- State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University Cancer Center, Guangzhou, China
Background: Radical resection is the only curative method for patients with pancreatic adenocarcinoma (PDAC). However, nearly 85% of PDAC patients suffer from local or distant recurrence within 5 years after curative resection. The progression of recurrent lesions accelerates the mortality rate in PDAC patients. However, the influence of clinicopathological factors on post-progression-free survival (PPFS), defined as the period from tumor recurrence to the timing of the progression of recurrent lesions, has rarely been discussed. The present study aimed to explore the independent prognostic factors for PPFS and construct a nomogram for PPFS prediction.
Materials and methods: The 200 recurrent PDAC patients were divided into training and validation groups by leave-one-out cross-validation. The patients’ clinicopathological characteristics were compared through a chi-square test. Meanwhile, these factors were enrolled in the univariate and multivariate COX regression to find the independent prognostic factors of PPFS. Moreover, the Kaplan–Meier survival analysis based on the independent prognostic factors was performed. Finally, we constructed a nomogram model for PPFS prediction, followed by an effectiveness examination.
Results: PDAC patients who received multi-agent chemotherapy after surgery showed a longer PPFS than the single-agent chemotherapy group. PDAC patients who received multi-agent chemotherapy after recurrence showed a similar PPFS compared to the single-agent chemotherapy group. Local recurrence with distant metastases, early recurrence, lympho-vascular invasion, higher T stage, and higher N stage predicted shorter PPFS in recurrent PDAC patients. Finally, a nomogram to indicate the progression of recurrent lesions was constructed.
Conclusion: Multi-agent chemotherapy is recommended for PDAC patients after surgery. Meanwhile, single-agent chemotherapy also deserves consideration after tumor recurrence. Moreover, the nomogram could be used in PPFS prediction.
Introduction
PDAC is the major component of pancreatic cancer (1, 2). As reported in previous research, the 5-year overall survival rate of PDAC is less than 10% (3). Moreover, PDAC was expected to become the second leading cause of cancer-related death by 2030 (4). So far, radical resection has been the only curative method for PDAC (5). Unfortunately, even though the adjuvant chemotherapy has been performed, nearly 85% of resected cases eventually experience tumor recurrence (6, 7). The relationship between the clinicopathological factors and tumor recurrence in PDAC has been explored previously. In detail, PDAC patients who received adjuvant chemotherapy achieved longer progression-free survival and overall survival compared to untreated patients (8, 9). However, the restriction effect of chemotherapy on recurrent PDAC has been rarely discussed.
The advancement of recurrent lesions was defined as follows: at least 20% increase in maximum diameter of the primary recurrent lesions, or detection of any new recurrent lesions in the distant tissue. Meanwhile, the period from tumor recurrence to the timing of the progression of recurrent lesions was defined as PPFS. The progression of relapse lesions reflects the weakness of the anti-tumor immune system and the production of circulating tumor cells, predicting poor prognosis (10–12). According to the NCCN guidelines, a change to an alternate chemotherapy regimen is usually recommended for recurrent PDAC patients. However, there lacked of a definite chemotherapeutic regimens for recurrent PDAC patients in the NCCN guidelines (13). Therefore, exploring independent prognostic factors of PPFS and further creating an analysis tool to predict the risk of PPFS is crucial for developing suitable adjuvant chemotherapy regimens for recurrent PDAC patients.
Several prediction models have been used to estimate the overall survival or progression-free survival of PDAC patients (14–16). Some independent factors in these models such as tumor markers or chemotherapy regimens were only collected before or soon after surgeries. The updated data after tumor recurrence were scarcely mentioned. Moreover, previous partial research only recorded whether PDAC patients received chemotherapy or not, the detailed classification of chemotherapy regimens has rarely been established. However, the PPFS of recurrent PDAC patients is greatly impacted by characteristics of relapse lesions rather than primary tumor features. Therefore, prior nomogram models may be less effective for PPFS prediction. Considering the lack of a specific predictive model for PPFS estimation, it was necessary to build a novel nomogram model for recurrent PDAC patients.
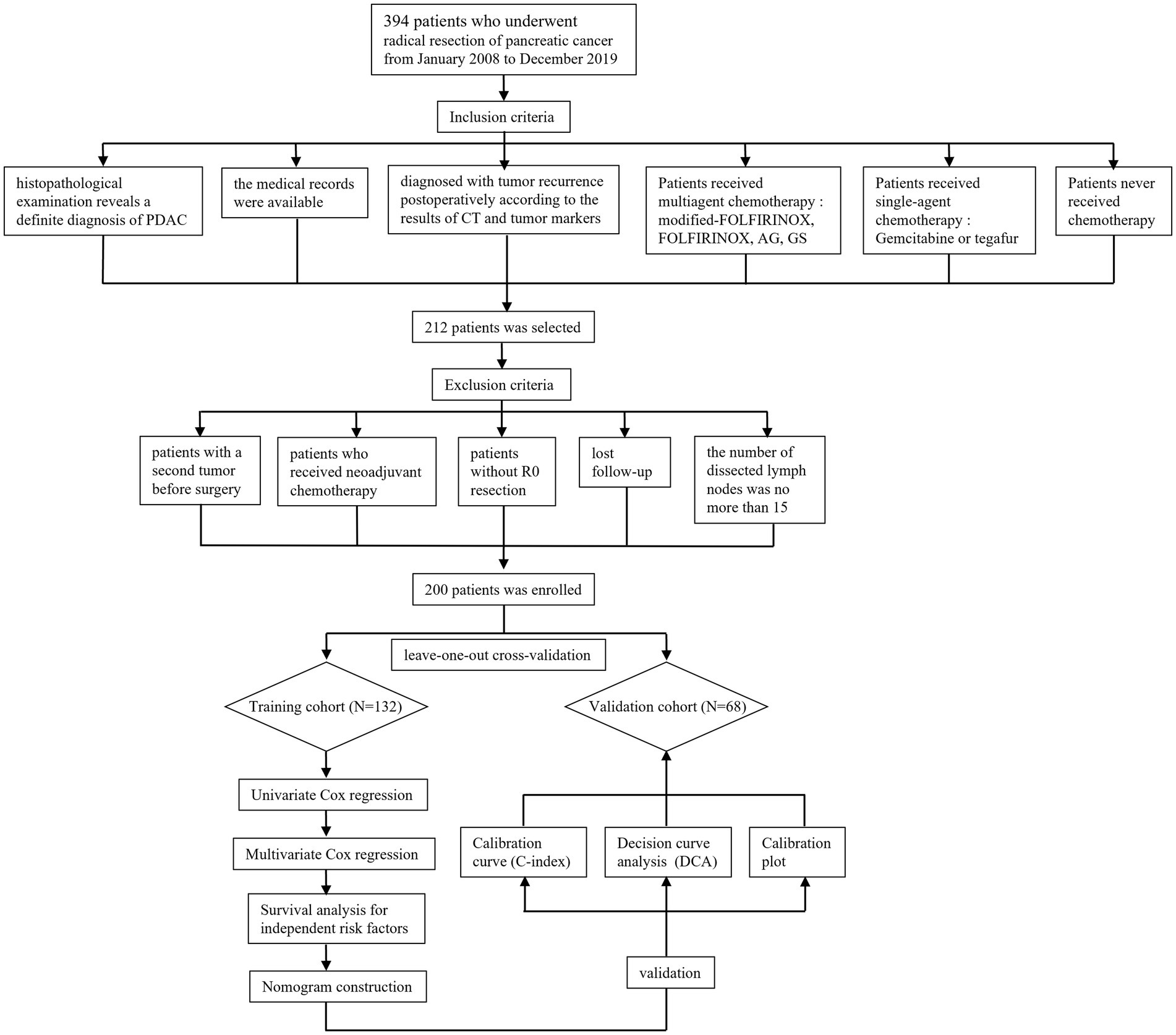
In order to ensure the quality and reliability of data analysis, all participants were screened by their clinical and pathologic features. The flow chart has been established in Figure 1. Finally, a total of 200 recurrent PDAC patients were collected from 394 surgically resected cases with pancreatic cancer.
Materials and methods
Patient’s enrollment and grouping
A total of 394 patients who underwent radical resection for PDAC from January 2008 to December 2019 were collected from medical records (Figure 1). Pancreaticoduodenectomy and distal pancreatectomy are the main type of radical resection (17, 18). Tumoral resectability was investigated by a professional multidisciplinary team for PDAC based on imaging findings from computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography-CT (PET-CT). Three chief physicians skilled in pancreaticoduodenectomy and distal pancreatectomy performed all of the surgeries included in this study. The attending clinician and resident physician were also required to participate in the surgical procedure. Furthermore, open, laparoscopic, and robotic surgery was chosen according to the clinical tumor state with consent obtained from patients.
