- 1Pharmacy Department, The 960th Hospital of PLA, Jinan, China
- 2State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
Background: While epidemiological studies have linked statin use to a reduced risk of advanced colorectal adenomas, its impact on colorectal cancer (CRC) risk in patients with inflammatory bowel disease (IBD) remains unclear. To our knowledge, no meta-analysis to date has specifically examined this association. Therefore, we conducted a systematic review and meta-analysis of the available observational studies to investigate the risk of CRC associated with statin use in IBD patients.
Methods: We searched three databases for articles published in English before September 2024, focusing on the protective effects of statins against CRC in IBD patients. We calculated multivariate odds ratios (ORs) and their 95% confidence intervals (CIs) to assess this association. A random-effects meta-analysis was conducted using the generic inverse variance method.
Results: The meta-analysis included 4 studies encompassing 22,250 IBD patients, 6,712 of whom were statin users. The methodological quality of three of the studies was deemed high. We found a significantly lower risk of CRC in statin users compared to non-users, with a pooled relative risk of 1.88 (95% CI 1.54–2.30). Sensitivity analyses confirmed the consistency of these findings.
Conclusion: Statin use appears to be associated with a reduced risk of CRC in patients with IBD. However, given the limited number of studies available, further prospective research with large sample size is necessary to confirm the potential chemopreventive role of statins in this population.
Introduction
Inflammatory bowel disease (IBD) encompasses a group of immune-mediated disorders that exhibit a relapsing–remitting course, including ulcerative colitis (UC), Crohn’s disease (CD), and IBD-unclassified colitis (IBD-U) (1). The global incidence of IBD is on the rise, presenting significant economic and social challenges to healthcare systems due to its high prevalence, early onset, and the requirement for lifelong treatment (2). IBD is associated with various complications such as anemia, stenosis, abscesses, and fistulas (3). Notably, colorectal cancer (CRC) represents a significant morbidity factor in IBD patients, with studies showing an estimated 2-fold increased risk compared to the general population (4). This risk is further elevated in patients with concomitant primary sclerosing cholangitis (5, 6). Recognizing the risk factors for CRC in IBD could aid in preventing the disease and guiding targeted interventions.
Statins are primarily prescribed to treat hypercholesterolemia and reduce cardiovascular morbidity and mortality (7). Beyond their lipid-lowering effects, statins have demonstrated anti-proliferative, anti-inflammatory, and anti-neoplastic properties in numerous preclinical studies (8–10). As a result, research has suggested a potential benefit of statins in reducing cancer incidence. In terms of CRC risk in the general population (11, 12), meta-analyses of observational studies have indicated a modest reduction in CRC risk, though randomized controlled trials have not shown significant benefits (13). Subsequently, several studies (14–17) have explored the link between statin use and CRC risk in patients with IBD. In the earliest study, Samadder et al. found no protective effect of statins against CRC. Similar results were reported in a U.S.-based hospital analysis. However, two larger population-based studies identified an inverse association between statin use and CRC. These findings raise the question of whether statin use is linked to a reduced risk of CRC in the IBD population.
In light of the growing prevalence of IBD and widespread use of statins, we conducted a systematic review and meta-analysis to evaluate the relationship between statin use and CRC risk among IBD patients. This review aimed to support the development of evidence-based clinical guidelines and inspire further research in this area.
Methods
Search strategy
This systematic review and meta-analysis was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. We conducted a comprehensive search in PubMed and Embase databases for studies published up to September 10, 2024. Our search terms included combinations of “inflammatory bowel disease,” “IBD,” “Crohn’s disease,” “CD,” “ulcerative colitis,” “UC,” “statin(s),” “HMG-CoA reductase inhibitor(s),” “simvastatin,” “atorvastatin,” “pravastatin,” “fluvastatin,” “rosuvastatin,” “lovastatin,” and terms related to colorectal conditions such as “colon,” “rectal,” “colorectal,” along with “cancer,” “tumor,” “carcinoma,” and “neoplasm.” In addition, a manual search of the reference lists of the retrieved articles was conducted.
Inclusion criteria
The inclusion criteria of our study followed the PICO (Population, Intervention, Comparison, Outcome) framework: (1) Population: patients diagnosed with IBD; (2) Intervention: statin use; (3) Comparison: non-use of statins; (4) Outcome: incidence of CRC. Randomized controlled trials (RCTs), cohort studies, and case–control studies were included, while case series, case reports, animal studies, editorials, and reviews were excluded.
Data extraction and quality assessment
Data from the included studies were extracted and summarized in an Excel spreadsheet. Extracted information included the first author, publication year, study design, study location, subject characteristics (age and IBD type), methods for assessing IBD, the number of IBD patients exposed and unexposed to statins, CRC definitions, statistical adjustments for confounders, and study quality assessment.
