- 1Department of Internal Medicine, Brugmann University Hospital, Université Libre de Bruxelles, Brussels, Belgium
- 2Department of Nephrology, Brugmann University Hospital, Université Libre de Bruxelles, Brussels, Belgium
- 3Department of Microbiology, Brugmann University Hospital, Université Libre de Bruxelles, Brussels, Belgium
- 4Department of Rheumatology, Brugmann University Hospital, Université Libre de Bruxelles, Brussels, Belgium
- 5Department of Infectious Diseases, Brugmann University Hospital, Université Libre de Bruxelles, Brussels, Belgium
Staphylococcus lugdunensis is a facultatively anaerobic gram-positive cocci of the coagulase-negative staphylococcus (CoNS) species. Initially considered as commensal, S. lugdunensis has been found to be responsible for a wide range of disseminated infections in humans (bacteriemia, foreign-body infection, endocarditis, arthritis, osteomyelitis, …) thereby often compared to Staphylococcus aureus in terms of virulence behavior. We present the case of a 62-year-old woman with end-stage renal disease, undergoing hemodialysis through an arteriovenous fistula (AVF) of the left forearm. She was diagnosed with S. lugdunensis bacteriemia and secondary native-knee septic arthritis. Endocarditis was ruled out and the patient evolved well with a 6-week course of IV cefazolin. Four months later, she consulted the rheumatology department with a recurrent right knee arthritis. Cultures came back positive for an identical multi-sensitive S. lugdunensis. Endocarditis was ruled out and full body 18F-FDG PET-CT showed no secondary location but a focal hypermetabolic activity in the left forearm fistula area. AVF Doppler showed no sign of collection nor thrombophlebitis around the fistula. We concluded in an infection of the fistula due to repeated punctures (recurrent cannulation) as entry point. She was treated with a 12-week oral combination of ciprofloxacin and trimethoprim-sulfamethoxazole, and her symptoms have not returned since. S. lugdunensis commonly causes prosthetic and arthroscopy-related joint infections. As native-joint septic arthritis is unusual, we conducted a review of the literature and discuss the burden of disseminated S. lugdunensis infections among dialysis patients.
Introduction
Staphylococcus lugdunensis is a coagulase-negative staphylococcus (CoNS) species, firstly described by Jean Freney et al. (1) at the Center National de Référence des Staphylocoques, Lyon, France (thereby giving its name lugdunensis, Latin adjective of lugdunum, Latin name of Lyon). The strains were isolated from different parts of the human body (blood, axillary lymph node, umbilicus, intrauterine device, thoracic and abscess drain), and were described as nonsporulating, nonmotile and facultatively anaerobic gram-positive cocci, with a negative coagulase test with rabbit and bovine plasma (1).
Human-associated CoNS are mainly represented by Staphylococcus epidermidis-like group (including S. epidermidis, S. haemolyticus, S. hominis, S. capitis, …) which often cause foreign body-infections and related bloodstream infections as well as preterm newborn infections (2). Other CoNS species include Staphylococcus saprophyticus (mostly found in urinary tract infections) and Staphylococcus lugdunensis.
S. lugdunensis is physiologically found in human skin and mucosa, principally in the perineum and groin area, axilla, lower limbs and toes regions (3). Initially considered as commensal, S. lugdunensis has proved to play an important role in a wide range of infections in humans (4, 5). Indeed, while most of CoNS species were historically considered as low virulence pathogens, S. lugdunensis is considered as more aggressive and often compared to Staphylococcus aureus in terms of virulence behavior. It is responsible for a relatively similar spectrum of invasive and disseminated infections in humans (6, 7). This can be explained by analogous features present in both species, namely regions of homology in the accessory gene regulator locus (regulating virulence factors), heat-stable δ-like hemolysin (avoiding killing and digestion from phagosomes) and OatA (O-acetyltransferase) explaining its resistance capacity to lysozymes (4). However, toxins production by S. aureus such as enterotoxins, TSST and exfoliatin have not been observed in S. lugdunensis. Differentiating S. lugdunensis from other CoNS species seems particularly important in numerous situations, as management of staphylococcal bacteriemia differs significantly following the involved subspecies. In spite of that, many laboratories did not routinely identify CoNS subspecies, therefore its pathogenic role in humans may be underestimated (2). S. lugdunensis commonly causes skin and soft tissue infections, endocarditis including on native and prosthetic valves, catheter-related bacteriemia, prosthetic joint infections or arthroscopy-related joint infections, osteomyelitis and discitis (2). Foreign body-associated infections are easily explained by its adherence capacity and biofilm formation (4, 8). However, some rarer cases of native joint infections have been described.
