- 1Xiyuan Hospital of China Academy of Chinese Medical Sciences, Beijing, China
- 2Shenzhen People's Hospital (The Second Clinical Medical College, Jinan University, The First Afiliated Hospital, Southern University of Science and Technology), Shen Zhen, China
Introduction: Acute ischemic stroke (AIS) poses a significant risk to human health. Intravenous thrombolysis and mechanical thrombectomy are essential treatments for AIS, offering substantial benefits for neurological recovery and brain protection. However, their efficacy is often limited by stringent time constraints and contraindications, restricting accessibility for certain patient populations. Investigating novel therapeutic strategies is, therefore, crucial. Our team developed Xiongzhitongluo granules specifically for AIS and is conducting a randomized controlled trial (RCT) to validate their effectiveness.
Methods and analysis: This multi-center, randomized, double-blind, placebo-controlled clinical trial includes 120 participants randomly allocated to the intervention or placebo group. Participants will receive a 14-day treatment alongside routine medications and will be monitored at multiple time points: days 1, 3, 5, 7, 14, 30, 60, and 90. The primary outcome is the change in the National Institutes of Health Stroke Scale (NIHSS) score from baseline to day 14. Secondary outcomes include the Scandinavian Stroke Scale (SSS), Barthel Index (BI), modified Rankin Scale (mRS), Brief Mini-Mental State Examination (MMSE), and traditional Chinese medicine (TCM) symptom assessment. Safety evaluations will include vital signs and laboratory tests. Data will be recorded using Epidata V3.1 and analyzed with SPSS 26.0.
Ethics and dissemination: This study received approval from the Ethics Committee of Xiyuan Hospital, China Academy of Chinese Medical Sciences (2021XLA102-2). Written informed consent was obtained from all participants.
Clinical trial registration: https://clinicaltrials.gov/, identifier, ChiCTR2200061859.
1 Introduction
Acute ischemic stroke (AIS) is characterized by a sudden interruption of cerebral blood flow, leading to localized ischemia, hypoxic necrosis of brain tissue, and subsequent neurological impairments (1). In China, AIS has become a prevalent non-communicable disease, significantly contributing to mortality and morbidity rates (2–4). Intravenous thrombolysis and mechanical thrombectomy are primary interventions for AIS, offering notable benefits in neurological restoration and brain preservation (5, 6). However, these treatments are often constrained by stringent time requirements and contraindications, limiting their accessibility for certain individuals.
AIS aligns with the traditional Chinese medicine (TCM) concept of “Zhong Feng” (stroke). Previous research has demonstrated the therapeutic potential of TCM in managing AIS, highlighting interventions such as Panax notoginseng saponins (PNS) and Xingnaojing Injection for mitigating cerebral ischemia/reperfusion injury and facilitating stroke rehabilitation (7–9). The integration of Western and TCM approaches in stroke rehabilitation has yielded promising results, suggesting the potential for synergistic outcomes.
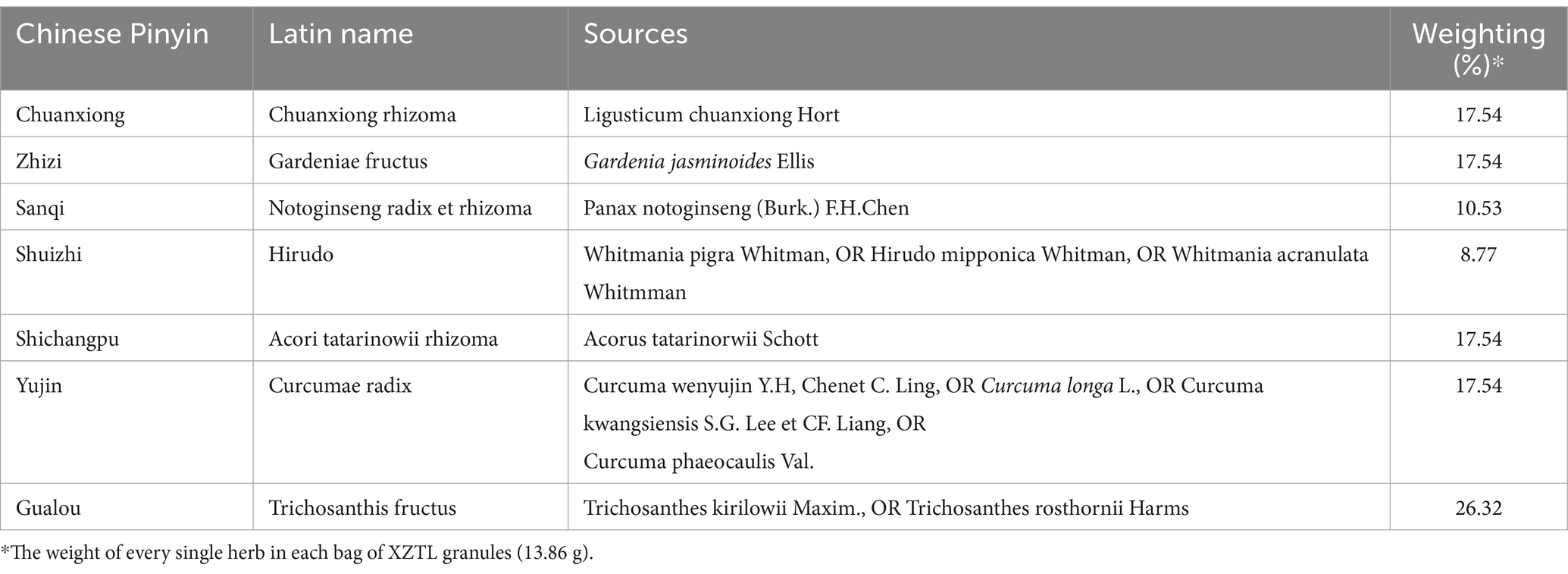
Our team’s research identified a strong correlation between AIS episodes and the TCM concepts of blood stasis and toxicity. We hypothesize that the interplay between blood stasis and toxicity, leading to cerebral collateral impairment, represents the fundamental mechanism driving AIS development (10–16). To address this, a standardized system for identifying stasis-toxicity syndromes in the acute phase of cerebral infarction has been implemented (17). Additionally, we proposed a treatment strategy emphasizing blood circulation promotion, stasis dispersion, collateral opening, and toxicity reduction. Building on these findings, our research led to the development of Xiongzhitongluo (XZTL) granules. XZTL granules comprise seven herbs (components detailed in Table 1), each with documented effects on inflammation, cerebrovascular diseases, and related conditions (18–22).
