- 1The First Clinical Medical College, Shanxi Medical University, Taiyuan, Shanxi, China
- 2Department of Dermatology, First Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
A 42 year-old male with prurigo nodularis treated with dupilumab showed a case of linear psoriasis, highlighting the potential of dupilumab to induce immune shift and chimeric gene expression.
Introduction
Linear psoriasis is a rare type of psoriasis characterized by a linear distribution of psoriatic lesions along the Blaschko’s line. Although the pathogenesis of linear psoriasis is not well-understood, the involvement of genetic chimerism has been proposed as a major driver of its occurrence. Prurigo nodularis (PN) is a chronic inflammatory skin disease which presents as a persistent solitary pruritic hard papules and nodules. Dupilumab has been approved by the Food and Drug Administration (FDA) for the treatment of adults with PN. In recent years, a subset of patients with PN was reported to experience immune shift following dupilumab treatment, resulting in psoriatic lesions (1, 2). A case of linear psoriasis in a patient with PN treated with dupilumab was presented. This case highlights the potential of dupilumab to induce not only immune shift but also chimeric gene expression.
Case reports
A 42 year-old male visited our department due to pruritic erythema and scattered nodules on his trunk and limbs for more than 10 years. Routine blood test, thyroid function, liver and kidney function tests, HIV, syphilis test, and the serum total IgE level found no abnormal results. The patient denied a personal and family history of atopic diseases and psoriasis. Based on the above background, a diagnosis of prurigo nodularis (PN) was made. Despite treatment with oral antihistamines, gabapentin, thalidomide, topical glucocorticoid, the patient’s condition did not improve. Three years ago, he was treated with dupilumab. After 4 months of use, erythematous scaly papules and plaques in a Blaschkoid distribution appeared on the right lower limb. Topical treatment with fluticasone propionate cream and calcipotriol ointment for 9 weeks completely resolved the right lower limb rash. Moreover, systemic erythema and nodules were cleared, and pruritus exhibited significant improvement, and hence, dupilumab treatment was gradually discontinued. The pruritic erythema and scattered nodules recurred 1 year ago. The patient was put on dupilumab treatment again at an initial dose of 600 mg iH, which was
