- 1Center of Integrative Medicine, Beijing Ditan Hospital, Capital Medical University, Beijing, China
- 2Center of Integrative Medicine, Peking University Ditan Teaching Hospital, Beijing, China
Background: Non-alcoholic fatty liver disease (NAFLD) or metabolic dysfunction-associated steatotic liver disease (MASLD) is closely associated with chronic inflammation and lipid metabolism disorders. The neutrophil-to-high-density lipoprotein cholesterol ratio (NHR) is an integrative marker reflecting inflammatory responses and lipid metabolism disorders and is associated with various diseases. This cross-sectional study aimed to determine the association between NHR and NAFLD, MASLD, and liver fibrosis.
Methods: Data for this study were obtained from the 2017–2020 National Health and Nutrition Examination Survey (NHANES), we employed weighted multiple regression and restricted cubic spline (RCS) analysis to assess the relationship between NHR and NAFLD, MASLD, and liver fibrosis. Additionally, we performed stratified analyses based on gender, age, body mass index, diabetes, hypertension, smoking status, and history of cardiovascular disease to evaluate the consistency of these associations across different subgroups.
Results: A total of 6,526 participants were included in the study. 2,839 (weighted 44.1%) participants were diagnosed with NAFLD and 2,813 (weighted 43.7%) participants were diagnosed with MASLD. After adjusting for confounders, NHR was positively associated with the risk of NAFLD/MASLD, and the correlation was particularly significant in the subgroups of females, those without hypertension, and those without diabetes (p < 0.05). By the NHR quartile, the risk of NAFLD/MASLD increased progressively with higher NHR levels (P for trend <0.001). In addition, RCS analysis showed a nonlinear association between NHR and NAFLD/MASLD and liver fibrosis (P-non-linear <0.05).
Conclusion: NHR may serve as a potential marker for NAFLD/MASLD and liver fibrosis, and lowering NHR levels could help reduce the incidence of these conditions.
Introduction
As modern lifestyles change, the incidence of metabolic diseases such as obesity and diabetes continue to rise, and the closely related problem of liver disease is becoming more and more prominent. Among them, non-alcoholic fatty liver disease (NAFLD) has become an important burden on global public health, affecting about a third of the world’s adult population (1, 2). As NAFLD is closely associated with multiple metabolic dysfunctions, to characterize this disease more accurately, in June 2023, an international consensus of experts decided to rename NAFLD as MASLD, emphasizing the central role of cardiometabolic risk factors in the development of the disease (3). The pathological features of NAFLD/MASLD include hepatocellular steatosis, inflammatory cell infiltration, and different degrees of hepatic fibrosis (4). For patients who have developed advanced hepatic fibrosis, the leading causes of death are liver-related complications and extrahepatic malignancies (5). For patients with NAFLD/MASLD who have not developed liver fibrosis, cardiovascular disease is the leading cause of death (6). Therefore, early detection of NAFLD/MASLD and liver fibrosis is crucial for timely intervention and effective management.
Inflammatory response and abnormal lipid metabolism are critical in causing and developing NAFLD/MASLD (7). As an essential component of the innate immune system, neutrophils play a central role in fighting infections. They are closely associated with various chronic inflammatory diseases, especially metabolic diseases such as obesity, type 2 diabetes, and NAFLD/MASLD (8). Several studies have shown that the degree of neutrophil infiltration in liver tissue of NAFLD/MASLD patients is positively correlated with the severity of the lesion. Neutrophil infiltration can directly lead to hepatocellular injury and accelerate the progression of NAFLD/MASLD to hepatic fibrosis and cirrhosis by promoting fibrosis (7–9). NAFLD/MASLD begins with excessive accumulation of triglycerides in the hepatocytes and is accompanied by a reduction in plasma cholesterol levels associated with antiatherosclerosis high-density lipoproteins (10). HDL-C is critical in developing NAFLD/MASLD through lipid metabolism modulation and reverse cholesterol promotion. HDL-C exerts anti-inflammatory and antioxidant effects by regulating lipid metabolism, promoting reverse cholesterol transport, and reducing the production of inflammatory mediators (11). Therefore, reducing HDL-C levels in NAFLD/MASLD may weaken its anti-inflammatory and antioxidant functions and thus exacerbate the occurrence and progression of NAFLD/MASLD (12). In recent years, NHR has been proposed as a comprehensive indicator of inflammation and lipid metabolic status. It has been shown that NHR is significantly associated with cardiovascular disease, metabolic syndrome, hepatocellular carcinoma, and Parkinson’s disease (13–16), but its potential relationship with NAFLD/MASLD has not been fully investigated.
This study used data from the 2017–2020 National Health and Nutrition Examination Survey (NHANES) in a cross-sectional analysis to investigate the association between NHR and both NAFLD/MASLD and liver fibrosis.
Materials and methods
Study population
NHANES is a long-term, large-scale health survey program initiated and conducted every 2 years by the National Center for Health Statistics (NCHS), a division of the Centers for Disease Control and Prevention (CDC). Through a complex, multistage sampling design, NHANES collects health and nutrition data representative of the United States population, including personal interviews, physical examinations, and laboratory test results. NHANES data are widely used to study public health trends, assess the burden of disease and nutritional status, and are freely available to researchers worldwide.
This study analyzed pre-epidemic data from the 2017–2020 NHANES survey, which initially included 15,560 participants. From this cohort, 8,317 individuals aged 18 years and older with vibration-controlled transient elastography (VCTE) results were selected. We excluded 219 participants with unreliable VCTE measurements (liver stiffness quartile/median ratio ≥ 30%), 283 with hepatitis B or C, 821 with excessive alcohol intake (defined as more than two standard drinks per day for women and more than three for men), and those with missing data on neutrophil or high-density lipoprotein cholesterol (HDL-C). Consequently, 6,526 participants were included in the final analysis.
Variables
Demographic and clinical data were extracted from the NHANES database. Age, gender, race, educational level, body mass index (BMI), diabetes, hypertension, history of cardiovascular disease (CVD), smoking status, and laboratory variables were included. Diabetes mellitus was defined as HbA1c ≥ 6.5% or fasting glucose ≥126 mg/dL; in addition, participants had diabetes if they answered “yes” to any of the following questions: “Do you use insulin?” or “Has your doctor told you that you have diabetes?” or “Do you take glucose-lowering medication?,” Hypertension was defined as a mean systolic blood pressure ≥ 140 mmHg or a mean diastolic blood pressure ≥ 90 mmHg on three consecutive measurements, and participants who responded to the questions “Have you been told you have high blood pressure on two or more occasions” or “Do you have to take prescription medication for high blood pressure?” A “yes” response was also defined as hypertension. A history of cardiovascular disease was described as a response confirming a physician’s diagnosis of myocardial infarction, angina pectoris, coronary heart disease, congestive heart failure, or stroke. Smoking status was categorized as a smoker or never smoker based on having smoked fewer than 100 cigarettes in their lifetime.
Laboratory tests included measurements of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), plasma triglycerides (TG), uric acid, albumin (Alb), glycosylated hemoglobin (HbA1c), γ-glutamyltranspeptidase (GGT) and high-density lipoprotein cholesterol (HDL-C). NHR was calculated as the ratio of neutrophil counts (109 cells /L) to HDL-C level (mmol/L).
