that antigen-presenting ILC3s play a crucial role by expressing a high amount of MHC II and effectively restricting the growth of allergen-specific CD4+T cells during airway challenge in experimental airway inflammation (58).
Hansel et al. reported that eosinophils expressed HLA-DR at sites of active disease processes (59). In addition, they have demonstrated that eosinophils are involved in antigen uptake, processing and presentation, and HLA-DR+eosinophils can present antigen to CD4+T lymphocytes. Additional confirmation was provided by the findings of Beninati W et al., which detected eosinophil HLA-DR expression at sites of human disease (60). This molecule was identified on eosinophils isolated from bronchoalveolar lavage (BAL) of patients with asthma and chronic eosinophilic pneumonia. This study primarily identified that mature eosinophils can express MHC II molecules, indicating a potential immunological function. Further research has shown that eosinophils in asthma patients and experimental models, it has been demonstrated that eosinophils express MHC II and costimulatory molecules that are crucial for APCs (61). The study conducted by Zhang and colleagues showed that eosinophils derived from mice infected with RSV can function as APCs and are responsible for triggering asthma (62).
Interstitial cells containing I-A-like MHC II were observed in the trachea and lungs of both conventional specific pathogen free (SPF) and germ-free rats (30). Nakano et al. found that mast cell expression of MHC II and OX40L is induced to increase through Notch signaling, thereby inducing a preferential shift of naïve CD4+T cells to Th2 cells with an antigen-presenting function and may play a role in allergic diseases (63).
In conclusion, the diverse roles of non-immune cells with antigen-presenting capabilities in the lung underscore their complex contributions to pulmonary diseases. While cells such as fibroblasts, ECs, eosinophils and mast cells can exhibit antigen-presenting functions, their effects vary significantly depending on their specific context and interactions within the lung microenvironment. These variations in antigen presentation not only influence the immune response but also impact the progression and outcome of pulmonary conditions.
The immune system, including the adaptive immune system, is considered to be the key to promote the regenerative response after injury (51). Recent studies have shown that the antigen pressing-adaptive immune axis plays a role in zebrafish heart regeneration, but this has not been studied in lung regeneration, which may be the light of future research on lung regeneration (64). The potential clinical transformation of non-professional APCs in pulmonary diseases has increasingly become a research focus. Recent studies highlight the significant role these cells may play in early diagnosis, therapeutic target development, and prognosis evaluation. Therefore, further exploration of non-professional APCs in pulmonary diseases is crucial for their clinical application.
Non-professional APCs express MHC II and costimulatory molecules under specific conditions. This enables them to perform antigen presentation functions in pulmonary diseases. These cells are integral to the local immune response by activating CD4+T cells. Understanding these functional changes in pathological environments helps elucidate disease mechanisms and offers new clinical insights.
From a clinical perspective, the expression patterns of non-professional APCs in pulmonary diseases present opportunities for early diagnosis. Monitoring MHC II and related molecule expression levels could help detect abnormal immune responses early, allowing for timely intervention. Additionally, the antigen-presenting ability of non-professional APCs at various disease stages may serve as biomarkers for disease progression and prognosis. For example, increased MHC II expression in fibroblasts during early pulmonary fibrosis could indicate immune dysregulation and tissue remodeling, aiding early diagnosis and disease classification.
Since COPD and IPF may often be multifactorial diseases, non-professional APCs may interact with other immune cells and factors in the pulmonary microenvironment. They not only play a key role in the immune response, but also may aggravate disease progression through the promotion of fibrosis. Although clinical translational research faces some challenges, it may become a new strategy for the treatment of COPD and IPF by regulating the function of these cells. Therefore, future treatment may require a multi-target strategy that combines the regulation of non-professional APCs with the intervention of other immune mechanisms to effectively improve the prognosis of patients. In-depth study of the specific role and mechanism of non-professional APCs in these diseases could help to develop new therapeutic strategies and alleviate the long-term effects of the disease. Moreover, non-professional APCs show potential as therapeutic targets in pulmonary diseases. Modulating their antigen-presenting functions could influence the immunopathological processes. For instance, inhibiting MHC II expression or costimulatory signaling in lung fibroblasts might reduce chronic inflammation and fibrosis. Immunomodulatory strategies targeting these cells could also benefit conditions like COPD, IPF and asthma by mitigating inflammatory responses and slowing disease progression. However, translating non-professional APCs research into clinical practice presents challenges. The functional heterogeneity of these cells across different diseases and microenvironments complicates targeted regulation. Non-professional APCs include a variety of cell types, such as epithelial cells, fibroblasts, and certain immune cells, among others, which may exhibit different functions in different pathological environments. For example, AECs may play different roles in healthy and diseased states, while their specific role in the immune response is not fully understood. Therefore, how to precisely identify and classify these cells, and how to assess their function in specific diseases, is a major challenge. The function of lay APCs is often spatio-temporal specific and may change with disease progression. These cells may exhibit different immunomodulatory effects in different immune environments in different lung diseases. How to identify and regulate the function of these cells, especially at different disease stages, remains a major challenge in translational medicine. The complex interactions between non-professional APCs and immune cells, especially the regulation of responsiveness by T cells, B cells, and macrophages, are not fully understood. How to regulate the interactions of these cells to achieve immune tolerance or immune activation and avoid excessive immune responses is a major problem in clinical applications.