Each article was independently evaluated using the Newcastle-Ottawa Quality Assessment Scale (NOS) (18), as recommended by the Cochrane Collaboration for assessing the quality of observational studies. A score higher than 7 points indicated a high-quality study.
Statistical analysis
Statistical analyses were conducted using Stata 12.0 software (Stata Corp., College Station, TX, USA). Heterogeneity was assessed using the I2 statistic, with I2 > 50% indicating significant heterogeneity (19). In cases of significant heterogeneity, random-effects models were applied. The risks of CRC were expressed as odds ratios (ORs) with 95% confidence intervals (CI) for case–control studies, and as relative risks (RRs) or hazard ratios (HRs) with 95% CIs for cohort studies (20). ORs were treated as approximations of RRs or HRs due to the rarity of CRC in all populations. Fixed-effect models were used when no significant heterogeneity was found. A funnel plot was not generated because <10 studies were included (21). A 2-sided test was performed, and p < 0.05 was considered statistically significant.
Results
Search results
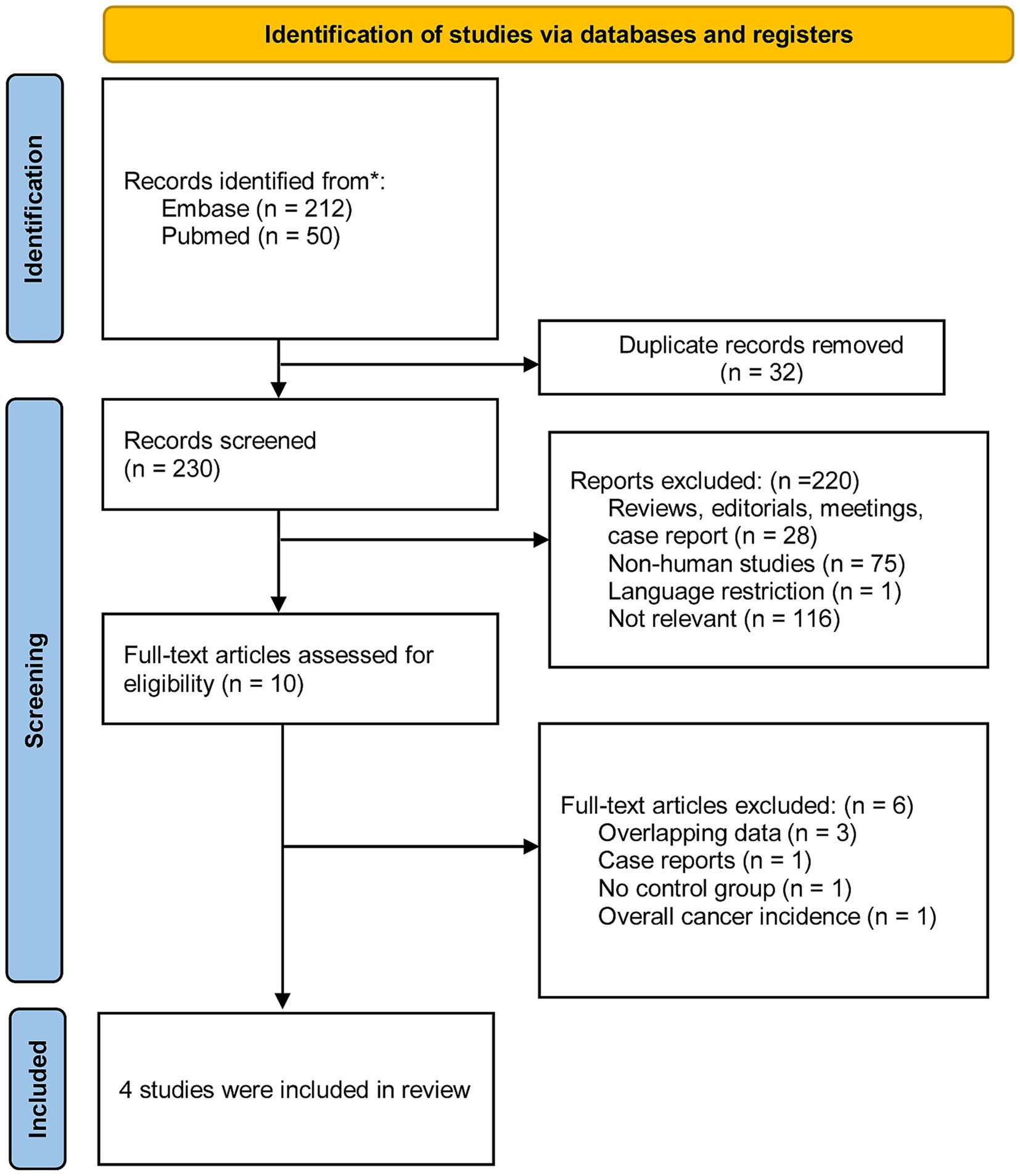
We identified 262 studies from two databases using relevant keywords. After screening titles and abstracts, 32 duplicates and 220 studies were excluded, leaving 10 for full-text review. Six studies were excluded due to multiple reasons. Ultimately, four studies involving patients with IBD were included in our analysis. The selection process was further refined by addressing potential duplicates (due to shared databases and common co-authors) and by excluding case reports. Figure 1 provides a flow diagram outlining the literature search and selection process.
