- 1Collaborative Innovation Center for Biomedicines, Shanghai University of Medicine and Health Sciences, Shanghai, China
- 2College of Nursing and Allied Health Sciences, St. Paul University Manila, Manila, Philippines
- 3College of Rehabilitation Sciences, Shanghai University of Medicine and Health Sciences, Shanghai, China
Over the past decade, the elderly Filipino population has significantly increased, rising from 4.6 million seniors, which was approximately 6% of the total population in 2000, to 6.5 million, or around 6.9% of the population in 2010. Projections suggest that by 2030, the percentage of the population aged 60 and above will increase to between 10 and 19%, indicating a significant demographic shift toward an aging population. This shift from a predominantly young population to a rapidly growing elderly demographic underscores the urgent need for effective health promotion and management programs targeting older adults. Sarcopenia, a muscle-wasting disorder, represents a significant global health challenge, particularly affecting the elderly. In the Philippines, the impact of sarcopenia is expected to become more pronounced, potentially straining both individuals and the national healthcare system over the next 15 years, despite the country’s relatively younger overall population. Despite the expanding research on sarcopenia in the Philippines, there remains an urgent need to raise awareness and implement proactive measures to address this escalating health issue.
1 Current state of research in the Philippines
The demographic shift toward aging populations in developing countries has intensified the prevalence of sarcopenia, creating unique challenges for healthcare systems already limited in resources (1). In regions like Southeast Asia, Latin America, and sub-Saharan Africa, life expectancy has risen steadily, leading to larger elderly populations than ever before. However, unlike in high-income countries, healthcare infrastructure in many lower- and middle-income nations often lacks adequate diagnostic facilities and trained personnel to address age-related conditions effectively (2, 3). This gap in healthcare capacity leaves older adults at greater risk for untreated muscle degeneration, which can lead to increased disability, decreased quality of life, and a greater financial burden on families and communities (4). The rising burden of sarcopenia in these regions underscores an urgent need for scalable, cost-effective interventions that consider local constraints.
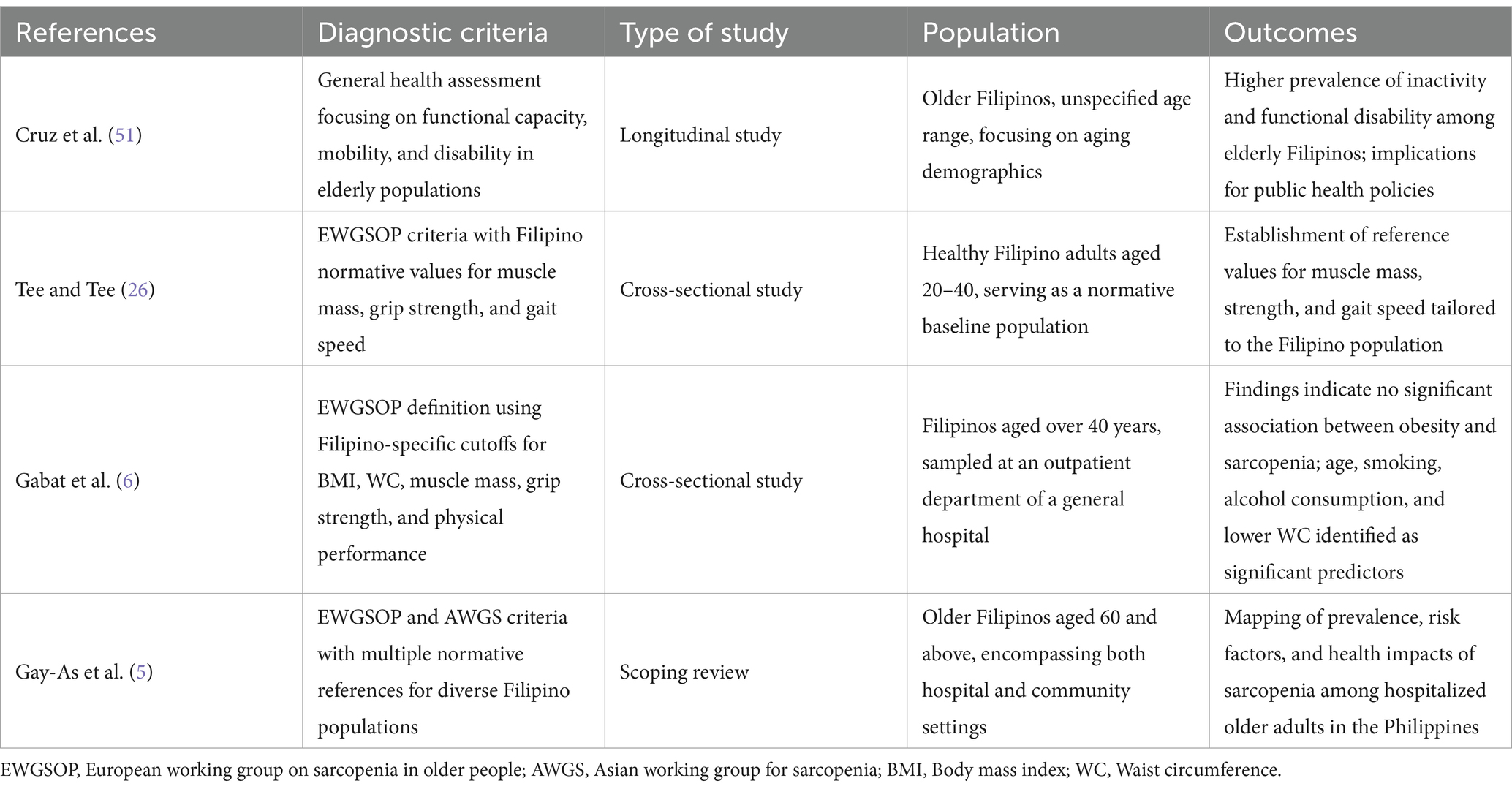
Research employing the Asian Working Group for Sarcopenia (AWGS) criteria has revealed a prevalence of 27.7%, while the revised European consensus (EWGSOP2) reported prevalence rates of 18.18%. A study from a tertiary hospital revealed a higher prevalence of 73.33% (5). Collectively, these findings suggest that sarcopenia is prevalent among Filipino adults aged 60 and older, influenced by various sociodemographic and physiological factors. Current research identifies age, waist circumference, smoking, and alcohol consumption as significant predictors of sarcopenia (6). Sarcopenia is closely associated with reduced physical function and diminished quality of life, underscoring the necessity for national guidelines to address this pressing issue. At the March 2023 Asia-Pacific conference on muscle health and sarcopenia, a multinational panel, including Filipino experts, proposed five core pillars: awareness, education, initiatives, engagement, and research. These pillars are designed to address the gaps and challenges in implementing muscle health assessments and management within clinical practice (7). Additionally, the country launched its first longitudinal study on health and aging (LSHAP) in 2018, using a nationally representative sample and recommending further research on sarcopenia and healthy life expectancy to enhance understanding of the health characteristics of Filipino older adults. A review of studies on sarcopenia in Filipinos aged 60 and above conducted between 2013 and 2023 revealed that most research has been hospital-based and involved patients with comorbidities. Sarcopenia remains inadequately integrated into routine clinical practice. The Geriatric Health-Research Institute (NCGH-RI) has incorporated sarcopenia screening into the Comprehensive Geriatric Assessment (CGA), emphasizing the need for further research and implementation to address this escalating public health concern. Table 1 summarized sarcopenia-related research conducted in the Philippines.
2 Mechanism
Sarcopenia is a complex muscle disorder characterized by the progressive loss of muscle mass, strength, and function, predominantly affecting older adults (8). This condition is influenced by a combination of genetic, biological, environmental, and lifestyle factors. The pathogenesis of sarcopenia is multifactorial, involving a combination of genetic predisposition, biological processes, environmental influences, and lifestyle factors. Research has shown that various signaling pathways, such as those involving protein metabolism, muscle regeneration, and inflammation, are crucial in the development of sarcopenia (9, 10). Aging results in the atrophy of fast-twitch type II muscle fibers, which are essential for generating force and power. This leads to a reduction in muscle size and strength, particularly in response to diminished satellite cell activity and impaired muscle regeneration (8). Concurrently, muscle protein synthesis declines with age, exacerbating the negative protein balance. This decline is partly due to reduced activity of the mTOR signaling pathway, which plays a key role in muscle protein synthesis and growth (9, 10). Additionally, aging promotes an increase in muscle protein breakdown, which is further exacerbated by chronic inflammation, oxidative stress, and physical inactivity. This process is mediated by upregulated proteolytic pathways, such as the ubiquitin-proteasome system and autophagy (11, 12). Hormonal changes, including declines in testosterone, growth hormone, and insulin-like growth factor 1 (IGF-1), further exacerbate the loss of muscle mass and strength. These hormonal alterations impair muscle regeneration and increase protein catabolism (13). Chronic low-grade inflammation, a hallmark of aging, is associated with sarcopenia. Pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), play a crucial role in muscle wasting by increasing muscle protein breakdown and inhibiting muscle protein synthesis. Inflammation also disrupts the balance of muscle repair and regeneration (14, 15). Physical inactivity is one of the most significant accelerators of sarcopenia, driving the rapid decline in muscle mass, strength, and functionality. It results in decreased muscle protein synthesis, impaired mitochondrial function, and muscle fiber atrophy, all of which contribute to the progression of sarcopenia (16, 17).