In conclusion, studying non-professional APCs in lung diseases reveals their crucial role in immune regulation and opens new avenues for clinical applications.
In the future, research on non-professional APCs will focus on several key issues and highlights. Addressing these will enhance our understanding of non-professional APCs in immune regulation and disease, and support their clinical applications.
Firstly, exploring the functional heterogeneity of non-professional APCs in various disease microenvironments is crucial. Non-professional APCs, such as AECs, ECs, and fibroblasts, exhibit diverse functions in diseases like COPD, IPF, and asthma. Their activation status and antigen presentation abilities are influenced by inflammatory factors, metabolic signals, and interactions with other immune cells. Future research must investigate how these cells behave in different pathological contexts. Secondly, the interaction between non-professional APCs and professional APCs warrants further study. Professional APCs, such as DCs and MACs, and non-professional APCs often work together in immune responses. In certain lung diseases, non-professional APCs may collaborate with professional APCs to regulate antigen presentation and T cell activation. Understanding these interactions is vital for unraveling complex immune networks, especially in chronic inflammatory and fibrotic conditions. Future studies should focus on elucidating these mechanisms.
Additionally, examining the dynamic changes of non-professional APCs in the immune microenvironment is important. These cells show functional changes throughout different disease stages, transitioning from a resting to an activated state, which can influence disease progression. This dynamic behavior reflects the plasticity of non-professional APCs. Advanced technologies, such as single-cell sequencing, time series analysis, and spatial omics, can map these changes across various pathological stages. This approach will clarify the specific roles of these cells in disease progression and inform targeted interventions. Furthermore, regulating MHC II expression on non-professional APCs could provide new strategies for vaccine development and immunotherapy. By enhancing MHC II expression, researchers could improve vaccine efficacy and design more effective strategies by targeting specific signaling pathways in these cells. Finally, emerging technologies will play a significant role in future research on non-professional APCs. Innovations such as single-cell multi-omics, spatial omics, mass spectrometry, and advanced flow cytometry will provide higher resolution and broader analysis of non-professional APCs functions. Additionally, new models like in vitro organoids and microfluidic chips will offer intuitive platforms for studying these cells in complex environments. These technologies will deepen our understanding of non-professional APCs and enhance their application in disease models, supporting their clinical translation.
In conclusion, although non-professional APCs do not play a dominant role in the immune response as professional APCs such as DCs, they can promote the proliferation and differentiation of T cells by presenting antigen, secreting cytokines, and expressing costimulatory molecules, and play an important auxiliary role in the immune response, especially in local immune and inflammatory reactions. To summarize, we have organized the documented non-professional lung APCs and their function in T cell reactions across various lung illnesses (Table 2; Figure 2). Gaining insight into the practical significance of non-professional APCs in presenting MHC II antigens in various situations could offer a basis for selectively adjusting this process to boost immune protection or alleviate harmful inflammation. Nevertheless, numerous domains necessitate additional examination, and the antigens they handle and exhibit in vivo under diverse circumstances remain mostly unidentified. These findings could potentially offer crucial insights into whether MHC II antigen presentation is employed to bolster the inflammatory response of anti-pathogen T cells or to facilitate tissue healing procedures. After viral damage, the diversity of the interaction between local T cells and both professional and non-professional APCs increases. The activation of memory T cells from various subsets of APCs may extend protective immune responses, allowing for the possibility of functional immune responses. The specific involvement of non-professional APCs in the display of MHC II antigens and the indirect control of T cell reactions through local means remains uncertain. Moreover, the unclear regulation of non-professional APCs expression involves the intricate interaction between microbiota, professional APC, and T cell responses.