Characteristics of included studies
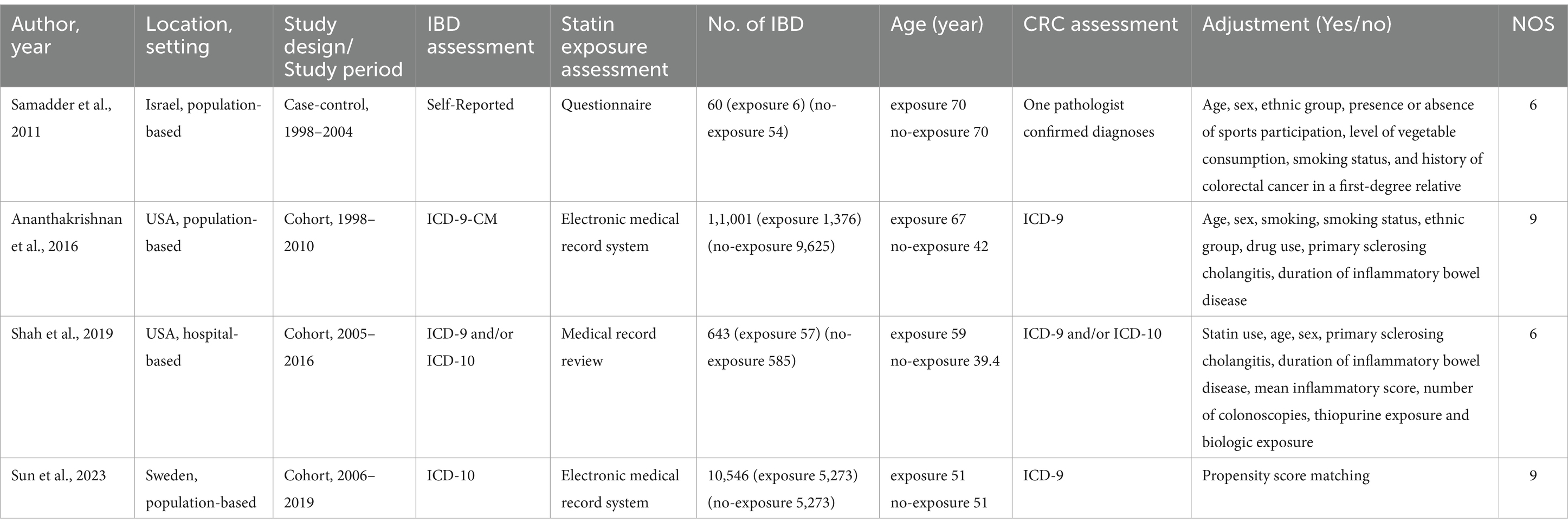
Table 1 summarizes the characteristics of the included studies: two were conducted in North America, one in Europe, and one in Asia. These studies were published between 2011 and 2023, with sample sizes ranging from 60 to 11,001 participants. Of the four studies, three were cohort studies and one was a case–control study. Based on methodological quality assessments, three studies were deemed high quality, while one was categorized as low quality. Supplementary Tables S1, S2 provide detailed score breakdowns.
Meta-analysis
We analyzed 4 studies, comprising 6,712 statin-exposed and 15,537 unexposed patients with IBD, to assess the risk of CRC associated with statin use. The combined OR for CRC was 0.53 (95% CI 0.31–0.92; p = 0.024), indicating a protective effect, although there was significant heterogeneity (I2 = 65.7%) (Figure 2). When the analysis was limited to three high-quality cohort studies, the protective effect of statins on CRC risk remained consistent (OR = 0.58, 95% CI 0.35–0.97, p = 0.037; I2 = 69.1%). Sensitivity analysis showed no substantial change in the pooled risk estimates; the pooled ORs for CRC ranged from 0.41 to 0.58.