The inclusion criteria concluded as follows: (1) histopathological examination reveals a definite diagnosis of PDAC, (2) diagnosed with tumor recurrence postoperatively according to the results of CT, tumor markers, and biopsy pathology, (3) the medical records were available, (4) Patients received multiagent chemotherapy: modified-FOLFIRINOX, FOLFIRINOX (oxaliplatin, Irinotecan, calcium folinate, fluorouracil), AG (nab-paclitaxel, gemcitabine), GS (gemcitabine, tegafur), (5) Patients received single-agent chemotherapy: Gemcitabine or tegafur, (6) Patients never received chemotherapy. On the contrary, the exclusion criteria were represented as follows: (1) patients with a second tumor before surgery, (2) patients who received neoadjuvant chemotherapy, (3) patients without R0 resection (the margin for R0 resection was described as 1.5–2 mm in the previous study) (19), (4) lost follow-up, (5) the number of dissected lymph nodes was no more than 15.
The patients enrolled in the present study were subsequently divided into training and validation group by leave-one-out cross-validation (LOOCV) method. In detail, one patient was enrolled into validation group each time, while the rest of patients served as a training group. Then, the accuracy of this model was recorded from analysis tool. The above procedure was repeated until all of the patients were tested as a validation sample. The final grouping schemes was determined by the average accuracy.
The consent to use the medical records has been obtained from all of patients enrolled in the present study. The Ethics Committee approved the retrospective study. This study was registered with www.researchregistry.com. This work has been reported according to the STROCSS (Strengthening the Reporting of Cohort Studies in Surgery) criteria (20).
Collection of clinicopathological characteristics
The clinicopathological factors included in this research were chosen based on the results of previous prognostic analyses (9, 14). It is worth mentioning that some of the factors were acquired at the time points after tumor recurrence because the present study focused on the progression of relapse lesion in PDAC patients. In detail, the pathological diagnosis was acquired from experienced pathologists, including the tumor size, tumor differentiation, lymph node metastasis, microvascular invasion, lymph vascular invasion, and adjacent organ invasion. Moreover, several inflammation indices, such as the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), were involved in this study. Besides, this study also registered clinical factors such as age, gender, serum levels of carbohydrate antigen 19-9 (CA19-9), and carcinoembryonic antigen (CEA) after confirmation of tumor recurrence. Moreover, the key features of tumor recurrence were also registered in this research, including time to recurrence (the cut-off value to define early and late recurrence was one year after surgery) and recurrence patterns (the definition of different relapse patterns referred from the research by Groot) (7, 21).
Development and classification criteria of chemotherapy regimens
The chemotherapy regimens mentioned in the present study were applied after radical surgery or tumor recurrence according to the recommendation from NCCN (2021 Ver2.0) guidelines for PDAC respectively (13). Meanwhile, the patients’ will and Eastern Cooperative Oncology Group Performance Status (ECGO PS) were considered before developing the chemotherapy schema. The modified-FOLFIRINOX or FOLFIRINOX were the preferred option for PDAC patients in better physical condition or ECGO PS range from 0 to 1 point. If patients had a poor overall physical condition (ECGO PS range from 2 to 5 point), gemcitabine or tegafur will be recommended. AG or GS chemotherapy schema were usually applied in recurrent PDAC patients who were not sensitive to FOLFIRINOX chemotherapy schema. Furthermore, the chemotherapy regimens were divided into three levels through the variety of drug utilization. Firstly, the patients who never received chemotherapy after surgery or tumor recurrence were classified as “untreated.” Then, the patients who got only gemcitabine or tegafur therapies were defined as “single-agent chemotherapy.” Finally, the patients who underwent modified-FOLFIRINOX or FOLFIRINOX, AG, GS chemotherapy schema were categorized as “multi-agent chemotherapy.”
Follow-up and outcome adjudication
The follow-up began at the time of tumor recurrence after radical surgery. The patients were recommended to undergo outpatient review every 3 months. Meanwhile, abdominal and chest CT, CA19-9, and CEA were performed regularly after surgery. If the outpatient review were unavailable for some patients, telephone contact would be the alternative method. The endpoint of the present study was progression in recurrent lesions, which is defined as follows: (A) ≥20% increase in maximum diameter of the primary recurrent lesions, (B) or detection of any new recurrent lesions in the distant tissue. The outcome adjudication was made after the imaging examination or pathology diagnosis during follow-up.
Statistical analysis
The comparison of clinicopathological characteristics between the early and late recurrent groups was conducted using the chi-square test. The relationship between clinicopathological factors and PPFS was investigated using Kaplan–Meier methods. In detail, the log-rank test was utilized when the survival curve was not crossed, while the landmark analysis was applied when the survival curve was crossed. Multivariate Cox regression analysis was used to detect the independent prognostic factors for PPFS after completing the study of univariate Cox regression. The concordance indexes (C-indexes), calibration plots, and decision curve analyses (DCA) were utilized to compare the predictive ability between the nomogram and TNM-stage prediction models. A two-tailed p < 0.05 was considered statistically significant in the present study. All statistical analyses were conducted using SPSS software version 22 and R software version 4.2.2 (R Development Core Team; http://www.r-project.org). Moreover, the R packages “getsummary, tidyverse, survival, plyr, broom, forestmodel, ggplot2, rms, survminer, and ggDCA” were used in this research.
Results
Patient’s clinicopathological characteristics
A total of 394 PDAC patients received radical surgery between January 2008 and December 2019, while 212 cases of them were eventually diagnosed with tumor recurrence. Meanwhile, 12 recurrent PDAC patients were eliminated from the research according to the exclusion criteria as follows: patients with a second tumor before surgery (2 cases), patients who received neo-adjuvant chemotherapy (1 case), patients without R0 resection (1 case), lost follow-up (6 cases), the number of dissected lymph nodes was less than 15 (2 cases). At last, 200 recurrent PDAC patients were enrolled in the present study. Subsequently, the 200 patients were divided into a training cohort (132 cases) and a validation cohort (68 cases) by the 200 recurrent PDAC patients were divided into training and validation groups by LOOCV. For the training cohort, the median PPFS was 5.25 months. For the validation cohort, the median PPFS was 5.25 months. The clinicopathological characteristics of the two groups were established in Table 1. Based on the results of the chi-square test, T stage, TNM stage, tumor differentiation, chemotherapy after recurrence, and recurrence patterns showed a significant difference between the training and validation cohorts.
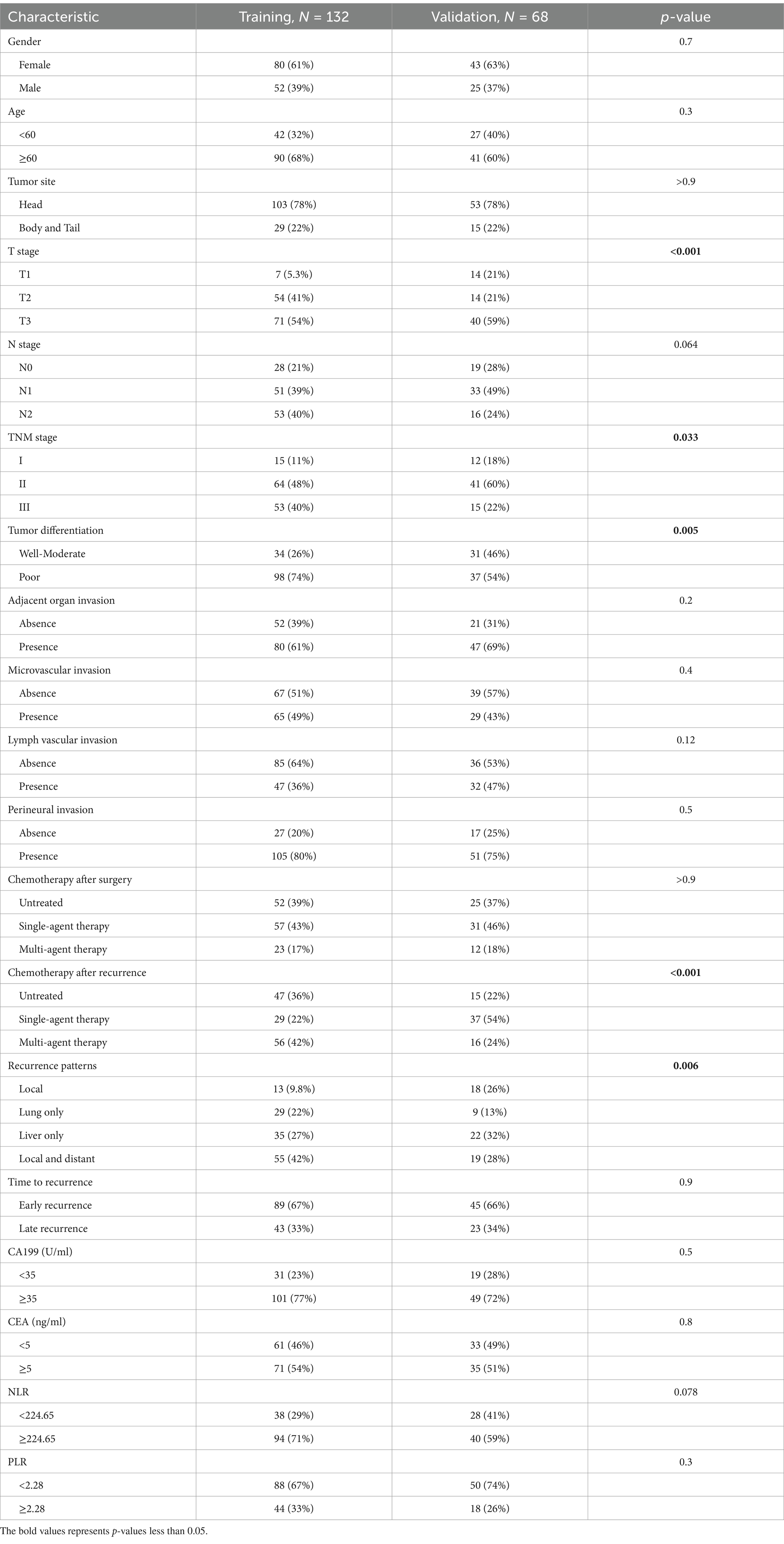
Table 1. Clinicopathological characteristics of PDAC patients in the training and validation cohort.