3 Diagnosis
The European Working Group on Sarcopenia in Older People (EWGSOP) and the Asian Working Group for Sarcopenia (AWGS) have established diagnostic algorithms for sarcopenia that include assessments of muscle mass, strength, and physical performance (18, 19). These algorithms are widely applied in clinical and research settings, including in countries like the Philippines, where sarcopenia research is becoming more prevalent. In EWGSOP1, the criteria for diagnosing sarcopenia include low muscle mass in conjunction with either low muscle strength or low physical performance (20), with muscle mass being the primary component of sarcopenia. These criteria have been widely studied in European and Asian populations, but there is a need to determine whether these criteria are appropriate for Filipino populations, considering potential ethnic and environmental differences. EWGSOP2, however, prioritizes muscle strength, defining sarcopenia as low muscle strength combined with low muscle quantity or low muscle quality (19), where muscle strength becomes the predominant component. This approach may need modification when applied to the Filipino population, as studies suggest variations in muscle strength and mass across ethnic groups. AWGS2019 also emphasizes the importance of muscle strength. Consequently, these differing criteria may result in variations in the reported prevalence of sarcopenia. A systematic review indicates that the prevalence of sarcopenia in older adults is lower when diagnosed using EWGSOP2 compared to EWGSOP1 (21). A similar trend has been observed in some studies conducted in Southeast Asia, but further research is needed to apply these findings to the Filipino population. A comparative study of sarcopenia prevalence in a large multinational European population sample using various diagnostic criteria (22) revealed similar findings, suggesting that prevalence estimates based on EWGSOP1 tend to be higher than those based on EWGSOP2. However, AWGS2 criteria show a similarly lower prevalence to EWGSOP2, likely due to their shared reliance on muscle mass and muscle strength. Baumgartner et al. (23) emphasized the significance of employing “population-specific diagnostic criteria” for sarcopenia. This is particularly relevant for the Filipino population, where unique genetic, dietary, and environmental factors may influence sarcopenia characteristics. They delineated a methodology for estimating the prevalence of “deficient” relative muscle mass, or sarcopenia, in population-based studies. Consequently, numerous sarcopenia criteria have incorporated the concept of “relative muscle mass.” Therefore, identifying suitable measurement tools and establishing cut-off values in the Philippines that align with international standards will enable researchers and policymakers to adapt actions and recommendations as proposed by organizations such as the EWGSOP.
Dual-energy X-ray absorptiometry (DXA) is a widely accepted and reliable method for assessing muscle mass in older adults and is endorsed by international organizations for diagnosing sarcopenia. However, the applicability of DXA in the Filipino population, due to differences in body composition, requires further validation. Bioelectrical impedance analysis (BIA) provides a portable, non-invasive, and cost-effective alternative to DXA. Recent studies have confirmed BIA as a dependable tool for diagnosing sarcopenia in older adults (23). Handgrip strength serves as a straightforward and cost-effective screening tool for muscle strength. Decreased handgrip strength is a significant predictor of adverse outcomes in older adults, including disability, falls, and mortality (24). Physical performance, especially gait speed, is another crucial diagnostic criterion for sarcopenia (25). Research indicates that gait speed is a dependable measure of muscle function in older adults and is correlated with adverse health outcomes, including disability, falls, and mortality.
Despite the availability of diagnostic tools, no established normative reference exists for diagnosing sarcopenia in the Philippines. A scoping review has highlighted the use of various assessment methods in sarcopenia studies among elderly Filipinos, including the EWGSOP and AWGS criteria, magnetic resonance imaging (MRI), and psoas muscle thickness divided by height (PMTH) (5). These studies have identified potential cut-off values for muscle mass, strength, and performance tailored to the Filipino population. Given the variability in population characteristics across countries, there is an urgent need for studies to establish appropriate cut-off points specific to the Filipino population. This would facilitate the development of local health promotion and sarcopenia management programs. Tee and Tee (26) aimed to establish normative data for muscle mass and physical performance among young Filipinos to enhance the applicability of EWGSOP criteria. Their study provides reference values for normal muscle mass, strength, and physical performance, thereby contributing to more accurate diagnosis and preventive strategies for sarcopenia. The cut-off points specific to Filipinos derived from this study have been cited in subsequent sarcopenia research in the Philippines, including studies on the association between obesity and sarcopenia and sarcopenia with osteoporosis in patients with chronic obstructive pulmonary disease (25, 26, 52). The diagnostic standards are as follows: (I) Low muscle mass is defined as a lean tissue index of <12.50 kg/m2 for males and < 8.33 kg/m2 for females using the Fresenius Body Composition Monitor. (II) Low muscle strength is defined as grip strength of <24.54 kg for males and < 16.10 kg for females, measured with a JAMAR dynamometer. (III) Low physical performance is assessed using Usual Gait Speed and Timed Get Up and Go. Usual Gait Speed is measured with the 4-m walk test, with values <0.55 m/s for males and < 0.65 m/s for females considered low. For the Timed Get Up and Go, times >8.31 s for males and > 8.74 s for females are considered indicative of low physical performance. Further research is needed to validate these reference values, specifically in diverse Filipino subpopulations, and to optimize diagnostic criteria.