The definition of NAFLD, MASLD, and liver fibrosis
NHANES staff use the FibroScan 502 Touch device to assess liver stiffness and fat content. The device measures liver elasticity and stiffness through vibration-controlled transient elastography (VCTE) technology to help determine the extent of liver fibrosis. At the same time, the device measures hepatic steatosis by ultrasound attenuation and records the Controlled Attenuation Parameter (CAP) as an indicator of hepatic fat content. A CAP value of ≥ 274 dB/m was defined as hepatic steatosis according to previous studies, and a CAP value of ≥ 302 dB/m indicated severe hepatic steatosis (17, 18). In addition, liver stiffness measurements (LSM) of ≥ 8.2 kPa, ≥ 9.7 kPa, and ≥ 13.7 kPa represented the F2 (significant fibrosis), F3 (advanced fibrosis), and F4 (cirrhosis) stages of liver fibrosis, respectively (19). NAFLD was defined as the presence of hepatic steatosis in the absence of excessive alcohol consumption or the absence of other chronic liver diseases. MASLD is defined as the presence of hepatic steatosis in the absence of other causes of hepatic steatosis or excessive alcohol consumption and meets at least one of the following adult cardiometabolic criteria (3): (1) BMI ≥ 25 kg/m2 or WC > 94 cm for males and > 80 cm for females; (2) fasting serum glucose ≥ 5.6 mmol/L (100 mg/dL) or 2-h post-load glucose level ≥ 7.8 mmol/L (140 mg/dL) or HbA1c ≥ 5.7% (39 mmol/L) or type 2 diabetes or treatment for type 2 diabetes. (3) Blood pressure ≥ 130/85 mmHg or specific antihypertensive drug treatment; (4) TG ≥ 1.70 mmol/L (150 mg/dL) or lipid-lowering treatment; (5) HDL-C ≤ 1.0 mmol/L (40 mg/dL) for males and ≤ 1.3 mmol/L (50 mg/dL) for females or lipid-lowering treatment.
Statistical analysis
Considering NHANES’s complicated multistage sampling design, sample weights were applied in all analyses to ensure that the results were representative of the US population. Participants were divided into four subgroups according to NHR quartiles. Continuous variables were reported as weighted mean and standard error, while categorical variables were reported as unweighted counts and weighted percentages. One-way analysis of variance (ANOVA) for continuous variables and weighted chi-squared tests for categorical variables were used to compare differences between the four subgroups.
NHR was analyzed as a continuous and categorical variable, with exposure variables grouped by quartiles (the first quartile served as the reference group). Outcome variables included CAP, NAFLD/MASLD, LSM, and liver fibrosis. We used weighted linear regression and weighted logistic regression models for the analysis. In addition, we assessed potential non-linear associations between NHR and the prevalence of NAFLD/MASLD and liver fibrosis using restricted cubic spline (RCS) analysis. The RCS model was adjusted for several confounders, including age, gender, race, smoking status, diabetes, hypertension, CVD, BMI, TC, ALT, and uric acid. Subgroup analyses were conducted by stratifying participants according to age, gender, BMI, hypertension, diabetes, smoking status, and CVD. All data analyses were done using R software (version 4.4.0), and the statistical significance level was set at p < 0.05.
Results
Baseline characteristics of study participants
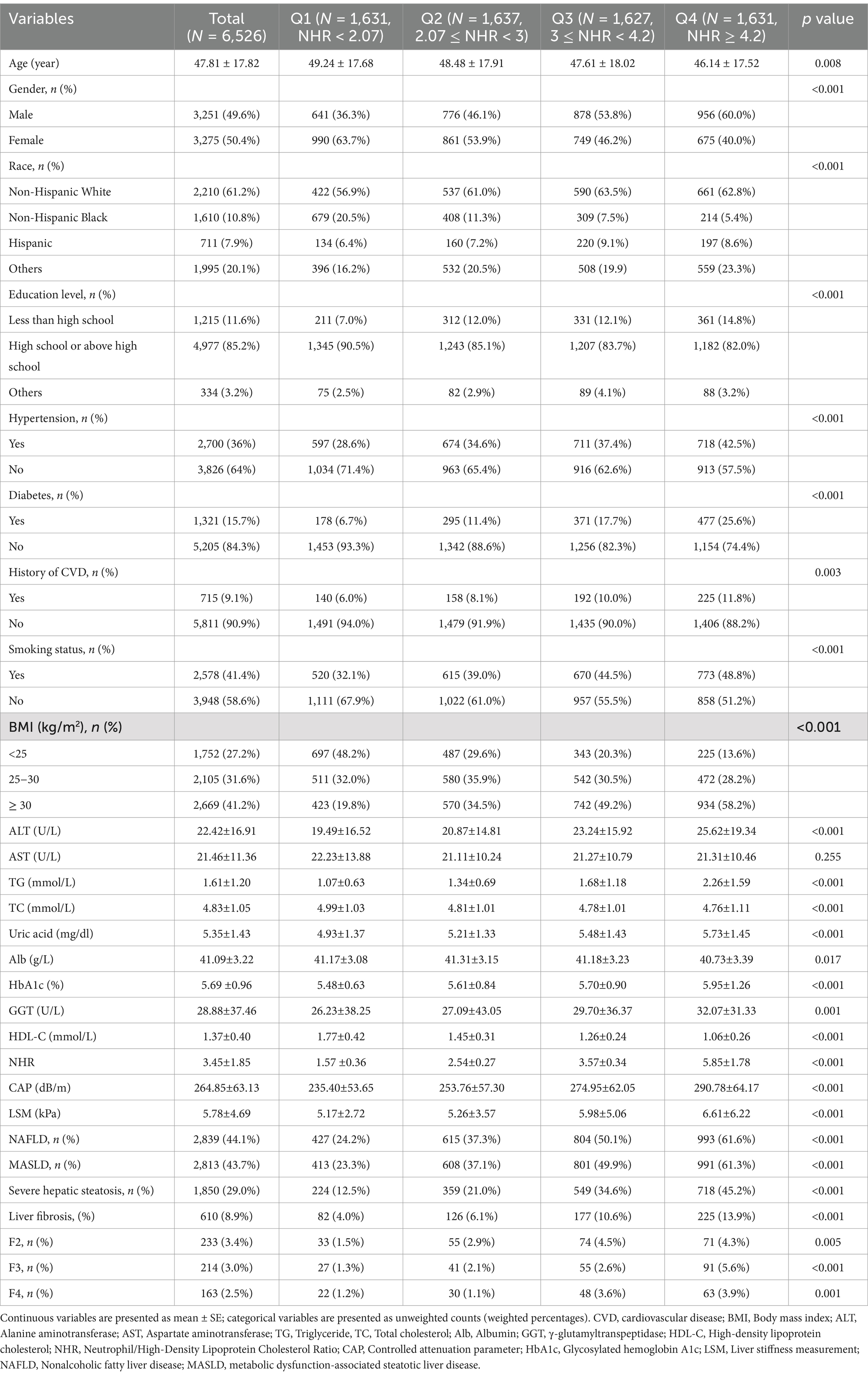
A total of 6,526 participants were included in this study. The mean age of the participants was 47.81 ± 17.82 years, with 49.6% males and 50.4% females. The weighted prevalence of NAFLD and MASLD was 44.1 and 43.7%, respectively, 8.9% of participants had liver fibrosis. The baseline characteristics of the study population, grouped according to quartiles of NHR, are presented in Table 1.
The results showed that the prevalence of NAFLD, MASLD, severe hepatic steatosis, liver fibrosis, and the severity of hepatic fibrosis gradually increased with increasing NHR (p < 0.05). In addition, it was found that there were significant differences between participants with different NHR levels in terms of age, gender, race, education level, hypertension, diabetes, smoking status, history of CVD, BMI, TG, TC, ALT, Alb, GGT, HbA1c, HDL-C, and uric acid (p < 0.05).
