- Department of Clinical Pharmacy, Traditional Chinese Medical Hospital of Zhuji, Shaoxing, China
Purpose: This meta-analysis aims to compare the diagnostic efficacy of [18F]FDG PET/CT and [18F]FDG PET/MRI in patients with non-small cell lung cancer (NSCLC).
Methods: An extensive literature search was conducted throughout the PubMed, Embase, and Web of Science databases for works accessible through September 2024. We included studies assessed the diagnostic efficacy of [18F]FDG PET/CT and [18F]FDG PET/MRI in NSCLC.
Results: The meta-analysis includes six studies with a total of 437 patients. The sensitivity and specificity of [18F]FDG PET/CT and [18F]FDG PET/MRI for detecting lymph node metastasis were similar, at 0.82 (0.68–0.94) vs. 0.86 (0.70–0.97) and 0.88 (0.76–0.96) vs. 0.90 (0.85–0.94), respectively, with no significant differences (p = 0.70 for sensitivity, p = 0.75 for specificity). For distant metastasis, the sensitivity of [18F]FDG PET/CT and [18F]FDG PET/MRI was 0.86 (0.60–1.00) and 0.93 (0.63–1.00), and specificity was 0.89 (0.65–1.00) vs. 0.90 (0.64–1.00), respectively, also showing no significant differences (p = 0.66 for sensitivity, p = 0.97 for specificity).
Conclusion: Our meta-analysis shows that [18F]FDG PET/MRI has similar sensitivity and specificity to [18F]FDG PET/CT in identifying lymph node and distant metastases in patients with NSCLC. Additional larger sample prospective studies are needed to confirm these findings.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023479817, CRD42023479817.
1 Introduction
Lung cancer is recognized as the most typical diagnosis malignancy globally, also notable for its high mortality rates (1). Lung cancer remains the most prevalent cancer globally in 2022, accounting for approximately 2.5 million new cases, which represents one in eight cancer diagnoses worldwide (12.4% of all global cancer incidences) (2). In this setting, roughly 80% of lung malignancies are categorized as non-small cell lung cancer (NSCLC), which is the main cancer diagnosis worldwide (3, 4). Surgery, radiation, chemotherapy, immunotherapy, and targeted therapy can all be used to treat NSCLC, depending on the stage of the tumor (5). The effectiveness of these treatments and the overall prognosis of the patient are profoundly impacted by the initial stage of the cancer (6). As a result, thorough and precise imaging-based staging is important for optimal care of NSCLC patients.
Currently, clinical methods used for NSCLC staging include computed tomography (CT), magnetic resonance imaging (MRI), and biopsy (7, 8). However, each of these modalities has its inherent limitations. While CT scanning excels in identifying the tumor’s location and determining lymph node enlargement, its limited ability to determine or exclude mediastinal metastasis imposes certain constraints on the accurate staging of lung cancer (9). MRI is often considered less effective than CT for detecting small cancer lesions, due to its sensitivity to cardiac and respiratory motion artifacts, extremely low T2 values, lung magnetic field heterogeneity, and the low proton density of lung parenchyma (10). Biopsies, although crucial for delivering definitive results, are associated with inherent risks and may not always be feasible. The most common complication encountered is pneumothorax, which occurs in 20–64% of all CT-guided biopsies (11, 12). Additionally, hemorrhage from the lung parenchyma stands as another notable complication, frequently resulting from the needle track crossing a pulmonary vessel (13).
Positron emission tomography (PET) plays a crucial role in diagnosing NSCLC, from initial detection to staging and monitoring tumor metastasis (14). Integrating PET with 18F-fluorodeoxyglucose ([18F]FDG) into PET/CT and PET/MRI systems has considerably revolutionized cancer imaging by integrating metabolic and anatomical information (15). [18F]FDG PET/CT plays an important role in managing NSCLC, notably in evaluating the nodal status and finding occult metastatic disease, where it outperforms the capabilities of CT scanning alone (16). The NCCN guidelines emphasize the importance of rapid access to PET/CT for accurate staging in NSCLC, highlighting its role in guiding management decisions and predicting prognosis across all stages of the disease, particularly in detecting metastases (17). One of its main benefits over traditional imaging approaches is its increased sensitivity for detecting extra-thoracic metastases (18, 19). Dahlsgaard-Wallenius et al. found that PET/MRI and PET/CT had comparable diagnostic capacities for N-staging in NSCLC (20). Combining the metabolic data from PET with the special characteristics of MRI—such as low radiation exposure and excellent soft tissue contrast—makes PET/MRI an advantageous test (21). In several studies, evidence suggested that PET/MRI may outperform PET/CT in detecting metastases within the pleura, brain, liver, and bone (22, 23). This is also in accordance with the results of a prospective single-center research of 330 exams, where PET/MRI found brain and liver metastases that were undetectable by PET/CT (24). Thus, the use of a hybrid PET/MRI in lung cancer patients may sometimes assist the detection of distant metastases, because NSCLC metastases are primarily situated in the brain, liver, and bone (25, 26). However, the included trials gave minimal data on extra-thoracic metastatic illness, making it unable to draw conclusions about the potential advantage of PET/MRI. Due to the relative novelty of PET/MRI and the limited availability of direct comparison studies, inconsistencies in the literature regarding their comparative efficacy warrant careful examination.
The goal of this meta-analysis is to comprehensively evaluate the diagnostic performance of [18F]FDG PET/MRI to [18F]FDG PET/CT in NSCLC through head-to-head comparison.
2 Methods
The meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Diagnostic Test Accuracy (PRISMA-DTA) standards (27). The protocol for this meta-analysis is registered with PROSPERO (CRD42023479817).
2.1 Search strategy
An extensive literature search was conducted in PubMed, Embase, and Web of Science to identify pertinent publications available up to September 2024. The search utilized the following keywords: (“PET/MRI” or “PET/CT”) AND (“lymph node metastasis”) AND (“distant metastasis”) AND (“non-small cell lung cancer”). Further details are available in Supplementary Table S1. The reference lists of the listed studies were meticulously manually examined to identify additional relevant literature.
2.2 Inclusion and exclusion criteria
This meta-analysis included studies that satisfied the PICOS framework: Population (P): patients diagnosed with NSCLC; Intervention (I): diagnostic imaging using [18F]FDG PET/CT and/or [18F]FDG PET/MRI; Comparison (C): studies comparing PET/CT and PET/MRI; Outcomes (O): studies that report diagnostic performance in assessing lymph node involvement and/or distant metastases; Study design (S): studies with a sample size greater than ten.
Studies were excluded if they were (1) animal studies, (2) non-research articles such as reviews, case reports, conference abstracts, meta-analyses, letters to the editor, or (3) non-randomized designs including case–control, cohort, and cross-sectional studies. Additionally, studies employing other radiotracers were also omitted. For studies utilizing the same data sets, only the most recent were considered.
2.3 Quality assessment
Two researchers independently evaluated the quality of the included studies using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool (28). This tool addresses four key domains: patient selection, index test, reference standard, and flow and timing. Each study was independently rated, and any disagreements were resolved through discussion to reach consensus. The QUADAS-2 tool allowed for a structured and transparent appraisal of study quality, highlighting areas with potential risk of bias or applicability concerns.
2.4 Data extraction
Two researchers extracted data separately from the selected papers. This data encompassed details as author, year of publication, imaging test type, study characteristics (country, study design, study duration, analysis, and reference standard), patient characteristics (number of patients, radiologists involved, and mean/median age), and technical specifics [scanner modality, ligand dose, image analysis, and true positives (TP), false positives (FP), false negatives (FN), true negatives (TN)].