4 Treatment
As highlighted in the review by Gay-as et al. (5), most of the existing research on sarcopenia among older Filipinos focuses on determining the prevalence and associated factors of sarcopenia in hospitalized individuals (secondary prevention). Some studies address the development of consensus on the prevention and management of sarcopenia and its associated factors, while few explore educational initiatives on sarcopenia for healthcare professionals. There is a significant gap in research on the treatment of sarcopenia within the Filipino population. Therefore, this review summarizes current global treatment approaches for sarcopenia to offer guidance for managing sarcopenia in the Philippines, with the goal of enabling older individuals to lead healthy and productive lives in accordance with the Philippine government’s vision for its elderly citizens.
4.1 Exercise
Physical activity can be an effective intervention for sarcopenia, although there is considerable variability in the types of physical activity (resistance, aerobic, balance, and combinations thereof) that target sarcopenia. Recent studies have shown that resistance exercise is a proven intervention for sarcopenia, improving muscle mass, strength and physical function in older adults (27). Among other things, high-intensity resistance training is more effective than low-intensity resistance training in improving muscle mass and strength in older adults with sarcopenia (27), as well as reducing body fat content and improving grip strength. Aerobic exercise also plays a significant role in the rehabilitation of sarcopenia. Some studies have demonstrated that aerobic exercise, such as walking or bicycling, improves cardiovascular fitness, endurance, and physical function in older adults with sarcopenia (27), as well as reducing body weight and fat mass. The results of a meta-analysis showed that a combination of aerobic exercise and resistance training reduced body fat percentage and increased walking speed in patients with sarcopenia (28). The combination of two or more types of exercise modalities may improve fitness in multiple ways (knee extension strength, gait speed, grip strength, standing chair time, physical performance, etc.) in patients with sarcopenia (29, 53). Current multicomponent exercise programs that combine resistance training, aerobic exercise, balance training, and flexibility training have been shown to be more effective than single-component exercise programs in improving physical functioning and reducing the risk of falls in older adults with sarcopenia (30). There is high-quality evidence that resistance training, aerobic exercise combined with balance training is effective in improving quality of life in patients with sarcopenia (31).
4.1.1 Resistance movement
Resistance training serves as a primary intervention for sarcopenia, recommending a frequency of 2–3 sessions per week, with each session lasting 30–60 min, for a minimum of 12 weeks. This training is structured in three progressive stages: beginner (40–60% of 1 Repetition Maximum, RM), intermediate, and advanced. Initially, participants should engage in beginner training for 1–2 weeks before progressing to intermediate and advanced stages. Resistance should be incrementally increased by 5–10% of 1 RM per session. The intermediate and advanced stages advocate for medium to high-intensity resistance training (60–80% of 1 RM). In the beginner stage, each exercise should be performed 8–10 times per set, with 1–2 sets per session, and a 1 to 2-min rest between sets. The intensity and volume of training should be escalated gradually. Common resistance exercises include the use of dumbbells, sandbags, elastic bands, grip devices, and bodyweight movements such as sit-ups, push-ups, squats, and jumps (32, 33).
4.1.2 Aerobic exercise
Aerobic exercise is characterized by energy generation through aerobic oxidation, where the respiratory system supplies sufficient oxygen to muscles for sustained activity. Heart rate should be monitored to maintain moderate-intensity exercise (50–80% of maximum heart rate), calculated as 220 minus ages. Aerobic exercise enhances cardiopulmonary function, endurance, immunity, and adaptability, complementing resistance training in a positive feedback loop. When performed alone, aerobic exercise should last 30–45 min, at least 3 times per week (17).
4.1.3 Balance training
Balance training aims to improve physical stability and reduce fall risk in individuals with sarcopenia. It includes: (1) Static balance training, where the body maintains a posture, such as the three-step potential balance (tandem, semi-tandem, and feet together standing), and single-leg stands (eyes open or closed, hands on waist or chair back). Each movement should gradually increase from 10 s to 1 to 2 min; (2) Dynamic balance training, focusing on the body’s balance during movement, through sit-to-stand transitions, walking exercises (forward, backward, sideways) (34).
4.1.4 Family training and educational training
This involves home-based rehabilitation to help patients adapt to the family environment, participate in family life, and interact with family members. Under professional guidance, family members act as trainers, conducting rehabilitation that includes disease education, use of rehabilitation equipment, medical exercises, and household activities. Training plans should be tailored to the patient’s abilities, specifying training time, venue, equipment, and content, utilizing everyday items. Situational training, such as daily routines and community activities, should have clear stage goals.
4.1.5 Exercise rehabilitation prescription
Exercise prescription should be personalized based on the FITT principle, considering the patient’s condition. FITT principle includes: (1) Frequency (F): Patients should increase daily physical activity, with at least three exercise sessions per week. (2) Intensity (I): Recommend moderate resistance exercise (<70% of 1 RM) and aerobic exercise (<70% of VO2peak), starting with low intensity and gradually increasing. (3) Time (T): The target duration is 30–60 min per session. (4) Type (T): Include aerobic, resistance, and flexibility exercises. During rehabilitation, the patient’s tolerance should be fully assessed to ensure exercise safety (28).
