- 1School of Optical Electrical and Computer Engineering, University of Shanghai for Science and Technology, Shanghai, China
- 2Affiliated Eye Hospital of Nanjing Medical University, Nanjing, China
- 3Department of Ophthalmology, Xinhua Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Purpose: This study compares the corneal temperature in dry eyes with normal eyes via high-resolution infrared thermography.
Methods: A total of 86 participants were enrolled, with 40 and 46 participants in the dry eye disease (DED) and control groups, respectively. All participants underwent non-invasive breakup time (NIBUT) measurement, an Ocular Surface Disease Index (OSDI) questionnaire and ocular thermography.
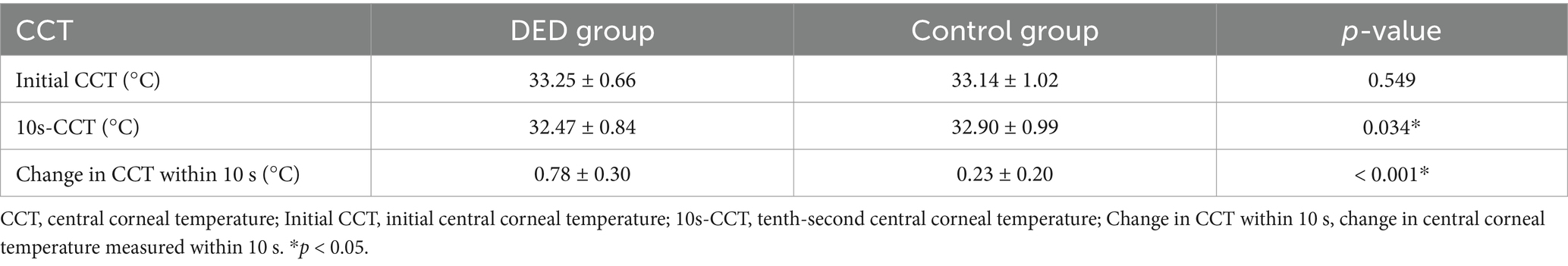
Results: In the DED group, the mean initial central corneal temperature (initial CCT) is 33.25 ± 0.66°C, the tenth-second central corneal temperature (10s-CCT) is 32.47 ± 0.84°C, and the mean change in central corneal temperature measured within 10 s (change in CCT within 10 s) is 0.78 ± 0.30°C. For the controls, the initial CCT, 10s-CCT, and change in CCT within 10 s are 33.14 ± 1.02°C, 32.90 ± 0.99°C, and 0.23 ± 0.20°C, respectively. Except for the initial CCT (p = 0.549), significant differences are observed in the 10s-CCT (p = 0.034) and the change in CCT within 10 s (p < 0.001) between the two groups. The standard deviation of the temperature values within the region of interest (SD of TVs within ROI) on the central cornea is calculated to compare the uniformity of corneal temperature. In the DED group, the mean standard deviation of the initial temperature values within the region of interest (SD of initial TVs within ROI) is similar to that in the control group (0.23 ± 0.07°C vs. 0.22 ± 0.05°C, p = 0.926). In contrast, the mean standard deviation of the tenth-second temperature values within the region of interest (SD of 10s-TVs within ROI) in the DED group is greater than that in the control group, and there is a significant difference (0.44 ± 0.20°C vs. 0.35 ± 0.15°C, p = 0.016). In the DED group, the mean change in CCT within 3 s after tear film break-up is significantly greater than that before tear film break-up (0.19 ± 0.08°C vs. 0.10 ± 0.10°C, p < 0.001).
Conclusion: As the time with eyes open increases, dry eyes present a significantly faster decrease in central corneal temperature (CCT) and a significantly worse uniformity of corneal temperature compared with normal eyes.
1 Introduction
Dry eye disease (DED) is a common ophthalmic disorder that can lead to ocular discomfort, reduced visual acuity, and a decline in both visual function and overall quality of life (1–3). It has been reported that 25% of patients attending ophthalmic clinics exhibit symptoms associated with dry eye syndrome (4, 5). According to the latest research updates, the Tear Film and Ocular Surface Society (TFOS) Dry Eye Workshop (DEWS) II has updated the definition of DED to describe it as a multifactorial disease affecting the ocular surface. This condition is marked by a disruption in the tear film’s homeostasis and is associated with ocular symptoms. Key factors in its development include tear film instability and hyperosmolarity, inflammation and damage to the ocular surface, as well as neurosensory abnormalities (6).
The impaired stability of the tear film is a key diagnostic criterion for identifying abnormalities in the tear film. The TFOS DEWS II Diagnostic Methodology report outlines various methods for assessing tear film stability, including fluorescein breakup time (FBUT) (7, 8), non-invasive tear breakup time (NIBUT) (9, 10), thermography (11, 12), osmolarity variability (13, 14), and tear evaporation rate (15, 16), among others. Among these ways, the thermography has facilitated assessments of ocular surface temperature (OST) through a non-invasive methodology (11, 12, 17–19). It is widely recognized that OST is associated primarily with the tear film (20). Studies have shown that OST and FBUT can be measured simultaneously (21, 22), revealing that the areas experiencing cooling and breakup of the ocular surface coincide (22). There is a direct correlation between FBUT and ocular surface cooling, suggesting that localized evaporation increases contribute to the thinning and breakup of the tear film (21). Furthermore, technological progress in instrumentation has enhanced the ability to measure OST with greater accuracy, resolution, and speed.
Recent and historical research has highlighted the distinctions in OST between normal eyes and those affected by DED (11, 12, 23–25). However, there has been limited analysis of the details of OST. The main reason for this is that previous infrared thermography devices had low pixel resolution, making it difficult to capture detailed differences in OST. Nevertheless, assessing these detailed differences in OST is also important for evaluating the differences between dry eyes and normal eyes (12, 26). Therefore, we compared the central corneal temperature (CCT) and uniformity of the corneal temperature between dry eyes and normal eyes via high-resolution infrared thermography to further explore the role of infrared thermography in the diagnosis of dry eyes.
2 Materials and methods
2.1 Participants
All procedures conducted in this study were in accordance with the Declaration of Helsinki. Approval for the research was granted by the Ethics Committee of Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Approval No. XHEC-D-2023-047). Informed consent was obtained from all participants before their enrolment in the study. After providing informed consent, a total of 86 consecutive Asian individuals, comprising 40 individuals with DED and 46 individuals with normal ocular conditions, participated in this observational study. The diagnosis of DED was established based on the criteria established by the Tear Film and Ocular Surface Society (TFOS) Dry Eye Workshop (DEWS) II Diagnostic Methodology Subcommittee (27).
The exclusion criteria for the study included individuals with a history of ocular surgery or trauma, those under the age of 18, patients who have undergone chalazion excision, individuals experiencing acute inflammation, and those with a documented history of blepharal or periorbital skin conditions or allergies within the past month. Additionally, participants with severe dry eyes accompanied by corneal epithelial defects, limbic keratitis, pterygium, corneal neovascularization, glaucoma, rheumatic autoimmune diseases, or a history of herpes zoster infection were excluded, as well as pregnant individuals, contact lens users, and those with a history of antihistamine or antidepressant medication use.