Association of NHR with NAFLD/MASLD
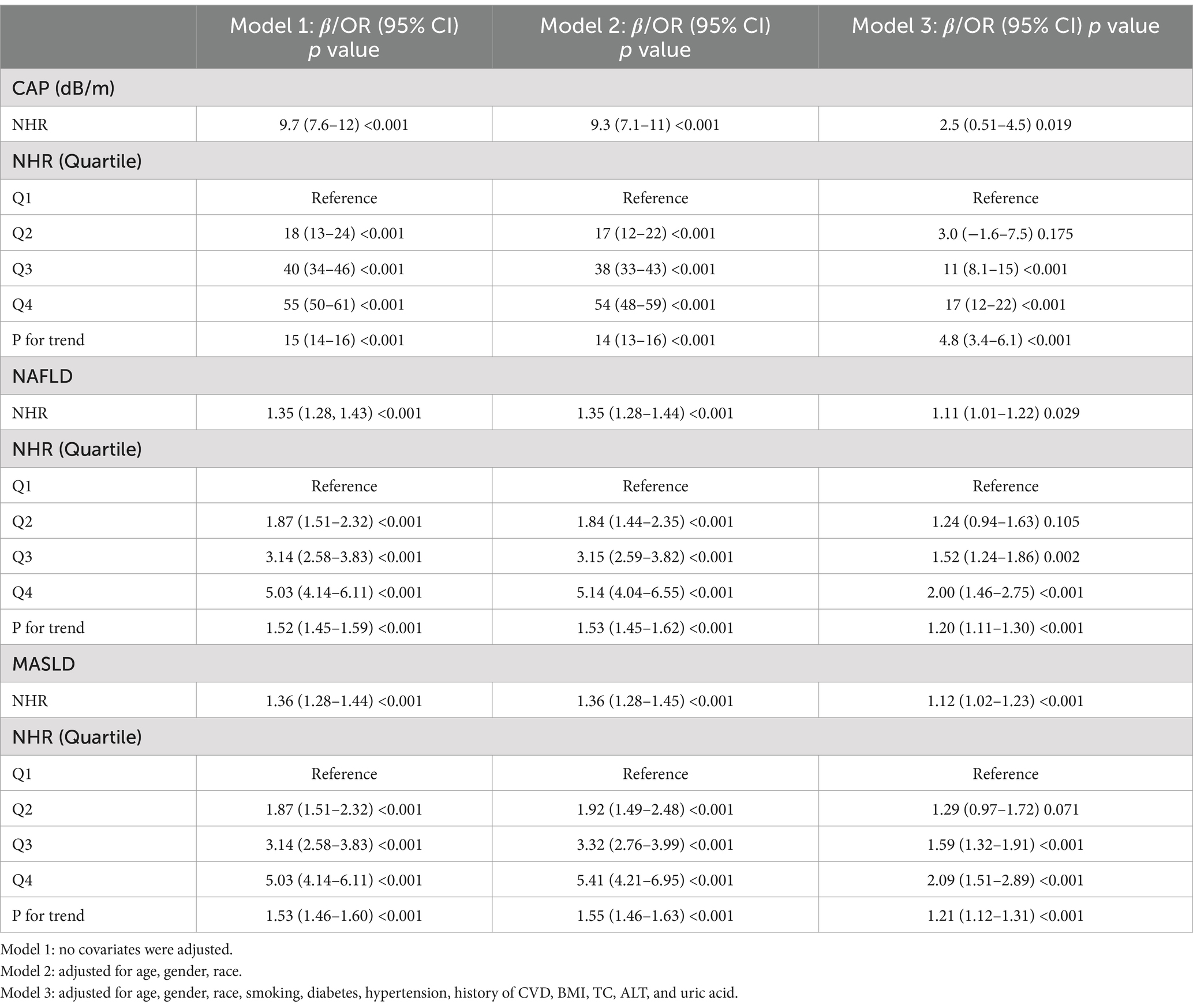
As shown in Table 2, we analyzed the effect of NHR on CAP and NAFLD/MASLD using weighted multiple regression models adjusted for all possible confounding variables (age, gender, race, smoking, diabetes, hypertension, history of CVD, BMI, TC, ALT, and uric acid).
We performed weighted linear regression analyses with CAP as the outcome. When NHR was included as a continuous variable in the model for analysis, the results showed that CAP increased by 2.5 dB/m for each unit increase in NHR in the fully adjusted model (β = 2.5; 95% CI (0.51–4.5); p = 0.019). NHR was included as a categorical variable (quartiles) in the analysis model, and after adjusting for all confounding variables, CAP values increased significantly with higher levels of NHR (P for trend <0.001), with participants in the fourth quartile group of NHR having the highest CAP values compared with the first quartile of NHR (β = 17; 95% CI (12–22); p < 0.001).
Subsequently, we performed weighted logistic regression analyses with NAFLD and MASLD as outcomes, which showed that higher NHR was associated with a higher prevalence of NAFLD and MASLD. After fully adjusting for confounding variables, each unit increase in NHR was associated with an 11% (OR = 1.11; 95% CI (1.01–1.22); p < 0.05) and 12% (OR = 1.12; 95% CI (1.02–1.23); p < 0.001) increase in the prevalence of NAFLD and MASLD, respectively. The inclusion of NHR as a categorical variable (quartiles) in the analysis model showed that the prevalence of NAFLD and MASLD increased with increasing levels of NHR after adjusting for all confounding variables (P for trend <0.001), and participants in the fourth quartile group of NHR had the highest risk of NAFLD and MASLD compared with the first quartile of NHR.
Relationship between NHR and liver fibrosis
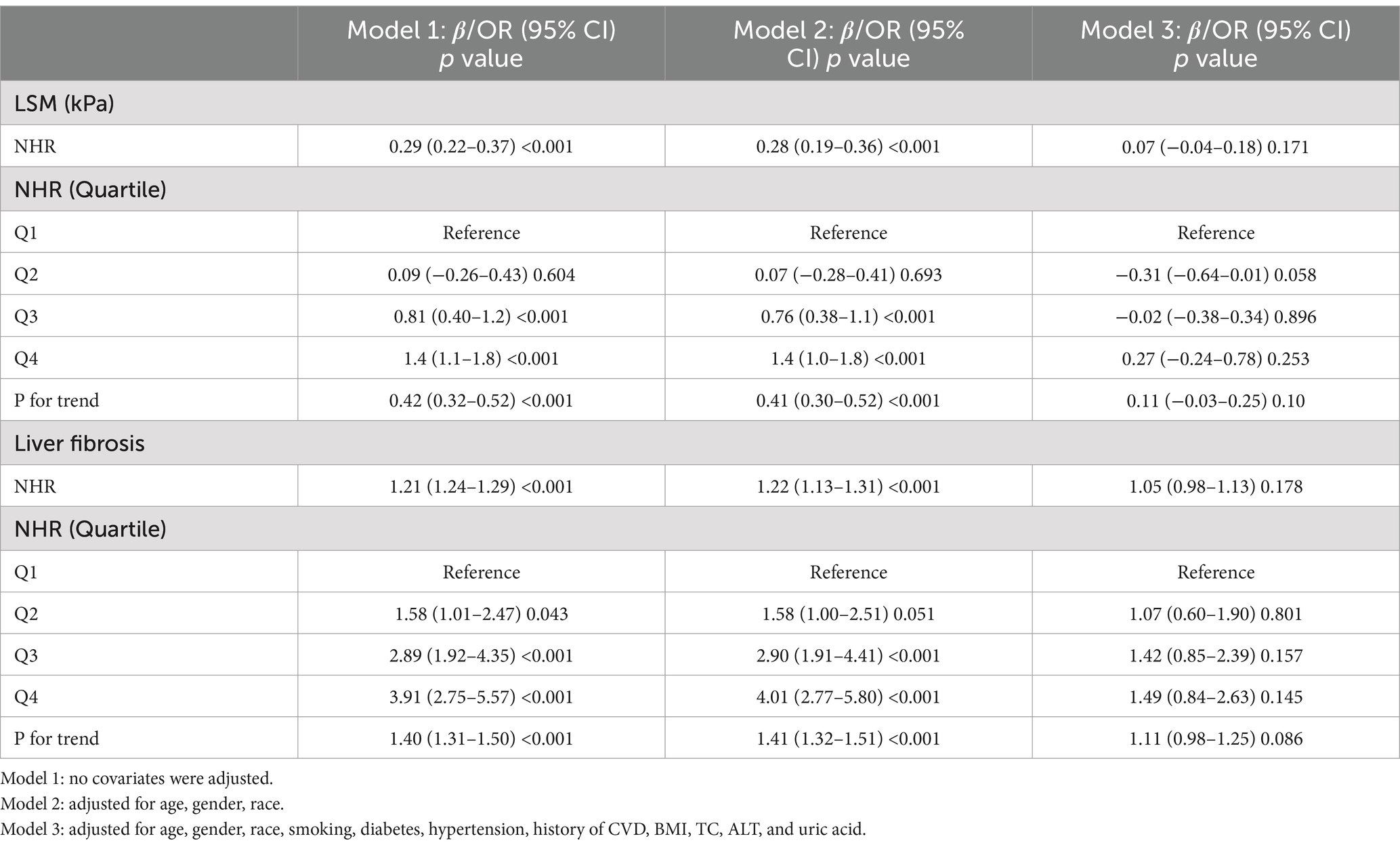
Similarly, we analyzed the effect of NHR on LSM and liver fibrosis using weighted multivariate regression models. As presented in Table 3, the unadjusted model indicated that each unit increase in NHR was associated with a 0.29 kPa increase in LSM (beta = 0.29; 95% CI: 0.22, 0.37; p < 0.001) and a 21% higher risk of liver fibrosis (OR = 1.21; 95% CI: 1.24, 1.29; p < 0.001). However, after adjustment for all confounders, the association between higher NHR and liver fibrosis was no longer statistically significant (Table 3).
