- Department of Hematology and Oncology, Anhui Provincial Children's Hospital (Anhui Hospital, Pediatric Hospital of Fudan University), Hefei, China
Objective: This study aims to identify key risk factors associated with the development of breakthrough invasive fungal infections (BIFI) in pediatric acute leukemia patients to improve early detection and intervention strategies.
Method: A retrospective analysis was conducted on 160 pediatric patients with acute leukemia admitted to Anhui Provincial Children's Hospital between October 2018 and June 2024. The study evaluated the impact of various clinical parameters on BIFI risk using univariate and multivariable analyses, with data including patient demographics, treatment regimens, and infection outcomes. The predictive model was assessed using receiver operating characteristic (ROC) curve analysis, calibration plots, and decision curve analysis (DCA).
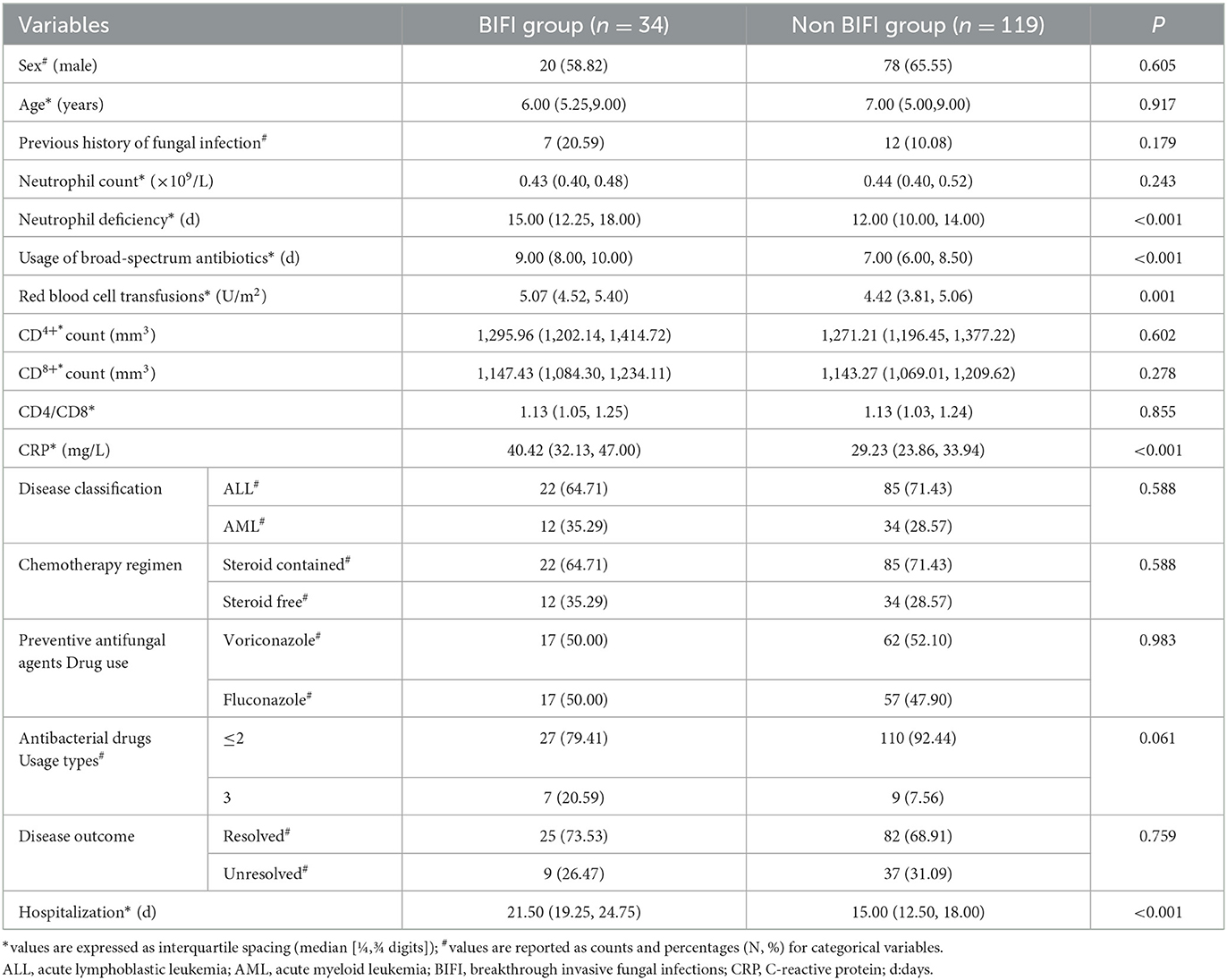
Result: Among the 160 pediatric acute leukemia patients, 34 (22.22%) developed BIFI. Univariate analysis identified longer durations of neutrophil deficiency (P < 0.001), broad-spectrum antibiotic use (P < 0.001), higher volumes of red blood cell transfusions (P = 0.001), and elevated C-reactive protein (CRP) levels (P < 0.001) as significant factors associated with BIFI. Multivariable analysis confirmed these as significant predictors, with odds ratios for neutrophil deficiency (OR = 1.38, 95% CI [1.15, 1.69]), antibiotic use (OR = 1.41, 95% CI [1.10, 1.84]), transfusions (OR = 2.54, 95% CI [1.39, 5.13]), and CRP levels (OR = 1.10, 95% CI [1.04, 1.17]). The model validation showed strong predictive performance with an AUC of 0.890 (95% CI: 0.828–0.952), good calibration (Brier score = 0.099), and demonstrated clinical utility across a range of risk thresholds.