Prognostic factors for PPFS
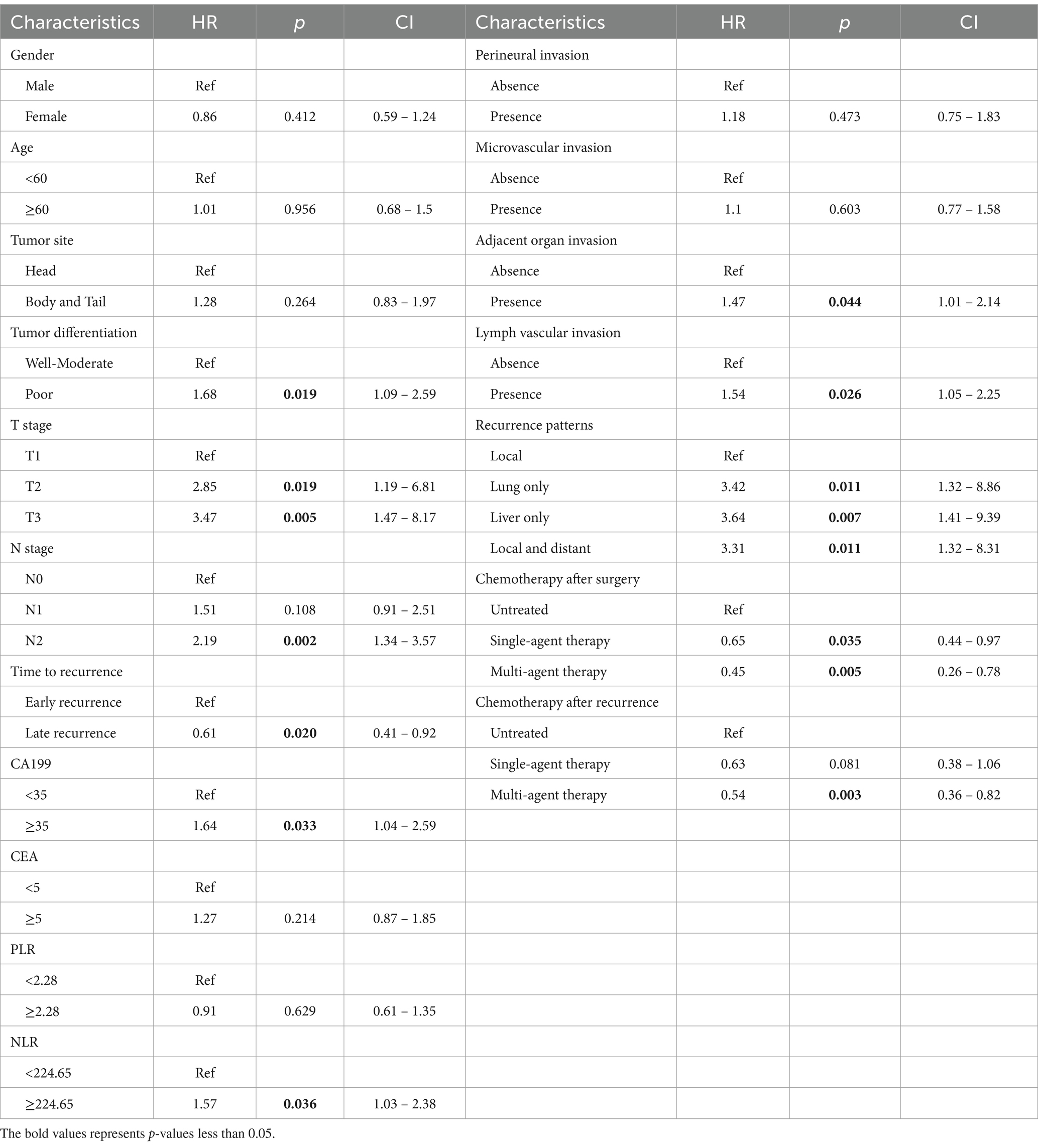
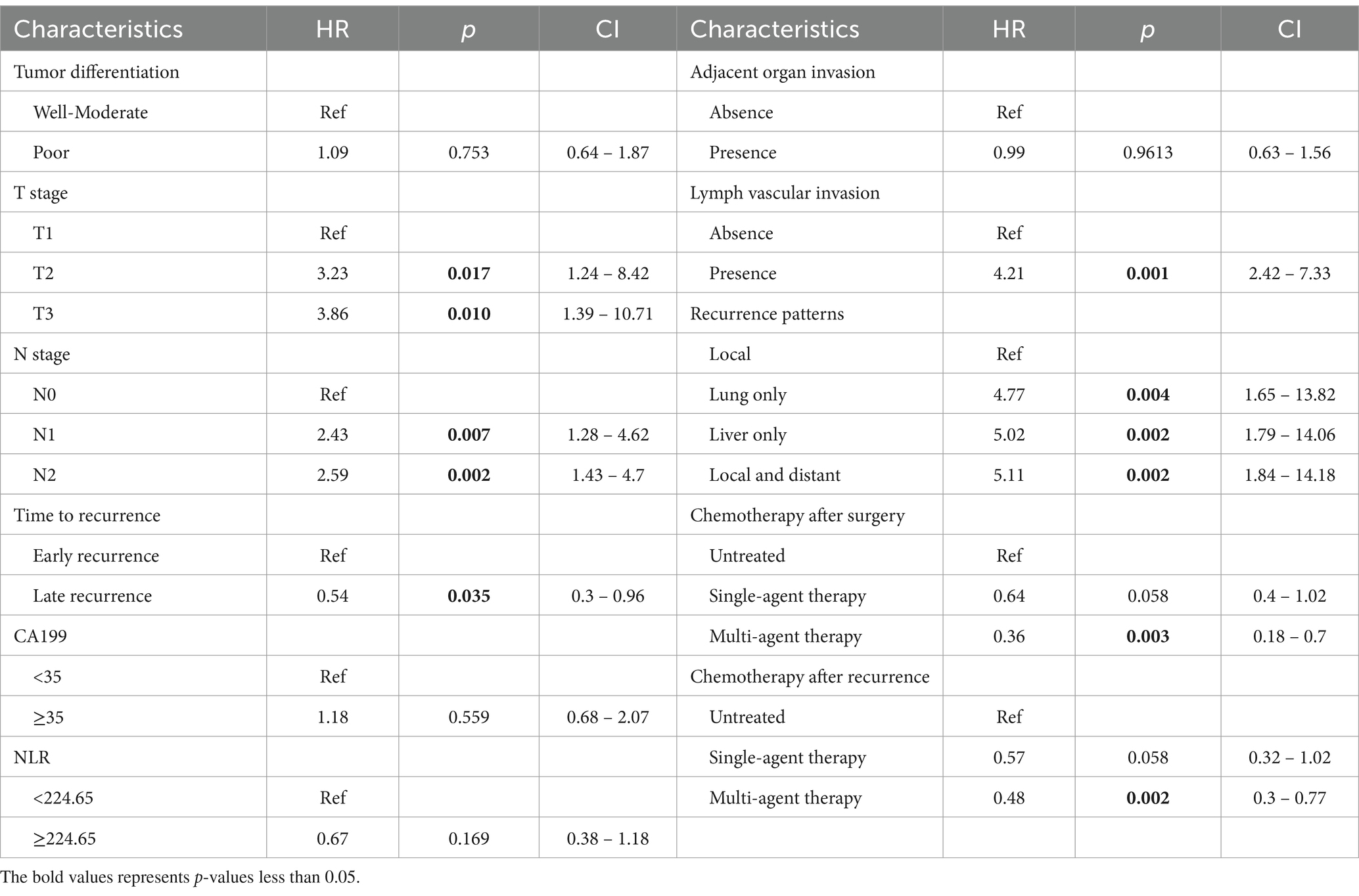
As shown in Table 2, 18 factors were enrolled into univariate Cox regression in the training cohort. As the results showed, 11 factors were described to be correlated with PPFS: tumor differentiation, T stage, N stage, time to recurrence, CA19-9, NLR, adjacent organ invasion, lymph vascular invasion, recurrence pattern, chemotherapy after surgery, chemotherapy after recurrence. Consequently, these factors were enrolled into the multivariate Cox regression, and the results were established in Table 3. The T stage, N stage, time to recurrence, Lymph vascular invasion, recurrence pattern, chemotherapy after surgery, chemotherapy after recurrence were considered as independent prognostic factors for PPFS in recurrent PDAC patients.