Case presentation
We present a 62-year-old woman diagnosed with recurrent right knee arthritis due to S. lugdunensis. Her medical history included end-stage renal disease (ESRD), type 2 diabetes, high blood pressure, bilateral gonarthrosis and cured B and C hepatitis. She underwent hemodialysis (HD) through a native arteriovenous fistula (AVF) of the left forearm surgically created in June 2019. AVF puncture was performed utilizing the buttonhole technique for hemodialysis.
She was admitted to the emergency department in April 2023 in a context of growing pain and swelling of her right knee for about a week, without any exterior trauma. She did not report fever nor chills over the past few days prior to the hospitalization. On physical examination, her right knee was warm and swollen, with a positive patellar tap test. Cardiac and pulmonary examinations were normal. The AVF exhibited a normal thrill, there was no pain nor sign of local inflammation.
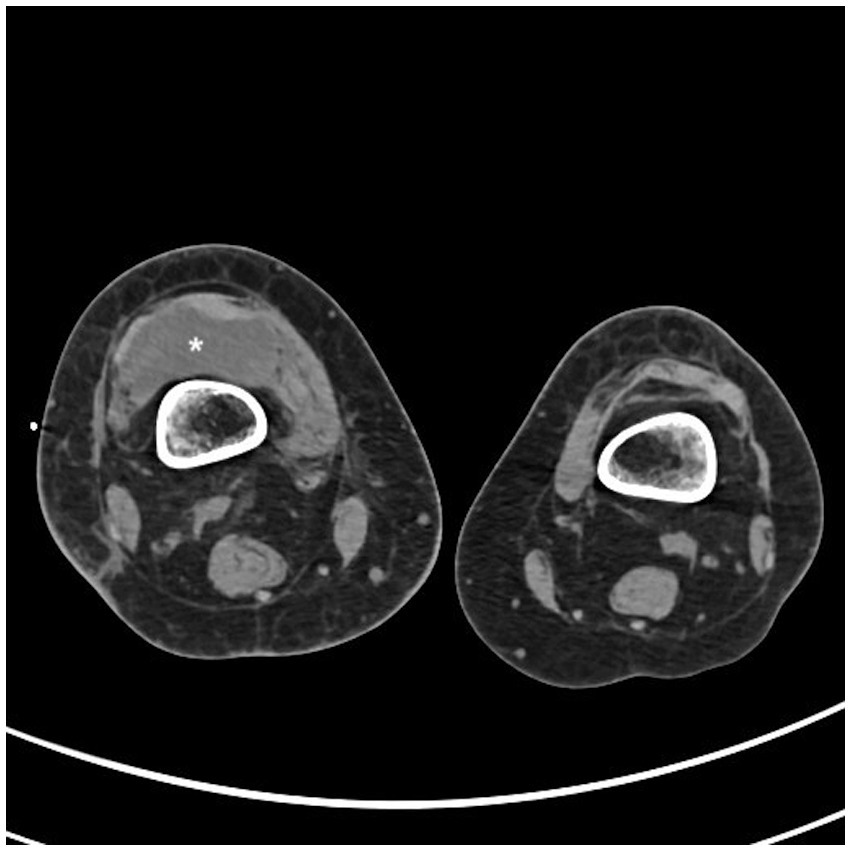
Conventional radiography of the right knee showed an irregular lateral tibial plateau. Computed tomography (CT) scan of the right knee showed a voluminous intra-articular effusion (Figure 1), internal femorotibial osteoarthritis and no sign of bone fracture. Knee ultrasound showed a large heterogeneous intra-articular compartmentalized effusion. Biology showed an elevated c-reactive protein (CRP) of 108.5 mg/L without hyperleukocytosis. Ultrasound-guided puncture of the right knee revealed 120.000 red blood cells (RBC)/μL, 91.675 white blood cells (WBC)/μL, including 99% neutrophils. Empiric antibiotherapy with IV oxacillin was initiated. Aerobic culture came back positive for Staphylococcus lugdunensis, which was sensitive to all the antibiotics tested (including oxacillin, clindamycin, trimethoprim-sulfamethoxazole, ciprofloxacin, linezolid, rifampicin, vancomycin). Blood cultures from the AVF came back positive for S. lugdunensis whereas three sets of blood cultures from peripheral veins at the same time remained negative. Cultures of repeated knee punctures to release effusion kept positive for S. lugdunensis for 5 days. She benefited from arthroscopic drainage and washout. Endocarditis was ruled out with transthoracic and transesophageal echocardiograms. AVF Doppler showed fibrous scarring at the venous puncture site but no sign of collection around the fistula nor thrombophlebitis.