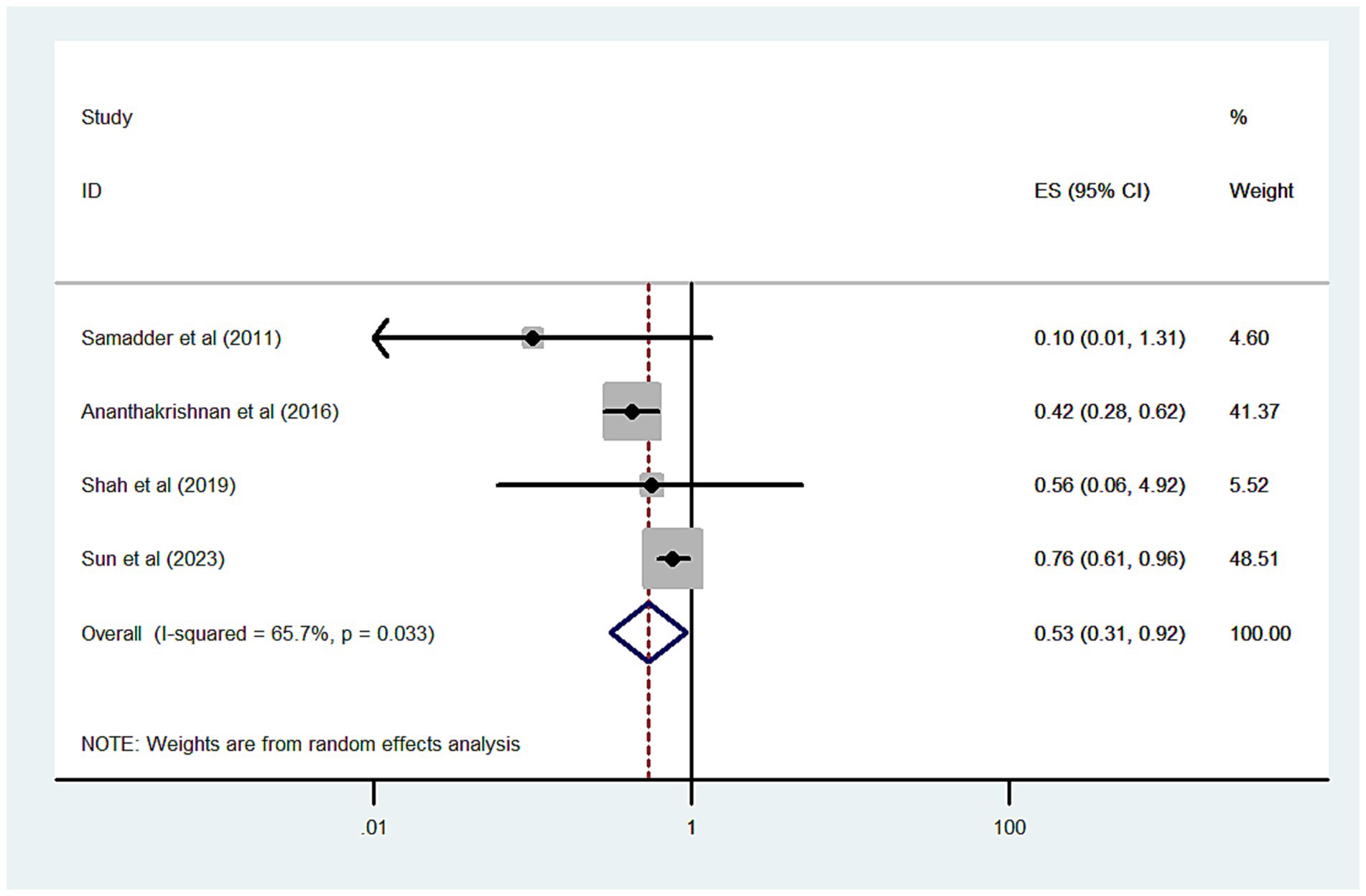
Figure 2. Forest plot of the overall risk of CRC in relation to exposure to statin among patients with IBD.
Further analysis by type of IBD revealed that statin-exposed UC patients had a lower risk of CRC (OR = 0.58, 95% CI 0.46–0.73, p < 0.001; I2 = 0%) (Figure 3A), whereas no significant protective effect was observed in CD patients (OR = 0.55, 95% CI 0.04–7.02, p = 0.649; I2 = 94.9%) (Figure 3B). In sex-based analyses, a protective effect was seen in male IBD patients exposed to statins (OR = 0.49, 95% CI 0.27–0.88, p = 0.018; I2 = 74.2%) (Figure 3C), but not in female patients (OR = 0.56, 95% CI 0.17–1.17, p = 0.122; I2 = 70.3%) (Figure 3D).
