
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 24 March 2025
Sec. Gastroenterology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1502849
This article is part of the Research TopicRecent Advances and New Biomarkers in Ulcerative Colitis - Volume IIView all 5 articles
 Da Zhao1†
Da Zhao1† Anqi Ge1†
Anqi Ge1† Cong Yan2
Cong Yan2 Xingci Liu1
Xingci Liu1 Kailin Yang1,3,4
Kailin Yang1,3,4 Yexing Yan3
Yexing Yan3 Moujia Hao3
Moujia Hao3 Junpeng Chen5,6,7
Junpeng Chen5,6,7 Pawan Daga8
Pawan Daga8 Charles C. Dai9,10
Charles C. Dai9,10 Changping Li11
Changping Li11 Hui Cao1*
Hui Cao1*Ulcerative colitis (UC) is a chronic relapsing inflammatory disease characterized by progressive mucosal damage. The incidence rate of UC is rising rapidly, which makes the burden of medical resources aggravated. In UC, due to various pathogenic factors such as mucosal immune system disorders, gene mutations and environmental factors disrupting the mucosal barrier function, the midgut pathogenic bacteria and exogenous antigens translocate into the lamina propria, thereby aggravating the inflammatory response and further damages the mucosal barrier. During the progression of UC, Th17 populations that cause inflammation generally increase, while Tregs that suppress Th17 activity decrease. Among them, Th17 mediates immune response, Treg mediates immunosuppression, and the coordinated balance of the two plays a key role in the inflammation and immune process of UC. Natural plant components can regulate biological processes such as immune inflammation from multiple levels of proinflammatory cytokines and signaling pathways. These characteristics have unique advantages and broad prospects in the treatment of UC. In immunomodulation, there is substantial clinical and experimental evidence for the modulatory role of natural plant products in restoring balance between Th17/Treg disturbances in UC. This review summarizes the previous studies on the regulation of Th17/Treg balance in UC by natural plant active ingredients, extracts, and traditional Chinese medicine prescriptions, and provides new evidence for the development and design of lead compounds and natural new drugs for the regulation of Th17/Treg balance in the future, and then provides ideas and evidence for future clinical intervention in the treatment of UC immune disorders and clinical trials.
Ulcerative colitis (UC) is a chronic idiopathic intestinal inflammatory disease (IBD) with multiple etiologies and unknown pathological mechanism (1). The lesion starts from the rectum and usually extends in a continuous manner to part of the colon or even the entire colon, and the inflammation is mainly limited to the mucosal surface. Bloody diarrhea and abdominal pain are characteristic symptoms of UC (2, 3). The clinical course is unpredictable, with periods of attack and remission alternating. The global prevalence of UC is estimated to be 5 million cases in 2023, and the incidence is increasing worldwide, with similar incidence in men and women (4, 5). Furthermore, the rise in IBD cases in regions like China and India has been notable, marking a transition from an uncommon pathology to a prevalent health concern (5). Individuals grappling with persistent and/or widespread UC face heightened susceptibility to colorectal cancer (CRC) when contrasted with the broader populace, given the intricate nature of the disease, its prolonged course, and inherent cancer predisposition (6). A meta-analysis showed that the cumulative probability of developing colorectal cancer among all patients with UC was 2% at 10 years, 8% at 20 years, and 18% at 30 years (7, 8).
The pathological factors causing UC mainly include heredity, mental psychology, external environment, and immune factors (3, 9). At present, it is believed that immune imbalance is the key factor of recurrent UC, protracted and difficult to heal (10). The research on the immune mechanism of UC has changed from the balance between Th1/Th2 in the early stage to the balance of Th17/Treg in recent years (11). Studies have shown that intestinal microbiota and their metabolites can affect the immune balance of Th17/Treg, and induce the body to produce an adaptive immune response under pathological conditions (12). The dysregulation between Th17 and Treg cells plays a pivotal role in the pathogenesis of UC. Particularly, Th17 cells are implicated in the initiation and advancement of diverse autoimmune conditions, including IBD (13). Activation of Th17 cells is facilitated by IL-23, while the transcription factor RORγt governs the differentiation and functional modifications of these cells. Th17 cells primarily secrete pro-inflammatory cytokines like IL-17 and IL-21. On the other hand, Foxp3 acts as a crucial transcription factor for Treg cells, regulating their development and functionality. Treg cells primarily release anti-inflammatory agents such as IL-10 and TGF-β, collectively orchestrating immune modulation (14, 15). Any disruption in the equilibrium between Th17 and Treg cells prompts alterations in their respective cytokine profiles, consequently triggering a cascade of immune responses and inflammatory reactions. Excessive immune reactions culminate in tissue injury and are intricately involved in both the onset and progression of UC (16).
At present, the clinical treatment of UC is mainly based on 5-amino acid salicylic acid maintenance therapy, glucocorticoids, immunosuppressants, etc. to control the acute attack. But its treatment time is long, easy to relapse, seriously reduces the quality of life of patients, and increases psychological and economic challenges (17–19). In recent years, the advantages of natural plant compounds in the treatment of UC have gradually become prominent, so the molecular mechanism of anti-inflammatory and anti-immune properties of natural plant compounds has been studied (20). In addition, there are many reports on the mechanism of natural plant components in regulating Th17/Treg cells (21). Our previous work also studied the molecular biological process of multi-component compounds intervening in the anti-inflammatory and anti-immune process of UC (22, 23). In order to provide lead compounds or natural plant components of parent nucleus structure for future drug development targeting the anti-inflammatory and anti-immune process of Th17/Treg cells, this review will not only summarize the research progress of the mechanism of Th17/Treg cells in UC, but also summarize the drugs of natural plant components regulating Th17/Treg cells.
The reviewers conducted a thorough literature search in databases including China National Knowledge Infrastructure (CNKI), Medline Complete, Web of Science, Wanfang Database, PubMed, Sinomed, Embase, VIP Database the Cochrane Library and ClinicalTrials.gov. The search covered the period from the inception of the databases until February 1, 2024. For instance, the search strategies for PubMed and Embase are outlined in Supplementary Table S1.
Inclusion criteria: (1) Participants: Patients diagnosed with UC, UC animal models, or UC-related cell models, regardless of type or race, must adhere to medical ethics. (2) Intervention: Natural plant components as intervention methods, with no restrictions on dosage type, route of administration, or preparation methods, including natural plant ingredient monomers, components, or active ingredients, as well as traditional Chinese medicine (TCM) prescriptions, but must be in accordance with medical ethics. (3) Outcomes: Including any changes in Th17/Treg. (4) Study Design: Basic or clinical trials.
Exclusion criteria: (1) Studies not adhering to medical ethics; (2) Retracted studies.
The reviewers initially conducted a preliminary search based on the search formula outlined in Supplementary Table S1. After removing duplicate publications from the preliminary search, two reviewers independently screened publications by reviewing titles, abstracts, and keywords according to the following principles: advancement of a publication to the next screening stage required agreement from only one reviewer, whereas exclusion of a publication necessitated agreement from both reviewers. Subsequently, the two reviewers further screened the articles based on search criteria, including articles that met the criteria (see Figure 1). The included articles are presented in Tables 1, 2.
The gut microbiota represents a complex assembly of microorganisms that inhabit the gastrointestinal tract. Alterations in the gut microbiota can impact populations of intestinal mucosal immune cells, including macrophages and dendritic cells, which can activate T and B cells through antigen presentation (24).
The pathogenesis of UC is strongly associated with disruptions in the intestinal microbiota. Normally, beneficial and pathogenic bacteria in the gut tract engage in reciprocal regulation to sustain the dynamic equilibrium of the immune-inflammatory system (25). Perturbations in the intestinal flora can prompt aberrant immune and inflammatory responses within the intestinal mucosa, leading to damage to the mucosal barrier, heightened epithelial permeability, bacterial and endotoxin accumulation in the lamina propria, anomalous immune responses, excessive inflammatory factor release, and the onset of UC (13, 26). Simultaneously, mucosal barrier impairment can alter pathogenic microorganism composition, exacerbating intestinal microbiota imbalance, thereby creating a detrimental feedback loop (27). Consequently, compromised intestinal mucosal barrier function stands as a pivotal pathological mechanism in UC pathogenesis.
Numerous studies have emphasized the pivotal role of balanced gut microbiota in upholding intestinal mucosal barrier integrity (28). Conversely, disruptions in the microbiota can disrupt normal mucosal barrier function, impact mucosal immune and inflammatory responses, and thus drive UC progression (29). Research indicates that interventions aimed at regulating the intestinal microbiota, such as probiotics, prebiotics, fecal transplantation, among others, can restore microbiota homeostasis, mend mucosal barrier integrity, and inhibit UC advancement, offering a promising avenue for UC treatment (30–32).
Mucosal immunity encompasses the entire internal surface of the body and serves as the primary defense mechanism against infections. The intestinal mucosal barrier, governed by the equilibrium of intestinal microbiota and its molecular sealing capacity, forms the foundation of mucosal immunity. Pathogen-associated molecular patterns released from the surface of intestinal bacteria bind to Toll-like receptors (TLR) on immune cell membranes and NOD-like receptors (NLR) in the intracellular environment, activating nuclear factor-kappa B (NF-κB) and transcription factor AP-1. This activation leads to the transcription of inflammatory genes, the release of inflammatory mediators, and the recruitment and activation of inflammatory cells, initiating the onset of UC (33). Inflammation generates an overabundance of reactive oxygen species, causing crypt abscesses and tissue damage, hallmarks of typical UC ulcers (34). Dendritic cells (DCs) facilitate and engage adaptive immunity through antigen presentation and cytokine secretion, activating CD4+ T effector cells, guiding and stimulating effector T cells, and subsequently inducing B cells to produce anti-inflammatory and mucosal protective secretory immunoglobulin A (SIgA). Ultimately, the subcellular imbalance between pro-inflammatory Th1, Th17 responses, and anti-inflammatory effects tips in favor of inflammation. The resulting mucosal damage from inflammation perpetuates ongoing colonic inflammation and ulceration (35, 36).
Regulatory T cells (Treg cells) are a subset of CD4+ T cells suppressing immune responses. Although Treg cells constitute less than 10% of CD4+ T cells, they are critical in preventing autoimmune disease and chronic inflammatory diseases, including gut inflammation (37, 38). Utilizing a murine model of UC, augmenting IL-10 and TGF-β production significantly alleviates diarrheal symptoms, illustrating the ability of Treg cells to dampen intestinal inflammatory cascades and exaggerated responses by modulating the secretion of anti-inflammatory factors like IL-10 and TGF-β, thus ameliorating UC’s clinical manifestations (39). Flow cytometry results demonstrated a reduction in the number of peripheral blood Treg cells in UC mice, aligning with the notion that Treg cells possess the capability to regulate intestinal inflammation (40, 41). Evidence also suggests that disruptions in intestinal tolerance resulting in deficiencies in Treg cell number and function may foster UC onset and progression (41). Clinical and animal studies emphasize Treg cells’ critical role in preventing gut inflammation, and their deficiencies may contribute to UC (37). Despite growing interest in Treg cells, research on their anomalies in human UC remains limited. Treg cells are recognized for producing essential cytokines like IL-10 and TGF-β, with IL-10 specifically regulating immune responses. By modulating the expression and release of pro-inflammatory molecules such as IL-1β, IL-6, and TNF-α, IL-10 effectively impedes antigen presentation, thereby restraining T cell-mediated immune responses (42). Studies involving IL-10 deficient mice revealed their susceptibility to developing colitis, mirroring human UC pathogenesis and highlighting IL-10’s crucial role in mitigating UC progression. Notably, administering IL-10-enriched bifidobacteria orally improved UC symptoms in mice, underscoring IL-10’s significance in UC management (43). Tao et al. observed reduced levels of peripheral blood Treg cells and IL-10 in UC-afflicted mice compared to controls in their research (44). Elevating serum and mucosal IL-10 levels, coupled with enhancing peripheral Treg cell counts, notably ameliorated colonic damage in UC mice, demonstrating the effective collaboration between IL-10 and Treg cells in reducing colon damage in UC mice (44). Furthermore, Treg cells also secrete TGF-β, a multifunctional cytokine with potent regulatory effects on inflammation, angiogenesis, immune response inhibition, and even tumor growth, invasion, and metastasis (45). Xu et al. discovered that Kuijie Granules could alleviate various UC symptoms by reducing TGF-β expression in observations of this medicine’s therapeutic effects on UC patients (46). Another researcher established a UC mouse model, noting a significant rise in TGF-β levels during active UC phases, positively correlating with disease severity, suggesting a role for TGF-β in promoting UC onset and progression (47).
Upon encountering antigen signals, naïve CD4+ T lymphocytes are stimulated and activated through co-stimulatory signals and molecules, leading to differentiation into various T lymphocyte subsets under specific conditions. These diverse cell types include T17 and Treg cells (48). Naïve CD4+ T cells transition into Treg cells under the influence of TGF-β, while differentiation into Th17 cells is facilitated by the combined effects of TGF-β and IL-6. Th17 and Treg cells have a reciprocal relationship during differentiation, mutually suppressing each other’s functions to maintain immune homeostasis within the body. Perturbation of this balance can result in the development of autoimmune disorders, including UC (49). Several studies have underscored the crucial role of the Th17/Treg balance in the inflammatory and immune processes of UC. An imbalance between Th17 and Treg cells leads to elevated serum levels of Th17-associated cytokines (such as IL-6, IL17A, and IL-17F) alongside diminished levels of Treg-related cytokines (like IL-10, IL-2, and TGF-β) (50, 51). Additionally, these cytokines are likely to act synergistically to induce damage to colonic epithelial cells, thereby contributing to local tissue inflammation. Restoring the immune balance between Th17 and Treg cells holds promise as a novel therapeutic avenue for managing UC (52). Gong et al. discovered that the upregulation of Th17 cells in UC patients correlates with increased serum IL-17 levels, while a decrease in Treg cells leads to reduced TGF-β levels, activating autoreactive T cells and decreasing immunosuppressive factors, thereby exacerbating colonic mucosal inflammation. This suggests the presence of a clear Th17/Treg imbalance in UC patients, which may play a pivotal role in UC development (53). Ma et al. observed an imbalance in the Th17/Treg cell ratio in UC patients, where a decrease in the Treg cell ratio was associated with serum indicators of inflammatory activity, indicating a link between Th17/Treg cell imbalance, UC disease activity, and inflammation (54). Luo et al. noted a significant decrease in the number of Treg cells in UC patients’ serum and colon tissue, accompanied by a considerable increase in Th17 cells, indicating a notable Treg/Th17 cell imbalance in UC patients, positively correlated with intestinal inflammation (55). The factors influencing UC pathogenesis are not solely the reduction of Treg cells or the increase of Th17 cells but rather the interplay between Treg and Th17 cells leading to immune imbalance (56). An evident imbalance exists in the Th17/Treg cell ratio in UC, potentially being a key aspect of UC pathogenesis. Throughout the progression of UC, inflammatory Th17 populations typically increase, while Tregs, responsible for suppressing Th17 activity, decrease. Among these, Th17 mediates immune responses, while Treg mediates immunosuppression, with the balanced interplay between the two being crucial in the inflammatory and immune processes of UC. Therefore, regulating the Th17/Treg cell balance to enhance Treg cells and suppress Th17 cells may play a significant role in protecting the intestinal mucosa in UC.
Currently, extensive research indicates that the modulation of the Th17/Treg equilibrium can effectively regulate UC. This modulation, in essence, establishes an immune regulatory framework by integrating diverse cytokines and transcription factors within intricate signaling pathways both upstream and downstream.
Cellular responses to IL-6 are orchestrated by a receptor complex comprising IL-6Rα and glycoprotein 130 (gp130) (57). Within the JAK–STAT signaling cascade, two JAK isoforms function as autophosphorylated homodimers or heterodimers, facilitating the recruitment and phosphorylation of various signaling molecules, including members of the STAT protein family, which are crucial for DNA-binding activities (58). Comprising four intracellular tyrosine protein kinases, the JAK family (JAK1-45I, JAK2, JAK3, and TYK2) activates STAT proteins—such as STAT1-4, STAT5a, STAT5b, and STAT6 (31)—sparking diverse downstream cellular responses (59). In the IL-6/JAK/STAT3 signaling axis, IL-6 binding to glycoprotein 130 receptors (gp130R) triggers tyrosine phosphorylation and activates JAKs linked to gp130R, promoting cytoplasmic STAT3 transcription factor activation (60). Phosphorylated STAT3 dimers are subsequently translocated into the nucleus to regulate gene transcription, participating in inflammatory responses (61). Activation through IL-6R/gp130 leads to a pro-inflammatory function for STAT3, contrasting with its anti-inflammatory role when stimulated by IL-10R (62). In gut-associated lymphoid tissues, dendritic cells and other antigen-presenting cells initiate antigen-specific immune responses, which are critical in determining B cell activation and the early differentiation of T helper cells through JAK–STAT-mediated cytokine-receptor interactions (63). These processes lead to the differentiation and expansion of TH1, TH2, TH9, and TH17 effector cells within the intestinal mucosa. In turn, TH cell subtypes regulate other immune cells, including CD8+ cytotoxic cells, regulatory T cells (Treg), macrophages, and dendritic cells, through cytokine-receptor interactions and JAK–STAT signaling pathways (60). Consequently, JAK inhibitors have the potential to broadly impact immune mechanisms underlying IBD, affecting inflammatory responses, the intestinal epithelial barrier, and fibrosis (60).
TLRs are a type I transmembrane receptor superfamily (64), which play an important role in the innate immune response against pathogenic microorganisms or tissue damage. After TLR is stimulated, it activates the intracellular cascade reaction and then transmits the stimulating signal to the lower signaling protein NF-KB coupled with MyD88 (65). Activated NF-κB can participate in the transcriptional regulation of various target genes, resulting in the release of various inflammatory factors (such as TNF-α, interferon-γ, IL-1β, IL-6) and antimicrobial peptides (66, 67), resulting in intestinal tissue damage and IBD (67, 68). In addition, the differentiation of Th17 and Treg is also regulated by NF-κB. NF-κB comprises a dimer of five proteins of the Rel transcription factor family: p105/p50, p100/p52, RelA (p65), RelB, and c-Rel, which shares N-terminal homology with the v-Rel oncogene (69). iTreg generation in c-Rel-deficient CD4+ T cells was severely hindered, and was associated with a decreased number of Foxp3* T cells in vivo (53). c-Rel is highly activated in the thymus regulatory T precursor cells (pre-tTreg) with high expression of CCR7, which initiates the transcription of Treg-related coding genes and promotes the differentiation of pre-tTreg into thymic regulatory T cells (tTreg) (70). Ablation of NF-KBc-Rel can specifically impair the generation and maintenance of activated regulatory T cells (aTreg) (71). In addition, c-Rel and p65 can drive Treg development by promoting the formation of Foxp3-specific enhancers (72). Rorg/Rorc are direct targets of the transcription factor p65 in Th17 cells. Therefore, when c-Rel or p65 is deficient, the expression of RORyt mRNA is reduced, the differentiation of CD4 + T to Th17 is weakened, and the expression of IL-17A/IL-17F of Th17 is reduced (73).
