- 1Faculty of Medicine, American University of Beirut, Beirut, Lebanon
- 2Department of Otorhinolaryngology-Head and Neck Surgery, American University of Beirut Medical Center, Beirut, Lebanon
- 3Division of Hematology-Oncology, Department of Internal Medicine, American University of Beirut Medical Center, Beirut, Lebanon
- 4Department of Radiation Oncology, American University of Beirut Medical Center, Beirut, Lebanon
Background: Anti-PD1 antibodies have gained popularity in the treatment of skin cancers. These drugs have been FDA approved for treatment of cutaneous melanoma and unresectable/metastatic squamous cell carcinoma of the skin. However, the use of anti-PD1 antibodies is not established for resectable cutaneous squamous cell carcinoma, as the mainstay treatment is surgical excision.
Case: A 49-year-old female with Xeroderma Pigmentosum presented with an ulcerating lateral nasal mass causing obstruction. Biopsy confirmed cutaneous squamous cell carcinoma and was staged as IVA (T2N2cM0) based on PET-CT findings, which showed a 2.7 × 2.3 cm left nasal mass and radiotracer-avid cervical lymph nodes. Despite surgical recommendations, the patient declined surgery due the expected morbidity and disfigurement. Instead, she received neoadjuvant Pembrolizumab (200 mg IV every 3 weeks). After two cycles, PET-CT and MRI showed significant reduction in the nasal mass and decreased cervical lymph node involvement. On physical exam, the nasal lesion had resolved. Multidisciplinary tumor board discussion recommended radiation therapy instead of neck dissection, considering the patient's clinical response and potential surgical morbidity. After a third Pembrolizumab cycle, she received 66 Gy in 33 fractions, followed by continued adjuvant immunotherapy.
Introduction
Cutaneous squamous cell carcinoma (cSCC) is the second most common non-melanoma skin cancer after basal cell carcinoma accounting for 20% of skin cancers (1). With 1 million new cases in the United States each year, it results in up to 9,000 estimated deaths annually (1–5). Importantly, cSCC incidence is increasing across the globe. For instance, the lifetime risk for development of this cancer is estimated at 4% to 9% for women and 9% to 14% for men in the United States (6). Currently, the incidence ranges from 5 to 499 per 100,000 patients depending on the latitude (1). In addition to morbidity, cSCC translates into a great cost burden on the health system. For example, a recent study in the UK estimated an annual cost of £33 to £46 million for diagnosis and treatment (7, 8).
Several therapies are being investigated for the treatment of cSCC, including laser, photodynamic therapy, topical therapy, curettage, and cryosurgery (9). However, the mainstay and widely approved treatment for cSCC is surgical excision and adjuvant radiation therapy. This conventional approach can carry an added morbidity when lesions are located in the head and neck region with increased anatomical intricacy and potential proximity to major vessels (10).
Historically, advanced and recurrent/metastatic cSCC of the head and neck (cSCC-HN) were treated with chemotherapy in addition to radiation therapy (11). Over the past decade, the advent of immune checkpoint inhibitors (ICIs), particularly PD-1 inhibitors, such as cemiplimab, pembrolizumab, and nivolumab, has led to significant improvement in cSCC outcomes. While ICIs gained (FDA) approval following their demonstrated efficacy and acceptable safety profile, their use remained limited to patients with unresectable, metastatic, or recurrent cSCC (12). Interestingly, recent data on neoadjuvant immunotherapy has resulted in a paradigm shift in the management of cutaneous melanoma (13, 14). However, evidence regarding the role of neoadjuvant immunotherapy for resectable cSCC remains limited to date. Importantly, experience and knowledge on the use of immunotherapy for patients with rare diseases, such as Xeroderma Pigmentosum (XP), remains limited despite high predisposition to develop skin cancers among this patient population.
In this article, we report the case of a 48-year-old female with XP presenting with resectable lateral nasal cSCC. Due to expected disfigurement and fear from surgical morbidity, the patient completely refused surgical excision even after multiple attempts to convince her that surgery might be inevitable. In respect of the patient's autonomy, the medical team explored other options and started the patient on pembrolizumab monotherapy with neoadjuvant intent, despite the lack of solid evidence on its efficacy in this setting. Surprisingly, the tumor disappeared completely exhibiting a complete clinical and radiologic response, and surgical excision was no longer warranted.