Conclusion: The study highlights the importance of considering these key predictors in the management of pediatric acute leukemia patients to mitigate the risk of BIFI. Incorporating these factors into personalized treatment strategies could enhance early intervention, reduce infection rates, and improve overall patient outcomes.
1 Introduction
Acute leukemia, a frequently encountered clinical condition characterized by the malignant proliferation of hematopoietic stem cells, includes both acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). Although ALL and AML are among the most common types of leukemia in children, their incidence varies by geographic region and demographic factors. For instance, in the United States, the incidence of ALL is estimated at 1.6 per 100,000 population, whereas in developing countries, the annual incidence ranges from 3 to 5 per 100,000. The incidence of AML is typically lower than that of ALL. The global incidence rates for both ALL and AML in children are influenced by multiple factors, including environmental and genetic predispositions (1–3). Recent studies in medical technology and continuous optimization of treatment protocols have significantly improved the five-year overall survival rate of children with acute leukemia, now exceeding 70% (3, 4).
Despite these advancements, chemotherapy for pediatric acute leukemia not only targets the proliferation of tumor cells but also inhibits the proliferation and differentiation of normal hematopoietic stem cells. A study from a developing country found that up to 70% of deaths in acute leukemia patients during the induction phase of treatment were associated with infections (5). However, these findings may not be generalizable to other settings or beyond the initial induction phase of therapy, as mortality rates and causes of death can vary significantly across different stages of treatment and geographical locations. Further research is needed to understand the role of infections in leukemia patient mortality in other clinical contexts. In previous study, the incidence of invasive fungal infection (IFI) episodes was 10.4% (6). IFIs, which occur when fungi invade the body and trigger an inflammatory response, continue to pose a high mortality risk—second only to leukemia relapse—despite the availability of new antifungal agents, with mortality rates reported between 20% and 70% in various studies (7–10).
In clinical practice, medications such as voriconazole and fluconazole are commonly used to prevent and treat IFI in children (11). These interventions have reduced the risk of IFI in pediatric acute leukemia patients to some extent. However, due to variations in prevention strategies, central treatment protocols, and the distribution of pathogenic fungi among immunocompromised populations, a subset of pediatric acute leukemia patients still develop breakthrough invasive fungal infections (BIFI) (12, 13). This phenomenon further elevates the mortality rate among these children.
Understanding the risk factors associated with BIFI in children with acute leukemia is crucial for early clinical intervention, improving survival rates, and enhancing the quality of life for affected patients. This study aims to explore these risk factors to inform and improve clinical practices.
2 Methods
2.1 Study population and data acquisition
A retrospective analysis was conducted, collecting clinical data from 160 pediatric patients with acute leukemia admitted to Anhui Provincial Children's Hospital between October 2018 and June 2024. The collected categorical data included gender, disease classification, chemotherapy regimen, disease outcome, usage of broad-spectrum antibiotics, prophylactic antifungal medication, types of antimicrobial agents used, and history of fungal infection. The continuous data collected included patient age, length of hospital stay, neutrophil count, duration of neutropenia, duration of broad-spectrum antibiotic use, red blood cell transfusion volume, cluster of differentiation 4 (CD4+) count, cluster of differentiation 8 (CD8+) count, CD4+/CD8+ ratio, and C-reactive protein (CRP) levels. In this retrospective study, neutrophil counts were measured every 2 days throughout the treatment process. CD4+ and CD8+ counts were primarily measured on the day following the identification of neutropenia in patients. For treatment protocols, refer to Supplementary material 1.
2.2 Inclusion and exclusion criteria
A database review was conducted for entries from October 2018 to June 2024. Patients were eligible for analysis if they received at least 4 days of systemic antifungal prophylaxis during AML and ALL induction or consolidation chemotherapy, with an anticipated duration of neutropenia (defined as an absolute neutrophil count ≤ 500/mL) exceeding seven days. Each patient was included only once.
2.3 Definitions
Based on the 2020 revised consensus by the European Organization for Research and Treatment of Cancer/Infectious Diseases Group and the National Institute of Allergy (14) and Infectious Diseases Mycoses Study Group, along with the sixth revision of China's diagnostic and treatment principles for invasive fungal disease in hematological disease/malignancy patients (15), the criteria for defining probable, possible, and proven BIFIs were established. Any IFI occurring during active antifungal prophylaxis was considered a BIFI. For invasive candidiasis/candidemia, the observation period is at least 4 weeks after starting treatment, and for invasive mold diseases, it is 6 to 12 weeks after initial treatment.