The mammalian target of rapamycin (mTOR) serves as a pivotal regulator of growth and development, modulated by an array of extracellular and intracellular factors like amino acids, energy levels, and hormonal cues (74). Notably, during inflammatory conditions, the hyperactivation of the PI3K/Akt–mTOR signaling pathway emerges as a significant player in the context of UC-associated carcinogenesis (75). In a study by Chi et al., heightened expression of the key transcription factor RORα, which oversees Th17 cell regulation, was notably observed in individuals with active UC, particularly in those unresponsive to TNF antagonist therapy. Utilizing a mouse model of T cell-triggered enteritis, they demonstrated that ablation of RORA in CD4 T cells substantially mitigated the severity of enteritis induced by T cells. RORα facilitated the migration of mesenteric lymph node T cells to the gut while impeding the apoptosis of resident intestinal T cells, thereby fostering T cell infiltration in the intestine. Furthermore, RORα prompted CD4 T cells to release potent cytokines like IL-17 and TNFα, thereby enhancing their pathogenicity. Through ChIP-seq and RNA-seq analyses, the researchers unearthed that RORα bolstered the activation of the mTORC1 signaling pathway, with the deletion of the mTORC1-specific component Rptor in T cells significantly curbing T cell pathogenicity in enteritis. Moreover, abnormal mTORC1 signaling was evident in active UC patients, showing a positive correlation with RORα expression levels. In essence, the pivotal interplay between the RORα-mTORC1 axis in modulating CD4 T cell enteritis pathogenicity may unveil a promising therapeutic target for managing IBD (76).
The mTOR signaling pathway plays a dual role in not only repairing intestinal mucosal damage but also sustaining the regular metabolism of intestinal epithelial cells. Often dysregulated in various conditions like tumors (75), leukemia (77), vascular and skin growth disorders (78), this pathway is intricately linked to the development of gastrointestinal malignancies stemming from prolonged IBD affliction. Within effector CD4+ T cells, mTOR facilitates the differentiation of Th1, Th2, and particularly Th17 cells, crucial for both in vitro and in vivo contexts, as T cells deficient in mTOR fail to mature into Th17 cells (79). In cell culture settings, microRNAs (miRNAs) exert control over mTOR signaling, prompting either the induction or suppression of autophagy in intestinal cells, releasing either anti-inflammatory or pro-inflammatory factors, respectively (80). By inhibiting mTOR-induced Foxp3 expression, Rapamycin (RAPA) fosters Treg generation from naïve CD4+ T cells, potentially through the restriction of essential amino acids (EAAs) (81). Evidently, the PI3K-AKT–mTOR axis tightly correlates with the Th17/Treg equilibrium, with this signaling pathway showing promise in regulating IBD via the modulation of Th17/Treg balance.
Initially identified for its role in modulating the toxicity and immune effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (82), the aryl hydrocarbon receptor (AhR) exhibits widespread distribution across various bodily tissues and cells. Specifically enriched in select CD4+ T cell subsets such as certain hematopoietic stem cells, bone marrow-derived dendritic cells, Langerhans cells, and Th17 cells, AhR displays diminished expression levels in B lymphocytes and Treg cells (83). AhR has a variety of ligands, which can be divided into endogenous and exogenous. Endogenous sources include 6-formylindolo (3,2-b) carbazole (FICZ), a photochemical product of tryptophan, bilirubin, a metabolite of heme via the liver, and lipoxin A4, a metabolite of arachidonic acid. Exogenous sources include environmental pollutants TCDD and benzopyrene formed during the combustion of organic matter inhaled through the respiratory tract, as well as quercetin, indole-3-carbinol, resveratrol, and curcumin in normal diets. Since different ligands can trigger different results, the biological functions and responses of AhR after binding to ligands with different structures are also different (83).
AhR’s role in regulating the differentiation of Th17 and Treg cells hinges on the activation of AhR ligands, failure of which can perturb the differentiation process of naïve T cells. AhR ligands modulate both innate and adaptive immune responses by engaging with AhR in immune cells (83). Recent investigations on T cell polarization in mammals reveal that T cells can fine-tune their differentiation trajectory through orchestrated expression of pivotal transcription factors and appropriate epigenetic alterations (84). Current studies delving into the mechanism of AhR-mediated regulation of Th17/Treg cell differentiation primarily scrutinize various components such as antigen-presenting cells, cytokines, transcription factors, epigenetic modifications, and other facets within activated T lymphocytes (85). As for the regulation of antigen-presenting cells, dendritic cells (DCs) actively engage in antigen uptake, processing, and presentation vital for immune defense. Renowned as the body’s most efficient antigen-presenting cells (APCs), DCs significantly influence T lymphocyte activation (86). Noteworthy is the pivotal immunomodulatory role played by AhR in DCs (87). Recent research points to indoleamine 2,3-dioxygenase (IDO) production dependency in DCs on AhR expression (88). IDO acts as a crucial rate-limiting enzyme in tryptophan breakdown, converting tryptophan into kynurenine. AhR modulates the metabolism of IDO in DCs, thereby influencing the differentiation and functionality of T lymphocytes. Upon TCDD-induced AhR activation, heightened IDO enzyme activation ensues, amplifying the mRNA expression levels of IDO1 and IDO2 (89). Tryptophan can promote the differentiation of Th17, while kynurenine can accelerate the apoptosis of effector T cells and induce the differentiation of Treg. Activated AhR promotes the expression of IDO in DCs, IDO decomposes tryptophan into kynurenine, and kynurenine further induces naïve T lymphocytes to differentiate into Tregs (90). In terms of regulating cytokine levels, Th17 and Treg depend on different cytokines during differentiation (91). The differentiation of induced regulatory T cells (iTreg) and Th17 cells through the aryl hydrocarbon receptor (AhR) may be contingent upon the presence of IL-6 and TGF-β. Lower concentrations of IL-6 and TGF-β have the capacity to heighten RORγt expression, thereby fostering Th17 differentiation. Conversely, elevated TGF-β levels can instigate Foxp3+ expression, consequently encouraging iTreg differentiation (73, 92). Research by Kimura and colleagues revealed that the standalone presence of TCDD or FICZ ligands fails to induce differentiation into Th17 and iTreg cells. However, in the presence of IL-6 and TGF-β cytokines, TCDD or FICZ can augment Th17 differentiation and IL-17 secretion (93). Additionally, under the influence of TGF-β cytokine, TCDD or FICZ can elevate Foxp3 expression. Regarding the modulation of transcription factor levels, in the context of inflammatory bowel disease (IBD), the JAK/STAT signaling pathway disrupts the T cell balance, thereby influencing the inflammatory response (94). IL-6-mediated STAT3 signaling pathway can upregulate Th17 differentiation. Chaudhry et al. believed that the activation of STAT3 in Treg cells could enable Treg to inhibit Th17 inflammatory response by increasing the expression of inhibitory cell molecules and chemokine receptors, and the loss of STAT3 in Treg cells could induce colitis (95). In addition to stimulating the STAT3 signaling pathway, Th17 differentiation can also inhibit Th17 differentiation through IFN-γ or IL-27-mediated STAT1 signaling pathway and IL-2-mediated STAT5 signaling pathway. After IFN-γ activates STAT1, or IL-2 activates STAT5, AhR interacts with STAT1 and STAT5, thereby inhibiting the differentiation of Th17. Experiments by Quintana et al. showed that activated AhR regulates STAT1 but contributes to the differentiation of iTreg (96). When TGF-β induces iTreg differentiation, AhR can also mediate the transcription factors Smad1 and Aiolos to promote Treg differentiation. Smad1 can regulate the +2079 to +2198 sequence of the Foxp3 promoter, and Aiolos forms a complex with Foxp3 to silence the expression of IL-2, thereby increasing the expression of Foxp3 to play an immune regulatory role (97).
In terms of regulating the epigenetic level, Kim et al. found that IL17A mRNA was highly expressed in peripheral blood mononuclear cells of IBD patients, and the IL-17A promoter was hypomethylated (98). Singh et al. found that TCDD can induce the differentiation of iTreg after activating AhR, but not the differentiation of Th17 (82). There are many studies on transcription factors and cytokines that regulate Th17 differentiation, but non-coding RNAs are relatively scarce. Among them, Th17-related microRNA, namely miR-326, is related to the occurrence of autoimmune diseases such as multiple sclerosis and autoimmune encephalomyelitis. Highly expressed miR-326 can promote the differentiation of Th17 and aggravate the occurrence of disease (99). However, Gasch et al. found that the expression of miR-326 was not related to the differentiation environment of T lymphocytes, but related to the AhR ligand FICZ, which down-regulated the expression of miR-326 in CD4+ T cells, thereby inhibiting the expression of IL-17A (100).
The mechanisms of UC are summarized in Figure 2.
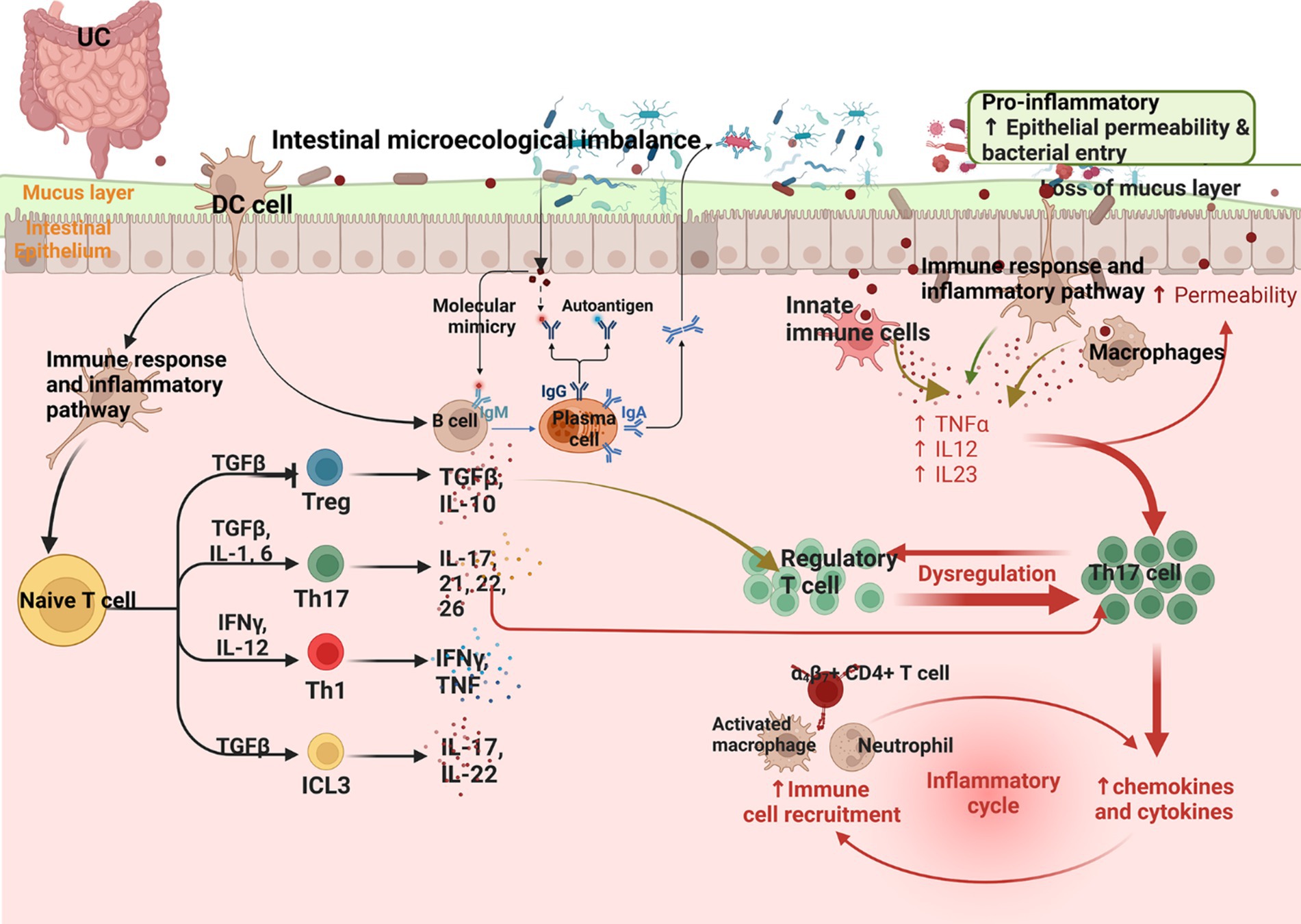
Figure 2. Summary of the mechanism of UC (Changes in the microbiota in UC and a reduction in the mucus layer lead to barrier breakdown, promoting access of the microbiota to the epithelial barrier, which in turn leads to infiltration of immune cells by binding to adhesion molecules expressed by the vascular endothelium. Infiltrating monocytes maturing into macrophages produce TNF, IL-12, IL-23, and IL-6, leading to Th1 cell polarization. Epithelial-derived IL-36γ suppresses Tregs and induces the polarization of IL-9-producing Th9, and IL-36 can induce fibrogenesis genes).
Monomers, components, or active ingredients of many natural plant active ingredients have regulatory effects on Th17/Treg balance in UC. These include extracts of various types of compounds such as glycosides, polysaccharides, volatile oils, alkaloids, flavonoids, polysaccharides, and natural plant active ingredients (Table 1; Figure 3).
TGP, derived from the dried root of Paeonia lactiflora Pall, encompasses active components like paeoniflorin, hydroxypaeoniflorin, and paeoniflorin. Known for its anti-inflammatory, immunomodulatory, antithrombotic, and hepatoprotective properties, TGP plays a crucial role in curbing autoimmune responses, thereby upholding immune tolerance within the body (101, 102). Lin et al. observed that TGP significantly curtails Th17-related cytokines like IL-17 and TNF-α in rats afflicted with UC while elevating Treg-related cytokines such as TNF-β, and IL-10, among others. This suggests that TGP mitigates intestinal mucosal damage in UC by orchestrating cytokine production, quelling the effector attributes of Th17 cells, and enhancing Treg responses (103). Basic research has revealed that paeoniflorin restores the balance of Th17/Treg, inhibits the activation of the NF-κB signaling pathway, reduces oxidative stress, suppresses the secretion of inflammatory factors such as IL-17, TNF-α, and ICAM, while simultaneously promoting the expression of autophagy-related factors such as LC3BII and Beclin1, thus ameliorating UC (104–106). Paeoniflorin also aids in improving TNBS-induced colitis by modulating the Th17/Treg balance mediated by DCs (107).
Mangiferin and neomangiferin (Figure 3) are present in Anemarrhena asphodeloides and mango pistils (from the Anacardiaceae family), commonly utilized in herbal remedies and functional foods to address inflammation, asthma, and pain management (108–110). Mangiferin exhibits antioxidants, analgesics, anti-inflammatories, and effects on colitis and diabetes (111–113). Neomangiferin, more hydrophilic than mangiferin, benefits osteoporosis and lipid issues (110, 114). In vitro studies indicate that neomangiferin restores the balance of Th17/Treg cell populations by downregulating IL-17 and RORγt expression while upregulating IL-10 and FOXP3 expression, ameliorating colitis (115). Meanwhile, mangiferin modulates innate immunity by inhibiting TLR4-NF-κB/MAPK signaling, particularly IRAK1 phosphorylation, exhibiting anti-colitis effects (116). In a TNBS-induced colitis mouse model, mangiferin significantly reduced colon shortening and myeloperoxidase activity, potentially by impeding Th17 cell differentiation, decreasing TNBS-induced IL-17 expression, promoting Treg cell differentiation, and enhancing TNBS-inhibited IL-10 expression. Additionally, mangiferin inhibited spleen cell differentiation into Th17 cells in vitro while promoting Treg cell differentiation. This may involve downregulating RORγt and IL-17 expression, inhibiting STAT3 activation in spleen cells, upregulating Foxp3 and IL-10 expression, and enhancing STAT5 activation (117). Overall, in both in vitro and in vivo settings, mangiferin has demonstrated its ability to mitigate inflammation by modulating the differentiation of adaptive immune-related T cells.
Timosaponin AIII (Figure 3), derived from Anemarrhena asphodeloides rhizomes and serving as sarsasapogenin, exhibits anti-inflammatory properties (118). Lim et al. identified that both timosaponin AIII and its metabolite sarsasapogenin effectively inhibited NF-κB and MAPK activation, as well as the phosphorylation of IRAK1, TAK1, and IκBα in LPS-stimulated macrophages. These compounds not only suppressed Th17 cell differentiation in the colonic lamina propria but also facilitated Treg cell differentiation. Additionally, timosaponin AIII and sarsasapogenin impeded the differentiation of splenic CD4+ T cells into Th17 cells in vitro (119).
Oleanolic acid (Figure 3) and its derivatives, natural pentacyclic triterpenoids extensively present in various plants, offer a range of preventive and therapeutic benefits across conditions such as UC, multiple sclerosis, and metabolic disorders (120–123). Research highlights the remarkable inhibitory prowess of a potent synthetic triterpene analog derived from oleanolic acid in interrupting cellular inflammatory pathways. Oleanolic acid showcases its inhibitory impact on induced nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 in macrophages (124). This compound also deters DSS-induced Th17 cell differentiation and lowers the expression of RORγt and IL-17 in the colon and colonic lamina propria concurrent with an upsurge in Treg cell differentiation markers, notably Foxp3 and IL-10 (125). These outcomes suggest that oleanolic acid holds promise in ameliorating inflammatory conditions like colitis by curtailing Th17 cell differentiation while enhancing Treg cell development.
The indigenous Nian hill tribes of Vietnam have traditionally harnessed the therapeutic potential of Panax vietnamensis Ha & Grushv., attributing it with significant anti-fatigue, life-preserving, and anti-inflammatory properties (126, 127). Notably rich in follistol saponins, particularly machonoside R2 (Figure 3), this plant showcases antistress, antidepressant, and anxiolytic effects (128). Lee et al. discovered that majonoside R2 can be metabolized into ocolitoll (Figure 3) through gut microbiota (129). Ocolitoll inhibited the TNBS-induced physiological damage, including colon shortening, increased macroscopic scores, heightened myeloperoxidase activity, and elevated production of nitric oxide and prostaglandin E2. Additionally, ocolitoll effectively suppressed Th17 cell differentiation triggered by TNBS within the colonic lamina propria, while modulating the expression levels of key markers such as T-bet, RORγt, IL-17, and IL-23. Concurrently, Treg cell differentiation was significantly enhanced, accompanied by increased expression of Foxp3 and IL-10. These findings suggest that oral administration of majonoside R2 leads to its conversion into ocolitoll through gut microbiota metabolism, thereby rebalancing the Th17/Treg ratio and offering relief in inflammatory conditions like colitis (129).