4.2 Nutritional interventions
Nutritional interventions play a crucial role in the rehabilitation of sarcopenia, with common supplements including protein, essential vitamins, leucine and its metabolites, and creatine. Protein and vitamin D supplementation, according to currently available studies, have been shown to be effective in improving muscle health in older patients with sarcopenia (35). However, a meta-analysis showed (36) that protein supplementation increased muscle mass but did not enhance muscle strength and physical performance. However, it may have a synergistic effect on muscle mass and strength in older patients with sarcopenia when combined with resistance training (37). Essential amino acids, which cannot be synthesized in the body, and some studies have shown that intake of complete essential amino acids improves the efficiency of energy conversion in the body more than supplementation of complete proteins, with leucine supplementation being effective in increasing muscle mass in patients with sarcopenia and having a weaker effect in muscle strength or physical performance. In muscle exercise, creatine is involved in the process of muscle contraction, and most studies have combined creatine supplementation with resistance exercise for interventions in patients with sarcopenia, which can increase muscle mass and strength, and also creatine has been considered as a preventive modality for elderly patients with sarcopenia (38).
The Chinese Consensus recommends that sarcopenic patients maintain a daily protein intake of 1.2–1.5 g/kg/day, with at least 50% being high-quality protein. For those with severe malnutrition, a minimum daily protein supplementation of 1.5 g/kg/day is advised (39). For instance, an elderly individual weighing 60 kg should consume 90 g of protein daily. Whey protein, abundant in essential amino acids like leucine and easily digestible, can effectively prevent muscle decay and enhance muscle strength when incorporated into the diet at 30–40 grams/day, alongside regular exercise. Protein intake should be evenly distributed across three meals to optimize muscle protein synthesis rates (36). Essential amino acids, particularly leucine, and L-carnitine are recommended for sarcopenic patients as they stimulate protein synthesis and ameliorate frailty, respectively (39, 40). β-Hydroxy-β-methylbutyric acid (HMB), a key metabolite in protein regulation, has been shown to have preventive and therapeutic effects on sarcopenia when used in conjunction with resistance training. It is suggested that elderly sarcopenic patients supplement with 3 g of HMB daily, particularly those with prolonged sedentary or bedridden periods (39). A study cited in the Asian Consensus demonstrated that oral nutritional supplements (ONS) containing 1.5–3 grams/day of the leucine derivative HMB, administered in two doses over 180 days, significantly improved nutritional outcomes and reduced the risk of malnutrition in the intervention group compared to the placebo group (40, 41).
4.3 Other rehabilitation
In the general population, hormonal imbalances may contribute to the development of sarcopenia, with hormones such as testosterone, growth hormone, insulin, thyroid hormones, and vitamin D potentially affecting its development. Hormone therapies that may improve the physical performance of sarcopenic patients, such as testosterone replacement therapy and selective androgen receptor modulators (SARMs), have been investigated for the treatment of sarcopenia, with promising results in terms of improved muscle mass and strength (42). Vitamin D is known to be associated with muscle strength, muscle size, and physical performance in patients with sarcopenia, but the outcomes of vitamin D replacement therapy remain controversial. Furthermore, it has also been suggested that the development of sarcopenia in patients with chronic kidney disease may be associated with an extended half-life of growth hormone (GH). In addition, it has been suggested that the development of sarcopenia may have a correlation with symptoms of insulin resistance, which may be related to reduced muscle glucose uptake, further impairs intracellular glucose metabolism and thus leads to muscle proteolysis, and may be a new avenue for targeted therapy (43). Gastric starvation hormone, estrogen, cortisol and dehydroepiandrosterone may be other factors in sarcopenia (44).
Targeted pharmacological interventions based on different hormonal pathways of action may alleviate further progression of sarcopenia, such as muscle growth inhibitor inhibitors and activin receptor type IIB (ActRIIB) ligand traps, which are being developed and tested for the treatment of sarcopenia, with potential benefits in terms of increasing muscle mass and strength (45).
Neuromuscular electrical stimulation (NMES) and whole-body vibration (WBV) are novel therapies, and recent research suggests that NMES and WBV may be useful adjuncts to traditional exercise interventions for sarcopenia (46, 47). Smith et al. (48) concluded that the improvements in balance and leg strength with WBV suggest that this physical intervention is beneficial in reducing the risk and incidence of falls. Machado et al. (49) suggest that adaptations to WBV may help offset the loss of muscle strength and mobility associated with age-induced sarcopenia by contributing to an increase in plantar flexor strength and power in older adults. In Paillard’s (50) study, NMES proved effective in improving postural balance function and reducing the risk of falls in older adults with muscle weakness, but there may be adverse effects of frequent interventions.
