- 1Department of Gastroenterology, The People’s Hospital of Yubei District of Chongqing City, Chongqing, China
- 2Department of Ultrasound, The People’s Hospital of Yubei District of Chongqing City, Chongqing, China
Background: Small intestinal bacterial overgrowth (SIBO) has been reported to be very common among individuals with inflammatory bowel disease (IBD), and the prevalence of SIBO is highly variable. We conducted this study to calculate the prevalence and identify predictors of SIBO in IBD.
Methods: PubMed, Cochrane Library, and EMBASE from inception to March 2024 were searched for studies evaluating the prevalence of SIBO in IBD. We calculated the pooled prevalence of SIBO among IBD patients and the odds ratio (OR) of SIBO in IBD compared with healthy controls. Besides, we also evaluated predictors of SIBO in IBD patients.
Results: Twenty-nine studies (3,250 IBD, 708 controls) were included in our study. The pooled prevalence of SIBO in IBD was 31.0% (95% CI 25.2–37.1), and the prevalence of SIBO was higher in IBD compared with healthy controls (OR 5.25, 95% CI 2.96–9.32). The pooled prevalence of SIBO was higher among CD patients (32.2, 95% CI 25.9–38.8) compared with UC patients (27.8, 95% CI 18.5–38.1). The odds of lower BMI (mean difference = −1.04; 95% CI −1.86 to −0.23), bloating (OR = 3.02, 95% CI 1.22–7.5), flatulence (OR = 4.70, 95% CI 1.44–15.35), history of abdominal surgery (OR = 2.05, 95% CI 1.35–3.11), and stricturing/penetrating disease behavior (OR = 3.51, 95% CI 1.67–7.40) increased significantly in IBD patients with SIBO compared to those without SIBO. Antibiotic treatment may be effective for SIBO in IBD patients.
Conclusion: Nearly one-third of IBD patients present with SIBO positive, and the odds of SIBO in IBD was increased by 5.25-fold compared with healthy controls. Lower BMI, bloating, flatulence, history of abdominal surgery, and stricturing/penetrating disease behavior were predictors of SIBO in IBD patients.
Introduction
Inflammatory bowel disease (IBD) is a chronic or remitting/relapsing inflammatory disease of the gastrointestinal tract characterized by abdominal pain, diarrhea, bloody stools, and weight loss, including ulcerative colitis (UC) and Crohn’s disease (CD) (1, 2). Over the past few decades, the prevalence of IBD has been increasing around the world (1, 3, 4). The recurrent symptoms of IBD require frequent medical evaluation and treatments, resulting in a substantial economic and psychological burden. Although the pathogenesis of IBD remains unclear, the abnormalities in disease susceptibility genes, environmental factors, and intestinal bacteria are associated with the development or progression of IBD (1).
Small intestinal bacterial overgrowth (SIBO) is a clinical syndrome caused by excessive numbers of bacteria and/or abnormal types of bacteria in the small intestinal tract (5, 6). The microbial investigation of jejunal aspirate culture (JAC) has been the gold standard for diagnosing SIBO. However, due to the invasiveness, expensiveness, and complexity of JAC, the breath test has become the mainstream method for diagnosing SIBO in real clinical practice (7). Many clinical symptoms of SIBO, such as abdominal pain, diarrhea, and weight loss, are similar to those of IBD. Hence, gastrointestinal (GI) symptoms seen in SIBO may be confused with IBD symptoms. The latest guidelines have suggested that dysbiosis of intestinal flora is also an important pathogenesis of IBD (1). Regrettably, the optimal role of SIBO in the development of IBD has not been established.
In this context, there is much interest in the possible association between SIBO and IBD. A series of clinical trials have assessed the prevalence of SIBO in IBD patients, but the reported result is highly variable, ranging from 9 to 62%. A previous meta-analysis published in 2019 concluded that the proportion of SIBO in IBD patients was 22.3% (8). However, the number of studies included in this research was very limited, and they did not quantitatively analyze the predictors of SIBO in IBD patients. Another meta-analysis published in 2021 reported that the prevalence of methane-positive SIBO in IBD patients was 5.6% (9). However, this meta-analysis did not include hydrogen-positive SIBO patients, which led to the result not truly demonstrating the condition of SIBO in IBD patients. Recognizing that previous studies might not be able to provide convincing data to affect practice, we conducted an updated meta-analysis.
The primary aim of our study was to evaluate the pooled prevalence rates of SIBO among individuals with IBD, the pooled odds ratio (OR) of SIBO among IBD patients compared with controls, and also to examine predictors associated with SIBO among IBD.
Methods
Search strategy and selection criteria
This meta-analysis is performed with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and was registered at the International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42024521031) (10).
We selected relevant studies published from inception to June 2024 by searching PubMed, Embase, and Cochrane Library. We applied no language restrictions. We used the following search strategy: [‘SIBO’ OR ‘small intestinal bacterial overgrowth’ OR ‘small intestine bacterial overgrowth’ OR ‘breath test’ OR ‘small bowel bacterial overgrowth’ OR ‘SBBO’] AND [‘IBD’ OR ‘inflammatory bowel disease’ OR ‘UC’ OR ‘Ulcerative colitis’ OR ‘CD’ OR ‘Crohn’s disease’]. We manually searched the reference lists of all the included articles to help identify additional potentially relevant studies. We tried to contact the authors if we could not get a full article.
Study selection and data extraction
The inclusion criteria were: (1) case-series study or case–control study; (2) studies recruiting subjects meeting diagnostic criteria for IBD, including clinical, radiological, colonoscopy, and histological diagnosis. (3) studies of SIBO being diagnosed using valid methods (breath test or JAC). (4) studies that reported the prevalence of SIBO in IBD patients or compared the prevalence of SIBO in IBD patients versus healthy controls. (5) studies included more than 40 individuals. The exclusion criteria were: (1) case reports, review articles, letters, and animal studies; (2) studies with inaccurate data. Two independent investigators (X Feng and J Hu) searched and assessed study titles, abstracts, and full-text.
Two investigators (X Feng and J Hu) extracted the following data from each selected study: first author, the year of study, study design, country, age, gender, sample sizes of IBD (UC, CD), and controls, diagnostic criteria for IBD, source of controls, a diagnostic test of SIBO including dose of substrate, cut off criteria for positive SIBO diagnosis and test duration, the proportion of SIBO in IBD (UC and CD) patients and controls, prior antibiotic use, concurrent PPI use, history of abdominal surgery, antibiotics treatment of SIBO positive patients and the improvements of main symptoms post-treatment.
The quality of the included case–control studies was assessed using the Newcastle-Ottawa scale (NOS) based on the following three domains: the selection of subjects, the comparability of groups, and the ascertainment of exposure of interest (11). The quality of the study was ranked as high when the study reached the score of 7 stars, moderate when the study reached the score of 4–6 stars, and low when the study was below the score of 4 stars. In addition, the quality of the included case-series studies was assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Tools (12). Studies with scores ≥7 “yes” were considered low risk of bias, scores of 5 “yes” or 6 “yes” were considered moderate risk of bias, and scores <5 “yes” were considered high risk of bias. A third investigator (X Zhang) resolved disagreements between the two investigators.
Statistical analysis
We estimated the pooled prevalence of SIBO in all individuals with IBD. For case–control studies, we calculated the pooled odds ratio (OR) and 95% confidence interval (CI) by comparing the prevalence of positive SIBO between the IBD group and the control group. Further, meta-analyses, according to predictors, such as demographic (gender, age), abdominal symptoms (abdominal pain, bloating), and history of abdominal operation, were also estimated.