Antibiotics were changed to post-dialysis cefazolin (schedule 2–2-3 grams) for a total duration of 6 weeks. She had a good clinical and biological evolution (CRP dropped to 0.6 mg/L). Follow-up blood cultures were negative. The patient pursued HD sessions three times weekly through her left forearm AVF.
Four months later, the patient consulted the rheumatology department with a recurrent right knee arthritis. Blood test showed an increased CRP of 96.8 mg/L, total WBC 4490/μL including 2,860 neutrophiles. Cytology from effusion of the right knee showed 6.200 RBC/μL and 183.772 WBC/μL including 87% neutrophils, 8% monocytes, 5% lymphocytes. Aerobic and anaerobic cultures from effusion of the right knee were negative but PCR 16S rRNA identified Staphylococcus lugdunensis. The day after admission she benefited from arthroscopic drainage and synovial biopsies were performed: bacterial cultures came back positive for S. lugdunensis with an identical antibiogram as during the first episode. Endocarditis was ruled out once again. Full body 18F-FDG PET-CT (Figure 2A) showed a hypermetabolic synovitis of the right knee (SUVmax 5.0). In addition, a discrete focal hypermetabolic activity in the left forearm fistula area (SUVmax 2.9) was revealed, with no visible peripheral soft tissue infiltration (Figure 2B). The hypothesis of the infection of the fistula by repeated punctures (recurrent cannulation) as entry point was evoked.
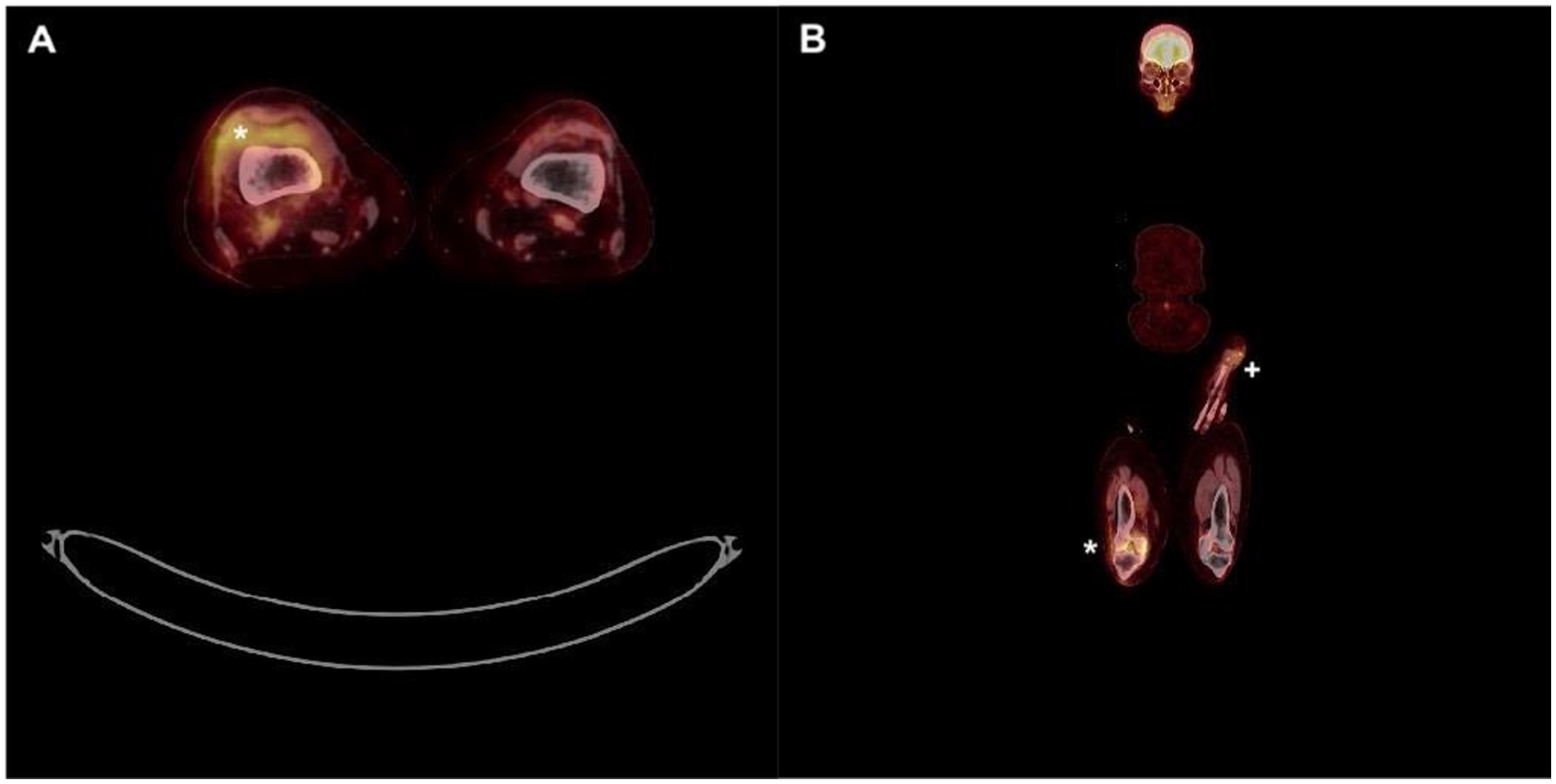
Figure 2. Full body 18F-FDG PET-CT. (A) Hypermetabolic synovitis of the right knee (*). (B) Focal hypermetabolic activity in the left forearm fistula area (+) as well as right knee (*).
Initial treatment consisted of ceftriaxone followed by flucloxacillin and then oral combination of ciprofloxacin (500 mg daily) together with trimethoprim-sulfamethoxazole (400/80 mg twice a day) for a total duration of 12 weeks. The patient evolved well and remained asymptomatic, more than 6 months after antibiotic cessation.
Discussion
Multiple cases of severe S. lugdunensis infections in the course of hemodialysis have been previously reported. In most of these patients, the presumed portal of entry is the vascular access device (9, 10). In our case, the patient had neither a foreign body nor a dialysis catheter. The main hypothesis of the entry point is repeated skin penetration through recurrent cannulation of the fistula for the dialysis access.
