- 1Department of Thoracic Surgery, Ganzhou People’s Hospital, Ganzhou, China
- 2Department of Oncology, Ganzhou People’s Hospital, Ganzhou, China
Background: Immune checkpoint inhibitors (ICIs) have been widely applicated for the treatment of patients with advanced esophageal cancer. Skin-related adverse reactions are frequent with ICIs, with toxic epidermal necrolysis (TEN) being a severe and potentially life-threatening cutaneous reaction.
Case presentation: We present a case of a 70-year-old male with locally advanced esophageal cancer who developed severe toxic epidermal necrolysis (TEN) after 18 days of tislelizumab combined with chemotherapy. The condition rapidly progressed to cover approximately 90% of his body. After treatment with intravenous methylprednisolone, immunoglobulin, and antibiotics, along with active nutritional support and wound care, the patient recovered from TEN induced by tislelizumab.
Conclusion: Treatment for TEN is complex, and no standardized guidelines currently exist. We propose an economical, safe, effective, and simple strategy for similar TEN patients.
1 Introduction
The widespread use of immune checkpoint inhibitors (ICIs) for treating esophageal squamous cell carcinoma (ESCC) has drawn attention to immune-related adverse events (ir-AEs) (1, 2). Skin toxicity is one of the most prevalent adverse reactions for ICIs (3). Toxic epidermal necrolysis (TEN) is a rare but potentially fatal reaction characterized by sudden widespread erythema and skin peeling, often accompanied by mucositis and fever, affecting more than 30% of the body surface area (4). Here, we presented a case of severe TEN that was induced by tislelizumab in a patient with esophageal squamous cell carcinoma.
2 Case report
A 70-year-old man was admitted to our hospital due to dysphagia. The patient had coronary stenting 2 weeks prior. The chest computed tomography scan and endoscopic ultrasonography revealed ESCC (Supplementary file 1) in the mid-chest area (Figure 1). For the patient’s locally advanced ESCC, neoadjuvant chemoradiotherapy or neoadjuvant immunotherapy plus chemotherapy was recommended. Tislelizumab (200 mg), carboplatin (450 mg) and docetaxel (110 mg) were administered on May 23, 2024. Nine days post-therapy, the patient suffered oral mucositis (Figure 2A), fever, rashes of the trunk (Figure 2B), and pruritus. Patient received oral prednisone (20–40 mg/d), loratadine (10 mg/d), levocetirizine (5 mg/d), and levofloxacin (500 mg/d). Skin toxicity still continued to progress, resulting in numerous blisters (Figure 2C) on the trunk after 16 days. After 18 days, the patient was admitted to our hospital due to the progression of skin toxicity to TEN (Figure 2D). The cutaneous lesions covered about 90% of the body’s surface. Following admission, urgent consultations were requested from the dermatology and oncology departments. Skin biopsies revealed subepidermal blisters with necrosis, apoptotic keratinocytes, and a lymphocytic inflammatory infiltrate (Supplementary file 2). The SCORTEN score (5) for severity assessment was calculated at 3 (including age above 40 y, malignancy and initial percentage of epidermal detachment above 10%). The ALDEN algorithm was utilized to evaluate drug causality in epidermal necrolysis (6). Tislelizumab had an ALDEN score of 6, and docetaxel’s score was 4. The patient was immediately given prophylactic intravenous antibiotics, intravenous methylprednisolone (2–3 mg/kg/day for 3 days) and intravenous immunoglobulin (400 mg/kg/day for 3 days). Active nutritional support and electrolyte balance were maintained simultaneously. After applying mupirocin ointment and recombinant bovine basic fibroblast growth factor gel to the limbs and trunk, cover with vaseline sand blocks (Figure 3A). Daily disinfect the skin using iodophor, aspirate the blister with a syringe, and softly debride the large detached epidermal segments. Recombinant bovine basic fibroblast growth factor eye drops and levofloxacin eye drops were applied to the eyes. Methylprednisolone was gradually reduced and discontinued after 11 days as symptoms improved. No skin infection developed during treatment, and re-epithelialization occurred two weeks after admission (Figure 3B). However, fever developed due to infection of the subclavian vein catheter. Escherichia coli was isolated from the vein catheter. The body temperature gradually normalized following the administration of sensitive antibiotics (ceftazidime). Abraxane (380 mg) and cis-platinum (100 mg) were administered on July 8, 2024. Figure 4 presented the patient’s treatment timeline. No new skin toxicity developed after the second chemotherapy. Radiotherapy (PTV 60GY/30F) was given on August 30, 2024. The patient was lost to follow-up after radiotherapy.