2.5 Outcome measures
The primary endpoints were the sensitivity and specificity of [18F]FDG PET/CT and [18F]FDG PET/MRI in detecting lymph node metastasis and distant metastasis. Sensitivity was defined as the proportion of TP scans relative to the sum of TP and FN scans, reported at either the patient or lesion level. Specificity was defined as the proportion of TN scans relative to the total of TN and FP scans, as documented.
2.6 Statistical analysis
The DerSimonian and Laird methods were used to assess sensitivity and specificity, which were then combined with the Freeman-Tukey double inverse sine transformation. Confidence intervals were calculated employing the Jackson method. Heterogeneity both within and across groups was evaluated using the Cochrane Q and I2 statistics (29). Significant heterogeneity (p < 0.10 or I2 > 50%) warranted sensitivity analysis and meta-regression to identify individual studies contributing to heterogeneity.
Both funnel plots and Egger’s test were used to investigate publication bias. For all statistical analyses, a significance level of p < 0.05 was set. R software version 4.1.2 was used for computation and graphical display of statistical analyses.
3 Results
3.1 Study selection
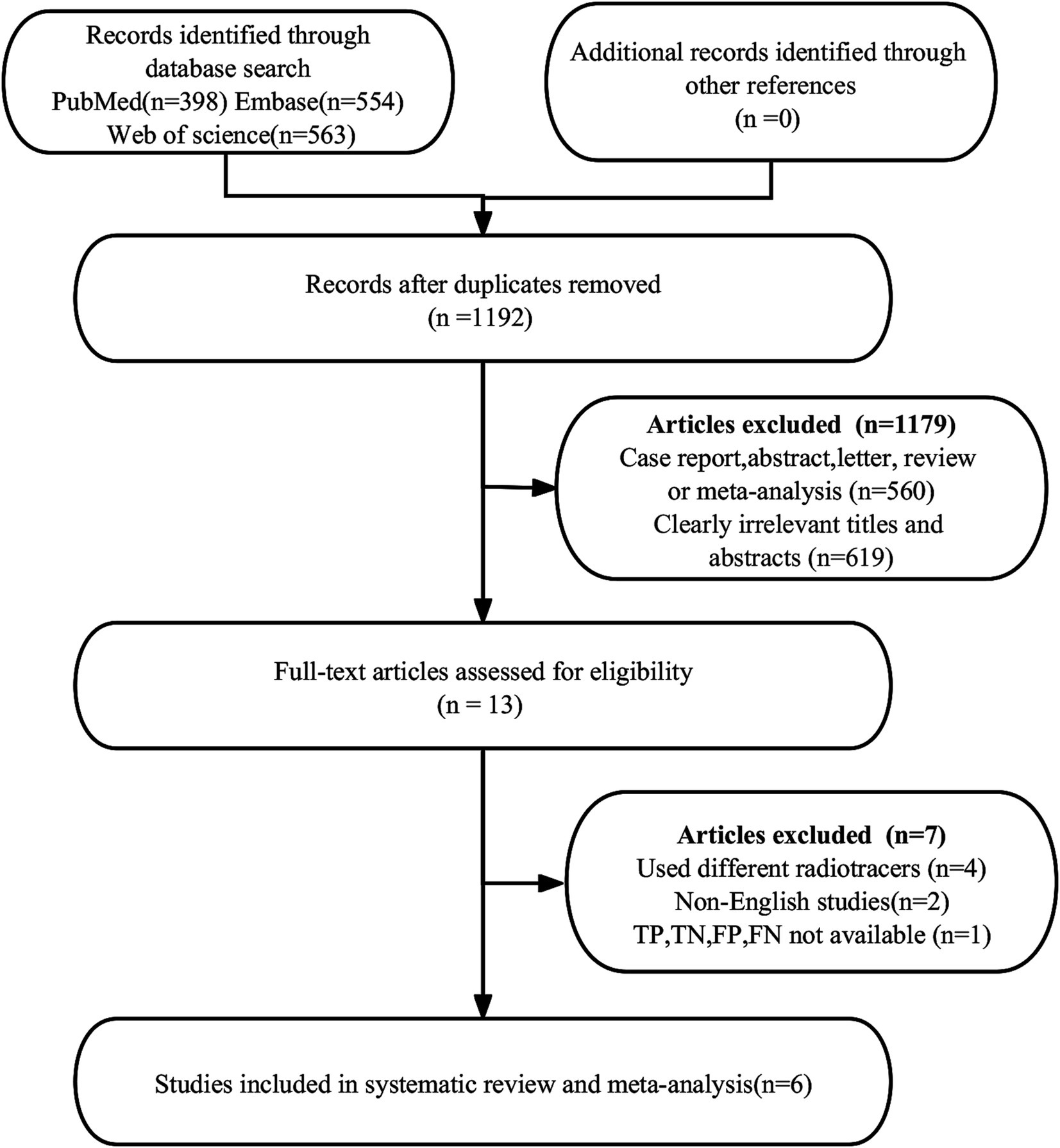
A total of 1,515 publications were found in the first search. Nevertheless, 323 studies were found to be duplicates and were not eligible for this study, leaving 1,192 studies for further analysis. After a thorough review of the remaining 13 articles, 7 more were deemed ineligible due to unavailable data (TP, FP, FN, and TN) (n = 1) or different radiotracers (n = 4). Additionally, non-English articles (n = 2) were excluded. Ultimately, the meta-analysis included 6 articles (23, 30–34) evaluating the diagnostic efficacy of [18F]FDG PET/CT and [18F]FDG PET/MRI. The article PRISMA selection process is illustrated in Figure 1.