reduced to 300 mg iH every 2 weeks. After 3 months of treatment, the original lesions disappeared completely, but pruritic linear lesions appeared in the left lower limb. Further examination revealed well-defined erythematous squamous papules and plaques on the posterior surface of the left thigh that were linearly arranged along the Blaschko’s line and extended to the ankle joint (Figure 1). Dermoscopic evaluation detected whitish scales and regularly distributed simple red loops, twisted red loops, and globules (Figure 2A). Skin biopsy was performed, and histopathology showed hyperkeratosis and parakeratosis, regular acanthosis, thin granular layer, dilated blood vessels at the tip of papillary dermis and perivascular infiltration of lymphocytes (Figure 2B). Therefore, the diagnosis of linear psoriasis was confirmed. Dupilumab was adjusted to 300 mg subcutaneous injection every 4 weeks. In addition, local topical treatment of fluticasone propionate cream and calcipotriol ointment was given once a day. Ten weeks later, the skin lesions gradually subsided. No recurrent rashes were seen after 6 months of follow-up.
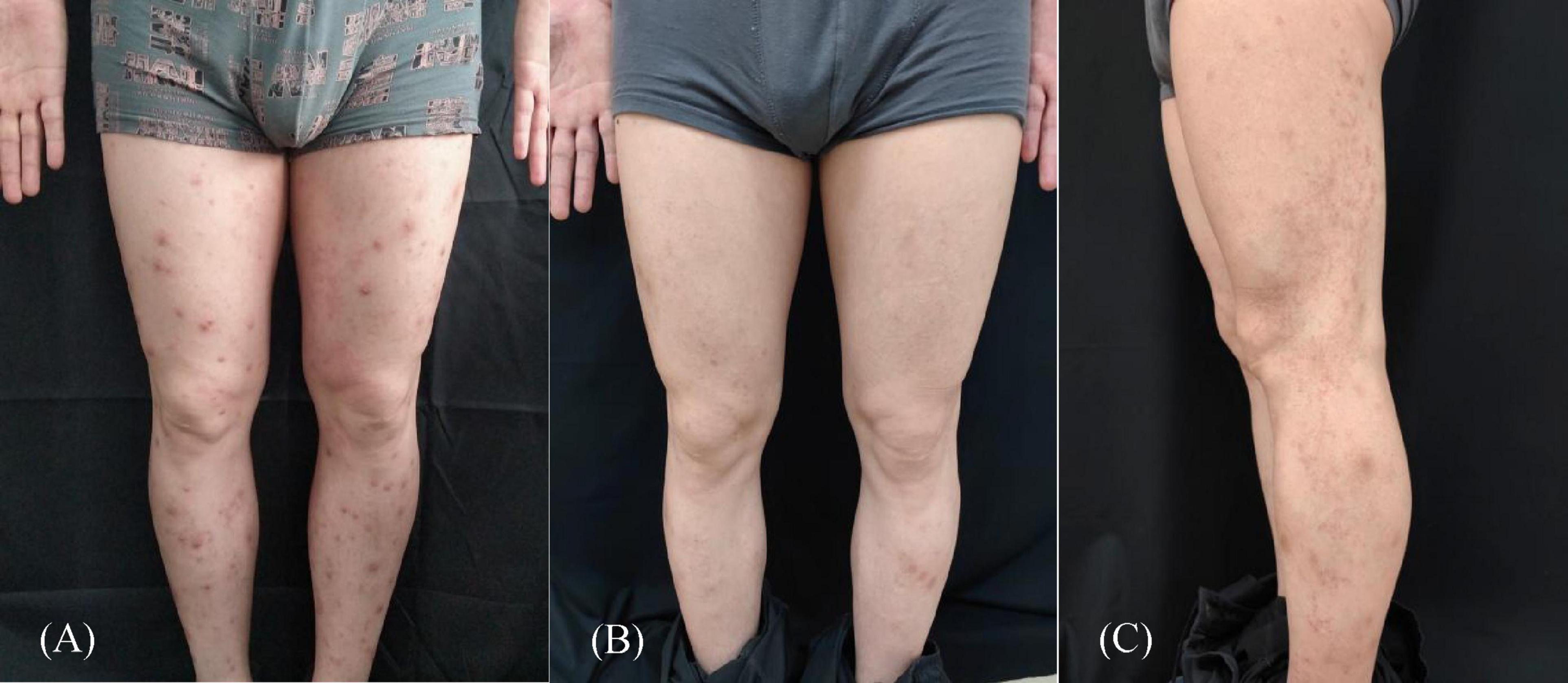
Figure 1. (A) Pruritic erythema and scattered nodules on the patient’s both lower limbs; (B) after 3 months of treatment, the pruritic erythema and scattered nodules disappeared completely; (C) well-defined erythematous squamous papules and plaques on the posterior surface of the left thigh that were linearly arranged along the Blaschko line and extended to the ankle joint.
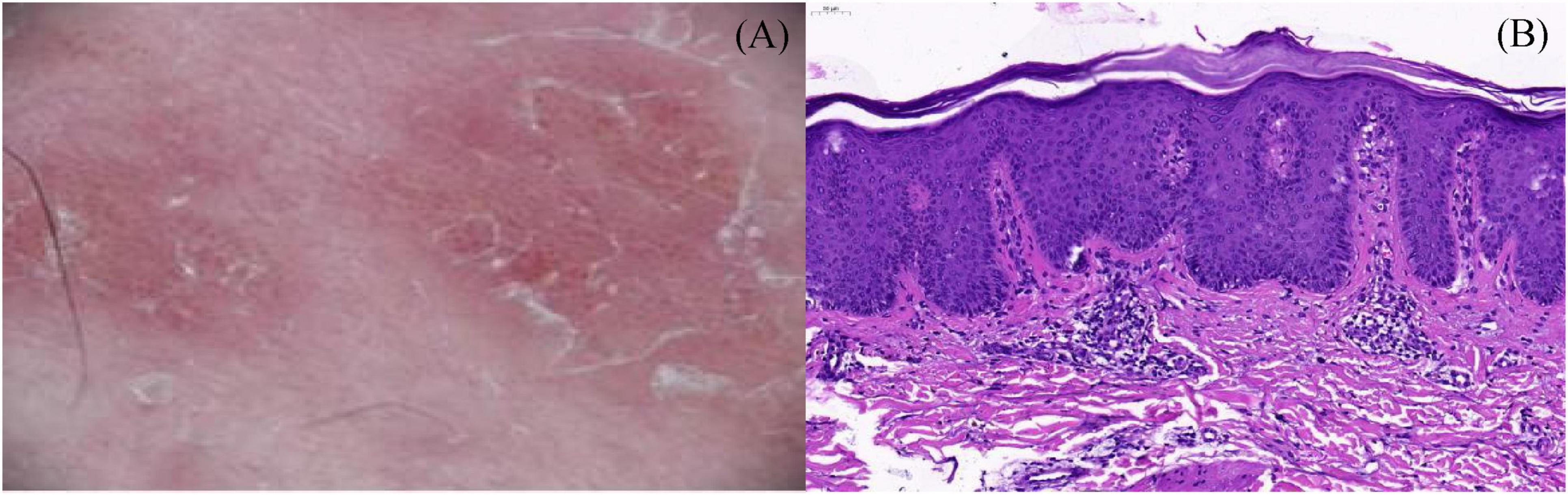
Figure 2. (A) Dermoscopic evaluation detected whitish scales and regularly distributed simple red loops, twisted red loops, and globules; (B) skin biopsy was performed, and histopathology showed hyperkeratosis and parakeratosis, regular acanthosis, thin granular layer, dilated blood vessels at the tip of papillary dermis and perivascular infiltration of lymphocytes (H&E, 200×).