A p value <0.05 was considered statistically significant. We used the Cochran Q test and I2 testing to assess heterogeneity between studies, with chi-squared test p < 0.10 or I2 ≥ 50% regarded as substantial heterogeneity (13, 14). We used the fixed-effects model in low heterogeneity (I2 < 50%) and the random-effects model in high heterogeneity (I2 ≥ 50%). We further conducted subgroup analyses stratified by diagnostic methods or quality of the studies to analyze the sources of heterogeneity among pooled studies. Furthermore, we constructed a funnel plot to assess the possibility of publication bias. We performed the Egger test to evaluate funnel plot asymmetry, with a p-value <0.05 indicating significant publication bias. We used RevMan 5.3 and Stata 12.0 for all statistical analyses.
Results
Study selection
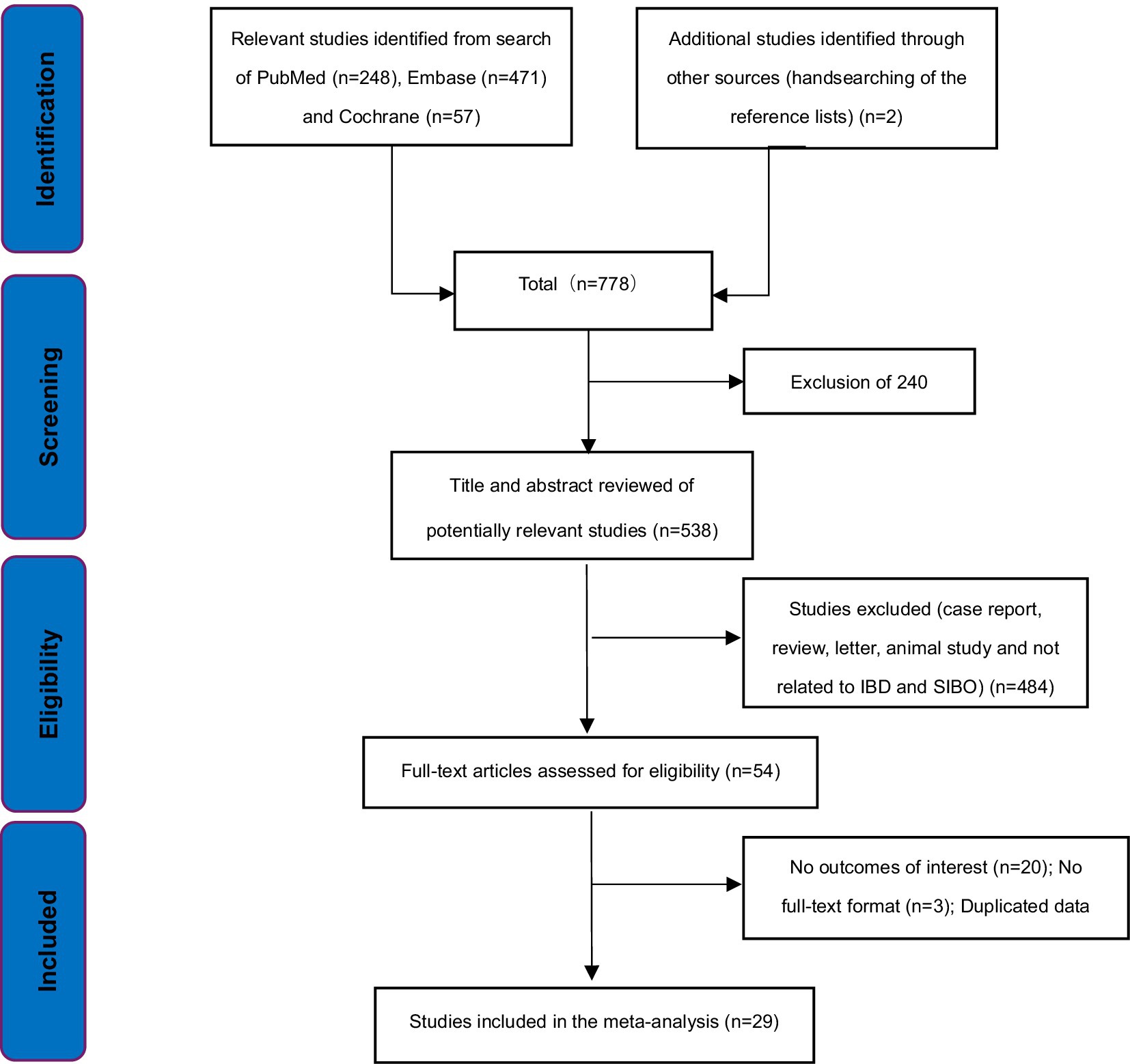
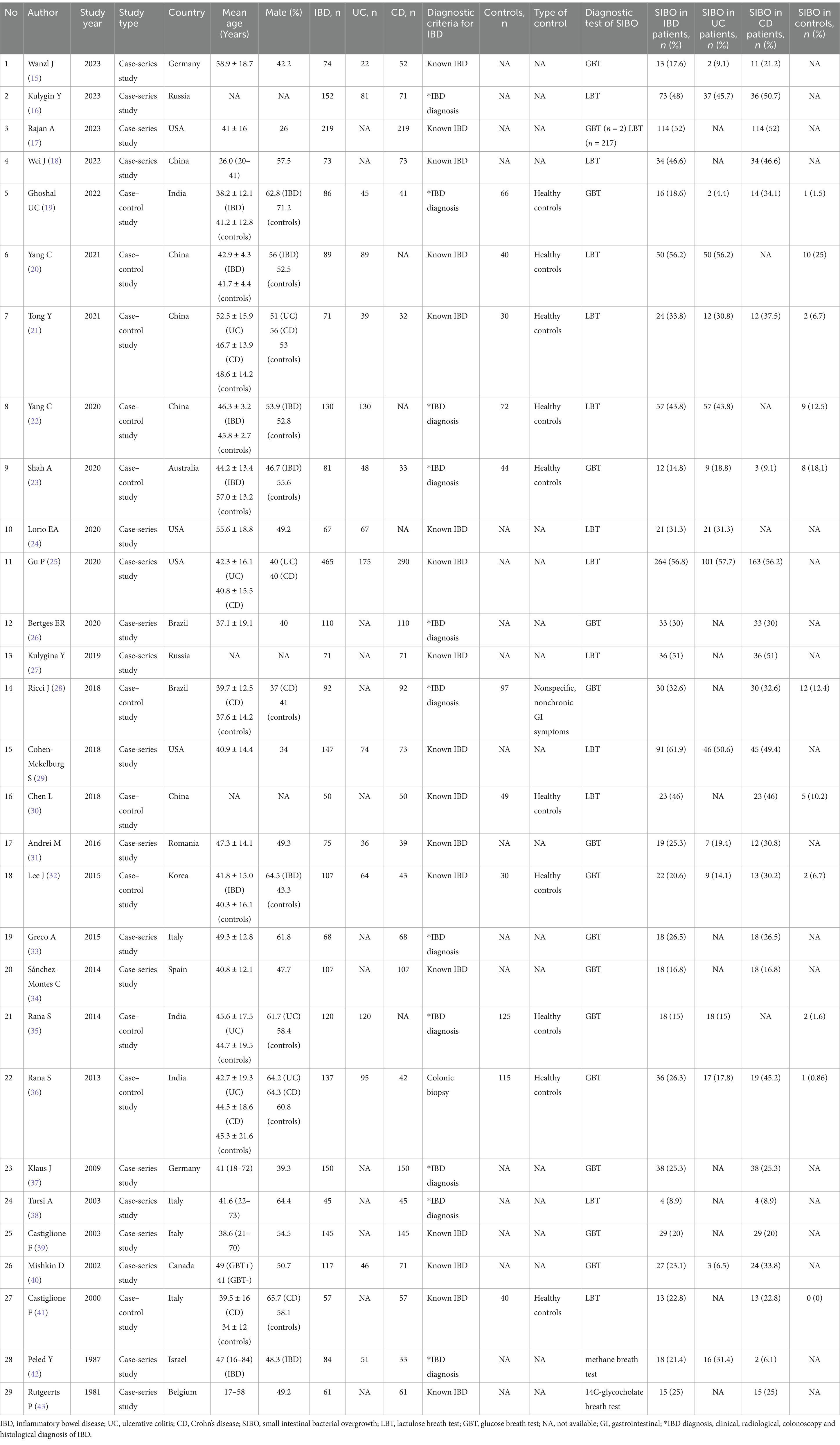
We identified 778 potentially relevant articles based on the search strategy. 240 articles were excluded for duplicates. 509 articles did not meet the inclusion criteria and were excluded after evaluation on the title/abstract/full-text level. Finally, 29 studies were included in our analysis (15–43) (Figure 1). 18 of the 29 studies were case-series studies (15–18, 24–27, 29, 31, 33, 34, 37–40, 42, 43) and the remaining 11 were case–control studies (19–23, 28, 30, 32, 35, 36, 41). Ten case–control studies included healthy volunteers as controls, and only one study included individuals undergoing nonspecific, nonchronic (duration <3 months) GI symptoms as controls (28). Four of these studies included UC patients (20, 22, 24, 35), 13 included CD patients (17, 18, 26–28, 30, 33, 34, 37–39, 41, 43), while 12 included both UC and CD patients (15, 16, 19, 21, 23, 25, 29, 31, 32, 36, 40, 42). In addition, 12 of the 18 case-series studies were considered low risk of bias (15–18, 25, 26, 29, 31, 33, 34, 37, 39), and six were considered moderate risk of bias (24, 27, 38, 40, 42, 43). Nine of the 11 case–control studies were ranked high quality (19–23, 28, 32, 35, 36), and two were considered moderate quality (30, 41). Twenty studies excluded patients who had previously received antibiotic treatment (15–17, 20–23, 26, 28, 31–37, 39–41, 43). Lastly, only one study was available in Russian (16), and the other 28 were in English. The main characteristics and quality evaluation of all the studies included in this study are shown in Table 1 and Supplementary Tables S1, S2.