5 Conclusion
The rising prevalence of sarcopenia in the Philippines, driven by the aging population, highlights the urgent need for effective diagnostic, preventive, and treatment strategies. Despite the growing body of research, there remains a significant gap in the application of sarcopenia research to clinical practice and public health initiatives. Establishing localized diagnostic criteria and expanding treatment options are vital steps in tackling this emerging health challenge. Enhanced awareness, research, and implementation of evidence-based practices are essential to improving outcomes for older adults with sarcopenia in the Philippines.
Author contributions
JS: Writing – original draft. WZ: Writing – review & editing. PH: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (82202814), Shanghai Municipal Health Commission (GWVI-11.2-YQ08), Academic Mentorship for Scientific Research Cadre Project (AMSCP-23-03-01), and Capacity Building project of Local Colleges of Shanghai Science and Technology Commission (23010502800).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mohammed, M, and Li, J. Stroke-related sarcopenia among two different developing countries with diverse ethnic backgrounds (cross-National Study in Egypt and China). Healthcare. (2022) 10:2336. doi: 10.3390/healthcare10112336
2. Boshnjaku, A. Is age-related sarcopenia a real concern for my developing country? J Cachexia Sarcopenia Muscle. (2022) 13:2589–92. doi: 10.1002/jcsm.13107
3. Jacob, L, Gyasi, RM, Oh, H, Smith, L, Kostev, K, López Sánchez, GF, et al. Leisure-time physical activity and sarcopenia among older adults from low- and middle-income countries. J Cachexia Sarcopenia Muscle. (2023) 14:1130–8. doi: 10.1002/jcsm.13215
4. Alghannam, AF, Alharbi, DS, and Al-Hazzaa, HM. Sarcopenia of ageing: does a healthier lifestyle matter in reversing the trajectory? A brief narrative review and a call for action in Saudi Arabia. Saudi J Med Med Sci. (2024) 12:10–6. doi: 10.4103/sjmms.sjmms_54_23
5. Gay-As, MU, Lee, S-C, and Lai, F-C. Sarcopenia among older people in the Philippines: a scoping review. Creat Nurs. (2024) 30:133–44. doi: 10.1177/10784535241239684
6. Gabat, JAL, Faltado, AL, Sedurante, MB, and Tee, ML. Association of obesity and sarcopenia among adult Filipinos. Osteoporosis Sarcopenia. (2018) 4:109–13. doi: 10.1016/j.afos.2018.08.001
7. Chew, STH, Nguyen, HTT, Joshi, S, Kamaruzzaman, SB, Landi, F, Manuel, MV, et al. The rising tsunami of poor muscle health and sarcopenia in Asia-Pacific: time for focused attention and targeted interventions. Arch Gerontol Geriatr. (2024) 117:105275. doi: 10.1016/j.archger.2023.105275
8. Malmgren, LT, Fisher, PJ, Bookman, LM, and Uno, T. Age‐related changes in muscle Fiber types in the human Thyroarytenoid muscle: an Immunohistochemical and stereological study using confocal laser scanning microscopy. Otolaryngol Head Neck Surg. (1999) 121:441–51. doi: 10.1016/S0194-5998(99)70235-4
9. Breen, L, and Phillips, SM. Skeletal muscle protein metabolism in the elderly: interventions to counteract the 'anabolic resistance' of ageing. Nutr Metab. (2011) 8:68. doi: 10.1186/1743-7075-8-68
10. Cuthbertson, D, Smith, K, Babraj, J, Leese, G, Waddell, T, Atherton, P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, Aging Muscle. FASEB. (2005) 19:1–22. doi: 10.1096/fj.04-2640fje
11. Dalle, S, Rossmeislova, L, and Koppo, K. The role of inflammation in age-related sarcopenia. Front Physiol. (2017) 8:1045. doi: 10.3389/fphys.2017.01045
12. Phillips, SM, Tipton, KD, Aarsland, A, Wolf, SE, and Wolfe, RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab. (1997) 273:E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99
13. Griggs, RC, Kingston, W, Jozefowicz, RF, Herr, BE, Forbes, G, and Halliday, D. Effect of testosterone on muscle mass and muscle protein synthesis. J Appl Physiol. (1989) 66:498–503. doi: 10.1152/jappl.1989.66.1.498
14. Pedersen, BK, Steensberg, A, Keller, P, Keller, C, Fischer, C, Hiscock, N, et al. Muscle-derived Interleukin-6: Lipolytic, anti-inflammatory and immune regulatory effects. Pflugers Arch. (2003) 446:9–16. doi: 10.1007/s00424-002-0981-z
15. Reid, MB, and Li, Y-P. Tumor necrosis factor-Α and muscle wasting: a cellular perspective. Respir Res. (2001) 2:269–72. doi: 10.1186/rr67
16. Booth, FW, Roberts, CK, and Laye, MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. (2012) 2:1143–211. doi: 10.1002/cphy.c110025
17. Montero-Fernández, N, and Serra-Rexach, JA. Role of exercise on sarcopenia in the elderly. Eur J Phys Rehabil Med. (2013) 49:131–43.