Case
A 49-year-old female, who is known to have Xeroderma Pigmentosum with no prior history of cancers, presented in August 2023 for evaluation and management of a growing ulcerating mass over the left side of the nose which was causing left-sided nasal obstruction (Figure 1A). Biopsy revealed moderately differentiated squamous cell carcinoma. On exam, the patient had an ulcerating 3 × 2 cm mass over the left nasal wall.
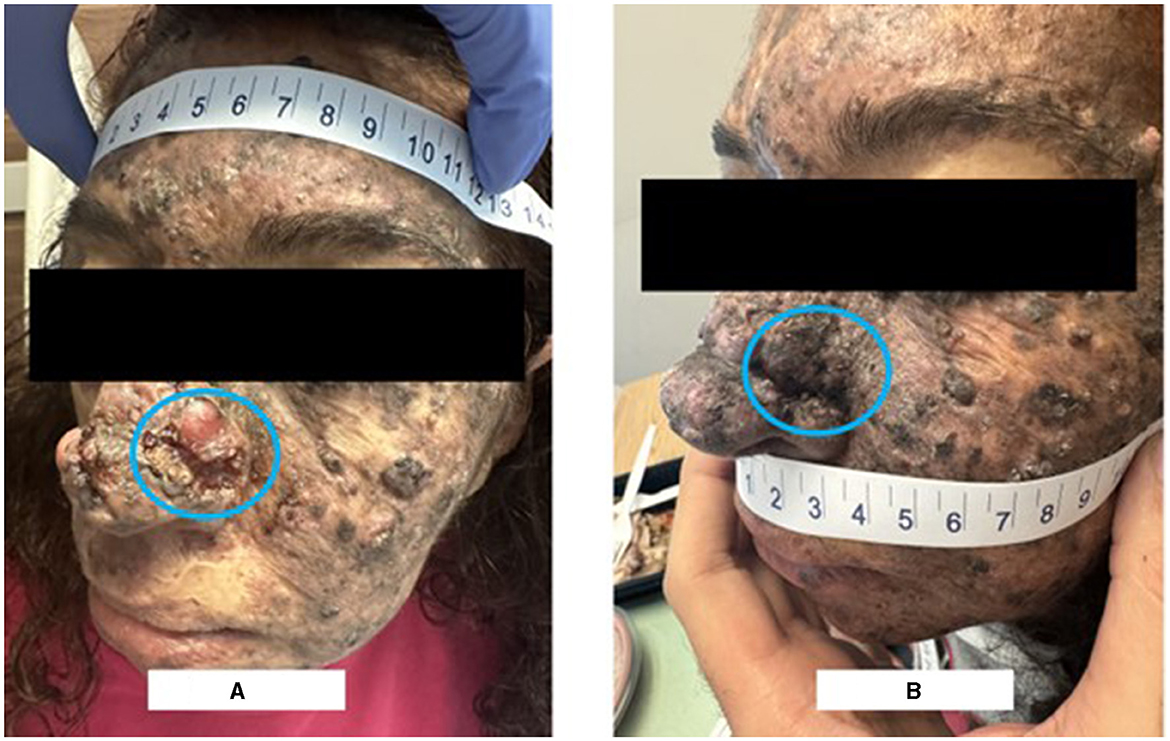
Figure 1. Clinical picture on presentation vs after 2 cycles of neoadjuvant pembrolizumab. (A) Shows the ulcerating mass over the left side of the nose. (B) Shows the disappearance of the left nasal lesion after 2 cycles of pembrolizumab.
PET-CT scan for the whole body with FDG was then performed (Figure 2A) and revealed a radiotracer avid ulcerating soft tissue mass involving the left side of the nose causing nasal obstruction, measuring 2.7 × 2.3 cm with SUV max of 16.2, representing the primary tumor. In addition, multiple radiotracer avid cervical lymph nodes were found, mainly submandibular, retromandibular, submental, jugulo-carotid, and mid and lower jugular lymph nodes. For example, a left submandibular lymph node found measured 1.2 × 0.7 cm with SUV max 5.7 and a right lower jugular lymph node found measured 0.8 × 0.7 cm with SUV max 4.5.