Study population and testing for SIBO
A total of 3,250 individuals with IBD (1,182 UC and 2,068 CD) and 708 healthy controls were included in the 29 studies. These together comprised 12 studies from Asia (16, 18–22, 27, 30, 32, 35, 36, 42), 9 from Europe (15, 31, 33, 34, 37–39, 41, 43), 7 from the Americas (17, 24–26, 28, 29, 40), 1 from Australia (23). SIBO was defined by using GBT in 14 (15, 19, 23, 26, 28, 31–37, 39, 40), LBT in 13 (16–18, 20–22, 24, 25, 27, 29, 30, 38, 41), methane breath test in one (42), and 14C-glycocholate breath test in one (43) (Table 1). Of the 14 studies that used GBT to diagnose SIBO, the substrate dose was 50 g glucose in seven studies, 75 g glucose in four, 80 g glucose in two, and 100 g glucose in one. Of the 13 studies that used LBT to diagnose SIBO, the substrate dose was 10 g lactulose in nine studies, and four studies did not specify the substrate dose. Multiple cut-off criteria to define a positive SIBO diagnosis were observed in the included studies. In the 14 studies that used GBT to measure hydrogen or methane production, positive SIBO was diagnosed by a rise of >12 parts per million (ppm) above baseline in 10 studies, a rise of >10 ppm above baseline in two studies, a rise of >20 ppm above baseline in two studies. In the 13 studies that used LBT to measure hydrogen or methane production, positive SIBO was diagnosed by a rise of >20 ppm above baseline in five studies, baseline >20 ppm in one study, a rise of >12 ppm above baseline and/or baseline >20 ppm in two studies, a rise of >10 ppm above baseline and/or baseline >20 ppm in one study, a rise of >12 ppm above baseline in one study, a rise of >10 ppm above baseline in one study, and criteria not specified in two studies. In the study that used methane breath test, positive SIBO was diagnosed when the methane gas level was at least 1 ppm above ambient air (42). The study that used 14C-glycocholate breath test did not specify criteria (43). The cut off criteria for diagnosing SIBO in IBD patients are included in Supplementary Table S3.
Prevalence of SIBO in IBD patients
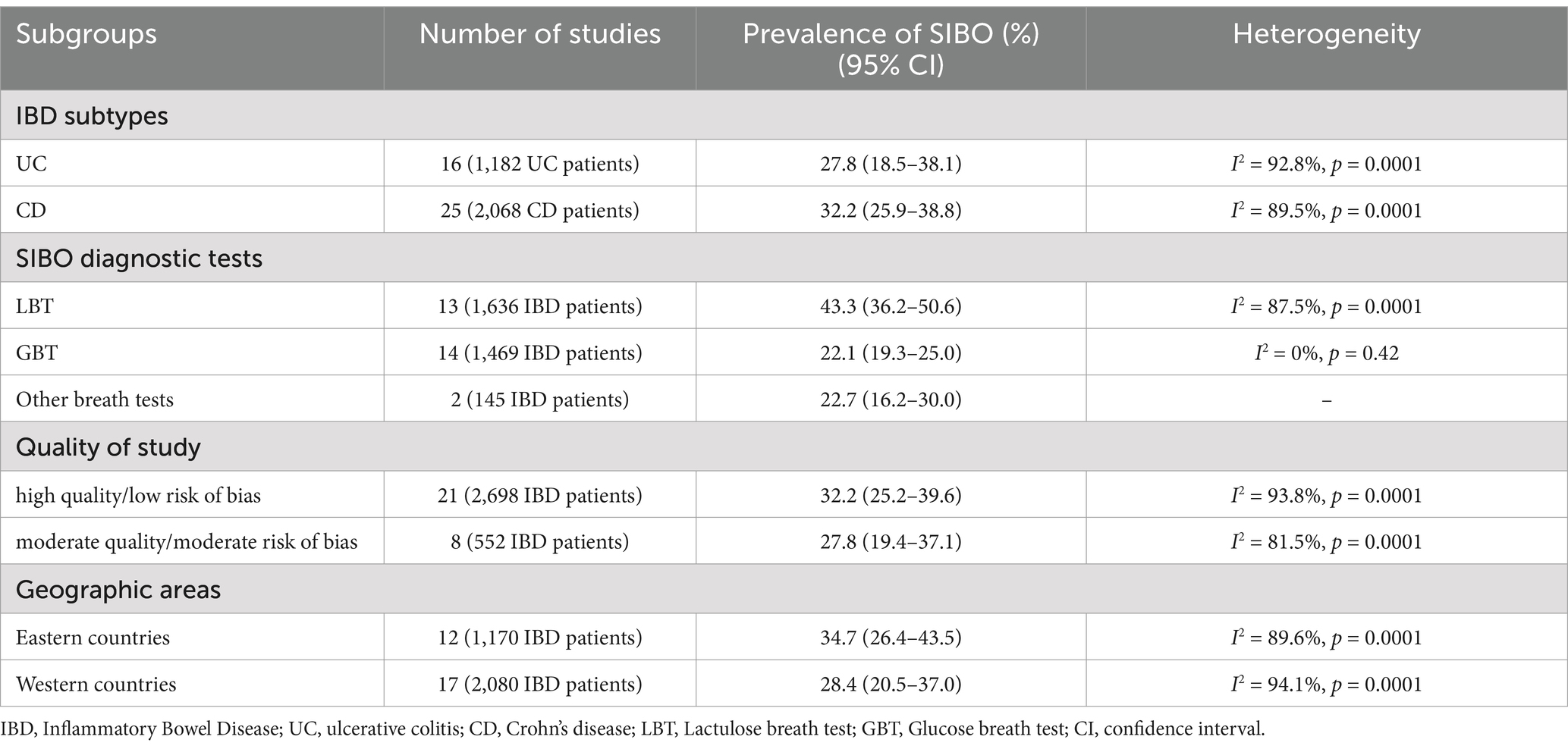
All 29 included studies reported the prevalence of positive SIBO in IBD patients. The pooled prevalence of SIBO in IBD population was 31.0% (95% CI 25.2–37.1) (Figure 2A). The highest prevalence of SIBO in IBD was 61.9% (29), and the lowest prevalence was 8.9% (38). Given the significantly high heterogeneity (I2 = 92.5%, p < 0.0001) detected among the included studies, we applied a random-effects model. The asymmetry of the funnel plot revealed the presence of publication bias (Figure 2B), which was further confirmed by the result of Egger’s test (p = 0.001) (Figure 2C). To explore the variability of the prevalence of SIBO among the studies, we conducted subgroup analyses based on IBD subtypes, SIBO diagnostic tests, quality of study, geographic area. The prevalence of SIBO in CD patients (32.2, 95% CI 25.9–38.8) was higher than in UC patients (27.8, 95% CI 18.5–38.1) (Supplementary Figure S1). The subgroup analysis also showed that the prevalence of SIBO was higher in studies using the LBT (43.3, 95% CI 36.2–50.6) than those using the GBT (22.1, 95% CI 19.3–25.0) or other breath tests (22.7, 95% CI 16.2–30.0) (Supplementary Figure S2). Furthermore, in subgroup analysis by quality of study, the prevalence of SIBO was 32.2% (95% CI 25.2–39.6) in studies with high quality or low risk of bias and 27.8% (95% CI 19.4–37.1) in studies with moderate quality or moderate risk of bias (Supplementary Figure S3). Finally, the prevalence of SIBO in Eastern countries (34.7, 95% CI 26.4–43.5) was greater than the prevalence in Western countries (28.4, 95% CI 20.5–37.0) (Supplementary Figure S4). The summary of the subgroup analyses is included in Table 2.