Survival analysis for independent prognostic factors
The patients who received multi-agent therapy showed better PPFS than patients treated with single-agent therapy (p = 0.009) (Figure 2A). Meanwhile, the patients treated with multi-agent or single-agent chemotherapy regiments after tumor relapse were estimated to have a similar prognosis (p = 0.011) (Figure 2B). The PDAC patients in the T1 stage had a finer prognosis than patients in the T2 or T3 stage (p = 0.011) (Figure 2C). Worse outcomes are observed in patients with higher N stages (p = 0.005) (Figure 2D). The patients with local recurrence had the best prognosis among the four relapse patterns mentioned in this study (p = 0.038) (Figure 2E). PDAC patients with late recurrence had a relatively better prognosis than early recurrent patients (p = 0.019) (Figure 2F). Finally, positive lymph vascular invasion in PDAC patients predicted worse outcomes when compared with negative lymph vascular invasion (p = 0.026) (Figure 2G).
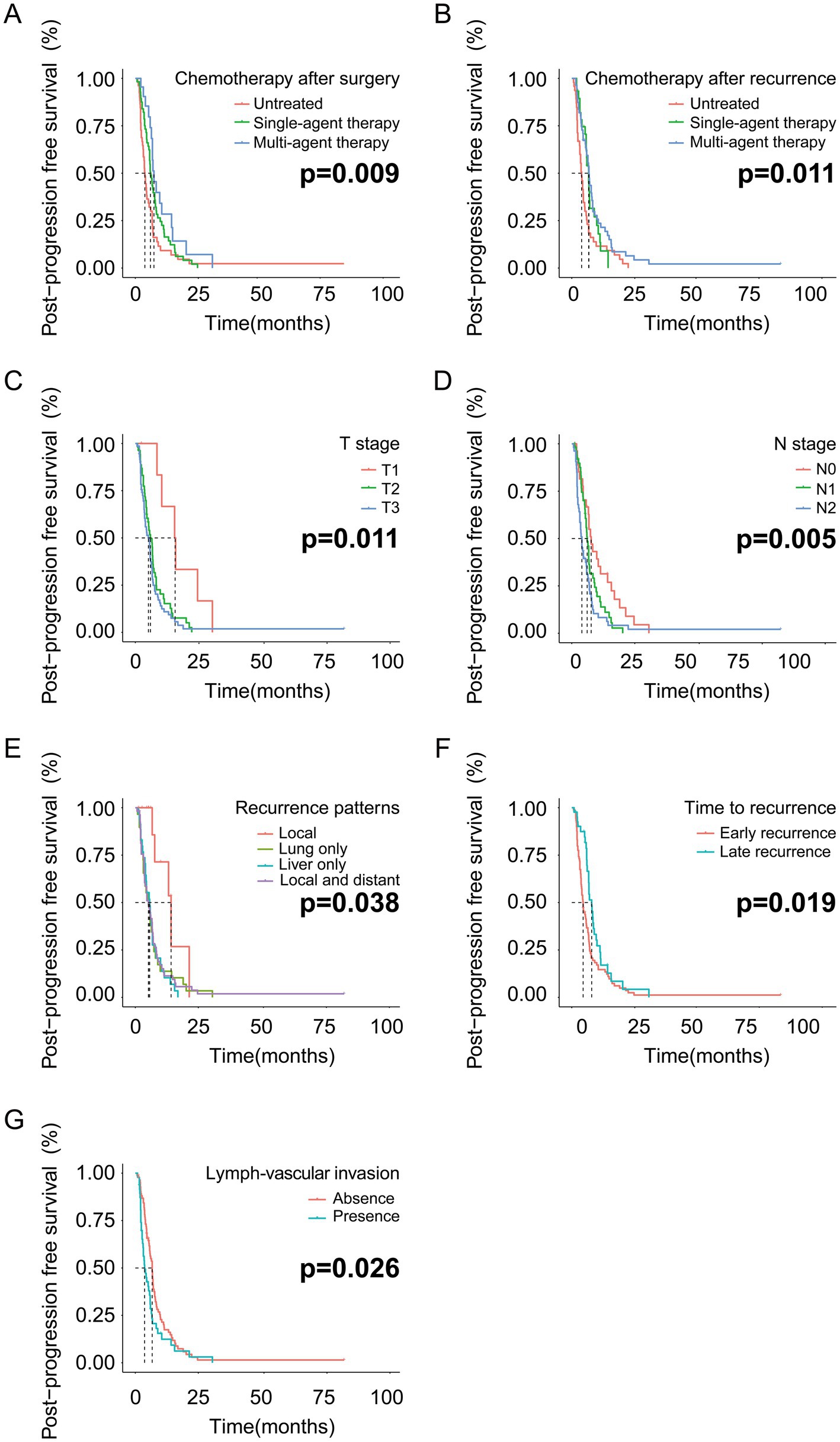
Figure 2. The PPFS analysis based on the independent risk factors in the training cohort. (A) PPFS analysis based on chemotherapy after surgery; (B) PPFS analysis based on chemotherapy after recurrence; (C) PPFS analysis based on T stage; (D) PPFS analysis based on N stage. (E) PPFS analysis based on recurrence patterns; (F) PPFS analysis based on time to recurrence; (G) PPFS analysis based on lymph vascular invasion.
Construction and validation of a nomogram for PPFS prediction
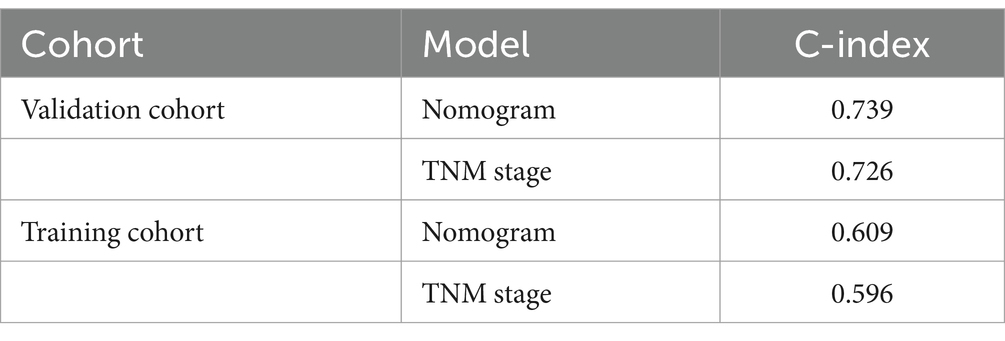
As shown in Figure 3, a specific nomogram for PPFS prediction in patients with PDAC was built on independent prognostic factors. The recurrence patterns are the factor that displayed the most prominent effect in this model, followed by the lymph vascular invasion, T-stage, N-stage, chemotherapy after recurrence, chemotherapy after surgery, and time to recurrence. Furthermore, to detect the accuracy of the nomogram model, the calibration plots were produced, demonstrating a high conformity between the actual and predictive PPFS in both training and validation groups (Figure 4). Meanwhile, to assess the discriminatory ability between the nomogram and TNM stage system, the C-index was calculated based on the training cohort and validation cohort; as shown in Table 4, the C-index of the nomogram model was significantly higher than that in the TNM stage in both training and validation cohort. Finally, the decision curve analysis was fabricated to compare the clinical benefits of the nomogram and the TNM stage system (Figure 5). It means that the nomogram built in the present study could be more beneficial for clinical prediction than the TNM stage system.