2.4 Statistical analysis
This study began by confirming the normality of the data distribution using the Kolmogorov-Smirnov test (16). Univariate analysis was then conducted using independent t-tests for continuous variables and chi-square tests for categorical variables to identify potential risk factors associated with BIFI (17). A P < 0.05 was considered statistically significant. Subsequently, all variables were then included in the stepwise (backward: conditional) multivariable logistic regression analysis model based on the Akaike Information Criterion, with odds ratios (OR) and 95% confidence intervals (CI) calculated to quantify these associations (18). The model's predictive performance was assessed using receiver operating characteristic (ROC) curve analysis (19) and brier score (20), the latter of which measures the accuracy of probabilistic predictions by evaluating the mean squared difference between predicted probabilities and actual outcomes. The performance of our model was evaluated through internal validation using 1,000 bootstrap resamples. To assess this, we calculated the Variance Inflation Factor (VIF) for each predictor variable. Variables with a VIF >5 were considered to indicate potential multicollinearity and were reviewed for inclusion in the final model. Finally, the model's clinical utility across various risk thresholds was further assessed through calibration plots (21) and decision curve analysis (DCA) (22). Model validation was assessed in terms of both discrimination and calibration. Additionally, we constructed a nomogram using variables with a P < 0.05 in the multivariable analysis to aid in clinical decision-making.
3 Results
3.1 Univariate analysis of BIFI in pediatric acute leukemia patients
Seven patients were excluded due to missing clinical data. A univariate analysis of 153 pediatric acute leukemia patients revealed that 34 patients (22.22%) developed BIFI in Table 1. Significant factors associated with BIFI included longer durations of neutrophil deficiency (median 15 days vs. 12 days, P < 0.001), extended use of broad-spectrum antibiotics (median 9 days vs. 7 days, P < 0.001), higher volumes of red blood cell transfusions (median 5.07 U/m2 vs. 4.42 U/m2, P = 0.001), elevated CRP levels (median 40.42 mg/L vs. 29.23 mg/L, P < 0.001), and longer hospital stays (median 21.50 days vs. 15.00 days, P < 0.001). No significant differences were observed in sex, age, previous history of fungal infection, neutrophil count, CD4+ and CD8+ counts, disease classification, chemotherapy regimen, preventive antifungal agents, or disease outcome between the groups.
3.2 Significant predictors of BIFI in pediatric acute leukemia identified by multivariable regression analysis