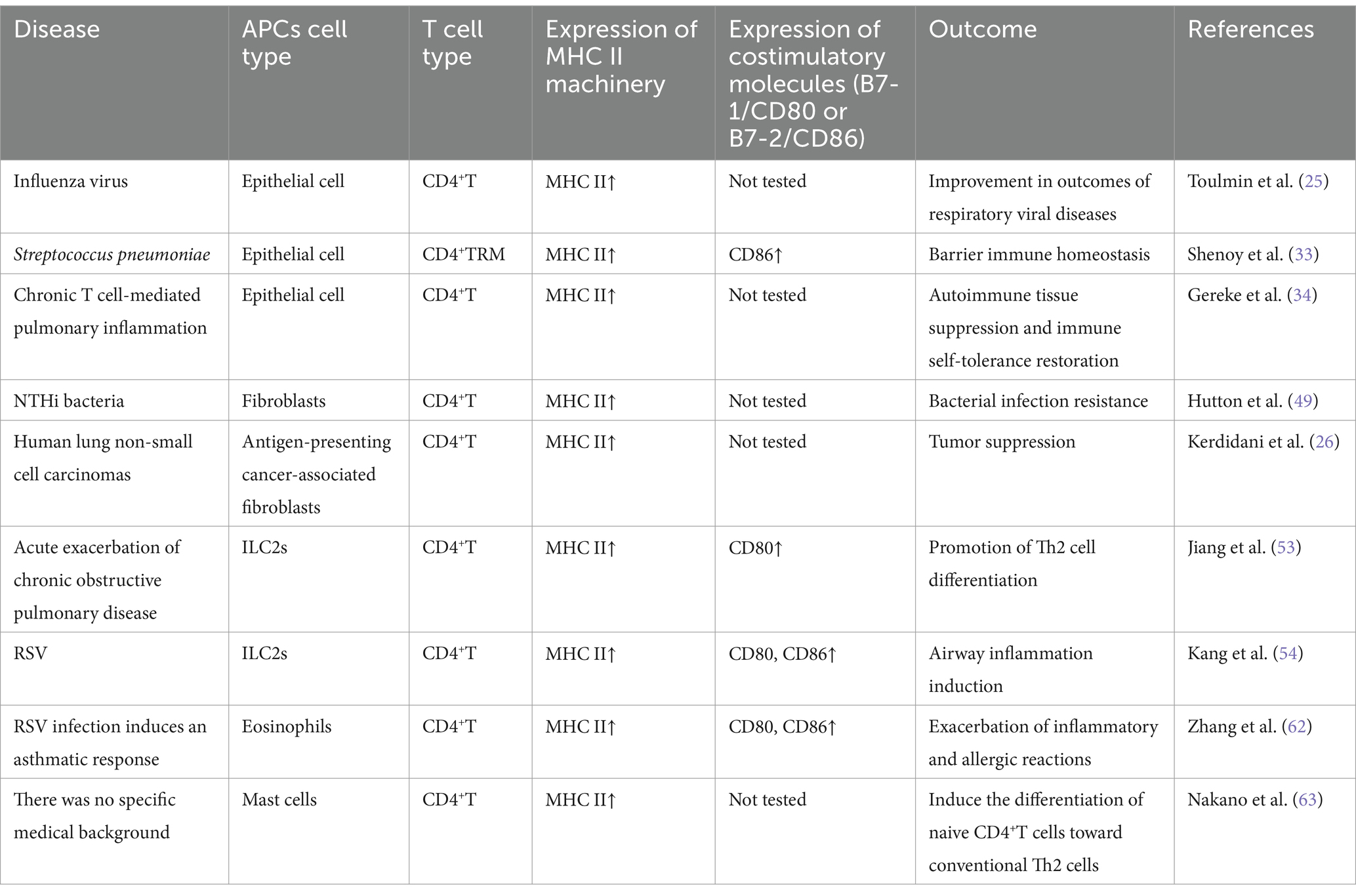
Table 2. Pulmonary non-professional APCs and their roles in T cell responses (current evidence suggests that non-professional APCs play an antigen-presenting role in respiratory diseases, including the expression of MHC complex genes and functional demonstration of antigen presentation in human and animal studies).
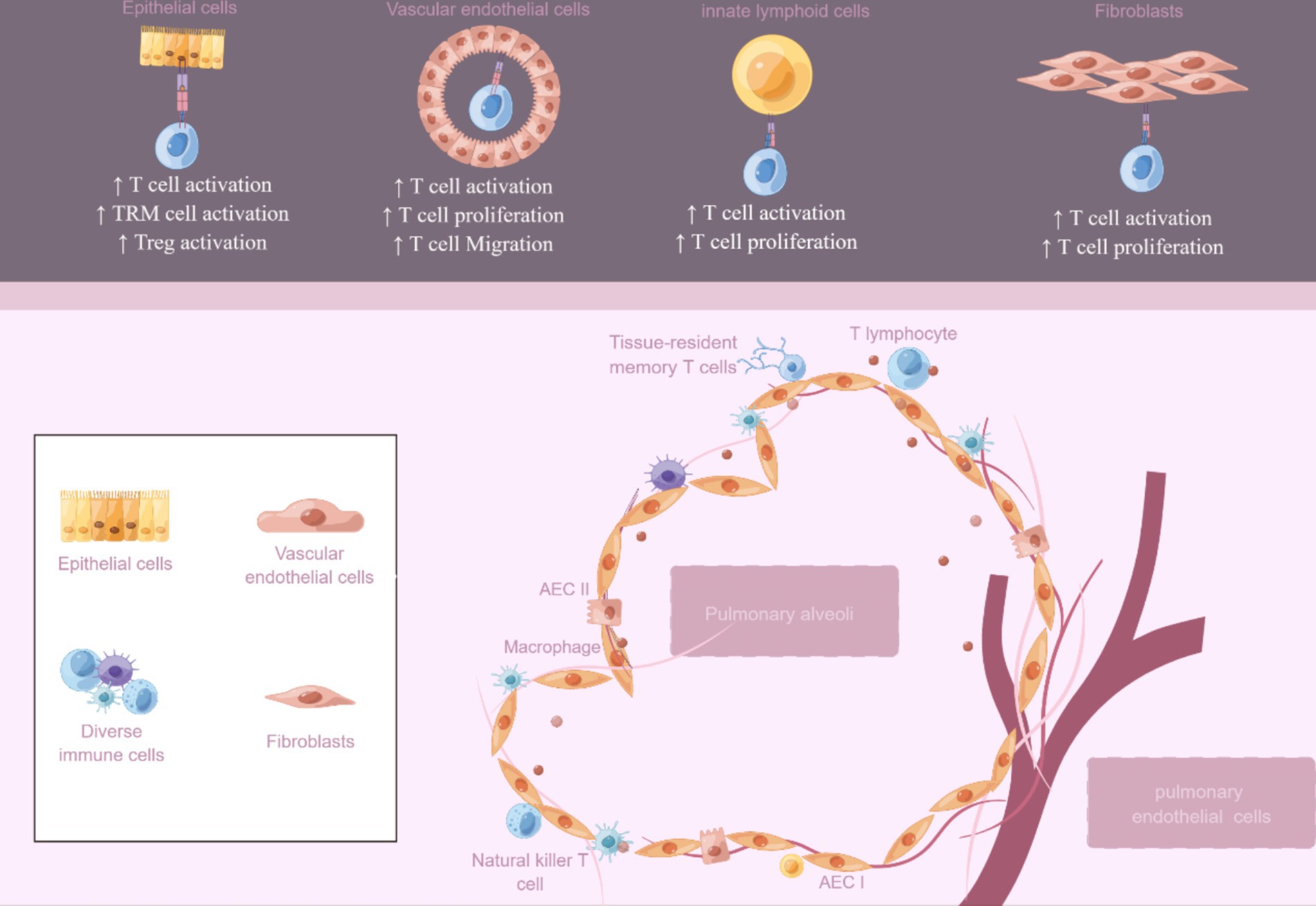
Figure 2. Non-professional antigen-presenting cells in the lung. An overview of the potential roles of non-professional APCs includes epithelial cells, vascular endothelial cells, innate lymphoid cells, and fibroblasts. The figure was created using Figdraw (www.figdraw.com).
The concept of non-hematopoietic cells playing a significant role in the initiation of the pulmonary immune response is currently under investigation. Nevertheless, our knowledge regarding the precise role of pulmonary non-professional APCs is restricted. One reason for this is due to various significant assumptions, including the observation that MHC I and II levels are increased in numerous illnesses. Nevertheless, these modifications by themselves do not ensure particular immune responses to antigens. Exposure to an inflammatory setting can alter the upregulation of MHC I and II expression. Nonetheless, the establishment of effective immune synapses between APCs and T cells necessitates supplementary costimulatory molecules, alongside the existence of MHC I and II machinery, and ultimately results in the activation of T cells (19). Hence, additional research is required to ascertain the impact of selective absence of MHC I and II presentation in non-professional APCs, like respiratory epithelial cells. This investigation aims to explore the influence of MHC II expression in non-professional APCs on local immune balance, addressing both APC-related inquiries and those unrelated to APCs function, thereby enhancing our comprehension of the role played by context and magnitude of MHC II expression in non-professional APCs.
In summary, future research on non-professional APCs will address functional heterogeneity, cell interactions, dynamic regulation, clinical translation challenges, and emerging technologies. Progress in these areas will establish a comprehensive understanding of non-professional APCs’ roles in immune regulation and disease, paving the way for their use in immunotherapy.
