- 1The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Traditional Chinese Medicine), Hangzhou, China
- 2The First School of Clinical Medicine, Zhejiang University of Traditional Chinese Medicine, Hangzhou, China
Background: Cytomegalovirus (CMV) infection remains a critical cause of mortality after allogeneic hematopoietic stem cell transplantation, despite significant advancements in CMV prevention and treatment with the introduction and widespread use of letermovir. However, in China, due to limitations in the availability and cost of medications, some patients still face challenges in accessing letermovir. For this subset of the population, exploring the risk factors for CMV infection remains significant in predicting its occurrence.
Methods: Therefore, a retrospective analysis was conducted on 88 haploidentical hematopoietic stem cell transplant recipients over 4 years.
Results: Our study results indicate that chronic graft-versus-host disease (cGVHD) is an independent risk factor for CMV infection following haploidentical hematopoietic stem cell transplantation (Haplo-HSCT). Survival analysis reveals lower survival rates in the refractory CMV infection (RCI) group compared to the non-RCI group, with patients having lower viral loads demonstrating higher rates of seroconversion and improved survival under the same treatment regimen.
Conclusion: Strengthening the monitoring of CMV-DNA in post-transplant patients, actively promoting hematopoietic recovery, preventing the occurrence of CMV infection, and controlling the development of CMV infection can lead to better survival outcomes for patients with aplastic anemia undergoing Haplo-HSCT.
1 Introduction
Cytomegalovirus (CMV) is a common and crucial viral infection following allogeneic hematopoietic stem cell transplantation (allo-HSCT) (1). Depending on the type of transplantation and geographical region, the incidence of CMV infection ranges from approximately 40%–70% (2, 3), significantly impacting both the survival and quality of life of affected patients. Despite significant advancements in CMV prevention and treatment with the introduction and widespread use of letermovir, in China, some patients still face challenges in accessing letermovir due to limitations in the availability and cost of medications. For these individuals, CMV infection remains associated with an increased risk of mortality (4), particularly in the case of refractory CMV infection (RCI) (4, 5), which can result in a mortality rate exceeding 80% (6), with CMV pneumonia being the most lethal manifestation. Additionally, the Chinese population resides in a high-risk zone for CMV infection, with an adult CMV serum positivity rate ranging from 80% to 93.7% (7, 8). This significantly increases the likelihood of CMV infection (9). For this subset of the population, exploring the risk factors for CMV infection remains meaningful in predicting its occurrence.
In the current study, we conducted a retrospective analysis of clinical data from 88 patients with aplastic anemia (AA) who underwent haploidentical hematopoietic stem cell transplantation (Haplo-HSCT). The aim was to investigate the incidence of CMV infection and its associated risk factors in haploidentical transplant recipients.
2 Materials and methods
2.1 Patients
In this study, we retrospectively analyzed the clinical data of 88 patients diagnosed with AA who underwent Haplo-HSCT at the Department of Hematology, Zhejiang Provincial Hospital of Traditional Chinese Medicine, from September 2018 to November 2022. The patients were actively followed up until July 2023. Among the 88 patients (44 males, 44 females), with a median age of 32 years (range: 9–55), there were 70 cases of severe aplastic anemia (SAA) and 18 cases of non-severe aplastic anemia (NSAA), all meeting the diagnostic criteria for AA (10). This study has been approved by the Institutional Review Board of the hospital. The baseline characteristics of the patients are presented in Table 1.
All patients underwent myeloablative conditioning, with 22 patients receiving the BUCY (busulfan/cyclophosphamide) conditioning regimen, and 66 patients undergoing the FCA (fludarabine/cyclophosphamide/antithymocyte globulin) conditioning regimen. A combination of antithymocyte globulin (ATG), mycophenolate mofetil (MMF), cyclosporine (CSA), and short-term methotrexate (MTX) was employed for graft-versus-host disease (GVHD) prophylaxis in all patients. Acute GVHD (aGVHD) and chronic GVHD (cGVHD) were diagnosed according to standard references (11–13). For patients experiencing aGVHD, immediate first-line treatment involved administering methylprednisolone at a dose of 1–2 mg⋅kg1⋅d1. In cases where methylprednisolone was ineffective or dependency occurred, second-line therapies such as ruxolitinib, anti-CD25 monoclonal antibodies, MMF, among others, were administered. The primary treatment for cGVHD involved the use of methylprednisolone and/or CSA as the first-line approach.
Follow-up for all 88 patients was conducted through methods such as phone interviews and hospital registration systems, with the follow-up deadline set at July 2023. Neutrophil engraftment was defined as a consecutive 3-day absolute neutrophil count (ANC) > 0.5 × 109/L, while platelet engraftment was defined as a consecutive 7-day platelet count (PLT) > 20 × 109/L without requiring platelet transfusions. Primary graft failure was defined according to established literature (14). Overall survival (OS) time post-transplantation was defined as the period from transplantation to either patient death or the last follow-up date.
2.2 CMV monitoring, definitions, and antiviral therapy
According to our internal standards, blood CMV-DNA positivity is defined as a quantitative PCR result with a CMV viral load > 1 × 102 copies/ml (15). CMV viremia is defined as two consecutive CMV-DNA tests showing levels exceeding 500 copies/ml, or a single CMV-DNA test result exceeding 1,000 copies/ml (16). In this study, the occurrence of viremia in patients was considered a confirmed CMV infection. RCI is defined as a situation where, after receiving reasonable anti-CMV treatment for 2 weeks, the CMV viral load remains unchanged or increases (17, 18). The definition of CMV-related diseases follows the literature reference (15), while the definitions of Epstein-Barr virus (EBV) viremia and related diseases adhere to the literature reference (19). Prophylaxis for CMV infection with ganciclovir was administered from day 9 to 2 during the pre-transplant period, and acyclovir prophylaxis for herpes virus infection was given from day 1 to 1-year post-transplant.