3.2 Study description and quality assessment
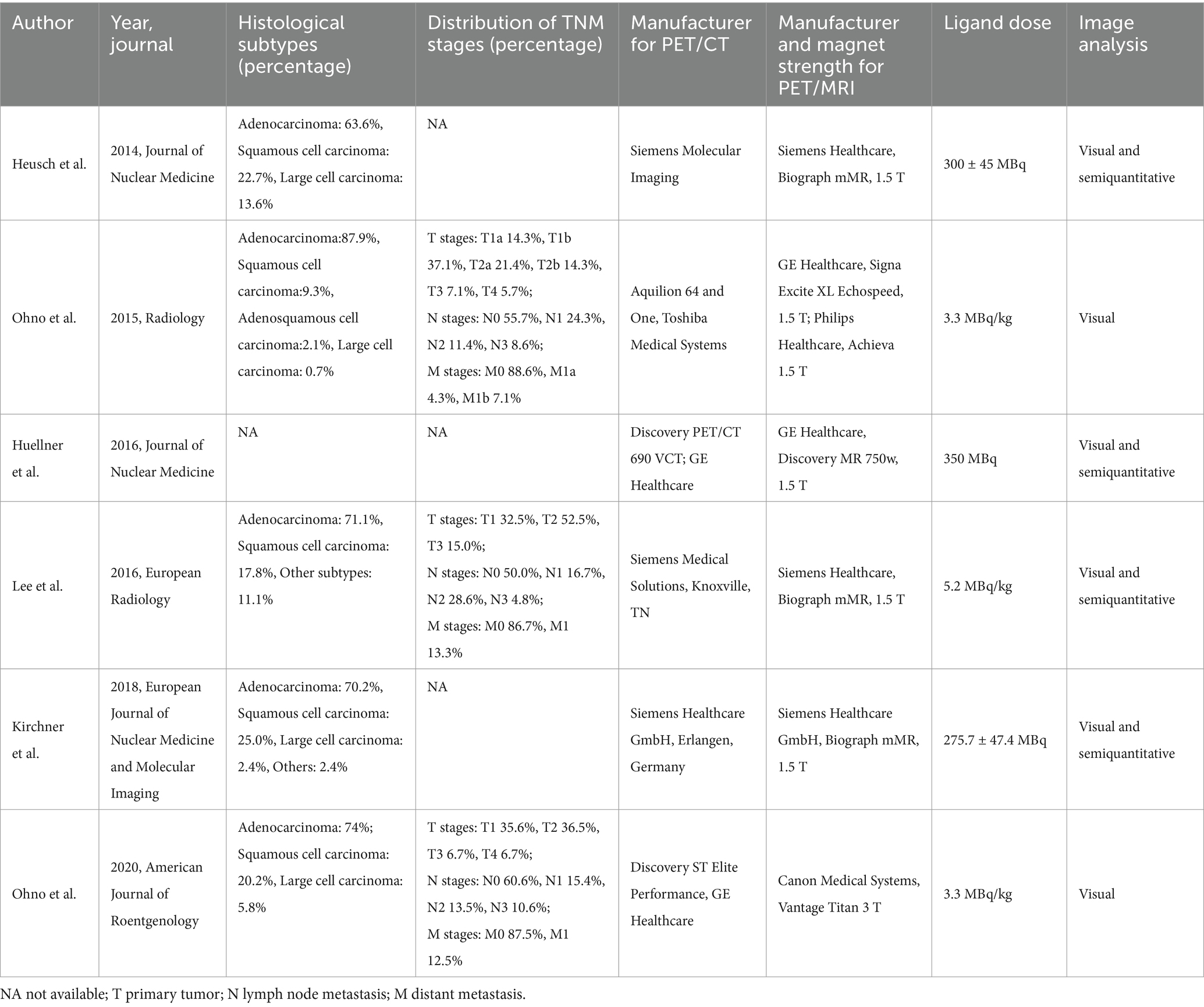
The six qualifying trials included a total of 437 NSCLC patients aged 35 to 89. All included articles were prospective design. All studies included N-stage evaluations, and three studies provided data regarding distant metastasis (23, 31, 32). Concerning analysis methods, each of the six articles employed patient-level analysis. Two articles adopted pathology as the reference standard, whereas four utilized either pathology or follow-up imaging for this purpose. Table 1 shows the study and patient characteristics for [18F]FDG PET/CT and [18F]FDG PET/MRI, whereas Tables 2, 3 describes the technical parameters.
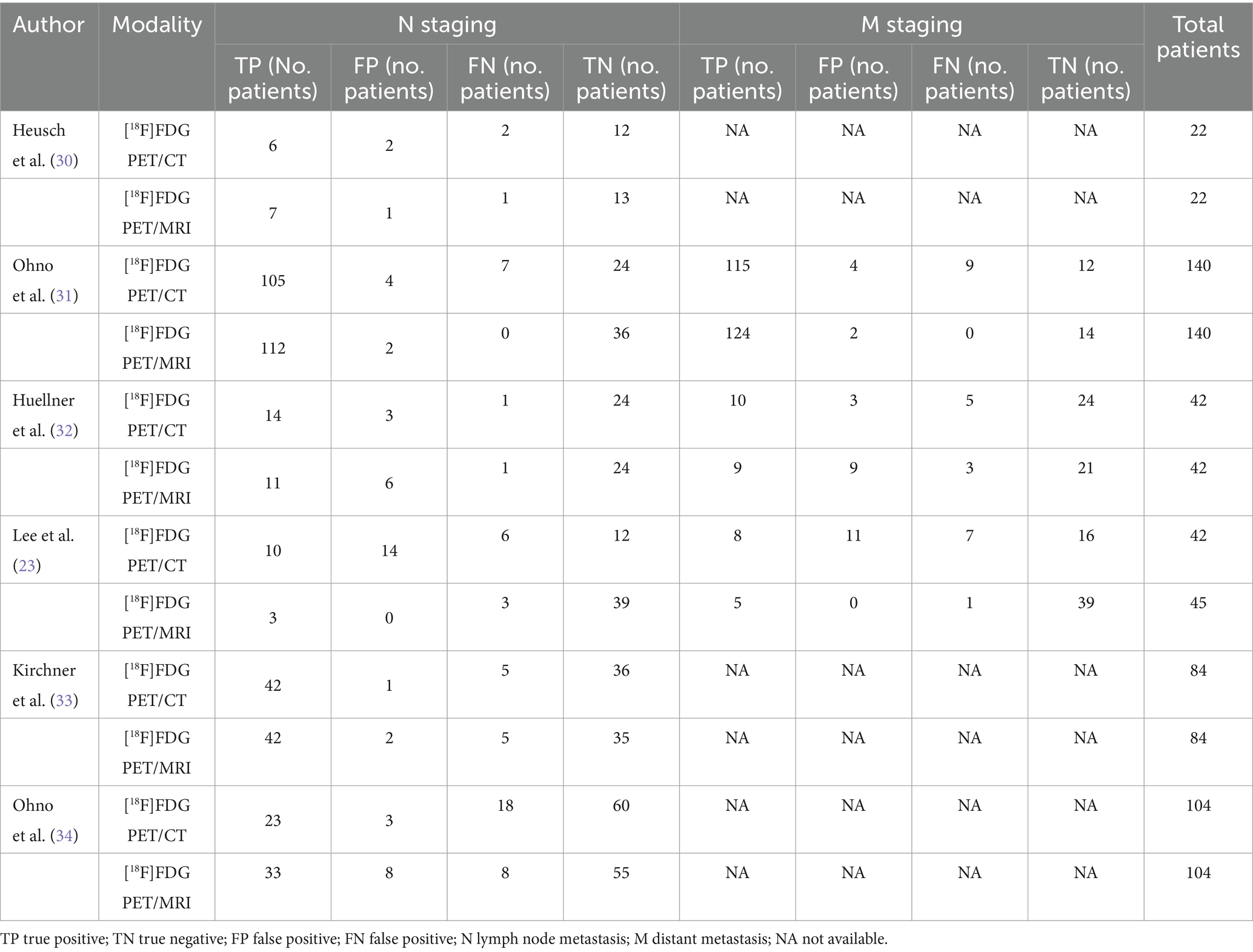
Table 3. Summary of 2×2 contingency table for diagnostic performance for N and M staging using [18F]FDG PET/CT and [18F]FDG PET/MRI.
Figure 2 depicts the risk of bias in each study, as assessed using the QUADAS-2 technique. When examining the risk of bias for patient selection, we discovered that one research was classified as “high risk” due to the absence of consecutive patients. One research classified the flow and timing criteria as “high risk” because certain subjects were excluded from the data analysis. The overall quality evaluation found that the included studies were good in quality.