The multivariable linear regression analysis identified four significant predictors of BIFI in pediatric acute leukemia patients. In Table 2, these predictors include the duration of neutrophil deficiency (OR = 1.38, 95% CI [1.15, 1.69], P = 0.001), the duration of broad-spectrum antibiotic use (OR = 1.41, 95% CI [1.10, 1.84], P = 0.009), red blood cell transfusions (OR = 2.54, 95% CI [1.39, 5.13], P = 0.005), and CRP levels (OR = 1.10, 95% CI [1.04, 1.17], P = 0.001). These findings highlight the importance of these factors in increasing the risk of developing BIFI. The Figure 1 demonstrates that prolonged neutrophil deficiency, extended use of broad-spectrum antibiotics, higher volumes of red blood cell transfusions, and elevated CRP levels are all significant predictors of increased risk for developing breakthrough invasive fungal infections in pediatric acute leukemia patients. The confidence intervals in each graph suggest some variability in predictions, especially at higher values of these predictors. Supplementary material 2 provides a multivariable linear regression analysis for BIFI.
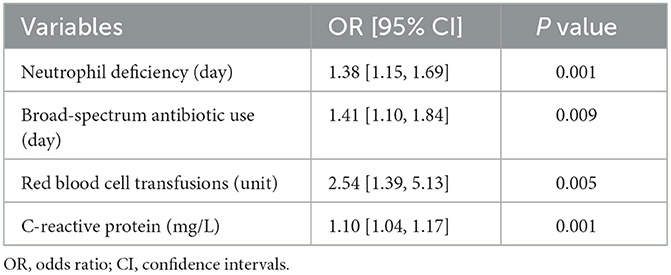
Table 2. Multivariable linear regression analysis identifying independent factors associated with BIFI.
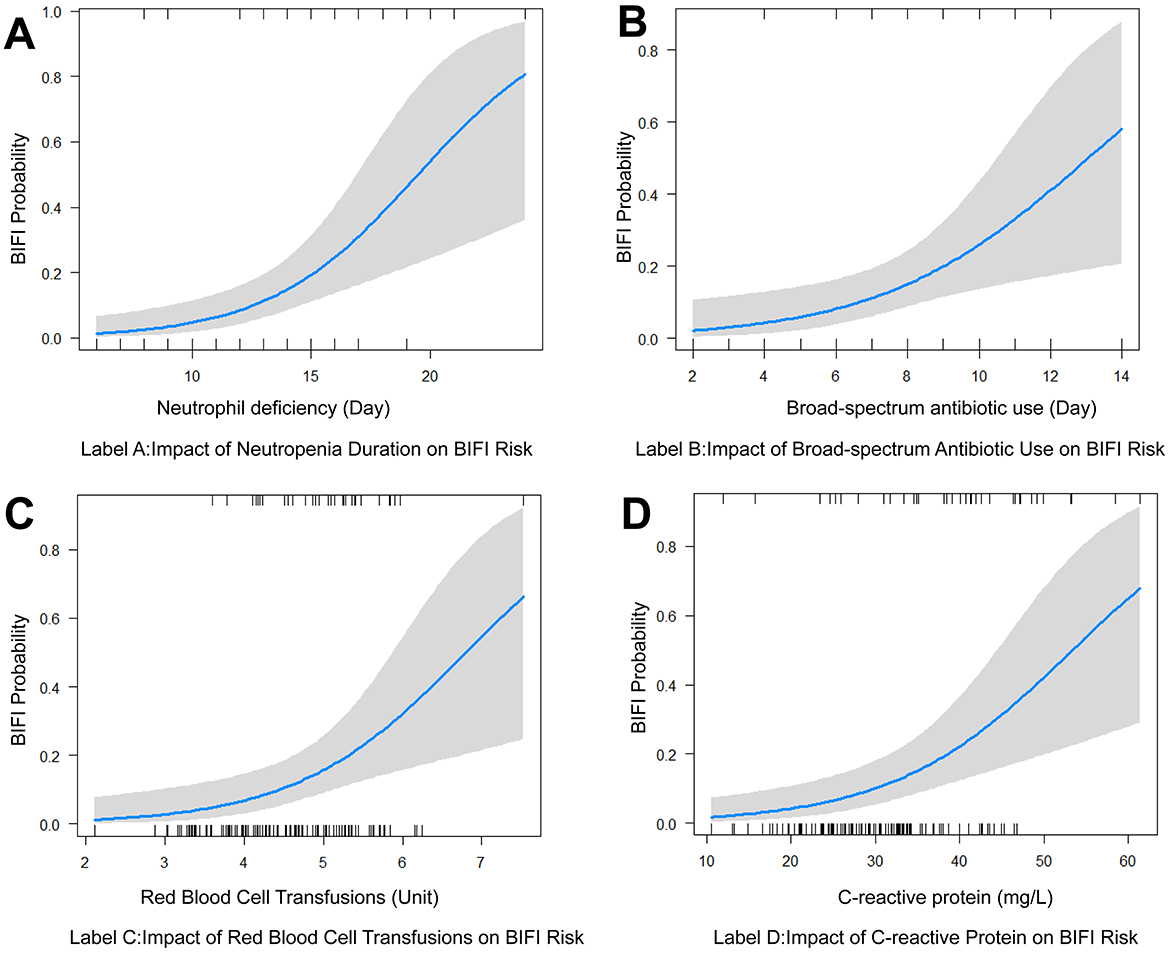
Figure 1. Visualization of key risk factors for breakthrough invasive fungal infections in pediatric acute leukemia. (A) Impact of neutrophil deficiency duration on the risk of BIFI. (B) Impact of broad-spectrum antibiotic use on BIFI risk. (C) Impact of red blood cell transfusions on BIFI risk. (D) Impact of C-reactive protein of BIFI risk. BIFI, breakthrough invasive fungal infections.
3.3 Model performance for BIFI in pediatric acute leukemia: ROC and calibration analysis
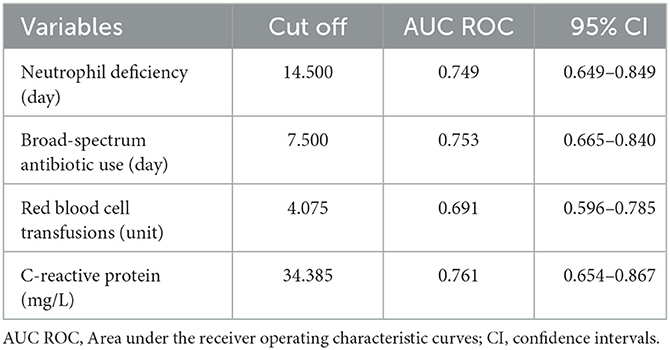
The Table 3 presents model variables along with their corresponding cut-off values, area under the curve (AUC), and 95% CI. The ROC curves are presented in Supplementary material 3. Neutrophil deficiency has a cut-off value of 14.500, an AUC of 0.749, and a 95% CI of 0.649–0.849. Broad-spectrum antibiotic use shows a cut-off value of 7.500, an AUC of 0.753, and a 95% CI of 0.665–0.840. Red blood cell transfusions have a cut-off value of 4.075, with an AUC of 0.691, supported by a 95% CI of 0.596–0.785. C-reactive protein is noted with a cut-off value of 34.385, an AUC of 0.761, and a 95% CI of 0.654–0.867. The VIF values for our model were as follows: Neutrophil deficiency: 1.10, Broad-spectrum antibiotic use: 1.01, Red blood cell transfusions: 1.10, C-reactive protein: 1.00.
In Figure 2, the ROC curve demonstrates that the model, which includes neutrophil deficiency, broad-spectrum antibiotic use, red blood cell transfusions, and CRP, has excellent predictive performance for BIFI in pediatric acute leukemia patients, with an AUC of 0.890 (95% CI: 0.828–0.952). At a threshold of 0.315, the model achieves a sensitivity of 0.866 and a specificity of 0.794.
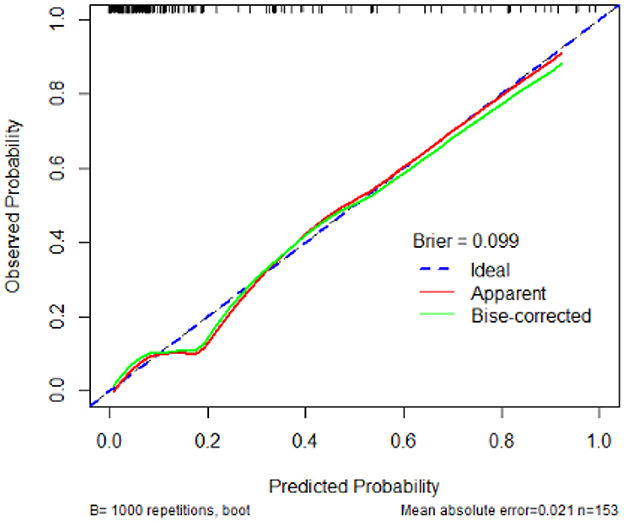
The calibration plot demonstrates that the predicted probabilities of developing BIFI align closely with the observed probabilities, with a Brier score of 0.099. The mean absolute error is 0.021, based on 1,000 bootstrap repetitions in Figure 3.