All patients underwent quantitative PCR monitoring of peripheral blood CMV-DNA and EBV-DNA twice a week from the initiation of pre-transplant conditioning until day +90. From day +90 onward, monitoring was conducted every 1–2 weeks until day +180. After day +180, in the presence of symptoms suggestive of a possible viral infection, simultaneous retesting of CMV-DNA and EBV-DNA was performed. If positivity occurred during this period, the monitoring frequency increased to twice a week until viral clearance. The first-line treatment options for CMV infection included either ganciclovir or sodium phosphonoformate. For RCI, drugs not used in the first-line regimen were selected for monotherapy or combination therapy. Once CMV-DNA became negative for two consecutive tests, acyclovir was administered orally for prophylaxis.
2.3 Statistical analyses
Inter-group continuous variables were subjected to two-tailed t-tests or Kruskal–Wallis tests, while categorical variables were analyzed using Chi-square tests or Fisher’s exact tests. Logistic regression models for binary variables were employed for both univariate and multivariate analyses, with the latter incorporating all factors from the univariate analysis with a p-value < 0.10. The cumulative incidence of CMV infection was computed using a competing risk model. Kaplan–Meier methodology was employed to determine the probability of OS, and comparisons were made using the log-rank test. A p-value < 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS 26.0 software, and graphical representations were created using GraphPad (Supplementary Table 1).
3 Results
3.1 Patient clinical characteristics and hematopoietic recovery
This study included a total of 88 patients with AA who underwent haploidentical transplantation, comprising 44 males and 44 females. The median age at the time of transplantation was 32 years (range: 9–55). Disease classification was as follows: NSAA in 18 cases, SAA in 65 cases, and very severe aplastic anemia (VSAA) in 5 cases. The median time for neutrophil engraftment in 85 patients was 12 days (range: 9–48), with 82 achieving engraftment within 28 days. For platelet engraftment, the median time for 81 patients was 16 days (range: 7–92), with 15 achieving engraftment within 28 days. Ultimately, hematopoietic recovery was achieved in 81 patients, while the remaining 7 patients experienced graft failure, adverse events, or early mortality (Table 1).
3.2 Overview of CMV infection, treatment, and outcome
Before transplantation, both donor and recipient CMV-DNA quantification levels were below the detection range (<1 × 102 copies/ml). Among the patients, 70 were CMV-IgG positive, and the remaining 18 were not assessed. By the end of the follow-up period, CMV infection occurred in 52 out of the 88 patients (59.1%). The median time to the first occurrence of CMV infection was 36.5 days (range: 11–189).
After the first-line treatment, 40 patients (76.9%) achieved CMV-DNA negativity, while the remaining patients experienced RCI. Among the 12 RCI patients, 5 (41.7%) achieved viral clearance after receiving second-line treatment, while 6 died with persistent CMV-DNA positivity. The overall rate of viral clearance after CMV infection treatment was 86.5% (45/52).
Among CMV-infected patients, there were 29 cases in the group with the highest viral load below 1 × 104 copies/ml, and the clearance rate was 96.6% (28/29). In the group with a viral load exceeding 1 × 104 copies/ml, there were 23 cases, and the clearance rate was 73.9% (17/23). The difference in clearance outcomes between the two groups was statistically significant (p = 0.035).
3.3 Risk factors for CMV infection
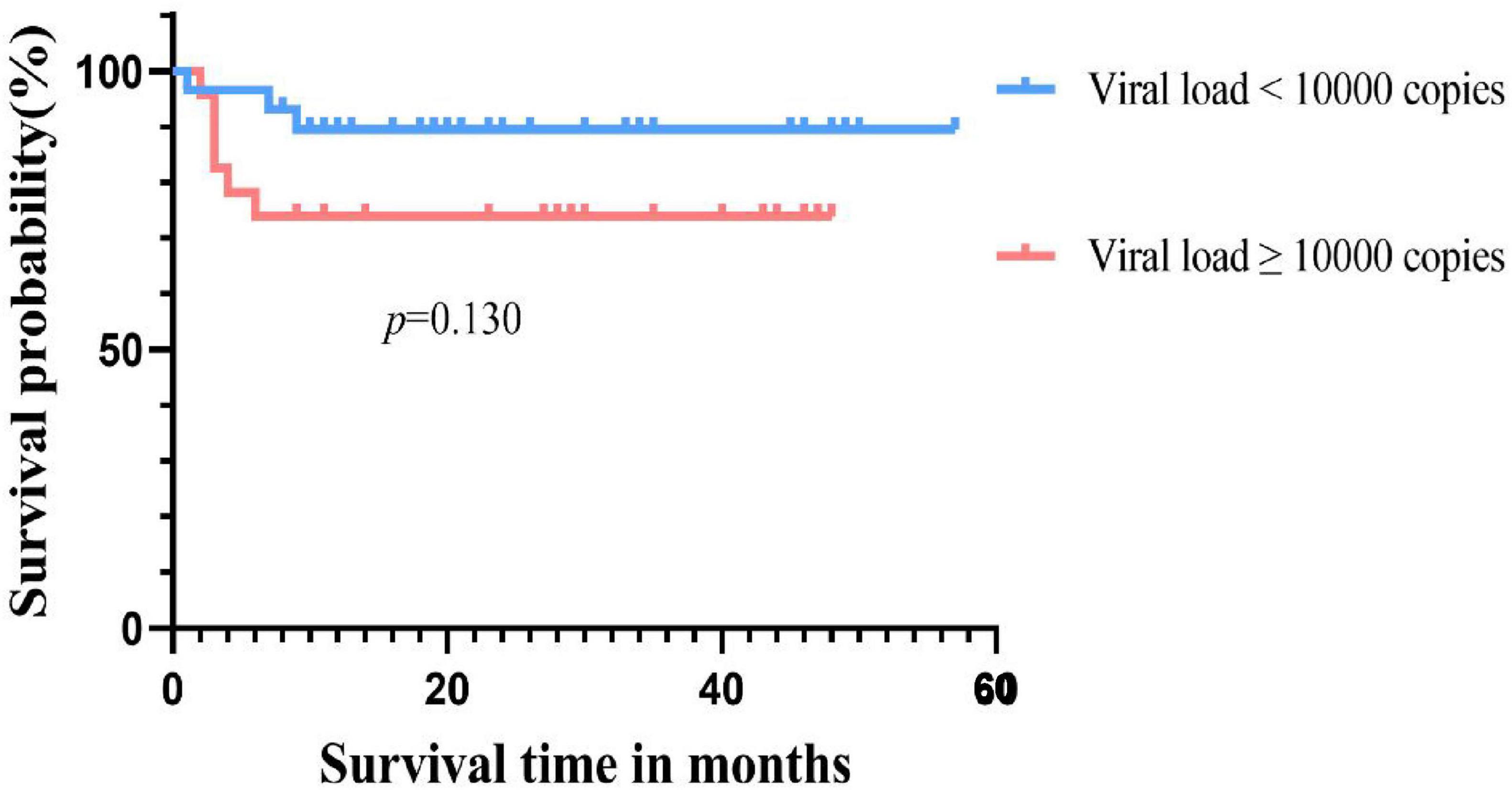
The results are shown in Table 2. Univariate analysis indicated that neutrophil engraftment beyond 28 days (p = 0.004), cGVHD (p = 0.007), and EBV infection (p = 0.044) were clinical risk factors for CMV infection in AA patients undergoing Haplo-HSCT. Multivariate analysis further identified cGVHD (p = 0.043) as an independent risk factor for the occurrence of CMV infection after Haplo-HSCT.