M-YW: Funding acquisition, Writing – original draft. YQ: Data curation, Investigation, Writing – original draft. S-JW: Data curation, Investigation, Writing – original draft. Z-LS: Supervision, Writing – review & editing. H-YL: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Natural Science Foundation of China (No.82171702), Zhenjiang Science and Technology Innovation Funds - Clinical Medicine Key Laboratory (SS2023012), Scientific Research Project of Health Commission of Jiangsu Province (M2022043), and Graduate student research and creative projects of Jiangsu Province (grant No. KYCX23_3755).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wang, P, Zhang, Q, Tan, L, Xu, Y, Xie, X, and Zhao, Y. The regulatory effects of mTOR complexes in the differentiation and function of CD4(+) T cell subsets. J Immunol Res. (2020) 2020:1–16. doi: 10.1155/2020/3406032
2. Low, JS, Farsakoglu, Y, Amezcua Vesely, MC, Sefik, E, Kelly, JB, Harman, CCD, et al. Tissue-resident memory T cell reactivation by diverse antigen-presenting cells imparts distinct functional responses. J Exp Med. (2020) 217:e20192291. doi: 10.1084/jem.20192291
3. Schenkel, JM, and Masopust, D. Tissue-resident memory T cells. Immunity. (2014) 41:886–97. doi: 10.1016/j.immuni.2014.12.007
4. Banchereau, J, Briere, F, Caux, C, Davoust, J, Lebecque, S, Liu, YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. (2000) 18:767–811. doi: 10.1146/annurev.immunol.18.1.767
5. van den Elsen, PJ, Holling, TM, Kuipers, HF, and van der Stoep, N. Transcriptional regulation of antigen presentation. Curr Opin Immunol. (2004) 16:67–75. doi: 10.1016/j.coi.2003.11.015
6. Claser, C, Nguee, SYT, Balachander, A, Wu Howland, S, Becht, E, Gunasegaran, B, et al. Lung endothelial cell antigen cross-presentation to CD8(+)T cells drives malaria-associated lung injury. Nat Commun. (2019) 10:4241. doi: 10.1038/s41467-019-12017-8
7. Ghasemi, F, Tessier, TM, Gameiro, SF, Maciver, AH, Cecchini, MJ, and Mymryk, JS. High MHC-II expression in Epstein-Barr virus-associated gastric cancers suggests that tumor cells serve an important role in antigen presentation. Sci Rep. (2020) 10:14786. doi: 10.1038/s41598-020-71775-4
8. Joffre, OP, Segura, E, Savina, A, and Amigorena, S. Cross-presentation by dendritic cells. Nat Rev Immunol. (2012) 12:557–69. doi: 10.1038/nri3254
9. Matsuda, JL, and Kronenberg, M. Presentation of self and microbial lipids by CD1 molecules. Curr Opin Immunol. (2001) 13:19–25. doi: 10.1016/S0952-7915(00)00176-X
10. Skold, M, Faizunnessa, NN, Wang, CR, and Cardell, S. CD1d-specific NK1.1+ T cells with a transgenic variant TCR. J Immunol. (2000) 165:168–74. doi: 10.4049/jimmunol.165.1.168
11. Steimle, V, Siegrist, CA, Mottet, A, Lisowska-Grospierre, B, and Mach, B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. (1994) 265:106–9. doi: 10.1126/science.8016643
12. Kreisel, D, Richardson, SB, Li, W, Lin, X, Kornfeld, CG, Sugimoto, S, et al. Cutting edge: MHC class II expression by pulmonary nonhematopoietic cells plays a critical role in controlling local inflammatory responses. J Immunol. (2010) 185:3809–13. doi: 10.4049/jimmunol.1000971
13. Krausgruber, T, Fortelny, N, Fife-Gernedl, V, Senekowitsch, M, Schuster, LC, Lercher, A, et al. Structural cells are key regulators of organ-specific immune responses. Nature. (2020) 583:296–302. doi: 10.1038/s41586-020-2424-4
14. Guo, M, Du, Y, Gokey, JJ, Ray, S, Bell, SM, Adam, M, et al. Single cell RNA analysis identifies cellular heterogeneity and adaptive responses of the lung at birth. Nat Commun. (2019) 10:37. doi: 10.1038/s41467-018-07770-1
15. Wayne, EC, Long, C, Haney, MJ, Batrakova, EV, Leisner, TM, Parise, LV, et al. Targeted delivery of si RNA Lipoplexes to Cancer cells using macrophage transient horizontal gene transfer. Adv Sci (Weinh). (2019) 6:1900582. doi: 10.1002/advs.201900582
16. Ma, D, Liu, S, Hu, L, He, Q, Shi, W, Yan, D, et al. Single-cell RNA sequencing identify SDCBP in ACE2-positive bronchial epithelial cells negatively correlates with COVID-19 severity. J Cell Mol Med. (2021) 25:7001–12. doi: 10.1111/jcmm.16714
17. Ngwenyama, N, Kaur, K, Bugg, D, Theall, B, Aronovitz, M, Berland, R, et al. Antigen presentation by cardiac fibroblasts promotes cardiac dysfunction. Nat Cardiovasc Res. (2022) 1:761–74. doi: 10.1038/s44161-022-00116-7
18. Griffin, TA, Nandi, D, Cruz, M, Fehling, HJ, Kaer, LV, Monaco, JJ, et al. Immunoproteasome assembly: cooperative incorporation of interferon gamma (IFN-gamma)-inducible subunits. J Exp Med. (1998) 187:97–104. doi: 10.1084/jem.187.1.97
19. Greenwald, RJ, Freeman, GJ, and Sharpe, AH. The B7 family revisited. Annu Rev Immunol. (2005) 23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611