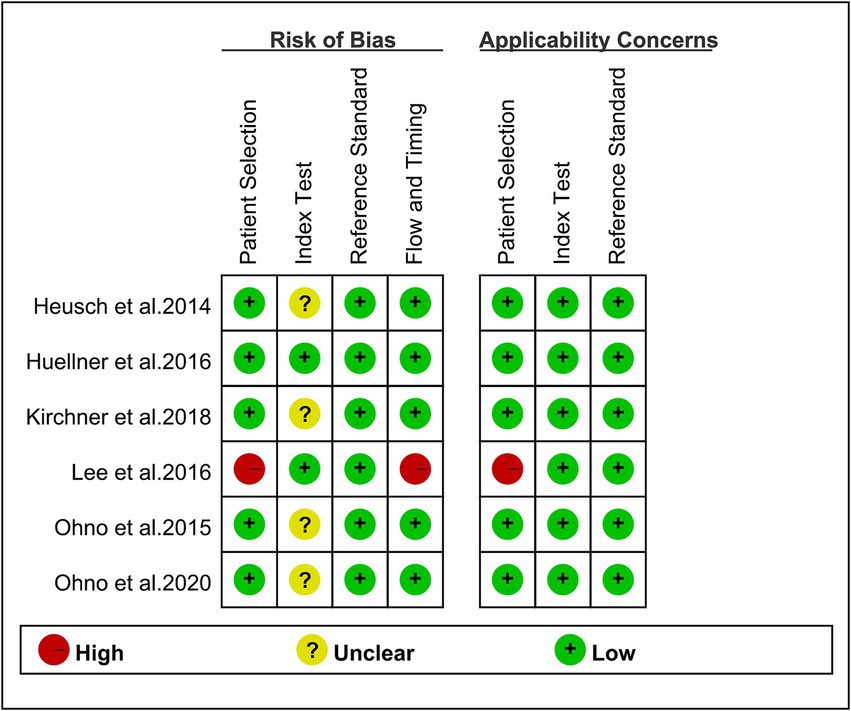
Figure 2. Risk of bias and applicability concerns of the included studies using the quality assessment of diagnostic performance studies QUADAS-2 tool.
3.3 Comparing the sensitivity of [18F]FDG PET/CT and [18F]FDG PET/MRI for detecting lymph node metastasis in NSCLC
The analysis incorporated six studies, revealing a pooled sensitivity of 0.82 (95% CI: 0.68–0.94) for [18F]FDG PET/CT in detecting lymph node metastases in NSCLC. On the other hand, [18F]FDG PET/MRI showed an overall sensitivity of 0.86 (95% CI: 0.70–0.97). As shown in Figure 3, there was no discernible change in sensitivity between [18F]FDG PET/CT and [18F]FDG PET/MRI (p = 0.70).

Figure 3. Forest plot of sensitivity comparison between [18F]FDG PET/CT and [18F]FDG PET/MRI in detecting lymph node metastasis in non-small cell lung cancer.
I2 values for [18F]FDG PET/CT and [18F]FDG PET/MRI were 84 and 90%, respectively. No discernible sources of heterogeneity were found using leave-one-out sensitivity analysis (Supplementary Figures S1, S2). The meta-regression analysis for [18F]FDG PET/CT also failed to find the origin of heterogeneity (Table 4). According to the meta-regression analysis for [18F]FDG PET/MRI, the difference in reference standard (p = 0.01) might be the source of heterogeneity (Table 5).
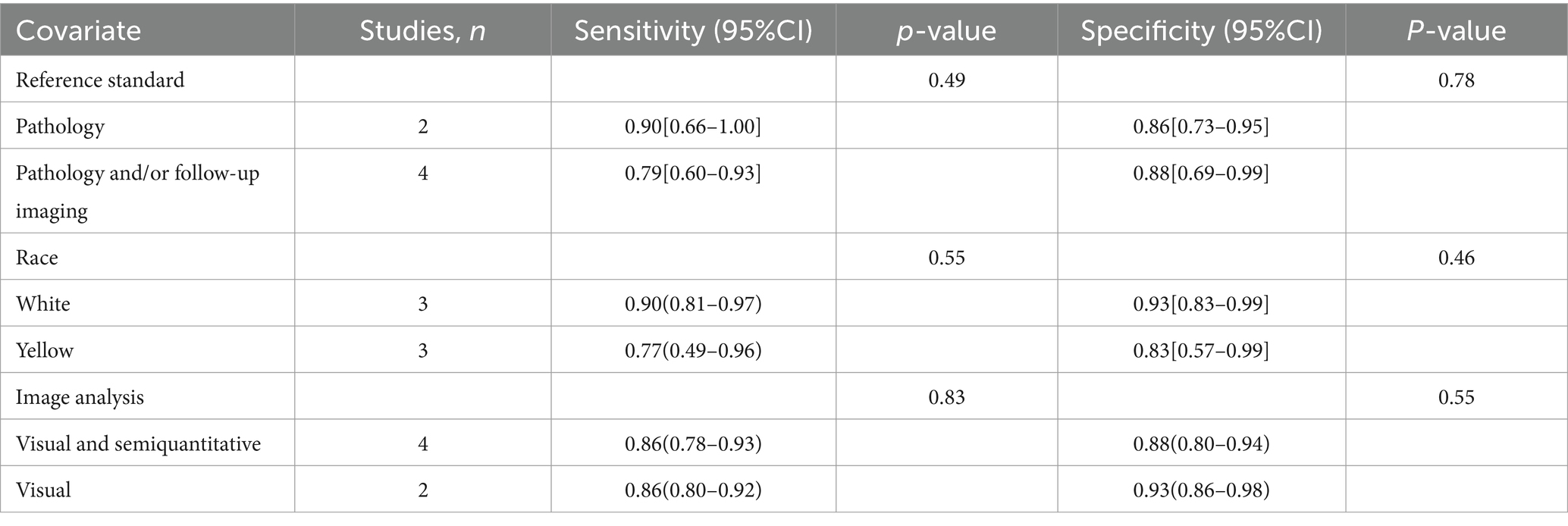
Table 4. Subgroup and meta-regression analysis of lymph node metastasis detection for [18F]FDG PET/CT.
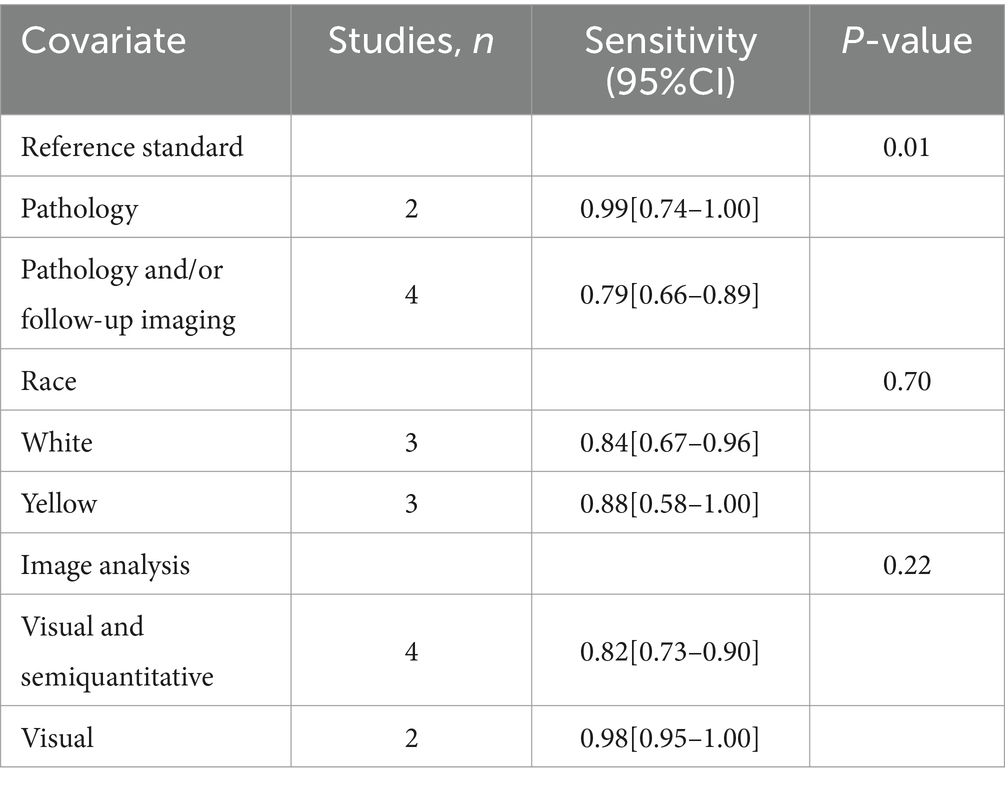
Table 5. Subgroup and meta-regression analysis of lymph node metastasis detection for [18F]FDG PET/MRI.