While individual herbal components have demonstrated efficacy in treating acute cerebral infarction, the effectiveness of their combination remains unclear. To bridge this gap, we designed a multi-center, randomized, double-blind, placebo-controlled clinical trial involving patients with AIS.
2 Methods and analysis
2.1 Objective
The objective of this study was to evaluate the clinical effectiveness and safety of XZTL granules, offering a novel therapeutic option based on traditional Chinese medicine.
2.2 Study design
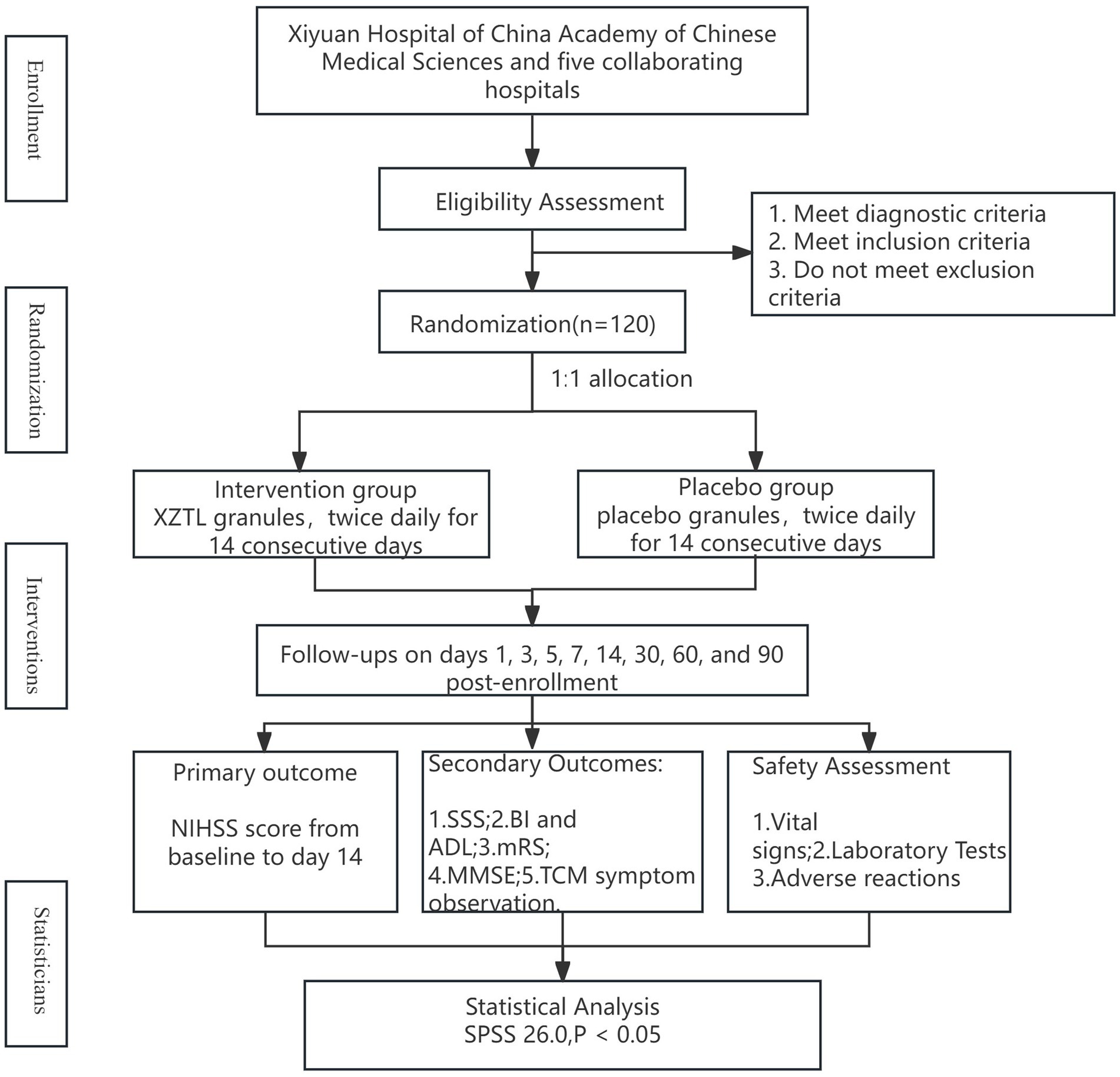
This multi-center, randomized, double-blind, placebo-controlled clinical trial aimed to recruit 120 participants diagnosed with AIS within 72 h of onset from six hospitals (details in Table 2). Participants were randomized in a 1:1 ratio to either the intervention or placebo group. In addition to routine medications, they received 13.86 g of XZTL granules or a placebo in granule form twice daily for 14 days. The study design flow chart is provided in Figure 1.