2.2 Non-invasive breakup time
Non-invasive breakup time (NIBUT) was evaluated noninvasively via the Keratograph 5 M (Oculus, Germany) topographer. Three consecutive measurements were obtained, and the median value was used for the diagnosis of DED (28).
2.3 Ocular surface disease index
The Ocular Surface Disease Index (OSDI) was used to evaluate and quantify the symptoms associated with DED. The questionnaire comprises 12 items, which can be aggregated into a score that spans from 0, indicating the absence of symptoms, to 100, indicating severe symptoms.
2.4 Ocular thermography
Thermal imaging and video recordings were obtained via an infrared thermal camera (Xcore LT640, IRay Tech Co., Ltd., China) operating within the long-wave spectrum (8–14 μm). The camera is characterized by a frame rate of 30 Hz and a spatial resolution of 640 × 512 pixels, with each pixel measuring 14 × 14 μm, and an accuracy of measurement of ±2%. Before the initiation of testing, the infrared thermal camera was preheated for 20 min, employing automatic non-uniformity correction with a correction interval of 1 min. Furthermore, each participant was instructed to rest for 10 min in the examination room. The environmental conditions, specifically temperature and humidity, were monitored and regulated to maintain a consistent range of 22 ± 1°C and 40 ± 5%, respectively. Measurements were conducted during standard hospital operating hours, specifically from 8 AM to 11 PM. The measurement protocol included the following steps: 1. The participants were directed to maintain a stable head position while their chin and forehead rested and their gaze was focused on a predetermined focal point; 2. The infrared thermal camera recorded the temperature of each participant’s forehead; 3. The participants were instructed to open their eyes, blink, and subsequently maintain their eyes in an open position for 10 s, during which the infrared thermal camera captured a video of the right eye.
2.5 Image acquisition and analysis methods
Image post-processing was conducted via custom-developed MATLAB scripts (MathWorks, Inc.). The average temperature readings were obtained from two specific locations: the forehead (Figure 1A) and the ocular surface (Figure 1B). The designated region of interest (ROI) on the ocular surface corresponds to the central cornea, which has a diameter of 8 mm (29, 30).
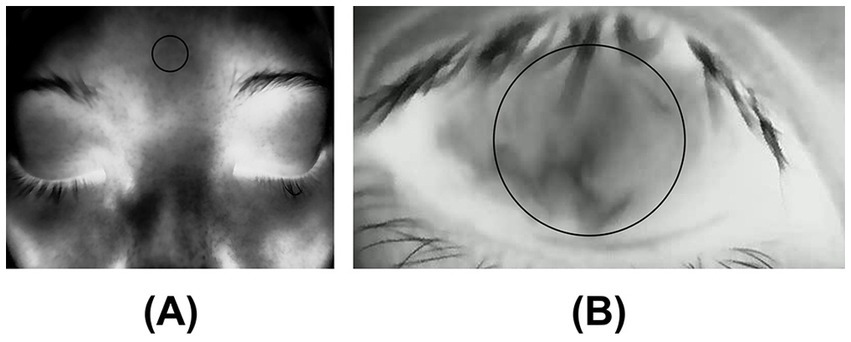
Figure 1. (A) Forehead temperature: The temperature was measured at the forehead; (B) Central corneal temperature (CCT): The average temperature was measured within ROI (8mmφ) on the central cornea.
The measurement of ROI is semiautomatic. This infrared thermal camera allows for customizable ROI, providing the average temperature within the region. Therefore, we predefined a circular ROI with a diameter of 160 pixels on the display interface of the infrared thermal camera. After focusing, this area corresponded to a circular region of approximately 8 mm in diameter on the central cornea. Data collection commenced once the eye was adequately open, ensuring that the upper eyelid was no longer elevated, thereby allowing the ROI on the ocular surface to be unobstructed by the eyelid or eyelashes. This approach facilitated the acquisition of the earliest possible measurements of central corneal temperature (CCT).
Upon achieving adequate eyelid elevation, the ROI was positioned at the center of the ocular surface, ensuring that there was no obstruction from the eyelids or eyelashes, to assess variations in the CCT. This initial measurement was designated 0 s. A total of 11 infrared thermal images of the ocular surface were subsequently extracted from an infrared thermal video at one-second intervals over 10 s for each eye. At each time point, the mean temperature of the central cornea was calculated and recorded. As a result, we were able to analyse the alterations in infrared thermal images of CCTs within the 10 s following the opening of the eye.
The thermogram was stored in irg format. According to the file in irg format, we calculated the standard deviation of the temperature values within the ROI (SD of TVs within ROI) to compare the uniformity of corneal temperature between the DED and control groups (29).
2.6 Statistical methods
Categorical variables were analysed through frequency rates and percentages, whereas continuous variables that followed a normal distribution were summarized using means and standard deviations. To assess the sex ratio between the DED group and the control group, a chi-square test was employed. Unpaired t-tests were used to evaluate differences in age, forehead temperature, non-invasive breakup time (NIBUT), Ocular Surface Disease Index (OSDI), initial central corneal temperature (initial CCT), tenth-second central corneal temperature (10s-CCT), change in central corneal temperature measured within 10 s (change in CCT within 10 s), standard deviation of the initial temperature values within the ROI (SD of initial TVs within ROI), and standard deviation of the tenth-second temperature values within the ROI (SD of 10s-TVs within ROI) between the DED and control groups. Unpaired t-tests were also used to evaluate the differences in initial CCT, 10s-CCT and change in CCT within 10 s between males and females in the only DED group, the only control group, and both groups. We used paired t-tests to evaluate the change in CCT within 1 s before and after tear film break-up, the change in CCT within 2 s before and after tear film break-up, and the change in CCT within 3 s before and after tear film break-up. All the statistical analyses were performed via the Statistical Package for the Social Sciences (SPSS) version 22.0, with a significance threshold set at p < 0.05 (two-tailed).
3 Results
3.1 Participants and baseline characteristics
A total of 86 participants were recruited for the study, comprising 40 individuals (17 males and 23 females) in the DED group and 46 individuals (24 males and 22 females) in the control group. Statistical analysis revealed no significant differences in age (p = 0.575), sex (p = 0.370), or forehead temperature (p = 0.076) between the two groups. Significant differences were observed in both the NIBUT (p < 0.001) and OSDI (p < 0.001) between the two groups, as detailed in Table 1.
3.2 Central corneal temperature in the DED and control groups
Compared with the control group, for participants diagnosed with DED, the mean initial CCT was greater, however, this difference did not reach statistical significance (p = 0.549). In contrast, the mean 10s-CCT of participants in the DED group was lower than that of participants in the control group, and there is a significant difference (p = 0.034). The change in CCT within 10 s of the DED group occurred faster than that of the control group, and this change was significantly different (p < 0.001). The alterations observed in the infrared thermal images of the ocular surface were conspicuous in the DED group (Figure 2A). In contrast, no obvious changes were observed in the control group (Figure 2B). The temperature scale ranges from 30°C to 38°C (Figure 2C). Table 2 and Figure 3 provide a detailed comparison of these findings, whereas Figure 4 illustrates the continuous variation in CCT over the 10 s for both the DED and control groups.