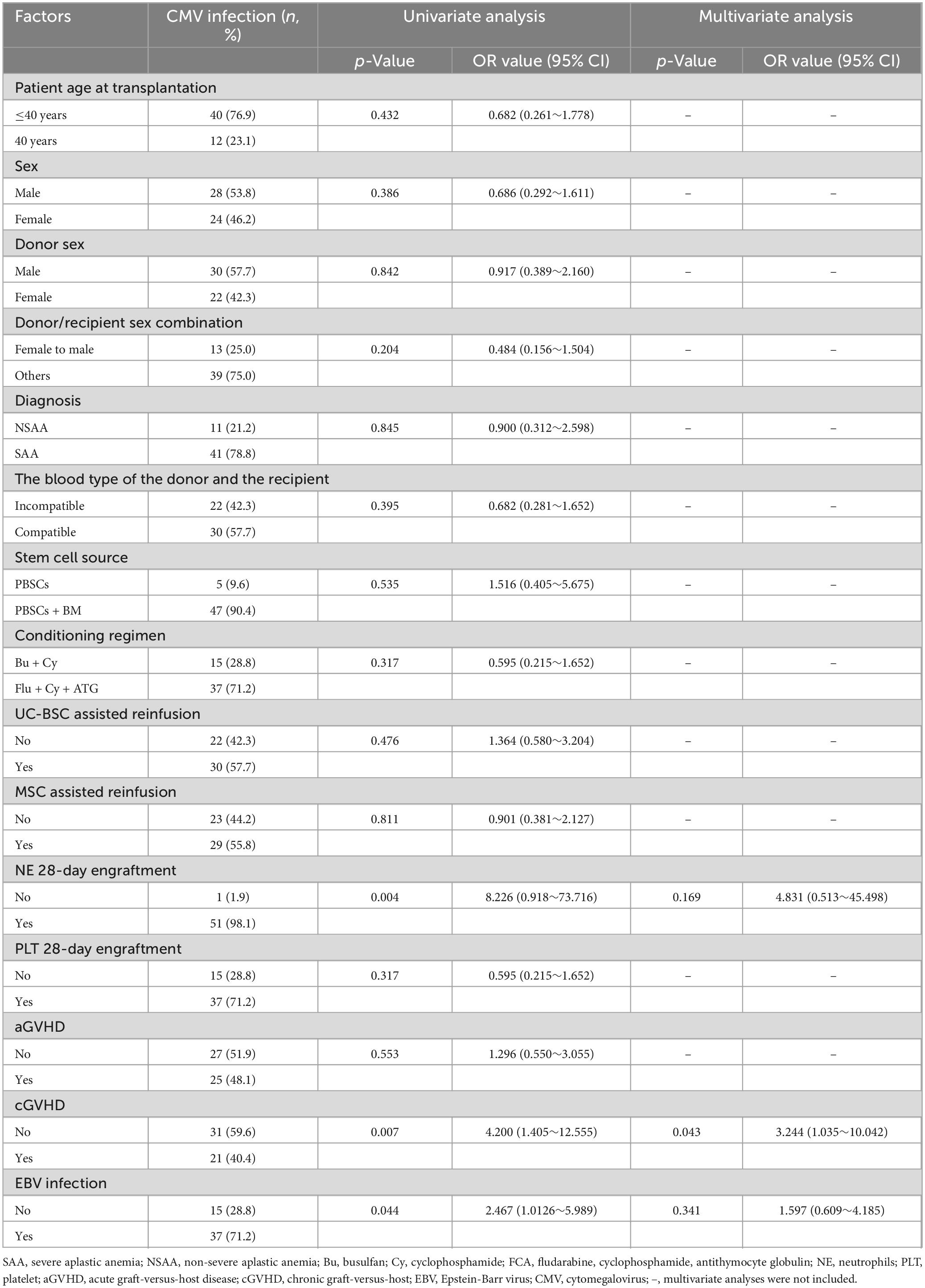
Table 2. Analysis of risk factors associated with CMV infection after allogeneic hematopoietic stem cell transplantation.