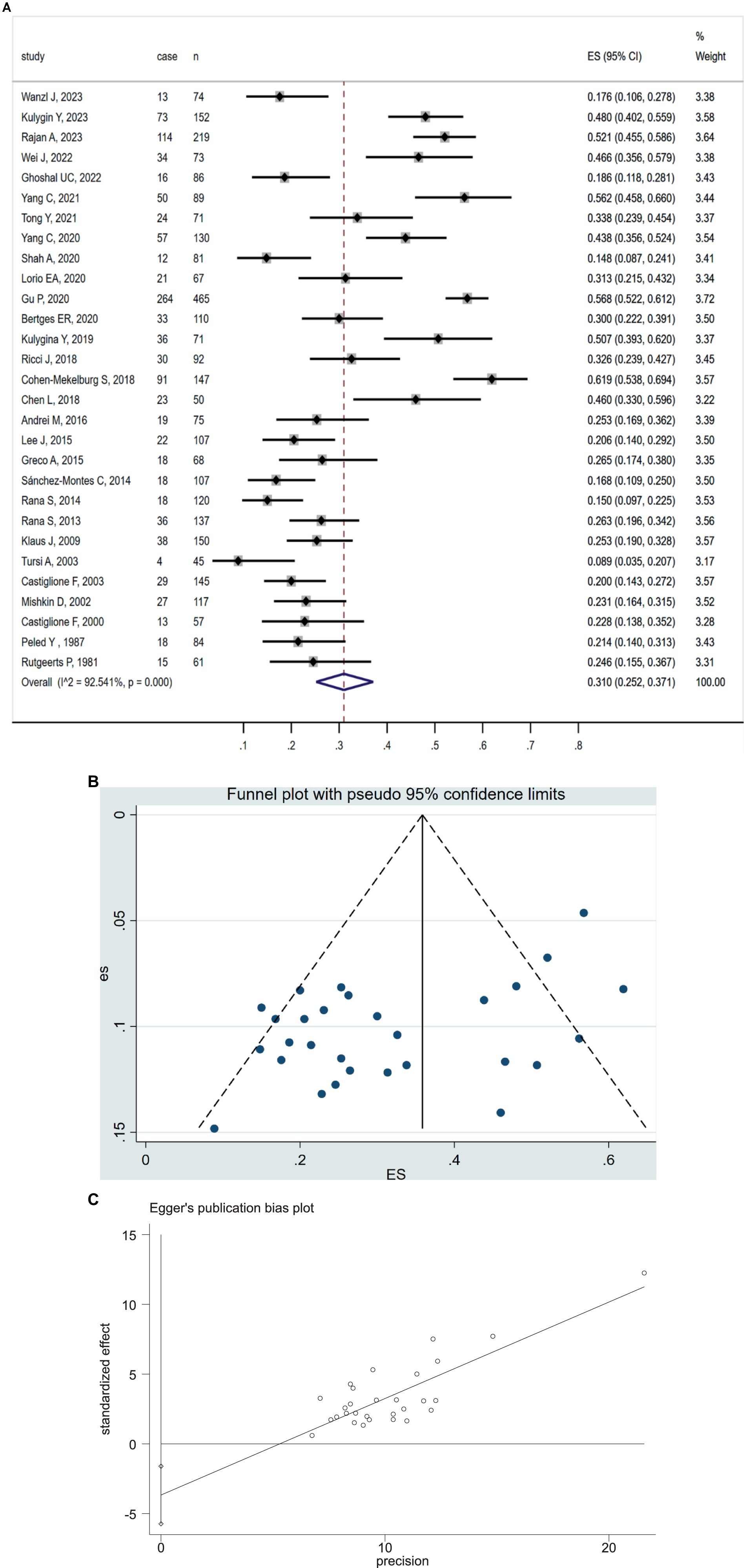
Figure 2. (A) Forest plot of studies showing pooled prevalence of SIBO in patients with IBD (31.0% [95% CI 25.2–37.1]), (I2 = 92.54, p = 0.0001). (B) Funnel plot of positive SIBO in patients with IBD. (C) Egger’s publication bias plot of positive SIBO in patients with IBD (p = 0.001).
Prevalence of SIBO in IBD patients and controls
The 11 case–control studies included 1,020 patients with IBD (630 UC and 390 CD) and 708 controls. The pooled OR of SIBO in IBD patients compared with healthy controls was 5.25 (95% CI 2.96–9.32, p < 0.00001) (Figure 3A), with moderate heterogeneity detected among the studies (I2 = 59%, p = 0.007). We performed a sensitivity analysis, which indicated that no single research was biasing the results (Figure 3B). The visual inspection of the funnel plot showed that no significant publication bias existed (Figure 3C), which is consistent with the result of Egger’s test (p = 0.014) (Figure 3D). Similarly, the subgroup analysis based on IBD subtype concluded the increased prevalence of SIBO in UC patients (OR = 4.22; 95% CI 2.26–7.85) and CD patients (OR = 7.34; 95% CI 2.69–20.05) (Supplementary Figures S5, S6).