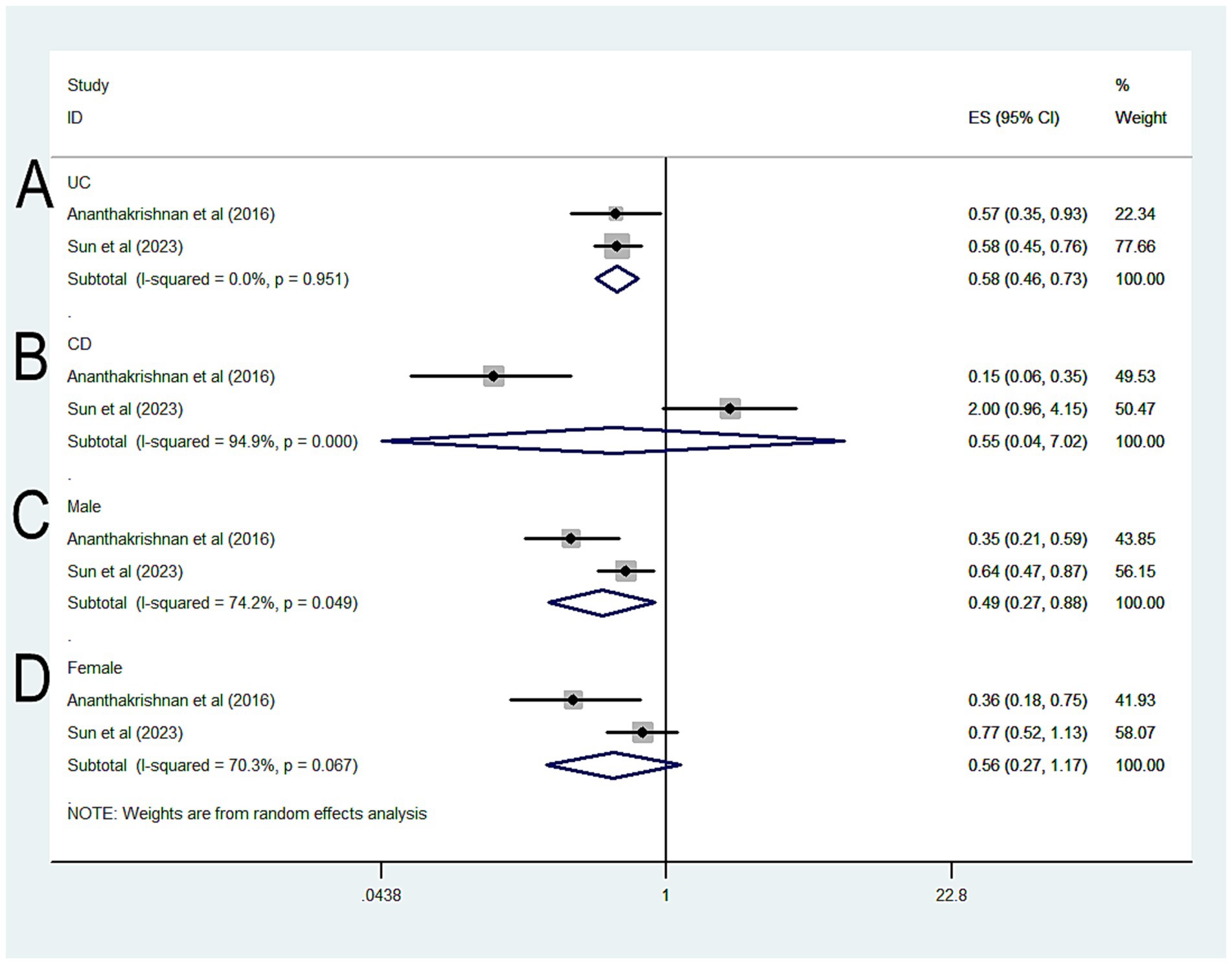
Figure 3. Forest plot of the overall risk of CRC in relation to exposure to statin among patients with IBD (A) UC; (B) CD; (C) Male; (D) Female.
Discussion
This is the first meta-analysis to investigate the association between statin use and the risk of CRC in patients with IBD. Our findings suggest a significantly reduced risk of CRC in statin users compared to non-users, even after adjusting for potential confounding factors. However, due to the limited number of included studies, caution is warranted when interpreting these results.