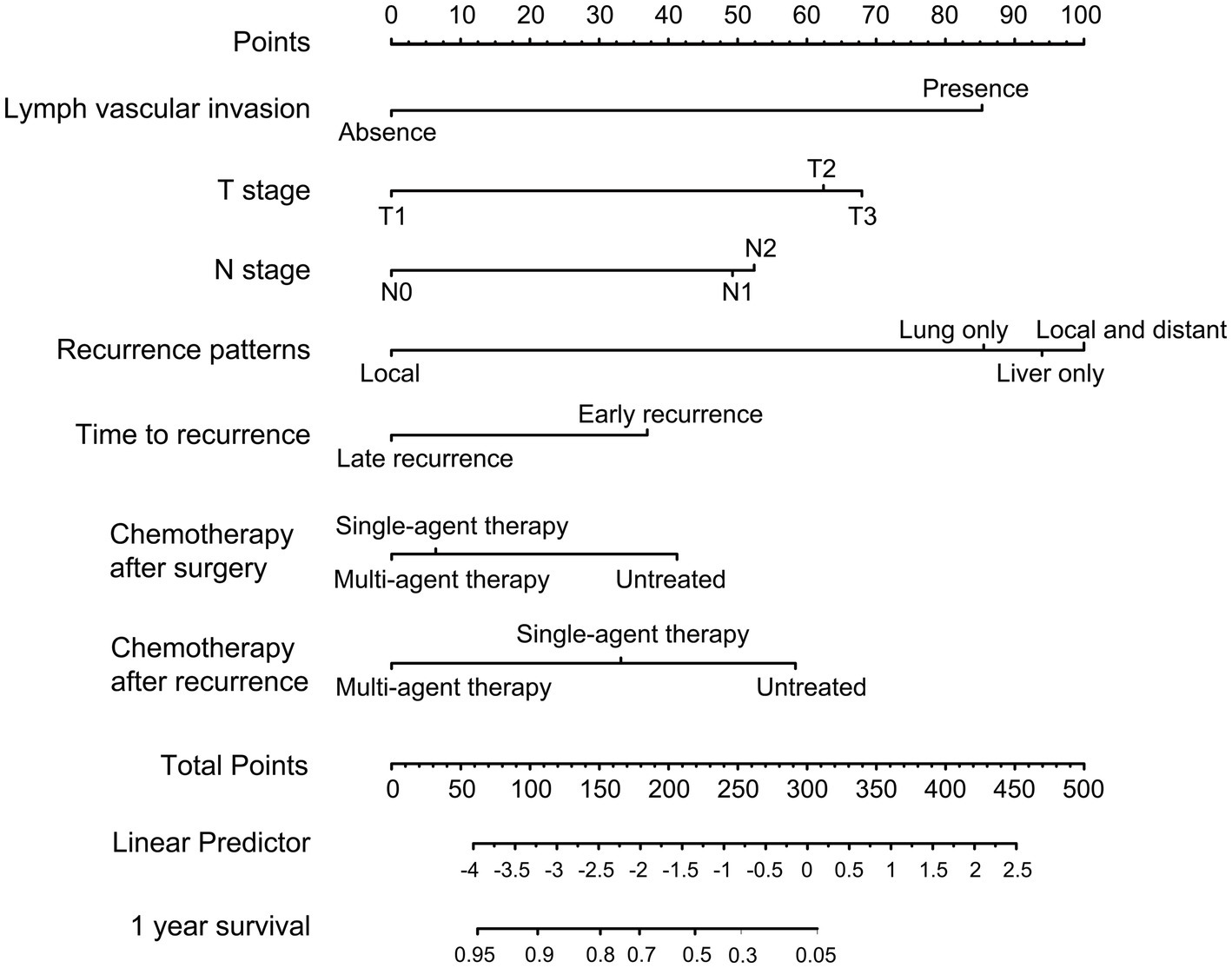
Figure 3. Nomogram for predicting the 1-year PPFS rates of PDAC patients after radical resection in the training cohort.
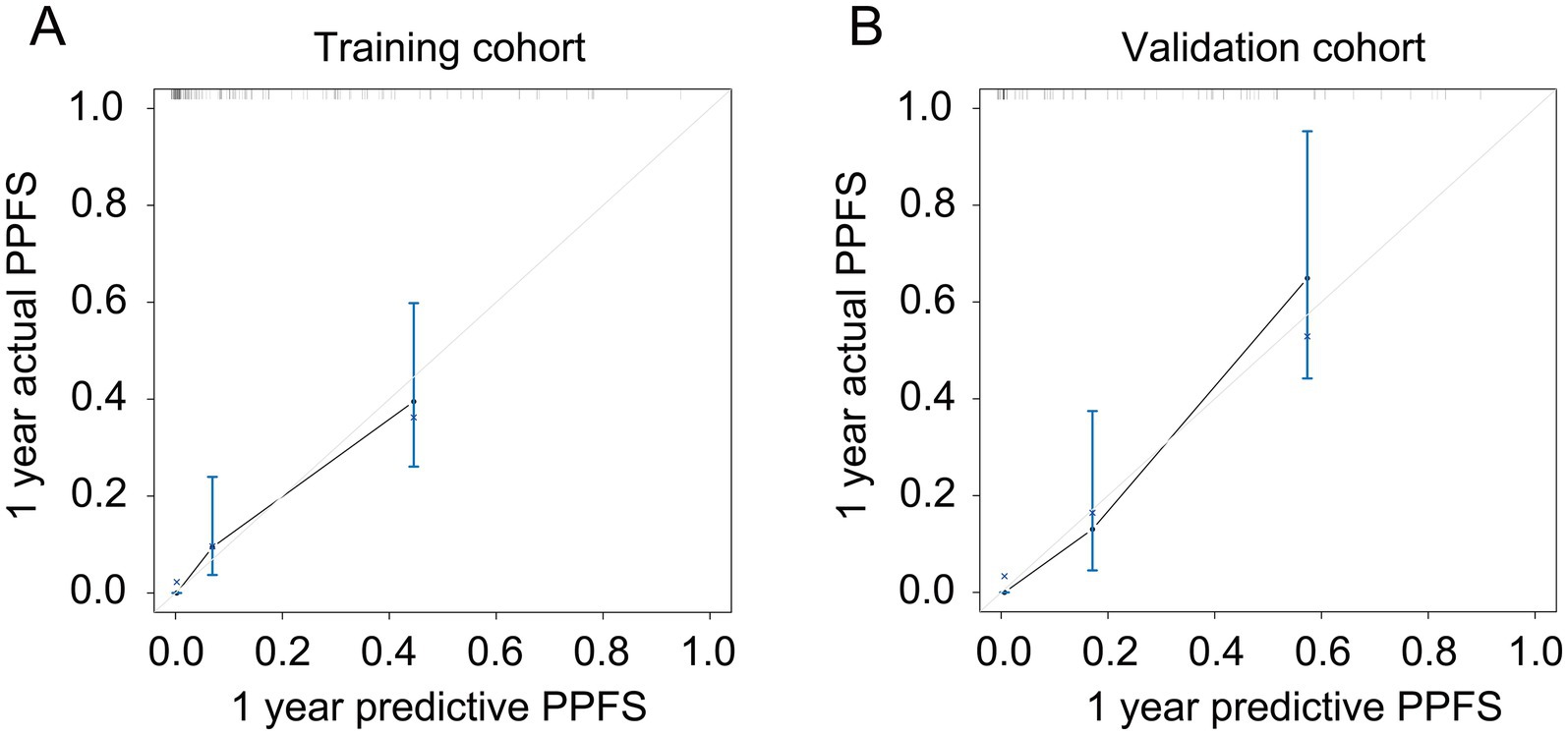
Figure 4. The calibration plot for predicting PPFS rates in recurrent PDAC. (A) The calibration plot for predicting the 1-year PPFS rates in the training cohort; (B) The calibration plot for predicting the 1-year PPFS rates in the validation cohort.