3.4 Overall prognosis and survival analysis of patients with CMV infection
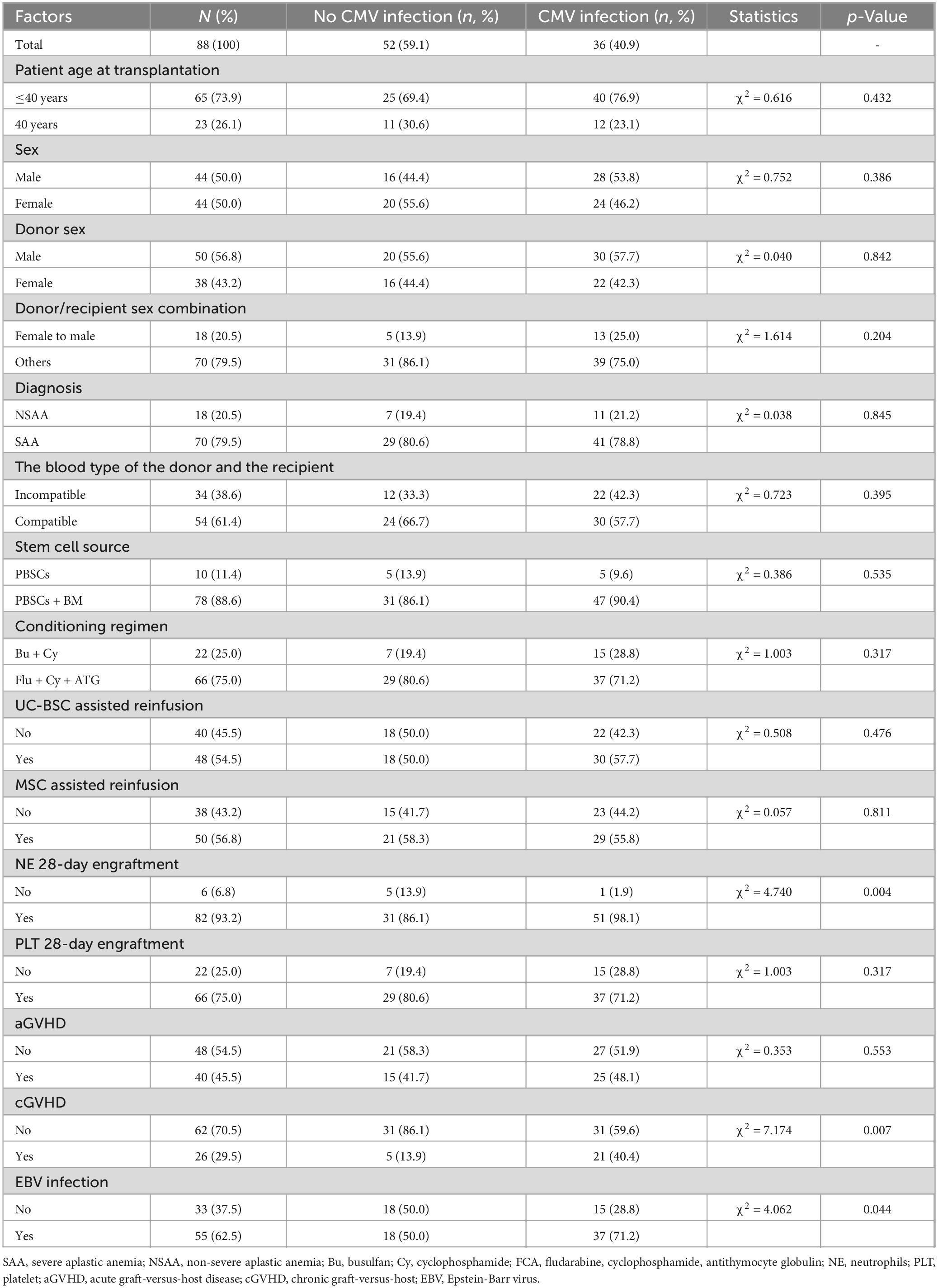
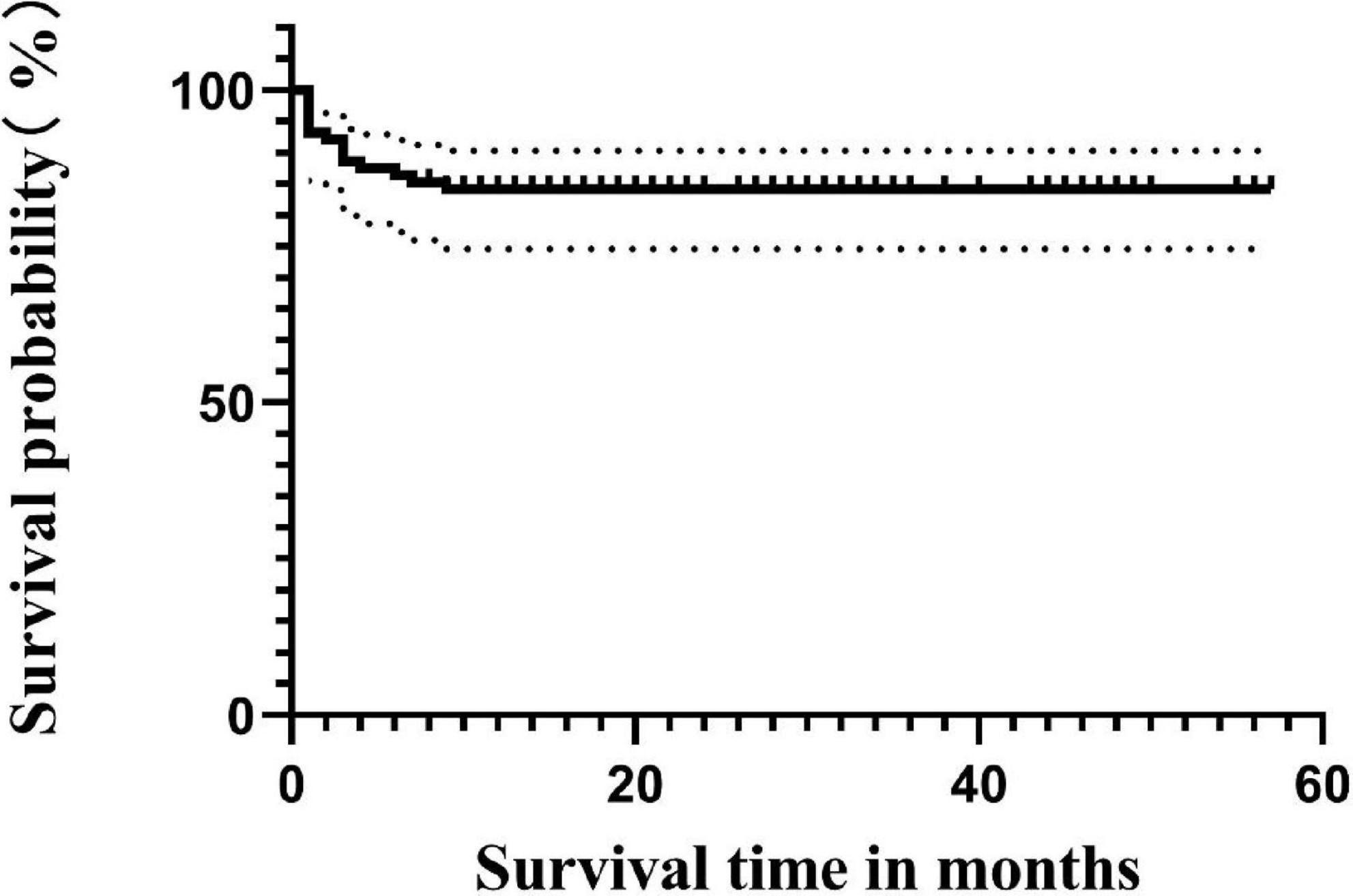
Until the follow-up endpoint, a total of 14 patients had died, with the specific causes as follows: 4 died from sepsis, 4 from severe pneumonia, 1 from cerebrovascular accident, 2 from aGVHD, 2 from post-transplant lymphoproliferative disorder (PTLD), and 1 from acute heart failure. The 4-year OS rate for all 88 patients was 84.1% (Figure 1). The survival rate for the non-CMV infection group was 86.1% (31/36), and for the CMV infection group, it was 82.7% (43/52), with no statistically significant difference in survival time between the two groups (p = 0.736) (Figure 2).