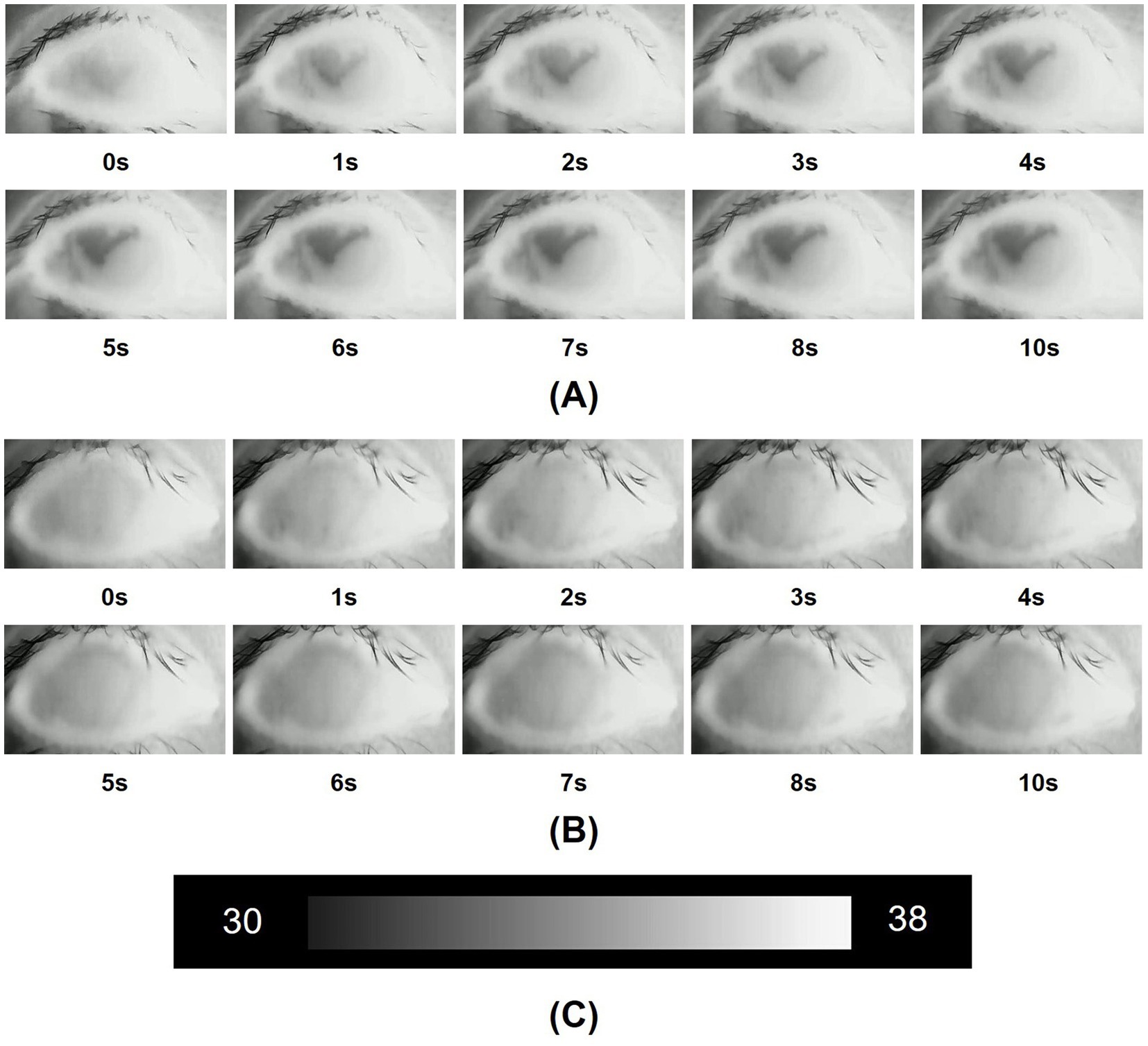
Figure 2. (A) Infrared thermal images of the dry eye were obtained over 10 s after the beginning of the measurements. In a dry eye, the ocular surface temperature (OST) gradually changes from a high gray level to a low gray level, signifying a drop in temperature over the 10-s period; (B) Infrared thermal images of the normal eye were obtained over 10 s after the beginning of the measurements. In a normal eye, the gray level of the images shows almost no variation, and the temperature stays stable over the 10-s period; (C) The temperature scale ranged from 30°C to 38°C.
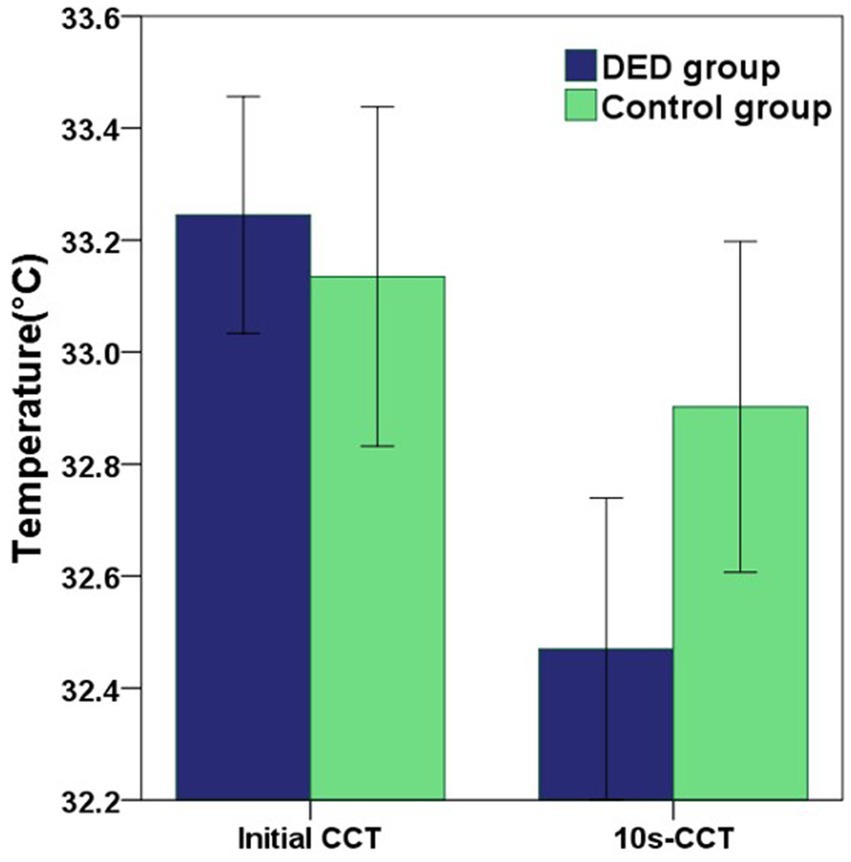
Figure 3. The graph showing the differences in initial CCT and 10s-CCT between the DED and control groups. Error bars = 95% confidence interval. The comparisons of mean values were performed using unpaired t-tests. Compared with the control group, the mean initial CCT in the DED group was greater, but there was no significant difference (p = 0.549). However, the mean 10s-CCT in the DED group was significantly lower than that in the control group (p = 0.034).