3.4 Nomogram and decision curve analysis for predicting BIFI risk in pediatric acute leukemia
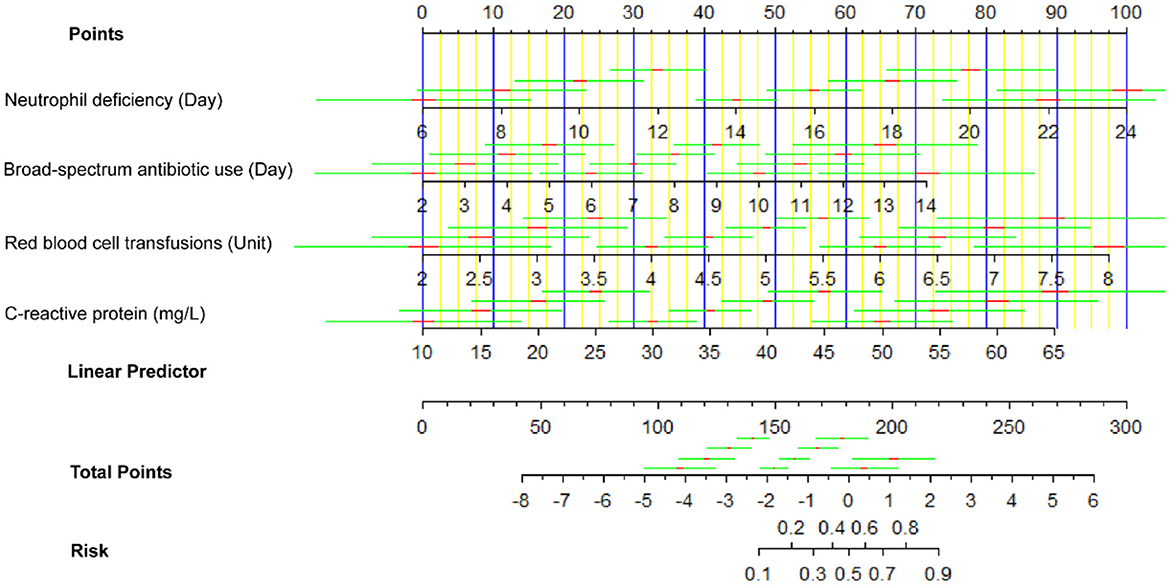
In Figure 4, the nomogram shown is designed to estimate the risk of BIFI in patients based on four predictor variables: neutrophil deficiency (days), broad-spectrum antibiotic use (days), red blood cell transfusions (units), and C-reactive protein (mg/L). For each predictor, specific values correspond to a point score on the top “Points” scale. For example, 10 days of neutrophil deficiency would yield approximately 15 points, while 7 days of broad-spectrum antibiotic use would yield around 40 points. These individual scores are then summed to obtain a total point score, which is located on the “Total Points” axis. The corresponding risk of BIFI is found by aligning the total score with the “Risk” scale at the bottom, which provides the predicted probability of BIFI for the patient. This nomogram allows clinicians to quantify individual patient risk, aiding in clinical decision-making by identifying patients who may benefit from closer monitoring or preventative interventions.
In Figure 5, the decision curve analysis curve shows that the predictive model for BIFI in pediatric acute leukemia patients provides a net benefit across a wide range of high-risk thresholds, particularly between 0.1 and 0.8. The red line representing the model demonstrates a higher standardized net benefit compared to treating all patients (gray line) or none (black line) within this threshold range. This indicates that the model is clinically useful and beneficial for decision-making in predicting BIFI risk, with the highest net benefit observed around the threshold of 0.2 to 0.3.
4 Discussion
The analysis of pediatric acute leukemia patients revealed significant predictors for the development of BIFI. Key factors include the duration of neutrophil deficiency, duration of broad-spectrum antibiotic use, red blood cell transfusions, and CRP levels.
4.1 Role of neutrophil deficiency in BIFI development
Neutrophils play a crucial role in the body's defense against fungal infections. As the first line of defense in innate immunity, neutrophils are responsible for identifying and neutralizing pathogens such as fungi. When neutrophil counts are significantly reduced, patients become highly susceptible to infections, particularly IFIs. In pediatric acute leukemia patients, prolonged neutropenia is a common side effect of chemotherapy, which significantly compromises the immune system. Previous research has established that there is a critical threshold in the duration of neutropenia, beyond which the risk of infection increases significantly (23). Additionally, a study by Marr et al. demonstrated that in hematopoietic stem cell transplant recipients, the longer the duration of neutropenia, the higher the incidence of mold infections, further supporting the strong association between prolonged neutropenia and the risk of invasive fungal infections (24). Our study further confirms the significant positive correlation between the duration of neutropenia and the risk of developing BIFI. Specifically, the longer the period of neutropenia, the greater the likelihood that the patient will develop breakthrough invasive fungal infections. This finding aligns with existing literature, emphasizing the critical role of neutropenia duration in the context of infection risk.