Centella asiatica (L.) Urb. is a perennial herb acclaimed for its bioactive compounds, which include glycosides such as madecassoside (Figure 3) and asiaticoside, alongside their corresponding aglycones asiatic acid and asiatic acid (130–132). Within investigations concerning collagen-induced arthritis in rats, the madecassoside was observed to play a crucial role in rebalancing Th17/Treg cell populations (133). Remarkably, a study revealed that oral administration of madecassic acid resulted in decreased proportions of Th17 cells, accompanied by reduced expression levels of pivotal markers like RORγt, IL-17A, IL-17F, IL-21, and IL-22, while concurrently increasing Treg cell counts and enhancing the expression of Foxp3 and IL-10 in the colons of mice with colitis (134). Interestingly, under Th17 polarized conditions, madecassic acid exhibited inhibitory effects on ACC1 expression and promoted the transition of Th17 cells to Treg cells, without impacting Treg cell differentiation under Treg-polarized conditions. Overall, the conversion of madecassoside to madecassic acid elucidates a regulatory mechanism involving the PPARγ/AMPK/ACC1 pathway, aimed at restoring the Th17/Treg balance to potentially mitigate conditions such as UC (134).
Curcumin (Figure 3), a naturally occurring non-toxic compound extracted from Curcuma longa L., possesses a myriad of biological properties, including anti-inflammatory, anti-infective, anticoagulant, and immunomodulatory actions (135, 136). Curcumin can reduce nitric oxide levels, myeloperoxidase activity, and TNF-α, inhibiting NF-κB activation to exert its anti-inflammatory effects, while providing protective benefits through antioxidative pathways in the treatment of DSS-induced colitis (137–139). Wei et al. observed that curcumin elevated the anti-inflammatory cytokine IL-10 while reducing pro-inflammatory cytokines like IL-23 to modulate the balance of Treg/Th17, thereby ameliorating DSS-induced colitis (140).
Derived from Scutellaria baicalensis Georgi, baicalin (Figure 3) is a flavonoid compound deeply entrenched in TCM for its versatile therapeutic properties, including anti-inflammatory, antibacterial, anti-allergic, and anti-cancer activities (141). Recent research underscores baicalin’s profound impact on TNBS-induced colitis, showcasing remarkable reductions in disease severity indicators like disease activity index, macroscopic and microscopic scores, while simultaneously ameliorating weight loss and colon shortening (142). Intriguingly, the salutary effects of baicalin appear intertwined with the modulation of Th17 and Treg cell dynamics. Treatment with baicalin notably dampened the population of Th17 cells, along with suppressing Th17-associated cytokines (IL-17 and IL-6) and repressing RORγt expression. Moreover, baicalin demonstrated the potential to enhance Treg cell numbers, elevate Treg-linked cytokines such as TGF-β and IL-10, and boost the expression of FOXP3 (142).
The primary constituents of Glycyrrhizae radix Et Rhizoma include Glycyrrhizin and flavonoids like liquiritigenin and isoliquiritigenin (Figure 3), along with their aglycones. Studies have shown that both Glycyrrhizae radix Et Rhizoma and isoliquiritigenin, a component thereof, possess the ability to impede NF-kB activation induced by LPS and the activation of the NLRP3 inflammasome (143–145). Notably, Glycyrrhizin aids in mitigating tissue inflammation by diminishing reactive oxygen species (ROS) production by neutrophils (146). In addition, Guo et al. uncovered that isoliquiritigenin and naringin facilitate the generation of Treg cells, both in laboratory settings and in live subjects. This observation suggests that the enhancement of regulatory T-cell development could serve as the fundamental mechanism behind the long-standing therapeutic efficacy of Glycyrrhizae radix Et Rhizoma, suggesting these two potent molecules as valuable resources in combatting autoimmune and inflammatory disorders (147).
Berberine (Figure 3), an alkaloid isolated from Rhizoma Coptidis, exhibits many biological functions and has been used in the treatment of diarrhea, gastroenteritis, diabetes, hyperlipidemia, and UC (148). Studies have shown that berberine can improve the balance of Treg/Th17 in DSS-induced UC model, and also reduce the diversity of intestinal microbiota, affecting the relative abundance of Desulfovibrio, Eubacterium and Bacteroides (149). Tang et al. found that berberine can regulate the expression of various inflammatory factors, proteins and miRNAs through the miR-31-5p-Th17/Treg immune network axis, and exert a therapeutic effect on TNBS/ethanol-induced UC (150).
Parthenolide (Figure 3), a sesquiterpene lactone extracted from Tanacetum balsamita branches, demonstrates robust bioactivity encompassing potent anticancer, anti-inflammatory, and antibacterial effects (151). Liu et al. reveal that mice treated with parthenolide exhibited marked improvements in colonic inflammation, accompanied by reductions in body weight, colon length, disease activity index, histological scores, and colonoscopy scores (152). Parthenolide exerts selective actions by increasing Treg cell levels while decreasing the proportion of colonic Th17 cells, thus enhancing the Treg/Th17 equilibrium crucial for intestinal stability. To validate this microbiota-dependent mechanism, experiments involving gut microbiota depletion and fecal microbiota transplantation (FMT) were conducted. The findings suggest that parthenolide substantially boosts the concentration of short-chain fatty acids in mice with colitis by regulating gut microbiota, specifically targeting short-chain fatty acid-associated bacteria such as Alloprevotella, Rikenella, and Fournierella, subsequently fostering Treg/Th17 balance (152).
Polydatin (Figure 3) is a natural component extracted from the dried rhizome of Polygonum cuspidatum Sieb. et Zucc., which has anti-fibrosis, anti-tumor, anti-atherosclerotic disease, anti-hepatitis, and prevention of multiple organ ischemia–reperfusion injury and other activities (153). Liu et al. found that Polydatin significantly alleviated DSS and TNBS-induced colitis in mice, and significantly reduced the proportion of Th17 cells in the spleen and mesenteric lymph nodes (154). Mechanistic studies have shown that polydatin can relieve colitis by directly binding to STAT3-specific inhibitory signal transducers and STAT3 phosphorylation activators, leading to the reduction of Th17 cells. These findings provide new insights into the anti-colitis effects of Polydatin, which may be a promising drug candidate for the treatment of IBD (154).
Resveratrol (Figure 3), a naturally derived active compound present in grapes, peanuts, and various plant sources, exhibits a diverse array of biological functions, including immunomodulation, anti-inflammatory properties, antioxidant effects, anti-angiogenic activity, and mitigation of tissue damage (155, 156). Notably, resveratrol showcases remarkable therapeutic potential in addressing UC (UC) by diminishing neutrophil exudation, thwarting adhesion molecules, and fine-tuning cytokine levels (157, 158). Clinical investigations underscore the efficacy of anti-inflammatory interventions in managing UC patients. Yao et al. found that resveratrol may restore Treg/Th17 equilibrium, elevate TGF-β1 and IL-10 concentrations, reduce IL-6 and IL-17 levels, and inhibit the hypoxia-mTOR-HIF-1α-Th17 as well as IL-6-STAT3-HIF-1α-Th17 pathways (159). Additionally, Gu et al. observed that resveratrol can rectify the Treg/Th17 immune imbalance and curb intestinal inflammatory responses in UC cases, highlighting its promising immunomodulatory properties (160).
Dihydromyricelin is a dihydroflavonol flavonoid compound, which has various pharmacological effects such as anti-oxidation, anti-tumor, anti-inflammation, anti-alcohol and liver protection, anti-pathogenic microorganisms and blood lipid regulation (161). Li et al. found that Dihydromyricelin may restore the balance of Treg/Th17 in peripheral blood and reduce the expression of MMP9 in colon tissue, so as to alleviate DSS-induced UC in mice (162).
Derived from daphne coumadin, DAPH is a coumarin derivative renowned for its robust anti-inflammatory and antioxidant characteristics, commonly employed in combatting inflammatory ailments (163–165). DAPH demonstrates the capacity to impede T h17 cell differentiation while fostering Treg cell maturation, thereby fostering immune equilibrium and ameliorating inflammation (166, 167). Previous research indicates that DAPH can shield against intestinal inflammatory disorders in a rat model of TNBS-induced colitis, with its intestinal anti-inflammatory efficacy linked to its antioxidant profile (165). A novel investigation reveals that this compound substantially boosts the population of gut microbiota capable of producing short-chain fatty acids (SCFAs), a shift associated with bolstering Treg development and diminishing the differentiation of pro-inflammatory T cells into h17 cells, ultimately quelling UC (168).
Stigmasterol (Figure 3), a phytosterol renowned for its anti-inflammatory, antioxidant, and antitumor attributes, and its positive impacts on metabolism (169, 170), serves as a key active component in traditional Chinese herbal mixtures utilized for managing inflammatory bowel disease (IBD) (171, 172). Prior investigations unveiled stigmasterol’s capacity to notably diminish cyclooxygenase-2 (COX-2) expression levels, leading to enhanced colonic inflammation scores and amelioration of colitis symptoms. Treatment with stigmasterol was shown to rebalance the Treg/Th17 equilibrium and induce alterations in gut microbiota composition in a colitis model induced by DSS. Moreover, stigmasterol treatment amplified the generation of short-chain fatty acids (SCFAs) by gut microbiota, particularly boosting butyrate production. Researchers hypothesized that stigmasterol might rectify the Treg/Th17 cell imbalance by activating PPARγ via butyrate, thus offering therapeutic benefits in addressing IBD concerns (173).
Citrus flavonoids, like psilocybin and tangeretin, are naturally occurring compounds found abundantly in the rinds of citrus fruits, particularly Citrus species (174). These compounds boast a spectrum of biological activities encompassing anti-inflammatory, anticancer, hypolipidemic, anti-obesity, and neuroprotective effects (175–179). They demonstrate efficacy in ameliorating dermal responses by mitigating histamine effects and triggering the activation of transcription factors NF-κB and AP-1 via protein kinase C pathways (180, 181). In UC, tangeretin effectively suppresses TNF-α, IL-12, and IL-23 expression, alongside NF-κB activation in lipopolysaccharide-stimulated dendritic cells. Moreover, Tangeretin impedes the differentiation of Th1 and Th17 cells induced by TNBS, restraining the expression of T-bet, RORγt, interferon-γ, IL-12, IL-17, and TNF-α, while concurrently promoting the differentiation of Treg cells suppressed by TNBS, upregulating Foxp3 and IL-10 expression levels (182).
Andrographolide (Figure 3), an therapeutic agent with clinical efficacy in treating bronchitis, tonsillitis, and bacillary dysentery (183, 184), has garnered attention in recent research for its ability to modulate macrophage activation, suppress Th1/Th17 immune responses, and diminish MAPK and NF-κB signaling pathways, beneficial for managing conditions like acute colitis and lung injuries (185, 186). Noteworthy findings indicate that andrographolide sulfonate notably ameliorated TNBS-induced symptoms including weight loss, myeloperoxidase activity, colon shortening, and colonic inflammation, while reducing the expression of pro-inflammatory cytokines at both mRNA and protein levels. Moreover, andrographolide sulfonate impeded the infiltration of CD4+ T cells and the differentiation of Th1 (CD4+ IFN-γ+) and Th17 (CD4+ IL17A+) subsets. Studies illustrated that andrographolide sulfonate successfully mitigated TNBS-induced colitis in mice through the inhibition of Th1/Th17 immune responses (187).
Astragaloside IV (Figure 3), a triterpene saponin derived from Astragalus membranaceus (Fisch.) Bge., is renowned for its wide-ranging beneficial effects, spanning antioxidant, cardioprotective, anti-inflammatory, antiviral, antibacterial, antifibrotic, antidiabetic, and immunomodulatory properties (188). Notably, in models of experimental colitis triggered by DSS and TNBS, astragaloside IV demonstrated the ability to modulate macrophage polarization via the STAT signaling pathway and influence energy metabolism, thereby effectively mitigating intestinal inflammation (189, 190). Furthermore, astragaloside IV exhibited the capacity to lower levels of key pro-inflammatory cytokines such as myeloperoxidase, TNF-α, IL-1β, IL-6, and nitric oxide in vitro (191). Recent research findings underscored that astragaloside IV not only ameliorated clinical symptoms associated with DSS-induced colitis but also significantly enhanced ulcer repair, reduced inflammatory cell infiltration and inflammation indices, and regulated the expression of inflammatory cytokines within colon tissues. Of significant note, early administration of astragaloside IV was found to restore Th17/Treg cell balance in mice with acute colitis, inhibiting Th17 responses while promoting Treg cell responses in cases of recurrent colitis (192, 193).
Astragalus polysaccharide, the primary bioactive compound derived from Astragalus membranaceus (Fisch.) Bge, showcases a diverse array of biological properties encompassing antiviral, antitumor, anti-aging, anti-radiation, anti-stress, and antioxidant activities (194, 195). In the context of DSS-induced colitis, Astragalus polysaccharides exhibited therapeutic effects by impeding the NF-κB and NRF2/HO-1 signaling pathways (196, 197). Recent investigations unveiled that these polysaccharides enhanced the survival rates, disease activity indices, body weight fluctuations, colon lengths, body weights, and mitigated colonic histopathological damage in a mouse model of UC. Notably, Astragalus polysaccharides orchestrated the expression levels of inflammatory cytokines (IL-2, IL-6, IL-12p70, IL-23, TNF-ɑ, and TGF-β1) in the colon tissues of colitis-afflicted mice. Moreover, Astragalus polysaccharide significantly upregulated Tfh10 and Tfr populations while downregulating Tfh1, Tfh17, and Tfh21. Additionally, these polysaccharides elevated Treg cell levels along with pertinent nuclear transcription factor Foxp3 and cytokine IL-10 expression in colitis models. In essence, Astragalus polysaccharide holds promise in alleviating UC by rebalancing the Tfh/Treg cell equilibria (198).
Derived from Epimedium brevicornu Maxim, icariin (Figure 3) is a natural flavonoid glucoside recognized for its diverse pharmacological attributes (199), spanning antioxidant, antidepressant, and anti-inflammatory properties (200, 201). Oral intake of icariin markedly decelerates disease advancement and mitigates pathological alterations in colitis conditions, and effectively curbs the generation of pro-inflammatory cytokines and the activation of p-p65, p-STAT1, and p-STAT3 within colon tissues (202). Further investigations unveiled the dose-dependent capacity of icariin to impede the proliferation and activation of T lymphocytes, along with lowering pro-inflammatory cytokine levels in stimulated T cells. Moreover, icariin administration was shown to suppress the phosphorylation of STAT1 and STAT3, pivotal transcription factors associated with Th1 and Th17 responses, within CD4+ T cells (202). Collectively, these outcomes underscore the potential of icariin as a viable therapeutic option for addressing IBD.
Hailing from green tea, EGCG (Figure 3) stands as a natural compound with a spectrum of beneficial attributes including antibacterial, anti-inflammatory, antitumor, antioxidant, and anti-aging properties (203, 204). Notably, research highlights its capacity to modulate the immune system, showcasing potential in the realm of autoimmune disease treatment (205, 206). EGCG interventions led to decreased IL-6 and IL-17 release, alongside the adjustment of Treg/Th17 ratios within mouse spleens. Concurrently, elevated plasma levels of IL-10 and TGF-β1 were observed, while the expression of HIF-1α and STAT3 proteins in the colon was curtailed. These findings hint at EGCG’s potential in ameliorating experimental colitis in mice by stifling IL-6 release and orchestrating the Treg/Th17 equilibrium in vivo (39).
Within the realm of herbal medicine lies Arctigenin (Figure 3), a bioactive dibenzylbutyrolactone lignan renowned for its array of properties. Its repertoire spans antimicrobial, anti-inflammatory, and immune-modulating capabilities, with a recent surge of interest stemming from its antitumor prowess (20, 207, 208). Deemed an ERβ agonist in a recent investigation, Arctigenin exhibits a moderate affinity for ERβ, initiating the dissociation of the ERβ/HSP90 complex, enhancing ERβ phosphorylation for nuclear translocation, and elevating transcriptional activity levels. Through ERβ activation, Arctic Aglycon impedes mTORC1 function by disrupting ERβ-constrained-mTOR complex interaction. Intriguingly, the absence of ERβ effectively annulled Arctigenin-induced suppression of Th17 cell differentiation, hinting at ERβ as a prime candidate for Arctigenin’s target protein. This regulatory mechanism obstructs mTORC1 activation, hence thwarting Th17 cell differentiation and the progression of colitis (209).
Emerging from the breakdown of glucosinolate in cruciferous vegetables, DIM is a natural compound known for inhibiting inflammatory responses in select mouse inflammation models (Figure 3) (210, 211). Recent investigations have spotlighted DIM’s protective role in inflammation mitigation, showcased by its ability to curb NF-κB activation, stifle prostaglandin E2 production, and modulate antioxidant levels (212, 213). A novel study underscored how DIM administration assuaged experimental colitis, suggesting its influence on the downstream signaling pathways of AhR could reduce Th2/Th17 cells while bolstering Treg populations. Demonstrating therapeutic efficacy in animal models of UC, DIM’s capability to suppress Th2/Th17 cells and boost Tregs positions it as a promising therapeutic avenue for UC patients (214).
Artemisinins (Figure 3), derived from Artemisia annua L., encompass a collection of sesquiterpene trioxanes lauded for their therapeutic potential (215, 216). Dihydroartemisinin (DHA), an artemisinin derivative, emerges as a robust immunomodulator with diverse applications. Studies indicate the efficacy of DHA and its derivatives in managing autoimmune conditions, such as experimental autoimmune encephalomyelitis (EAE) (217) and collagen-induced arthritis (218). Yan et al. discovered that DHA inhibited Th1, Th17, Th9, and Th22 cell populations in TNBS- or OXA-induced colitis, while enhancing Tregs (219). Remarkably, DHA induced HO-1 production, promoted CD4+ T cell apoptosis, and restored the Th17/Treg balance, effects that were blocked by the HO-1 inhibitor Sn-protoporphyrin IX. Overall, these results suggest that DHA has the potential to serve as a novel therapeutic agent for managing inflammatory bowel disease (IBD) or Th17/Treg immune modulation (219).
Derived from the green walnut shell of Juglans regia L., juglone (Figure 3) stands out as a pivotal naphthoquinone compound recognized for its manifold properties (220, 221). Recent investigations have highlighted juglone’s anti-inflammatory prowess, showcasing efficacy in conditions like hepatitis, neuroinflammation, and allergic pulmonary fibrosis (222, 223). Juglone has been shown to improve the disease activity index and pathological features of UC, significantly reducing the protein levels of IL-6, TNF-α, and IL-1β, while enhancing IL-10 expression (224). Moreover, Juglone induces changes in microbial diversity and gut microbiota composition, promoting the ratio of Firmicutes to Bacteroidetes and the abundance of Actinobacteria, while inhibiting the levels of Proteobacteria (224). This compound also inhibits the protein levels of IL-6, STAT3, and RORγt, thereby enhancing FOXP3 expression. Additionally, Juglone impedes the progression of Th17 cells and promotes the generation of Tregs, essential for maintaining the Th17/Treg balance (224). These findings suggest that Juglone can protect mice from UC by shaping the dynamics of gut microbiota and restoring the balance of Th17/Treg.