20. Chung, KP, Hsu, CL, Fan, LC, Huang, Z, Bhatia, D, Chen, YJ, et al. Mitofusins regulate lipid metabolism to mediate the development of lung fibrosis. Nat Commun. (2019) 10:3390. doi: 10.1038/s41467-019-11327-1
21. Kobayashi, Y, Tata, A, Konkimalla, A, Katsura, H, Lee, RF, Ou, J, et al. Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat Cell Biol. (2020) 22:934–46. doi: 10.1038/s41556-020-0542-8
22. Ghaedi, M, Mendez, JJ, Bove, PF, Sivarapatna, A, Raredon, MS, and Niklason, LE. Alveolar epithelial differentiation of human induced pluripotent stem cells in a rotating bioreactor. Biomaterials. (2014) 35:699–710. doi: 10.1016/j.biomaterials.2013.10.018
23. Tamimi, F, Altigani, S, and Sanz, M. Periodontitis and coronavirus disease 2019. Periodontol 2000. (2022) 89:207–14. doi: 10.1111/prd.12434
24. Cunningham, AC, Zhang, JG, Moy, JV, Ali, S, and Kirby, JA. A comparison of the antigen-presenting capabilities of class II MHC-expressing human lung epithelial and endothelial cells. Immunology. (1997) 91:458–63. doi: 10.1046/j.1365-2567.1997.d01-2249.x
25. Toulmin, SA, Bhadiadra, C, Paris, AJ, Lin, JH, Katzen, J, Basil, MC, et al. Type II alveolar cell MHCII improves respiratory viral disease outcomes while exhibiting limited antigen presentation. Nat Commun. (2021) 12:3993. doi: 10.1038/s41467-021-23619-6
26. Kerdidani, D, Aerakis, E, Verrou, KM, Angelidis, I, Douka, K, Maniou, MA, et al. Lung tumor MHCII immunity depends on in situ antigen presentation by fibroblasts. J Exp Med. (2022) 219:e20210815. doi: 10.1084/jem.20210815
27. Badve, S, Deshpande, C, Hua, Z, and Logdberg, L. Expression of invariant chain (CD 74) and major histocompatibility complex (MHC) class II antigens in the human fetus. J Histochem Cytochem. (2002) 50:473–82. doi: 10.1177/002215540205000404
28. Peters, U, Papadopoulos, T, and Muller-Hermelink, HK. MHC class II antigens on lung epithelial of human fetuses and neonates. Ontogeny and expression in lungs with histologic evidence of infection. Lab Investig. (1990) 63:38–43.
29. Cunningham, AC, Milne, DS, Wilkes, J, Dark, JH, Tetley, TD, and Kirby, JA. Constitutive expression of MHC and adhesion molecules by alveolar epithelial cells (type II pneumocytes) isolated from human lung and comparison with immunocytochemical findings. J Cell Sci. (1994) 107:443–9. doi: 10.1242/jcs.107.2.443
30. Steiniger, B, and Sickel, E. Class II MHC molecules and monocytes/macrophages in the respiratory system of conventional, germ-free and interferon-gamma-treated rats. Immunobiology. (1992) 184:295–310. doi: 10.1016/S0171-2985(11)80588-7
31. Reith, W, Leibund Gut-Landmann, S, and Waldburger, JM. Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol. (2005) 5:793–806. doi: 10.1038/nri1708
32. Stephens, WZ, Kubinak, JL, Ghazaryan, A, Bauer, KM, Bell, R, Buhrke, K, et al. Epithelial-myeloid exchange of MHC class II constrains immunity and microbiota composition. Cell Rep. (2021) 37:109916. doi: 10.1016/j.celrep.2021.109916
33. Shenoy, AT, Lyon De Ana, C, Arafa, EI, Salwig, I, Barker, KA, Korkmaz, FT, et al. Antigen presentation by lung epithelial cells directs CD4(+) T (RM) cell function and regulates barrier immunity. Nat Commun. (2021) 12:5834. doi: 10.1038/s41467-021-26045-w
34. Gereke, M, Jung, S, Buer, J, and Bruder, D. Alveolar type II epithelial cells present antigen to CD4(+) T cells and induce Foxp 3(+) regulatory T cells. Am J Respir Crit Care Med. (2009) 179:344–55. doi: 10.1164/rccm.200804-592OC
35. Ahmadvand, N, Khosravi, F, Lingampally, A, Wasnick, R, Vazquez-Armendariz, AI, Carraro, G, et al. Identification of a novel subset of alveolar type 2 cells enriched in PD-L1 and expanded following pneumonectomy. Eur Respir J. (2021) 58:2004168. doi: 10.1183/13993003.04168-2020
36. Mock, JR, Garibaldi, BT, Aggarwal, NR, Jenkins, J, Limjunyawong, N, Singer, BD, et al. Foxp 3+ regulatory T cells promote lung epithelial proliferation. Mucosal Immunol. (2014) 7:1440–51. doi: 10.1038/mi.2014.33
37. Lo, B, Hansen, S, Evans, K, Heath, JK, and Wright, JR. Alveolar epithelial type II cells induce T cell tolerance to specific antigen. J Immunol. (2008) 180:881–8. doi: 10.4049/jimmunol.180.2.881
38. Sacco, O, Lantero, S, Scarso, L, Galietta, LJ, Spallarossa, D, Silvestri, M, et al. Modulation of HLA-DR antigen and ICAM-1 molecule expression on airway epithelial cells by sodium nedocromil. Ann Allergy Asthma Immunol. (1999) 83:49–54. doi: 10.1016/S1081-1206(10)63512-0
39. Chang, SC, Hsu, HK, Perng, RP, Shiao, GM, and Lin, CY. Increased expression of MHC class II antigens in rejecting canine lung allografts. Transplantation. (1990) 49:1158–63. doi: 10.1097/00007890-199006000-00026
40. Kaneko, Y, Kuwano, K, Kunitake, R, Kawasaki, M, Hagimoto, N, and Hara, N. B7-1, B7-2 and class II MHC molecules in idiopathic pulmonary fibrosis and bronchiolitis obliterans-organizing pneumonia. Eur Respir J. (2000) 15:49–55.