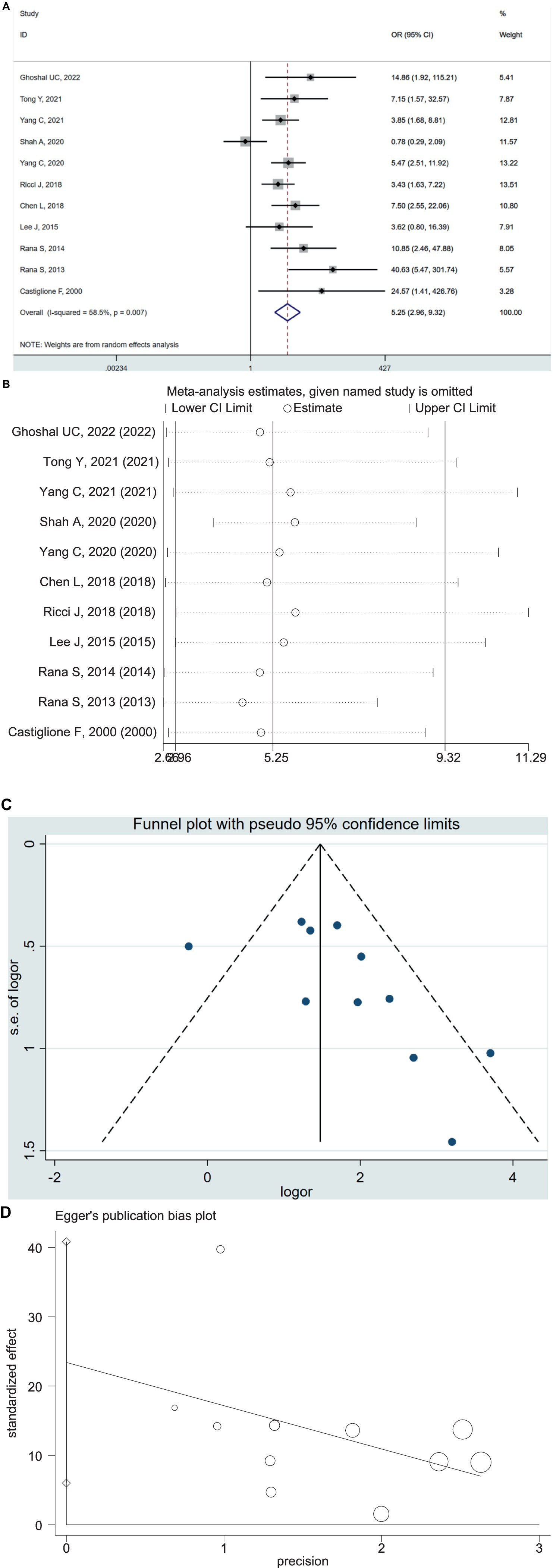
Figure 3. (A) Forest plot of odds ratios of SIBO in IBD patients compared with healthy controls (OR = 5.25 [95% CI 2.96–9.32]), (I2 = 58.5, p = 0.007). (B) Sensitivity analysis plot of odds ratios of SIBO in IBD patients compared with healthy controls. (C) Funnel plot showing the publication bias of odds ratios of SIBO in IBD compared with healthy controls. (D) Egger’s publication bias plot of odds ratios of SIBO in IBD compared with healthy controls (p = 0.014).
Predictors of SIBO in patients with IBD
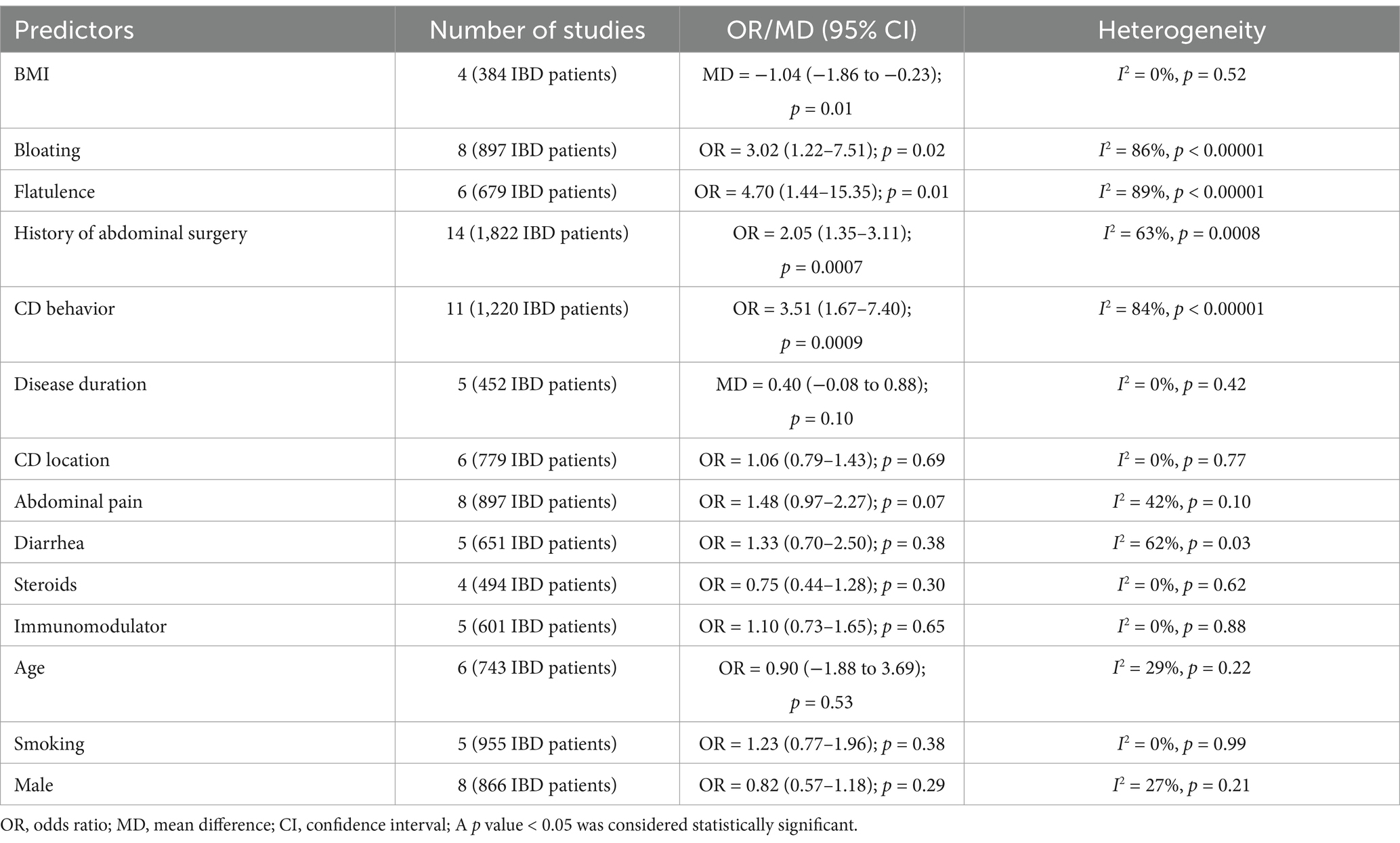
We conducted quantitative analyses to examine demographic and clinical factors that potentially impact the prevalence of SIBO in the IBD population. Four studies (N = 384 IBD patients) assessed the difference in BMI between patients with and without SIBO. Pooling the data of these studies demonstrated that IBD patients with SIBO had a lower BMI than those without SIBO (mean difference (MD) = −1.04; 95% CI −1.86 to −0.23), which was statistically significant (p = 0.01) (Supplementary Figure S7). Eight studies (N = 897 IBD patients) assessed the OR of bloating between patients with and without SIBO. Pooling the data of these studies showed that the prevalence of bloating was greater in patients with SIBO than those without SIBO (OR = 3.02, 95% CI 1.22–7.51; p = 0.02) (Supplementary Figure S8). Similarly, the OR of flatulence was also assessed by six studies (N = 679 IBD patients). The result showed that IBD patients with SIBO had a greater prevalence of flatulence than those without SIBO (OR = 4.70, 95% CI 1.44–15.35; p = 0.01) (Supplementary Figure S9). Pooled analysis of 14 studies (N = 1,822 IBD patients) showed a higher likelihood of a history of abdominal surgery in IBD patients with SIBO compared to those without SIBO (OR = 2.05, 95% CI 1.35–3.11; p = 0.0007) (Supplementary Figure S10). Pooled analysis of 11 studies (N = 1,220 IBD patients) that assessed the CD behavior (B1: Inflammatory; B2: Structuring; B3: Penetrating) showed that CD patients with SIBO had a higher likelihood of B2 or B3 behavior than those without SIBO (OR = 3.51, 95% CI 1.67–7.40; p = 0.0009) (Supplementary Figure S11). Furthermore, other meta-analyses demonstrated that the differences in the mean age, gender, disease duration, disease location (L1: ileal; L2: colonic; L3: ileocolonic), abdominal pain, diarrhea, steroids, immunomodulator, and smoking were not statistically significant between IBD patients with and without SIBO (Supplementary Figures S12–S20). The summary of the meta-analyses for the predictors of SIBO in IBD is included in Table 3.