Potential non-linear relationship between NHR and NAFLD/MASLD and liver fibrosis
The potential non-linear associations between NHR and NAFLD/MASLD and liver fibrosis were analyzed using the RCS model. As shown in Figure 1, the NHR was nonlinearly linked with the prevalence of NAFLD and MASLD (P-non-linear <0.001). Figure 1 reveals that at NHR < 3.013, the smaller the NHR, the lower the risk of NAFLD and MASLD. Figure 2 shows that lower NHR levels were not significantly associated with the risk of liver fibrosis, but the risk of liver fibrosis increased significantly when the NHR exceeded 3.013.
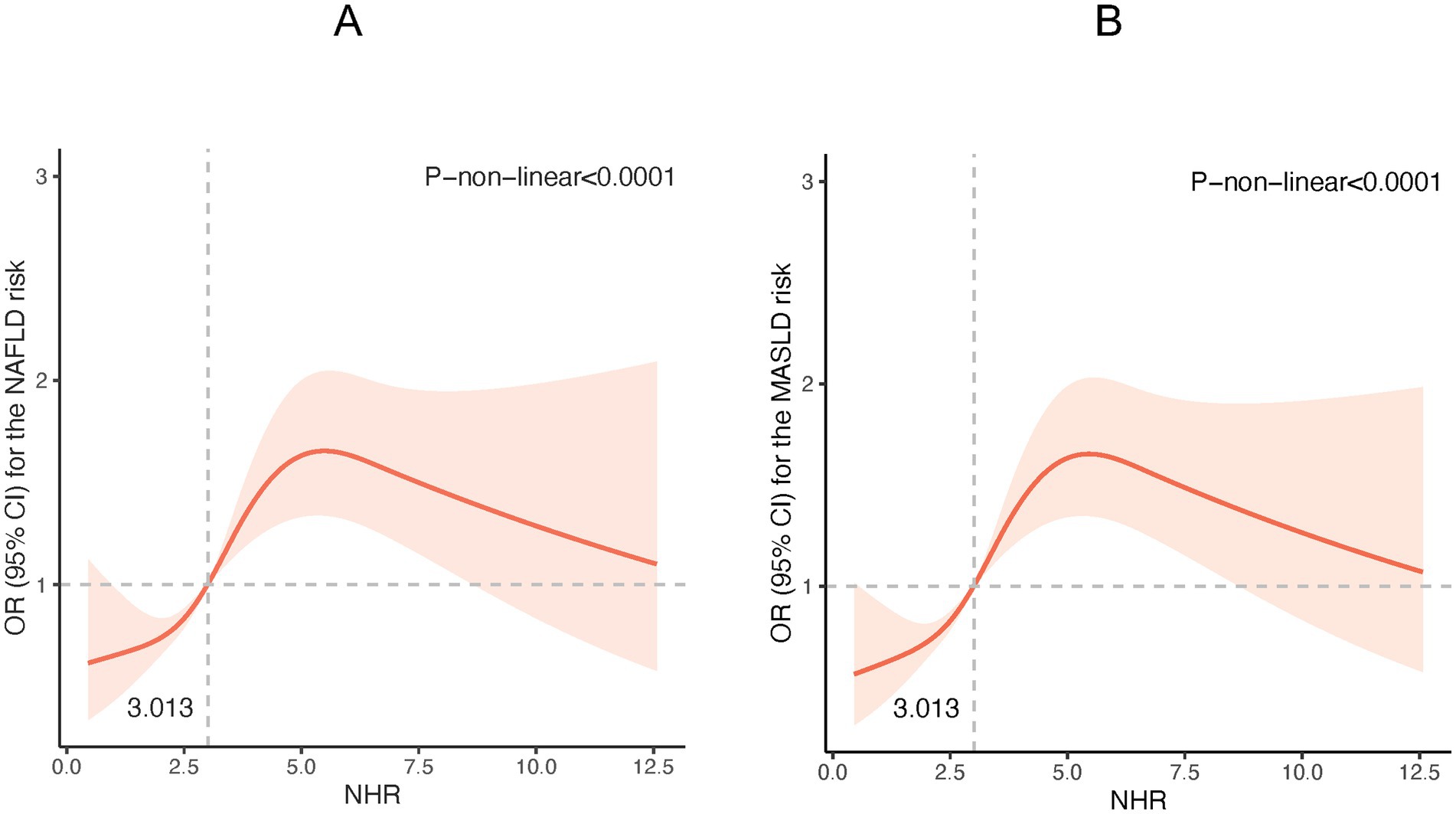
Figure 1. Restricted cubic spline (RCS) plot of the non-linear relationship between NHR and NAFLD /MASLD risk. (A) NHR - NAFLD; (B) NHR – MASLD. (Nonlinear relationships were detected after age, gender, race, smoking, diabetes, hypertension, history of CVD, BMI, TC, ALT, and uric acid).
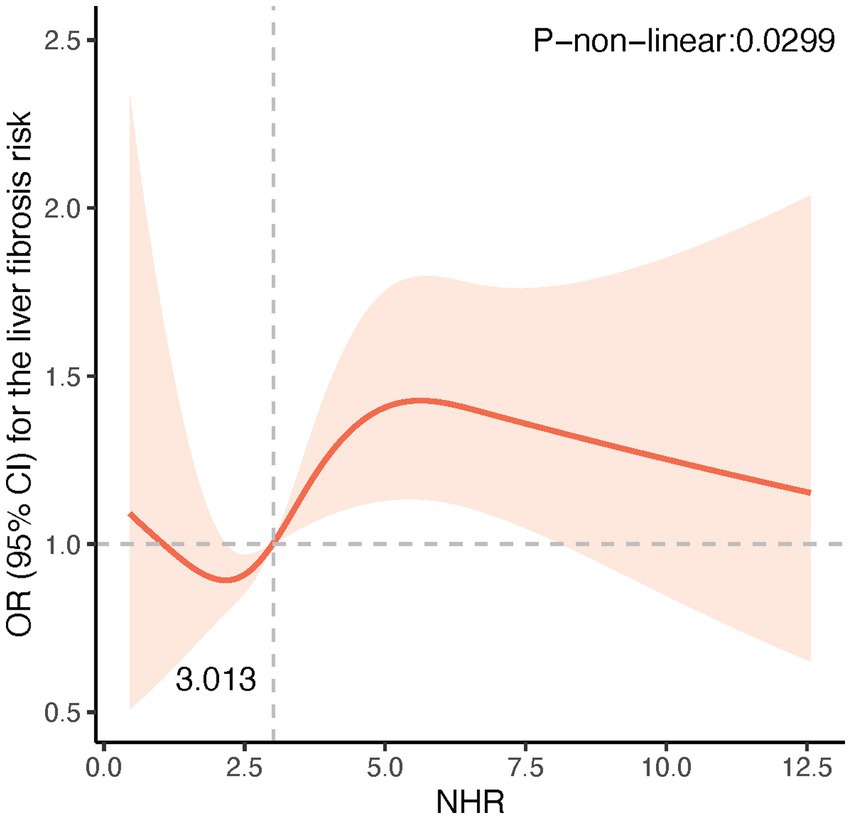
Figure 2. Restricted cubic spline (RCS) plot of the non-linear relationship between NHR and risk of liver fibrosis. A nonlinear relationship was detected after age, gender, race, smoking, diabetes, hypertension, history of CVD, BMI, TC, ALT, and uric acid.