41. Niethamer, TK, Stabler, CT, Leach, JP, Zepp, JA, Morley, MP, Babu, A, et al. Defining the role of pulmonary endothelial cell heterogeneity in the response to acute lung injury. eLife. (2020) 9:9. doi: 10.7554/eLife.53072
42. Ley, K, Laudanna, C, Cybulsky, MI, and Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. (2007) 7:678–89. doi: 10.1038/nri2156
43. Krupnick, AS, Gelman, AE, Barchet, W, Richardson, S, Kreisel, FH, Turka, LA, et al. Murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp 3+ regulatory T cells. J Immunol. (2005) 175:6265–70. doi: 10.4049/jimmunol.175.10.6265
44. Anderson, KG, Sung, H, Skon, CN, Lefrancois, L, Deisinger, A, Vezys, V, et al. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol. (2012) 189:2702–6. doi: 10.4049/jimmunol.1201682
45. Steuerman, Y, Cohen, M, Peshes-Yaloz, N, Valadarsky, L, Cohn, O, David, E, et al. Dissection of influenza infection in vivo by single-cell RNA sequencing. Cell Syst. (2018) 6:679–691.e4. doi: 10.1016/j.cels.2018.05.008
46. Davidson, S, Coles, M, Thomas, T, Kollias, G, Ludewig, B, Turley, S, et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat Rev Immunol. (2021) 21:704–17. doi: 10.1038/s41577-021-00540-z
47. Denton, AE, Innocentin, S, Carr, EJ, Bradford, BM, Lafouresse, F, Mabbott, NA, et al. Type I interferon induces CXCL13 to support ectopic germinal center formation. J Exp Med. (2019) 216:621–37. doi: 10.1084/jem.20181216
48. Silva-Sanchez, A, and Randall, TD. Role of iBALT in respiratory immunity. Curr Top Microbiol Immunol. (2020) 426:21–43. doi: 10.1007/82_2019_191
49. Hutton, AJ, Polak, ME, Spalluto, CM, Wallington, JC, Pickard, C, Staples, KJ, et al. Human lung fibroblasts present bacterial antigens to autologous lung Th cells. J Immunol. (2017) 198:110–8. doi: 10.4049/jimmunol.1600602
50. Neill, DR, Wong, SH, Bellosi, A, Flynn, RJ, Daly, M, Langford, TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. (2010) 464:1367–70. doi: 10.1038/nature08900
51. Shanley, LC, Mahon, OR, Kelly, DJ, and Dunne, A. Harnessing the innate and adaptive immune system for tissue repair and regeneration: considering more than macrophages. Acta Biomater. (2021) 133:208–21. doi: 10.1016/j.actbio.2021.02.023
52. Halim, TY, Steer, CA, Matha, L, Gold, MJ, Martinez-Gonzalez, I, McNagny, KM, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. (2014) 40:425–35. doi: 10.1016/j.immuni.2014.01.011
53. Jiang, M, Liu, H, Li, Z, Wang, J, Zhang, F, Cao, K, et al. ILC2s induce adaptive Th2-type immunity in acute exacerbation of chronic obstructive pulmonary disease. Mediat Inflamm. (2019) 2019:1–12. doi: 10.1155/2019/3140183
54. Kang, L, Wang, S, Wang, D, Wang, J, Zheng, R, Jiang, X, et al. Group 2 innate lymphoid cells mediate the activation of CD4(+) T cells and aggravate Th1/Th2 imbalance via MHC II molecules during respiratory syncytial virus infection. Int Immunopharmacol. (2022) 113:109306. doi: 10.1016/j.intimp.2022.109306
55. Einenkel, R, Ehrhardt, J, Zygmunt, M, and Muzzio, DO. Oxygen regulates ILC3 antigen presentation potential and pregnancy-related hormone actions. Reprod Biol Endocrinol. (2022) 20:109. doi: 10.1186/s12958-022-00979-2
56. von Burg, N, Chappaz, S, Baerenwaldt, A, Horvath, E, Bose Dasgupta, S, Ashok, D, et al. Activated group 3 innate lymphoid cells promote T-cell-mediated immune responses. Proc Natl Acad Sci USA. (2014) 111:12835–40. doi: 10.1073/pnas.1406908111
57. Lehmann, FM, von Burg, N, Ivanek, R, Teufel, C, Horvath, E, Peter, A, et al. Microbiota-induced tissue signals regulate ILC3-mediated antigen presentation. Nat Commun. (2020) 11:1794. doi: 10.1038/s41467-020-15612-2
58. Teng, F, Tacho-Pinot, R, Sung, B, Farber, DL, Worgall, S, Hammad, H, et al. ILC3s control airway inflammation by limiting T cell responses to allergens and microbes. Cell Rep. (2021) 37:110051. doi: 10.1016/j.celrep.2021.110051