This trial was registered with the Chinese Clinical Trial Registry (ChiCTR) under registration number ChiCTR2200061859 and adhered to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 Statement. Additional details are available in Supplementary material 1.
2.3 Eligibility criteria
2.3.1 Diagnostic criteria
2.3.1.1 Western diagnostic criteria
The Western diagnostic criteria for AIS were based on the “Chinese Guidelines for Diagnosis and Treatment of Acute Ischemic Stroke 2018” (23). The criteria included:
(1) Acute onset of the disease.
(2) Presence of focal neurological deficits such as weakness or numbness of one side of the face or limbs, speech disorders, or, in some cases, comprehensive neurological deficits.
(3) Identification of responsible foci on imaging studies or symptoms/signs persisting for more than 24 h.
(4) Exclusion of non-vascular etiologies.
(5) Confirmation of the absence of brain hemorrhage using CT/MRI imaging.
2.3.1.2 Chinese medical diagnostic criteria
The diagnostic criteria for Blood Stasis Toxin Syndrome in AIS were based on the “Diagnostic Criteria for Ischemic Cardiovascular and Cerebrovascular Diseases” (16), as detailed in Table 3.
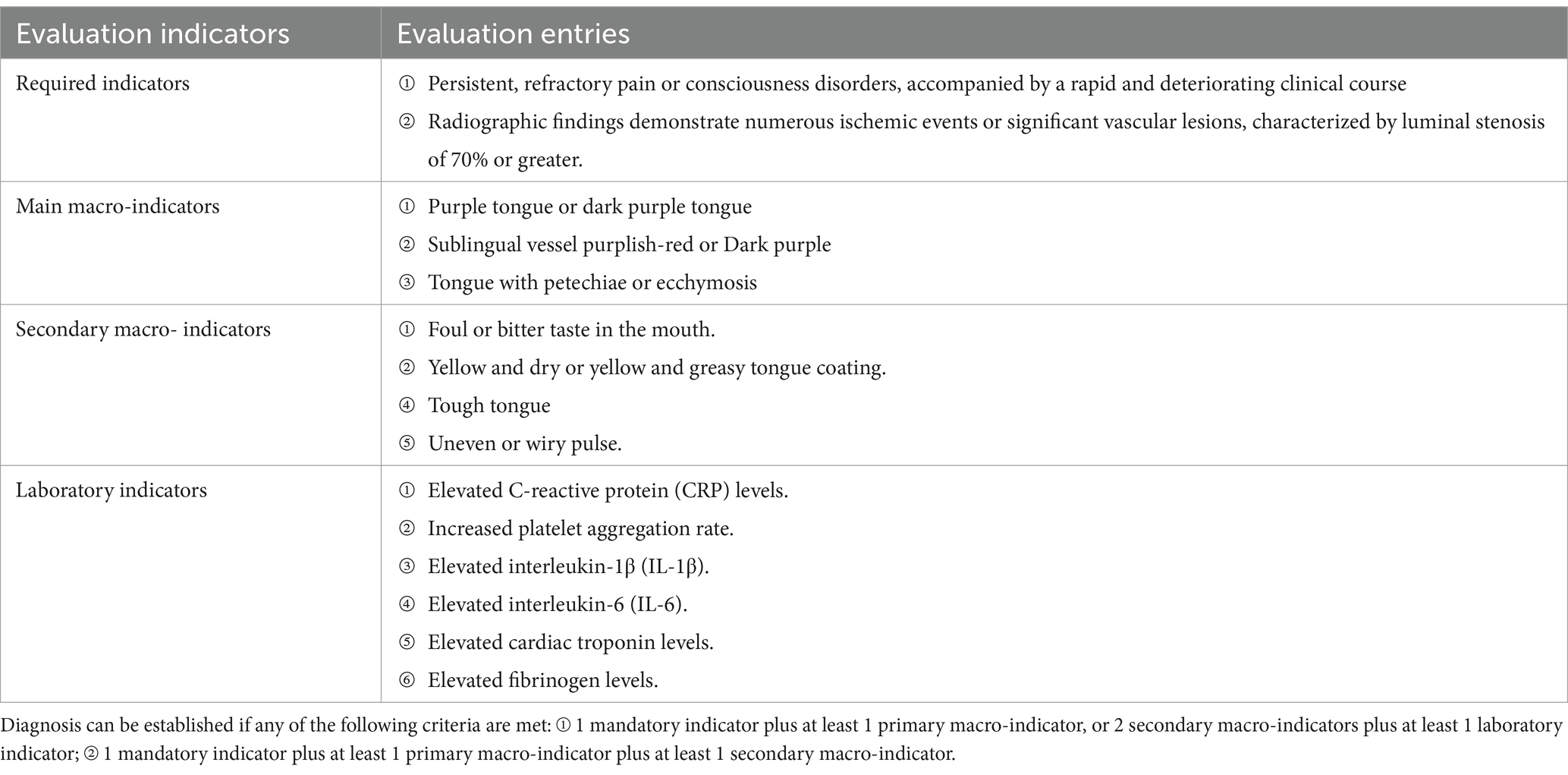
Table 3. Diagnostic criteria for ischemic cardiovascular and cerebrovascular diseases with stasis and toxicity interaction.