The exact pharmacological mechanisms behind the antitumor effects of statins are not yet fully understood, but several explanations have been proposed. One of the primary mechanisms involves the inhibition of HMG-CoA reductase, the enzyme statins target to lower cholesterol. This inhibition reduces mevalonate synthesis, which is essential for cholesterol production (22). Interestingly, disruptions caused by statins in the mevalonate synthesis pathway inhibit cancer growth and lead to apoptotic cell death, and the depletion of cholesterol may inhibit cancer cell growth (23). Additionally, statins may inhibit the synthesis of isoprenoids, which are essential lipid attachments for intracellular signaling molecules, such as Rho, Rac, and Cdc42 (24). These related-proteins are overpresented in CRC and are associated with tumor invasion. Furthermore, statins may reduce the formation of aberrant crypt foci and polyps, and reduce tumor metastasis (25). Non-HMG-CoA-related effects of statins include anti-proliferative actions, regulation of cell adhesion, antioxidant properties, and anti-inflammatory effects (26). Finally, several reports have demonstrated that the use of statins may reshape the balance of gut microbiota in patients with hyperlipidemia and favors the growth of species whose metabolites may exert anti-inflammatory effects as Bifidobacterium (27). The anti-tumor effect of Bifidobacterium has been proved in in vitro and in vivo (28, 29).
Chronic intestinal inflammation is thought to play a critical role in the development of CRC (30). Persistent inflammation in IBD patients increases the risk of colorectal neoplasia and its long-term consequences, including CRC. A meta-analysis by Lutgens et al. (4) reported that IBD patients have a 70% higher risk of CRC compared to the general population. Since 2010, several meta-analyses (11, 12) based on observational studies have shown a lower CRC risk in the general population. Given the high incidence of CRC in IBD and the potential antitumor effects of statins, it is important to explore further the association between statin use and CRC risk in IBD patients.
Although the potential protective effects of statins on CRC in IBD are biologically plausible, the studies included in our meta-analysis reported inconsistent results, which is reflected in the significant clinical heterogeneity observed. A key source of this heterogeneity is the variation in sample sizes. Two studies reporting no protective effect of statins enrolled only 703 IBD patients and identified 44 CRC cases, making it reasonable to speculate that their findings may be influenced by small sample sizes. In contrast, the other 2 studies, with a combined total of 21,545 IBD patients, observed a protective role for statins in CRC prevention.
Previous meta-analysis (31) have demonstrated that colonoscopy can effectively reduce the incidence of CRC. In one of the included studies, Shah et al. explored the relationship between statin use and CRC in a cohort of IBD patients undergoing regular colorectal surveillance, which may have minimized the observed protective effect of statins. Additionally, the statin-exposed group in this study were older than the unexposed group, which is notable since CRC incidence increases with age. This uneven age distribution may have masked the effect of statins.
Other factors, such as exposure to chemopreventive agents, IBD medications, IBD severity, and the presence of primary sclerosing cholangitis, have also been associated with CRC development in IBD patients (32–34). However, the studies included in our meta-analysis adjusted for these confounding variables to varying degrees. The failure to account for these important factors in some studies may have influenced the strength and reliability of their conclusions.
This is the first and most comprehensive systematic review and meta-analysis to investigate the risk of CRC in statin users with IBD. However, our study has some limitations, particularly regarding unknown confounders. Given ethical and practical constraints, conducting RCTs to evaluate the chemopreventive effects of statins on CRC risk is not feasible. Future well-designed studies that account for additional variables, such as smoking status, chronic comorbidities, and other medication use, are needed to examine further this association. Second, another limitation is the high heterogeneity with respect to the characteristics of the included studies, and finding sources of heterogeneity is one of the most important goals of meta-analysis. In the present meta-analysis, this heterogeneity could not be explained by the sensitivity, or subgroup analyses based on type of IBD or gender. The existence of clinical heterogeneity would be the source of statistical heterogeneity in the results. One included study (14) observed a significant duration-dependent benefit. With respect to statin type, previous meta-analysis (35) showed a significant association between lipophilic statin use and CRC risk and a null association between hydrophilic statin use and CRC risk among the general population. However, the included studies provided limited data on the dose, duration, and type of statins used, preventing us from conducting further analyses about how these factors might influence CRC risk. Third, the number of eligible studies and the sample size of IBD patients and CRC cases were relatively small, which may have affected the accuracy of our findings. These results should be interpreted with caution, and more clinical and basic research is needed to confirm the potential protective effects of statins in IBD patients. Fourth, we were unable to assess the causal relationship between statin use and CRC risk, which would provide a deeper understanding of the association. Finally, most of the included studies were conducted in Western countries, limiting the generalizability of our findings to other populations.