Discussion
Dupilumab is a fully human monoclonal antibody generated against the alpha chain of the interleukin-4 receptor, which inhibits both IL-4 and IL-13 signaling. It was approved by the FDA for the treatment of PN in September 2022 (3). Studies have demonstrated its efficacy and safety, also during COVID-19 (4–6). However, it has been linked to occurrence of paradoxical reactions, such as facial and neck dermatitis (7), urticaria (8), and lichen planus (9).
In recent years, several cases of dupilumab associated with psoriasis have been reported. Psoriasis is a well-known T cell-mediated disease characterized by increased expression of Th1 and Th17 cytokines. Since IL-4 and IL-13 are key mediators of Th2-mediated inflammation, their blockade by dupilumab may induce a shift from Th2-mediated inflammation to Th1/Th17 subsets, causing psoriasis (10). Some of the dupilumab induced psoriasis are classified as vulgaris (10), erythrodermic (11), and pustular (12) types. The recurrence of lesions following the reintroduction of dupilumab strongly supports a causal relationship. To our best knowledge, this is the first case of linear psoriasis, induced by dupilumab.
Linear psoriasis is a rare subtype of psoriasis which exhibits a linear distribution along the Blaschko’s line and mainly involves the extremities. The pathogenesis of linear psoriasis may involve a genetic mosaic phenomenon. Happle proposed that the loss of heterozygosity in somatic cells during the early stages of embryogenesis leads to somatic recombination, resulting in homozygosity for one of the genes associated with psoriasis. In this scenario, one of the daughter cells may become homozygous for a psoriasis-related gene, subsequently serving as the stem cell for a clone that proliferates in a linear fashion throughout the embryonic development of the skin, indicating that cells harboring somatic mutations linked to psoriasis migrate following the lines of Blaschko (13).
However, in order to manifest linear psoriasis, an external/environmental factor or trigger may be involved besides the genetic component. Because linear psoriasis is usually not present at birth and often occurs later in life, about one-third of patients report exogenous triggers or aggravating factors, including medications [e.g., lithium (14) and pabolizumab (15)], climate (e.g., aggravation in winter, resolution in summer), and upper digestive tract infections. Therefore, the following hypothesis was put forth: in our case, the dupilumab induced the expression of chimeric genes in linear psoriasis probably.
Currently, there are no guidelines for the treatment of linear psoriasis. It is recommended that patients with this subtype be treated following the management guidelines for psoriasis vulgaris. For the dupilumab-induced psoriasis, mild to moderate cases respond well to local treatment, including corticosteroids or vitamin D derivatives, and discontinuation of dupilumab is recommended in severe cases (16). Some reports proposed the use of Janus kinase (JAK) inhibitors as potential treatments for atopic dermatitis (AD) and psoriasis complications, such as baricitinib (17) and upadacitinib (18). The safety profile of dupilumab appears to be an important issue and, therefore, long-term monitoring is warranted. In our case, since the skin lesions were not severe, the administration of dupilumab was not terminated but rather extended the interval between injections and, prescribed topical therapy, which successfully controlled the lesions of linear psoriasis.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the studies involving humans because we only retrospectively summarized the patient information. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from patients who come to the hospital of Shanxi Medical University. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WS: Conceptualization, Writing–original draft. KW: Formal analysis, Writing–original draft. YR: Data curation, Writing–original draft. HY: Methodology, Writing–original draft. XL: Formal analysis, Writing–original draft. SG: Writing–review and editing. HL: Data curation, Methodology, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hawsawi K, AlDoboke AW, Alsulami SA, Alamri GE, Alsufi RF. Dupilumab-induced scalp psoriasis in a patient with prurigo nodularis: A case report. Cureus. (2023) 15:e37992. doi: 10.7759/cureus.37992