In a retrospective study of 36 cases of S. lugdunensis bacteriemia, seven patients (19%) were on hemodialysis (11). Although arteriovenous fistulas are less frequently associated with infections compared to dialysis catheters placed in the internal jugular vein, multiple cases of disseminated S. lugdunensis infections on hemodialysis patients with AVF have been described, including bacteriemia and endocarditis (12, 13).
The buttonhole technique consists in creating a permanent puncture site, by using a sharp needle in the same location for cannulation during each dialysis session until the creation of a tract of scar tissue. This allows for easier and more consistent needle insertion. The buttonhole cannulation is associated with less bleeding complication and aneurysm formation. However, this technic appears to be associated with an increased infectious risk (14). Constant observance of reinforced hygiene protocols by trained staff and central coordination can significantly reduce the infectious risk associated with buttonhole cannulation (14). Regular assessment and monitoring of the access site are necessary to detect any signs of complications. AVF has been proposed as a potential source for S. lugdunensis infections even without localized signs of infection as in our patient. The buttonhole method was proposed as a major contributor to the development and persistence of bacteremia (13). Numerous cases of S. lugdunensis peritonitis have been published in the context of peritoneal dialysis as well, showcasing the burden of S. lugdunensis infections among patients undergoing dialysis (15).
In these patients, disseminated infections are feared as S. lugdunensis bacteriemia are frequently associated with endocarditis. In a retrospective study of 28 cases of patients with positive S. lugdunensis blood culture, 13 (46%) patients had endocarditis from which 85% underwent cardiac surgery and 23% of them died (16). Fifteen patients (54%) without endocarditis had no complications related to the bacteriemia. Among these patients, 73% had an indwelling medical device. In another retrospective study of 21 cases of S. lugdunensis bacteriemia, among those who appeared to have clinically significant bacteriemia, five patients had catheter-related infections. Among these five patients, three were on hemodialysis (17).