2.3.2 Inclusion criteria
(1) Fulfilled the diagnostic criteria for AIS.
(2) Met the diagnostic criteria for stroke disease with stasis and toxicity interconnection.
(3) Onset time ≤ 72 h.
(4) National Institutes of Health Stroke Scale (NIHSS) score ≥ 3.
(5) Glasgow Coma Scale (GCS) score ≥ 12 (24).
(6) Age between 18 and 85 years.
(7) Provided informed consent and signed the consent form.
2.3.3 Exclusion criteria
(1) Patients eligible for and prepared to receive thrombolysis.
(2) Patients who had already undergone thrombolytic therapy.
(3) Presence of severe circulatory, respiratory, urinary, or digestive system diseases or cancer.
(4) Abnormal liver or kidney function exceeding two or more times the normal value.
(5) Recent bleeding or bleeding tendency.
(6) Pregnant or lactating women.
(7) Patients with mental disorders who could not cooperate with efficacy assessments.
(8) Patients allergic to any herbal ingredients used in the study protocol.
(9) Participants enrolled in other clinical trials.
(10) Individuals unable to comply with the study protocol.
2.3.4 Termination criteria
(1) The participant’s condition continued to worsen or deteriorated rapidly during the trial, and the attending physician judged that continuation of the clinical trial was inappropriate.
(2) The participant developed comorbidities, complications, or specific physiological changes during the trial that made continued participation unsuitable.
(3) The participant experienced a serious adverse reaction or event during the study period, necessitating withdrawal.
2.3.5 Shedding criteria
Participants were classified as withdrawn under the following circumstances:
(1) Poor compliance with study requirements.
(2) Voluntary withdrawal by the participant.
(3) Failure to adhere to the medication regimen specified in the study protocol.
2.4 Sample size calculation
This study was designed as a parallel, multi-center, randomized, double-blind, placebo-controlled trial. The sample size was calculated based on the difference between the NIHSS score for neurological deficits on day 14 of enrollment and the baseline score, which served as the primary efficacy endpoint. The calculation used a test of difference with data from a previous clinical study. In the experimental group, the NIHSS score improved by 4.11 ± 1.34 points, while in the control group, the improvement was 3.42 ± 1.29 points. The test level (α) was set at 0.05 (two-sided), with a desired power (1-β) of 80%. Based on these parameters, the required sample size for each group was estimated to be 58 participants. To account for potential enrollment deviations in clinical practice, the total planned enrollment was set at 120 participants, with 60 in each group (Figure 1).
2.5 Randomization, allocation, and blinding
2.5.1 Randomization and allocation
Randomization was conducted using a block randomization method overseen by statisticians from the Good Clinical Practice (GCP) Center at Xiyuan Hospital, China Academy of Chinese Medical Sciences. The drug coding and randomization table were generated using SAS V9.4 software. Drug codes were assigned from 1 to 160, and the randomization table and scheme were sealed and securely stored at the GCP Center of Xiyuan Hospital.
Before the trial commenced, pre-prepared blinded drugs and corresponding emergency letters were allocated to each center in consecutive number segments. Drug dispensing at each center began with the lowest available number, and drugs were sequentially provided to eligible participants based on their enrollment time.
2.5.2 Blinding
The blinding process was overseen by statistical experts from the GCP Center at Xiyuan Hospital, China Academy of Chinese Medical Sciences. Blinding was conducted by personnel not directly involved in the trial from the randomization and administering units. Initially, blinded personnel verified the consistency of the appearance and packaging of the study drugs across all groups. Subsequently, drug labels corresponding to the assigned drug numbers were affixed to the external packaging of the respective drug groups. The entire blinding process was documented, and records were securely preserved.
Emergency letters containing grouping information corresponding to the drug codes were sealed and distributed to each center along with the pre-prepared blinded drugs. In emergency situations, if the investigator determined it necessary to identify the drug administered for adverse event management, unblinding could be conducted. The unblinding investigator was required to document the reason, date, and signature on the emergency letter upon opening it.
2.5.3 Intervention
All patients enrolled in the study received standard basic treatment as outlined in the “2018 Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke.” Additionally, participants were randomly assigned to receive either XZTL or placebo granules as an adjunct to standard treatment. The treatment regimen consisted of administering one sachet of granules twice daily for 14 consecutive days.
Both XZTL and placebo granules were produced by Beijing Kangrentang Pharmaceutical Co., Ltd. The placebo was formulated with 95% pure dextrin and 5% of the original herbs used in XZTL, ensuring similarity in properties, odor, color, and appearance to the XZTL granules.
2.5.4 Drugs contraindicated in the study
Participants were required to discontinue the following medications prior to enrollment. Use of these medications during the study was considered a protocol violation:
(1) Chinese medicine injections with the efficacy of activating blood circulation and removing blood stasis or clearing heat and toxins, such as Xuesaitong Injection, Xingnaojing Injection, and Kudiezi Injection.
(2) Proprietary Chinese medicines with the efficacy of activating blood circulation and removing blood stasis, such as Xueshuantong capsules and Naoxintong capsules.
(3) TCM tonics or other preparations with the effect of activating blood circulation, removing blood stasis, detoxifying, or clearing collaterals.
2.6 Outcome indicator
2.6.1 Primary outcome
The primary efficacy endpoint of this study was the difference in the National Institutes of Health Stroke Scale (NIHSS) score between baseline and day 14 after enrollment.