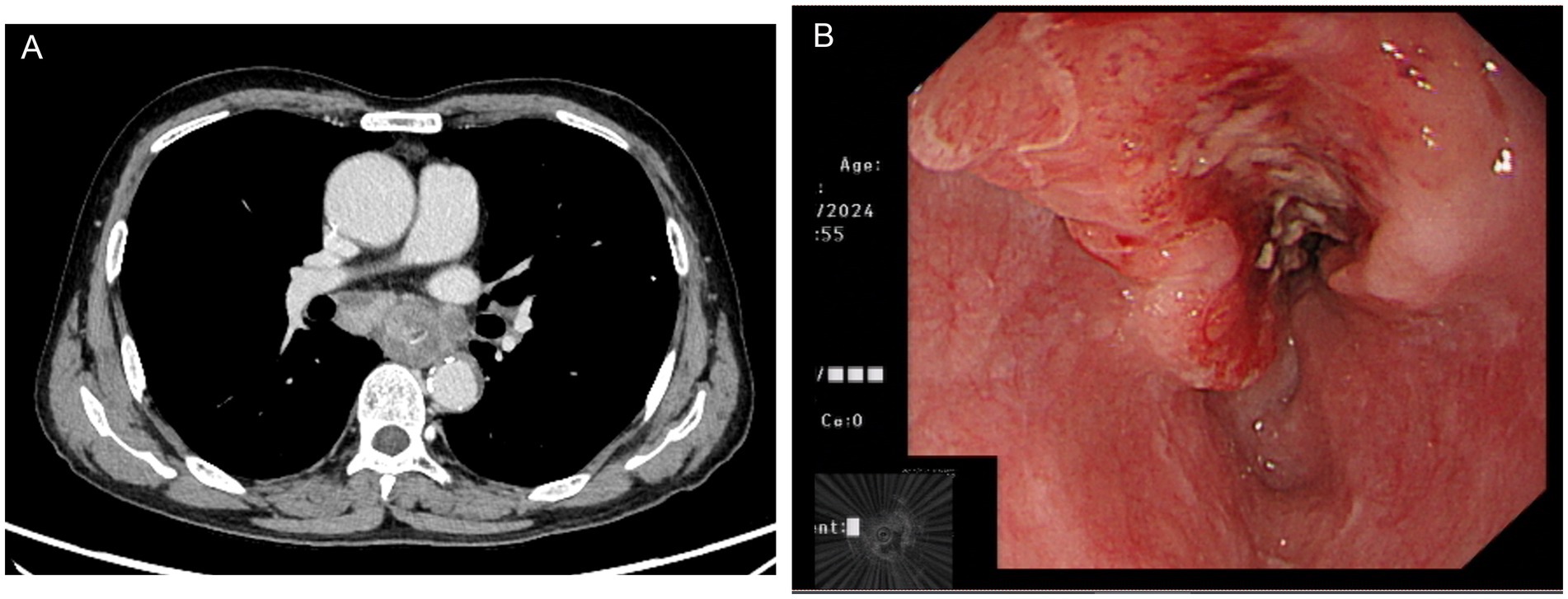
Figure 1. Chest computed tomography (A) and endoscopic ultrasonography (B) showed esophageal squamous cell carcinoma in the mid-chest area.