Effect of antibiotic treatment on IBD patients with SIBO
Six studies evaluated the effect of antibiotic treatment in IBD patients with SIBO (20, 25, 29, 33, 38, 39) (Supplementary Table S4). Yang et al. (20) divided 50 UC patients into group A (mesalazine) and group B (mesalazine + rifaximin) to compare the clinical efficacy. They found that group B presented a greater total effective rate than group A (92.86% vs. 63.64%, p < 0.05) and led to a greater reduction in the level of ESR and CRP (all p < 0.05). Gu et al. (25) treated 117 IBD patients with antibiotics for 2 weeks, and 57.3% of patients showed symptomatic improvement significantly. Cohen-Mekelburg et al. (29) observed a significant reduction in both the median Mayo Score and the median HBI score after the treatment with rifaximin and probiotics. Greco et al. (33) treated 15 CD patients with various antibiotic treatments (ciprofloxacin, metronidazole, or rifaximin). After treatment, normalization of GBT in 13/15 patients and a significant increase in vitamin B12 levels (p = 0.011) were reported. Similarly, Castiglione (39) reported normalization of GBT in 27/29 CD patients and significant improvement in GI symptoms after antibiotic treatment (metronidazole or ciprofloxacin). Tursi (38) also found that 87% of CD patients had normalized orocaecal transit time after the treatment with rifaximin. In conclusion, antibiotic treatment may improve GI symptoms and normalize breath tests in SIBO-positive patients.
Discussion
The development or progression of IBD was widely suggested to be significantly associated with gut microbiota (1, 44–46). The link between IBD and SIBO has been previously presented in two meta-analyses (8, 9). Since then, increasing studies have been implemented to clarify the relationship between SIBO and IBD further. We exhaustively summarized the relevant data from 29 studies to conduct the updated meta-analysis. The included studies were conducted in 14 countries around the world. The sample size of our meta-analysis is about triple that of the previous meta-analysis (8).
Dysbiosis is one of the main pathogenesis of IBD (1, 45). The gut microbiota of a healthy population is mainly composed of the phyla Firmicutes, Bacteroidetes, Actinobacteria, and Verrucomicrobia (47). The dysbiosis in IBD patients is mainly characterized by a reduction of Firmicutes and Bacteroidetes and a relative increase of Proteobacteria (48). The gut microbiota produces local neurotransmitters, biologically active catecholamines, and metabolites and influences the gut-brain axis, thus playing an essential role in the pathogenesis of IBD (49–51). In our study, the pooled prevalence of SIBO among IBD patients was 31.0%, ranging from 8.9 to 61.9%. The odds of SIBO were 5.25-fold higher in IBD patients as compared to healthy controls. These conclusions are inconsistent with the previous studies (8, 9). Subgroup analysis showed that the odds of SIBO were higher in patients with CD (32.2%) compared to those with UC (27.8%), but the odds of SIBO were increased in both UC (OR = 4.22) and CD patients (OR = 7.34) compared to healthy controls. One possible reason is that the impaired ileocaecal valve in CD patients cannot prevent retrograde translocation of colonic bacteria. Thus, SIBO occurs more easily (52, 53). Differences in SIBO diagnostic tests may account for the variance of reported SIBO prevalence among IBD. In our study, the prevalence of SIBO diagnosed by LBT was prominently higher than that of GBT (43.3% vs. 22.1%). Glucose is a monosaccharide rapidly absorbable in the proximal small bowel, resulting in a low sensitivity for diagnosing SIBO (54). In contrast, lactulose is a disaccharide delivered early to colonic bacteria, producing excess hydrogen gas and a higher false-positive result (55). In addition, SIBO is more common among IBD patients in Eastern countries than in Western countries (34.7% vs. 28.4%). This might be a result of the differences in diets and metabolism among different geographic areas. In Eastern countries, the mainstream diet is carbohydrates (starch, sugars), which would increase the relative abundance of Bifidobacteria (56). Finally, the prevalence of SIBO among IBD patients from high-quality studies was higher than those from moderate-quality studies. Although uncertain, a greater number of studies using LBT in the high-quality group may explain the high prevalence of SIBO.
Gastrointestinal symptoms seen in IBD may overlap with those associated with SIBO, such as abdominal pain, diarrhea, and flatulence. According to previous studies (25, 33), antibiotic treatment can effectively improve symptoms of IBD among those with SIBO by altering the gut microbiota. Given the high prevalence of SIBO in individuals with IBD, identifying predictors of SIBO is important for IBD patients to achieve the maximum possible gain from antibiotic treatment. In our meta-analysis, IBD patients with SIBO had a lower BMI as compared to those without SIBO (MD = −1.04, p = 0.01). A reasonable explanation is that bacteria and/or their metabolites in SIBO will damage the epithelial barrier, leading to Inflammatory response and enhanced intestinal permeability, ultimately resulting in significant weight loss and malnutrition (57, 58). The luminal competition with the host for nutrients in SIBO may further contribute to malnutrition (59). Furthermore, the risks of bloating (OR = 3.02, p = 0.02) and flatulence (OR = 4.70, p = 0.01) were increased in SIBO-positive IBD patients. Bloating and flatulence were typical symptoms of SIBO, which may be associated with excessive production of hydrogen and intestinal motility disorders (5). Lastly, SIBO was positively associated with a history of abdominal surgery (OR = 2.05, p = 0.0007) and stricturing/penetrating disease (OR = 3.51, p = 0.0009) in IBD patients. Altered GI anatomy damages the integrality of the ileocecal valve and antegrade motility of the ileum, leading to retrograde translocation of colonic bacteria, which may predispose them to SIBO (6, 60).
Strengths of our study include an exhaustive literature search, careful analysis of variability of SIBO prevalence, and exploration of predictors of SIBO in IBD. There are limitations to our research. None of the included studies used jejunal aspirate and culture, the gold standard to diagnose SIBO. Copious definitions of positive breath tests in the included studies may contribute to heterogeneity in estimating SIBO prevalence in IBD. In addition, the asymmetry of the funnel plot calculating the pooled prevalence of SIBO suggested that the prevalence of SIBO in IBD may have been overestimated.