18. Chen, LK, Woo, J, Assantachai, P, Auyeung, TW, Chou, MY, Iijima, K, et al. Asian working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7. doi: 10.1016/j.jamda.2019.12.012
19. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
20. Fernandes, LV, Paiva, AEG, Silva, ACB, and de Castro, IC. Prevalence of sarcopenia according to EWGSOP1 and EWGSOP2 in older adults and their associations with unfavorable health outcomes: a systematic review. Aging Clin Exp Res. (2022) 34:505–14. doi: 10.1007/s40520-021-01951-7
21. Stuck, AK, Tsai, LT, Freystaetter, G, Vellas, B, Kanis, JA, Rizzoli, R, et al. Comparing prevalence of sarcopenia using twelve sarcopenia definitions in a large multinational European population of community-dwelling older adults. J Nutr Health Aging. (2023) 27:205–12. doi: 10.1007/s12603-023-1888-y
22. Baumgartner, RN, Koehler, KM, Gallagher, D, Romero, L, Heymsfield, SB, Ross, RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. (1998) 147:755–63. doi: 10.1093/oxfordjournals.aje.a009520
23. Petermann-Rocha, F, Balntzi, V, Gray, SR, Lara, J, Ho, FK, Pell, JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and Meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:86–99. doi: 10.1002/jcsm.12783
24. Bohannon, RW, Wang, Y-C, Yen, S-C, and Grogan, KA. Handgrip strength: a comparison of values obtained from the Nhanes and Nih toolbox studies. Am J Occup Ther. (2019) 73:7302205080p1–9. doi: 10.5014/ajot.2019.029538
25. Studenski, S, Perera, S, Patel, K, Rosano, C, Faulkner, K, Inzitari, M, et al. Gait speed and survival in older adults. JAMA. (2011) 305:50–8. doi: 10.1001/jama.2010.1923
26. Tee, ML, Tee, CA, and Montemayor, EB. Determination of normative reference for the definition of sarcopenia among Filipinos. Osteoporosis Sarcopenia. (2016) 2:186–90. doi: 10.1016/j.afos.2016.07.004
27. Liu, CJ, and Latham, NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Datab Syst Rev. (2009) 3:1–10. doi: 10.1002/14651858.CD002759.pub2
28. Izquierdo, M, Merchant, RA, Morley, JE, Anker, SD, Aprahamian, I, Arai, H, et al. International exercise recommendations in older adults (Icfsr): expert consensus guidelines. J Nutr Health Aging. (2021) 25:824–53. doi: 10.1007/s12603-021-1665-8
29. Hsu, K-J, Liao, C-D, Tsai, M-W, and Chen, C-N. Effects of exercise and nutritional intervention on body composition, metabolic health, and physical performance in adults with Sarcopenic obesity: a meta-analysis. Nutrients. (2019) 11:2163. doi: 10.3390/nu11092163
30. Yoon, DH, Lee, J-Y, and Song, W. Effects of resistance exercise training on cognitive function and physical performance in cognitive frailty: a randomized controlled trial. J Nutr Health Aging. (2018) 22:944–51. doi: 10.1007/s12603-018-1090-9
31. Shen, Y, Shi, Q, Nong, K, Li, S, Yue, J, Huang, J, et al. Exercise for sarcopenia in older people: a systematic review and network meta-analysis. J Cachexia Sarcopenia Muscle. (2023) 14:1199–211. doi: 10.1002/jcsm.13225
32. Kirkman, DL, Lee, DC, and Carbone, S. Resistance exercise for cardiac rehabilitation. Prog Cardiovasc Dis. (2022) 70:66–72. doi: 10.1016/j.pcad.2022.01.004
33. Hurst, C, Robinson, SM, Witham, MD, Dodds, RM, Granic, A, Buckland, C, et al. Resistance exercise as a treatment for sarcopenia: prescription and delivery. Age Ageing. (2022) 51:afac003. doi: 10.1093/ageing/afac003
34. Sayer, AA, and Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing. (2022) 51:afac220. doi: 10.1093/ageing/afac220
35. Deutz, NEP, Bauer, JM, Barazzoni, R, Biolo, G, Boirie, Y, Bosy-Westphal, A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the Espen expert group. Clin Nutr. (2014) 33:929–36. doi: 10.1016/j.clnu.2014.04.007
36. Gielen, E, Beckwée, D, Delaere, A, De Breucker, S, Vandewoude, M, and Bautmans, I. Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: an umbrella review of systematic reviews and Meta-analyses. Nutr Rev. (2021) 79:121–47. doi: 10.1093/nutrit/nuaa011
37. Bauer, J, Biolo, G, Cederholm, T, Cesari, M, Cruz-Jentoft, AJ, Morley, JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the Prot-age study group. J Am Med Dir Assoc. (2013) 14:542–59. doi: 10.1016/j.jamda.2013.05.021