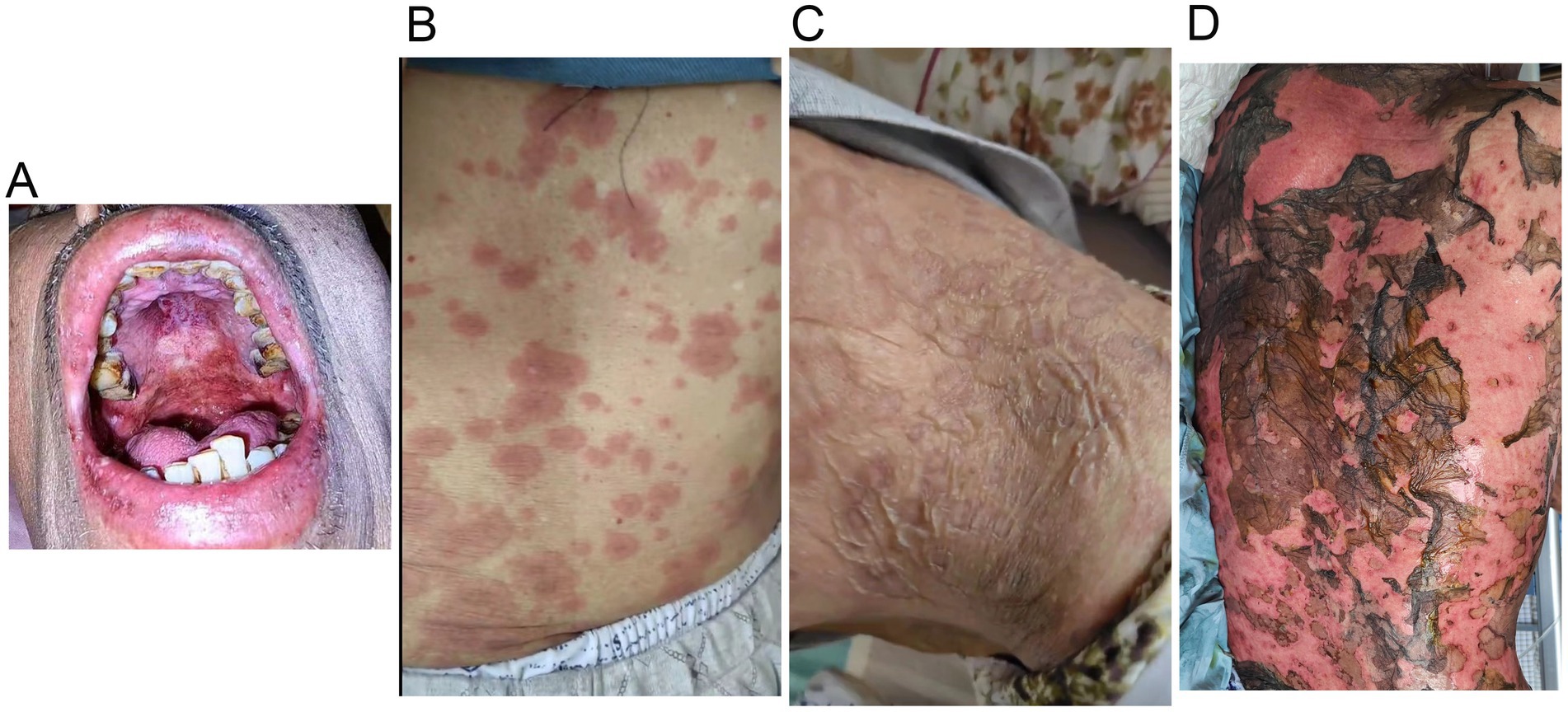
Figure 2. Tislelizumab-related skin toxicity. (A) Oral mucositis; (B) Rashes of the trunk; (C) Blisters; (D) Toxic epidermal necrolysis.