However, the clinical implications of single S. lugdunensis blood culture positivity remain unclear. A retrospective study was published about clinical outcomes in 41 patients with single or multiple positive blood cultures (18). Overall 30-day mortality rates were 13.3% for single-set patients and 18.2% for multiple-set patients, but 90-day mortality rates were 36.7% for single-set and 18.2% for multiple-set patients. In that study, five patients with a single positive blood culture, who did not meet the criteria for true bacteriemia, and who did not receive antibiotic therapy, were followed up and evolved well. This indicates that in some low-risk clinical situations, single S. lugdunensis blood culture may represent contamination or low bacteriemia that might be considered clinically insignificant. Proposed criteria to better identify such patients include the absence of an apparent source of infection, time between culture collection and positivity, a low Pitt bacteriemia score (based on body temperature, hypotension, altered mental status, mechanical ventilation and cardiac arrest) and the SIRS criteria (based on body temperature, heart and respiratory rates, total WBC count) (18).
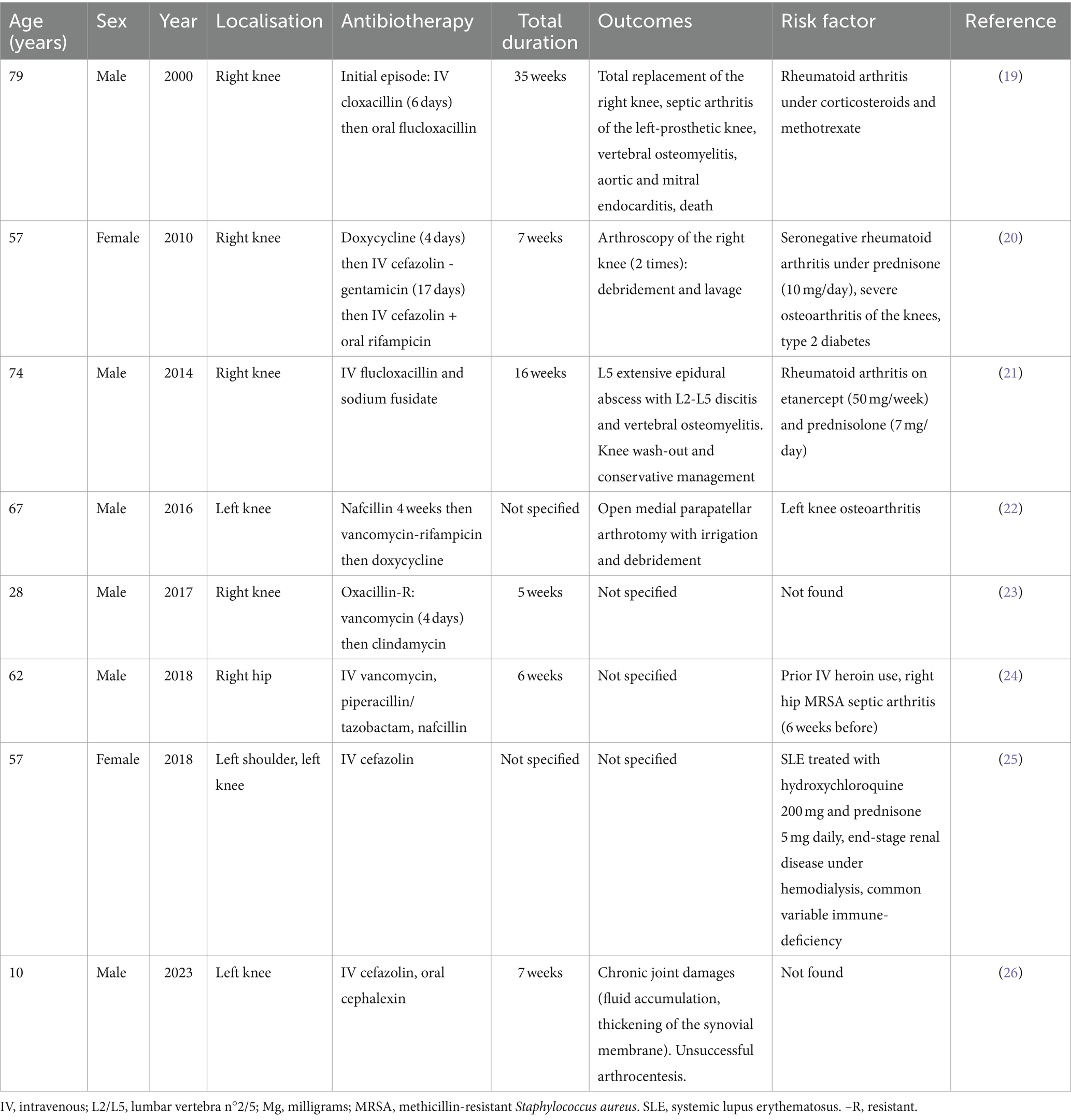
Because native joint S. lugdunensis septic arthritis are uncommon, we conducted a review of the literature (Table 1). We found eight previously published cases on PubMed (19–26). There were six males and two females, the median age was 54.2 years. There were seven cases of knee arthritis, one hip and one shoulder. Underlying factors such as IV drug abuse, immunosuppressive medications and immune disorders were identified most of the times. One of the eight patients was on hemodialysis as well but we found no details on the assessment of underlying bacteriemia or catheter infection. Interestingly, six patients had suffered from joint conditions before the episode of septic arthritis: three had rheumatoid arthritis, one had systematic lupus erythematosus, two had underlying osteoarthritis of the infected joint, and one had septic arthritis in the past. Regarding outcomes, two patients underwent arthroscopy washout, one had open arthrotomy debridement, one had total knee replacement and one patient had significant joint damage. Two patients were diagnosed with associated discitis and vertebral osteomyelitis. One of these patients died of acute respiratory and circulatory failure in the course of the infection. He was the only patient among the eight to be diagnosed with infectious endocarditis. Total antibiotic duration ranged from 5 to 35 weeks (two not specified). Most patients were treated with long-term IV cefazolin. Two were treated with nafcillin and two others with flucloxacillin. Only one was oxacillin-resistant and was treated with vancomycin followed by prolonged oral clindamycin.