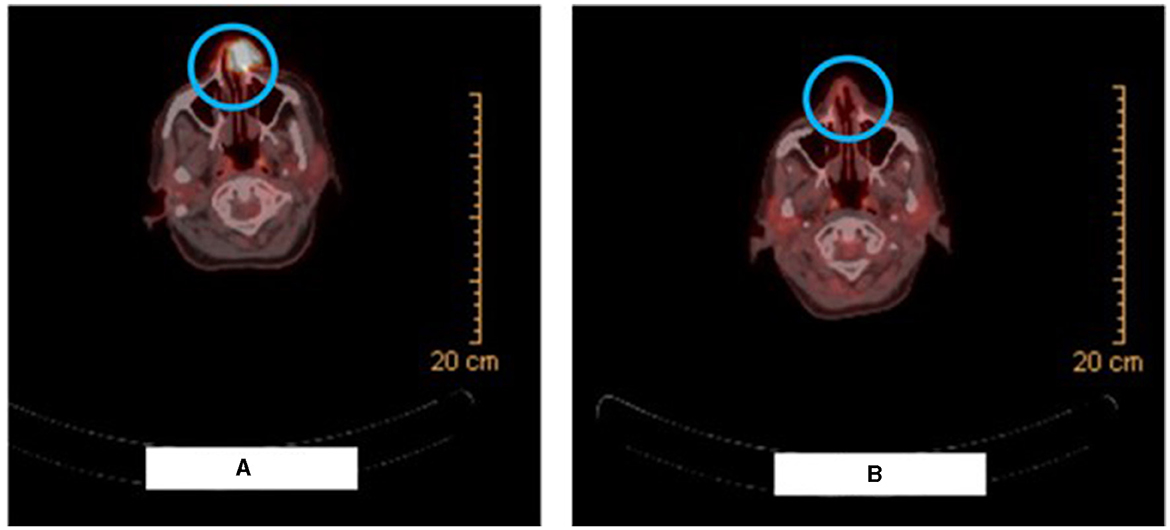
Figure 2. Radiologic picture on presentation vs after 2 cycles of neoadjuvant pembrolizumab. (A) Shows the PET-CT scan image demonstrating the radiotracer avid ulcerating soft tissue mass involving the left side of the nose causing nasal obstruction. (B) Shows the PET-CT scan image demonstrating resolution of the large radiotracer avid ulcerating soft tissue mass involving the left side of the nose and an interval decrease in activity, number, and size of the bilateral cervical lymph nodes.
The patient's disease was considered as locally advanced, yet potentially resectable, nasal cSCC, stage IVA (T2N2cM0) according to the American Joint Committee on Cancer (AJCC), eighth edition. Her case was discussed at the multidisciplinary tumor board. Two treatment approaches were discussed: therapeutic intent dual immunotherapy with ipilimumab and nivolumab, considering her stage IVA disease, vs. surgical intent approach, considering her potentially resectable disease according to the locally advanced lymph nodes involvement and her young age coupled with her excellent performance status. The patient was adamant that she becomes disease-free. With her locally advanced disease, upfront surgery was not feasible. In respect to her wishes, the medical team had to explore other options and decided to resort to immunotherapy as a neoadjuvant treatment after which surgery can be rediscussed with the patient following 2 cycles of immunotherapy. As such, the patient was planned to receive 200 mg IV of the humanized monoclonal anti-PD1 antibody, Pembrolizumab, every 3 weeks for 2 years as well as radiation therapy.