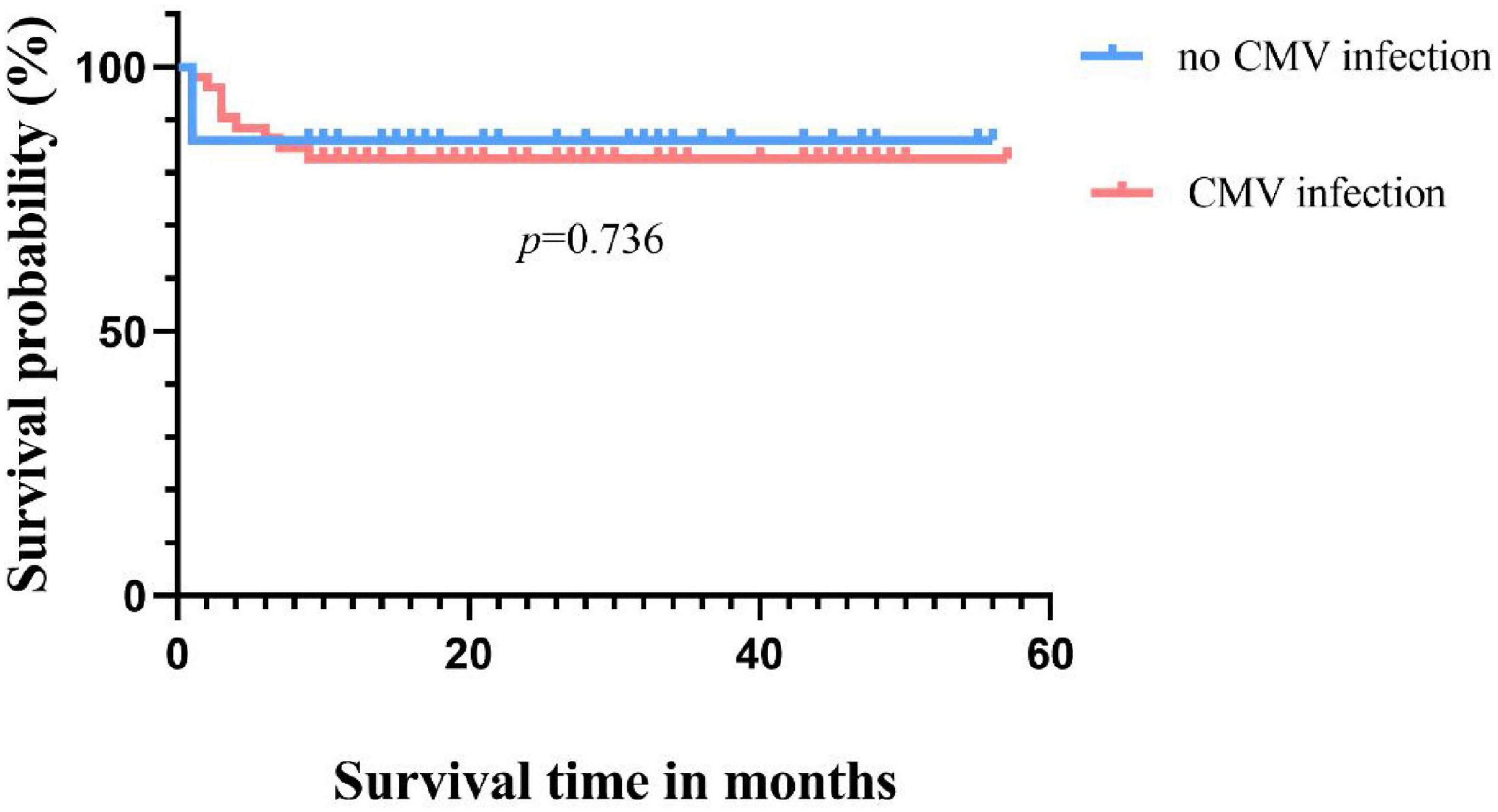
Among the 52 patients with CMV infection, the OS rate was 50% (6/12) in the RCI group and 92.5% (37/40) in the non-RCI group, with a statistically significant difference in survival outcomes between the two groups (p = 0.000). For the group with the highest viral load above 1.0 × 104 copies/ml, the survival rate was 73.9%, while for the group with a load below 1.0 × 104 copies/ml, the survival rate was 89.5%, with no statistically significant difference in survival time between the two groups (p = 0.130) (Figure 3).