Nelumbo nucifera Gaertn. is used in TCM for fever, diarrhea and bleeding (225). Nuciferine, an alkaloid containing an aromatic ring, is the main bioactive component derived from Nelumbo nucifera Gaertn. (226). Studies reveal nuciferine’s potential in counteracting high-fat diet-induced obesity in mice through gut microbiota modulation (227, 228), mitigating inflammation triggered by fructose or uric acid in HK-2 cells, among other beneficial effects (229). In research, nuciferine showcased its efficacy in alleviating symptoms in DSS-induced UC mice, such as mitigating histological damage and colon shortening. Notably, nuciferine played a role in enhancing the Th1/Th2 and Treg/Th17 equilibrium, alongside influencing gut microbiota composition within DSS-induced inflammatory bowel disease (IBD) models (230).
In this section, different TCM prescriptions (Table 2) for treatment of UC will be discussed.
KJL, a TCM formulation deeply rooted in empirical practices, addresses UC by employing a therapeutic approach that involves heat dissipation, spleen fortification, and blood circulation enhancement (231). Noteworthy outcomes in clinical assessments underscore its effectiveness (232). Experimental investigations reveal that KJL can mitigate ulceration, ameliorate pathological tissue damage, and diminish levels of inflammatory markers such as IL-1β, TNF-α, and IL-6 in UC-induced rats (232–234). KJL enhances the levels of blood TGF-β1, IL-2, IL-10, increases colonic Foxp3, STAT5, and IL-2 levels, while decreasing the levels of IL-6, IL-23, IL-17, IL-21 in the blood and inhibiting colonic-related RORγt, STAT3, IL-23, IL-17, and IL-21, thereby restoring the balance of Th17/Treg (235, 236).
BTWT, a prevalent TCM concoction majorly compounded with pulsatilla, has gained substantial traction in UC therapy across China (237–239). Tan et al. discovered that BTWT could downregulate RORγt expression, upregulate Foxp3 expression, increase the proportion of Treg cells in patients, decrease the proportion of Th17 cells, thereby restoring the Th17/Treg balance in the body (240).
With a historical backdrop spanning millennia, FZLZT stands as a stalwart remedy against gastrointestinal maladies, encompassing conditions such as enteritis, diarrhea, and gastritis (241–243). Shedding light on its therapeutic potential, Li et al. unveiled FZLZT’s capacity to ameliorate UC symptoms linked to spleen-kidney yang deficiency, attenuate colonic tissue inflammation in rat models, and facilitate mucosal regeneration within the colon (244). Furthermore, FZZLT bolsters the expression of Treg-associated factors like IL-10, TGF-β1, Foxp3, and STAT5, thereby contributing significantly to the management of UC characterized by spleen-kidney yang deficiency (244).
QCWZT has showcased clinical efficacy in alleviating symptoms of UC and has obtained authorization for further clinical investigations. Research findings underscore its notable capacity to effectively suppress the active phase of UC, exhibiting comparable outcomes to mesalamine (245). Moreover, QCWZT demonstrates the ability to diminish the modified Mayo score in patients grappling with mild to moderate UC, enhance pathological repair, curtail inflammatory cellular infiltration in the intestinal mucosa, promote colonic goblet cell proliferation, elevate mucin MUC2 expression in colonic mucosal tissue, and impede STAT6 expression (245). In a parallel discovery, Sun et al. proposed that QCWZT may modulate the VDR signaling pathway by downregulating miR-675-5p expression, thereby harmonizing the Th17/Treg immune equilibrium in UC, remedying intestinal mucosal barrier impairments, and aiding in UC management (246). Recent investigations have indicated that the bioactive constituents of QCWZT possess the capability to suppress the IL-6-STAT3 pathway, ultimately impeding the differentiation of Th17 lymphocytes, thereby diminishing colonic inflammation (247).
Clinical evaluations have affirmed the therapeutic efficacy of FFKST in managing UC, showcasing a capacity to diminish intestinal mucosal inflammation in afflicted individuals. This herbal formulation has proven instrumental in alleviating abdominal pain and hematochezia symptoms (248). Primarily constituted of active components like matrine, gallic acid, indigo, notoginsenoside R1, ginsenoside Rb1, and glycyrrhizic acid, FFKST has demonstrated inhibitory properties at a molecular level across various UC models. Matrine, for instance, mitigated colitis manifestations and curbed inflammatory cytokine expression of IFN-γ and IL-17 in TNBS-induced colitis within IL-10-deficient mice (249). Oxymatrine, when tested in a UC model, ameliorated intestinal damage by modulating T cell-secreted inflammatory cytokines and inhibiting NF-κB activation (250, 251). Moreover, researchers noted significant enhancements in colitis symptoms and pathological mitigation in mice treated with FFKST, alongside notable effects on immune modulation through the regulation of Th17/Treg cell balance in DSS-induced colitis models (252, 253).
Originally documented in the ancient text Jingui Yaolüe, DHMDT emerges as a viable solution for intestinal abscesses (254). Discoveries by Luo et al. unveiled the remarkable efficacy of DHMDT in mitigating pathological alterations in UC-afflicted mice. This included the restoration of colon length, amelioration of weight loss, and tissue repair in conjunction with inflammation reduction. Additionally, DHMDT precipitated a shift in gut microbiota composition, enhancing alpha diversity while notably elevating Firmicutes and Actinobacteria populations and diminishing Proteobacteria and Bacteroidetes numbers. Noteworthy was the substantial augmentation of butyrate-producing Butyricoccus elongatum colonies and the restoration of short-chain fatty acid levels in the gut. DHMDT also fostered an improved Th17 cell/Treg cell ratio in mesenteric lymph nodes and a decline in various inflammatory cytokines like IL-6, TNF-α, IFNγ, IL-10, IL-17A, IL-21, and IL-22 within the colon (255).
Utilized clinically for acute enteritis, chronic diarrhea, and bacillary dysentery, GQT harbors a diverse array of potent bioactive compounds like berberine, baicalin, and puerarin. Recent studies suggest that these constituents possess the ability to modulate T cell differentiation individually, offering promise in mitigating T cell-associated inflammatory disorders across various animal models (256–258). Hu et al. reported significant symptom relief in UC-afflicted mice following GQT treatment, alongside noteworthy inhibition of myeloperoxidase activity. Notably, the administration of GQT led to a substantial decrease in pro-inflammatory factors like IL-1β, TNF-α, and IL-6. This herbal formulation facilitated the infiltration of Treg and Th17 cells into the colon while concurrently reducing the expression of inflammatory mediators such as TGF-β1 and IL-17. Through the suppression of IL-6/JAK2/STAT3 signaling, GQT is postulated to restore Treg and Th17 cell equilibrium within colon tissue, ultimately easing DSS-induced UC symptoms (259). Wang et al. additionally observed that modified GQT modulates the Treg/Th17 balance and effectively addresses DSS-triggered acute experimental colitis in mice by reshaping the gut microbiota composition (260).
In a study by Tian et al., it was observed that when SLBZS was administered in conjunction with standard therapy, patients exhibited significantly lower rates of colonoscopic congestion, edema, erosion, and ulcers compared to those in the control group. This indicates the potential benefits of this combined approach in aiding the restoration of intestinal mucosal integrity in individuals with UC. The purported efficacy of SLBZS on mucosal repair appears connected to its capability to address Th17/Treg imbalances and temper inflammatory responses in UC patients. Notably, the post-treatment recurrence rate in the observation group was notably lower than in the control group, underscoring the potential role of SLBZS in rectifying immune irregularities among patients (261). The therapeutic mechanisms of SLBZS in UC management could entail modulating the expression levels of RORγt/FoxP3 and rectifying the Th17/Treg immune imbalance. Furthermore, Qi et al. highlighted that SLBZS’s protective influence in UC rats might also be linked to adjusting the RORγt/FoxP3 expression levels and correcting Th17/Treg immune discrepancies (262).
Extracts from Panax ginseng C. A. Mey. contain essential components like ginsenosides Rb1, Rb2, Re, and Rg1, utilized in TCM to address conditions such as inflammation, cancer, and diabetes (263, 264). These ginsenosides are known for their anti-inflammatory properties and immune-regulating abilities (265–267). For instance, ginsenoside Rb1 undergoes gut microbiota metabolism to produce 20-O-(β-D-glucopyranosyl)-20(S)-protopanaxadiol (compound K), which has demonstrated suppressive effects on LPS-induced inflammation by targeting IRAK1 in macrophages and enhancing outcomes in TNBS-induced colitis models in mice (268).
In the realm of ginsenoside research, ginsenoside Re, belonging to the prototalol-type ginsenosides, was found effective in ameliorating TNBS-induced colitis by impeding the LPS-TLR4 binding on macrophages, thus showcasing its potential as an anti-colitis agent (249). Recent investigations unveiled that oral administration of ginsenosides Rg1, Rh1, or 20(S)-protopanaxadiol could counter TNBS-induced colon narrowing, suppress myeloperoxidase activity, and alleviate the expression of pro-inflammatory cytokines like IL-1β, IL-17, and TNFα. Additionally, these compounds exhibited the capability to hinder NF-κB activation, restore the Th17/Treg balance disrupted by TNBS, and revive the expression of key regulatory molecules like IL-10 and Foxp3. In vitro studies indicated their ability to inhibit Th17 cell differentiation, with 20(S)-protopanaxadiol showcasing the most potent anti-inflammatory effects, followed by Rh1. The transformation of ginsenoside Rg1 into 20(S)-protopanaxadiol by ginsenoside Rh1 and F1 unveiled a potential route for therapeutic benefits (269).
These metabolites, particularly 20(S)-protopanaxadiol, were found effective in ameliorating inflammatory conditions like colitis by impeding LPS-TLR4 interaction on macrophages and restoring Th17/Treg balance. Long et al. further highlighted the potential of ginsenoside Rg1 in mitigating DSS-induced UC in mice by modulating the Treg/Th9 cell equilibrium (270).
Originating from the Jin and Yuan Dynasties, SYT represents a traditional Chinese medicinal formula known for its therapeutic benefits in managing UC (271). Recent investigations have shed light on its potential mechanisms, including the regulation of immune factors within immune cells, inflammation inhibition, and mitigation of oxidative stress (272–274). In a study conducted by Lu et al., it was observed that SYT led to a significant reduction in serum IL-17 levels and an increase in serum IFN-γ and IL-27 levels among rats afflicted with gastroenteric fever-type UC. This suggests that the therapeutic efficacy of SYT could be attributed to the modulation of serum IL-17 levels alongside elevated concentrations of IL-27 and IFN-γ (275).
Our previous research has highlighted the effectiveness of SYT in UC treatment, indicating a possible mechanism of action linked to the restoration of Treg/Th17 balance through the inhibition of HIF-1α (22). Furthermore, findings by Yao et al. unveiled that SYT exhibited significant relief of intestinal symptoms in UC patients characterized by damp-heat syndrome in the large intestine. By fostering intestinal mucosa recovery and enhancing patient quality of life, SYT was shown to regulate the Th17/Treg cell balance effectively (276).
Although the research on natural plant components mentioned above has demonstrated potential advantages in treating UC, there are still some shortcomings.
(1) Most studies are animal or cell experiments lacking clinical research. While animal studies can provide valuable insights into potential therapeutic effects, they may not always directly apply to humans as the efficacy and safety observed in animal models may not be replicated in clinical trials involving human participants.
(2) Inconsistent handling of compounds in different studies. Due to factors such as plant variability, processing methods, and storage conditions, the components, potency, and quality of traditional herbal medicines may vary. The lack of standardization and quality control measures may lead to inconsistent treatment outcomes and potential safety issues.
(3) Compared to single-agent drugs, some traditional plant therapies (e.g., TCM prescriptions) lack rigorous scientific validation and may have side effects as well as interactions with other medications or health conditions. Without comprehensive clinical trials and post-market monitoring, identifying and managing potential risks may be challenging.
(4) Integrating traditional medicine into modern healthcare systems may pose challenges in cultural, ethical, and regulatory aspects. Concerns may arise regarding cultural appropriation, sustainability of plant resources, and equitable access to healthcare opportunities.
In addressing the above limitations, future research could involve more clinical trials (e.g., randomized controlled trials) focusing on natural plant components (including plant extracts and TCM prescriptions), comprehensively studying their mechanisms of action and safety, exploring ways to integrate them into modern healthcare systems to establish safety, efficacy, and optimal dosing regimens. Additionally, future researchers should emphasize quality control and standardization of natural compounds to ensure reproducibility of efficacy.
In summary, IBD is a common disorder associated with a shift in the Th17/Treg balance towards a pro-inflammatory Th17 program, which negatively affects quality of life. Th17/Treg, as two closely related aspects of the immune responses, is crucial in maintaining the body’s immune homeostasis. A large amount of clinical and experimental evidence clearly shows that the regulation of Th17/Treg is an important mechanism of action of natural plant ingredients in the treatment of UC, such as the regulation of Th17/Treg regulation of transcription factor differentiation (TRORγt, STAT3, Foxp3 and STAT5), and regulate Th17/Treg signaling pathways (mTOR signaling pathway, PI3K/ Akt signaling pathway, AMPK signaling pathway, Nrf-2/HO-1 signaling pathway, HIF-1α signaling pathway, Notch signaling pathway, Wnt signaling pathway). This review mainly summarizes and analyzes the previous studies on the regulation of Th17/Treg balance in UC by natural plant components and TCM compounds and provides references for clinical rational design and treatment of UC. After summarizing, it was found that glycosides, polysaccharides, volatile oils, alkaloids, flavonoids, polysaccharides, and other types of compounds in TCM and some TCM extracts have unique regulatory advantages in regulating Th17/Treg in UC.
Currently, the impact of TCM on Th17/Treg balance in UC is underscored through several key aspects: Firstly, it works to reduce the proportion of Th17 cells, suppressing the secretion and expression of pro-inflammatory markers to alleviate intestinal inflammation and facilitate mucosal repair. Secondly, TCM aids in augmenting Treg production and differentiation, bolstering the population of anti-inflammatory cells and enhancing the secretion of related factors to reinforce intestinal immune responses. Thirdly, there is a concerted effort to diminish the presence of Th17 pro-inflammatory factors while concurrently boosting Treg anti-inflammatory factors. This synchronized modulation aims to restore balance in the Th17/Treg equilibrium, safeguarding the intestinal mucosa by reinstating immune tolerance and normal response patterns. TCM is renowned for its multifaceted, targeted effects, exhibiting minimal toxicity and side effects, thus harboring immense research and developmental value. Nevertheless, the intricate composition of TCM and its compounds presents challenges, as the precise mechanisms of action remain inadequately understood. Currently, the intricate relationship between Th17/Treg equilibrium, UC pathology, and the interventions of TCM remains elusive. Consequently, further in-depth investigations are imperative to elucidate the precise mechanisms through which TCM modulates Th17/Treg balance in UC. Advancing this understanding is essential for propelling the utilization and efficacy of TCM in addressing UC and other challenging ailments.
DZ: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. AG: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. CY: Data curation, Formal analysis, Writing – original draft. XL: Data curation, Formal analysis, Writing – original draft. KY: Data curation, Formal analysis, Writing – original draft. YY: Data curation, Formal analysis, Writing – original draft. MH: Data curation, Formal analysis, Writing – original draft. JC: Data curation, Formal analysis, Writing – original draft. PD: Data curation, Formal analysis, Writing – original draft. CD: Data curation, Formal analysis, Writing – original draft. CL: Data curation, Formal analysis, Writing – original draft. HC: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by the Natural Science Foundation of Hunan Province, China (No. 2023JJ60487).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1502849/full#supplementary-material
1. Segal, JP, LeBlanc, JF, and Hart, AL. Ulcerative colitis: an update. Clin Med (Lond). (2021) 21:135–9. doi: 10.7861/clinmed.2021-0080
2. Radziszewska, M, Smarkusz-Zarzecka, J, Ostrowska, L, and Pogodziński, D. Nutrition and supplementation in ulcerative colitis. Nutrients. (2022) 14:2469. doi: 10.3390/nu14122469
3. Adams, SM, Close, ED, and Shreenath, AP. Ulcerative colitis: rapid evidence review. Am Fam Physician. (2022) 105:406–11.