2.6.2 Secondary outcome
2.6.2.1 Neurological deficit assessment
The Scandinavian Stroke Scale (SSS) (25) was used to evaluate neurological functions, including consciousness, orientation, eye movements, speech, facial paralysis, upper limb muscle strength, hand muscle strength, lower limb muscle strength, and walking ability. SSS scores range from 0 to 58, with higher scores indicating better neurological status.
2.6.2.2 Daily life ability assessment
The Barthel Index (BI) (26) assessed self-care ability across 10 basic aspects, with a maximum score of 100. Lower BI scores indicated greater incapacity. A BI score > 60 suggested independence with assistance, while a score ≤ 60 indicated dependence on others for daily activities. Additionally, the Ability to Daily Life Scale (ADL) evaluated somatic self-care and instrumental daily life activities, with a total score ranging from 20 to 80. Scores ≤26 were considered normal, while scores >26 indicated varying degrees of functional decline.
2.6.2.3 Disease disability assessment
The modified Rankin Scale (mRS) (26) measured functional independence and disability severity, graded on a scale of 0–6. Higher scores indicated greater disability, while an mRS score ≤ 2 indicated functional independence.
2.6.2.4 Cognitive function evaluation
The Brief Mini-Mental State Examination Scale (MMSE) (27) evaluated cognitive functions, including orientation, recall, attention, calculation, language, and visuospatial abilities. Scores ranged from 0 to 30, with lower scores indicating worse cognitive dysfunction.
2.6.2.5 TCM symptom observation
The TCM Symptom Observation Form for AIS comprised 16 core TCM symptoms, 44 peripheral symptoms, 18 tongue signs, and 12 pulse signs. This multidimensional assessment was conducted by uniformly trained neurologists and incorporated insights from ancient and modern literature, pre-surveys, and expert verification.
2.7 Safety assessment
(1) Vital Signs: Temperature, blood pressure, respiration, and heart rate.
(2) Laboratory Tests: Blood routine, seven indicators of liver and kidney function (AST, ALT, GGT, TBIL, BUN, CREA, UA), urine routine, stool routine, coagulation function, and ECG examination.
(3) Adverse reactions/events occurred.
2.8 Data management and monitoring
2.8.1 Follow-up program
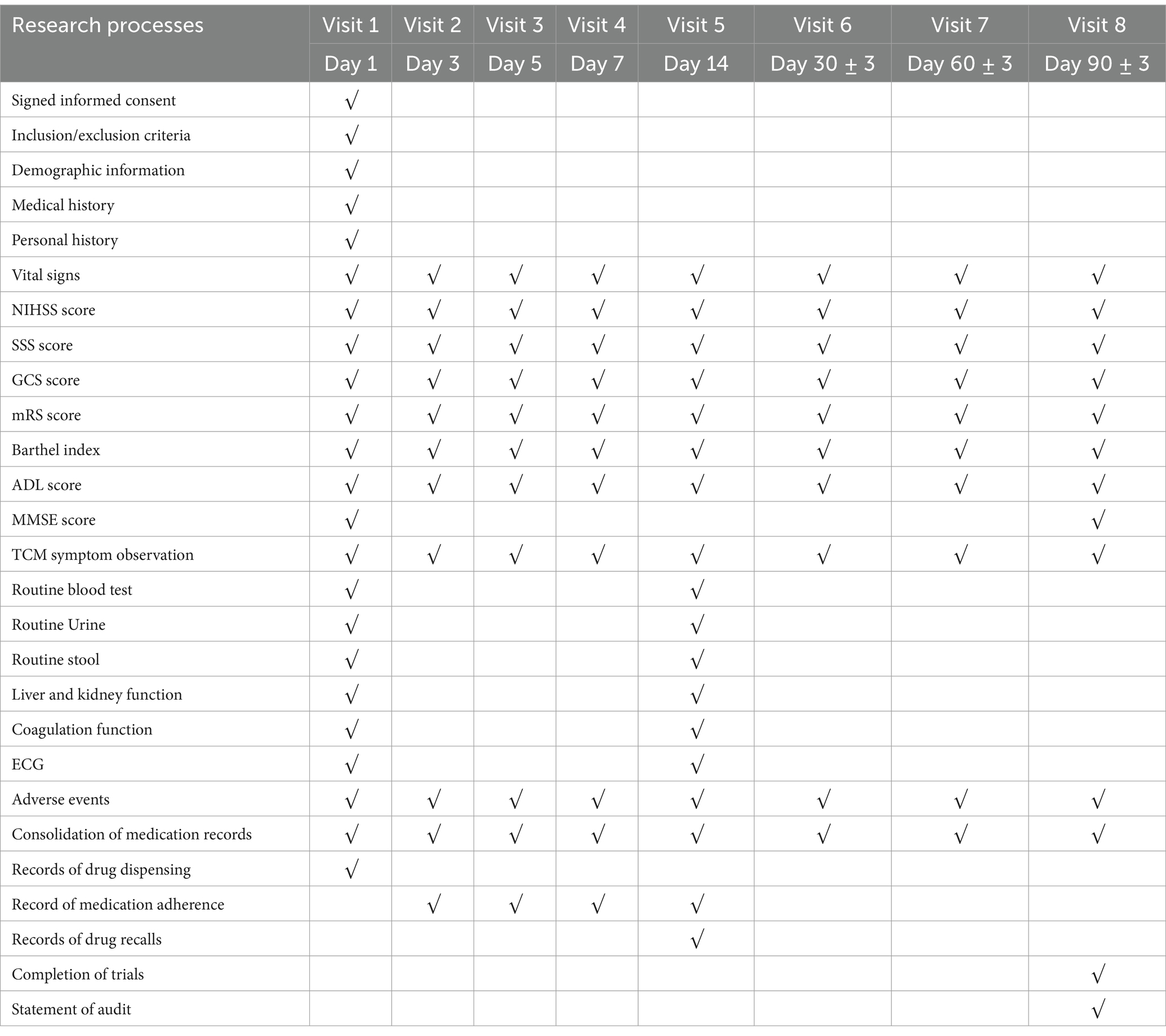
Visits were conducted on days 1, 3, 5, 7, 14, 30, 60, and 90 post-enrollment. Tasks during visits included data registration, public education, scale assessments, drug distribution, evaluation of efficacy indicators, observation of TCM symptoms, recording of vital signs, and documentation of adverse reactions/events.