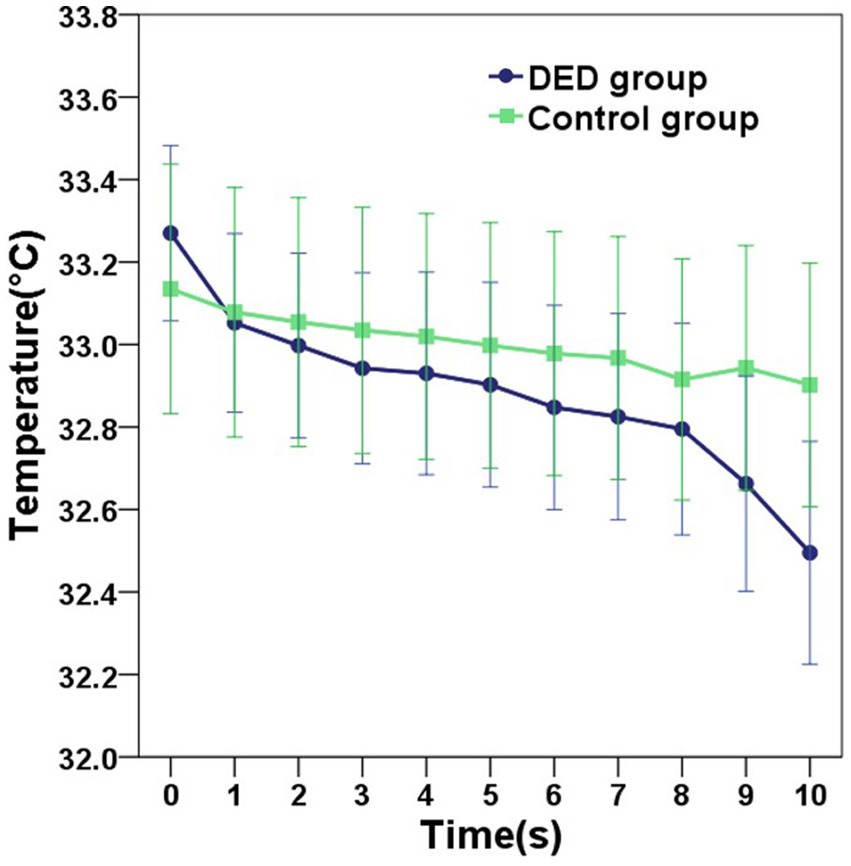
Figure 4. The graph showing the continuous variation in the central corneal temperature (CCT) of the DED and control groups within 10 s. Error bars = 95% confidence interval. Compared with the control group, the mean CCT in the DED group decreased more rapidly over the 10-s period.
3.3 Comparison of the standard deviation of the temperature values within the ROI between the DED and control groups
In the DED group, the mean standard deviation of the initial temperature values within the ROI (SD of initial TVs within ROI) was similar to that in the control group (p = 0.926). In contrast, the mean standard deviation of the tenth-second temperature values within the ROI (SD of 10s-TVs within ROI) in the DED group was greater than that in the control group, and there is a significant difference (p = 0.016). Figures 5A,B are the initial and tenth-second infrared thermal images of dry eyes, respectively. Figures 5C,D are the corresponding ROIs. Figures 6A,B are the initial and tenth-second infrared thermal images of normal eyes, respectively. Figures 6C,D are the corresponding ROIs. A detailed comparison of these findings is shown in Table 3.
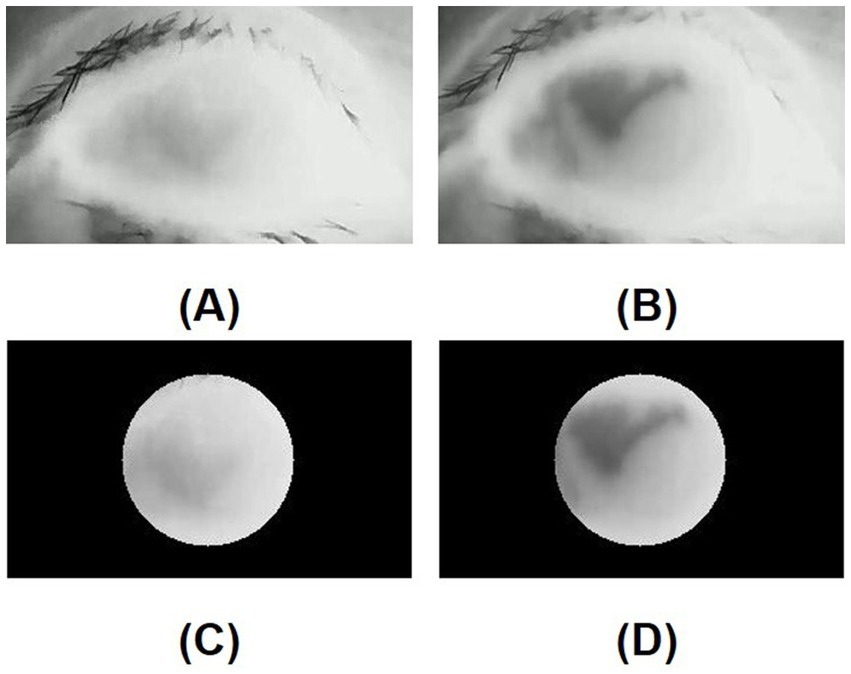
Figure 5. (A) Initial infrared thermal image of the dry eye; (B) Tenth-second infrared thermal image of the dry eye; (C) Region of interest (ROI, 8mmφ) of the initial infrared thermal image on the central cornea of the dry eye; (D) Region of interest (ROI, 8mmφ) of tenth-second infrared thermal image on the central cornea of the dry eye; In a dry eye, after opening the eye, a lower temperature area is generated due to the instability of the tear film.
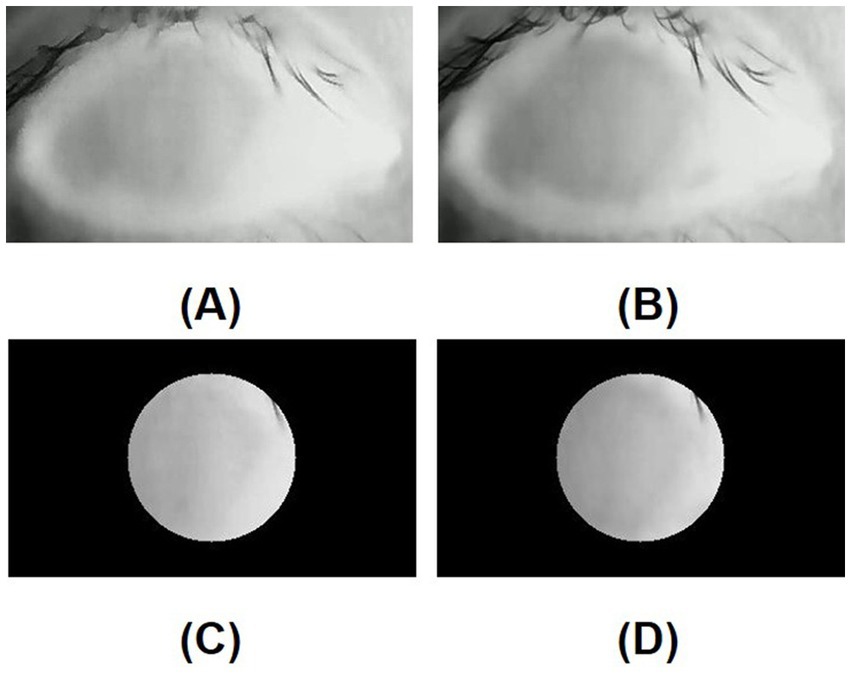
Figure 6. (A) Initial infrared thermal image of the normal eye; (B) Tenth-second infrared thermal image of the normal eye; (C) Region of interest (ROI, 8mmφ) of initial infrared thermal image on the central cornea of the normal eye; (D) Region of interest (ROI, 8mmφ) of tenth-second infrared thermal image on the central cornea of the normal eye; In a normal eye, after opening the eye, a lower temperature area is not generated due to the stability of the tear film.