Staphylococcus lugdunensis is considered to be sensitive to most antimicrobial agents, including cefazolin, daptomycin, linezolid, moxifloxacin, nafcillin, rifampicin, quinupristin-dalfopristin, tetracycline, trimethoprim-sulfamethoxazole and vancomycin (27). Mechanisms of antibiotic therapy failure include biofilm formation (8). In a recent study by de Oliveira et al. (28), S. lugdunensis isolates did not show in vitro resistance to oxacillin, vancomycin, erythromycin, gentamicin, linezolid and sulfamethoxazole-trimethoprim. In that study, the presence of a biofilm was associated with a small increase in the MIC for linezolid and vancomycin, but did not cause resistance to these antibiotics, which was the case in Frank et al. study (27). Vancomycin was not bactericidal against 93% of S. lugdunensis isolates growing in biofilm, thereby suggesting vancomycin tolerance in this species. Moxifloxacin is one of the most efficient agents against S. lugdunensis biofilms (27). Resistance to beta-lactams and macrolides have been reported in the presence of a biofilm but remain infrequent (28).
The total recommended duration of antibiotic therapy for S. lugdunensis septic arthritis is at least 6 weeks according to the guidelines from the French Infectious Diseases Society (SPLIF) (29). Treatment and management should be similar to that of S. aureus septic arthritis. IV cefazolin or penicillin such as cloxacillin and oxacillin are the reference for the initial treatment of methicillin-sensitive strains. In the case of methicillin-resistant staphylococcus, daptomycin in monotherapy is recommended as first-line therapy with vancomycin and teicoplanin as alternatives. In our case, a first 6-week regimen of IV cefazolin was completed and led to remission. However, to treat the recurrence of S. lugdunensis septic arthritis, a second therapy of a 12-week oral combination of ciprofloxacin and trimethoprim-sulfamethoxazole was proposed and led to clinical and biological remission.
Conclusion
This case report and review of the literature showcase the virulent behavior of S. lugdunensis and its ability to cause septic arthritis of native joints, outside the well-known context of prosthetic joint infections or arthroscopy-related joint infections.