PET-CT scan and MRI of the face were performed after 2 cycles of pembrolizumab. The PET-CT scan (Figure 2B) demonstrated resolution of the large radiotracer avid ulcerating soft tissue mass involving the left side of the nose and an interval decrease in activity, number, and size of the bilateral cervical lymph nodes. Similarly, the MRI showed a significant decrease in the size of the ulcerating mass in the left side of the nose and detected only a few prominent level IIa submandibular and submental lymph nodes (for example, a left level IIa lymph node measuring 1 × 0.8 cm). Furthermore, physical examination revealed the disappearance of the left nasal lesion which was previously present at the time of presentation (Figure 1B). The case was discussed at our multidisciplinary tumor boards discussion. Taking into consideration the significant morbidity that would arise from neck lymph node dissection, coupled with the complete clinical response of the cutaneous lesion itself, decision was to perform radiation therapy after an additional (third) cycle of pembrolizumab. She received a total of 66 Gray in 33 fractions and continued adjuvant pembrolizumab following the radiation therapy course with no adverse events.
Discussion
In this report, we discussed the case of a 48-year-old female with xeroderma pigmentosum (XP) presenting with locally advanced potentially resectable nasal SCC. The tumor's location and size, coupled with its locally advanced lymph nodes involvement, would have necessitated a traumatic and challenging surgery with expected disfigurement post-operatively. At first, the medical team were reluctant to start the patient on pembrolizumab monotherapy due to limited evidence on its efficacy in resectable cSCC-HN and its limited evidence on XP patients. But, after thorough discussion with the patient and her family, and after a multidisciplinary discussion, the team proceeded with neoadjuvant intent pembrolizumab. Interestingly, this led to complete remission without the need for surgery.
ICIs efficacy in advanced and metastatic cSCC was investigated in several multicohort studies and clinical trials (15), the two main PD-1 inhibitors studied were cemiplimab (16–23) and pembrolizumab (24, 25).
The efficacy of cemiplimab in cSCC was studied in two phases. Initially, a phase I multicohort study involved 26 patients (20, 21), followed by a larger phase II EMPOWER-CSCC 1 trial with 193 patients (16–19, 22). Both studies had an open-label, multicenter design. The phase I trial demonstrated that cemiplimab was safe and effective, showing a 50% response rate [95% CI (30, 70)]. Among the 13 responding patients, 7 had responses lasting more than 6 months (20). These results were consistent in the phase II trial (18), where cemiplimab was given at different doses across three groups, with median times to response of 1.9 months for groups 1 and 2, and 2.1 months for group 3. About one-third of the patients had prior systemic treatments, most had surgery, and many had radiotherapy. In the metastatic cSCC cohort, 22 out of 29 responders had a duration of response (DOR) of 12 months or more, and in the locally advanced cSCC cohort, 12 out of 34 responders had a DOR of 12 months or more.
Pembrolizumab was evaluated in two phase II trials for cSCC: KEYNOTE 629 with 105 patients (24) and the CARSKIN trial with 57 patients in the expansion cohort (25). Both trials were open-label, single-arm, and multicenter. In KEYNOTE 629, the objective response rate was 34%, with complete responses in 4% and partial responses in 31% of patients. Among the 36 patients with a confirmed response, approximately 69% experienced durable responses longer than 6 months. With a median follow-up of about 10 months, the median progression-free survival (PFS) was 7 months, and the 1-year overall survival (OS) was 60% (24). Finally, in the CARSKIN trial, which only included treatment-naive patients, the objective response rate was 42%, with complete responses in 7% and partial responses in 35% of patients. In the expansion cohort, the response rate was higher in patients with PD-L1-positive disease (55%) compared to those with PD-L1-negative disease (17%), with a significant difference (P = 0.02) (25). After a median follow-up of 22.4 months in the primary cohort, the median PFS was 7 months, and the median OS was 25 months.
Thus, this led to the FDA approval of cemiplimab and pembrolizumab for the treatment of advanced and metastatic cSCC. We could not perform immunohistochemistry test for PD-L1 due to financial restrains, but this did not hinder initiation of immunotherapy as the benefit from pembrolizumab in cSCC was observed regardless of PD-L1 combined positive score (CPS) (24). However, a gray zone remains about the role of ICIs in resectable cSCC. For long, the mainstay of treatment of resectable cSCC-HN is surgical resection and adjuvant radiation therapy.