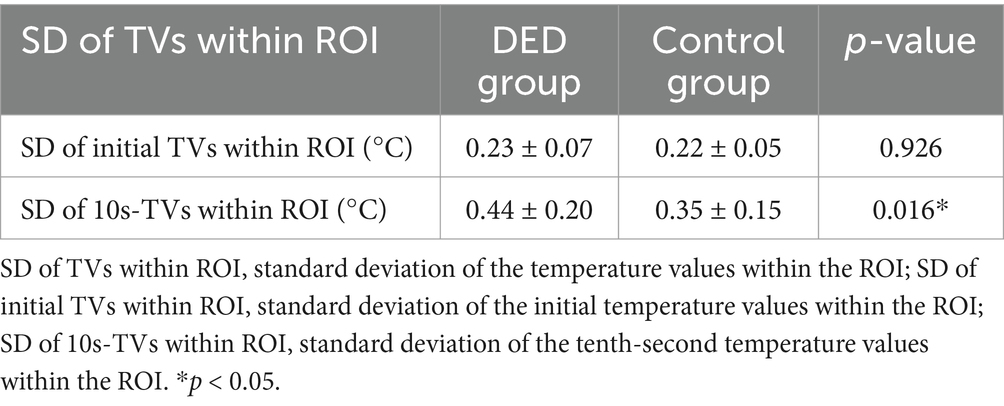
Table 3. Standard deviation of the temperature values within the ROI between the DED and control groups.
3.4 Comparisons of the change in central corneal temperature before and after tear film break-up in the DED group
In the DED group, the mean change in CCT within 1 s after tear film break-up was greater than that before tear film break-up, but there was no significant difference (p = 0.281). The mean changes in CCT within 2 s before and after tear film break-up showed the same result (p = 0.277). The mean change in CCT within 3 s after tear film break-up was significantly greater than that before tear film break-up, and there was a significant difference (p < 0.001). Detailed comparisons can be found in Figure 7 and Table 4.
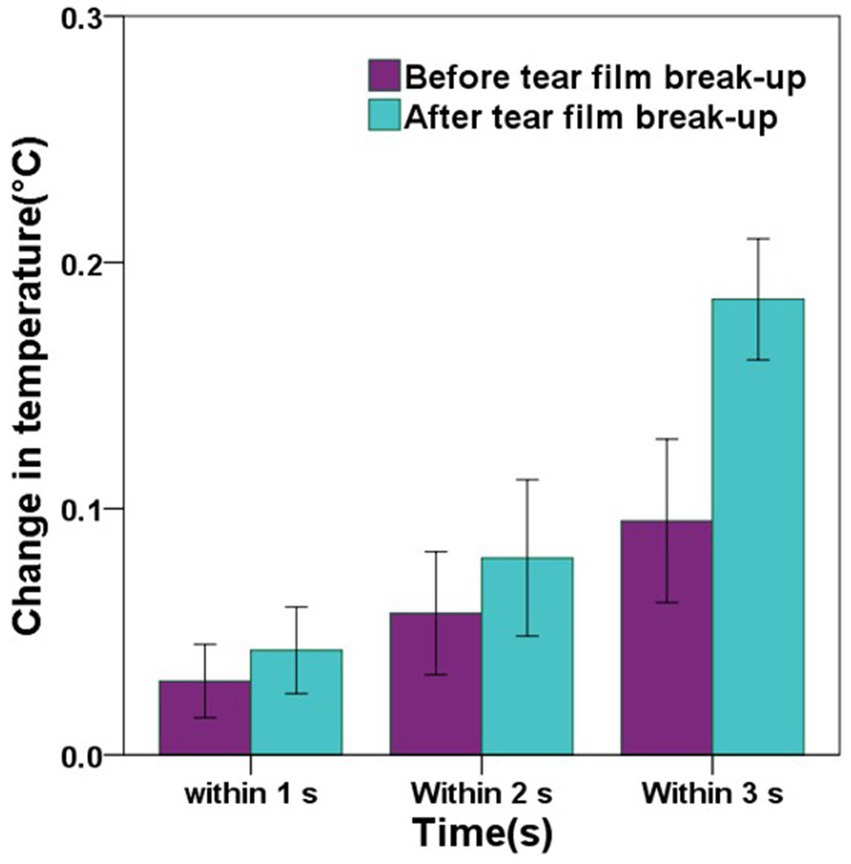
Figure 7. The graph showing the comparisons of the change in central corneal temperature (CCT) before and after tear film break-up in the DED group. Error bars = 95% confidence interval. The comparisons of mean values were performed using paired t-tests. In the DED group, the changes in CCT within 1 s, 2 s, and 3 s after tear film break-up were greater than those before tear film break-up, but only the change in CCT within 3 s before and after tear film break-up has a significant difference (p < 0.001).
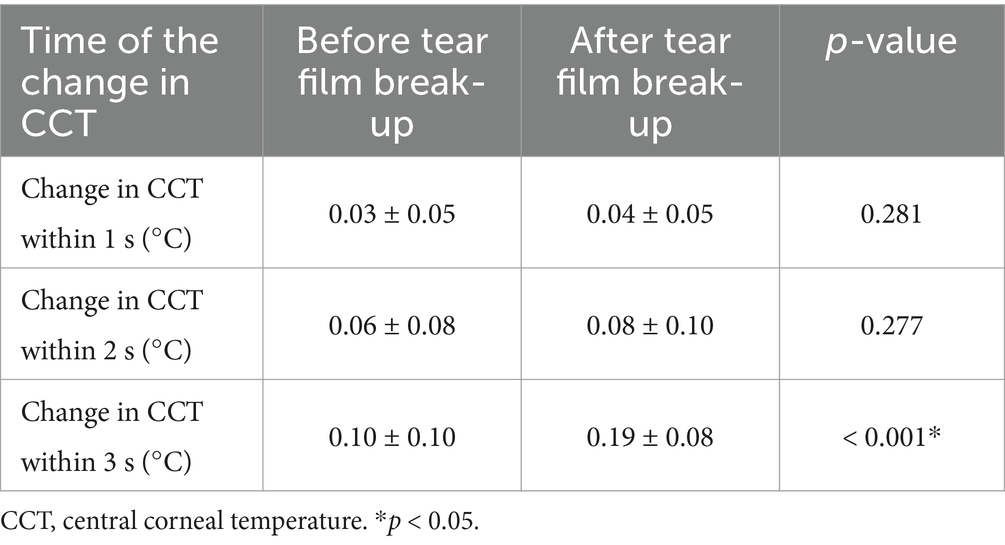
Table 4. Comparisons of the change in central corneal temperature before and after tear film break-up in the DED group.