S. lugdunensis is responsible for a wide range of infections in humans and should be given full consideration when isolated in bacterial cultures. Due to its aggressive behavior, some authors have drawn a parallel with S. aureus in terms of therapeutic management. However, it is important to bear in mind that in some cases, the identification of S. lugdunensis may represent contamination or be of minor clinical significance. This case also highlights the burden of disseminated and relapsing S. lugdunensis infections among dialysis patients. It emphasizes the need for a comprehensive and in-depth disease assessment as well as the need for adapted and extensive antibiotic therapies. Finally, it is crucial to ensure compliance with good practice guidelines for the prevention of vascular access infections in such fragile patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
A-RW: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. MT: Writing – review & editing. JN: Writing – review & editing. BM: Writing – review & editing. IR: Writing – review & editing. EM: Writing – review & editing. PC: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Freney, J, Brun, Y, Bes, M, Meugnier, H, Grimont, F, Grimont, PAD, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clincial specimens. Int J Syst Bacteriol. (1988) 38:168–72. doi: 10.1099/00207713-38-2-168
2. Becker, K, Heilmann, C, and Peters, G. Coagulase-negative staphylococci. Clin Microbiol Rev. (2014) 27:870–926. doi: 10.1128/CMR.00109-13
3. Bieber, L, and Kahlmeter, G. Staphylococcus lugdunensis in several niches of the normal skin flora. Clin Microbiol Infect. (2010) 16:385–8. doi: 10.1111/j.1469-0691.2009.02813.x
4. Babu, E, and Oropello, J. Staphylococcus lugdunensis: the coagulase-negative staphylococcus you don't want to ignore. Expert Rev Anti-Infect Ther. (2011) 9:901–7. doi: 10.1586/eri.11.110
5. Argemi, X, Hansmann, Y, Riegel, P, and Prévost, G. Is Staphylococcus lugdunensis significant in clinical samples? J Clin Microbiol. (2017) 55:3167–74. doi: 10.1128/JCM.00846-17
6. Ravaioli, S, Selan, L, Visai, L, Pirini, V, Campoccia, D, Maso, A, et al. Staphylococcus lugdunensis, an aggressive coagulase-negative pathogen not to be underestimated. Int J Artif Organs. (2012) 35:742–53. doi: 10.5301/ijao.5000142
7. Non, LR, and Santos, CA. The occurrence of infective endocarditis with Staphylococcus lugdunensis bacteremia: a retrospective cohort study and systematic review. J Infect. (2017) 74:179–86. doi: 10.1016/j.jinf.2016.10.003
8. Argemi, X, Hansmann, Y, Prola, K, and Prévost, G. Coagulase-negative staphylococci Pathogenomics. Int J Mol Sci. (2019) 20:1215. doi: 10.3390/ijms20051215
9. Kamaraju, S, Nelson, K, Williams, DN, Ayenew, W, and Modi, KS. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. (1999) 19:605–8. doi: 10.1159/000013528
10. Michelen, YE, Lobo, Z, Walshon-Dipillo, D, and Psevdos, G. Recurrent Staphylococcus lugdunensis osteomyelitis of the lumbar spine in a patient on chronic hemodialysis. J Glob Infect Dis. (2020) 12:231–3. doi: 10.4103/jgid.jgid_17_20
11. Hauser, N, Kim, JJ, Luethy, PM, Schmalzle, SA, and Bork, JT. Multicenter retrospective cohort study of the clinical significance of Staphylococcus lugdunensis isolated from a single blood culture set. Diagn Microbiol Infect Dis. (2021) 99:115261. doi: 10.1016/j.diagmicrobio.2020.115261
12. Mousa, A, Ghazy, A, Kakhktsyan, T, Chepenko, K, and Young, K. Staphylococcus lugdunensis infective endocarditis with mitral valve Vegetations in a hemodialysis patient with recurrent arteriovenous fistula cannulation: a case report. Cureus. (2023) 15:e39853. doi: 10.7759/cureus.39853
13. Saito, AK, and Wu, S. High-grade Staphylococcus lugdunensis bacteremia in a patient on home hemodialysis. Fed Pract. (2023) 40:123–7. doi: 10.12788/fp.0361
14. Labriola, L, Crott, R, Desmet, C, Romain, C, and Jadoul, M. Infectious complications associated to buttonhole cannulation of native arteriovenous fistulas: a 22-year follow-up. Nephrol Dial Transplant. (2023) 39:1000–7. doi: 10.1093/ndt/gfad229