3.5 Analysis of factors influencing survival
A univariate and multivariate Cox regression analysis was conducted to explore potential factors influencing the survival time of patients post-transplantation. The results are presented in Table 3. Univariate analysis indicated that recipient age >40 years (p = 0.017), unassisted infusion of mesenchymal stem cells (p = 0.019), neutrophil engraftment beyond 28 days (p = 0.005), platelet engraftment beyond 28 days (p = 0.000), and non-EBV infection (p = 0.017) were risk factors affecting patient survival. Multivariate Cox regression analysis identified platelet engraftment beyond 28 days (HR = 0.132, 95% CI 0.036∼0.481, p = 0.002) as an independent risk factor influencing the survival time of patients.

Table 3. Univariate and multivariate analysis of factors influencing overall survival after allogeneic hematopoietic stem cell transplantation.
4 Discussion
Following the initial infection, CMV establishes a lifelong latent infection in the host under the control of the immune response. Reactivation of CMV is a common event in recipients of allo-HSCT. In this study, 59.1% (52/88) of patients experienced CMV infection during the postoperative follow-up period, with 23.1% (12/52) of CMV-infected patients developing RCI. The incidence of CMV infection and RCI in this study is similar to previous reports (4, 5). Without the use of letermovir, exploring risk factors for CMV infection may provide new insights into treatment strategies.
In previous studies, recipient seropositive status, graft source, transplantation type, HLA compatibility, and GVHD have been identified as common risk factors for CMV infection (20–22). We observed that among the 26 patients who developed cGVHD, there was an 81% incidence of CMV infection. This observation leads us to infer that cGVHD is a significant risk factor for CMV infection, and our study confirms this hypothesis. Our data indicate that cGVHD is an independent risk factor for CMV infection. This finding is not entirely consistent with previous research results (22). On the one hand, there may be a reciprocal interaction between CMV virus and cGVHD. This could be related to the type of disease, as the CMV virus is more likely to infect when T cells are deficient or impaired. In the case of AA transplantation, long-term use of immunosuppressive agents is required, leading to a slower immune reconstitution compared to other hematological malignancies after transplantation, thus providing opportunities for extended periods of immune reconstitution recovery, which may increase the risk of infection. On the other hand, the EBV may contribute to CMV infection by influencing aGVHD and cGVHD.
The main pathophysiological process of cGVHD is immune-mediated inflammatory response. Chronic inflammation causes thymus damage and B cell and T cell immune disorder, which eventually leads to tissue fibrosis. T lymphocytes can cause tissue damage and fibrosis through direct cytolysis and cytokine secretion (23, 24), especially CD4+ T lymphocytes, whose interaction with B cells promotes B cell differentiation and the production of autoantibodies. These include antibodies against cytoskeletal intermediate filaments, cytoplasmic squamous epithelial cells, and nucleolar B23, These antibodies participate in inflammation and activate signal transduction pathways, leading to increased expression of type I collagen genes, promoting fibroblast activation, and inducing typical cGVHD clinical symptoms such as skin sclerosis and pulmonary fibrosis. In addition, T cell subsets play a crucial role in the immune regulation of cGVHD. Activation of the NOTCH2 signaling pathway in B cells has a profound effect on T cell subsets, including helper T cells (Th) and regulatory T cells (Treg) (25–27). This results in delayed immune reconstitution after allo-HSCT, increased risk of death and cGVHD, and increased risk of CMV reactivation. In addition, since patients with AA use immunosuppressants longer after transplantation than those with other hematological malignancies, it is more likely to cause delayed immune reconstitution after transplantation.
There is limited research on the correlation between cGVHD and CMV infection, but the relationship between cGVHD and CMV infection is not absent. Olkinuora et al. (28) found that mild aGVHD and cGVHD can promote the recovery of cellular and humoral immunity, while moderate to severe cGVHD hurts immune recovery after transplantation. Furthermore, active CMV infection can contribute to the occurrence and exacerbation of cGVHD by increasing levels of IL-2 and tumor necrosis factor-alpha in peripheral blood (29).
Previous research results indicate that the influence of CMV infection on aGVHD is affirmative (30, 31). The study by Styczynski (32) indicates that the incidence of CMV infection in patients with aGVHD is almost twice that of patients without aGVHD [p < 0.0001, 60.1% (885/1,472) vs. 32.1% (892/2,780)]. Cantoni et al. (33) found that GVHD and its treatment can induce CMV replication, and CMV replication increases the risk of GVHD occurrence (34, 35). It is noteworthy that in our study, there was no significant difference in the incidence of CMV infection between patients with and without aGVHD.
As is well-known, EBV, as one of the common viral infections after allo-HSCT, is also a routine monitoring indicator. Previous studies have suggested that EBV infection increases the incidence of II–IV degree aGVHD and cGVHD (34). Since CMV infection is influenced by aGVHD, EBV infection may indirectly affect CMV infection by influencing post-transplant immune reconstitution. There is a complex interrelationship between CMV infection, EBV infection, and the occurrence of GVHD. However, current research on the impact of EBV infection on CMV infection is limited, and the relationship among these three factors is not yet clear. Interestingly, in this study, univariate analysis found an association between the occurrence of CMV infection and cGVHD as well as EBV infection, which warrants further in-depth investigation.