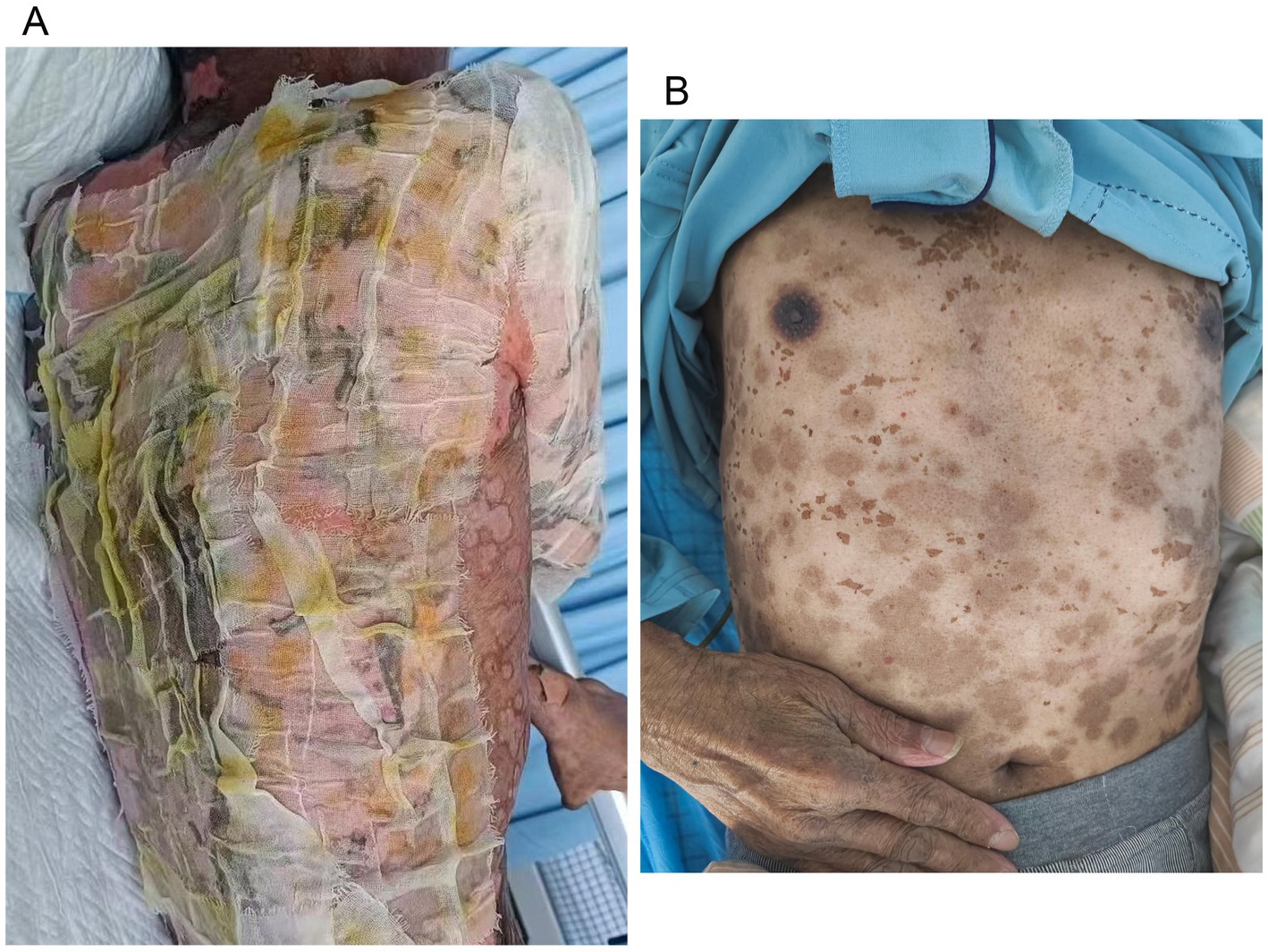
Figure 3. Wound care for toxic epidermal necrolysis (A). An image of the skin after recovery from toxic epidermal necrolysis (B).
3 Discussion
Immune checkpoint inhibitor therapy has now become the standard treatment for advanced ESCC. Recent trials have indicated that the use of ICI combination chemotherapy in the perioperative settings may enable a high PCR rate, complete resection rate, acceptable safety profiles, and thus lead to better survival outcomes (2, 7). Although the safety of ICIs was manageable in most studies, severe cardiovascular, respiratory, endocrine disorders and severe skin toxicity have been reported (2). To the best of our knowledge, this is the first report of TEN induced by tislelizumab in a patient with ESCC.
A recent meta-analysis found that ICIs significantly increased the rates of ir-AEs in esophageal or gastroesophageal junction cancer, but did not significantly raise the rates of serious treatment-related adverse events (TRAEs). The most common irAEs were skin reaction, with varying toxicity manifestations among different ICIs (8). As we know, no study has reported TEN induced by tislelizumab in a patient with ESCC. Among single ICI therapies, sintilimab had the highest incidence of TEN (9). The RATIONALE-306 study showed that 97% of patients in the tislelizumab plus chemotherapy group experienced TRAEs, with myelosuppression being the most common grade 3 or 4 TRAE. The study indicated that skin toxicity with stomatitis and pruritis, but without the occurrence of TEN (10).