3.4 Comparing the specificity of [18F]FDG PET/CT and [18F]FDG PET/MRI for detecting lymph node metastases in NSCLC
Six studies were included, and a pooled specificity of 0.88 (95% CI: 0.76–0.96) for [18F]FDG PET/CT in identifying lymph node metastases in NSCLC. In contrast, [18F]FDG PET/MRI had a pooled specificity of 0.90 (95% CI: 0.85–0.94) (Figure 4). There was no significant difference in specificity between [18F]FDG PET/CT and [18F]FDG PET/MRI (p = 0.75).
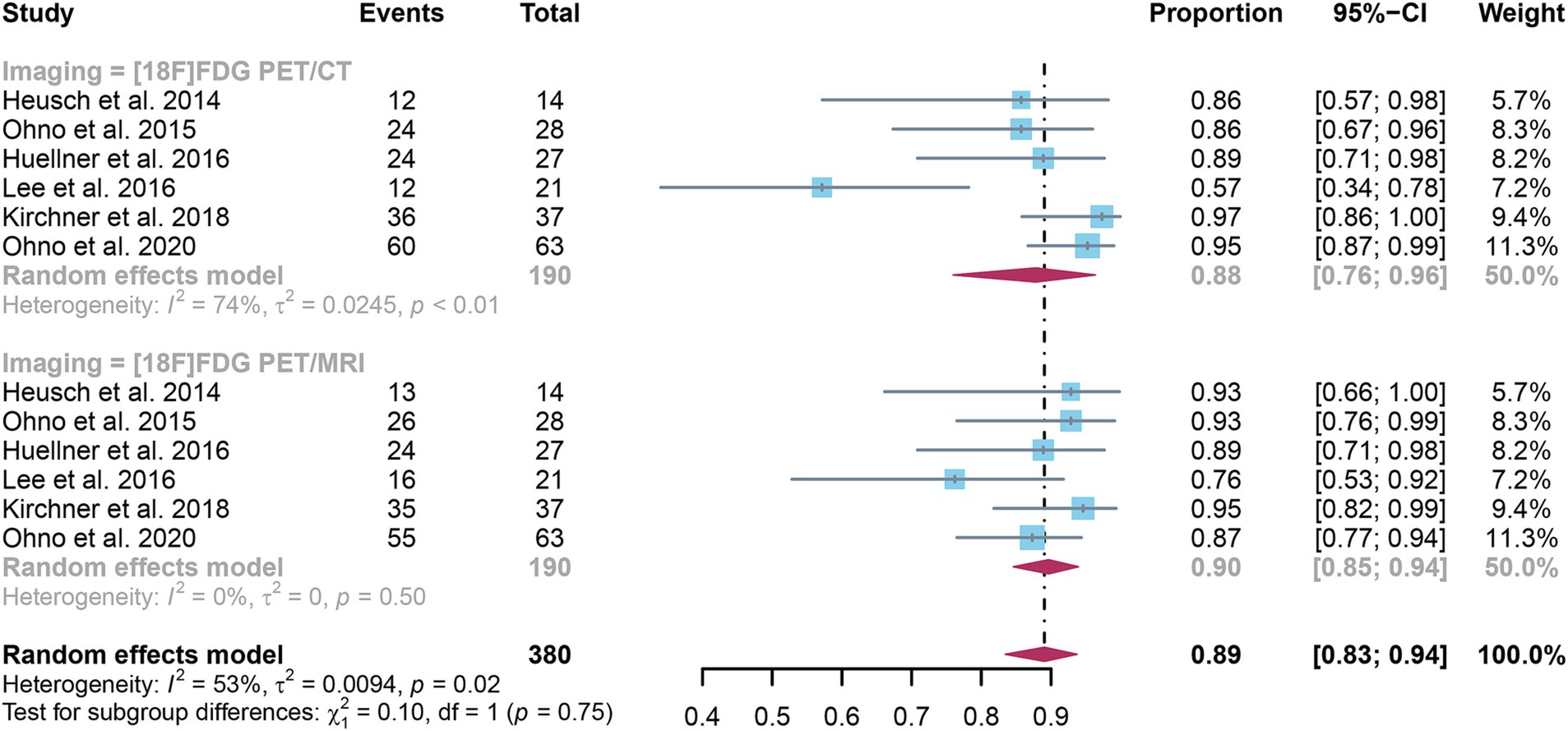
Figure 4. Forest plot of specificity comparison between [18F]FDG PET/CT and [18F]FDG PET/MRI in detecting lymph node metastasis in non-small cell lung cancer.
The I2 for sensitivity of [18F]FDG PET/CT was 74%. After omitting Lee et al.’s study, the I2 value reduced to 21%, indicating that this study might be a source of heterogeneity. Nonetheless, the findings of the specificity study were similar, with only modest differences between 0.85 and 0.93, as shown in Supplementary Figure S3.
3.5 Comparing the sensitivity of [18F]FDG PET/CT and [18F]FDG PET/MRI for detecting distant metastases in NSCLC
The analysis incorporated three studies, revealing a pooled sensitivity of 0.86 (95% CI: 0.60–1.00) for [18F]FDG PET/CT in detecting distant metastases in NSCLC. In contrast, [18F]FDG PET/MRI had an overall sensitivity of 0.93 (95% CI: 0.63–1.00) (Figure 5). There was no significant difference in sensitivity between [18F]FDG PET/CT and [18F]FDG PET/MRI (p = 0.66).