Cytomegalovirus infection has a significant impact on the prognosis of patients, especially with a higher mortality rate in CMV disease and RCI, significantly affecting patient survival (36, 37). In our study, although there was no significant difference in survival rates between CMV-infected and uninfected patients (82.7% vs. 86.1%, p = 0.736), the occurrence of RCI was associated with shorter OS compared to the non-RCI group (50% vs. 96.6%, p = 0.000), consistent with previous reports (4, 5). The direct and indirect effects of CMV in this study may negatively influence patient prognosis in different ways. On the other hand, consistent with Green et al., a higher CMV viral load after transplantation was associated with an increased risk of death (adjusted hazard ratio [HR] 19.8, 95% CI 9.6–41.1) (38). However, in our cohort, the peak viral load of CMV reactivation in transplant recipients was used as a qualitative parameter. The CMV-infected patients were divided into two groups based on the highest viral load, with a threshold of 1.0 × 104 copies/ml. Regarding the final survival rate, no significant difference was observed between the low viral load group and the high viral load group (89.5% vs. 73.9%, p = 0.130). This may be influenced by sample size and other factors. However, under the same treatment, the low viral load group had a higher rate of turning negative compared to the high viral load group (96.6% vs. 73.9%, p = 0.035). This suggests that patients with lower viral loads are more likely to turn negative and have a higher survival rate under the same treatment regimen, thereby improving the prognosis. This also emphasizes the importance of closely monitoring CMV, and with the advent and clinical application of letermovir (39). These patients may benefit from letermovir. Therefore, early intervention, especially after discontinuing prophylaxis, may be considered if necessary. On the other hand, the quantitative definition of pre-transplant CMV serostatus, rather than qualitative, influences the 3-year survival rate after allo-HSCT. This provides new insights into the negative prognostic impact of CMV on transplant recipients (35). However, the lack of pre-transplant serostatus in some patients in this cohort is a limitation of this study. The CMV seropositivity rate of Chinese HSCT patients is as high as 80%–93.7%, which is much higher than that in European and American countries. Therefore, although some patients in this study lack serological status, we can still speculate that they are at risk of CMV reactivation.
Furthermore, we attempted survival analysis, indicating that CMV infection was not statistically significant. Factors such as hematopoietic reconstruction and age may influence patient OS, and failure of platelet engraftment within 28 days (p = 0.002) emerged as an independent risk factor affecting patient OS. This differs from previous studies reporting CMV infection as an independent prognostic factor. It is considered that this discrepancy may be due to the combined influence of other factors, and further studies with an expanded sample size are needed to validate these findings.
5 Conclusion
In summary, further emphasis on monitoring CMV-DNA in transplant recipients is warranted, particularly in patients developing cGVHD, necessitating proactive prevention of CMV infection. High viral load patients should receive more aggressive treatment to prevent RCI occurrence, early combination therapy when necessary. Once CMV infection progresses to RCI, the prognosis is poor. Actively promoting hematopoietic reconstruction, preventing the occurrence of CMV infection, and controlling the development of CMV infection can lead to improved survival in AA patients undergoing Haplo-HSCT. In addition, we should pay close attention to the level of T lymphocyte subsets to evaluate cellular immune reconstitution, and rationally adjust immunosuppressants to further reduce CMV reactivation. For patients who use letermovir to prevent CMV infection, we can also further study its effect on the level of T lymphocyte subsets and cellular immune reconstitution.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Zhejiang University of Traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
JF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. XZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. ZT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. YZ: Resources, Supervision, Validation, Writing – review & editing. HH: Resources, Supervision, Validation, Writing – review & editing. JC: Resources, Supervision, Validation, Writing – review & editing. LW: Resources, Supervision, Validation, Writing – review & editing. QY: Resources, Supervision, Validation, Writing – review & editing. DW: Resources, Supervision, Validation, Writing – review & editing. BY: Funding acquisition, Resources, Supervision, Validation, Writing – review & editing. WL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank the support in The First Affiliated Hospital of Zhejiang Chinese Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1523909/full#supplementary-material
The supplementary material is the original data table.
References
1. Einsele H, Ljungman P, Boeckh M. How i treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood. (2020) 135:1619–29.
2. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. (2010) 20:202–13.
3. Cho SY, Lee DG, Kim HJ. Cytomegalovirus infections after hematopoietic stem cell transplantation: current status and future immunotherapy. Int J Mol Sci. (2019) 20:2666.
4. Leserer S, Bayraktar E, Trilling M, Bogdanov R, Arrieta-Bolaños E, Tsachakis-Mück N, et al. Cytomegalovirus kinetics after hematopoietic cell transplantation reveal peak titers with differential impact on mortality, relapse and immune reconstitution. Am J Hematol. (2021) 96:436–45.
5. Liu J, Kong J, Chang YJ, Chen H, Chen YH, Han W, et al. Patients with refractory cytomegalovirus (CMV) infection following allogeneic haematopoietic stem cell transplantation are at high risk for CMV disease and non-relapse mortality. Clin Microbiol Infect. (2015) 21:1121.e9–15.
6. Chan ST, Logan AC. The clinical impact of cytomegalovirus infection following allogeneic hematopoietic cell transplantation: why the quest for meaningful prophylaxis still matters. Blood Rev. (2017) 31:173–83.
7. Ljungman P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. (2019) 19:e260–72.
8. Adland E, Klenerman P, Goulder P, Matthews PC. Ongoing burden of disease and mortality from HIV/CMV coinfection in Africa in the antiretroviral therapy era. Front Microbiol. (2015) 6:1016. doi: 10.3389/fmicb.2015.01016
9. Eberhardt KA, Jung V, Knops E, Heger E, Wirtz M, Steger G, et al. CMV-IgG pre-allogeneic hematopoietic stem cell transplantation and the risk for CMV reactivation and mortality. Bone Marrow Transplant. (2023) 58:639–46.
10. Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. (2016) 172:187–207.
11. Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. (1995) 15:825–8.
12. Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. (2018) 53:1401–15.
13. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. (2015) 21:389–401.e1.
14. Xiong YY, Fan Q, Huang F, Zhang Y, Wang Y, Chen XY, et al. Mesenchymal stem cells versus mesenchymal stem cells combined with cord blood for engraftment failure after autologous hematopoietic stem cell transplantation: a pilot prospective, open-label, randomized trial. Biol Blood Marrow Transplant. (2014) 20:236–42.
15. Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. (2017) 64:87–91.
16. Sun YQ, Wang Y, Zhang XH, Xu LP, Liu KY, Yan CH, et al. Virus reactivation and low dose of CD34+ cell, rather than haploidentical transplantation, were associated with secondary poor graft function within the first 100 days after allogeneic stem cell transplantation. Ann Hematol. (2019) 98:1877–83.