4.2 Role of broad-spectrum antibiotics in BIFI development
The duration of broad-spectrum antibiotic use emerged as a significant predictor of BIFI in pediatric acute leukemia patients. Prolonged antibiotic use disrupts the natural gut microbiota, reducing bacterial competition and creating an environment conducive to fungal overgrowth. This finding aligns with existing research that highlights the impact of antibiotic-induced dysbiosis on increasing the risk of fungal infections (25). The balance between effective bacterial infection control and the risk of inducing fungal infections presents a critical challenge in managing immunocompromised patients. Incorporating these findings into personalized treatment plans can improve outcomes and reduce the incidence of BIFI, as supported by both the study and existing research (26).
It is important to note that the study did not directly assess the impact of microbiota-preserving strategies, which represents a potential area for future research. Investigating these interventions could provide valuable insights into more holistic approaches to infection prevention in this vulnerable population.
4.3 Role of red blood cell transfusions in BIFI development
Our study identified a significant positive correlation between the volume of red blood cell transfusions and the risk of BIFI in pediatric acute leukemia patients. This finding aligns with existing literature that highlights the immunosuppressive effects of transfusions, known as transfusion-related immunomodulation, which can increase susceptibility to infections (27). Moreover, repeated transfusions can lead to iron overload, which is a known risk factor for fungal infections due to iron's role in promoting the growth of pathogens like Candida and Aspergillus (28).
To mitigate these risks, clinicians should carefully assess the need for transfusions and consider strategies such as using leukoreduced or irradiated blood products to minimize immunosuppression (29). Close monitoring of transfusion-dependent patients for early signs of infection and iron overload is essential, along with exploring alternatives to transfusions, such as erythropoiesis-stimulating agents (30). Personalized transfusion strategies, guided by the patient's risk profile, can help balance the immediate benefits of transfusions with the long-term goal of reducing infection risks, including BIFI.
The study did not explore the long-term outcomes of different transfusion strategies, nor did it account for potential variations in transfusion practices across different institutions. Future studies should aim to address these gaps by examining the impact of specific transfusion protocols on BIFI incidence and outcomes in larger, multi-center cohorts.
4.4 Role of CRP in BIFI development
The study identified a strong correlation between elevated CRP levels and the risk of BIFI in pediatric acute leukemia patients, suggesting that CRP can serve as a valuable predictive biomarker for these infections. This finding is supported by existing literature, which highlights the role of CRP as an indicator of systemic inflammation and immune dysregulation, both of which increase susceptibility to fungal infections in immunocompromised patients (31). Notably, CRP demonstrates a cut-off value of 34.385, an area under the curve (AUC) of 0.761, and a 95% confidence interval (CI) of 0.654–0.867, confirming its strong predictive effectiveness in this context.
In addition to CRP, our analysis also revealed that neutrophil deficiency (cut-off value: 14.500, AUC: 0.749, 95% CI: 0.649–0.849) and broad-spectrum antibiotic use (cut-off value: 7.500, AUC: 0.753, 95% CI: 0.665–0.840) serve as important predictive factors for BIFI risk. However, red blood cell transfusions exhibited a weaker predictive power, with a cut-off value of 4.075 and an AUC of 0.691 (95% CI: 0.596–0.785).
Given its predictive value, CRP levels can be used to guide antifungal therapy, with elevated levels prompting early initiation or escalation of treatment (32). Additionally, CRP monitoring can help assess the effectiveness of ongoing antifungal therapy, with decreasing levels indicating a favorable response. However, due to its lack of specificity, CRP should be interpreted alongside other clinical and diagnostic information to ensure accurate risk assessment and management of BIFI in this vulnerable population.
4.5 Interrelationship of key risk factors for BIFI
The three identified risk factors for BIFI (duration of neutropenia, length of antibiotic use, and RBC transfusion) are inherently interrelated, as patients experiencing prolonged myelosuppression from myelotoxic therapies will inevitably require extended periods of neutropenia, necessitating longer courses of broad-spectrum antibiotic prophylaxis and more frequent RBC transfusions to manage treatment-induced anemia; this self-reinforcing cycle, where each factor contributes to and exacerbates the others, underscores the mutually dependent nature of these key risk determinants for BIFI, and recognizing this interdependence is crucial for developing comprehensive prevention and management strategies that consider the complex interactions between these variables, with interventions targeting one factor potentially having downstream benefits on the others, though the precise quantitative relationships between these factors require further investigation. Recent findings have highlighted the high rate of breakthrough invasive aspergillosis among patients receiving caspofungin for persistent fever and neutropenia, underscoring the need for vigilant monitoring and possibly adjusting antifungal therapy based on CRP levels and other biomarkers (33, 34).