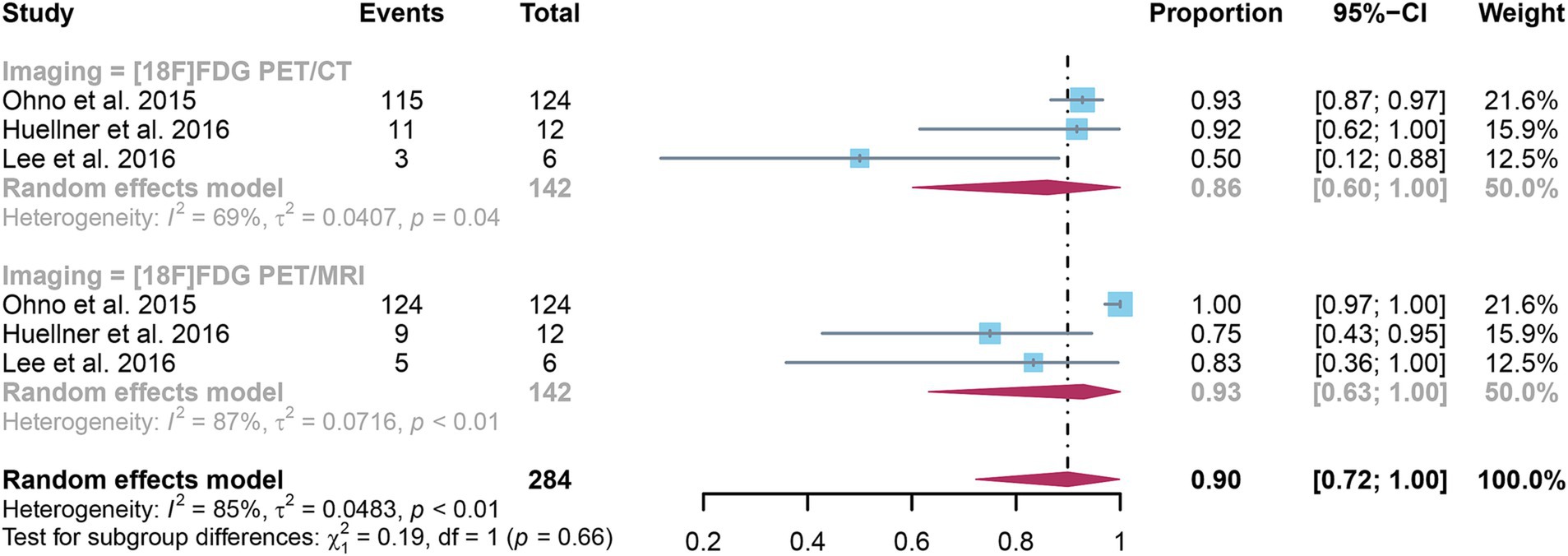
Figure 5. Forest plot of sensitivity comparison between [18F]FDG PET/CT and [18F]FDG PET/MRI in detecting distant metastasis in non-small cell lung cancer.
3.6 Comparing the specificity of [18F]FDG PET/CT and [18F]FDG PET/MRI for detecting distant metastases in NSCLC
The analysis incorporated three studies, revealing a pooled specificity of 0.89 (95% CI: 0.65–1.00) for [18F]FDG PET/CT in detecting distant metastases in NSCLC. In contrast, [18F]FDG PET/MRI had an overall specificity of 0.90 (95% CI: 0.64–1.00) (Figure 6). There was no significant difference in specificity between [18F]FDG PET/CT and [18F]FDG PET/MRI (p = 0.97).
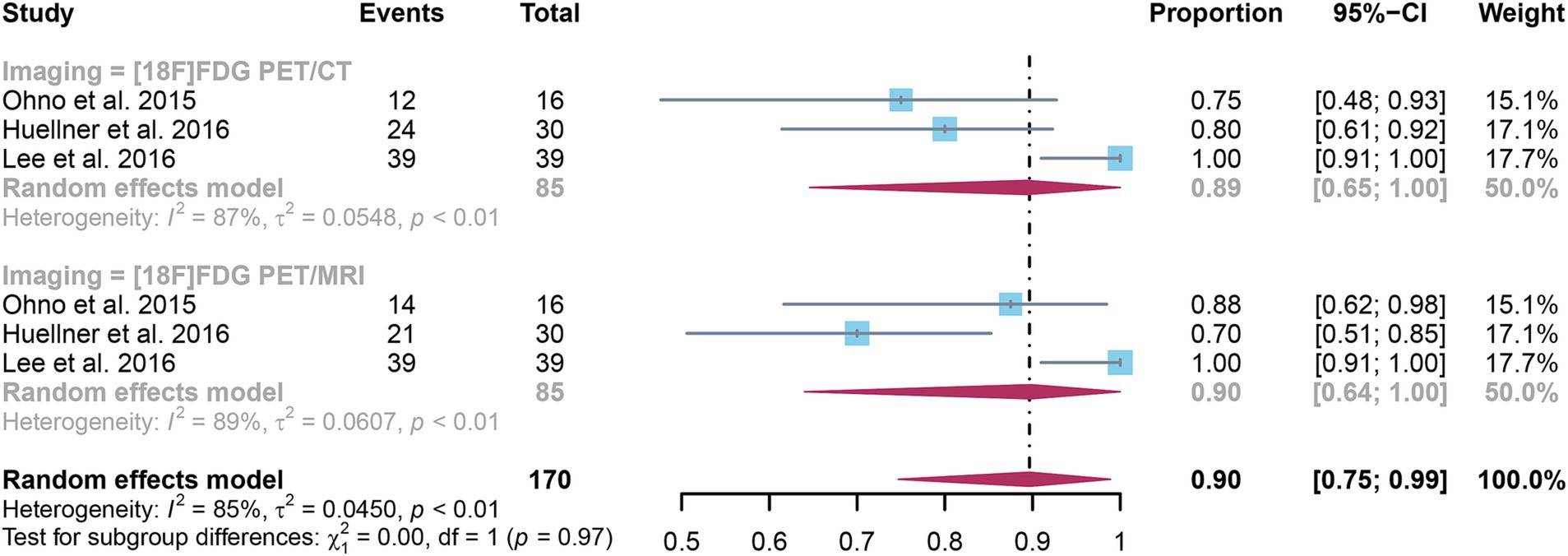
Figure 6. Forest plot of specificity comparison between [18F]FDG PET/CT and [18F]FDG PET/MRI in detecting distant metastasis in non-small cell lung cancer.
3.7 Publication bias
Funnel plot asymmetry tests were conducted to assess publication bias in [18F]FDG PET/CT and PET/MRI. For PET/CT, results indicated no significant bias for sensitivity (Egger’s p = 0.33, Supplementary Figure S4) or specificity (Egger’s p = 0.13, Supplementary Figure S5). For PET/MRI, significant bias was found in sensitivity (Egge’s p = 0.04, Supplementary Figure S6), while specificity showed no substantial bias (Egger’s p = 0.84, Supplementary Figure S7).
4 Discussion
The continuing controversy in the field of nuclear medicine regarding the comparative usefulness of [18F]FDG PET/CT and [18F]FDG PET/MRI in the assessment of lymph node and distant metastases in NSCLC requires a comprehensive meta-analysis (20, 22, 23). This analysis is critical for elucidating the diagnostic accuracy of these modalities, thereby informing clinical decision-making.
Our meta-analysis incorporated six studies to compare these imaging techniques. We discovered that [18F]FDG PET/CT had a pooled sensitivity of 0.82 and specificity of 0.88 in identifying lymph node metastases, whereas [18F]FDG PET/MRI had a sensitivity of 0.86 and specificity of 0.90, with no significant differences identified. Similarly, in identifying distant metastases, [18F]FDG PET/CT had a sensitivity of 0.86 and specificity of 0.89, whereas [18F]FDG PET/MRI had a sensitivity of 0.93 and specificity of 0.90, with no significant differences found. The slightly higher sensitivity of PET/MRI may be attributed to its superior soft tissue contrast provided by MRI, which enables better differentiation between tissues, especially in complex anatomical areas such as the lungs and lymph nodes (35). Unlike PET/CT, which uses X-ray imaging, MRI offers much higher resolution for soft tissue, allowing for more accurate detection of small or ambiguous lesions (36). However, the overlapping confidence intervals suggest that these differences might not be clinically significant.