59. Hansel, TT, Braunstein, JB, Walker, C, Blaser, K, Bruijnzeel, PL, Virchow, JC Jr, et al. Sputum eosinophils from asthmatics express ICAM-1 and HLA-DR. Clin Exp Immunol. (1991) 86:271–7. doi: 10.1111/j.1365-2249.1991.tb05809.x
60. Beninati, W, Derdak, S, Dixon, PF, Grider, DJ, Strollo, DC, Hensley, RE, et al. Pulmonary eosinophils express HLA-DR in chronic eosinophilic pneumonia. J Allergy Clin Immunol. (1993) 92:442–9. doi: 10.1016/0091-6749(93)90123-W
61. Farhan, RK, Vickers, MA, Ghaemmaghami, AM, Hall, AM, Barker, RN, and Walsh, GM. Effective antigen presentation to helper T cells by human eosinophils. Immunology. (2016) 149:413–22. doi: 10.1111/imm.12658
62. Zhang, D, Yang, J, Zhao, Y, Shan, J, Wang, L, Yang, G, et al. RSV infection in neonatal mice induces pulmonary eosinophilia responsible for asthmatic reaction. Front Immunol. (2022) 13:817113. doi: 10.3389/fimmu.2022.817113
63. Nakano, N, Nishiyama, C, Yagita, H, Koyanagi, A, Akiba, H, Chiba, S, et al. Notch signaling confers antigen-presenting cell functions on mast cells. J Allergy Clin Immunol. (2009) 123:74–81.e1. doi: 10.1016/j.jaci.2008.10.040
64. Cardeira-da-Silva, J, Wang, Q, Sagvekar, P, Mintcheva, J, Latting, S, Gunther, S, et al. Antigen presentation plays positive roles in the regenerative response to cardiac injury in zebrafish. Nat Commun. (2024) 15:3637. doi: 10.1038/s41467-024-47430-1
65. Kallenberg, CG, Schilizzi, BM, Beaumont, F, Poppema, S, De Leij, L, and The, TH. Expression of class II MHC antigens on alveolar epithelium in fibrosing alveolitis. Clin Exp Immunol. (1987) 67:182–90.
66. Vignola, AM, Campbell, AM, Chanez, P, Bousquet, J, Paul-Lacoste, P, Michel, FB, et al. HLA-DR and ICAM-1 expression on bronchial epithelial cells in asthma and chronic bronchitis. Am Rev Respir Dis. (1993) 148:689–94. doi: 10.1164/ajrccm/148.3.689
67. Dias, AA, Silva, C, da Silva, CO, Linhares, NRC, Santos, JPS, Vivarini, AC, et al. TLR-9 plays a role in Mycobacterium leprae-induced innate immune activation of A549 alveolar epithelial cells. Front Immunol. (2021) 12:657449. doi: 10.3389/fimmu.2021.657449
68. Guo, N, Wen, Y, Wang, C, Kang, L, Wang, X, Liu, X, et al. Lung adenocarcinoma-related TNF-alpha-dependent inflammation upregulates MHC-II on alveolar type II cells through CXCR-2 to contribute to Treg expansion. FASEB J. (2020) 34:12197–213. doi: 10.1096/fj.202000166RR
69. Shen, H, Liu, C, Shao, P, Yi, L, Wang, Y, Mills Ko, E, et al. Enhanced phenotypic alterations of alveolar type II cells in response to aflatoxin G1-induced lung inflammation. J Cell Physiol. (2015) 230:1199–211. doi: 10.1002/jcp.24852
70. Hasegawa, K, Sato, A, Tanimura, K, Uemasu, K, Hamakawa, Y, Fuseya, Y, et al. Fraction of MHCII and EpCAM expression characterizes distal lung epithelial cells for alveolar type 2 cell isolation. Respir Res. (2017) 18:150. doi: 10.1186/s12931-017-0635-5
Keywords: alveolar epithelial cells, major histocompatibility complex, antigen-presenting cells, lung disease, T cells
Citation: Wang M-Y, Qiao Y, Wei S-J, Su Z-L and Lu H-Y (2025) MHC class II of different non-professional antigen-presenting cells mediate multiple effects of crosstalk with CD4+T cells in lung diseases. Front. Med. 12:1388814. doi: 10.3389/fmed.2025.1388814
Received: 20 February 2024; Accepted: 06 January 2025;
Published: 17 January 2025.
Edited by:
Pau Serra Devecchi, August Pi i Sunyer Biomedical Research Institute (IDIBAPS), SpainReviewed by:
Aditi Murthy, University of Pennsylvania, United StatesCopyright © 2025 Wang, Qiao, Wei, Su and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhao-Liang Su, c3psMzBAdWpzLmVkdS5jbg==; Hong-Yan Lu, aHlsdUB1anMuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.