Currently, clinical trials are being conducted on the efficacy of ICIs in resectable cSCC. For instance, a phase II trial of 20 patients with resectable stage III/IV cSCC of the head and neck showed that 85% of participants achieved a pathologic response on cemiplimab, with 55% achieving a complete pathologic response, 20% achieving a major pathologic response and 10% achieving a partial pathologic response (26). Additionally, the 12 month disease-specific survival (DSS), disease-free survival (DFS), and OS are 95% [95% CI, (85.9, 100)], 89.5% [95% CI, (76.7, 100)], and 95% [95% CI, (85.9, 100)], respectively (27). Another cohort study of 27 patients with resectable cSCC (mainly in the head and neck region) treated with neoadjuvant cemiplimab or pembrolizumab showed an overall pathologic response rate of 47.4% and the overall radiologic response rate was 50.0%. Further, the 1-year recurrence-free survival rate, progession free survival, DSS, and OS were 90.9% (95% CI, 50.8%−98.7%), 83.3% (95% CI, 27.3%−97.5%), 91.7% (95% CI, 53.9%−98.8%), and 84.6% (95% CI, 51.2%−95.9%) respectively (28). However, further research is needed to establish the benefits of ICIs monotherapy in cSCC-HN.
In addition to immunotherapy, our decision to use radiation therapy for this patient was based on two main factors: preventing tumor recurrence and reducing the incidence of skin cancers in the treated area, as studies have shown radiation therapy to be effective for XP patients (29).
Deinlein et al. were the first to report the efficacy of pembrolizumab among patients with XP-associated cSCC with rapid response observed after only three cycles (30). A few other reports also suggested the safety and effectiveness of immunotherapy and radiation therapy for metastatic cSCC (30–32). However, there are no clinical studies or randomized-control trials to date that confirm the safety and efficacy of immunotherapy and radiation among patients with XP. With high predisposition to skin cancers and immunogenicity based on high tumor mutation burden, this patient population is actually a suitable target for future studies on the effectiveness of immunotherapy. Our patient serves as a good model for the study of efficacy of immunotherapy and radiation on skin cancers in XP patients with cSCC.
To our knowledge, this is the first documented case outside of a clinical trial of a patient with XP with locally resectable cSCC-HN who was treated with pembrolizumab monotherapy. This regimen led to a complete response of the tumor and thus spared the patient from any surgical intervention and disfigurement. Future follow-up is needed to document potential recurrence.
In light of early clinical trials and this case report, it is crucial to acknowledge that surgery is not the only option for resectable cSCC-HN and thus we should consider the option of neoadjuvant ICIs for patients reluctant on undergoing surgery, because this might lead to complete response. Phase III trials are needed to compare ICI monotherapy to the standard of care. Also, clinical trials are needed to investigate the potential role of prophylactic ICIs in patients with XP who are at higher risk for cSCC.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because ethical approval by IRB is not required for case reports. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NZ: Conceptualization, Writing – original draft, Writing – review & editing. LK: Conceptualization, Writing – original draft, Writing – review & editing. BY: Writing – original draft, Writing – review & editing. MM: Writing – original draft, Writing – review & editing. FK: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin. (2019) 33:1–12. doi: 10.1016/j.hoc.2018.08.001
2. Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. (2015) 151:1081–6. doi: 10.1001/jamadermatol.2015.1187
3. Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. (2013) 68:957–66. doi: 10.1016/j.jaad.2012.11.037
4. Schmults CD, Karia PS, Carter JB, Han J, Qureshi AA. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol. (2013) 149:541–7. doi: 10.1001/jamadermatol.2013.2139
5. Giese AP, Weng WH, Kindt KS, Chang HH, Montgomery JS, Ratzan EM, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. (2008) 9:713–20. doi: 10.1016/S1470-2045(08)70178-5
6. Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. (1994) 30:774–8. doi: 10.1016/S0190-9622(08)81509-5
7. Green AC, Olsen C. Cutaneous squamous cell carcinoma: an epidemiological review. Br J Dermatol. (2017) 177:373–81. doi: 10.1111/bjd.15324
8. Vallejo-Torres L, Morris S, Kinge JM, Poirier V, Verne J. Measuring current and future cost of skin cancer in England. J Public Health. (2014) 36:140–8. doi: 10.1093/pubmed/fdt032
9. Alam M, Armstrong A, Baum C, Bordeaux JS, Brown M, Busam KJ, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. (2018) 78:560–78. doi: 10.1016/j.jaad.2017.10.007
10. Gurudutt VV, Genden EM. Cutaneous squamous cell carcinoma of the head and neck. J Skin Cancer. (2011) 2011:502723. doi: 10.1155/2011/502723
11. Iancu D, Fulga A, Vesa D, Zenovia A, Fulga I, Sarbu MI, et al. Metastatic patterns and treatment options for head and neck cutaneous squamous cell carcinoma. Molec Clin Oncol. (2024) 20:1–11. doi: 10.3892/mco.2024.2739
12. Boutros A, Cecchi F, Tanda ET, Croce E, Gili R, Arecco L, et al. Immunotherapy for the treatment of cutaneous squamous cell carcinoma. Front Oncol. (2021) 11:733917. doi: 10.3389/fonc.2021.733917
13. Hieken TJ, Kreidieh F, Aedo-Lopez V, Block MS, McArthur GA, Amaria RN. Neoadjuvant immunotherapy in melanoma: the paradigm shift. Am Soc Clin Oncol Educ Book. (2023) 43:e390614. doi: 10.1200/EDBK_390614
14. Patel SP, Othus M, Chen Y, Wright GP Jr, Yost KJ, Hyngstrom JR, et al. Neoadjuvant–adjuvant or adjuvant-only pembrolizumab in advanced melanoma. New England J Med. (2023) 388:813–23. doi: 10.1056/NEJMoa2211437
15. Alberti A, Bossi P. Immunotherapy for cutaneous squamous cell carcinoma: results and perspectives. Front Oncol. (2022) 11:727027. doi: 10.3389/fonc.2021.727027
16. Migden MR, Khushalani NI, Chang ALS, Lewis KD, Schmults CD, Hernandez-Aya L, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol. (2020) 21:294–305. doi: 10.1016/S1470-2045(19)30728-4
17. Guminski AD, Lim AM, Khushalani NI, Schmults CD, Hernandez-Aya LF, Modi B, et al. Phase 2 study of cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with metastatic cutaneous squamous cell carcinoma (mCSCC; Group 1): 12-month follow-up. Am Soc Clin Oncol. (2019) 37:e9526. doi: 10.1200/JCO.2019.37.15_suppl.9526
18. Lee A, Duggan S, Deeks ED. Cemiplimab: a review in advanced cutaneous squamous cell carcinoma. Drugs. (2020) 80:813–9. doi: 10.1007/s40265-020-01302-2
19. Migden MR, Khushalani NI, Chang AL, Rischin D, Schmults CD, Hernandez-Aya L, et al. Primary analysis of phase 2 results of cemiplimab, a human monoclonal Anti–PD-1, in patients with locally advanced cutaneous squamous cell carcinoma. Head Neck. (2019) 62:79–5. doi: 10.25251/skin.2.supp.79
20. Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. New Engl J Med. (2018) 379:341–51. doi: 10.1056/NEJMoa1805131
21. Owonikoko TK, Papadopoulos KP, Johnson ML. Phase 1 study of cemiplimab, a human monoclonal anti-PD-1, in patients with unresectable locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC): final efficacy and safety data. SKIN J Cutaneous Med. (2018) 2:S78–S78. doi: 10.25251/skin.2.supp.78