4.6 Clinical utility of nomogram and DCA for BIFI risk stratification
In our study, we developed a nomogram based on key predictors to estimate the risk of BIFI in pediatric acute leukemia patients. The nomogram assigns scores to specific values of each predictor, allowing clinicians to calculate an individualized risk score for BIFI. By visually mapping each variable's impact, the nomogram aids in translating complex statistical results into an accessible tool for clinical decision-making. This tool has significant potential for clinical application, as it allows healthcare providers to assess BIFI risk in real-time and personalize preventive and therapeutic strategies based on individual patient risk. For instance, patients identified as high-risk may benefit from enhanced monitoring, timely antifungal interventions, or additional preventive measures. However, while the nomogram shows promise in risk stratification, its applicability should be further validated in independent cohorts to confirm its generalizability and clinical utility.
In this study, decision curve analysis indicated a potential benefit of the predictive model relative to treating all patients or none, which suggests its utility in identifying patients at higher risk of BIFI. While all patients in this cohort received antifungal prophylaxis, our findings support the idea that, the model could help stratify risk levels, guiding personalized prophylactic strategies. Importantly, we do not propose the removal of prophylaxis in low-risk patients solely based on this model. Instead, we suggest that such a risk-based approach could refine prophylactic intensity in clinical practice, potentially optimizing outcomes. Additionally, most risk factors included in the model, such as patient demographics, initial disease status, and baseline laboratory values, would generally be available early in the treatment process, supporting its potential application in real-time decision-making.
A limitation of this study is that it did not evaluate other inflammatory markers that may also be predictive of BIFI. Furthermore, the study did not include the length of hospital stay in the analysis, as BIFI may also contribute to prolonged hospitalization, leading to a potential bidirectional causality between the two variables. Additionally, the relatively small sample size limits the generalizability of our findings. Future research would benefit from a larger cohort, ideally with external data for validation, to enhance both the predictive accuracy and robustness of the model. A broader investigation into the role of multiple biomarkers and factors such as hospital stay duration could provide more comprehensive tools for clinical decision-making.
5 Conclusion
The study identifies key predictors of BIFI in pediatric acute leukemia patients, offering valuable insights for early identification and personalized management strategies. By leveraging these findings, clinicians can improve patient outcomes and reduce the burden of invasive fungal infections in this vulnerable population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Anhui Provincial Children's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/Institutional Review Board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because informed consent was waived as all patient records and information were anonymized and de-identified before analysis.
Author contributions
YL: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. LQ: Writing – original draft, Validation, Methodology. JW: Writing – original draft, Methodology, Formal analysis, Data curation. PC: Writing – original draft, Methodology, Formal analysis, Data curation. AJ: Writing – original draft, Validation, Software, Methodology, Data curation. HL: Writing – original draft, Writing – review & editing, Resources, Investigation, Funding acquisition, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Anhui Provincial Key Research and Development Program (Clinical Medicine Research and Translational Application Special Project), Project Number: 202304295107020064.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fmed.2025.1692510.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1488514/full#supplementary-material
References
1. Ivanov AV, Alecsa MS, Popescu R, Starcea MI, Mocanu AM, Rusu C, et al. Pediatric acute lymphoblastic leukemia emerging therapies—from pathway to target. Int J Mol Sci. (2023) 24:4661. doi: 10.3390/ijms24054661
2. Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. (2017) 7:e577. doi: 10.1038/bcj.2017.53
3. Ssenyonga N, Stiller C, Nakata K, Shalkow J, Redmond S, Bulliard J-L, et al. Worldwide trends in population-based survival for children, adolescents, and young adults diagnosed with leukaemia, by subtype, during 2000–14 (CONCORD-3): analysis of individual data from 258 cancer registries in 61 countries. Lancet Child Adolescent Health. (2022) 6:409–31. doi: 10.1016/S2352-4642(22)00095-5
4. Ehrhardt MJ, Krull KR, Bhakta N, Liu Q, Yasui Y, Robison LL, et al. Improving quality and quantity of life for childhood cancer survivors globally in the twenty-first century. Na Rev Clini Oncol. (2023) 20:678–96. doi: 10.1038/s41571-023-00802-w
5. Hafez HA, Soliaman RM, Bilal D, Hashem M, Shalaby LM. Early deaths in pediatric acute leukemia: a major challenge in developing countries. J Pediatr Hematol Oncol. (2019) 41:261–6. doi: 10.1097/MPH.0000000000001408
6. Sezgin Evim M, Tüfekçi Ö, Baytan B, Ören H, Çelebi S, Ener B, et al. Invasive fungal infections in children with leukemia: clinical features and prognosis. Turkish J Haematol. (2022) 39:94–102. doi: 10.4274/tjh.galenos.2021.2021.0203
7. Bossù G, Di Sario R, Muratore E, Leardini D, Pession A, Esposito S, et al. Novel insights into fungal infections prophylaxis and treatment in pediatric patients with cancer. Antibiotics. (2022) 11:1316. doi: 10.3390/antibiotics11101316
8. Wurster S, Watowich SS, Kontoyiannis DP. Checkpoint inhibitors as immunotherapy for fungal infections: Promises, challenges, and unanswered questions. Front Immunol. (2022) 13:1018202. doi: 10.3389/fimmu.2022.1018202
9. Ruijters VJ, Oosterom N, Wolfs TFW, van den Heuvel-Eibrink MM, van Grotel M. Frequency and determinants of invasive fungal infections in children with solid and hematologic malignancies in a nonallogeneic stem cell transplantation setting: a narrative review. J Pediatr Hematol Oncol. (2019) 41:345–54. doi: 10.1097/MPH.0000000000001468
10. Lashkari HP, Fernandes N, Alva K, Rai S. Central nervous system fungal infection and acute lymphoblastic leukemia in children: what is the optimal duration of antifungal therapy? J Pediatr Hematol Oncol. (2017) 39:e312–7. doi: 10.1097/MPH.0000000000000855
11. Bhatt VR, Viola GM, Ferrajoli A. Invasive fungal infections in acute leukemia. Ther Adv Hematol. (2011) 2:231–47. doi: 10.1177/2040620711410098
12. Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. (2006) 91:1068−75.