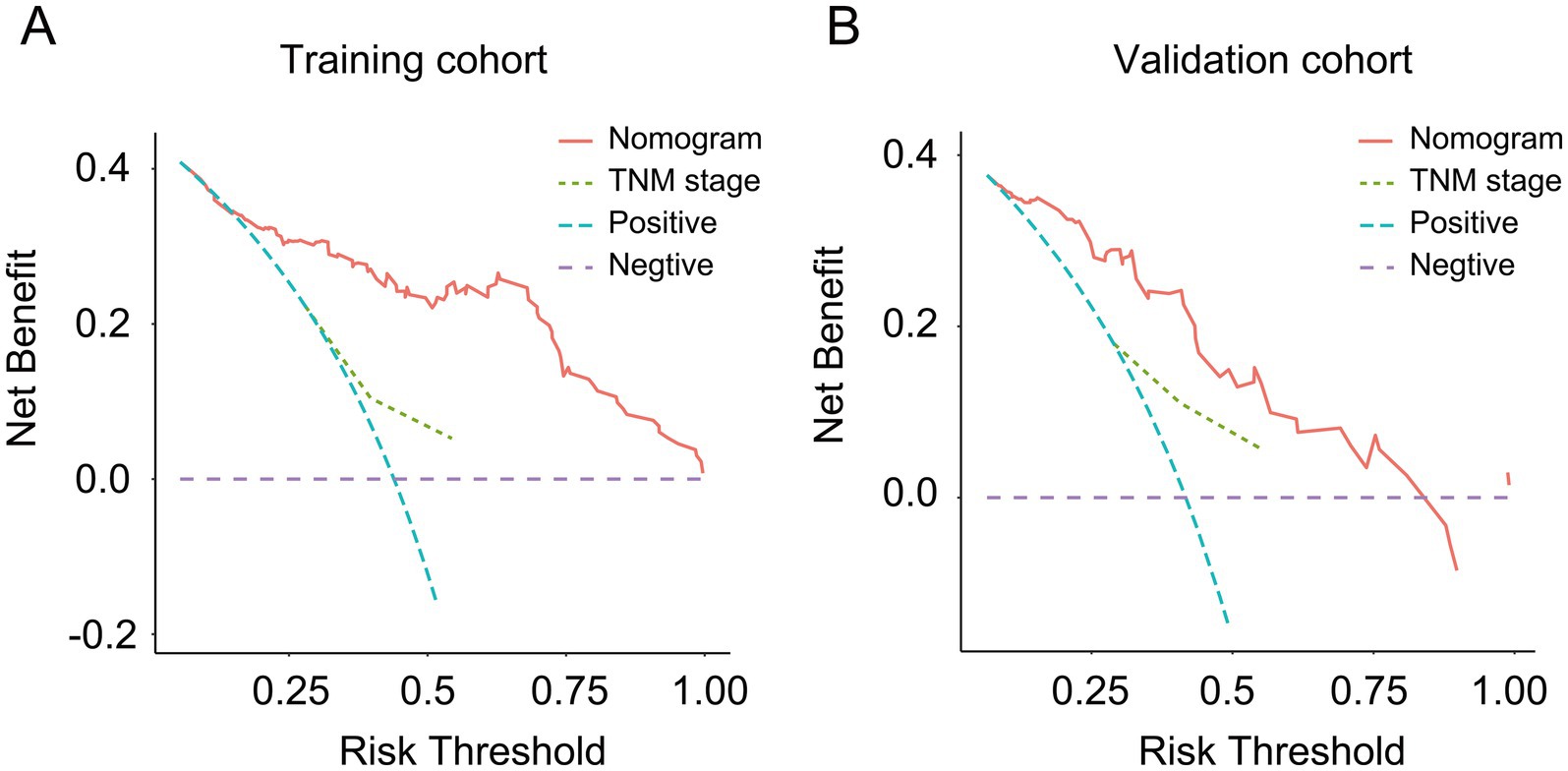
Figure 5. The decision curve analysis for the nomogram and TNM stage. (A) Decision curve analysis for the nomogram and TNM stage in the training cohort; (B) decision curve analysis for the nomogram and TNM stage in the validation cohort.
Discussion
PDAC was a lethal disease in which micro-metastatic lesions occurred in the early stage of carcinogenesis (22). Therefore, nearly 85% of the PDAC patients who received radical surgery would suffer from tumor relapse in the early postoperative period (6, 7). Tumor recurrence was portrayed as a crucial time point for PDAC patients since 80% of them may die from tumor recurrence or metastasis during the next five years (23, 24). Moreover, the advancement of recurrent lesions was also demonstrated as another primary concern, from which the alternative chemotherapy regimen should be considered for restricting the growth of recurrent lesions (13, 25). Therefore, exploring the factors independently influencing the PPFS and precisely predicting the PPFS through the nomogram model was essential for developing alternative chemotherapy regimens and improving patient outcomes. However, the correlation between the clinicopathological factors and PPFS has not been explored clearly. The present study determined that lymph vascular invasion, N stage, T stage, recurrence patterns, time to recurrence, chemotherapy after surgery, and chemotherapy after recurrence were independent prognostic factors for PPFS. Furthermore, we also constructed a nomogram based on those independent prognostic factors with favorable predictive capacity.
Since the high invasive capacity of PDAC, micro-metastatic lesions and residual tumor foci commonly co-existed in the same patient (22, 26, 27), adjuvant chemotherapy was imminent after radical resection (28–31). The preceding research declared that chemotherapy inhibited tumor progression and metastasis (32–35). However, the tumor-inhibiting effect of chemotherapy on the relapse lesions had been rarely discussed before. The present study found that PDAC patients received multi-agent chemotherapy after surgery showed better PPFS than patients received single-agent chemotherapy. Meanwhile, we also found that multi-agent chemotherapy and single-agent chemotherapy had similar efficacy in limiting the progression of relapse foci. Drug-tolerant tumor cells was fundamental for drug-resistant clones and contribute to relapse and disease progression (36). It means that the resistance to chemotherapy was significantly strengthened in the recurrent lesion compared with the primary tumor. Meanwhile, the progression of recurrent lesions was too fast to be restricted by the chemotherapeutic agent (37, 38). Therefore, the superiority of the multi-agent regimen was masked when compared with the single-agent schema in recurrent PDAC patients (39–43). Based on the results of the present study, we argue that multi-agent chemotherapy brings more survival benefits to PDAC patients after radical surgery compared with a single-agent scheme. However, single-agent chemotherapy regimens should also be recommended for recurrent PDAC patients with poor chemotherapy tolerance to reduce the toxic side effects of chemotherapeutic agents (44, 45).
In previous research, lymph node metastasis was explained as an essential predictor for tumor progression (46–49). Meanwhile, the consensus in the Japanese Pancreatic Society also cited that confirmation of N9 and N16 lymph node metastasis was intimately linked with tumor relapse and distant metastasis (14). Similarly, the higher N stage and positive lymph vascular invasion portended early advancement of recurrent lesions in the present research. Dissemination into the lymphatic system was the major routes for PDAC metastasis (50). Meanwhile, lymph node metastasis was an initial step in PDAC metastasis. It was also crucial for determination of clinical staging, prognosis and survival in PDAC patients (51, 52). Therefore, recurrent PDAC patients with lymph vascular invasion or lymph node metastasis may benefit from chemotherapy for inhibiting the relapse lesion progression.
The T stage represented the tumor diameters measured by the pathologists, indicating the tumor burden. Moreover, it has also been estimated as the reference standard for chemotherapeutic efficacy (13, 53, 54). The probability that a cancer contains drug-resistant clones depended on the size of the tumor (55). In this study, we found that the higher T stage was one of the independent prognostic factors for PPFS, portending a shorter PPFS. The residual disease of PDAC patients after surgery my cause tumor recurrence or relapse lesions progression. Hence, the radiotherapy or nano knife against the resection margin may reduce the local recurrence rate in PDAC patients with large tumor volumes.
Different recurrence patterns of PDAC patients are usually linked with diverse post-progression survival (7, 21, 56). In the earlier study, the local and distant recurrence pattern heralded the poorest post-progression survival among the four abovementioned recurrence patterns (7). The present study also estimated that the local and distant recurrence patterns predicted poorer PPFS compared to the local recurrence patterns. The late tumor stage may be one of the most important causes leading to this result (7, 57). Therefore, PDAC patients with local and distant recurrence are warranted to received chemotherapy when compared with local recurrent cases.
Last but not least, we investigated the relationship between the time to recurrence and PPFS. The results declared that PDAC patients with late relapse had exclusively longer PPFS compared with the early relapse patients. In the previous research, early recurrence reflected the malignant phenotype of PDAC (8, 14, 58). Most of the early relapse patients were combine with late tumor stage, poorer tumor differentiation, and stronger drug-resistant effect, causing a more rapid progression of recurrent lesions (7, 59, 60). Therefore, chemotherapy-only may be not enough to restrain the tumor progression in early recurrent PDAC patients. Combination therapies may be more suitable for early recurrent cases. In detail, secondary surgery could be performed in PDAC patients with isolated local recurrent or oligometastatic lesions. Besides this, radiofrequency ablation or radiation therapy combined with chemotherapy could be another option for early recurrent patients with multiple metastases.
The development of recurrent lesions was a crucial time point for replacing chemotherapy schemes according to the NCCN guidelines (13). Furthermore, individualized intervention for PDAC patients should be managed based on the risk of relapse lesions advancement. Therefore, precise prediction for PPFS is essential for clinical decision-making. After statistical analysis, our research selected several independent prognostic factors from high-dimensional radiological and pathological variables. Furthermore, we also constructed a nomogram for PPFS prediction based on those independent prognostic factors. The results of contrast analysis (the calibration curve, DCA, and C-index) between the training and validation cohort demonstrated this nomogram system’s strong predictive and discriminative power. Thus, clinicians can precisely assess the risk of progression in relapse lesions for PDAC patients by using the nomogram system fabricated in this study.