4. Wei, SC, Sollano, J, Hui, YT, Yu, W, Santos Estrella, PV, Llamado, LJQ, et al. Epidemiology, burden of disease, and unmet needs in the treatment of ulcerative colitis in Asia. Expert Rev Gastroenterol Hepatol. (2021) 15:275–89. doi: 10.1080/17474124.2021.1840976
5. Le Berre, C, Honap, S, and Peyrin-Biroulet, L. Ulcerative colitis. Lancet. (2023) 402:571–84. doi: 10.1016/S0140-6736(23)00966-2
6. Bergemalm, D, Andersson, E, Hultdin, J, Eriksson, C, Rush, S, Kalla, R, et al. Systemic inflammation in preclinical ulcerative colitis. Gastroenterology. (2021) 161:1526–1539.e9. doi: 10.1053/j.gastro.2021.07.026
7. Kobayashi, T, Siegmund, B, Le Berre, C, Wei, SC, Ferrante, M, Shen, B, et al. Ulcerative colitis. Nat Rev Dis Primers. (2020) 6:74. doi: 10.1038/s41572-020-0205-x
8. Bopanna, S, Ananthakrishnan, AN, Kedia, S, Yajnik, V, and Ahuja, V. Risk of colorectal cancer in Asian patients with ulcerative colitis: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2017) 2:269–76. doi: 10.1016/S2468-1253(17)30004-3
9. Martí-Aguado, D, Ballester, MP, and Mínguez, M. Risk factors and management strategies associated with non-response to aminosalicylates as a maintenance treatment in ulcerative colitis. Rev Esp Enferm Dig. (2021) 113:447–53. doi: 10.17235/reed.2021.7797/2021
10. D’Alessio, S, Ungaro, F, Noviello, D, Lovisa, S, Peyrin-Biroulet, L, and Danese, S. Revisiting fibrosis in inflammatory bowel disease: the gut thickens. Nat Rev Gastroenterol Hepatol. (2022) 19:169–84. doi: 10.1038/s41575-021-00543-0
11. Kałużna, A, Olczyk, P, and Komosińska-Vassev, K. The role of innate and adaptive immune cells in the pathogenesis and development of the inflammatory response in ulcerative colitis. J Clin Med. (2022) 11:400. doi: 10.3390/jcm11020400
12. Nabavi-Rad, A, Sadeghi, A, AsadzadehAghdaei, H, Yadegar, A, Smith, SM, and Zali, MR. The double-edged sword of probiotic supplementation on gut microbiota structure in Helicobacter pylori management. Gut Microbes. (2022) 14:2108655. doi: 10.1080/19490976.2022.2108655
13. Noviello, D, Mager, R, Roda, G, Borroni, RG, Fiorino, G, and Vetrano, S. The IL23-IL17 immune Axis in the treatment of ulcerative colitis: successes, defeats, and ongoing challenges. Front Immunol. (2021) 12:611256. doi: 10.3389/fimmu.2021.611256
14. Shahini, A, and Shahini, A. Role of interleukin-6-mediated inflammation in the pathogenesis of inflammatory bowel disease: focus on the available therapeutic approaches and gut microbiome. J Cell Commun Signal. (2022) 17:55–74. doi: 10.1007/s12079-022-00695-x
15. Nakase, H, Sato, N, Mizuno, N, and Ikawa, Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun Rev. (2022) 21:103017. doi: 10.1016/j.autrev.2021.103017
16. Saez, A, Gomez-Bris, R, Herrero-Fernandez, B, Mingorance, C, Rius, C, and Gonzalez-Granado, JM. Innate lymphoid cells in intestinal homeostasis and inflammatory bowel disease. Int J Mol Sci. (2021) 22:7618. doi: 10.3390/ijms22147618
17. Hirten, RP, and Sands, BE. New therapeutics for ulcerative colitis. Annu Rev Med. (2021) 72:199–213. doi: 10.1146/annurev-med-052919-120048
18. López-Sanromán, A, Esplugues, JV, and Domènech, E. Pharmacology and safety of tofacitinib in ulcerative colitis. Gastroenterol Hepatol. (2021) 44:39–48. doi: 10.1016/j.gastrohep.2020.04.012
19. Alsoud, D, Verstockt, B, Fiocchi, C, and Vermeire, S. Breaking the therapeutic ceiling in drug development in ulcerative colitis. Lancet Gastroenterol Hepatol. (2021) 6:589–95. doi: 10.1016/S2468-1253(21)00065-0
20. Chang, Y, Zhai, L, Peng, J, Wu, H, Bian, Z, and Xiao, H. Phytochemicals as regulators of Th17/Treg balance in inflammatory bowel diseases. Biomed Pharmacother. (2021) 141:111931. doi: 10.1016/j.biopha.2021.111931
21. Xie, F, Xiong, Q, Li, Y, Yao, C, Wu, R, Wang, Q, et al. Traditional Chinese medicine regulates Th17/Treg balance in treating inflammatory bowel disease. Evid Based Complement Alternat Med. (2022) 2022:6275136–13. doi: 10.1155/2022/6275136
22. Dongsheng, W, Hui, C, Zhang, Y, Zihan, L, and Yi, L. Shaoyao decoction treats ulcerative colitis by inhibiting HIF-1α and regulating Th17/Treg balance. Chin J Exp Formulas. (2021) 27:9–15. doi: 10.13422/j.cnki.syfjx.20211503
23. Wu, D, Hui, C, Yu, Z, Bo, Z, Qihua, H, Nianjia, X, et al. Shaoyaotang alleviates ulcerative colitis by regulating th17/treg balance through il-6/stat3 signaling pathway. Chin J Exp Formulas. (2022) 29:1–9. doi: 10.13422/j.cnki.syfjx.20221602
24. Zhou, B, Yuan, Y, Zhang, S, Guo, C, Li, X, Li, G, et al. Intestinal Flora and Disease mutually shape the regional immune system in the intestinal tract. Front Immunol. (2020) 11:575. doi: 10.3389/fimmu.2020.00575
25. Wan, Y, Yang, L, Jiang, S, Qian, D, and Duan, J. Excessive apoptosis in ulcerative colitis: crosstalk between apoptosis, ROS, ER stress, and intestinal homeostasis. Inflamm Bowel Dis. (2022) 28:639–48. doi: 10.1093/ibd/izab277
26. Jakubczyk, D, Leszczyńska, K, and Górska, S. The effectiveness of probiotics in the treatment of inflammatory bowel disease (IBD)-a critical review. Nutrients. (2020) 12:1973. doi: 10.3390/nu12071973
27. Nascimento, RPD, Machado, APDF, Galvez, J, Cazarin, CBB, and Maróstica, M. Ulcerative colitis: Gut microbiota, immunopathogenesis and application of natural products in animal models. Life Sci. (2020) 258:118129. doi: 10.1016/j.lfs.2020.118129
28. Tavakoli, P, Vollmer-Conna, U, Hadzi-Pavlovic, D, and Grimm, MC. A review of inflammatory bowel disease: a model of microbial, immune and neuropsychological integration. Public Health Rev. (2021) 42:1603990. doi: 10.3389/phrs.2021.1603990
29. Candelli, M, Franza, L, Pignataro, G, Ojetti, V, Covino, M, Piccioni, A, et al. Interaction between lipopolysaccharide and Gut microbiota in inflammatory bowel diseases. Int J Mol Sci. (2021) 22:6242. doi: 10.3390/ijms22126242
30. Hart, L, Verburgt, CM, Wine, E, Zachos, M, Poppen, A, Chavannes, M, et al. Nutritional therapies and their influence on the intestinal microbiome in pediatric inflammatory bowel disease. Nutrients. (2021) 14:4. doi: 10.3390/nu14010004
31. Oka, A, and Sartor, RB. Microbial-based and microbial-targeted therapies for inflammatory bowel diseases. Dig Dis Sci. (2020) 65:757–88. doi: 10.1007/s10620-020-06090-z
32. Niu, W, Chen, X, Xu, R, Dong, H, Yang, F, Wang, Y, et al. Polysaccharides from natural resources exhibit great potential in the treatment of ulcerative colitis: a review. Carbohydr Polym. (2021) 254:117189. doi: 10.1016/j.carbpol.2020.117189
33. Shan, Y, Lee, M, and Chang, EB. The Gut microbiome and inflammatory bowel diseases. Annu Rev Med. (2022) 73:455–68. doi: 10.1146/annurev-med-042320-021020
34. Caruso, R, Lo, BC, and Núñez, G. Host-microbiota interactions in inflammatory bowel disease. Nat Rev Immunol. (2020) 20:411–26. doi: 10.1038/s41577-019-0268-7
35. Schirmer, M, Garner, A, Vlamakis, H, and Xavier, RJ. Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol. (2019) 17:497–511. doi: 10.1038/s41579-019-0213-6
36. Uchiyama, K, Naito, Y, and Takagi, T. Intestinal microbiome as a novel therapeutic target for local and systemic inflammation. Pharmacol Ther. (2019) 199:164–72. doi: 10.1016/j.pharmthera.2019.03.006
37. Jacobse, J, Li, J, Rings, EHHM, Samsom, JN, and Goettel, JA. Intestinal regulatory T cells as specialized tissue-restricted immune cells in intestinal immune homeostasis and disease. Front Immunol. (2021) 12:716499. doi: 10.3389/fimmu.2021.716499
38. Cassinotti, A, Passamonti, F, and Segato, S. Cell therapy in inflammatory bowel disease. Pharmacol Res. (2021) 163:105247. doi: 10.1016/j.phrs.2020.105247
39. Xu, Z, Wei, C, Zhang, RU, Yao, J, Zhang, D, and Wang, L. Epigallocatechin-3-gallate-induced inhibition of interleukin-6 release and adjustment of the regulatory T/T helper 17 cell balance in the treatment of colitis in mice. Exp Ther Med. (2015) 10:2231–8. doi: 10.3892/etm.2015.2824
40. Negi, S, Saini, S, Tandel, N, Sahu, K, Mishra, RPN, and Tyagi, RK. Translating Treg therapy for inflammatory bowel disease in humanized mice. Cells. (2021) 10:1847. doi: 10.3390/cells10081847
41. Elshal, MF, Aldahlawi, AM, Saadah, OI, and McCoy, JP. Reduced dendritic cells expressing CD200R1 in children with inflammatory bowel disease: correlation with Th17 and regulatory T cells. Int J Mol Sci. (2015) 16:28998–9010. doi: 10.3390/ijms161226143
42. Heo, YJ, Joo, YB, Oh, HJ, Park, MK, Heo, YM, Cho, ML, et al. IL-10 suppresses Th17 cells and promotes regulatory T cells in the CD4+ T cell population of rheumatoid arthritis patients. Immunol Lett. (2010) 127:150–6. doi: 10.1016/j.imlet.2009.10.006
43. Zhang, D, Wei, C, Yao, J, Cai, X, and Wang, L. Interleukin-10 gene-carrying bifidobacteria ameliorate murine ulcerative colitis by regulating regulatory T cell/T helper 17 cell pathway. Exp Biol Med (Maywood). (2015) 240:1622–9. doi: 10.1177/1535370215584901
44. Tao, M, Wang, X, Wang, A, Gao, N, Pan, J, Liu, X, et al. Effect of jiaweiwumel decoction on regulatory T cells and interleukin-10 in a rat model of ulcerative colitis. J Tradit Chin Med. (2015) 35:312–5. doi: 10.1016/s0254-6272(15)30103-5
45. Principe, DR, DeCant, B, Staudacher, J, Vitello, D, Mangan, RJ, Wayne, EA, et al. Loss of TGFβ signaling promotes colon cancer progression and tumor-associated inflammation. Oncotarget. (2017) 8:3826–39. doi: 10.18632/oncotarget.9830
46. Xu, X, Xu, C, Saud, SM, Lu, X, Liu, L, Fang, L, et al. Effect of Kuijie granule on the expression of TGF-β/Smads signaling pathway in patients with ulcerative colitis. Evid Based Complement Alternat Med. (2016) 2016:2601830. doi: 10.1155/2016/2601830
47. Iboshi, Y, Nakamura, K, Fukaura, K, Iwasa, T, Ogino, H, Sumida, Y, et al. Increased IL-17A/IL-17F expression ratio represents the key mucosal T helper/regulatory cell-related gene signature paralleling disease activity in ulcerative colitis. J Gastroenterol. (2017) 52:315–26. doi: 10.1007/s00535-016-1221-1
48. Gomez-Bris, R, Saez, A, Herrero-Fernandez, B, Rius, C, Sanchez-Martinez, H, and Gonzalez-Granado, JM. CD4 T-cell subsets and the pathophysiology of inflammatory bowel disease. Int J Mol Sci. (2023) 24:2696. doi: 10.3390/ijms24032696
49. Jiang, P, Zheng, C, Xiang, Y, Malik, S, Su, D, Xu, G, et al. The involvement of TH17 cells in the pathogenesis of IBD. Cytokine Growth Factor Rev. (2023) 69:28–42. doi: 10.1016/j.cytogfr.2022.07.005
50. Hisamatsu, T, Erben, U, and Kühl, AA. The role of T-cell subsets in chronic inflammation in celiac disease and inflammatory bowel disease patients: more common mechanisms or more differences? Inflamm Intest Dis. (2016) 1:52–62. doi: 10.1159/000445133
51. Fu, SH, Chien, MW, Hsu, CY, Liu, YW, and Sytwu, HK. Interplay between cytokine circuitry and transcriptional regulation shaping helper T cell pathogenicity and plasticity in inflammatory bowel disease. Int J Mol Sci. (2020) 21:3379. doi: 10.3390/ijms21093379
52. Akitsu, A, and Iwakura, Y. Interleukin-17-producing γδ T (γδ17) cells in inflammatory diseases. Immunology. (2018) 155:418–26. doi: 10.1111/imm.12993
53. Tindemans, I, Joosse, ME, and Samsom, JN. Dissecting the heterogeneity in T-cell mediated inflammation in IBD. Cells. (2020) 9:110. doi: 10.3390/cells9010110
54. Ma, HQ, Zhao, SJ, and Zhang, HJ. Alterations of serum inflammatory activity index, Th17/Treg cells in patients with ulcerative colitis and their clinical significance. J Gastroenterol Hepatol. (2018) 27:885–90. doi: 10.3969/j.issn.1006-5709.2018.08.011
55. Luo, A, Leach, ST, Barres, R, Hesson, LB, Grimm, MC, and Simar, D. The microbiota and epigenetic regulation of T helper 17/regulatory T cells: in search of a balanced immune system. Front Immunol. (2017) 8:417. doi: 10.3389/fimmu.2017.00417
56. Rahimi, K, Ahmadi, A, Hassanzadeh, K, Soleimani, Z, Sathyapalan, T, Mohammadi, A, et al. Targeting the balance of T helper cell responses by curcumin in inflammatory and autoimmune states. Autoimmun Rev. (2019) 18:738–48. doi: 10.1016/j.autrev.2019.05.012
57. Philips, RL, Wang, Y, Cheon, H, Kanno, Y, Gadina, M, Sartorelli, V, et al. The JAK-STAT pathway at 30: much learned, much more to do. Cell. (2022) 185:3857–76. doi: 10.1016/j.cell.2022.09.023
58. Nishimura, R, Hata, K, Takahata, Y, Murakami, T, Nakamura, E, Ohkawa, M, et al. Role of signal transduction pathways and transcription factors in cartilage and joint diseases. Int J Mol Sci. (2020) 21:1340. doi: 10.3390/ijms21041340
59. Traidl, S, Freimooser, S, and Werfel, T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. (2021) 5:293–304. doi: 10.5414/ALX02272E
60. Harris, C, and Cummings, JRF. JAK1 inhibition and inflammatory bowel disease. Rheumatology (Oxford). (2021) 60:ii45–51. doi: 10.1093/rheumatology/keaa896
61. McLornan, DP, Pope, JE, Gotlib, J, and Harrison, CN. Current and future status of JAK inhibitors. Lancet. (2021) 398:803–16. doi: 10.1016/S0140-6736(21)00438-4
62. Li, Y, de Haar, C, Peppelenbosch, MP, and van der Woude, CJ. New insights into the role of STAT3 in IBD. Inflamm Bowel Dis. (2012) 18:1177–83. doi: 10.1002/ibd.21884
63. Salas, A, Hernandez-Rocha, C, Duijvestein, M, Faubion, W, McGovern, D, Vermeire, S, et al. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. (2020) 17:323–37. doi: 10.1038/s41575-020-0273-0
64. Lu, Y, Li, X, Liu, S, Zhang, Y, and Zhang, D. Toll-like receptors and inflammatory bowel disease. Front Immunol. (2018) 9:72. doi: 10.3389/fimmu.2018.00072
65. Wang, FY, and Chi, CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and Gut-skin Axis of Rosacea. Adv Ther. (2021) 38:1415–24. doi: 10.1007/s12325-021-01624-x
66. Dai, W, Long, L, Wang, X, Li, S, and Xu, H. Phytochemicals targeting toll-like receptors 4 (TLR4) in inflammatory bowel disease. Chin Med. (2022) 17:53. doi: 10.1186/s13020-022-00611-w
67. Giuffrida, P, and Di Sabatino, A. Targeting T cells in inflammatory bowel disease. Pharmacol Res. (2020) 159:105040. doi: 10.1016/j.phrs.2020.105040
68. Dejban, P, Nikravangolsefid, N, Chamanara, M, Dehpour, A, and Rashidian, A. The role of medicinal products in the treatment of inflammatory bowel diseases (IBD) through inhibition of TLR4/NF-kappaB pathway. Phytother Res. (2021) 35:835–45. doi: 10.1002/ptr.6866
69. Efferth, T, and Oesch, F. The immunosuppressive activity of artemisinin-type drugs towards inflammatory and autoimmune diseases. Med Res Rev. (2021) 41:3023–61. doi: 10.1002/med.21842
70. Britton, GJ, Contijoch, EJ, Mogno, I, Vennaro, OH, Llewellyn, SR, Ng, R, et al. Microbiotas from humans with inflammatory bowel disease Alter the balance of Gut Th17 and RORγt+ regulatory T cells and exacerbate colitis in mice. Immunity. (2019) 50:212–224.e4. doi: 10.1016/j.immuni.2018.12.015
71. Soukou, S, Brockmann, L, Bedke, T, Gagliani, N, Flavell, RA, and Huber, S. Role of IL-10 receptor signaling in the function of CD4+ T-regulatory type 1 cells: T-cell therapy in patients with inflammatory bowel disease. Crit Rev Immunol. (2018) 38:415–31. doi: 10.1615/CritRevImmunol.2018026850
72. Sanmarco, LM, Chao, CC, Wang, YC, Kenison, JE, Li, Z, Rone, JM, et al. Identification of environmental factors that promote intestinal inflammation. Nature. (2022) 611:801–9. doi: 10.1038/s41586-022-05308-6
73. Zhang, W, Liu, X, Zhu, Y, Liu, X, Gu, Y, Dai, X, et al. Transcriptional and posttranslational regulation of Th17/Treg balance in health and disease. Eur J Immunol. (2021) 51:2137–50. doi: 10.1002/eji.202048794
74. Geier, C, and Perl, A. Therapeutic mTOR blockade in systemic autoimmunity: implications for antiviral immunity and extension of lifespan. Autoimmun Rev. (2021) 20:102984. doi: 10.1016/j.autrev.2021.102984
75. Arab, HH, Al-Shorbagy, MY, and Saad, MA. Activation of autophagy and suppression of apoptosis by dapagliflozin attenuates experimental inflammatory bowel disease in rats: targeting AMPK/mTOR, HMGB1/RAGE and Nrf2/HO-1 pathways. Chem Biol Interact. (2021) 335:109368. doi: 10.1016/j.cbi.2021.109368
76. Chi, X, Jin, W, Bai, X, Zhao, X, Shao, J, Li, J, et al. RORα is critical for mTORC1 activity in T cell-mediated colitis. Cell Rep. (2021) 36:109682. doi: 10.1016/j.celrep.2021.109682
77. Zhou, M, Xu, W, Wang, J, Yan, J, Shi, Y, Zhang, C, et al. Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine. (2018) 35:345–60. doi: 10.1016/j.ebiom.2018.08.035
78. He, F, Wu, C, Li, P, Li, N, Zhang, D, Zhu, Q, et al. Functions and signaling pathways of amino acids in intestinal inflammation. Biomed Res Int. (2018) 2018:9171905–13. doi: 10.1155/2018/9171905
79. Jiang, Q, Huang, X, Yu, W, Huang, R, Zhao, X, and Chen, C. mTOR signaling in the regulation of CD4+ T cell subsets in periodontal diseases. Front Immunol. (2022) 13:827461. doi: 10.3389/fimmu.2022.827461