13. Bartlett AW, Cann MP, Yeoh DK, Bernard A, Ryan AL, Blyth CC, et al. Epidemiology of invasive fungal infections in immunocompromised children; an Australian national 10-year review. Pediatr Blood Cancer. (2019) 66:e27564. doi: 10.1002/pbc.27564
14. Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clini Infect Dis. (2020) 71:1367–76. doi: 10.1093/cid/ciz1008
15. Hematologists CA, Group CIFIW. The Chinese guidelines for the diagnosis and treatment of invasive fungal disease in patients with hematological disorders and cancers (the 6th revision). Zhonghua nei ke za zhi. (2020) 59:754–63. doi: 10.3760/cma.j.cn112138-20200627-00624
16. Massey FJ Jr. The Kolmogorov-Smirnov test for goodness of fit. J Am Stat Assoc. (1951) 46:68–78. doi: 10.1080/01621459.1951.10500769
18. Hosmer Jr DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. Hoboken, NJ: John Wiley & Sons (2013).
19. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. (1982) 143:29–36. doi: 10.1148/radiology.143.1.7063747
20. Brier GW. Verification of forecasts expressed in terms of probability. Monthly Weather Rev. (1950) 78:1–3. doi: 10.1021/acs.accounts.0c00636
21. Van Calster B, Vickers AJ. Calibration of risk prediction models: impact on decision-analytic performance. Med Deci Making. (2015) 35:162–9. doi: 10.1177/0272989X14547233
22. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Deci Making. (2006) 26:565–74. doi: 10.1177/0272989X06295361
23. Groll AH, Castagnola E, Cesaro S, Dalle J-H, Engelhard D, Hope W, et al. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. Lancet Oncol. (2014) 15:e327–40. doi: 10.1016/S1470-2045(14)70017-8
24. Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. (2002) 34:909–17. doi: 10.1086/339202
25. Dunn KA, MacDonald T, Rodrigues GJ, Forbrigger Z, Bielawski JP, Langille MGI, et al. Antibiotic and antifungal use in pediatric leukemia and lymphoma patients are associated with increasing opportunistic pathogens and decreasing bacteria responsible for activities that enhance colonic defense. Front Cell Infect Microbiol. (2022) 12:924707. doi: 10.3389/fcimb.2022.924707
26. Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Ellis D, Tullio V, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2005: an 8.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol. (2006) 44:3667–72. doi: 10.1128/JCM.00409-07
27. Vamvakas EC. Transfusion-related immunomodulation. Transfusion. (2009) 49:715–27. doi: 10.1111/j.1537-2995.2009.02175.x
28. Hershko C. Pathogenesis and management of iron toxicity in thalassemia. Ann N Y Acad Sci. (2010) 1202:1–9. doi: 10.1111/j.1749-6632.2010.05544.x
29. Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet. (2012) 381:1845–54. doi: 10.1016/S0140-6736(13)60650-9
30. Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. Correction of anemia with epoetin alfa in chronic kidney disease. Kidney Int. (2006) 69:399–407. doi: 10.1056/NEJMoa065485
31. Arvanitis M, Anagnostou T, Fuchs BB, Caliendo AM, Mylonakis E. Molecular and nonmolecular diagnostic methods for invasive fungal infections. Clin Microbiol Rev. (2015) 28:579–634. doi: 10.1128/CMR.00091-13
32. Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. (2018) 62:e1–e50. doi: 10.1093/cid/civ933
33. Puerta-Alcalde P, Monzó-Gallo P, Aguilar-Guisado M, Ramos JC, Laporte-Amargós J, Machado M, et al. Breakthrough invasive fungal infection among patients with haematologic malignancies: a national, prospective, and multicentre study. J Infection. (2023) 87:46–53. doi: 10.1016/j.jinf.2023.05.005
Keywords: pediatric acute leukemia, breakthrough invasive fungal infections, predictive factors, neutropenia, risk model development
Citation: Li Y, Qu L, Wang J, Chen P, Jiang A and Liu H (2024) Predictors of breakthrough invasive fungal infections (BIFI) in pediatric acute leukemia: a retrospective analysis and predictive model development. Front. Med. 11:1488514. doi: 10.3389/fmed.2024.1488514
Received: 30 August 2024; Accepted: 25 November 2024;
Published: 10 December 2024; Corrected: 19 September 2025.
Edited by:
Michele Redell, Baylor College of Medicine, United StatesReviewed by:
Michael James Burke, Medical College of Wisconsin, United StatesWesley Smith, University of Kentucky, United States
Austin Brown, Baylor College of Medicine, United States
Copyright © 2024 Li, Qu, Wang, Chen, Jiang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongjun Liu, MTM1MTU2NTc3NTlAMTI2LmNvbQ==
 Yan Li
Yan Li Lijun Qu
Lijun Qu