Safety evaluations were performed on days 1 and 14 of enrollment. The intervention treatment lasted for 14 days, followed by a follow-up period on days 30, 60, and 90 post-enrollment. The study’s overall flow is summarized in Table 4.
2.8.2 Data management
The research history form was managed by designated personnel conducting one-on-one assessments based on the observation form’s content. Results were truthfully recorded, and entries were signed for confirmation. Any modifications were marked with a red pen, noting the date and the initials of the modifier, ensuring traceability.
Epidata V3.1 software was used to establish the database. Before data entry, thorough checks of the case history forms ensured completeness, absence of missing pages, accuracy of recorded information, and logical consistency.
Data entry employed a double-entry method to ensure timeliness and accuracy. Computerized audits were conducted during data pre-processing to identify missing values, logical errors, and inconsistencies. Based on audit results, original cases were reviewed, or the data collectors were contacted to verify and ensure traceability of the original data.
Research medical records and related data were sealed and securely stored by the primary research unit.
2.9 Statistical analysis
Statistical analyses were performed using SPSS 26.0 software, with two-tailed tests and a significance threshold set at p < 0.05. Measurement data were summarized using basic descriptive statistical methods. For normally distributed data, the arithmetic mean and standard deviation were calculated. Intergroup comparisons were conducted using the independent sample t-test, while intragroup comparisons were performed using the paired sample t-test.
For data with a non-normal distribution, the median and interquartile range were calculated, and the non-parametric rank-sum test was employed. Count and rank data were summarized as frequencies and percentages within their respective categories. Statistical analyses of these data sets utilized the chi-square test, Fisher’s exact test, or the Monte Carlo method for exact significance testing.
Analysis of covariance (ANCOVA) was used to evaluate between-group differences in the change from baseline NIHSS scores on day 14. Grouping was included as a fixed factor, and baseline NIHSS scores were used as a covariate. Results were reported as least squares means, standard errors, 95% confidence intervals, F-values, and p-values.
Repeated-measures analysis of variance (ANOVA) was used to assess changes in repeated-measures data, including neurological deficits, daily living activities, and disease-associated disability levels.
For data that deviated from a normal distribution, a natural logarithmic transformation was applied to achieve approximate normality before statistical analysis. Two-factor ANOVA was performed for data satisfying Mauchly’s sphericity test. Data failing this test underwent Greenhouse–Geisser correction.
Descriptive statistics were applied to analyze the incidence of adverse reactions/events.
3 Discussion
The pathological mechanism of AIS is a complex process involving various factors, including blood vessels, cells, and inflammation (28). Occlusion of cerebral blood vessels leads to local ischemia and hypoxia in brain tissues. These conditions disrupt the energy metabolism of nerve cells, resulting in cellular imbalance, degeneration, necrosis, and apoptosis (29). Simultaneously, ischemia- and hypoxia-induced brain tissue damage activates an inflammatory response, triggering the release of inflammatory mediators that exacerbate nerve cell injury (30).
While advancements in intravenous thrombolysis and mechanical thrombectomy techniques have reduced disability and mortality rates in AIS to some extent, their strict time windows and usage restrictions limit their applicability, leaving many patients unable to receive timely and effective treatment. For these patients, who cannot undergo conventional treatments, TCM offers a potential alternative.
XZTL granules, a traditional TCM formulation, have demonstrated multiple therapeutic effects in previous studies, including anti-inflammatory, antiplatelet aggregation, antioxidative stress, and neuroprotective properties. This study aims to evaluate the potential benefits of XZTL granules for AIS treatment through a multicenter, randomized, double-blind, placebo-controlled clinical trial, recruiting patients who have not undergone intravenous thrombolysis or thrombectomy within 72 h of onset.
If the trial results are positive, XZTL granules may provide a new treatment option, offering broader therapeutic opportunities for AIS patients. Furthermore, combining TCM with Western medical treatments may yield more comprehensive therapeutic effects, enhancing recovery rates and improving the quality of life for patients.
4 Study status
This study is ongoing and currently in the patient recruitment phase.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Xiyuan Hospital, China Academy of Chinese Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YC: Data curation, Investigation, Writing – original draft, Writing – review & editing, Validation. JW: Project administration, Supervision, Writing – review & editing. XL: Investigation, Project administration, Writing – review & editing. YL: Data curation, Investigation, Project administration, Writing – review & editing. LM: Data curation, Writing – review & editing. DZ: Data curation, Project administration, Writing – review & editing. YFZ: Data curation, Validation, Writing – review & editing. HL: Data curation, Investigation, Writing – original draft, Writing – review & editing. YLZ: Conceptualization, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Innovation Team and Talents Cultivation Program of the National Administration of Traditional Chinese Medicine (No. ZYYCXTD-C-202007), the Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (No. CI2021B006), the National TCM Leading Personnel Support Program NATCM Personnel and Education Department (2018) (No. 12).