15. Ludlam, H, and Phillips, I. Staphylococcus lugdunensis peritonitis. Lancet. (1989) 334:1394. doi: 10.1016/s0140-6736(89)92001-1
16. Zinkernagel, AS, Zinkernagel, MS, Elzi, MV, Genoni, M, Gubler, J, Zbinden, R, et al. Significance of Staphylococcus lugdunensis bacteremia: report of 28 cases and review of the literature. Infection. (2008) 36:314–21. doi: 10.1007/s15010-008-7287-9
17. Ebright, JR, Penugonda, N, and Brown, W. Clinical experience with Staphylococcus lugdunensis bacteremia: a retrospective analysis. Diagn Microbiol Infect Dis. (2004) 48:17–21. doi: 10.1016/j.diagmicrobio.2003.08.008
18. Sasaki, T, and Doi, Y. Staphylococcus lugdunensis bacteremia: clinical implications of single set positive blood cultures. Diagn Microbiol Infect Dis. (2023) 105:115835. doi: 10.1016/j.diagmicrobio.2022.115835
19. Kragsbjerg, P, Bomfim-Loogna, J, Törnqvist, E, and Söderquist, B. Development of antimicrobial resistance in Staphylococcus lugdunensis during treatment-report of a case of bacterial arthritis, vertebral osteomyelitis and infective endocarditis. Clin Microbiol Infect. (2000) 6:496–9. doi: 10.1046/j.1469-0691.2000.00103.x
20. Grupper, M, Potasman, I, Rosner, I, Slobodin, G, and Rozenbaum, M. Septic arthritis due to Staphylococcus lugdunensis in a native joint. Rheumatol Int. (2010) 30:1231–3. doi: 10.1007/s00296-009-1044-y
21. Rose, AM, Barnett, J, Morris-Jones, S, and Marks, DJ. Staphylococcus lugdunensis septic arthritis and epidural abscess in a patient with rheumatoid arthritis receiving anti-tumour necrosis factor therapy. Rheumatology (Oxford). (2014) 53:2231. doi: 10.1093/rheumatology/keu365
22. Begly, JP, Sobieraj, M, Liporace, FA, and Dayan, A. Staphylococcus lugdunensis septic arthritis of a native knee a case report. Bull Hosp Jt Dis 2013. (2016) 74:314–7.
23. Tan, CD, Moritz, D, and Lora, AJM. S. lugdunensis native-joint septic arthritis: case report and review of the literature. Case Rep Infect Dis. (2017) 2017:8903907. doi: 10.1155/2017/8903907
24. Gaglani, B, Dahdouh, M, and Shah, K. Septic arthritis of native hip joint by Staphylococcus lugdunensis: a case report. Rev Soc Bras Med Trop. (2018) 51:554–6. doi: 10.1590/0037-8682-0169-2017
25. Laloo, A, and Kyttaris, VC. Polyarticular septic arthritis caused by Staphylococcus lugdunensis in a patient with systemic lupus erythematosus. Eur J Rheumatol. (2018) 5:266–8. doi: 10.5152/eurjrheum.2018.18037
26. Greenboim Kraushar, I, Livni, G, and Pasternak, Y. Staphylococcus lugdunensis septic arthritis in a pediatric patient. Pediatr Infect Dis J. (2023) 42:e426–7. doi: 10.1097/INF.0000000000004030
27. Frank, KL, Reichert, EJ, Piper, KE, and Patel, R. In vitro effects of antimicrobial agents on planktonic and biofilm forms of Staphylococcus lugdunensis clinical isolates. Antimicrob Agents Chemother. (2007) 51:888–95. doi: 10.1128/AAC.01052-06
28. de Oliveira, A, Cataneli Pereira, V, Pinheiro, L, Moraes Riboli, DF, Benini Martins, K, de Souza, R, et al. Antimicrobial resistance profile of planktonic and biofilm cells of Staphylococcus aureus and coagulase-negative staphylococci. Int J Mol Sci. (2016) 17:1423. doi: 10.3390/ijms17091423
Keywords: Staphylococcus lugdunensis, septic arthritis, bacteriemia, hemodialysis, arteriovenous fistula
Citation: Wery AR, Taghavi M, Nortier J, Mahadeb B, Raftakis I, Maillart E and Clevenbergh P (2024) Staphylococcus lugdunensis: an unusual cause of relapsing hematogenous septic arthritis of a native knee. Case report and review of the literature. Front. Med. 11:1494449. doi: 10.3389/fmed.2024.1494449
Edited by:
Sam Donta, Falmouth Hospital, United StatesReviewed by:
Anna Budzyńska, Nicolaus Copernicus University in Toruń, PolandGeraldine Durand, BioMérieux (France), France
Copyright © 2024 Wery, Taghavi, Nortier, Mahadeb, Raftakis, Maillart and Clevenbergh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandre-Raphael Wery, QWxleGFuZHJlLndlcnlAdWxiLmJl
 Alexandre-Raphael Wery
Alexandre-Raphael Wery Maxime Taghavi
Maxime Taghavi Joelle Nortier2
Joelle Nortier2