Subgroup analysis
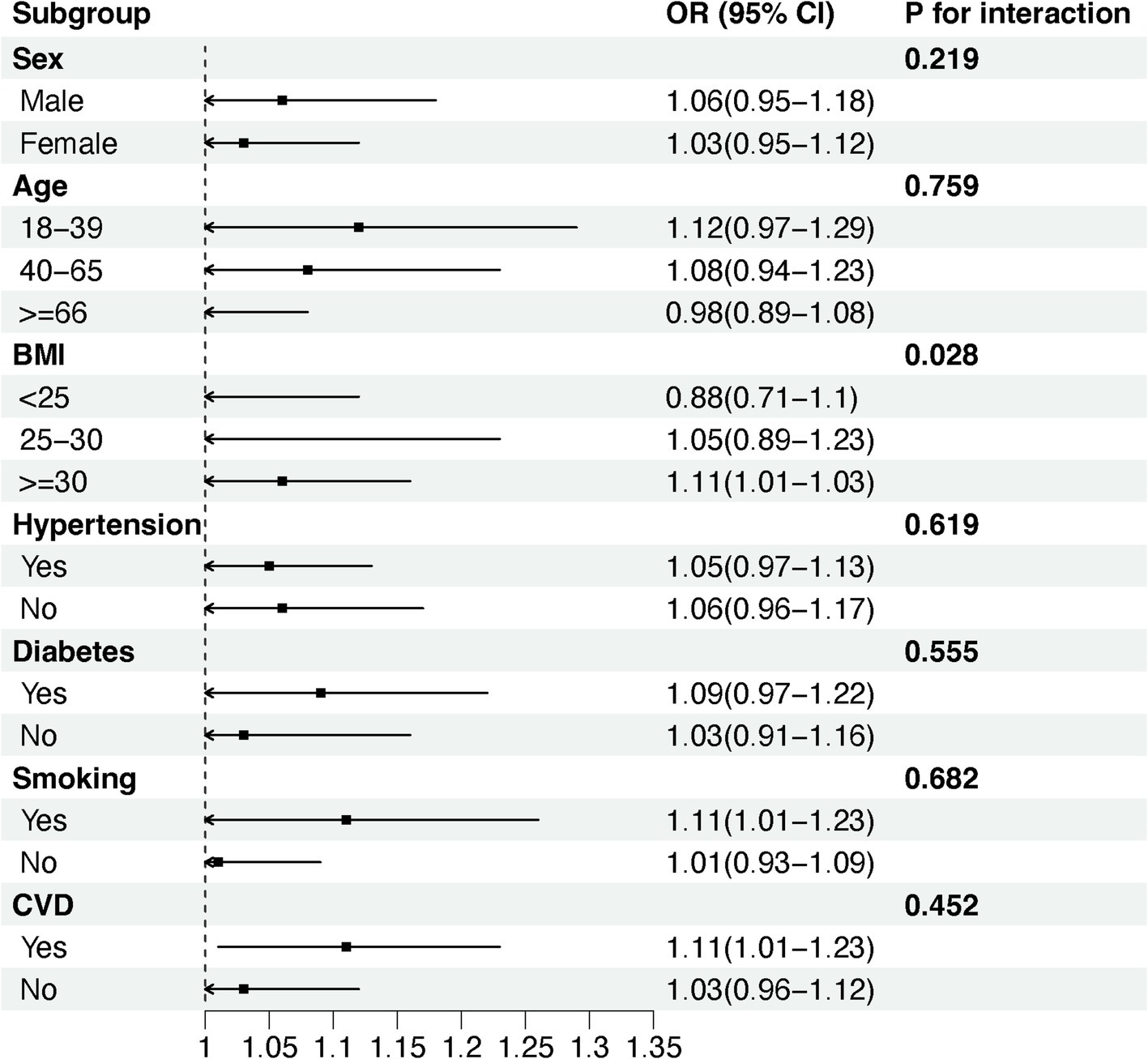
We used stratified weighted multiple regression analysis to investigate the association of NHR with NAFLD/MASLD and liver fibrosis in different populations, dividing participants into subgroups based on gender, age, BMI, hypertension, diabetes, smoking status, and history of CVD for the analyses and interaction tests as shown in Figure 3, there was a significant interaction between NHR and gender, hypertension, and diabetes (P for interaction <0.05), and the positive correlation between NHR and NAFLD/MASLD was stronger in female, non-diabetic, and hypertensive participants. In addition, BMI was the factor that significantly interacted with the relationship between NHR and hepatic fibrosis in different population subgroups, and the positive association between NHR and hepatic fibrosis was more pronounced, especially in the obese population (BMI ≥ 30) (Figure 4).
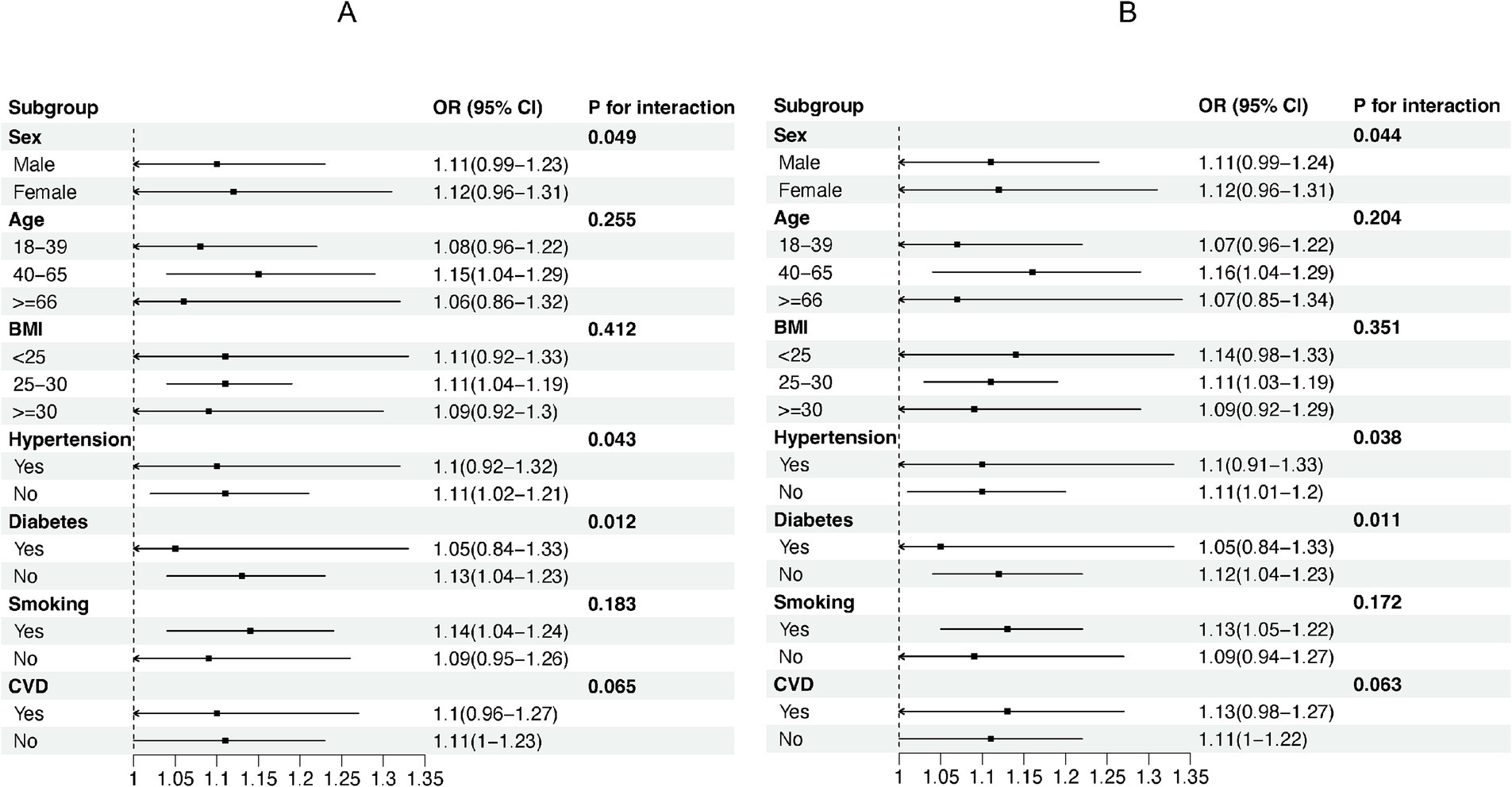
Figure 3. Subgroup analyses of the association between NHR and NAFLD/MASLD. (A) NHR - NAFLD; (B) NHR - MASLD.