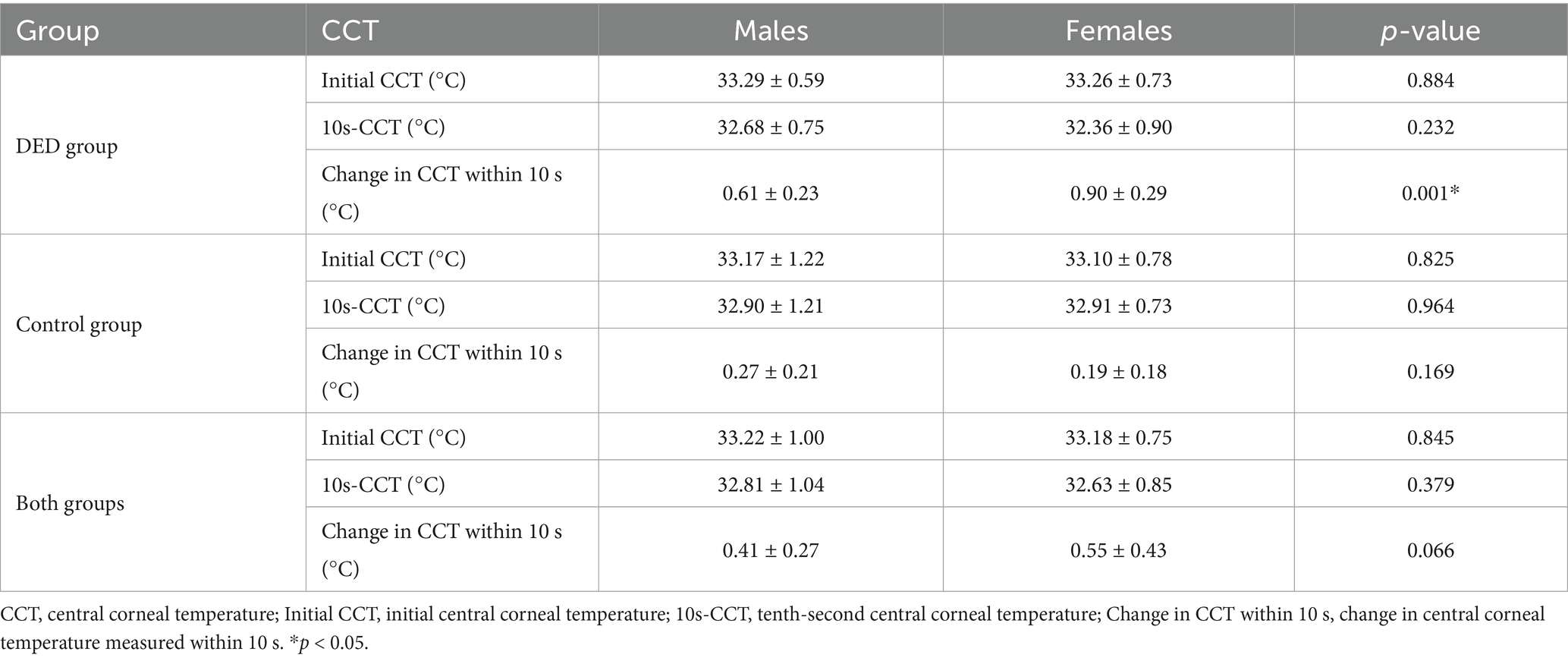
3.5 Differences in the central corneal temperature between males and females
For the participants in the DED group, the mean initial CCT and mean 10s-CCT of males were similar to those of females, with no significant difference (p = 0.884, p = 0.232, respectively). However, the mean change in CCT within 10 s of females was greater than that of males, and this difference was significant (p = 0.001). In the control group, there were no significant differences in initial CCT, 10s-CCT, and change in CCT within 10 s between males and females (p = 0.825, p = 0.964, and p = 0.169, respectively). A comparison of all males and females in both groups showed no significant differences in initial CCT and 10s-CCT (p = 0.845, p = 0.379, respectively). Although there was also no significant difference in change in CCT within 10 s between males and females in both groups, the mean change in CCT within 10 s of females was greater than that of males. Detailed comparisons are shown in Table 5.
4 Discussion
Compared with that of normal eyes, the mean initial central corneal temperature (initial CCT) of dry eyes was greater, although there was no significant difference between the two groups. This research result is consistent with the findings of several previous studies (31, 32). The underlying factors contributing to this outcome may be associated with inflammation (33). In dry eyes, there may be inflammatory manifestations on the ocular surface (34, 35), which can potentially result in an elevated central corneal temperature (CCT). Additionally, the thickness of the tear film may be one of the reasons for this result. It has been noted in the literature that infrared radiation within the wavelength range of 8 to 13 μm is absorbed by water, with the extent of absorption being contingent upon the thickness of the water layer (31). Because the tear film is less thick in dry eyes (36, 37), less infrared radiation is absorbed by the tear film, resulting in a higher CCT. This finding may also be related to the stability of the tear film (25, 38). However, some researchers have reported that the initial CCT of dry eyes is lower than that of normal eyes (11, 30). This difference could be attributed to a combination of technical factors, such as the precision of the instruments, the conditions under which the measurements are taken, and the methodologies utilized (18).
Compared with normal eyes, dry eyes presented a significantly faster decrease in CCT. This result is consistent with the results of other studies (11, 12, 25, 30). Due to the rapid decrease in CCT of dry eyes, the mean tenth-second central corneal temperature (10s-CCT) of participants in the DED group was significantly lower than that of participants in the control group (11). As previously reported in the literature, DED is characterized by two primary pathogenic mechanisms: diminished aqueous tear production resulting from lacrimal gland dysfunction and increased evaporation of the tear film (24, 27, 39, 40). Consequently, both the rate of tear secretion and the stability of the tear film may influence the changes in CCT following a blink (25). Mapstone posits that the thermal dynamics associated with the ocular surface can be elucidated through the mechanisms of convection, radiation, and evaporation. Specifically, the movement of air across a surface results in heat dissipation via convection, whereas radiant heat loss transpires when thermal energy is transferred to cooler surrounding temperatures (41). When the eye is open and the tear film evaporates, the ocular surface experiences a decrease in temperature. This cooling effect occurs as a result of the positive latent heat of vaporization, whereby heat is dissipated into the surrounding environment during the transition from liquid to gas (42). Compared with that of normal eyes, the tear film stability of dry eyes was lower, increasing the tear evaporation rate and resulting in a greater change in the CCT.
In this study, the standard deviation of the temperature values within the ROI (SD of TVs within ROI) on the central cornea was assessed to evaluate the uniformity of corneal temperature between the DED group and the control group. The standard deviation of the initial temperature values within the ROI (SD of initial TVs within ROI) of dry eyes was not significantly different from that of normal eyes. However, the mean standard deviation of the tenth-second temperature values within the ROI (SD of 10s-TVs within ROI) in the DED group was significantly greater than that in the control group. When the tear film is abnormal, it affects the rate of evaporation in some areas of the ocular surface. This disrupts the uniformity of the corneal temperature, manifested as an increase in the SD of TVs within ROI. For normal eyes, the tear film is stable, and the variation in CCT is small and smooth during the evaporation process. For dry eyes, the tear film remains relatively intact shortly after opening the eyes and the uniformity of the corneal temperature shows little difference compared to normal eyes. However, as the time with eyes open increases, the instability of the tear film results in a greater degree of variation in the CCT and the generation of a lower temperature area on the tear film. In other words, tear film break-up compromised the uniformity of the corneal temperature (12, 26).