Compared to the previous studies by Mojahed et al. (37) and Zhang et al. (35), which evaluated the diagnostic accuracy of [18F]FDG PET/CT versus [18F]FDG PET/MRI in T and N staging, our analysis reveals equivalent effectiveness of these modalities in detecting N and M stages in NSCLC patients. However, unlike Mojahed et al. and Zhang et al., we included evaluations of distant metastases, a crucial aspect of NSCLC staging. In addition to building on the previous analyses, our meta-analysis incorporates four new studies (23, 31, 32, 34), particularly those focusing on M stage (distant metastasis) assessment (23, 31, 32). This addition provides a more comprehensive understanding of [18F]FDG PET/MRI’s capabilities, addressing both nodal and metastatic assessments in NSCLC staging.
Zhang et al.’s (21) meta-analysis included 14 papers, five of which focused on lung cancer. In an analysis of five lung cancer trials including 429 patients, [18F]FDG PET/CT exhibited better sensitivity (0.87 vs. 0.84) and slightly worse specificity (0.95 vs. 0.96) than PET/MRI. In contrast, our meta-analysis found that [18F]FDG PET/MRI had similar sensitivity and specificity to [18F]FDG PET/CT in detecting lymph nodes and distant metastases in NSCLC patients. The discrepancy may stem from several factors. One key reason could be that Zhang et al.’s meta-analysis included patients with small cell lung cancer in addition to those with NSCLC. Small cell lung cancer generally presents with different patterns of lymph node and metastatic involvement compared to NSCLC, which may affect the diagnostic performance of [18F]FDG PET/CT and PET/MRI.
While PET/CT and PET/MRI modalities offer similar diagnostic efficacy, their cost and accessibility differ markedly, influencing their clinical integration. PET/CT, significantly more affordable, emerges as a cost-effective solution for healthcare providers (38). Its broader availability enhances its utility across diverse medical environments, proving especially advantageous in areas lacking advanced medical infrastructure. In instances where both techniques yield comparable sensitivity and specificity, PET/CT is frequently the preferred option. This preference stems not only from its cost-efficiency and wider accessibility, which promote extensive use, but also from its role in fostering more equitable healthcare access, particularly in under-resourced regions. Thus, balancing sophisticated diagnostic capabilities for practicalities such as affordability and accessibility, PET/CT distinctly outperforms when both modalities present equivalent diagnostic outcomes (36). A comprehensive comparison of the pros and cons of both imaging modalities is detailed in Supplementary Table S2.
Our study has some limitations. The inclusion of only six studies, and our analysis limited by the lack of detailed diagnostic performance data for different organ sites of metastasis, suggests a need for more extensive research in this area. Additionally, not all patients underwent pathological biopsy, some diagnoses were based on a combination of biopsy and clinical imaging follow-up. Future research should focus on studies using pathology as the sole gold standard to further validate these findings.
5 Conclusion
Our meta-analysis shows that [18F]FDG PET/MRI has similar sensitivity and specificity to [18F]FDG PET/CT in identifying lymph node and distant metastases in patients with NSCLC. Additional larger sample prospective studies are needed to confirm these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
DY: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. CC: Conceptualization, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1517805/full#supplementary-material
References
1. Li, C, Lei, S, Ding, L, Xu, Y, Wu, X, Wang, H, et al. Global burden and trends of lung cancer incidence and mortality. Chin Med J. (2023) 136:1583–90. doi: 10.1097/cm9.0000000000002529
2. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
3. Barta, JA, Powell, CA, and Wisnivesky, JP. Global epidemiology of lung Cancer. Ann glob. Health. (2019) 85, 1–16. doi: 10.5334/aogh.2419
4. Abdo, M, Belloum, Y, Heigener, D, Welker, L, von Weihe, S, Schmidt, M, et al. Comparative evaluation of PD-L1 expression in cytology imprints, circulating tumour cells and tumour tissue in non-small cell lung cancer patients. Mol Oncol. (2023) 17:737–46. doi: 10.1002/1878-0261.13415
5. Hendriks, LE, Remon, J, Faivre-Finn, C, Garassino, MC, Heymach, JV, Kerr, KM, et al. Non-small-cell lung cancer. Nat Rev Dis Prim. (2024) 10:71. doi: 10.1038/s41572-024-00551-9
6. Bi, JH, Tuo, JY, Xiao, YX, Tang, DD, Zhou, XH, Jiang, YF, et al. Observed and relative survival trends of lung cancer: a systematic review of population-based cancer registration data. Thoracic Cancer. (2024) 15:142–51. doi: 10.1111/1759-7714.15170
7. Owens, C, Hindocha, S, Lee, R, Millard, T, and Sharma, B. The lung cancers: staging and response, CT, (18)F-FDG PET/CT, MRI, DWI: review and new perspectives. Br J Radiol. (2023) 96:20220339. doi: 10.1259/bjr.20220339
8. Amicizia, D, Piazza, MF, Marchini, F, Astengo, M, Grammatico, F, Battaglini, A, et al. Systematic review of lung cancer screening: advancements and strategies for implementation. Healthcare. (2023), 11. doi: 10.3390/healthcare11142085
9. Silvestri, GA, Gonzalez, AV, Jantz, MA, Margolis, ML, Gould, MK, Tanoue, LT, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2013) 143:e211S–50S. doi: 10.1378/chest.12-2355
10. Kim, TJ, Kim, CH, Lee, HY, Chung, MJ, Shin, SH, Lee, KJ, et al. Management of incidental pulmonary nodules: current strategies and future perspectives. Expert Rev Respir Med. (2020) 14:173–94. doi: 10.1080/17476348.2020.1697853
11. Sabatino, V, Russo, U, D'Amuri, F, Bevilacqua, A, Pagnini, F, Milanese, G, et al. Pneumothorax and pulmonary hemorrhage after CT-guided lung biopsy: incidence, clinical significance and correlation. Radiol Med. (2021) 126:170–7. doi: 10.1007/s11547-020-01211-0
12. Nour-Eldin, NE, Alsubhi, M, Naguib, NN, Lehnert, T, Emam, A, Beeres, M, et al. Risk factor analysis of pulmonary hemorrhage complicating CT-guided lung biopsy in coaxial and non-coaxial core biopsy techniques in 650 patients. Eur J Radiol. (2014) 83:1945–52. Epub 2014/07/27. doi: 10.1016/j.ejrad.2014.06.023
13. Philip, B, Jain, A, Wojtowicz, M, Khan, I, Voller, C, Patel, RSK, et al. Current investigative modalities for detecting and staging lung cancers: a comprehensive summary. Indian J Thorac Cardiovasc Surg. (2023) 39:42–52. doi: 10.1007/s12055-022-01430-2