In conclusion, our results suggest that statin use is associated with a reduced risk of CRC in patients with IBD, indicating potential for statins as a chemopreventive agent in this population. However, these findings need to be confirmed through larger, well-designed prospective studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
A-jL: Conceptualization, Data curation, Investigation, Software, Writing – original draft. H-yJ: Methodology, Project administration, Resources, Supervision, Writing – review & editing. Y-hJ: Formal analysis, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1507739/full#supplementary-material
References
1. Baumgart, DC, and Sandborn, WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. (2007) 369:1641–57. doi: 10.1016/S0140-6736(07)60751-X
2. Ng, SC, Shi, HY, Hamidi, N, Underwood, FE, Tang, W, Benchimol, EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. (2017) 390:2769–78. doi: 10.1016/S0140-6736(17)32448-0
3. Argollo, M, Gilardi, D, Peyrin-Biroulet, C, Chabot, JF, Peyrin-Biroulet, L, and Danese, S. Comorbidities in inflammatory bowel disease: a call for action. Lancet Gastroenterol Hepatol. (2019) 4:643–54. doi: 10.1016/S2468-1253(19)30173-6
4. Lutgens, MW, van Oijen, MG, van der Heijden, GJ, Vleggaar, FP, Siersema, PD, and Oldenburg, B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. (2013) 19:789–99. doi: 10.1097/MIB.0b013e31828029c0
5. Brentnall, TA, Haggitt, RC, Rabinovitch, PS, Kimmey, MB, Bronner, MP, Levine, DS, et al. Risk and natural history of colonic neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. (1996) 110:331–8. doi: 10.1053/gast.1996.v110.pm8566577
6. Soetikno, RM, Lin, OS, Heidenreich, PA, Young, HS, and Blackstone, MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. (2002) 56:48–54. doi: 10.1067/mge.2002.125367
7. Chou, R, Cantor, A, Dana, T, Wagner, J, Ahmed, AY, Fu, R, et al. Statin use for the primary prevention of cardiovascular disease in adults: updated evidence report and systematic review for the US preventive services task force. JAMA. (2022) 328:754–71. doi: 10.1001/jama.2022.12138
8. Bedi, O, Dhawan, V, Sharma, PL, and Kumar, P. Pleiotropic effects of statins: new therapeutic targets in drug design. Naunyn Schmiedeberg's Arch Pharmacol. (2016) 389:695–712. doi: 10.1007/s00210-016-1252-4
9. Bryniarski, KL, den Dekker, W, Legutko, J, Gasior, P, Tahon, J, Diletti, R, et al. Role of lipid-lowering and anti-inflammatory therapies on plaque stabilization. J Clin Med. (2024) 13:3096. doi: 10.3390/jcm13113096
10. Ji, L, Liu, C, Yuan, Y, Gao, H, Tang, ZX, Yang, Z, et al. Key roles of rho GTPases, YAP, and mutant P53 in anti-neoplastic effects of statins. Fundam Clin Pharmacol. (2020) 34:4–10. doi: 10.1111/fcp.12495
11. Jeong, GH, Lee, KH, Kim, JY, Eisenhut, M, Kronbichler, A, van der Vliet, HJ, et al. Effect of statin on Cancer incidence: an umbrella systematic review and Meta-analysis. J Clin Med. (2019) 8:819. doi: 10.3390/jcm8060819
12. Lytras, T, Nikolopoulos, G, and Bonovas, S. Statins and the risk of colorectal cancer: an updated systematic review and meta-analysis of 40 studies. World J Gastroenterol. (2014) 20:1858–70. doi: 10.3748/wjg.v20.i7.1858
13. Chen, Z, Wu, P, Wang, J, Chen, P, Fang, Z, and Luo, F. The association of statin therapy and cancer: a meta-analysis. Lipids Health Dis. (2023) 22:192. doi: 10.1186/s12944-023-01955-4
14. Sun, J, Halfvarson, J, Bergman, D, Ebrahimi, F, Roelstraete, B, Lochhead, P, et al. Statin use and risk of colorectal cancer in patients with inflammatory bowel disease. EClinicalMedicine. (2023) 63:102182. doi: 10.1016/j.eclinm.2023.102182
15. Samadder, NJ, Mukherjee, B, Huang, SC, Ahn, J, Rennert, HS, Greenson, JK, et al. Risk of colorectal cancer in self-reported inflammatory bowel disease and modification of risk by statin and NSAID use. Cancer. (2011) 117:1640–8. doi: 10.1002/cncr.25731
16. Shah, SC, Glass, J, Giustino, G, Hove, JRT, Castaneda, D, Torres, J, et al. Statin exposure is not associated with reduced prevalence of colorectal neoplasia in patients with inflammatory bowel disease. Gut Liver. (2019) 13:54–61. doi: 10.5009/gnl18178