In summary, our meta-analysis has shown that nearly one-third of individuals with IBD present with SIBO positive, and the prevalence of SIBO varied according to the SIBO diagnostic methods performed. The odds of SIBO in IBD was increased by 5.25-fold compared with healthy individuals. Lower BMI, bloating, flatulence, history of abdominal surgery, and stricturing/penetrating disease behavior were predictors of SIBO in IBD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XF: Writing – original draft. JH: Writing – review & editing. XZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1490506/full#supplementary-material
References
1. Nakase, H, Uchino, M, Shinzaki, S, Matsuura, M, Matsuoka, K, Kobayashi, T, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. (2021) 56:489–526. doi: 10.1007/s00535-021-01784-1
2. Lamb, CA, Kennedy, NA, Raine, T, Hendy, PA, Smith, PJ, Limdi, JK, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. (2019) 68:s1–s106. doi: 10.1136/gutjnl-2019-318484
3. Vălean, D, Zaharie, R, Țaulean, R, Usatiuc, L, and Zaharie, F. Recent trends in non-invasive methods of diagnosis and evaluation of inflammatory bowel disease: a short review. Int J Mol Sci. (2024) 25:2077. doi: 10.3390/ijms25042077
4. Balderramo, D, Quaresma, AB, Olivera, PA, Savio, MC, Villamil, MPG, Panaccione, R, et al. Challenges in the diagnosis and treatment of inflammatory bowel disease in Latin America. Lancet Gastroenterol Hepatol. (2024) 9:263–72. doi: 10.1016/S2468-1253(23)00284-4
5. Pimentel, M, Saad, RJ, Long, MD, and Rao, SSC. ACG clinical guideline: small intestinal bacterial overgrowth. Am J Gastroenterol. (2020) 115:165–78. doi: 10.14309/ajg.0000000000000501
6. Bushyhead, D, and Quigley, EMM. Small intestinal bacterial overgrowth-pathophysiology and its implications for definition and management. Gastroenterology. (2022) 163:593–607. doi: 10.1053/j.gastro.2022.04.002
7. Rezaie, A, Buresi, M, Lembo, A, Lin, H, McCallum, R, Rao, S, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: the North American consensus. Am J Gastroenterol. (2017) 112:775–84. doi: 10.1038/ajg.2017.46
8. Shah, A, Morrison, M, Burger, D, Martin, N, Rich, J, Jones, M, et al. Systematic review with meta-analysis: the prevalence of small intestinal bacterial overgrowth in inflammatory bowel disease. Aliment Pharmacol Ther. (2019) 49:624–35. doi: 10.1111/apt.15133
9. Gandhi, A, Shah, A, Jones, MP, Koloski, N, Talley, NJ, Morrison, M, et al. Methane positive small intestinal bacterial overgrowth in inflammatory bowel disease and irritable bowel syndrome: a systematic review and meta-analysis. Gut Microbes. (2021) 13:1933313. doi: 10.1080/19490976.2021.1933313
10. Moher, D, Liberati, A, Tetzlaff, J, Altman, DG, and S,. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
11. Deeks, JJ, Dinnes, J, D'Amico, R, Sowden, A, Sakarovitch, C, Song, F, et al. Evaluating non-randomised intervention studies. Health Technol Assess. (2003) 7:iii–173. doi: 10.3310/hta7270
12. Munn, Z, Moola, S, Lisy, K, Riitano, D, and Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. (2015) 13:147–53. doi: 10.1097/XEB.0000000000000054
13. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
14. Higgins, JP, and Thompson, SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
15. Wanzl, J, Gröhl, K, Kafel, A, Nagl, S, Muzalyova, A, Gölder, SK, et al. Impact of small intestinal bacterial overgrowth in patients with inflammatory bowel disease and other gastrointestinal disorders-a retrospective analysis in a tertiary single center and review of the literature. J Clin Med. (2023) 12:935. doi: 10.3390/jcm12030935
16. Kulygin, YUA, and Osipenko, MF. Excessive bacterial growth syndrome in small intestine burdens the clinical picture of inflammatory bowel diseases. Exp Clin Gastroenterol. (2023) 4:49–54. doi: 10.31146/1682-8658-ecg-212-4-49-54
17. Rajan, A, Pan, Y, Mahtani, P, Niec, R, Longman, R, Gerber, J, et al. The impact of confounders on symptom-endoscopic discordances in Crohn's disease. Crohns Colitis 360. (2023) 5:otad017. doi: 10.1093/crocol/otad017
18. Wei, J, Feng, J, Chen, L, Yang, Z, Tao, H, Li, L, et al. Small intestinal bacterial overgrowth is associated with clinical relapse in patients with quiescent Crohn's disease: a retrospective cohort study. Ann Transl Med. (2022) 10:784. doi: 10.21037/atm-22-3335
19. Ghoshal, UC, Yadav, A, Fatima, B, Agrahari, AP, and Misra, A. Small intestinal bacterial overgrowth in patients with inflammatory bowel disease: a case-control study. Indian J Gastroenterol. (2022) 41:96–103. doi: 10.1007/s12664-021-01211-6
20. Yang, C, Zhang, X, Wang, S, Huo, X, and Wang, J. Small intestinal bacterial overgrowth and evaluation of intestinal barrier function in patients with ulcerative colitis. Am J Transl Res. (2021) 13:6605–10.
21. Tong, YL, Yu, XF, Yu, Y, Mi, L, Zhang, ZY, Bao, Z, et al. Small intestinal bacterial overgrowth and low-grade systemic inflammation in patients with inflammatory bowel disease. Acta Med Mediterr. (2021) 37:2941–6. doi: 10.19193/0393-6384_2021_5_454
22. Yang, C, Guo, X, Wang, J, Fan, H, Huo, X, Dong, L, et al. Relationship between small intestinal bacterial overgrowth and peripheral blood ET, TLR2 and TLR4 in ulcerative colitis. J Coll Physicians Surg Pak. (2020) 30:245–9. doi: 10.29271/jcpsp.2020.03.245
23. Shah, A, Talley, NJ, Koloski, N, Macdonald, GA, Kendall, BJ, Shanahan, ER, et al. Duodenal bacterial load as determined by quantitative polymerase chain reaction in asymptomatic controls, functional gastrointestinal disorders and inflammatory bowel disease. Aliment Pharmacol Ther. (2020) 52:155–67. doi: 10.1111/apt.15786
24. Lorio, EA, Wisniewski, N, Brown, ED, and Vizuete, JA. An analysis of small intestine bacterial overgrowth rates in ulcerative colitis. Am J Gastroenterol. (2020) 115:S439–40. doi: 10.14309/01.ajg.0000705456.97559.1b
25. Gu, P, Patel, D, Lakhoo, K, Ko, J, Liu, X, Chang, B, et al. Breath test gas patterns in inflammatory bowel disease with concomitant irritable bowel syndrome-like symptoms: a controlled large-scale database linkage analysis. Dig Dis Sci. (2020) 65:2388–96. doi: 10.1007/s10620-019-05967-y
26. Bertges, ER, and Chebli, JMF. Prevalence and factors associated with small intestinal bacterial overgrowth in patients with CROHN'S disease: a retrospective study at a referral center. Arq Gastroenterol. (2020) 57:283–8. doi: 10.1590/S0004-2803.202000000-64
27. Kulygina, Y, Osipenko, M, Skalinskaya, M, Alikina, T, Kabilov, M, Lukinov, V, et al. Small intestinal bacterial overgrowth in patients with Crohn's disease is not only associated with a more severe disease, but is also marked by dramatic changes in the gut microbiome. J Crohn's Colitis. (2019) 13:S544–S4479. doi: 10.1093/ecco-jcc/jjy222.965
28. Ricci,, Chebli, LA, Ribeiro, TCDR, Castro, ACS, Gaburri, PD, Pace, FHDL, et al. Small-intestinal bacterial overgrowth is associated with concurrent intestinal inflammation but not with systemic inflammation in Crohn's disease patients. J Clin Gastroenterol. (2018) 52:530–6. doi: 10.1097/MCG.0000000000000803