14. Zhu, J, Pan, F, Cai, H, Pan, L, Li, Y, Li, L, et al. Positron emission tomography imaging of lung cancer: an overview of alternative positron emission tomography tracers beyond F18 fluorodeoxyglucose. Front Med (Lausanne). (2022) 9:945602. doi: 10.3389/fmed.2022.945602
15. Yap, JT, Carney, JP, Hall, NC, and Townsend, DW. Image-guided cancer therapy using PET/CT. Cancer J. (2004) 10:221–33. doi: 10.1097/00130404-200407000-00003
16. Gao, SJ, Kim, AW, Puchalski, JT, Bramley, K, Detterbeck, FC, Boffa, DJ, et al. Indications for invasive mediastinal staging in patients with early non-small cell lung cancer staged with PET-CT. Lung Cancer. (2017) 109:36–41. doi: 10.1016/j.lungcan.2017.04.018
17. Riely, GJ, Wood, DE, Ettinger, DS, Aisner, DL, Akerley, W, Bauman, JR, et al. Non–small cell lung Cancer, version 4.2024, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. (2024) 22:249–74. doi: 10.6004/jnccn.2204.0023
18. Farsad, M . FDG PET/CT in the staging of lung Cancer. Curr Radiopharm. (2020) 13:195–203. doi: 10.2174/1874471013666191223153755
19. Schaarschmidt, BM, Grueneisen, J, Metzenmacher, M, Gomez, B, Gauler, T, Roesel, C, et al. Thoracic staging with (18)F-FDG PET/MR in non-small cell lung cancer—does it change therapeutic decisions in comparison to (18)F-FDG PET/CT? Eur Radiol. (2017) 27:681–8. doi: 10.1007/s00330-016-4397-0
20. Dahlsgaard-Wallenius, SE, Hildebrandt, MG, Johansen, A, Vilstrup, MH, Petersen, H, Gerke, O, et al. Hybrid PET/MRI in non-small cell lung cancer (NSCLC) and lung nodules-a literature review. Eur J Nucl Med Mol Imaging. (2021) 48:584–91. doi: 10.1007/s00259-020-04955-z
21. Zhang, C, Liang, Z, Liu, W, Zeng, X, and Mo, Y. Comparison of whole-body 18F-FDG PET/CT and PET/MRI for distant metastases in patients with malignant tumors: a meta-analysis. BMC Cancer. (2023) 23:37. doi: 10.1186/s12885-022-10493-8
22. Fraioli, F, Screaton, NJ, Janes, SM, Win, T, Menezes, L, Kayani, I, et al. Non-small-cell lung cancer resectability: diagnostic value of PET/MR. Eur J Nucl Med Mol Imaging. (2015) 42:49–55. doi: 10.1007/s00259-014-2873-9
23. Lee, SM, Goo, JM, Park, CM, Yoon, SH, Paeng, JC, Cheon, GJ, et al. Preoperative staging of non-small cell lung cancer: prospective comparison of PET/MR and PET/CT. Eur Radiol. (2016) 26:3850–7. doi: 10.1007/s00330-016-4255-0
24. Mayerhoefer, ME, Prosch, H, Beer, L, Tamandl, D, Beyer, T, Hoeller, C, et al. PET/MRI versus PET/CT in oncology: a prospective single-center study of 330 examinations focusing on implications for patient management and cost considerations. Eur J Nucl Med Mol Imaging. (2020) 47:51–60. doi: 10.1007/s00259-019-04452-y
25. Popper, HH . Progression and metastasis of lung cancer. Cancer Metastasis Rev. (2016) 35:75–91. doi: 10.1007/s10555-016-9618-0
26. Milovanovic, IS, Stjepanovic, M, and Mitrovic, D. Distribution patterns of the metastases of the lung carcinoma in relation to histological type of the primary tumor: an autopsy study. Ann Thorac Med. (2017) 12:191–8. doi: 10.4103/atm.ATM_276_16
27. McInnes, MDF, Moher, D, Thombs, BD, McGrath, TA, Bossuyt, PM, Clifford, T, et al. Preferred reporting items for a systematic review and Meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. (2018) 319:388–96. doi: 10.1001/jama.2017.19163
28. Whiting, PF, Rutjes, AW, Westwood, ME, Mallett, S, Deeks, JJ, Reitsma, JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
29. Higgins, JP, and Thompson, SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
30. Heusch, P, Buchbender, C, Köhler, J, Nensa, F, Gauler, T, Gomez, B, et al. Thoracic staging in lung cancer: prospective comparison of 18F-FDG PET/MR imaging and 18F-FDG PET/CT. J Nucl Med. (2014) 55:373–8. doi: 10.2967/jnumed.113.129825
31. Ohno, Y, Koyama, H, Yoshikawa, T, Takenaka, D, Seki, S, Yui, M, et al. Three-way comparison of whole-body MR, Coregistered whole-body FDG PET/MR, and integrated whole-body FDG PET/CT imaging: TNM and stage assessment capability for non-small cell lung Cancer patients. Radiology. (2015) 275:849–61. doi: 10.1148/radiol.14140936
32. Huellner, MW, de Galiza, BF, Husmann, L, Pietsch, CM, Mader, CE, Burger, IA, et al. TNM staging of non-small cell lung Cancer: comparison of PET/MR and PET/CT. J Nucl Med. (2016) 57:21–6. doi: 10.2967/jnumed.115.162040
33. Kirchner, J, Sawicki, LM, Nensa, F, Schaarschmidt, BM, Reis, H, Ingenwerth, M, et al. Prospective comparison of (18)F-FDG PET/MRI and (18)F-FDG PET/CT for thoracic staging of non-small cell lung cancer. Eur J Nucl Med Mol Imaging. (2019) 46:437–45. doi: 10.1007/s00259-018-4109-x
34. Ohno, Y, Takeshi, Y, Takenaka, D, Koyama, H, Aoyagi, K, and Yui, M. Comparison of diagnostic accuracy for TNM stage among whole-body MRI and Coregistered PET/MRI using 1.5-T and 3-T MRI systems and integrated PET/CT for non-small cell lung Cancer. AJR Am J Roentgenol. (2020) 215:1191–8. doi: 10.2214/ajr.19.22565
35. Zhang, M, Liu, Z, Yuan, Y, Yang, W, Cao, X, Ma, M, et al. Head-to-head comparison of 18F-FDG PET/CT and 18F-FDG PET/MRI for lymph node metastasis staging in non-small cell lung cancer: a meta-analysis. Diagn Interv Radiol. (2024) 30:99–106. doi: 10.4274/dir.2023.232280
36. Weber, W . Clinical PET/MR. Recent Results Cancer Res. (2020) 216:747–64. doi: 10.1007/978-3-030-42618-7_22
37. Shahraki Mojahed, B, Saravani, K, and Parooie, F. Thoracic staging in patients with non-small cell lung cancer: a systematic review and meta-analysis on diagnostic accuracy of [18F]FDG PET/MRI and [18F]FDG PET/CT. Nucl Med Rev Cent East Eur. (2022) 26:11–9. doi: 10.5603/NMR.a2022.0037
38. Evangelista, L, Cuocolo, A, Pace, L, Mansi, L, Del Vecchio, S, Miletto, P, et al. Performance of FDG-PET/CT in solitary pulmonary nodule based on pre-test likelihood of malignancy: results from the ITALIAN retrospective multicenter trial. Eur J Nucl Med Mol Imaging. (2018) 45:1898–907. doi: 10.1007/s00259-018-4016-1
Keywords: [18F]FDG PET/CT, [18F]FDG PET/MRI, non-small cell lung cancer, meta-analaysis, staging
Citation: Yu D and Chen C (2025) [18F]FDG PET/CT versus [18F]FDG PET/MRI in staging of non-small cell lung cancer: a head-to-head comparative meta-analysis. Front. Med. 11:1517805. doi: 10.3389/fmed.2024.1517805
Edited by:
Nataliya Lutay, Skåne University Hospital, SwedenReviewed by:
Salvatore Annunziata, Fondazione Policlinico Universitario A. Gemelli IRCCS, ItalySilvia Taralli, Fondazione Policlinico Universitario A. Gemelli IRCCS, Italy
Quaovi Sodji, University of Wisconsin-Madison, United States
Copyright © 2025 Yu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaolin Chen, bHVja3kwMTA0MjAyM0AxNjMuY29t
 Dandan Yu
Dandan Yu Chaolin Chen
Chaolin Chen