Acknowledgments
We would like to express our gratitude to all the medical staff in the Neurology Department of Xiyuan Hospital, China Academy of Chinese Medical Sciences, for their assistance in this study. We also extend our appreciation to all the participants for their cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1507278/full#supplementary-material
Abbreviations
AIS, Acute ischemic stroke; TCM, Traditional Chinese medicine; XZTL Granules, Xiongzhitongluo granules; RCT, Randomized controlled trial; GCP, Good Clinical Practice; PNS, Panax notoginseng saponins; NIHSS, The National Institutes of Health Stroke Scale; SSS, The Scandinavian Stroke Scale; BI, The Barthel Index; mRS, The Modified Rankin Scale; MMSE, The Brief Mini-Mental State Examination Scale; ANCOVA, Analysis of covariance; ANOVA, A repeated-measures analysis of variance.
References
1. Campbell, BCV, De Silva, DA, Macleod, MR, Coutts, SB, Schwamm, LH, Davis, SM, et al. Ischaemic stroke. Nat Rev Dis Primers. (2019) 5:70. doi: 10.1038/s41572-019-0118-8
2. Wang, YJ, Li, ZX, Gu, HQ, Zhai, Y, Zhou, Q, Jiang, Y, et al. China stroke statistics: an update on the 2019 report from the National Center for healthcare quality Management in Neurological Diseases, China National Clinical Research Center for neurological diseases, the Chinese Stroke Association, National Center for chronic and non-communicable disease control and prevention, Chinese Center for Disease Control and Prevention and institute for global neuroscience and stroke collaborations. Stroke Vasc Neurol. (2022) 7:415–50. doi: 10.1136/svn-2021-001374
3. Wang, Y, Jing, J, Meng, X, Pan, Y, Wang, Y, Zhao, X, et al. The third China National Stroke Registry (CNSR-III) for patients with acute ischaemic stroke or transient ischaemic attack: design, rationale and baseline patient characteristics. Stroke Vasc Neurol. (2019) 4:158–64. doi: 10.1136/svn-2019-000242
4. Tu, WJ, and Wang, LD. China stroke surveillance report 2021. Mil Med Res. (2023) 10:33. doi: 10.1186/s40779-023-00463-x
5. Yoshimura, S, Sakai, N, Yamagami, H, Uchida, K, Beppu, M, Toyoda, K, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. (2022) 386:1303–13. doi: 10.1056/NEJMoa2118191
6. Powers, WJ, Rabinstein, AA, Ackerson, T, Adeoye, OM, Bambakidis, NC, Becker, K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
7. Zhang, J, Guo, F, Zhou, R, Xiang, C, Zhang, Y, Gao, J, et al. Proteomics and transcriptome reveal the key transcription factors mediating the protection of Panax notoginseng saponins (PNS) against cerebral ischemia/reperfusion injury. Phytomedicine. (2021) 92:153613. doi: 10.1016/j.phymed.2021.153613
8. Tian, ZY, Feng, LD, Xie, Y, Xu, DH, Zhang, CY, Kong, LB, et al. Chinese herbal medicine Xingnaojing injection for acute ischemic stroke: an overview of systematic reviews and meta-analyses. Front Pharmacol. (2021) 12:659408. doi: 10.3389/fphar.2021.659408
9. Zhong, LL, Zheng, Y, Lau, AY, Wong, N, Yao, L, Wu, X, et al. Would integrated Western and traditional Chinese medicine have more benefits for stroke rehabilitation? A systematic review and meta-analysis. Stroke Vasc Neurol. (2022) 7:77–85. doi: 10.1136/svn-2020-000781
10. Zhang, YL, Chang, FY, Wang, YY, Yang, BQ, and Huang, QF. The significance of the doctrine on the etiology and pathogenesis of internal toxicity injury to the collateral vessels. Beijing J Tradit Chin Med. (2006) 29:514–6.
11. Liu, C, Zhang, YL, Tao, Y, Chen, ZG, and Guo, RJ. Mechanism of brain collateral lesion by toxin of acute cerebral infarction. Beijing J Tradit Chin Med. (2008) 31:221–4.
12. Zhang, ZC, Zhang, YL, Cao, XL, Zhang, YL, Zhao, JJ, Chen, ZQ, et al. Correlation between syndrome characteristics of brain collateral damaged by internal toxin and blood pressure-temperature ganged fluctuation of acute cerebral infarction based on data mining. Beijing J Tradit Chin Med. (2011) 34:309–12.
13. Zhang, J, Zhang, YL, Guo, RJ, Chen, ZG, and Wang, YY. From toxin damaging brain collaterals to toxin damaging collaterals. Beijing Med J. (2013) 32:483–6. doi: 10.16025/j.1674-1307.2013.07.003
14. Wang, FL, Guo, RJ, Chen, ZG, and Zhang, YL. Theoretical foundation and practical basis for the treatment of acute cerebral infarction from the theory of fire toxicity. Beijing Med J. (2015) 34:797–800. doi: 10.16025/j.1674-1307.2015.10.010
15. Liu, XM, and Zhang, YL. Historical evolution and clinical identification of stroke and fire toxin syndrome. Beijing Med J. (2017) 36:600–6. doi: 10.16025/j.1674-1307.2017.07.005
16. Ju, JQ, Liang, X, Gao, J, Liu, NY, Tian, WD, Gao, ZY, et al. Study on the diagnostic criteria of ischemic cardiovascular and cerebrovascular disease with stasis and toxicity syndrome. Chin J Integr Med Card Cerebrovasc Dis. (2023) 21:577–80.