17. Stem Cell Application Group, Chinese Society of Hematology, Chinese Medical Association. [The Chinese consensus on the management of cytomegalovirus infection in allogeneic hematopoietic stem cell transplantation patients (2022)]. Zhonghua Xue Ye Xue Za Zhi. (2022) 43:617–23.
18. Chemaly RF, Chou S, Einsele H, Griffiths P, Avery R, Razonable RR, et al. Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials. Clin Infect Dis. (2019) 68:1420–6.
19. Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN, et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant. (2009) 43:757–70.
20. Kawamura S, Nakasone H, Takeshita J, Kimura SI, Nakamura Y, Kawamura M, et al. Prediction of cytomegalovirus reactivation by recipient cytomegalovirus-IgG titer before allogeneic hematopoietic stem cell transplantation. Transplant Cell Ther. (2021) 27:683.e1–7.
21. Arcuri LJ, Schirmer M, Colares M, Maradei S, Tavares R, Moreira MCR, et al. Impact of Anti-CMV IgG titers and CD34 count prior to hematopoietic stem cell transplantation from alternative donors on CMV reactivation. Biol Blood Marrow Transplant. (2020) 26:e275–9.
22. Cui J, Zhao K, Sun Y, Wen R, Zhang X, Li X, et al. Diagnosis and treatment for the early stage of cytomegalovirus infection during hematopoietic stem cell transplantation. Front Immunol. (2022) 13:971156. doi: 10.3389/fimmu.2022.971156
23. Miklos DB, Abu Zaid M, Cooney JP, Albring JC, Flowers M, Skarbnik AP, et al. Ibrutinib for first-line treatment of chronic graft-versus-host disease: results from the randomized phase III iNTEGRATE study. J Clin Oncol. (2023) 41:1876–87.
24. Li X, Gao Q, Feng Y, Zhang X. Developing role of B cells in the pathogenesis and treatment of chronic GVHD. Br J Haematol. (2019) 184:323–36.
25. Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. (2005) 105:2973–8.
26. Nguyen JT, Jessri M, Costa-da-Silva AC, Sharma R, Mays JW, Treister NS. Oral chronic graft-versus-host disease: pathogenesis, diagnosis, current treatment, and emerging therapies. Int J Mol Sci. (2024) 25:10411.
27. Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. (2017) 130:2243–50.
28. Olkinuora H, von Willebrand E, Kantele JM, Vainio O, Talvensaari K, Saarinen-Pihkala U, et al. The impact of early viral infections and graft-versus-host disease on immune reconstitution following paediatric stem cell transplantation. Scand J Immunol. (2011) 73:586–93.
29. Xie WX, Huang WF, Tu SF, Li YH. [Research progres on relationship between the graft-versus-host disease and cytomegalovirus infection–Review]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. (2016) 24:303–6.
30. Takenaka K, Onishi Y, Mori T, Hirakawa T, Tada Y, Uchida N, et al. Negative impact of cytomegalovirus reactivation on survival in adult patients with aplastic anemia after an allogeneic hematopoietic stem cell transplantation: a report from transplantation-related complication and adult aplastic anemia working groups of the japan society for hematopoietic cell transplantation. Transplant Cell Ther. (2021) 27:82.e1–8.
31. Dziedzic M, Sadowska-Krawczenko I, Styczynski J. Risk factors for cytomegalovirus infection after allogeneic hematopoietic cell transplantation in malignancies: proposal for classification. Anticancer Res. (2017) 37:6551–6.
32. Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. (2018) 7:1–16.
33. Cantoni N, Hirsch HH, Khanna N, Gerull S, Buser A, Bucher C, et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. (2010) 16: 1309–14.
34. Janeczko M, Mielcarek M, Rybka B, Ryczan-Krawczyk R, Noworolska-Sauren D, Kałwak K. Immune recovery and the risk of CMV/ EBV reactivation in children post allogeneic haematopoietic stem cell transplantation. Cent Eur J Immunol. (2016) 41:287–96.
35. Chen X, Das R, Komorowski R, Beres A, Hessner MJ, Mihara M, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. (2009) 114: 891–900.
36. Yong MK, Gottlieb D, Lindsay J, Kok J, Rawlinson W, Slavin M, et al. New advances in the management of cytomegalovirus in allogeneic haemopoietic stem cell transplantation. Intern Med J. (2020) 50(3):277–84.
37. Erard V, Guthrie KA, Seo S, Smith J, Huang M, Chien J, et al. Reduced mortality of cytomegalovirus pneumonia after hematopoietic cell transplantation due to antiviral therapy and changes in transplantation practices. Clin Infect Dis. (2015) 61:31–9.
38. Green ML, Leisenring W, Xie H, Mast TC, Cui Y, Sandmaier BM, et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: A retrospective cohort study. Lancet Haematol. (2016) 3:e119–e1. doi: 10.1016/S2352-3026(15)00289-627
Keywords: Keyword: cytomegalovirus infection, aplastic anemia, haploidentical hematopoietic stem cell transplantation, chronic graft-versus-host disease, immunology
Citation: Feng J, Zhang X, Tan Z, Zhao Y, Hu H, Chen J, Wu L, Yu Q, Wu D, Ye B and Liu W (2025) Risk factors and clinical outcomes of cytomegalovirus infection following haploidentical hematopoietic stem cell transplantation in patients with aplastic anemia: a single-center retrospective study. Front. Med. 11:1523909. doi: 10.3389/fmed.2024.1523909
Received: 06 November 2024; Accepted: 13 December 2024;
Published: 29 January 2025.
Edited by:
Alessandro Perrella, Hospital of the Hills, ItalyReviewed by:
Boyuan Wang, Beijing Xiaotangshan Hospital, ChinaPankaj Dipankar, National Institutes of Health (NIH), United States
Copyright © 2025 Feng, Zhang, Tan, Zhao, Hu, Chen, Wu, Yu, Wu, Ye and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbin Liu, c3p5eWJsb29kQDE2My5jb20=
†These authors have contributed equally to this work
 Jia Feng1,2†
Jia Feng1,2† Xinhe Zhang
Xinhe Zhang Wenbin Liu
Wenbin Liu