17. Ananthakrishnan, AN, Cagan, A, Cai, T, Gainer, VS, Shaw, SY, Churchill, S, et al. Statin use is associated with reduced risk of colorectal Cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. (2016) 14:973–9. doi: 10.1016/j.cgh.2016.02.017
18. Higgins, J.P., Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane collaboration. Available at: https://cochrane-handbook.org/. (2014)
19. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
20. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
21. Lau, J, Ioannidis, JP, Terrin, N, Schmid, CH, and Olkin, I. The case of the misleading funnel plot. BMJ. (2006) 333:597–600. doi: 10.1136/bmj.333.7568.597
22. Pikoulis, E, Margonis, GA, Angelou, A, Zografos, GC, and Antoniou, E. Statins in the chemoprevention of colorectal cancer in established animal models of sporadic and colitis-associated cancer. Eur J Cancer Prev. (2016) 25:102–8. doi: 10.1097/CEJ.0000000000000152
23. Wong, WW, Dimitroulakos, J, Minden, MD, and Penn, LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. (2002) 16:508–19. doi: 10.1038/sj.leu.2402476
24. Lochhead, P, and Chan, AT. Statins and colorectal cancer. Clinical gastroenterology and hepatology. J Am Gastroenterol Ass. (2013) 11:109–18. doi: 10.1016/j.cgh.2012.08.037
25. Terdiman, JP, Steinbuch, M, Blumentals, WA, Ullman, TA, and Rubin, DT. 5-Aminosalicylic acid therapy and the risk of colorectal cancer among patients with inflammatory bowel disease. Inflamm Bowel Dis. (2007) 13:367–71. doi: 10.1002/ibd.20074
26. Demierre, MF, Higgins, PD, Gruber, SB, Hawk, E, and Lippman, SM. Statins and cancer prevention. Nat Rev Cancer. (2005) 5:930–42. doi: 10.1038/nrc1751
27. She, J, Sun, L, Yu, Y, Fan, H, Li, X, Zhang, X, et al. A gut feeling of statin. Gut Microbes. (2024) 16:2415487. doi: 10.1080/19490976.2024.2415487
28. Kosumi, K, Hamada, T, Koh, H, Borowsky, J, Bullman, S, Twombly, TS, et al. The amount of Bifidobacterium genus in colorectal carcinoma tissue in relation to tumor characteristics and clinical outcome. Am J Pathol. (2018) 188:2839–52. doi: 10.1016/j.ajpath.2018.08.015
29. Lee, DK, Jang, S, Kim, MJ, Kim, JH, Chung, MJ, Kim, KJ, et al. Anti-proliferative effects of Bifidobacterium adolescentis SPM0212 extract on human colon cancer cell lines. BMC Cancer. (2008) 8:310. doi: 10.1186/1471-2407-8-310
30. Laine, L, Kaltenbach, T, Barkun, A, McQuaid, KR, Subramanian, V, and Soetikno, R. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc. (2015) 81:489–501.e26. doi: 10.1016/j.gie.2014.12.009
31. Wang, D, Xu, Q, Dai, S, Zhang, Y, Ding, F, and Ji, L. Effects of sigmoidoscopy screening (including colonoscopy) on colorectal cancer: a meta-analysis based on randomized controlled trials. Prev Med Rep. (2024) 39:102636. doi: 10.1016/j.pmedr.2024.102636
32. Brandaleone, L, Dal Buono, A, Gabbiadini, R, Marcozzi, G, Polverini, D, Carvello, M, et al. Hereditary colorectal Cancer syndromes and inflammatory bowel diseases: risk management and surveillance strategies. Cancers. (2024) 16:2967. doi: 10.3390/cancers16172967
33. Axelrad, JE, and Rubin, DT. The Management of Colorectal Neoplasia in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. (2024) 22:1181–5. doi: 10.1016/j.cgh.2024.01.030
34. Fatakhova, K, and Rajapakse, R. From random to precise: updated colon cancer screening and surveillance for inflammatory bowel disease. Transl Gastroenterol Hepatol. (2024) 9:27. doi: 10.21037/tgh-23-36
Keywords: lipid-lowering, colon, rectum, neoplasm, cancer
Citation: Li A-j, Jiang H-y and Jia Y-h (2024) Statin exposure and risk of colorectal cancer in patients with inflammatory bowel disease: a systematic review and meta-analysis. Front. Med. 11:1507739. doi: 10.3389/fmed.2024.1507739
Edited by:
Hua Zhong, University of Hawaii at Manoa, United StatesReviewed by:
Laixing Zhang, University of California, Los Angeles, United StatesNan Zhang, St. Jude Children’s Research Hospital, United States
Copyright © 2024 Li, Jiang and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-hui Jia, amlheW9uZ2h1aTcyOUAxNjMuY29t
 Ai-juan Li
Ai-juan Li Hai-yin Jiang2
Hai-yin Jiang2 Yong-hui Jia
Yong-hui Jia