29. Cohen-Mekelburg, S, Tafesh, Z, Coburn, E, Weg, R, Malik, N, Webb, C, et al. Testing and treating small intestinal bacterial overgrowth reduces symptoms in patients with inflammatory bowel disease. Dig Dis Sci. (2018) 63:2439–44. doi: 10.1007/s10620-018-5109-1
30. Chen, LY, Yuan, BS, Wei, J, and Wang, FY. Abstracts published only. J Dig Dis. (2018) 19:49–146. doi: 10.1111/1751-2980.12665
31. Andrei, M, Gologan, S, Stoicescu, A, Ionescu, M, Nicolaie, T, and Diculescu, M. Small intestinal bacterial overgrowth syndrome prevalence in Romanian patients with inflammatory bowel disease. Curr Health Sci J. (2016) 42:151–6. doi: 10.12865/CHSJ.42.02.06
32. Lee, JM, Lee, KM, Chung, YY, Lee, YW, Kim, DB, Sung, HJ, et al. Clinical significance of the glucose breath test in patients with inflammatory bowel disease. J Gastroenterol Hepatol. (2015) 30:990–4. doi: 10.1111/jgh.12908
33. Greco, A, Caviglia, GP, Brignolo, P, Ribaldone, DG, Reggiani, S, Sguazzini, C, et al. Glucose breath test and Crohn's disease: diagnosis of small intestinal bacterial overgrowth and evaluation of therapeutic response. Scand J Gastroenterol. (2015) 50:1376–81. doi: 10.3109/00365521.2015.1050691
34. Sánchez-Montes, C, Ortiz, V, Bastida, G, Rodríguez, E, Yago, M, Beltrán, B, et al. Small intestinal bacterial overgrowth in inactive Crohn's disease: influence of thiopurine and biological treatment. World J Gastroenterol. (2014) 20:13999–4003. doi: 10.3748/wjg.v20.i38.13999
35. Rana, SV, Sharma, S, Kaur, J, Prasad, KK, Sinha, SK, Kochhar, R, et al. Relationship of cytokines, oxidative stress and GI motility with bacterial overgrowth in ulcerative colitis patients. J Crohns Colitis. (2014) 8:859–65. doi: 10.1016/j.crohns.2014.01.007
36. Rana, SV, Sharma, S, Malik, A, Kaur, J, Prasad, KK, Sinha, SK, et al. Small intestinal bacterial overgrowth and orocecal transit time in patients of inflammatory bowel disease. Dig Dis Sci. (2013) 58:2594–8. doi: 10.1007/s10620-013-2694-x
37. Klaus, J, Spaniol, U, Adler, G, Mason, RA, Reinshagen, M, and von Tirpitz, CC. Small intestinal bacterial overgrowth mimicking acute flare as a pitfall in patients with Crohn's disease. BMC Gastroenterol. (2009) 9:61. doi: 10.1186/1471-230X-9-61
38. Tursi, A, Brandimarte, G, Giorgetti, G, and Nasi, G. Assessment of orocaecal transit time in different localization of Crohn's disease and its possible influence on clinical response to therapy. Eur J Gastroenterol Hepatol. (2003) 15:69–74. doi: 10.1097/00042737-200301000-00012
39. Castiglione, F, Rispo, A, di, E, Cozzolino, A, Manguso, F, Grassia, R, et al. Antibiotic treatment of small bowel bacterial overgrowth in patients with Crohn's disease. Aliment Pharmacol Ther. (2003) 18:1107–12. doi: 10.1046/j.1365-2036.2003.01800.x
40. Mishkin, D, Boston, FM, Blank, D, Yalovsky, M, and Mishkin, S. The glucose breath test: a diagnostic test for small bowel stricture (s) in Crohn's disease. Dig Dis Sci. (2002) 47:489–94. doi: 10.1023/a:1017991313789
41. Castiglione, F, del, G, Rispo, A, Petrelli, G, Amalfi, G, Cozzolino, A, et al. Orocecal transit time and bacterial overgrowth in patients with Crohn's disease. J Clin Gastroenterol. (2000) 31:63–6. doi: 10.1097/00004836-200007000-00015
42. Peled, Y, Weinberg, D, Hallak, A, and Gilat, T. Factors affecting methane production in humans. Dig Dis Sci. (1987) 32:267–71. doi: 10.1007/BF01297052
43. Rutgeerts, P, Ghoos, Y, Vantrappen, G, and Eyssen, H. Ileal dysfunction and bacterial overgrowth in patients with Crohn's disease. Eur J Clin Investig. (1981) 11:199–206. doi: 10.1111/j.1365-2362.1981.tb01841.x
44. Banfi, D, Moro, E, Bosi, A, Bistoletti, M, Cerantola, S, Crema, F, et al. Impact of microbial metabolites on microbiota-gut-brain axis in inflammatory bowel disease. Int J Mol Sci. (2021) 22:1623. Published 2021 Feb 5. doi: 10.3390/ijms22041623
45. Sinagra, E, Utzeri, E, Morreale, GC, Fabbri, C, Pace, F, and Anderloni, A. Microbiota-gut-brain axis and its affect inflammatory bowel disease: pathophysiological concepts and insights for clinicians. World J Clin Cases. (2020) 8:1013–25. doi: 10.12998/wjcc.v8.i6.1013
46. Ye, X, Zhang, M, Zhang, N, Wei, H, and Wang, B. Gut-brain axis interacts with immunomodulation in inflammatory bowel disease. Biochem Pharmacol. (2024) 219:115949. doi: 10.1016/j.bcp.2023.115949
47. Jandhyala, SM, Talukdar, R, Subramanyam, C, Vuyyuru, H, Sasikala, M, and Nageshwar, RD. Role of the normal gut microbiota. World J Gastroenterol. (2015) 21:8787–803. doi: 10.3748/wjg.v21.i29.8787
48. Zuo, T, and Ng, SC. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front Microbiol. (2018) 9:2247. doi: 10.3389/fmicb.2018.02247
49. Iyer, LM, Aravind, L, Coon, SL, Klein, DC, and Koonin, EV. Evolution of cell–cell signaling in animals: did late horizontal gene transfer from bacteria have a role? Trends Genet. (2004) 20:292–9. doi: 10.1016/j.tig.2004.05.007
50. Asano, Y, Hiramoto, T, Nishino, R, Aiba, Y, Kimura, T, Yoshihara, K, et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. (2012) 303:G1288–95. doi: 10.1152/ajpgi.00341.2012
51. Lavelle, A, and Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. (2020) 17:223–37. doi: 10.1038/s41575-019-0258-z
52. Sorathia, SJ, Chippa, V, and Rivas, JM. Small intestinal bacterial overgrowth In: Stat pearls [Internet].. Treasure Island (FL): Stat Pearls Publishing (2023).
53. Bures, J, Cyrany, J, Kohoutova, D, Förstl, M, Rejchrt, S, Kvetina, J, et al. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. (2010) 16:2978–90. doi: 10.3748/wjg.v16.i24.2978
54. Saad, RJ, and Chey, WD. Breath testing for small intestinal bacterial overgrowth: maximizing test accuracy. Clin Gastroenterol Hepatol. (2014) 12:1964–72. doi: 10.1016/j.cgh.2013.09.055
55. Yu, D, Cheeseman, F, and Vanner, S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut. (2011) 60:334–40. doi: 10.1136/gut.2009.205476
56. Singh, RK, Chang, HW, Yan, D, Lee, KM, Ucmak, D, Wong, K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. (2017) 15:73. Published 2017 Apr 8. doi: 10.1186/s12967-017-1175-y
57. Shawki, A, and McCole, DF. Mechanisms of intestinal epithelial barrier dysfunction by adherent-invasive Escherichia Coli. Gastroenterol Hepatol. (2017) 3:41–50. Published 2016 Oct 22. doi: 10.1016/j.jcmgh.2016.10.004
58. Ghosh, S, Whitley, CS, Haribabu, B, and Jala, VR. Regulation of intestinal barrier function by microbial metabolites. Cell Mol Gastroenterol Hepatol. (2021) 11:1463–82. doi: 10.1016/j.jcmgh.2021.02.007
59. Putnam, EE, and Goodman, AL. B vitamin acquisition by gut commensal bacteria. PLoS Pathog. (2020) 16:e1008208. doi: 10.1371/journal.ppat.1008208
Keywords: small intestinal bacterial overgrowth, inflammatory bowel disease, meta-analysis, ulcerative colitis, Crohn’s disease
Citation: Feng X, Hu J and Zhang X (2025) Prevalence and predictors of small intestinal bacterial overgrowth in inflammatory bowel disease: a meta-analysis. Front. Med. 11:1490506. doi: 10.3389/fmed.2024.1490506
Edited by:
Farah Al Marzooq, United Arab Emirates University, United Arab EmiratesReviewed by:
Catherine Chaput, NEC Laboratories Europe, GermanyEwa Malecka-Wojciesko, Medical University of Lodz, Poland
Copyright © 2025 Feng, Hu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Zhang, MTg3MTYyNTU4MjhAMTYzLmNvbQ==
 Xin Feng1
Xin Feng1 Xin Zhang
Xin Zhang