Discussion
This study evaluated the association between NHR and NAFLD, MASLD, and liver fibrosis in the American population. The results of the study showed that NHR was positively and nonlinearly associated with NAFLD and MASLD. In addition, although lower NHR levels were not significantly associated with the risk of liver fibrosis, the risk of liver fibrosis increased significantly when NHR exceeded 3.013. Subgroup analyses further revealed that the association between NHR and NAFLD/MASLD was more significant in women and individuals without hypertension and diabetes, and the association between NHR and liver fibrosis was more prominent in participants with BMI >30.
Our study extends and supports previous findings. An earlier study involving 936 individuals from a Chinese population demonstrated that NHR was positively associated with the risk of ultrasound-diagnosed NAFLD, suggesting that NHR may be a valid predictor of NAFLD (20). Our study validated this association and assessed the prevalence and severity of NAFLD using data from a large-scale U.S. general population, incorporating the VCTE technology of the FibroScan device. Studies have shown that the accuracy of VCTE in diagnosing hepatic steatosis and fibrosis is comparable to liver biopsy (21, 22), which enhances the broad applicability and reliability of the results.
The main features of NAFLD/MASLD include hepatic lipid accumulation, inflammatory response, fibrosis formation, and hepatocyte injury (2). Hepatic steatosis is the pathological basis for the progression of NAFLD/MASLD, often accompanied by the onset of chronic inflammatory responses (23). Studies have shown that chronic inflammation plays a vital role in the development of NAFLD/MASLD (24, 25). Notably, chronic inflammation in the liver may result in dysfunction of hepatic sinusoidal endothelial cells and vascular endothelium, leading to an increased risk of cardiovascular disease, which is not directly related to the presence or absence of diabetes mellitus, hypertension, and obesity (26). An analysis based on NHANES 2017–2018 showed that the neutrophil-to-albumin ratio (NPAR), a systemic marker of inflammation, was significantly associated with NAFLD and advanced liver fibrosis (27). When immune cells such as neutrophils and lymphocytes are activated, they release pro-inflammatory cytokines that promote the development of NAFLD (28). Neutrophils are the first immune cells to respond to inflammation, producing cytokines to promote lymphocyte activation and recruit macrophages, ultimately leading to chronic inflammation (29, 30). In addition, HDL-C reduces neutrophil activation, adhesion, spreading, and migration, inhibiting oxidized LDL production and exerting anti-inflammatory and antioxidant effects (31, 32). Therefore, NHR, as a combination of neutrophil numbers and HDL-C levels, may reflect the state of chronic inflammation and oxidative stress and serve as a sensitive indicator of the pathological process of NAFLD/MASLD.
Recent studies have shown that NHR is associated with the progression of several diseases, particularly cardiovascular and metabolic diseases (13, 14, 33), Our analysis revealed a non-linear association of NHR with NAFLD and liver fibrosis, which may be related to the complex interaction of neutrophils and HDL-C in the development of metabolic diseases. In in vitro experiments, mice fed a high-fat diet showed increased neutrophil infiltration in the liver, accompanied by the development of hepatic steatosis and inflammation. Neutrophil depletion in mice using the 1A8 antibody significantly reduced liver triglyceride accumulation, hepatic inflammation, and fibrosis (34). Thus, NHR may play an essential role in the progression of NAFLD/MASLD, with high NHR levels strongly associated with increased severity of hepatic steatosis and fibrosis.
In particular, the risk of liver fibrosis increases significantly when the NHR exceeds 3.013, probably due to exacerbation of chronic inflammation. Neutrophil accumulation has been linked to the progression of liver fibrosis and cirrhosis. During the development of liver inflammation and fibrosis, immature neutrophils with pro-inflammatory properties are released into the circulation, further exacerbating the inflammatory response and liver fibrosis (35). In addition, several studies have shown that neutrophil elastase (NE), neutrophil granule protein (PR3), tissue protease G (CSTG), and other neutrophil-derived proteases play a critical role in the progression of hepatic steatosis and inflammation in NAFLD (36–38). The role of myeloperoxidase (MPO) in the progression of liver fibrosis has also been demonstrated (39). These mechanisms may explain the association between NHR and hepatic steatosis and fibrosis.
Subgroup analyses in this study revealed a significant interaction between gender and the association of NHR with NAFLD/MASLD, The positive correlation between NHR and NAFLD/MASLD was more pronounced in the female population. This gender difference may be attributed to the unique physiological and endocrine characteristics of females. Studies have shown that higher levels of estrogen in women can exert anti-inflammatory and antioxidant effects through multiple pathways and to some extent, mitigate the liver damage caused by inflammatory responses and lipid metabolism disorders (40). However, after menopause, this protective effect is weakened by decreasing levels of estrogen, and the inflammatory response is relatively enhanced (41), which may make the relationship between NHR and NAFLD/MASLD more significant. Additionally, the association between the NHR and NAFLD/MASLD was modified by hypertension and diabetic status, both of which are recognized risk factors for the development and progression of NAFLD/MASLD (42, 43), They also strongly correlate with systemic inflammation and disorders of lipid metabolism (44, 45). Therefore, co-morbid diabetes and hypertension may mask the association between NHR and NAFLD/MASLD. In the obese population, the NHR index was significantly associated with the risk of liver fibrosis, which may be due to the accumulation of fat and metabolic abnormalities caused by obesity, which triggered a series of inflammatory reactions and then promoted the development of liver fibrosis (46), making NHR an important influence factor of liver fibrosis in the state of obesity.
Study strengths and limitations
The main strength of this study is the use of large-scale data from the general population of the United States, with a large and nationally representative sample size. In addition, we used VCTE to assess hepatic steatosis and hepatic fibrosis, which provided greater diagnostic accuracy. However, this study has some limitations. Firstly, as a cross-sectional study, it was impossible to establish a causal relationship between NHR and hepatic steatosis and fibrosis. Secondly, although we adjusted for confounders as much as possible, there may still be potential confounding variables that were not considered. Thirdly, although VCTE demonstrated high accuracy in non-invasive diagnosis, its diagnostic accuracy compared to liver biopsy requires further validation. Finally, this study only used data from the US population, and its applicability to Asian populations cannot be directly inferred. Future studies need to include data from different ethnic populations to further validate and expand the findings. In addition, a longitudinal design should be used to assess the long-term association between NHR and NAFLD/MASLD progression and to validate its predictive value.