38. Kreider, RB, Kalman, DS, Antonio, J, Ziegenfuss, TN, Wildman, R, Collins, R, et al. International Society of Sports Nutrition Position Stand: safety and efficacy of Creatine supplementation in exercise, sport, and medicine. J Int Soc Sports Nutr. (2017) 14:18. doi: 10.1186/s12970-017-0173-z
39. Cui, H, Wang, Z, Wu, J, Liu, Y, Zheng, J, Xiao, W, et al. Chinese expert consensus on prevention and intervention for elderly with sarcopenia (2023). Aging Med. (2023) 6:104–15. doi: 10.1002/agm2.12245
40. Chen, LK, Arai, H, Assantachai, P, Akishita, M, Chew, STH, Dumlao, LC, et al. Roles of nutrition in muscle health of community-dwelling older adults: evidence-based expert consensus from Asian working Group for Sarcopenia. J Cachexia Sarcopenia Muscle. (2022) 13:1653–72. doi: 10.1002/jcsm.12981
41. Abe, S, Ezaki, O, and Suzuki, M. Medium-chain triglycerides (8:0 and 10:0) are promising nutrients for sarcopenia: a randomized controlled trial. Am J Clin Nutr. (2019) 110:652–65. doi: 10.1093/ajcn/nqz138
42. Bhasin, S, Krishnan, V, Storer, TW, Steiner, M, and Dobs, AS. Androgens and selective androgen receptor modulators to treat functional limitations associated with aging and chronic disease. J Gerontol Series A. (2023) 78:25–31. doi: 10.1093/gerona/glad027
43. Kumagai, H, Coelho, AR, Wan, J, Mehta, HH, Yen, K, Huang, A, et al. Mots-C reduces Myostatin and muscle atrophy signaling. Am J Physiol Endocrinol Metab. (2021) 320:E680–90. doi: 10.1152/ajpendo.00275.2020
44. Gungor, O, Ulu, S, Hasbal, NB, Anker, SD, and Kalantar-Zadeh, K. Effects of hormonal changes on sarcopenia in chronic kidney disease: where are we now and what can we do? J Cachexia Sarcopenia Muscle. (2021) 12:1380–92. doi: 10.1002/jcsm.12839
45. Rolland, Y, Dray, C, Vellas, B, and Barreto, PDS. Current and investigational medications for the treatment of sarcopenia. Metabolism. (2023) 149:155597. doi: 10.1016/j.metabol.2023.155597
46. Doucet, BM, Lam, A, and Griffin, L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J Biol Med. (2012) 85:201–15.
47. Gonçalves de Oliveira, R, Coutinho, HMEL, Martins, MNM, Bernardo-Filho, M, de Sa-Caputo, DC, Campos de Oliveira, L, et al. Impacts of whole-body vibration on muscle strength, power, and endurance in older adults: a systematic review and meta-analysis. J Clin Med. (2023) 12:4467. doi: 10.3390/jcm12134467
48. Smith, DT, Judge, S, Malone, A, Moynes, RC, Conviser, J, and Skinner, JS. Effects of biodensity training and power plate whole-body vibration on strength, balance, and functional Independence in older adults. J Aging Phys Act. (2016) 24:139–48. doi: 10.1123/japa.2015-0057
49. Machado, A, García-López, D, González-Gallego, J, and Garatachea, N. Whole-body vibration training increases muscle strength and mass in older women: a randomized-controlled trial. Scand J Med Sci Sports. (2010) 20:200–7. doi: 10.1111/j.1600-0838.2009.00919.x
50. Paillard, T. Acute and chronic neuromuscular electrical stimulation and postural balance: a review. Eur J Appl Physiol. (2020) 120:1475–88. doi: 10.1007/s00421-020-04383-9
51. Cruz, G, Saito, Y, and Natividad, JN. Active life expectancy and functional health transition among Filipino older people. Can Stud Popul. (2007) 34:29–47. doi: 10.25336/P62K6R
52. Chua, JR, and Tee, ML. Association of Sarcopenia with osteoporosis in patients with chronic obstructive pulmonary disease. Osteoporosis Sarcopenia. (2020) 6:129–32. doi: 10.1016/j.afos.2020.07.004
Keywords: sarcopenia, Philippines, rehabilitation, older people, diagnosis
Citation: Sun J, Zhang W and Han P (2025) Prospects for the diagnosis and treatment of sarcopenia in the Philippines. Front. Med. 11:1501501. doi: 10.3389/fmed.2024.1501501
Edited by:
Marios Kyriazis, National Gerontology Centre, CyprusReviewed by:
Arben Boshnjaku, University of Gjakova “Fehmi Agani,” AlbaniaCopyright © 2025 Sun, Zhang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peipei Han, aGFucHBAc3VtaHMuZWR1LmNu
 Ji Sun
Ji Sun Weixin Zhang
Weixin Zhang Peipei Han
Peipei Han