There are still some limitations to the present study. First, this study adopted a single-center retrospective design. The regional difference of clinical practices might affect the extrapolation of the results in our current analysis. A multi-center control study was needed to better estimate the predictive capacity of the nomogram constructed in the present study. Meanwhile, a major potential bias in this analysis is selection bias. For example, there was a lower readmission probability in the PDAC patients with history of stroke or coronary artery disease because of the higher surgical risk when compared with PDAC patients with better physical condition. It may limit the predictive power of the nomogram in the PDAC patients with poor physical status.
Hence, to minimize such bias, we inflated the sample size by including the resected PDAC patients from 2008 to 2019, and we also set strict inclusion criteria and exclusion criteria for screening. On the other hand, some variables, such as serum total bilirubin levels, C-reactive protein, and albumin, had not been included in this research. It was beneficial to explore the impact of nutritional status and inflammatory response on the progression of relapse lesions in PDAC patients. Meanwhile, some of the characteristics were only vaguely described during the information collection. For example, the specific chemotherapy regimens and the lymph node metastasis group had not been displayed in detail. The involvement of the characteristics mentioned above could further refine the predictive effect of the nomogram. For instance, more precise results would be established in future exploration when a larger sample size was available. Finally, some modified described methods for lymph node metastasis, such as lymph node ratio and log odds of positive lymph nodes, have already been proposed in some research (61). However, we still referred to the description of lymph node metastasis from the AJCC guidelines in the present study. More persuasive results would be available if future research could apply the modified methods to lymph node metastasis discrimination.
Conclusion
Multi-agent chemotherapy is recommended for PDAC patients after surgery. The single-agent chemotherapy also deserves consideration after tumor recurrence. The nomogram model provided a new way for PPFS prediction in recurrent PDAC patients. Moreover, the predictive value and accuracy of the present nomogram will be improved if validated in a larger and multi-center cohort.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Sun Yat-sen University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The manuscript presents research on animals that do not require ethical approval for their study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DQ: Writing – original draft. PX: Data curation, Formal analysis, Writing – original draft. KH: Methodology, Resources, Writing – original draft. LJ: Visualization, Writing – original draft. ZY: Software, Writing – original draft. RW: Writing – review & editing. SL: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Funds (Nos. 81972299 and 81672390) and National Key Research and Development Plan (No. 2017YFC0910002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1486750/full#supplementary-material
References
1. Siegel, R, Miller, K, and Jemal, A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
2. Rawla, P, Sunkara, T, and Gaduputi, V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. (2019) 10:10–27. doi: 10.14740/wjon1166
4. Rahib, L, Smith, B, Aizenberg, R, Rosenzweig, A, Fleshman, J, and Matrisian, L. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. (2014) 74:2913–21. doi: 10.1158/0008-5472.Can-14-0155
5. Ben-Josef, E, Guthrie, K, el-Khoueiry, AB, Corless, CL, Zalupski, MM, Lowy, AM, et al. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol. (2015) 33:2617–22. doi: 10.1200/jco.2014.60.2219
6. Suenaga, M, Fujii, T, Kanda, M, Takami, H, Okumura, N, Inokawa, Y, et al. Pattern of first recurrent lesions in pancreatic cancer: hepatic relapse is associated with dismal prognosis and portal vein invasion. Hepatogastroenterology. (2014) 61:1756–61.
7. Groot, V, Rezaee, N, Wu, W, Cameron, JL, Fishman, EK, Hruban, RH, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. (2018) 267:936–45. doi: 10.1097/sla.0000000000002234
8. He, C, Cai, Z, Zhang, Y, and Lin, X. Comparative recurrence analysis of pancreatic adenocarcinoma after resection. J Oncol. (2021) 2021:3809095–18. doi: 10.1155/2021/3809095
9. He, C, Huang, X, Zhang, Y, Cai, Z, Lin, X, and Li, S. A quantitative clinicopathological signature for predicting recurrence risk of pancreatic ductal adenocarcinoma after radical resection. Front Oncol. (2019) 9:1197. doi: 10.3389/fonc.2019.01197
10. Lei, P, Pereira, E, Andersson, P, Amoozgar, Z, van Wijnbergen, JW, O’Melia, MJ, et al. Cancer cell plasticity and MHC-II-mediated immune tolerance promote breast cancer metastasis to lymph nodes. J Exp Med. (2023) 220:e20221847. doi: 10.1084/jem.20221847
11. Goldfarb, Y, Sorski, L, Benish, M, Levi, B, Melamed, R, and Ben-Eliyahu, S. Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg. (2011) 253:798–810. doi: 10.1097/SLA.0b013e318211d7b5
12. Miyamoto, D, Lee, R, Kalinich, M, LiCausi, JA, Zheng, Y, Chen, T, et al. An RNA-based digital circulating tumor cell signature is predictive of drug response and early dissemination in prostate cancer. Cancer Discov. (2018) 8:288–303. doi: 10.1158/2159-8290.Cd-16-1406
13. Tempero, M, Malafa, M, al-Hawary, M, Behrman, SW, Benson, AB, Cardin, DB, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. (2021) 19:439–57. doi: 10.6004/jnccn.2021.0017
14. He, C, Sun, S, Zhang, Y, Lin, X, and Li, S. A Novel Nomogram to Predict Survival in Patients With Recurrence of Pancreatic Ductal Adenocarcinoma After Radical Resection. Front Oncol. (2020) 10:1564. doi: 10.3389/fonc.2020.01564
15. Tiansong, X, Xuebin, X, Wei, L, Lei, C, Kefu, L, and Zhengrong, Z. Prediction of postoperative recurrence in resectable pancreatic body/tail adenocarcinoma: a novel risk stratification approach using a CT-based nomogram. Eur Radiol. (2023) 33:7782–93. doi: 10.1007/s00330-023-10047-x
16. Tang, TY, Li, X, Zhang, Q, Guo, CX, Zhang, XZ, Lao, MY, et al. Development of a Novel Multiparametric MRI Radiomic Nomogram for Preoperative Evaluation of Early Recurrence in Resectable Pancreatic Cancer. J Magn Reson Imaging. (2020) 52:231–45. doi: 10.1002/jmri.27024
17. Ruiqiu, C, Chaohui, X, Shaoming, S, Lin, Z, Tianchen, Z, and Rong, L. The optimal choice for patients underwent minimally invasive pancreaticoduodenectomy: a systematic review and meta-analysis including patient subgroups. Surg Endosc. (2024) 38:6237–53. doi: 10.1007/s00464-024-11289-6
18. Jiang, L, Ning, D, and Chen, XP. Improvement in distal pancreatectomy for tumors in the body and tail of the pancreas. World J Surg Oncol. (2021) 19:49. doi: 10.1186/s12957-021-02159-9
19. Chang, D, Johns, A, Merrett, N, Gill, AJ, Colvin, EK, Scarlett, CJ, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol. (2009) 27:2855–62. doi: 10.1200/jco.2008.20.5104
20. Mathew, G, Agha, R, Albrecht, J, Goel, P, Mukherjee, I, Pai, P, et al. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg. (2021) 96:106165. doi: 10.1016/j.ijsu.2021.106165
21. Groot, V, Gemenetzis, G, Blair, A, Rivero-Soto, RJ, Yu, J, Javed, AA, et al. Defining and Predicting Early Recurrence in 957 Patients With Resected Pancreatic Ductal Adenocarcinoma. Ann Surg. (2019) 269:1154–62. doi: 10.1097/sla.0000000000002734
22. Bednar, F, and Pasca di Magliano, M. Chemotherapy and Tumor Evolution Shape Pancreatic Cancer Recurrence after Resection. Cancer Discov. (2020) 10:762–4. doi: 10.1158/2159-8290.Cd-20-0359
23. Siegel, R, Miller, K, and Jemal, A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
24. Gillen, S, Schuster, T, Meyer Zum Büschenfelde, C, Friess, H, and Kleeff, J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. (2010) 7:e1000267. doi: 10.1371/journal.pmed.1000267