In the DED group, the mean change in CCT within 3 s after tear film break-up was significantly greater than that before tear film break-up, and there was a significant difference. When the eyes are open, the tear film undergoes evaporation, causing it to become thinner and eventually break up. Tear film break-up results in a lower temperature area on the tear film (26). As the area of tear film break-up continues to increase, the decrease in CCT also accelerates.
For the participants in the DED group, the mean change in CCT within 10 s of females was significantly greater than that of males. In previous studies, it has been found that DED is more prevalent in females compared to males (43, 44). The gender differences in corneal temperature in the DED group may help us further understand the pathogenesis of the dry eye, which is a topic worth studying.
This study also has some limitations. Firstly, it does not classify DED, and different types of the DED may exhibit variations in high-resolution ocular thermography, which warrants further investigation. Secondly, although we explored the gender differences in corneal temperature, the sample size was small, and a larger sample study is needed for validation.
According to this study, there are significant differences in CCT between dry eyes and normal eyes. The evaporation of the tear film results in a cooling of the ocular surface (42), so measuring the changes in CCT during the inter-blink period can be used as an index of tear film stability. This index may have greater clinical value for the diagnosis of evaporative dry eyes. At the same time, the uniformity of the corneal temperature can indicate whether there is a tear film break-up. Additionally, doctors can observe high-resolution infrared thermography images and videos to assist in diagnosing dry eyes. Compared with the fluorescein breakup time (FBUT), ocular thermography does not require fluorescein staining and is relatively simple to perform. Compared with the non-invasive tear breakup time (NIBUT), ocular thermography allows for direct observation of tear film changes over time without the Placido disk. These aspects highlight the potential of ocular thermography in the diagnosis of DED. However, the sensitivity of ocular thermography to environmental conditions and the need for precise calibration present challenges for its clinical use.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CW: Writing – original draft. YuH: Methodology, Software, Writing – review & editing. BX: Methodology, Software, Writing – review & editing. BL: Methodology, Supervision, Writing – review & editing. SZ: Methodology, Supervision, Writing – review & editing. GC: Formal analysis, Writing – review & editing. YaH: Data curation, Writing – review & editing. ZG: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
DED, Dry eye disease; FBUT, Fluorescein breakup time; NIBUT, Non-invasive breakup time; OSDI, Ocular Surface Disease Index; ROI, Region of interest; OST, Ocular surface temperature; CCT, Central corneal temperature; Initial CCT, Initial central corneal temperature; 10s-CCT, Tenth-second central corneal temperature; Change in CCT within 10 s, Change in central corneal temperature measured within 10 s; SD of TVs within ROI, Standard deviation of the temperature values within the ROI; SD of initial TVs within ROI, Standard deviation of the initial temperature values within the ROI; SD of 10s-TVs within ROI, Standard deviation of the tenth-second temperature values within the ROI.
References
1. Gayton, J. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. (2009) 2009:405. doi: 10.2147/OPTH.S5555
2. O’Brien, PD, and Collum, LMT. Dry eye: diagnosis and current treatment strategies. Curr Allergy Asthma Rep. (2004) 4:314–9. doi: 10.1007/s11882-004-0077-2
3. Bron, AJ, de Paiva, CS, Chauhan, SK, Bonini, S, Gabison, EE, Jain, S, et al. TFOS DEWS II pathophysiology report. Ocul Surf. (2017) 15:438–510. doi: 10.1016/j.jtos.2017.05.011
4. Tsubota, K, Yokoi, N, Shimazaki, J, Watanabe, H, Dogru, M, Yamada, M, et al. New perspectives on dry eye definition and diagnosis: a consensus report by the Asia dry eye society. Ocul Surf. (2017) 15:65–76. doi: 10.1016/j.jtos.2016.09.003
5. Rosenthal, P, and Borsook, D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf. (2012) 10:2–14. doi: 10.1016/j.jtos.2012.01.002
6. Craig, JP, Nichols, KK, Akpek, EK, Caffery, B, Dua, HS, Joo, C-K, et al. TFOS DEWS II definition and classification report. Ocul Surf. (2017) 15:276–83. doi: 10.1016/j.jtos.2017.05.008
7. Cardona, G, Serés, C, Quevedo, L, and Augé, M. Knowledge and use of tear film evaluation tests by Spanish practitioners. Optom Vis Sci. (2011) 88:1106–11. doi: 10.1097/OPX.0b013e3182231b1a
8. Downie, LE, Keller, PR, and Vingrys, AJ. An evidence-based analysis of Australian optometrists’ dry eye practices. Optom Vis Sci. (2013) 90:1385–95. doi: 10.1097/OPX.0000000000000087
9. Best, N, Drury, L, and Wolffsohn, JS. Clinical evaluation of the oculus Keratograph. Contact Lens Anterior Eye. (2012) 35:171–4. doi: 10.1016/j.clae.2012.04.002
10. Gumus, K, Crockett, CH, Rao, K, Yeu, E, Weikert, MP, Shirayama, M, et al. Noninvasive assessment of tear stability with the tear stability analysis system in tear dysfunction patients. Investig Opthalmol Visual Sci. (2011) 52:456–61. doi: 10.1167/iovs.10-5292
11. Kamao, T, Yamaguchi, M, Kawasaki, S, Mizoue, S, Shiraishi, A, and Ohashi, Y. Screening for dry eye with newly developed ocular surface thermographer. Am J Ophthalmol. (2011) 151:782–791.e1. doi: 10.1016/j.ajo.2010.10.033
12. Su, TY, Hwa, CK, Liu, PH, Wu, MH, Chang, DO, Su, PF, et al. Noncontact detection of dry eye using a custom designed infrared thermal image system. J Biomed Opt. (2011) 16:046009. doi: 10.1117/1.3562964
13. Keech, A, Senchyna, M, and Jones, L. Impact of time between collection and collection method on human tear fluid Osmolarity. Curr Eye Res. (2013) 38:428–36. doi: 10.3109/02713683.2013.763987
14. Gilbard, JP, Farris, RL, and Santamaria, J. Osmolarity of tear microvolumes in Keratoconjunctivitis Sicca. Arch Ophthalmol. (1978) 96:677–81. doi: 10.1001/archopht.1978.03910050373015