2. Sørensen JA, Johansen CB, Egeberg A, Thyssen JP, Thomsen SF. Paradoxical psoriasis after dupilumab treatment of prurigo nodularis treated with adalimumab. JAAD Case Rep. (2024) 52:146–9. doi: 10.1016/j.jdcr.2024.06.044
3. Liao V, Cornman HL, Ma E, Kwatra SG. Prurigo nodularis: New insights into pathogenesis and novel therapeutics. Br J Dermatol. (2024) 190:798–810. doi: 10.1093/bjd/ljae052
4. Cao P, Xu W, Jiang S, Zhang L. Dupilumab for the treatment of prurigo nodularis: A systematic review. Front Immunol. (2023) 14:1092685. doi: 10.3389/fimmu.2023.1092685
5. Napolitano M, Fabbrocini G, Neri I, Stingeni L, Boccaletti V, Piccolo V, et al. Dupilumab treatment in children aged 6-11 years with atopic dermatitis: A multicentre, real-life study. Paediatr Drugs. (2022) 24:671–8. doi: 10.1007/s40272-022-00531-0
6. Patruno C, Stingeni L, Fabbrocini G, Hansel K, Napolitano M. Dupilumab and COVID-19: What should we expect? Dermatol Ther. (2020) 33:e13502. doi: 10.1111/dth.13502
7. Jo CE, Finstad A, Georgakopoulos JR, Piguet V, Yeung J, Drucker AM. Facial and neck erythema associated with dupilumab treatment: A systematic review. J Am Acad Dermatol. (2021) 84:1339–47. doi: 10.1016/j.jaad.2021.01.012
8. Mastorino L, Ortoncelli M, Virginia B, Rolla G, Avallone G, Cavaliere G, et al. Dupilumab-induced Urticaria. Dermatol Ther. (2021) 34:e15117. doi: 10.1111/dth.15117
9. Mastorino L, Ortoncelli M, Giura MT, Avallone G, Viola R, Quaglino P, et al. Lichen ruber planus arising during dupilumab treatment for atopic dermatitis. Ital J Dermatol Venerol. (2022) 157:449–50. doi: 10.23736/S2784-8671.21.07070-5
10. Safa G, Paumier V. Psoriasis induced by dupilumab therapy. Clin Exp Dermatol. (2019) 44:e49–50. doi: 10.1111/ced.13901
11. Tracey EH, Elston C, Feasel P, Piliang M, Michael M, Vij A. Erythrodermic presentation of psoriasis in a patient treated with dupilumab. JAAD Case Rep. (2018) 4:708–10. doi: 10.1016/j.jdcr.2018.05.014
12. Liu Y, Liu L, Zhou H, Chen G, Wen C, Wu R. Pustular psoriasis induced by dupilumab: A case report. J Inflamm Res. (2024) 17:6389–94. doi: 10.2147/JIR.S476297
13. Happle R. Somatic recombination may explain linear psoriasis. J Med Genet. (1991) 28:337. doi: 10.1136/jmg.28.5.337
14. Garg S, Kumar A, Bhalla M, Kaur A, Punia RPS. Lithium-induced linear psoriasis: A rare presentation. J Clin Aesthet Dermatol. (2019) 12:38–9.
15. Huang PW, Chu CY. Pembrolizumab-induced linear psoriasis. Lung Cancer. (2020) 146:378–9. doi: 10.1016/j.lungcan.2020.06.012
16. Su Z, Zeng YP. Dupilumab-associated psoriasis and psoriasiform manifestations: A scoping review. Dermatology. (2023) 239:646–57. doi: 10.1159/000530608
17. Ali K, Wu L, Qiu Y, Li M. Case report: Clinical and histopathological characteristics of psoriasiform erythema and de novo IL-17A cytokines expression on lesioned skin in atopic dermatitis children treated with dupilumab. Front Med (Lausanne). (2022) 9:932766. doi: 10.3389/fmed.2022.932766
Keywords: dupilumab, linear psoriasis, prurigo nodularis, case report, chimeric gene
Citation: Sun W, Wang K, Ren Y, Yuan H, Lang X, Guo S and Liu H (2025) Case report: Dupilumab-induced linear psoriasis: a rare presentation. Front. Med. 11:1527257. doi: 10.3389/fmed.2024.1527257
Received: 13 November 2024; Accepted: 20 December 2024;
Published: 27 January 2025.
Edited by:
Maddalena Napolitano, University of Molise, ItalyReviewed by:
Miquel Armengot-Carbó, Hospital General Universitari de Castelló (HGUCS), SpainLuca Potestio, University of Naples Federico II, Italy
Copyright © 2025 Sun, Wang, Ren, Yuan, Lang, Guo and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuping Guo, Z3NwNjY4OEBzaW5hLmNvbQ==; Hongye Liu, Ymxld3Nub3cxNjhAc2luYS5jb20=
 Wen Sun
Wen Sun Kexin Wang1
Kexin Wang1 Xiaoqing Lang
Xiaoqing Lang Shuping Guo
Shuping Guo Hongye Liu
Hongye Liu