Conclusion
In conclusion, this study demonstrated a significant non-linear association between NHR and NAFLD/MASLD and liver fibrosis. NHR can be used as a potential marker for NAFLD/MASLD and liver fibrosis, thus aiding in the early detection and intervention of NAFLD/MASLD.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Ethics statement
The studies involving humans were approved by NCHS Research Ethics Review Board (ERB), National Center for Health Statistics (NCHS). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NZ: Conceptualization, Writing – review & editing, Data curation, Formal analysis, Investigation, Writing – original draft. YaL: Data curation, Formal analysis, Investigation, Writing – review & editing. YiL: Data curation, Investigation, Writing – review & editing. XC: Data curation, Investigation, Writing – review & editing. XL: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Capital’s Funds for Health Improvement and Research (2024-1-1203); Dengfeng Talent Support Program of Beijing Municipal Administration of Hospitals (No. DFL20221601); High-level Public Health Technical Personnel Construction Project (Subject leaders-03-21).
Acknowledgments
We are grateful for the dedication of the NHANES team and the valuable participation of all survey participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NAFLD, Non-alcoholic fatty liver disease; MASLD, Metabolic dysfunction-associated steatotic liver disease; NHR, Neutrophil-to-high-density lipoprotein cholesterol ratio; NHANES, National Health and Nutrition Examination Survey; VCTE, Vibration-controlled transient elastography; CAP, Controlled attenuation parameter; LSM, Liver stiffness measurement; CVD, Cardiovascular disease; BMI, Body mass index; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; TG, Triglyceride; TC, Total cholesterol; Alb, Albumin; GGT, γ-glutamyltranspeptidase; HDL-C, High-density lipoprotein cholesterol; HbA1c, Glycosylated hemoglobin A1c.
References
1. Cusi, K, Isaacs, S, Barb, D, Basu, R, Caprio, S, Garvey, WT, et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the diagnosis and Management of Nonalcoholic Fatty Liver Disease in primary care and endocrinology clinical settings: co-sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. (2022) 28:528–62. doi: 10.1016/j.eprac.2022.03.010
2. Riazi, K, Azhari, H, Charette, JH, Underwood, FE, King, JA, Afshar, EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2022) 7:851–61. doi: 10.1016/S2468-1253(22)00165-0
3. Rinella, ME, Lazarus, JV, Ratziu, V, Francque, SM, Sanyal, AJ, Kanwal, F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. (2023) 78:1966–86. doi: 10.1097/HEP.0000000000000520
4. Friedman, SL, Neuschwander-Tetri, BA, Rinella, M, and Sanyal, AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. (2018) 24:908–22. doi: 10.1038/s41591-018-0104-9
5. Simon, TG, Roelstraete, B, Khalili, H, Hagström, H, and Ludvigsson, JF. Mortality in biopsy-confirmed nonalcoholic fatty liver disease. Gut. (2021) 70:1375–82. doi: 10.1136/gutjnl-2020-322786
6. Targher, G, Byrne, CD, and Tilg, H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. (2020) 69:1691–705. doi: 10.1136/gutjnl-2020-320622
7. Luci, C, Bourinet, M, Leclère, PS, Anty, R, and Gual, P. Chronic inflammation in non-alcoholic Steatohepatitis: molecular mechanisms and therapeutic strategies. Front Endocrinol. (2020) 11:597648. doi: 10.3389/fendo.2020.597648
8. Herrero-Cervera, A, Soehnlein, O, and Kenne, E. Neutrophils in chronic inflammatory diseases. Cell Mol Immunol. (2022) 19:177–91. doi: 10.1038/s41423-021-00832-3
9. Antonucci, L, Porcu, C, Timperi, E, Santini, SJ, Iannucci, G, and Balsano, C. Circulating neutrophils of nonalcoholic Steatohepatitis patients show an activated phenotype and suppress T lymphocytes activity. J Immunol Res. (2020) 2020:4570219–5. doi: 10.1155/2020/4570219
10. Karami, S, Poustchi, H, Sarmadi, N, Radmard, AR, Ali Yari, F, Pakdel, A, et al. Association of anti-oxidative capacity of HDL with subclinical atherosclerosis in subjects with and without non-alcoholic fatty liver disease. Diabetol Metab Syndr. (2021) 13:121. doi: 10.1186/s13098-021-00741-5
11. Deprince, A, Haas, JT, and Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol Metab. (2020) 42:101092. doi: 10.1016/j.molmet.2020.101092
12. Li, R, Kong, D, Ye, Z, Zong, G, Hu, K, Xu, W, et al. Correlation of multiple lipid and lipoprotein ratios with nonalcoholic fatty liver disease in patients with newly diagnosed type 2 diabetic mellitus: a retrospective study. Front Endocrinol. (2023) 14:1127134. doi: 10.3389/fendo.2023.1127134
13. Kou, T, Luo, H, and Yin, L. Relationship between neutrophils to HDL-C ratio and severity of coronary stenosis. BMC Cardiovasc Disord. (2021) 21:127. doi: 10.1186/s12872-020-01771-z
14. Chen, T, Chen, H, Xiao, H, Tang, H, Xiang, Z, Wang, X, et al. Comparison of the value of neutrophil to high-density lipoprotein cholesterol ratio and lymphocyte to high-density lipoprotein cholesterol ratio for predicting metabolic syndrome among a population in the southern coast of China. Diabetes Metab Syndr Obes. (2020) 13:597–605. doi: 10.2147/DMSO.S238990
15. Shi, K, Hou, J, Zhang, Q, Bi, Y, Zeng, X, and Wang, X. Neutrophil-to-high-density-lipoprotein-cholesterol ratio and mortality among patients with hepatocellular carcinoma. Front Nutr. (2023) 10:1127913. doi: 10.3389/fnut.2023.1127913
16. Liu, Z, Fan, Q, Wu, S, Wan, Y, and Lei, Y. Compared with the monocyte to high-density lipoprotein ratio (MHR) and the neutrophil to lymphocyte ratio (NLR), the neutrophil to high-density lipoprotein ratio (NHR) is more valuable for assessing the inflammatory process in Parkinson’s disease. Lipids Health Dis. (2021) 20:35. doi: 10.1186/s12944-021-01462-4
17. Xie, R, Xiao, M, Li, L, Ma, N, Liu, M, Huang, X, et al. Association between SII and hepatic steatosis and liver fibrosis: a population-based study. Front Immunol. (2022) 13:925690. doi: 10.3389/fimmu.2022.925690
18. Xie, R, and Liu, M. Relationship between non-alcoholic fatty liver disease and degree of hepatic steatosis and bone mineral density. Front Endocrinol. (2022) 13:857110. doi: 10.3389/fendo.2022.857110
19. Eddowes, PJ, Sasso, M, Allison, M, Tsochatzis, E, Anstee, QM, Sheridan, D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. (2019) 156:1717–30. doi: 10.1053/j.gastro.2019.01.042
20. Jia, Z, Li, Z, and Chen, S. The association between neutrophil/high-density lipoprotein cholesterol ratio and non-alcoholic fatty liver disease in a healthy population. Diabetes Metab Syndr Obes. (2024) 17:2597–605. doi: 10.2147/DMSO.S464406