17. Jin, W, Zhao, J, Yang, E, Wang, Y, Wang, Q, Wu, Y, et al. Neuronal STAT3/HIF-1α/PTRF axis-mediated bioenergetic disturbance exacerbates cerebral ischemia-reperfusion injury via PLA2G4A. Theranostics. (2022) 12:3196–216. doi: 10.7150/thno.71029
18. Zeng, P, Yi, Y, Su, HF, Ye, CY, Sun, YW, Zhou, XW, et al. Key phytochemicals and biological functions of chuanxiong Rhizoma against ischemic stroke: a network pharmacology and experimental assessment. Front Pharmacol. (2021) 12:758049. doi: 10.3389/fphar.2021.758049
19. Tian, J, Qin, S, Han, J, Meng, J, and Liang, A. A review of the ethnopharmacology, phytochemistry, pharmacology, and toxicology of fructus gardeniae (Zhi-zi). J Ethnopharmacol. (2022) 289:114984. doi: 10.1016/j.jep.2022.114984
20. Zhang, Y, Long, Y, Yu, S, Li, D, Yang, M, Guan, Y, et al. Natural volatile oils derived from herbal medicines: a promising therapy way for treating depressive disorder. Pharmacol Res. (2021) 164:105376. doi: 10.1016/j.phrs.2020.105376
21. Yavarpour-Bali, H, Ghasemi-Kasman, M, and Pirzadeh, M. Curcumin-loaded nanoparticles: a novel therapeutic strategy in treatment of central nervous system disorders. Int J Nanomedicine. (2019) 14:4449–60. doi: 10.2147/IJN.S208332
22. Yu, X, Tang, L, Wu, H, Zhang, X, Luo, H, Guo, R, et al. Trichosanthis fructus: botany, traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. (2018) 224:177–94. doi: 10.1016/j.jep.2018.05.034
23. Chan, AW, Tetzlaff, JM, Altman, DG, Laupacis, A, Gøtzsche, PC, Krle, AJK, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Rev Panam Salud Publica. (2015) 38:506–14. doi: 10.7326/0003-4819-158-3-201302050-00583
24. Neurology CSo, Society CS. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chin J Neurol. (2018) 51:666–82. doi: 10.3760/cma.j.issn.1006-7876.2018.09.004
25. Mehta, R, and Chinthapalli, K. Glasgow coma scale explained. BMJ. (2019) 365:l1296. doi: 10.1136/bmj.l1296
26. Stubbs, PW, and Mortensen, J. Clinimetrics: the Scandinavian stroke scale. J Physiother. (2020) 66:132. doi: 10.1016/j.jphys.2019.08.010
27. Liu, X, Zhou, M, Zhao, J, Gao, Y, Wang, Y, Zhou, J, et al. Functional independence and disability evaluation in stroke patients: optimal cutoff scores for a pictorial-based Longshi scale, Barthel index, and modified Rankin scale. Front Neurol. (2022) 13:710852. doi: 10.3389/fneur.2022.710852
28. Zhu, Y, Zhao, S, Fan, Z, Li, Z, He, F, Lin, C, et al. Evaluation of the mini-mental state examination and the Montreal cognitive assessment for predicting post-stroke cognitive impairment during the acute phase in Chinese minor stroke patients. Front Aging Neurosci. (2020) 12:236. doi: 10.3389/fnagi.2020.00236
29. Qin, C, Yang, S, Chu, YH, Zhang, H, Pang, XW, Chen, L, et al. Signaling pathways involved in ischemic stroke: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. (2022) 7:215. doi: 10.1038/s41392-022-01064-1
Keywords: acute ischemic stroke, Xiongzhitongluo granules, randomized controlled study, traditional Chinese medicine, study protocol, efficacy and safety
Citation: Chen Y, Wei J, Liang X, Liu Y, Miao L, Zhao D, Zhang Y, Liu H and Zhang Y (2025) Efficacy and safety of Xiongzhitongluo granules in the treatment of acute ischemic stroke: study protocol for a randomized controlled trial. Front. Med. 11:1507278. doi: 10.3389/fmed.2024.1507278
Edited by:
Sergei V. Fedorovich, Belarusian State University, BelarusReviewed by:
Xun Luo, Kerry Rehabilitation Medicine Research Institute, ChinaMengnan Liu, Southwest Medical University, China
Copyright © 2025 Chen, Wei, Liang, Liu, Miao, Zhao, Zhang, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongxi Liu, MTUzMjQ0Mzk4MUBxcS5jb20=; Yunling Zhang, eXVubGluZ3poYW5nMjAwNEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Yunmeng Chen
Yunmeng Chen Jingjing Wei
Jingjing Wei Xiao Liang
Xiao Liang Yue Liu
Yue Liu Lina Miao
Lina Miao Di Zhao
Di Zhao Yunfan Zhang
Yunfan Zhang Hongxi Liu
Hongxi Liu Yunling Zhang
Yunling Zhang