Stevens–Johnson syndrome (SJS) and TEN is a type IV hypersensitivity reaction triggered by an immunologic response, most often to drugs (11). SJS/TEN occurs in 1–5 cases per million individuals annually, with a higher incidence in adults than in pediatric patients (12). Identifying the drug causing SJS/TEN is essential, and ALDEN is a valuable tool for assessing drug causality in this condition (6). Compared to Non-ICI drugs, patients with ICI-induced SJS/TEN had a smaller affected body surface area, a trend of prolonged latency, and milder oral and ocular mucositis (13). SJS/TEN usually occurs early in treatment, with a median onset of 23 days after starting ICI, typically following 1–2 cycles (14). We could not definitively determine if immunotherapy or chemotherapy led to TEN before the second round of chemotherapy. The ALDEN score was highest with tislelizumab and decreased to 2 with docetaxel after the second round of chemotherapy. So we concluded that tislelizumab caused TEN. The patient developed TEN on the 18th day following the initial immunotherapy in our case. The overall mortality rate of TEN was 32%, primarily due to infections and tumor progression, with a median interval of 28 days from SJS/TEN onset to death. Deceased patients had a notably higher proportion of TEN and a greater epidermal detachment area than survivors (14). TEN leads to the permanent withdrawal of immunotherapy and the delay of anti-tumor therapy in this patient.
There are no standardized guidelines for treatment SJS/TEN, some organizations recommend permanently discontinuing ICIs, using corticosteroids and intravenous immunoglobulins, and providing comprehensive supportive care (15, 16). Given the rapid progression of SJS/TEN, with peak skin detachment typically occurring within 8 days, prompt initiation of corticosteroids is essential due to the prognostic significance of skin loss extent (17). Methylprednisolone was administered at an elevated dose due to the rapid progression of TEN in our case. The extent of epidermal detachment is an important prognostic factor in ICI-related cases, likely due to the heightened risk of infection and metabolic disturbances linked to extensive skin involvement (14). There is currently no consensus on optimal wound care. Gentle debridement of large detached epidermal segments, aspiration of bulla fluid, and anti-shear measures, along with adjunct immunomodulatory therapies, is the preferred approach for most cases (18). A recent systematic review showed that patients with ICI-related SJS/TEN typically experienced re-epithelization within 30 days. In our case, re-epithelization occurred in 14 days, presenting a simple and effective wound care method. What’s more, managing intravenous transfusions was challenging.
4 Conclusion
In conclusion, tislelizumab-related TEN in esophageal cancer treatment is a very rare but serious adverse event. It is crucial to monitor skin reactions during and after treatment with anti-PD-1 agents. Active systemic therapy, supportive treatment, and wound care are crucial. We present a cost-effective, safe, efficient, and simple strategy that we recommend for similar TEN patients. However, the limited number of cases prevents us from verifying the strategy’s universality.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual (s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SW: Data curation, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. CX: Resources, Supervision, Writing – review & editing. HL: Data curation, Investigation, Resources, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1522525/full#supplementary-material
Abbreviations
ICIs, Immune checkpoint inhibitors; ESCC, Esophageal squamous cell carcinoma; ir-AEs, Immune-related adverse events; TEN, Toxic epidermal necrolysis; TRAEs, Treatment-related adverse events; SJS, Stevens–Johnson syndrome.