80. Shao, BZ, Yao, Y, Zhai, JS, Zhu, JH, Li, JP, and Wu, K. The role of autophagy in inflammatory bowel disease. Front Physiol. (2021) 12:621132. doi: 10.3389/fphys.2021.621132
81. Grover, P, Goel, PN, and Greene, MI. Regulatory T cells: regulation of identity and function. Front Immunol. (2021) 12:750542. doi: 10.3389/fimmu.2021.750542
82. Pernomian, L, Duarte-Silva, M, and de Barros Cardoso, CR. The aryl hydrocarbon receptor (AHR) as a potential target for the control of intestinal inflammation: insights from an immune and Bacteria sensor receptor. Clin Rev Allergy Immunol. (2020) 59:382–90. doi: 10.1007/s12016-020-08789-3
83. Esser, C, Rannug, A, and Stockinger, B. The aryl hydrocarbon receptor in immunity. Trends Immunol. (2009) 30:447–54. doi: 10.1016/j.it.2009.06.005
84. Hall, BM. CD4+CD25+ T regulatory cells in transplantation tolerance: 25 years on. Transplantation. (2016) 100:2533–47. doi: 10.1097/TP.0000000000001436
85. Dean, JW, and Zhou, L. Cell-intrinsic view of the aryl hydrocarbon receptor in tumor immunity. Trends Immunol. (2022) 43:245–58. doi: 10.1016/j.it.2022.01.008
86. Habano, W, Miura, T, Terashima, J, and Ozawa, S. Aryl hydrocarbon receptor as a DNA methylation reader in the stress response pathway. Toxicology. (2022) 470:153154. doi: 10.1016/j.tox.2022.153154
87. Collins, SL, Stine, JG, Bisanz, JE, Okafor, CD, and Patterson, AD. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat Rev Microbiol. (2022) 21:236–47. doi: 10.1038/s41579-022-00805-x
88. Sondermann, NC, Faßbender, S, Hartung, F, Hätälä, AM, Rolfes, KM, Vogel, CFA, et al. Functions of the aryl hydrocarbon receptor (AHR) beyond the canonical AHR/ARNT signaling pathway. Biochem Pharmacol. (2023) 208:115371. doi: 10.1016/j.bcp.2022.115371
89. Salminen, A. Aryl hydrocarbon receptor (AhR) reveals evidence of antagonistic pleiotropy in the regulation of the aging process. Cell Mol Life Sci. (2022) 79:489. doi: 10.1007/s00018-022-04520-x
90. Lin, L, Dai, Y, and Xia, Y. An overview of aryl hydrocarbon receptor ligands in the last two decades (2002–2022): a medicinal chemistry perspective. Eur J Med Chem. (2022) 244:114845. doi: 10.1016/j.ejmech.2022.114845
91. Zhang, L, Zhang, Y, Zhong, W, Di, C, Lin, X, and Xia, Z. Heme oxygenase-1 ameliorates dextran sulfate sodium-induced acute murine colitis by regulating Th17/Treg cell balance. J Biol Chem. (2014) 289:26847–58. doi: 10.1074/jbc.M114.590554
92. Vuerich, M, Wang, N, Kalbasi, A, Graham, JJ, and Longhi, MS. Dysfunctional immune regulation in autoimmune hepatitis: from pathogenesis to novel therapies. Front Immunol. (2021) 12:746436. doi: 10.3389/fimmu.2021.746436
93. Bock, KW. Aryl hydrocarbon receptor (AHR) functions: balancing opposing processes including inflammatory reactions. Biochem Pharmacol. (2020) 178:114093. doi: 10.1016/j.bcp.2020.114093
94. Wang, L, Hu, Y, Song, B, Xiong, Y, Wang, J, and Chen, D. Targeting JAK/STAT signaling pathways in treatment of inflammatory bowel disease. Inflamm Res. (2021) 70:753–64. doi: 10.1007/s00011-021-01482-x
95. Chaudhry, A, Rudra, D, Treuting, P, Samstein, RM, Liang, Y, Kas, A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. (2009) 326:986–91. doi: 10.1126/science.1172702
96. Chang, JH, Chuang, HC, Hsiao, G, Hou, TY, Wang, CC, Huang, SC, et al. Acteoside exerts immunomodulatory effects on dendritic cells via aryl hydrocarbon receptor activation and ameliorates Th2-mediated allergic asthma by inducing Foxp3+ regulatory T cells. Int Immunopharmacol. (2022) 106:108603. doi: 10.1016/j.intimp.2022.108603
97. Gandhi, R, Kumar, D, Burns, EJ, Nadeau, M, Dake, B, Laroni, A, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. (2010) 11:846–53. doi: 10.1038/ni.1915
98. McGonagle, D, Watad, A, Sharif, K, and Bridgewood, C. Why inhibition of IL-23 lacked efficacy in ankylosing spondylitis. Front Immunol. (2021) 12:614255. doi: 10.3389/fimmu.2021.614255
99. Hiltensperger, M, and Korn, T. The interleukin (IL)-23/T helper (Th)17 Axis in experimental autoimmune encephalomyelitis and multiple sclerosis. Cold Spring Harb Perspect Med. (2018) 8:a029637. doi: 10.1101/cshperspect.a029637
100. Gasch, M, Goroll, T, Bauer, M, Hinz, D, Schütze, N, Polte, T, et al. Generation of IL-8 and IL-9 producing CD4+ T cells is affected by Th17 polarizing conditions and AHR ligands. Mediat Inflamm. (2014) 2014:182549. doi: 10.1155/2014/182549
101. Li, H, Cao, XY, Dang, WZ, Jiang, B, Zou, J, and Shen, XY. Total glucosides of Paeony protects against collagen-induced mouse arthritis via inhibiting follicular helper T cell differentiation. Phytomedicine. (2019) 65:153091. doi: 10.1016/j.phymed.2019.153091
102. Xiang, J, Hu, R, Li, Q, Zhang, Y, Li, S, Wang, X, et al. Total glucosides of Paeony attenuates ulcerative colitis via inhibiting TLR4/NF-κB signaling pathway. Tohoku J Exp Med. (2022) 258:225–36. doi: 10.1620/tjem.2022.J073
103. Lin, H, Zhang, W, Jiang, X, Chen, R, Huang, X, and Huang, Z. Total glucosides of paeony ameliorates TNBS induced colitis by modulating differentiation of Th17/Treg cells and the secretion of cytokines. Mol Med Rep. (2017) 16:8265–76. doi: 10.3892/mmr.2017.7598
104. Wei, Z, Chunying, Y, and Rong, Z. Paeoniflorin regulates Th17/Treg balance by targeting NF-κB signaling pathway in ulcerative colitis. J Shanxi Med Univ. (2022) 53:192–201. doi: 10.13753/j.issn.1007-6611.2022.02.011
105. Yong, Z, Kai, Z, and Huang, L. Regulatory effect of paeoniflorin on Treg/Th17 balance and oxidative stress response in mice with ulcerative colitis. Drug Eval. (2021) 18:1306–10. doi: 10.19939/j.cnki.1672-2809.2021.21.08
106. Si, X, Wang, Y, Wang, Z, Li, L, and Zhu, X. Effect of Paeoniflorin on DSS-induced IL-17 expression in rats with chronic ulcerative colitis. Chin J Immunol. (2021) 37:946–50. doi: 10.3969/j.issn.1000-484X.2021.08.011
107. Zheng, K, Jia, J, Yan, S, Shen, H, Zhu, P, and Yu, J. Paeoniflorin ameliorates ulcerative colitis by modulating the dendritic cell-mediated TH17/Treg balance. Inflammopharmacology. (2020) 28:1705–16. doi: 10.1007/s10787-020-00722-6
108. Wang, Y, Dan, Y, Yang, D, Hu, Y, Zhang, L, Zhang, C, et al. The genus Anemarrhena Bunge: a review on ethnopharmacology, phytochemistry and pharmacology. J Ethnopharmacol. (2014) 153:42–60. doi: 10.1016/j.jep.2014.02.013
109. Chi, X, Zhang, F, Gao, Q, Xing, R, and Chen, S. A review on the Ethnomedicinal usage, Phytochemistry, and pharmacological properties of Gentianeae (Gentianaceae) in Tibetan medicine. Plants (Basel). (2021) 10:2383. doi: 10.3390/plants10112383
110. Zhou, C, Zhou, J, Han, N, Liu, Z, Xiao, B, and Yin, J. Beneficial effects of neomangiferin on high fat diet-induced nonalcoholic fatty liver disease in rats. Int Immunopharmacol. (2015) 25:218–28. doi: 10.1016/j.intimp.2015.01.027
111. Lei, LY, Wang, RC, Pan, YL, Yue, ZG, Zhou, R, Xie, P, et al. Mangiferin inhibited neuroinflammation through regulating microglial polarization and suppressing NF-κB, NLRP3 pathway. Chin J Nat Med. (2021) 19:112–9. doi: 10.1016/S1875-5364(21)60012-2
112. Chen, L, Li, S, Zhu, J, You, A, Huang, X, Yi, X, et al. Mangiferin prevents myocardial infarction-induced apoptosis and heart failure in mice by activating the Sirt1/FoxO3a pathway. J Cell Mol Med. (2021) 25:2944–55. doi: 10.1111/jcmm.16329
113. Walia, V, Chaudhary, SK, and Kumar, SN. Therapeutic potential of mangiferin in the treatment of various neuropsychiatric and neurodegenerative disorders. Neurochem Int. (2021) 143:104939. doi: 10.1016/j.neuint.2020.104939
114. Qin, L, Han, T, Zhang, Q, Cao, D, Nian, H, Rahman, K, et al. Antiosteoporotic chemical constituents from Er-Xian decoction, a traditional Chinese herbal formula. J Ethnopharmacol. (2008) 118:271–9. doi: 10.1016/j.jep.2008.04.009
115. Lim, SM, Kang, GD, Jeong, JJ, Choi, HS, and Kim, DH. Neomangiferin modulates the Th17/Treg balance and ameliorates colitis in mice. Phytomedicine. (2016) 23:131–40. doi: 10.1016/j.phymed.2016.01.002
116. Jeong, JJ, Jang, SE, Hyam, SR, Han, MJ, and Kim, DH. Mangiferin ameliorates colitis by inhibiting IRAK1 phosphorylation in NF-κB and MAPK pathways. Eur J Pharmacol. (2014) 740:652–61. doi: 10.1016/j.ejphar.2014.06.013
117. Lim, SM, Jeong, JJ, Choi, HS, Chang, HB, and Kim, DH. Mangiferin corrects the imbalance of Th17/Treg cells in mice with TNBS-induced colitis. Int Immunopharmacol. (2016) 34:220–8. doi: 10.1016/j.intimp.2016.03.004
118. Lee, B, Trinh, HT, Jung, K, Han, SJ, and Kim, DH. Inhibitory effects of steroidal timosaponins isolated from the rhizomes of Anemarrhenaasphodeloides against passive cutaneous anaphylaxis reaction and pruritus. Immunopharmacol Immunotoxicol. (2010) 32:357–63. doi: 10.3109/08923970903383889
119. Lim, SM, Jeong, JJ, Kang, GD, Kim, KA, Choi, HS, and Kim, DH. Timosaponin AIII and its metabolite sarsasapogenin ameliorate colitis in mice by inhibiting NF-κB and MAPK activation and restoring Th17/Treg cell balance. Int Immunopharmacol. (2015) 25:493–503. doi: 10.1016/j.intimp.2015.02.016
120. Castellano, JM, Ramos-Romero, S, and Perona, JS. Oleanolic acid: extraction, characterization and biological activity. Nutrients. (2022) 14:623. doi: 10.3390/nu14030623
121. Wang, W, Li, Y, Li, Y, Sun, D, Li, H, and Chen, L. Recent Progress in Oleanolic acid: structural modification and biological activity. Curr Top Med Chem. (2022) 22:3–23. doi: 10.2174/1568026621666211105101231
122. Yang, YH, Dai, SY, Deng, FH, Peng, LH, Li, C, and Pei, YH. Recent advances in medicinal chemistry of oleanolic acid derivatives. Phytochemistry. (2022) 203:113397. doi: 10.1016/j.phytochem.2022.113397
123. Gupta, N. A review on recent developments in the anticancer potential of Oleanolic acid and its analogs (2017-2020). Mini Rev Med Chem. (2022) 22:600–16. doi: 10.2174/1389557521666210810153627
124. Fernández-Aparicio, Á, Correa-Rodríguez, M, Castellano, JM, Schmidt-RioValle, J, Perona, JS, and González-Jiménez, E. Potential molecular targets of Oleanolic acid in insulin resistance and underlying oxidative stress: a systematic review. Antioxidants (Basel). (2022) 11:1517. doi: 10.3390/antiox11081517
125. Kang, GD, Lim, S, and Kim, DH. Oleanolic acid ameliorates dextran sodium sulfate-induced colitis in mice by restoring the balance of Th17/Treg cells and inhibiting NF-κB signaling pathway. Int Immunopharmacol. (2015) 29:393–400. doi: 10.1016/j.intimp.2015.10.024
126. Banskota, AH, Tezuka, Y, Le Tran, Q, and Kadota, S. Chemical constituents and biological activities of Vietnamese medicinal plants. Curr Top Med Chem. (2003) 3:227–48. doi: 10.2174/1568026033392516
127. Thu, VT, Yen, NTH, Tung, NH, Bich, PT, Han, J, and Kim, HK. Majonoside-R2 extracted from Vietnamese ginseng protects H9C2 cells against hypoxia/reoxygenation injury via modulating mitochondrial function and biogenesis. Bioorg Med Chem Lett. (2021) 36:127814. doi: 10.1016/j.bmcl.2021.127814
128. Nguyen, TT, Matsumoto, K, Yamasaki, K, Nguyen, MD, Nguyen, TN, and Watanabe, H. Crude saponin extracted from Vietnamese ginseng and its major constituent majonoside-R2 attenuate the psychological stress- and foot-shock stress-induced antinociception in mice. Pharmacol Biochem Behav. (1995) 52:427–32. doi: 10.1016/0091-3057(95)00133-h
129. Lee, SY, Jeong, JJ, Le, TH, Eun, SH, Nguyen, MD, Park, JH, et al. Ocotillol, a Majonoside R2 metabolite, ameliorates 2,4,6-Trinitrobenzenesulfonic acid-induced colitis in mice by restoring the balance of Th17/Treg cells. J Agric Food Chem. (2015) 63:7024–31. doi: 10.1021/acs.jafc.5b02183
130. Chandrika, UG, and Prasad Kumarab, PA. Gotu Kola (Centella asiatica): nutritional properties and plausible health benefits. Adv Food Nutr Res. (2015) 76:125–57. doi: 10.1016/bs.afnr.2015.08.001
131. James, JT, and Dubery, IA. Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) urban. Molecules. (2009) 14:3922–41. doi: 10.3390/molecules14103922
132. Azerad, R. Chemical structures, production and enzymatic transformations of sapogenins and saponins from Centella asiatica (L.) urban. Fitoterapia. (2016) 114:168–87. doi: 10.1016/j.fitote.2016.07.011
133. Wang, T, Wei, Z, Dou, Y, Yang, Y, Leng, D, Kong, L, et al. Intestinal interleukin-10 mobilization as a contributor to the anti-arthritis effect of orally administered madecassoside: a unique action mode of saponin compounds with poor bioavailability. Biochem Pharmacol. (2015) 94:30–8. doi: 10.1016/j.bcp.2015.01.004
134. Xu, X, Wang, Y, Wei, Z, Wei, W, Zhao, P, Tong, B, et al. Madecassic acid, the contributor to the anti-colitis effect of madecassoside, enhances the shift of Th17 toward Treg cells via the PPARγ/AMPK/ACC1 pathway. Cell Death Dis. (2017) 8:e2723. doi: 10.1038/cddis.2017.150
135. Zeng, L, Yang, T, Yang, K, Yu, G, Li, J, Xiang, W, et al. Curcumin and Curcuma longa extract in the treatment of 10 types of autoimmune diseases: a systematic review and Meta-analysis of 31 randomized controlled trials. Front Immunol. (2022) 13:896476. doi: 10.3389/fimmu.2022.896476
136. Billerey-Larmonier, C, Uno, JK, Larmonier, N, Midura, AJ, Timmermann, B, Ghishan, FK, et al. Protective effects of dietary curcumin in mouse model of chemically induced colitis are strain dependent. Inflamm Bowel Dis. (2008) 14:780–93. doi: 10.1002/ibd.20348
137. Yao, J, Wang, JY, Lai, MG, Li, YX, Zhu, HM, Shi, RY, et al. Treatment of mice with dextran sulfate sodium-induced colitis with human interleukin 10 secreted by transformed Bifidobacterium longum. Mol Pharm. (2011) 8:488–97. doi: 10.1021/mp100331r
138. Arafa, HM, Hemeida, RA, El-Bahrawy, AI, and Hamada, FM. Prophylactic role of curcumin in dextran sulfate sodium (DSS)-induced ulcerative colitis murine model. Food Chem Toxicol. (2009) 47:1311–7. doi: 10.1016/j.fct.2009.03.003
139. Shi, H, Wang, D, Chen, W, Li, Y, Si, G, and Yang, T. Quality of evidence supporting the role of supplement curcumin for the treatment of ulcerative colitis: An overview of systematic reviews. Gastroenterol Res Pract. (2022) 2022:3967935–13. doi: 10.1155/2022/3967935
140. Wei, C, Wang, JY, Xiong, F, Wu, BH, Luo, MH, Yu, ZC, et al. Curcumin ameliorates DSS induced colitis in mice by regulating the Treg/Th17 signaling pathway. Mol Med Rep. (2021) 23:34. doi: 10.3892/mmr.2020.11672
141. Liu, P, Xiao, Z, Yan, H, Lu, X, Zhang, X, Luo, L, et al. Baicalin suppresses Th1 and Th17 responses and promotes Treg response to ameliorate sepsis-associated pancreatic injury via the RhoA-ROCK pathway. Int Immunopharmacol. (2020) 86:106685. doi: 10.1016/j.intimp.2020.106685
142. Zou, Y, Dai, SX, Chi, HG, Li, T, He, ZW, Wang, J, et al. Baicalin attenuates TNBS-induced colitis in rats by modulating the Th17/Treg paradigm. Arch Pharm Res. (2015) 38:1873–87. doi: 10.1007/s12272-014-0486-2
143. Honda, H, Nagai, Y, Matsunaga, T, Okamoto, N, Watanabe, Y, Tsuneyama, K, et al. Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. J Leukoc Biol. (2014) 96:1087–100. doi: 10.1189/jlb.3A0114-005RR
144. Honda, H, Nagai, Y, Matsunaga, T, Saitoh, S, Akashi-Takamura, S, Hayashi, H, et al. Glycyrrhizin and isoliquiritigenin suppress the LPS sensor toll-like receptor 4/MD-2 complex signaling in a different manner. J Leukoc Biol. (2012) 91:967–76. doi: 10.1189/jlb.0112038
145. Zhang, Z, Yung, KK, and Ko, JK. Therapeutic intervention in Cancer by Isoliquiritigeninfrom licorice: a natural antioxidant and redox regulator. Antioxidants (Basel). (2022) 11:1349. doi: 10.3390/antiox11071349
146. Akamatsu, H, Komura, J, Asada, Y, and Niwa, Y. Mechanism of anti-inflammatory action of glycyrrhizin: effect on neutrophil functions including reactive oxygen species generation. Planta Med. (1991) 57:119–21. doi: 10.1055/s-2006-960045
147. Guo, A, He, D, Xu, HB, Geng, CA, and Zhao, J. Promotion of regulatory T cell induction by immunomodulatory herbal medicine licorice and its two constituents. Sci Rep. (2015) 5:14046. doi: 10.1038/srep14046
148. Cheng, Z, Kang, C, Che, S, Su, J, Sun, Q, Ge, T, et al. Berberine: a promising treatment for neurodegenerative diseases. Front Pharmacol. (2022) 13:845591. doi: 10.3389/fphar.2022.845591
149. Cui, H, Cai, Y, Wang, L, Jia, B, Li, J, Zhao, S, et al. Berberine regulates Treg/Th17 balance to treat ulcerative colitis through modulating the Gut microbiota in the Colon. Front Pharmacol. (2018) 9:571. doi: 10.3389/fphar.2018.00571
150. Tang, M. Research on the molecular mechanism of berberine regulating miR-31-5p-Th17/Treg immune network in the treatment of ulcerative colitis. Shaanxi University of Traditional Chinese Medicine (2019).