15. Rolando, M, and Refojo, MF. Tear evaporimeter for measuring water evaporation rate from the tear film under controlled conditions in humans. Exp Eye Res. (1983) 36:25–33. doi: 10.1016/0014-4835(83)90086-6
16. TREES, GR, and TOMLINSON, A. Effect of artificial tear solutions and saline on tear film evaporation. Optom Vis Sci. (1990) 67:886–90. doi: 10.1097/00006324-199012000-00002
17. Su, T-Y, Ho, W-T, Chiang, S-C, Lu, C-Y, Chiang, HK, and Chang, S-W. Infrared thermography in the evaluation of meibomian gland dysfunction. J Formos Med Assoc. (2017) 116:554–9. doi: 10.1016/j.jfma.2016.09.012
18. Tan, LL, Sanjay, S, and Morgan, PB. Repeatability of infrared ocular thermography in assessing healthy and dry eyes. Contact Lens Anterior Eye. (2016) 39:284–92. doi: 10.1016/j.clae.2016.01.010
19. Tan, LL, Sanjay, S, and Morgan, PB. Screening for dry eye disease using infrared ocular thermography. Contact Lens Anterior Eye. (2016) 39:442–9. doi: 10.1016/j.clae.2016.08.004
20. Purslow, C, and Wolffsohn, J. The relation between physical properties of the anterior eye and ocular surface temperature. Optom Vis Sci. (2007) 84:197–201. doi: 10.1097/OPX.0b013e3180339f6e
21. Li, W, Graham, AD, Selvin, S, and Lin, MC. Ocular surface cooling corresponds to tear film thinning and breakup. Optom Vis Sci. (2015) 92:e248–e256. doi: 10.1097/OPX.0000000000000672
22. Su, TY, Ho, WT, Chang, SW, and Chiang, HK. Thermographic evaluation of tear film break-up time to study tear film stability. Int J Therm Sci. (2016) 99:36–40. doi: 10.1016/j.ijthermalsci.2015.07.032
23. Azharuddin, M, Bera, SK, Datta, H, and Dasgupta, AK. Thermal fluctuation based study of aqueous deficient dry eyes by non-invasive thermal imaging. Exp Eye Res. (2014) 120:97–102. doi: 10.1016/j.exer.2014.01.007
24. Matteoli, S, Favuzza, E, Mazzantini, L, Aragona, P, Cappelli, S, Corvi, A, et al. Ocular surface temperature in patients with evaporative and aqueous-deficient dry eyes: a thermographic approach. Physiol Meas. (2017) 38:1503–12. doi: 10.1088/1361-6579/aa78bd
25. Su, T-Y, and Chang, S-W. Normalized ocular surface temperature models for tear film characteristics and dry eye disease evaluation. Ocul Surf. (2021) 19:74–82. doi: 10.1016/j.jtos.2020.04.002
26. Su, TY, Chang, SW, Yang, CJ, and Chiang, HK. Direct observation and validation of fluorescein tear film break-up patterns by using a dual thermal-fluorescent imaging system. Biomed Opt Express. (2014) 5:2614–9. doi: 10.1364/boe.5.002614
27. Wolffsohn, JS, Arita, R, Chalmers, R, Djalilian, A, Dogru, M, Dumbleton, K, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. (2017) 15:539–74. doi: 10.1016/j.jtos.2017.05.001
28. Hong, J, Sun, X, Wei, A, Cui, X, Li, Y, Qian, T, et al. Assessment of tear film stability in dry eye with a newly developed Keratograph. Cornea. (2013) 32:716–21. doi: 10.1097/ICO.0b013e3182714425
29. Tan, J-H, Ng, EYK, Rajendra Acharya, U, and Chee, C. Study of normal ocular thermogram using textural parameters. Infrared Phys Technol. (2010) 53:120–6. doi: 10.1016/j.infrared.2009.10.006
30. Abreau, K, Callan, C, Kottaiyan, R, Zhang, A, Yoon, G, Aquavella, JV, et al. Temperatures of the ocular surface, lid, and periorbital regions of Sjögren’s, evaporative, and aqueous-deficient dry eyes relative to Normals. Ocular Surface. (2016) 14:64–73. doi: 10.1016/j.jtos.2015.09.001
31. Tullo, AB, and Efron, N. Infrared thermography of the tear film in dry eye. Eye. (1995) 9:615–8. doi: 10.1038/eye.1995.149
32. Zhang, Q, Wu, Y, Song, Y, Qin, G, Yang, L, Talwar, SS, et al. Screening evaporative dry eyes severity using an infrared image. J Ophthalmol. (2021) 2021:1–8. doi: 10.1155/2021/8396503
33. Kawali, AA. Thermography in ocular inflammation. Indian J Radiol Imag. (2013) 23:281–3. doi: 10.1055/s-0041-1734381
34. Stern, ME, and Pflugfelder, SC. Inflammation in dry eye. Ocul Surf. (2004) 2:124–30. doi: 10.1016/S1542-0124(12)70148-9
35. Baudouin, C, Irkeç, M, Messmer, EM, Benítez-del-Castillo, JM, Bonini, S, Figueiredo, FC, et al. Clinical impact of inflammation in dry eye disease: proceedings of the ODISSEY group meeting. Acta Ophthalmol. (2018) 96:111–9. doi: 10.1111/aos.13436
36. Montés-Micó, R, Cerviño, A, Ferrer-Blasco, T, García-Lázaro, S, and Madrid-Costa, D. The tear film and the optical quality of the eye. Ocular Surface. (2010) 8:185–92. doi: 10.1016/S1542-0124(12)70233-1
37. King-Smith, PE, Fink, BA, Hill, RM, Koelling, KW, and Tiffany, JM. The thickness of the tear film. Curr Eye Res. (2004) 29:357–68. doi: 10.1080/02713680490516099
38. Tan, JH, Ng, EYK, and Acharya, UR. Evaluation of topographical variation in ocular surface temperature by functional infrared thermography. Infrared Phys Technol. (2011) 54:469–77. doi: 10.1016/j.infrared.2011.07.010
39. Stern, ME, Gao, J, Siemasko, KF, Beuerman, RW, and Pflugfelder, SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. (2004) 78:409–16. doi: 10.1016/j.exer.2003.09.003
40. Messmer, EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. (2015) 112:71–81; quiz 82. doi: 10.3238/arztebl.2015.0071
41. Mapstone, R. Determinants of corneal temperature. Br J Ophthalmol. (1968) 52:729–41. doi: 10.1136/bjo.52.10.729
42. Craig, JP, Singh, I, Tomlinson, A, Morgan, PB, and Efron, N. The role of tear physiology in ocular surface temperature. Eye. (2000) 14:635–41. doi: 10.1038/eye.2000.156
43. Farrand, KF, Fridman, M, Stillman, IÖ, and Schaumberg, DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. (2017) 182:90–8. doi: 10.1016/j.ajo.2017.06.033
Keywords: dry eye disease, DED, ocular surface temperature, corneal temperature, ocular thermography, high-resolution infrared thermography
Citation: Wu C, Huang Y, Xu B, Li B, Zhuang S, Cao G, Hu Y and Gu Z (2025) Comparing the corneal temperature of dry eyes with that of normal eyes via high-resolution infrared thermography. Front. Med. 11:1526165. doi: 10.3389/fmed.2024.1526165
Edited by:
Shujun Wang, Hong Kong Polytechnic University, Hong Kong SAR, ChinaReviewed by:
Driss Zoukhri, Tufts University, United StatesShangkun Ou, Affiliated Hospital of Guizhou Medical University, China
Xiangqian Hong, Shenzhen Eye Hospital, China
Copyright © 2025 Wu, Huang, Xu, Li, Zhuang, Cao, Hu and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhensheng Gu, MTM5MDE5MTI5ODNAMTYzLmNvbQ==
 Chunbo Wu
Chunbo Wu Yuanshen Huang1
Yuanshen Huang1 Zhensheng Gu
Zhensheng Gu