References
1. Waters, JK, and Reznik, SI. Update on Management of Squamous Cell Esophageal Cancer. Curr Oncol Rep. (2022) 24:375–85. doi: 10.1007/s11912-021-01153-4
2. Shoji, Y, Koyanagi, K, Kanamori, K, Tajima, K, Ogimi, M, Ninomiya, Y, et al. Immunotherapy for esophageal cancer: where are we now and where can we go. World J Gastroenterol. (2024) 30:2496–501. doi: 10.3748/wjg.v30.i19.2496
3. Geisler, AN, Phillips, GS, Barrios, DM, Wu, J, Leung, DYM, Moy, AP, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. (2020) 83:1255–68. doi: 10.1016/j.jaad.2020.03.132
4. Bastuji-Garin, S, Rzany, B, Stern, RS, Shear, NH, Naldi, L, and Roujeau, JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. (1993) 129:92–6. doi: 10.1001/archderm.1993.01680220104023
5. Bastuji-Garin, S, Fouchard, N, Bertocchi, M, Roujeau, JC, Revuz, J, and Wolkenstein, P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. (2000) 115:149–53. doi: 10.1046/j.1523-1747.2000.00061.x
6. Sassolas, B, Haddad, C, Mockenhaupt, M, Dunant, A, Liss, Y, Bork, K, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. (2010) 88:60–8. doi: 10.1038/clpt.2009.252
7. Fick, CN, Dunne, EG, Sihag, S, Molena, D, Cytryn, SL, Janjigian, YY, et al. Immunotherapy for Resectable locally advanced esophageal carcinoma. Ann Thorac Surg. (2024) 118:130–40. doi: 10.1016/j.athoracsur.2024.02.021
8. Zheng, J, Huang, B, Xiao, L, Wu, M, and Li, J. Treatment-and immune-related adverse events of immune checkpoint inhibitors in esophageal or gastroesophageal junction cancer: a network meta-analysis of randomized controlled trials. Front Oncol. (2022) 12:821626. doi: 10.3389/fonc.2022.821626
9. Zhou, J, Wang, CP, Li, J, Zhang, HL, and He, CX. Stevens-Johnson syndrome and toxic epidermal necrolysis associated with immune checkpoint inhibitors: a systematic review. Front Immunol. (2024) 15:1414136. doi: 10.3389/fimmu.2024.1414136
10. Xu, J, Kato, K, Raymond, E, Hubner, RA, Shu, Y, Pan, Y, et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): a global, randomised, placebo-controlled, phase 3 study. Lancet Oncol. (2023) 24:483–95. doi: 10.1016/S1470-2045(23)00108-0
11. Wasuwanich, P, So, JM, Chakrala, TS, Chen, J, and Motaparthi, K. Epidemiology of Stevens-Johnson syndrome and toxic epidermal necrolysis in the United States and factors predictive of outcome. JAAD Int. (2023) 13:17–25. doi: 10.1016/j.jdin.2023.06.014
12. Marks, ME, Botta, RK, Abe, R, Beachkofsky, TM, Boothman, I, Carleton, BC, et al. Updates in SJS/TEN: collaboration, innovation, and community. Front Med. (2023) 10:1213889. doi: 10.3389/fmed.2023.1213889
13. Qin, K, Gong, T, Ruan, SF, Lin, M, Su, X, Lv, X, et al. Clinical features of Stevens-Johnson syndrome and toxic epidermal necrolysis induced by immune checkpoint inhibitor versus non-immune checkpoint inhibitor drugs in China: a cross-sectional study and literature review. J Inflamm Res. (2024) 17:7591–605. doi: 10.2147/JIR.S491791
14. Maloney, NJ, Ravi, V, Cheng, K, Bach, DQ, and Worswick, S. Stevens-Johnson syndrome and toxic epidermal necrolysis-like reactions to checkpoint inhibitors: a systematic review. Int J Dermatol. (2020) 59:e183–8. doi: 10.1111/ijd.14811
15. Thompson, JA, Schneider, BJ, Brahmer, J, Andrews, S, Armand, P, Bhatia, S, et al. Management of Immunotherapy-Related Toxicities, version 1.2019. JNCCN. (2019) 17:255–89. doi: 10.6004/jnccn.2019.0013
16. Haanen, J, Obeid, M, Spain, L, Carbonnel, F, Wang, Y, Robert, C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Annals Oncol. (2022) 33:1217–38. doi: 10.1016/j.annonc.2022.10.001
17. Heng, YK, Lee, HY, and Roujeau, JC. Epidermal necrolysis: 60 years of errors and advances. Br J Dermatol. (2015) 173:1250–4. doi: 10.1111/bjd.13989
Keywords: toxic epidermal necrolysis, tislelizumab, esophageal squamous cell carcinoma, immune-related adverse events, skin toxicity
Citation: Wu S, Xu C and Liu H (2024) Case report: Toxic epidermal necrolysis induced by tislelizumab in a patient with esophageal squamous cell carcinoma. Front. Med. 11:1522525. doi: 10.3389/fmed.2024.1522525
Edited by:
Zhenhua Chen, Jinzhou Medical University, ChinaReviewed by:
Rolando Perez-Lorenzo, Columbia University, United StatesMariateresa Rossi, University of Brescia, Italy
Copyright © 2024 Wu, Xu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huafeng Liu, aHVhZmVuZ19sMTk4MUAxNjMuY29t
 Shilong Wu
Shilong Wu Chenyang Xu1
Chenyang Xu1