151. Ren, Y, Li, Y, Lv, J, Guo, X, Zhang, J, Zhou, D, et al. Parthenolide regulates oxidative stress-induced mitophagy and suppresses apoptosis through p53 signaling pathway in C2C12 myoblasts. J Cell Biochem. (2019) 120:15695–708. doi: 10.1002/jcb.28839
152. Liu, YJ, Tang, B, Wang, FC, Tang, L, Lei, YY, Luo, Y, et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics. (2020) 10:5225–41. doi: 10.7150/thno.43716
153. Jin, Y, Zhan, X, Zhang, B, Chen, Y, Liu, C, and Yu, L. Polydatin exerts an antitumor effect through regulating the miR-382/PD-L1 Axis in colorectal Cancer. Cancer Biother Radiopharm. (2020) 35:83–91. doi: 10.1089/cbr.2019.2999
154. Liu, YJ, Xu, WH, Fan, LM, Zhang, YQ, Xu, W, Chen, YP, et al. Polydatin alleviates DSS- and TNBS-induced colitis by suppressing Th17 cell differentiation via directly inhibiting STAT3. Phytother Res. (2022) 36:3662–71. doi: 10.1002/ptr.7533
155. Yao, J, Wang, JY, Liu, L, Li, YX, Xun, AY, Zeng, WS, et al. Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch Med Res. (2010) 41:288–94. doi: 10.1016/j.arcmed.2010.05.002
156. Grzeczka, A, and Kordowitzki, P. Resveratrol and SIRT1: antiaging cornerstones for oocytes? Nutrients. (2020) 14:5101. doi: 10.3390/nu14235101
157. Singh, UP, Singh, NP, Singh, B, Hofseth, LJ, Taub, DD, Price, RL, et al. Role of resveratrol-induced CD11b(+) gr-1(+) myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3(+) T cells and amelioration of chronic colitis in IL-10(−/−) mice. Brain Behav Immun. (2012) 26:72–82. doi: 10.1016/j.bbi.2011.07.236
158. Shi, Y, Zhou, J, Jiang, B, and Miao, M. Resveratrol and inflammatory bowel disease. Ann N Y Acad Sci. (2017) 1403:38–47. doi: 10.1111/nyas.13426
159. Gowd, V, Kanika,, Jori, C, Chaudhary, A, Rudayni, H, Rashid, S, et al. Resveratrol and resveratrol nano-delivery systems in the treatment of inflammatory bowel disease. J Nutr Biochem. (2022) 109:109101. doi: 10.1016/j.jnutbio.2022.109101
160. Qiuping, G, Fangqing, Z, Jinjin, L, and XieJunfeng, TJ. Experimental study of resveratrol regulating Treg/Th17 and inhibiting intestinal mucosal inflammation in ulcerative colitis. Drug. Evaluation. (2021) 18:591–3. doi: 10.19939/j.cnki.1672-2809.2021.10.05
161. Sun, Y, Liu, S, Yang, S, Chen, C, Yang, Y, Lin, M, et al. Mechanism of Dihydromyricetin on inflammatory diseases. Front Pharmacol. (2022) 12:794563. doi: 10.3389/fphar.2021.794563
162. Li, P. Li P. Effects of Dihydromyricetin on Treg/Th17 balance in peripheral blood and MMP9 expression in intestinal tissue of mice with ulcerative colitis. Lanzhou University (2017).
163. Yu, W, Wang, H, Ying, H, Yu, Y, Chen, D, Ge, W, et al. Daphnetin attenuates microglial activation and proinflammatory factor production via multiple signaling pathways. Int Immunopharmacol. (2014) 21:1–9. doi: 10.1016/j.intimp.2014.04.005
164. Wang, D, Lu, Z, Zhang, H, Jin, SF, Yang, H, Li, YM, et al. Daphnetin alleviates experimental autoimmune encephalomyelitis via regulating dendritic cell activity. CNS Neurosci Ther. (2016) 22:558–67. doi: 10.1111/cns.12537
165. Witaicenis, A, Seito, LN, da Silveira, CA, de Almeida, LD, Jr, LAC, Rodrigues-Orsi, P, et al. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine. (2014) 21:240–6. doi: 10.1016/j.phymed.2013.09.001
166. Yao, R, Fu, Y, Li, S, Tu, L, Zeng, X, and Kuang, N. Regulatory effect of daphnetin, a coumarin extracted from Daphne odora, on the balance of Treg and Th17 in collagen-induced arthritis. Eur J Pharmacol. (2011) 670:286–94. doi: 10.1016/j.ejphar.2011.08.019
167. Tu, L, Li, S, Fu, Y, Yao, R, Zhang, Z, Yang, S, et al. The therapeutic effects of daphnetin in collagen-induced arthritis involve its regulation of Th17 cells. Int Immunopharmacol. (2012) 13:417–23. doi: 10.1016/j.intimp.2012.04.001
168. Ji, J, Ge, X, Chen, Y, Zhu, B, Wu, Q, Zhang, J, et al. Daphnetin ameliorates experimental colitis by modulating microbiota composition and T/T17 balance. FASEB J. (2019) 33:9308–22. doi: 10.1096/fj.201802659RR
169. Bakrim, S, Benkhaira, N, Bourais, I, Benali, T, Lee, LH, El Omari, N, et al. Health benefits and pharmacological properties of Stigmasterol. Antioxidants (Basel). (2022) 11:1912. doi: 10.3390/antiox11101912
170. Kangsamaksin, T, Chaithongyot, S, Wootthichairangsan, C, Hanchaina, R, Tangshewinsirikul, C, and Svasti, J. Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-α. PLoS One. (2017) 12:e0189628. doi: 10.1371/journal.pone.0189628
171. Zhang, B, Liu, K, Yang, H, Jin, Z, Ding, Q, and Zhao, L. Gut microbiota: the potential key target of TCM's therapeutic effect of treating different diseases using the same method-UC and T2DM as examples. Front Cell Infect Microbiol. (2022) 12:855075. doi: 10.3389/fcimb.2022.855075
172. Wen, S, Zhong, Z, He, L, Zhao, D, Chen, X, Mi, H, et al. Network pharmacology dissection of multiscale mechanisms for jiaoqi powder in treating ulcerative colitis. J Ethnopharmacol. (2021) 275:114109. doi: 10.1016/j.jep.2021.114109
173. Wen, S, He, L, Zhong, Z, Zhao, R, Weng, S, Mi, H, et al. Stigmasterol restores the balance of Treg/Th17 cells by activating the butyrate-PPARγ Axis in colitis. Front Immunol. (2021) 12:741934. doi: 10.3389/fimmu.2021.741934
174. Wang, Y, Liu, XJ, Chen, JB, Cao, JP, Li, X, and Sun, CD. Citrus flavonoids and their antioxidant evaluation. Crit Rev Food Sci Nutr. (2022) 62:3833–54. doi: 10.1080/10408398.2020.1870035
175. Alam, F, Mohammadin, K, Shafique, Z, Amjad, ST, and Asad, MHHB. Citrus flavonoids as potential therapeutic agents: a review. Phytother Res. (2022) 36:1417–41. doi: 10.1002/ptr.7261
176. Goh, JXH, Tan, LT, Goh, JK, Chan, KG, Pusparajah, P, Lee, LH, et al. Nobiletin and derivatives: functional compounds from Citrus fruit Peel for Colon Cancer chemoprevention. Cancers (Basel). (2019) 11:867. doi: 10.3390/cancers11060867
177. Yasuda, N, Ishii, T, Oyama, D, Fukuta, T, Agato, Y, Sato, A, et al. Neuroprotective effect of nobiletin on cerebral ischemia-reperfusion injury in transient middle cerebral artery-occluded rats. Brain Res. (2014) 1559:46–54. doi: 10.1016/j.brainres.2014.02.007
178. Wu, Y, Cheng, CS, Li, Q, Chen, JX, Lv, LL, Xu, JY, et al. The application of Citrus folium in breast Cancer and the mechanism of its Main component Nobiletin: a systematic review. Evid Based Complement Alternat Med. (2021) 2021:2847466–15. doi: 10.1155/2021/2847466
179. NebaAmbe, GNN, Breda, C, Bhambra, AS, and Arroo, RRJ. Effect of the Citrus flavone Nobiletin on circadian rhythms and metabolic syndrome. Molecules. (2022) 27:7727. doi: 10.3390/molecules27227727
180. Feng, D, Fang, Z, and Zhang, P. The melanin inhibitory effect of plants and phytochemicals: a systematic review. Phytomedicine. (2022) 107:154449. doi: 10.1016/j.phymed.2022.154449
181. Herfarth, H, and Vavricka, SR. 5-Aminosalicylic acid chemoprevention in inflammatory bowel diseases: is it necessary in the age of biologics and small molecules? Inflamm Intest Dis. (2021) 7:28–35. doi: 10.1159/000518865
182. Eun, SH, Woo, JT, and Kim, DH. Tangeretin inhibits IL-12 expression and NF-κB activation in dendritic cells and attenuates colitis in mice. Planta Med. (2017) 83:527–33. doi: 10.1055/s-0042-119074
183. Zhang, G, Jiang, C, Xie, N, Xu, Y, Liu, L, and Liu, N. Treatment with andrographolide sulfonate provides additional benefits to imipenem in a mouse model of Klebsiella pneumoniae pneumonia. Biomed Pharmacother. (2019) 117:109065. doi: 10.1016/j.biopha.2019.109065
184. Guo, W, Liu, W, Chen, G, Hong, S, Qian, C, Xie, N, et al. Water-soluble andrographolide sulfonate exerts anti-sepsis action in mice through down-regulating p38 MAPK, STAT3 and NF-kappaB pathways. Int Immunopharmacol. (2012) 14:613–9. doi: 10.1016/j.intimp.2012.09.002
185. Guo, W, Sun, Y, Liu, W, Wu, X, Guo, L, Cai, P, et al. Small molecule-driven mitophagy-mediated NLRP3 inflammasome inhibition is responsible for the prevention of colitis-associated cancer. Autophagy. (2014) 10:972–85. doi: 10.4161/auto.28374
186. Geng, J, Liu, J, Yuan, X, Liu, W, and Guo, W. Andrographolide triggers autophagy-mediated inflammation inhibition and attenuates chronic unpredictable mild stress (CUMS)-induced depressive-like behavior in mice. Toxicol Appl Pharmacol. (2019) 379:114688. doi: 10.1016/j.taap.2019.114688
187. Liu, W, Guo, W, Guo, L, Gu, Y, Cai, P, Xie, N, et al. Andrographolide sulfonate ameliorates experimental colitis in mice by inhibiting Th1/Th17 response. Int Immunopharmacol. (2014) 20:337–45. doi: 10.1016/j.intimp.2014.03.015
188. Zaman, Q, Zhang, D, Reddy, OS, Wong, WT, and Lai, WF. Roles and mechanisms of Astragaloside IV in combating neuronal aging. Aging Dis. (2022) 13:1845–61. doi: 10.14336/AD.2022.0126
189. Jiang, XG, Sun, K, Liu, YY, Yan, L, Wang, MX, Fan, JY, et al. Astragaloside IV ameliorates 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis implicating regulation of energy metabolism. Sci Rep. (2017) 7:41832. doi: 10.1038/srep41832
190. Tian, L, Zhao, JL, Kang, JQ, Guo, SB, Zhang, N, Shang, L, et al. Astragaloside IV alleviates the experimental DSS-induced colitis by remodeling macrophage polarization through STAT signaling. Front Immunol. (2021) 12:740565. doi: 10.3389/fimmu.2021.740565
191. Liu, Y. Research on the effect and mechanism of Astragaloside IV regulating the differentiation of CD4+T cells in treating EAE. Shanghai University of Traditional Chinese Medicine (2020).
192. Zhong, Y, Liu, W, Xiong, Y, Li, Y, Wan, Q, Zhou, W, et al. Astragaloside IV alleviates ulcerative colitis by regulating the balance of Th17/Treg cells. Phytomedicine. (2022) 104:154287. doi: 10.1016/j.phymed.2022.154287
193. Shengyan, X, Xiangdang, H, Zongliang, Y, An, L, Yongheng, H, and Haiyan, L. Study on the anti-inflammatory effect of astragaloside IV on colitis mice and the effect on the proportion of peripheral blood Th17 cells. China J Tradit Chin Med. (2022) 47:469–75. doi: 10.19540/j.cnki.cjcmm.20210827.401
194. Zheng, W, Huang, T, Tang, QZ, Li, S, Qin, J, and Chen, F. Astragalus polysaccharide reduces blood pressure, renal damage, and dysfunction through the TGF-β1-ILK pathway. Front Pharmacol. (2021) 12:706617. doi: 10.3389/fphar.2021.706617
195. Zhou, L, Liu, Z, Wang, Z, Yu, S, Long, T, Zhou, X, et al. Astragalus polysaccharides exerts immunomodulatory effects via TLR4-mediated MyD88-dependent signaling pathway in vitro and in vivo. Sci Rep. (2017) 7:44822. doi: 10.1038/srep44822
196. Chen, Y, Wang, J, Li, J, Zhu, J, Wang, R, Xi, Q, et al. Astragalus polysaccharide prevents ferroptosis in a murine model of experimental colitis and human Caco-2 cells via inhibiting NRF2/HO-1 pathway. Eur J Pharmacol. (2021) 911:174518. doi: 10.1016/j.ejphar.2021.174518
197. Lv, J, Zhang, Y, Tian, Z, Liu, F, Shi, Y, Liu, Y, et al. Astragalus polysaccharides protect against dextran sulfate sodium-induced colitis by inhibiting NF-κВ activation. Int J Biol Macromol. (2017) 98:723–9. doi: 10.1016/j.ijbiomac.2017.02.024
198. Zhong, Y, Xiao, Q, Kang, Z, Huang, J, Ge, W, Wan, Q, et al. Astragalus polysaccharide alleviates ulcerative colitis by regulating the balance of Tfh/Treg cells. Int Immunopharmacol. (2022) 111:109108. doi: 10.1016/j.intimp.2022.109108
199. Zhang, J, Fan, F, Liu, A, Zhang, C, Li, Q, Zhang, C, et al. Icariin: a potential molecule for treatment of knee osteoarthritis. Front Pharmacol. (2022) 13:811808. doi: 10.3389/fphar.2022.811808
200. Bi, Z, Zhang, W, and Yan, X. Anti-inflammatory and immunoregulatory effects of icariin and icaritin. Biomed Pharmacother. (2022) 151:113180. doi: 10.1016/j.biopha.2022.113180
201. Zeng, Y, Xiong, Y, Yang, T, Wang, Y, Zeng, J, Zhou, S, et al. Icariin and its metabolites as potential protective phytochemicals against cardiovascular disease: from effects to molecular mechanisms. Biomed Pharmacother. (2022) 147:112642. doi: 10.1016/j.biopha.2022.112642
202. Tao, F, Qian, C, Guo, W, Luo, Q, Xu, Q, and Sun, Y. Inhibition of Th1/Th17 responses via suppression of STAT1 and STAT3 activation contributes to the amelioration of murine experimental colitis by a natural flavonoid glucoside icariin. Biochem Pharmacol. (2013) 85:798–807. doi: 10.1016/j.bcp.2012.12.002
203. Mokra, D, Adamcakova, J, and Mokry, J. Green tea polyphenol (−)-Epigallocatechin-3-Gallate (EGCG): a time for a new player in the treatment of respiratory diseases? Antioxidants (Basel). (2022) 11:1566. doi: 10.3390/antiox11081566
204. Farabegoli, F, and Pinheiro, M. Epigallocatechin-3-Gallate delivery in lipid-based nanoparticles: potentiality and perspectives for future applications in Cancer chemoprevention and therapy. Front Pharmacol. (2022) 13:809706. doi: 10.3389/fphar.2022.809706
205. Wang, Y, Wu, S, Li, Q, Lang, W, Li, W, Jiang, X, et al. Epigallocatechin-3-gallate: a phytochemical as a promising drug candidate for the treatment of Parkinson's disease. Front Pharmacol. (2022) 13:977521. doi: 10.3389/fphar.2022.977521
206. Brückner, M, Westphal, S, Domschke, W, Kucharzik, T, and Lügering, A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J Crohns Colitis. (2012) 6:226–35. doi: 10.1016/j.crohns.2011.08.012
207. Shabgah, AG, Suksatan, W, Achmad, MH, Bokov, DO, Abdelbasset, WK, Ezzatifar, F, et al. Arctigenin, an anti-tumor agent; a cutting-edge topic and up-to-the-minute approach in cancer treatment. Eur J Pharmacol. (2021) 909:174419. doi: 10.1016/j.ejphar.2021.174419
208. Mottaghi, S, and Abbaszadeh, H. A comprehensive mechanistic insight into the dietary and estrogenic lignans, arctigenin and sesamin as potential anticarcinogenic and anticancer agents. Current status, challenges, and future perspectives. Crit Rev Food Sci Nutr. (2022) 62:7301–18. doi: 10.1080/10408398.2021.1913568
209. Wu, X, Tong, B, Yang, Y, Luo, J, Yuan, X, Wei, Z, et al. Arctigenin functions as a selective agonist of estrogen receptor β to restrict mTORC1 activation and consequent Th17 differentiation. Oncotarget. (2016) 7:83893–906. doi: 10.18632/oncotarget.13338
210. Cho, HJ, Seon, MR, Lee, YM, Kim, J, Kim, JK, Kim, SG, et al. 3,3'-Diindolylmethane suppresses the inflammatory response to lipopolysaccharide in murine macrophages. J Nutr. (2008) 138:17–23. doi: 10.1093/jn/138.1.17
211. Kim, YH, Kwon, HS, Kim, DH, Shin, EK, Kang, YH, Park, JH, et al. 3,3′-diindolylmethane attenuates colonic inflammation and tumorigenesis in mice. Inflamm Bowel Dis. (2009) 15:1164–73. doi: 10.1002/ibd.20917
212. Kim, JY, Le, TAN, Lee, SY, Song, DG, Hong, SC, Cha, KH, et al. 3,3'-Diindolylmethane improves intestinal permeability dysfunction in cultured human intestinal cells and the model animal Caenorhabditis elegans. J Agric Food Chem. (2019) 67:9277–85. doi: 10.1021/acs.jafc.9b03039
213. Huang, Z, Zuo, L, Zhang, Z, Liu, J, Chen, J, Dong, L, et al. 3,3'-Diindolylmethane decreases VCAM-1 expression and alleviates experimental colitis via a BRCA1-dependent antioxidant pathway. Free Radic Biol Med. (2011) 50:228–36. doi: 10.1016/j.freeradbiomed.2010.10.703
214. Huang, Z, Jiang, Y, Yang, Y, Shao, J, Sun, X, Chen, J, et al. 3,3'-Diindolylmethane alleviates oxazolone-induced colitis through Th2/Th17 suppression and Treg induction. Mol Immunol. (2013) 53:335–44. doi: 10.1016/j.molimm.2012.09.007
215. Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med. (2011) 17:1217–20. doi: 10.1038/nm.2471
216. Tu, Y. Artemisinin-a gift from traditional Chinese medicine to the world (Nobel lecture). Angew Chem Int Ed Engl. (2016) 55:10210–26. doi: 10.1002/anie.201601967
217. Zhao, YG, and Wang, Y. Dihydroartemisinin ameliorates inflammatory disease by its reciprocal effects on Th and regulatory T cell function via modulating the mammalian target of rapamycin pathway. J Immunol. (2012) 189:4417–25. doi: 10.4049/jimmunol.1200919
218. Fan, M, and Li, Y. DC32, a Dihydroartemisinin derivative, ameliorates collagen-induced arthritis through an Nrf2-p62-Keap1 feedback loop. Front Immunol. (2018) 9:2762. doi: 10.3389/fimmu.2018.02762
219. Yan, SC, Wang, YJ, Li, YJ, Cai, WY, Weng, XG, Li, Q, et al. Dihydroartemisinin regulates the Th/Treg balance by inducing activated CD4+ T cell apoptosis via Heme Oxygenase-1 induction in mouse models of inflammatory bowel disease. Molecules. (2019) 24:2475. doi: 10.3390/molecules24132475
220. Dos, S, Moreira, C, Santos, TB, Freitas, RHCN, Pacheco, PAF, and da Rocha, DR. Juglone: a versatile natural platform for obtaining new bioactive compounds. Curr Top Med Chem. (2021) 21:2018–45. doi: 10.2174/1568026621666210804121054
221. Delaviz, H, Mohammadi, J, Ghalamfarsa, G, Mohammadi, B, and Farhadi, N. A review study on Phytochemistry and pharmacology applications of Juglans Regia plant. Pharmacogn Rev. (2017) 11:145–52. doi: 10.4103/phrev.phrev_10_17
222. Peng, X, Nie, Y, Wu, J, Huang, Q, and Cheng, Y. Juglone prevents metabolic endotoxemia-induced hepatitis and neuroinflammation via suppressing TLR4/NF-kappaB signaling pathway in high-fat diet rats. Biochem Biophys Res Commun. (2015) 462:245–50. doi: 10.1016/j.bbrc.2015.04.124
223. Shen, ZJ, Esnault, S, Rosenthal, LA, Szakaly, RJ, Sorkness, RL, Westmark, PR, et al. Pin1 regulates TGF-beta1 production by activated human and murine eosinophils and contributes to allergic lung fibrosis.J. Clin Investig. (2008) 118:479–90. doi: 10.1172/JCI32789
224. Hua, Y, Liu, R, Lu, M, Guan, X, Zhuang, S, Tian, Y, et al. Juglone regulates gut microbiota and Th17/Treg balance in DSS-induced ulcerative colitis. Int Immunopharmacol. (2021) 97:107683. doi: 10.1016/j.intimp.2021.107683
225. Sharma, BR, Gautam, LN, Adhikari, D, and Karki, R. A comprehensive review on chemical profiling of Nelumbo Nucifera: potential for drug development. Phytother Res. (2017) 31:3–26. doi: 10.1002/ptr.5732
226. Wang, Y, Yao, WF, Li, B, Qian, SY, Wei, BB, Gong, SQ, et al. Nuciferine modulates the gut microbiota and prevents obesity in high-fat diet-fed rats. Exp Mol Med. (2020) 52:1959–75. doi: 10.1038/s12276-020-00534-2
227. Shi, Z, Fang, ZY, Gao, XX, Yu, H, Zhu, YW, Ouyang, HL, et al. Nuciferine improves high-fat diet-induced obesity via reducing intestinal permeability by increasing autophagy and remodeling the gut microbiota. Food Funct. (2021) 12:5850–61. doi: 10.1039/D1FO00367D
228. Xiong, WT, Liao, JB, Yang, ZX, Cui, HT, Zhang, ZY, Wen, WB, et al. Effect of nuciferine on gut microbiota and inflammatory response in obese model mice. China J Chin Mater Med. (2021) 46:2104–11. doi: 10.19540/j.cnki.cjcmm.20201207.401
229. Wang, MX, Liu, YL, Yang, Y, Zhang, DM, and Kong, LD. Nuciferine restores potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Eur J Pharmacol. (2015) 747:59–70. doi: 10.1016/j.ejphar.2014.11.035
230. Zhu, Y, Zhao, Q, Huang, Q, Li, Y, Yu, J, Zhang, R, et al. Nuciferine regulates immune function and Gut microbiota in DSS-induced ulcerative colitis. Front Vet Sci. (2022) 9:939377. doi: 10.3389/fvets.2022.939377
231. Zhuang, KH, and Liu, FB. Professor Lao Shaoxian’s idea of treatment based on syndrome differentiation in ulcerative colitis. J Guangzhou Univ Tradit Chin Med. (2013) 30:914–6.