22. Rischin D, Migden MR, Lim AM, Schmults CD, Khushalani NI, Hughes BGM, et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J Immunother Cancer. (2020) 8:e000775. doi: 10.1136/jitc-2020-000775
23. Larkin J, Vecchio MD, Mandalá M, Gogas H, Fernandez AMA, Dalle S, et al. Adjuvant nivolumab (NIVO) versus ipilimumab (IPI) in resected stage III/IV melanoma: 3-year efficacy and biomarker results from the phase III CheckMate 238 trial. Ann Oncol. (2019) 30:v533–4. doi: 10.1093/annonc/mdz255
24. Grob JJ, Gonzalez R, Basset-Seguin N, Vornicova O, Schachter J, Joshi A, et al. Pembrolizumab monotherapy for recurrent or metastatic cutaneous squamous cell carcinoma: a single-arm phase II trial (KEYNOTE-629). J Clin Oncol. (2020) 38:2916. doi: 10.1200/JCO.19.03054
25. Maubec E, Boubaya M, Petrow P, Beylot-Barry M, Basset-Seguin N, Deschamps L, et al. Phase II study of pembrolizumab as first-line, single-drug therapy for patients with unresectable cutaneous squamous cell carcinomas. J Clin Oncol. (2020) 38:3051–61. doi: 10.1200/JCO.19.03357
26. Gross ND, Ferrarotto R, Amit M, Nagarajan P, Yuan Y, Bell D, et al. Long-term outcomes of a phase II trial of neoadjuvant immunotherapy for advanced, resectable cutaneous squamous cell carcinoma of the head and neck (CSCC-HN). Am Soc Clin Oncol. (2022) 40:e9519. doi: 10.1200/JCO.2022.40.16_suppl.9519
27. Ferrarotto R, Amit M, Nagarajan P, Rubin ML, Yuan Y, Bell D, et al. Pilot phase II trial of neoadjuvant immunotherapy in locoregionally advanced, resectable cutaneous squamous cell carcinoma of the head and neck. Clin Cancer Res. (2021) 27:4557–65. doi: 10.1158/1078-0432.CCR-21-0585
28. Kim EY, Ruiz ES, DeSimone MS, Shalhout SZ, Hanna GJ, Miller DM, et al. Neoadjuvant-intent immunotherapy in advanced, resectable cutaneous squamous cell carcinoma. JAMA Otolaryngol–Head Neck Surg. (2024) 150:414–20. doi: 10.1001/jamaoto.2024.0259
29. Schaffer JV, Orlow SJ. Radiation therapy for high-risk squamous cell carcinomas in patients with xeroderma pigmentosum: report of two cases and review of the literature. Dermatology. (2011) 223:97–103. doi: 10.1159/000324509
30. Deinlein T, Lax SF, Schwarz T, Giuffrida R, Schmid-Zalaudek K, Zalaudek I. Rapid response of metastatic cutaneous squamous cell carcinoma to pembrolizumab in a patient with xeroderma pigmentosum: case report and review of the literature. Eur J Cancer. (2017) 83:99–102. doi: 10.1016/j.ejca.2017.06.022
31. Steineck A, Krumm N, Sarthy JF, Pritchard CC, Chapman T, Stacey AW, et al. Response to pembrolizumab in a patient with xeroderma pigmentosum and advanced squamous cell carcinoma. JCO Precis Oncol. (2019) 3:28. doi: 10.1200/PO.19.00028
Keywords: anti-PD1, pembrolizumab, cutaneous squamous cell carcinoma, cSCC, xeroderma pigmentosa
Citation: Zalaquett NG, Kreidieh L, Youssef B, Mourad M and Kreidieh F (2024) Case report: Neoadjuvant-intent pembrolizumab resulted in complete response in a xeroderma pigmentosum patient with locally advanced resectable cutaneous squamous cell carcinoma of the nose. Front. Med. 11:1488400. doi: 10.3389/fmed.2024.1488400
Received: 29 August 2024; Accepted: 30 September 2024;
Published: 11 October 2024.
Edited by:
Andreas Recke, University of Lübeck, GermanyReviewed by:
Albert Ruebben, University Hospital RWTH Aachen, GermanyLars Boeckmann, University Hospital Rostock, Germany
Copyright © 2024 Zalaquett, Kreidieh, Youssef, Mourad and Kreidieh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Firas Kreidieh, ZmszMEBhdWIuZWR1Lmxi
 Nader G. Zalaquett
Nader G. Zalaquett Lara Kreidieh
Lara Kreidieh Bassem Youssef
Bassem Youssef Marc Mourad2
Marc Mourad2 Firas Kreidieh
Firas Kreidieh