21. Selvaraj, EA, Mózes, FE, Jayaswal, ANA, Zafarmand, MH, Vali, Y, Lee, JA, et al. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: a systematic review and meta-analysis. J Hepatol. (2021) 75:770–85. doi: 10.1016/j.jhep.2021.04.044
22. Boursier, J, Hagström, H, Ekstedt, M, Moreau, C, Bonacci, M, Cure, S, et al. Non-invasive tests accurately stratify patients with NAFLD based on their risk of liver-related events. J Hepatol. (2022) 76:1013–20. doi: 10.1016/j.jhep.2021.12.031
23. Bessone, F, Razori, MV, and Roma, MG. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell Mol Life Sci. (2018) 76:99–128. doi: 10.1007/s00018-018-2947-0
24. Petermann-Rocha, F, Wirth, MD, Boonpor, J, Parra-Soto, S, Zhou, Z, Mathers, JC, et al. Associations between an inflammatory diet index and severe non-alcoholic fatty liver disease: a prospective study of 171,544 UK biobank participants. BMC Med. (2023) 21:123. doi: 10.1186/s12916-023-02793-y
25. Gong, H, He, Q, Zhu, L, Feng, Z, Sun, M, Jiang, J, et al. Associations between systemic inflammation indicators and nonalcoholic fatty liver disease: evidence from a prospective study. Front Immunol. (2024) 15:1389967. doi: 10.3389/fimmu.2024.1389967
26. Nasiri-Ansari, N, Androutsakos, T, Flessa, C-M, Kyrou, I, Siasos, G, Randeva, HS, et al. Endothelial cell dysfunction and nonalcoholic fatty liver disease (NAFLD): a concise review. Cells. (2022) 11:2511. doi: 10.3390/cells11162511
27. Liu, CF, and Chien, LW. Predictive role of neutrophil-percentage-to-albumin ratio (NPAR) in nonalcoholic fatty liver disease and advanced liver fibrosis in nondiabetic US adults: evidence from NHANES 2017–2018. Nutrients. (2023) 15:1892. doi: 10.3390/nu15081892
28. Hwang, S, Yun, H, Moon, S, Cho, YE, and Gao, B. Role of neutrophils in the pathogenesis of nonalcoholic Steatohepatitis. Front Endocrinol. (2021) 12:751802. doi: 10.3389/fendo.2021.751802
29. Mantovani, A, Cassatella, MA, Costantini, C, and Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. (2011) 11:519–31. doi: 10.1038/nri3024
30. Borregaard, N. Neutrophils, from marrow to microbes. Immunity. (2010) 33:657–70. doi: 10.1016/j.immuni.2010.11.011
31. Murphy, AJ, Woollard, KJ, Suhartoyo, A, Stirzaker, RA, Shaw, J, Sviridov, D, et al. Neutrophil activation is attenuated by high-density lipoprotein and Apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler Thromb Vasc Biol. (2011) 31:1333–41. doi: 10.1161/ATVBAHA.111.226258
32. Curcic, S, Holzer, M, Frei, R, Pasterk, L, Schicho, R, Heinemann, A, et al. Neutrophil effector responses are suppressed by secretory phospholipase A2 modified HDL. Biochim Biophys Acta. (2015) 1851:184–93. doi: 10.1016/j.bbalip.2014.11.010
33. Huang, J-B, Chen, Y-S, Ji, H-Y, Xie, W-M, Jiang, J, Ran, L-S, et al. Neutrophil to high-density lipoprotein ratio has a superior prognostic value in elderly patients with acute myocardial infarction: a comparison study. Lipids Health Dis. (2020) 19:59. doi: 10.1186/s12944-020-01238-2
34. Ou, R, Liu, J, Lv, M, Wang, J, Wang, J, Zhu, L, et al. Neutrophil depletion improves diet-induced non-alcoholic fatty liver disease in mice. Endocrine. (2017) 57:72–82. doi: 10.1007/s12020-017-1323-4
35. Maretti-Mira, AC, Salomon, MP, Chopra, S, Yuan, L, and Golden-Mason, L. Circulating neutrophil profiles undergo a dynamic shift during metabolic dysfunction-associated Steatohepatitis (MASH) progression. Biomedicines. (2024) 12:1105. doi: 10.3390/biomedicines12051105
36. Talukdar, S, Oh, DY, Bandyopadhyay, G, Li, D, Xu, J, McNelis, J, et al. Neutrophils mediate insulin resistance in high fat diet fed mice via secreted elastase. Nat Med. (2012) 18:1407–12. doi: 10.1038/nm.2885
37. Mirea, AM, Toonen, EJM, van den Munckhof, I, Munsterman, ID, Tjwa, ETTL, Jaeger, M, et al. Increased proteinase 3 and neutrophil elastase plasma concentrations are associated with non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes. Mol Med. (2019) 25:16. doi: 10.1186/s10020-019-0084-3
38. Toonen, EJ, Mirea, AM, Tack, CJ, Stienstra, R, Ballak, DB, van Diepen, JA, et al. Activation of proteinase 3 contributes to nonalcoholic fatty liver disease and insulin resistance. Mol Med. (2016) 22:202–14. doi: 10.2119/molmed.2016.00033
39. Pulli, B, Ali, M, Iwamoto, Y, Zeller, MW, Schob, S, Linnoila, JJ, et al. Myeloperoxidase–hepatocyte–stellate cell cross talk promotes hepatocyte injury and fibrosis in experimental nonalcoholic Steatohepatitis. Antioxid Redox Signal. (2015) 23:1255–69. doi: 10.1089/ars.2014.6108
40. Lee, C, Kim, J, and Jung, Y. Potential therapeutic application of estrogen in gender disparity of nonalcoholic fatty liver disease/nonalcoholic Steatohepatitis. Cells. (2019) 8:1259. doi: 10.3390/cells8101259
41. Yang, JD, Abdelmalek, MF, Pang, H, Guy, CD, Smith, AD, Diehl, AM, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic Steatohepatitis. Hepatology. (2014) 59:1406–14. doi: 10.1002/hep.26761
42. Yuan, M, He, J, Hu, X, Yao, L, Chen, P, Wang, Z, et al. Hypertension and NAFLD risk: insights from the NHANES 2017–2018 and Mendelian randomization analyses. Chin Med J. (2023) 137:457–64. doi: 10.1097/CM9.0000000000002753
43. Loosen, SH, Demir, M, Kunstein, A, Jördens, M, Qvarskhava, N, Luedde, M, et al. Variables associated with increased incidence of non-alcoholic fatty liver disease (NAFLD) in patients with type 2 diabetes. BMJ Open Diabetes Res Care. (2021) 9:e002243. doi: 10.1136/bmjdrc-2021-002243
44. Wu, W, Zhang, Z, Qi, Y, Zhang, H, Zhao, Y, and Li, J. Association between dietary inflammation index and hypertension in participants with different degrees of liver steatosis. Ann Med. (2023) 55:2195203. doi: 10.1080/07853890.2023.2195203
45. Rohm, TV, Meier, DT, Olefsky, JM, and Donath, MY. Inflammation in obesity, diabetes and related disorders. Immunity. (2022) 55:31–55. doi: 10.1016/j.immuni.2021.12.013
Keywords: non-alcoholic fatty liver disease, metabolic dysfunction-associated steatotic liver disease, neutrophil-to-high-density lipoprotein cholesterol ratio, inflammation, lipid metabolism disorders, liver fibrosis
Citation: Zhu N, Li Y, Lin Y, Cui X and Li X (2025) Association between neutrophil-to-high-density lipoprotein cholesterol ratio and non-alcoholic fatty liver disease or metabolic dysfunction-associated steatotic liver disease: evidence from NHANES 2017–2020. Front. Med. 11:1491858. doi: 10.3389/fmed.2024.1491858
Edited by:
Zheng Li, Jiangsu Normal University, ChinaReviewed by:
Theodoros Androutsakos, National and Kapodistrian University of Athens, GreeceSuxian Zhao, Third Hospital of Hebei Medical University, China
Copyright © 2025 Zhu, Li, Lin, Cui and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Li, bGVheGluQGNjbXUuZWR1LmNu
 Na Zhu
Na Zhu Yanyan Li
Yanyan Li Yingying Lin
Yingying Lin XinYu Cui1
XinYu Cui1 Xin Li
Xin Li