232. Pan, G, Liu, B, Li, S, Han, M, Gao, L, Xu, G, et al. Kuijieling, a Chinese medicine alleviates DSS-induced colitis in C57BL/6Jmouse by improving the diversity and function of gut microbiota. FEMS Microbiol Lett. (2020) 367:fnaa082. doi: 10.1093/femsle/fnaa082
233. Jie, F, Xiao, S, Qiao, Y, You, Y, Feng, Y, Long, Y, et al. Kuijieling decoction suppresses NLRP3-mediated pyroptosis to alleviate inflammation and experimental colitis in vivo and in vitro. J Ethnopharmacol. (2021) 264:113243. doi: 10.1016/j.jep.2020.113243
234. Wang, F, Wu, X, Gan, L, Long, Y, Li, Y, Wu, Y, et al. The regulatory mechanism of Kuijieling decoction on Th17 differentiation in ulcerative colitis rats Pharmacol. Pharmacology and Clinics of Chinese Materia Medica. (2016) 32:177–81.
235. Liu, Z, Guo,, Li, Y, Wu, Y, Li, F, Zou, J, et al. Effects of Kuijieling on balance of peripheral blood Treg/Th17 in experimental ulcerative colitis rats. Tradit Chin Drug Res Clin Pharmacol. (2015) 26:1–5. doi: 10.3969/j.issn.1003-9783.2015.01.001
236. Long, Y, Li, S, Qin, J, Xie, L, Gan, L, Jie, F, et al. Kuijieling regulates the differentiation of Treg and Th17 cells to ameliorate experimental colitis in rats. Biomed Pharmacother. (2018) 105:781–8. doi: 10.1016/j.biopha.2018.06.011
237. Muluye, RA, Bian, Y, and Alemu, PN. Anti-inflammatory and antimicrobial effects of heat-clearing Chinese herbs: a current review. J Tradit Complement Med. (2014) 4:93–8. doi: 10.4103/2225-4110.126635
238. Yang, L, Wu, H, Qiu, W, Guo, L, Du, X, Yu, Q, et al. Pulsatilla decoction inhibits Candida albicans proliferation and adhesion in a mouse model of vulvovaginal candidiasis via the Dectin-1 signaling pathway. J Ethnopharmacol. (2018) 223:51–62. doi: 10.1016/j.jep.2018.05.018
239. Xu, QM, Shu, Z, He, WJ, Chen, LY, Yang, SL, Yang, G, et al. Antitumor activity of Pulsatilla chinensis (Bunge) regel saponins in human liver tumor 7402 cells in vitro and in vivo. Phytomedicine. (2012) 19:293–300. doi: 10.1016/j.phymed.2011.08.066
240. Tan, Z, Zhang, S, Liu, R, Zou, L, and Hu, H. Regulatory effect of Pulsatilla decoction on Th17/Treg cell imbalance in patients with ulcerative colitis. J Liaoning Univ Tradit Chin Med. (2017) 19:191–4. doi: 10.13194/j.issn.1673-842x.2017.07.052
241. Zhang, Z, Yang, S, Lin, X, Huang, Y, Wei, X, Zhou, J, et al. Metabolomics of spleen-Yang deficiency syndrome and the therapeutic effect of FuziLizhong pill on regulating endogenous metabolism. J Ethnopharmacol. (2021) 278:114281. doi: 10.1016/j.jep.2021.114281
242. Luo, Z, Xia, LY, Tang, YQ, Huang, L, Liu, D, Huai, WY, et al. Action mechanism underlying improvement effect of FuziLizhong decoction on nonalcoholic fatty liver disease: a study based on network pharmacology and molecular docking. Evid Based Complement Alternat Med. (2022) 2022:1670014. doi: 10.1155/2022/1670014
243. Wu, HN. Basic information and clinical application of FuziLizhong pill. Chin J Clin Rat Drug Use. (2020) 13:179–80. doi: 10.15887/j.cnki.13-1389/r.2020.01.101
244. Yao, L. Mechanism of FuziLizhong decoction on Treg cell related factors in spleen and kidney Yang deficiency rats with ulcerative colitis. Shaanxi University of Traditional Chinese Medicine (2021).
245. Han, X, Li, J, Chen, X, Wang, Z, Yuan, Y, Xing, Y, et al. Study on the regulatory effect of intestinal cleansing and warming Chinese formula on mucosa barrier function in patients with ulcerative colitis. Chin J Integr Tradit Chin West Med. (2022) 30:713–8.
246. Sun, Z, Yuan, W, Mao, T, Din, P, Wang, W, Shi, R, et al. Qingchang Wenzhong formula regulates ulcerative colitis via miR-675-5p/VDR signaling pathway study on the mechanism of Th17/Treg immune balance and intestinal mucosal barrier in colitis. Chin Med Emerg. (2019) 28:94–97+108.
247. Xia, S, Chen, L, Li, Z, Li, Y, Zhou, Y, Sun, S, et al. QingchangWenzhong decoction reduce ulcerative colitis in mice by inhibiting Th17 lymphocyte differentiation. Phytomedicine. (2022) 107:154460. doi: 10.1016/j.phymed.2022.154460
248. Dan, L, and Shaohua, X. The clinical efficacy of compound Kushen decoction in the treatment of chronic ulcerative colitis and its influence on inflammatory factors. J Chronic Dis. (2019) 20:1044–6. doi: 10.16440/j.cnki.1674-8166.2019.07.030
249. Lee, IA, Hyam, SR, Jang, SE, Han, MJ, and Kim, DH. Ginsenoside re ameliorates inflammation by inhibiting the binding of lipopolysaccharide to TLR4 on macrophages. J Agric Food Chem. (2012) 60:9595–602. doi: 10.1021/jf301372g
250. Fan, H, Chen, R, Shen, L, Lv, J, Xiong, P, Shou, Z, et al. Oxymatrine improves TNBS-induced colitis in rats by inhibiting the expression of NF-kappaB p65.J. Huazhong Univ. Sci. Technol. Med Sci. (2008) 28:415–20. doi: 10.1007/s11596-008-0409-x
251. Guzman, JR, Koo, JS, Goldsmith, JR, Muhlbauer, M, Narula, A, and Jobin, C. Oxymatrine prevents NF-kappaB nuclear translocation and ameliorates acute intestinal inflammation. Sci Rep. (2013) 3:1629. doi: 10.1038/srep01629
252. Pandurangan, AK, Mohebali, N, Esa, NM, Looi, CY, Ismail, S, and Saadatdoust, Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: possible mechanisms. Int Immunopharmacol. (2015) 28:1034–43. doi: 10.1016/j.intimp.2015.08.019
253. Xu, M, Duan, XY, Chen, QY, Fan, H, Hong, ZC, Deng, SJ, et al. Effect of compound sophorae decoction on dextran sodium sulfate (DSS)-induced colitis in mice by regulating Th17/Treg cell balance. Biomed Pharmacother. (2019) 109:2396–408. doi: 10.1016/j.biopha.2018.11.087
254. Zhang, CG. IllustratedZhongjing Zhang's compound formula: interpretation and application. Beijing: China Traditional Chinese Medicine Press (2013). 373 p.
255. Luo, S, Wen, R, Wang, Q, Zhao, Z, Nong, F, Fu, Y, et al. Rhubarb Peony decoction ameliorates ulcerative colitis in mice by regulating gut microbiota to restoring Th17/Treg balance. J Ethnopharmacol. (2019) 231:39–49. doi: 10.1016/j.jep.2018.08.033
256. Cui, G, Qin, X, Zhang, Y, Gong, Z, Ge, B, and Zang, YQ. Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J Biol Chem. (2009) 284:28420–9. doi: 10.1074/jbc.M109.012674
257. Lu, JZ, Ye, D, and Ma, BL. Constituents, pharmacokinetics, and pharmacology of Gegen-Qinlian decoction. Front Pharmacol. (2021) 12:668418. doi: 10.3389/fphar.2021.668418
258. Li, Q, Cui, Y, Xu, B, Wang, Y, Lv, F, Li, Z, et al. Main active components of Jiawei GegenQinlian decoction protects against ulcerative colitis under different dietary environments in a gut microbiota-dependent manner. Pharmacol Res. (2021) 170:105694. doi: 10.1016/j.phrs.2021.105694
259. Hu, J, Huang, H, Che, Y, Ding, C, Zhang, L, Wang, Y, et al. QingchangHuashi formula attenuates DSS-induced colitis in mice by restoring gut microbiota-metabolism homeostasis and goblet cell function. J Ethnopharmacol. (2021) 266:113394. doi: 10.1016/j.jep.2020.113394
260. Wang, Y, Zhang, J, Xu, L, Ma, J, Lu, M, Ma, J, et al. Modified GegenQinlian decoction regulates Treg/Th17 balance to ameliorate DSS-induced acute experimental colitis in mice by altering the Gut microbiota. Front Pharmacol. (2021) 12:756978. doi: 10.3389/fphar.2021.756978
261. Tian, Y, Chen, X, Zhang, H, Li, J, Li, H, and Zhang, X. The effect of Shenling Baizhu granule combined with mesalazine on Th17/Treg balance in patients with ulcerative colitis and its clinical efficacy. Chin J Integr Tradit Chin West Med Dig. (2019) 27:703–6. doi: 10.3969/j.issn.1671-038X.2019.09.13
262. Yunfei, Q, Mingliao, N, and Longjiang, Z. Effect of Shenling Baizhu powder on Th_(17)/Treg immune balance in rats with ulcerative colitis. Chin Folk Med. (2022) 30:88–92. doi: 10.19621/j.cnki.11-3555/r.2022.0631
263. Zha, W, Sun, Y, Gong, W, Li, L, Kim, W, and Li, H. Ginseng and ginsenosides: therapeutic potential for sarcopenia. Biomed Pharmacother. (2022) 156:113876. doi: 10.1016/j.biopha.2022.113876
264. Goodwin, PH. The rhizosphere microbiome of ginseng. Microorganisms. (2022) 10:1152. doi: 10.3390/microorganisms10061152
265. Chen, J, Huang, Q, Li, J, Yao, Y, Sun, W, Zhang, Z, et al. Panax ginseng against myocardial ischemia/reperfusion injury: a review of preclinical evidence and potential mechanisms. J Ethnopharmacol. (2023) 300:115715. doi: 10.1016/j.jep.2022.115715
266. de, B, de, A, Miola, V, Guissoni, L, Spilla, C, and Barbalho, S. Panax ginseng and aging related disorders: a systematic review. Exp Gerontol. (2022) 161:111731. doi: 10.1016/j.exger.2022.111731
267. Zhang, H, Hu, C, Xue, J, Jin, D, Tian, L, Zhao, D, et al. Ginseng in vascular dysfunction: a review of therapeutic potentials and molecular mechanisms. Phytother Res. (2022) 36:857872:857–72. doi: 10.1002/ptr.7369
268. Joh, EH, Lee, IA, Jung, IH, and Kim, DH. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation--the key step of inflammation. Biochem Pharmacol. (2011) 82:278–86. doi: 10.1016/j.bcp.2011.05.003
269. Lee, SY, Jeong, JJ, Eun, SH, and Kim, DH. Anti-inflammatory effects of ginsenoside Rg1 and its metabolites ginsenoside Rh1 and 20(S)-protopanaxatriol in mice with TNBS-induced colitis. Eur J Pharmacol. (2015) 762:333–43. doi: 10.1016/j.ejphar.2015.06.011
270. Jian, L, Zengping, K, Youbao, Z, Xin, D, Mengxue, W, Haimei, Z, et al. Regulatory effect of ginsenoside Rg1 on Treg/Th9 cell balance in DSS-induced ulcerative colitis mice. New Drugs Clin Pharmacol Tradit Chin Med. (2022) 33:20–6. doi: 10.19378/j.issn.1003-9783.2022.01.004
271. Wei, YY, Fan, YM, Ga, Y, Zhang, YN, Han, JC, and Hao, ZH. Shaoyao decoction attenuates DSS-induced ulcerative colitis, macrophage and NLRP3 inflammasome activation through the MKP1/NF-κB pathway. Phytomedicine. (2021) 92:153743. doi: 10.1016/j.phymed.2021.153743
272. Wang, X, Saud, SM, Wang, F, He, S, Zhang, X, Hua, B, et al. Protective effect of ShaoYao decoction on colitis-associated colorectal cancer by inducing Nrf2 signaling pathway. J Ethnopharmacol. (2020) 252:112600. doi: 10.1016/j.jep.2020.112600
273. Wu, J, Luo, Y, Shen, Y, Hu, Y, Zhu, F, Wu, J, et al. Integrated Metabonomics and network pharmacology to reveal the action mechanism effect of Shaoyao decoction on ulcerative colitis. Drug Des Devel Ther. (2022) 16:3739–76. doi: 10.2147/DDDT.S375281
274. Lin, X, Yi, Z, Diao, J, Shao, M, Zhao, L, Cai, H, et al. ShaoYao decoction ameliorates colitis-associated colorectal cancer by downregulating proinflammatory cytokines and promoting epithelial-mesenchymal transition. J Transl Med. (2014) 12:105. doi: 10.1186/1479-5876-12-105
275. Lu, L, He, Y, Luo, M, Luo, Y, Tan, Z, and Liu, J. Shaoyao decoction treated 30 cases of damp-heat intrinsic ulcerative colitis. Chin Mod Dist Educ Tradit Chin Med. (2010) 8:11–2. doi: 10.3969/j.issn.1672-2779.2010.19.010
276. Yao, C, Li, Y, Luo, L, and Feng, P. Study on the mechanism of Shaoyao decoction regulating the balance of Th17Treg cells and improving the inflammatory response in ulcerative colitis with damp-heat syndrome of large intestine. World Sci Technol Mod Tradit Chin Med. (2021) 23:2635–42. doi: 10.11842/wst.20210116008
Keywords: Th17/Treg balance, ulcerative colitis, immune inflammation, natural plant components, inflammatory disease
Citation: Zhao D, Ge A, Yan C, Liu X, Yang K, Yan Y, Hao M, Chen J, Daga P, Dai CC, Li C and Cao H (2025) T helper cell 17/regulatory T cell balance regulates ulcerative colitis and the therapeutic role of natural plant components: a review. Front. Med. 11:1502849. doi: 10.3389/fmed.2024.1502849
Received: 27 September 2024; Accepted: 23 December 2024;
Published: 24 March 2025.
Edited by:
Angel Lanas, University of Zaragoza, SpainReviewed by:
Cheng-Rong Yu, National Eye Institute (NIH), United StatesCopyright © 2025 Zhao, Ge, Yan, Liu, Yang, Yan, Hao, Chen, Daga, Dai, Li and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Cao, Y2FvaDM0NTZAaG90bWFpbC5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.