25. Taniyama, T, Morizane, C, Nakachi, K, Nara, S, Ueno, H, Kondo, S, et al. Treatment outcome for systemic chemotherapy for recurrent pancreatic cancer after postoperative adjuvant chemotherapy. Pancreatology. (2012) 12:428–33. doi: 10.1016/j.pan.2012.07.016
26. Akhtar, M, Haider, A, Rashid, S, and Al-Nabet, A. Paget's "Seed and Soil" Theory of Cancer Metastasis: An Idea Whose Time has Come. Adv Anat Pathol. (2019) 26:69–74. doi: 10.1097/pap.0000000000000219
27. Psaila, B, and Lyden, D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. (2009) 9:285–93. doi: 10.1038/nrc2621
28. Herrmann, R, Bodoky, G, Ruhstaller, T, Glimelius, B, Bajetta, E, Schüller, J, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. (2007) 25:2212–7. doi: 10.1200/jco.2006.09.0886
29. Conroy, T, Desseigne, F, Ychou, M, Bouché, O, Guimbaud, R, Bécouarn, Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. (2011) 364:1817–25. doi: 10.1056/NEJMoa1011923
30. Mavros, M, Moris, D, Karanicolas, P, Katz, M, O'Reilly, E, and Pawlik, T. Clinical Trials of Systemic Chemotherapy for Resectable Pancreatic Cancer: A Review. JAMA Surg. (2021) 156:663–72. doi: 10.1001/jamasurg.2021.0149
31. von Hoff, DD, Ervin, T, Arena, F, Chiorean, EG, Infante, J, Moore, M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. (2013) 369:1691–703. doi: 10.1056/NEJMoa1304369
32. Sohal, D, Duong, M, Ahmad, S, Gandhi, NS, Beg, MS, Wang-Gillam, A, et al. Efficacy of Perioperative Chemotherapy for Resectable Pancreatic Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. (2021) 7:421–7. doi: 10.1001/jamaoncol.2020.7328
33. Xiong, X, Mao, Q, Yang, J, Chen, S, and Li, X. Clinical effectiveness of fluorouracil and cisplatin intraperitoneal perfusion combined with intravenous chemotherapy for peritoneal metastasis in gastric cancer. Eur Rev Med Pharmacol Sci. (2023) 27:8716–31. doi: 10.26355/eurrev_202309_33794
34. Inworn, N, Senavat, P, Aleenajitpong, N, Chingchaimaneesri, M, Siripoon, T, Srirattanapong, S, et al. Predictive Factors for the Survival Outcomes of Preoperative Chemotherapy in Patients with Resectable and Borderline Resectable Colorectal Cancer with Liver Metastasis. Asian Pac J Cancer Prev. (2023) 24:3037–47. doi: 10.31557/apjcp.2023.24.9.3037
35. Zeng, Y, Zhang, S, Li, S, Song, G, Meng, T, Yuan, H, et al. Normalizing Tumor Blood Vessels to Improve Chemotherapy and Inhibit Breast Cancer Metastasis by Multifunctional Nanoparticles. Mol Pharm. (2023) 20:5078–89. doi: 10.1021/acs.molpharmaceut.3c00381
36. Zhao, X, Ren, Y, Lawlor, M, Shah, BD, Park, PMC, Lwin, T, et al. BCL2 Amplicon Loss and Transcriptional Remodeling Drives ABT-199 Resistance in B Cell Lymphoma Models. Cancer Cell. (2019) 35:752–766.e9. doi: 10.1016/j.ccell.2019.04.005
37. Orgaz, JL, Crosas-Molist, E, Sadok, A, Perdrix-Rosell, A, Maiques, O, Rodriguez-Hernandez, I, et al. Myosin II Reactivation and Cytoskeletal Remodeling as a Hallmark and a Vulnerability in Melanoma Therapy Resistance. Cancer Cell. (2020) 37:85–103.e9. doi: 10.1016/j.ccell.2019.12.003
38. Mollaoglu, G, Guthrie, MR, Böhm, S, Brägelmann, J, Can, I, Ballieu, PM, et al. MYC Drives Progression of Small Cell Lung Cancer to a Variant Neuroendocrine Subtype with Vulnerability to Aurora Kinase Inhibition. Cancer Cell. (2017) 31:270–85. doi: 10.1016/j.ccell.2016.12.005
39. Yan, W, Si, L, Ding, Y, Qiu, S, Zhang, Q, and Liu, L. Neoadjuvant chemotherapy does not improve the prognosis and lymph node metastasis rate of locally advanced cervical squamous cell carcinoma: A retrospective cohort study in China. Medicine. (2019) 98:e17234. doi: 10.1097/md.0000000000017234
40. Inoue, Y, Fujii, K, Tashiro, K, Ishii, M, Masubuchi, S, Yamamoto, M, et al. Preoperative Chemotherapy May Not Influence the Remnant Liver Regenerations and Outcomes After Hepatectomy for Colorectal Liver Metastasis. World J Surg. (2018) 42:3316–30. doi: 10.1007/s00268-018-4590-1
41. Hoyle, M, Crathorne, L, Peters, J, Jones-Hughes, T, Cooper, C, Napier, M, et al. The clinical effectiveness and cost-effectiveness of cetuximab (mono- or combination chemotherapy), bevacizumab (combination with non-oxaliplatin chemotherapy) and panitumumab (monotherapy) for the treatment of metastatic colorectal cancer after first-line chemotherapy (review of technology appraisal No.150 and part review of technology appraisal No. 118): a systematic review and economic model. Health Technol Assess. (2013) 17:1–237. doi: 10.3310/hta17140
42. Ranson, M, Hersey, P, Thompson, D, Beith, J, McArthur, GA, Haydon, A, et al. Randomized trial of the combination of lomeguatrib and temozolomide compared with temozolomide alone in chemotherapy naive patients with metastatic cutaneous melanoma. J Clin Oncol. (2007) 25:2540–5. doi: 10.1200/jco.2007.10.8217
43. Rosenberg, S, Yang, J, Schwartzentruber, D, Hwu, P, Marincola, FM, Topalian, SL, et al. Prospective randomized trial of the treatment of patients with metastatic melanoma using chemotherapy with cisplatin, dacarbazine, and tamoxifen alone or in combination with interleukin-2 and interferon alfa-2b. J Clin Oncol. (1999) 17:968–75. doi: 10.1200/jco.1999.17.3.968
44. Varol, U, Dirican, A, Yildiz, I, Oktay, E, Degirmenci, M, Alacacioglu, A, et al. First-line mono-chemotherapy in frail elderly patients with metastatic colorectal cancer. Asian Pac J Cancer Prev. (2014) 15:3157–61. doi: 10.7314/apjcp.2014.15.7.3157
45. Yhim, H, Lee, J, Kim, K, Kim, SA, Lee, JY, Hwang, HG, et al. Increased risk of venous and arterial thromboembolism in patients with colorectal cancer receiving cetuximab-based combination chemotherapy: A population-based study in Korea. Thromb Res. (2023) 231:50–7. doi: 10.1016/j.thromres.2023.10.005
46. Sahin, T, Fujii, T, Kanda, M, Nagai, S, Kodera, Y, Kanzaki, A, et al. Prognostic implications of lymph node metastases in carcinoma of the body and tail of the pancreas. Pancreas. (2011) 40:1029–33. doi: 10.1097/MPA.0b013e3182207893
47. Murata, Y, Ogura, T, Hayasaki, A, Gyoten, K, Ito, T, Iizawa, Y, et al. Predictive risk factors for early recurrence in patients with localized pancreatic ductal adenocarcinoma who underwent curative-intent resection after preoperative chemoradiotherapy. PLoS One. (2022) 17:e0264573. doi: 10.1371/journal.pone.0264573
48. Kadera, B, Sunjaya, D, Isacoff, W, Li, L, Hines, OJ, Tomlinson, JS, et al. Locally advanced pancreatic cancer: association between prolonged preoperative treatment and lymph-node negativity and overall survival. JAMA Surg. (2014) 149:145–53. doi: 10.1001/jamasurg.2013.2690
49. Asiyanbola, B, Gleisner, A, Herman, J, Choti, MA, Wolfgang, CL, Swartz, M, et al. Determining pattern of recurrence following pancreaticoduodenectomy and adjuvant 5-flurouracil-based chemoradiation therapy: effect of number of metastatic lymph nodes and lymph node ratio. J Gastrointest Surg. (2009) 13:752–9. doi: 10.1007/s11605-008-0762-x
50. Åkerberg, D, Ansari, D, and Andersson, R. Re-evaluation of classical prognostic factors in resectable ductal adenocarcinoma of the pancreas. World J Gastroenterol. (2016) 22:6424–33. doi: 10.3748/wjg.v22.i28.6424
51. Sun, Y, Chen, Y, Li, J, Huo, YM, Liu, DJ, Hua, R, et al. A novel long non-coding RNA ENST00000480739 suppresses tumour cell invasion by regulating OS-9 and HIF-1α in pancreatic ductal adenocarcinoma. Br J Cancer. (2014) 111:2131–41. doi: 10.1038/bjc.2014.520
52. Liu, C, Cheng, H, Jin, K, Fan, Z, Gong, Y, Qian, Y, et al. Resected Pancreatic Cancer With N2 Node Involvement Is Refractory to Gemcitabine-Based Adjuvant Chemotherapy. Cancer Control. (2020) 27:1073274820915947. doi: 10.1177/1073274820915947
53. Zhao, Y, and Wang, C. Clinicopathological Features, Recurrence Patterns, and Prognosis of Pancreatic Adenocarcinoma with Normal Serum CA19-9. A Consecutive Series of 154 Cases from a Single Institute. J Gastrointest Surg. (2020) 24:855–65. doi: 10.1007/s11605-019-04209-w
54. Kim, N, and Kim, H. Preoperative risk factors for early recurrence in patients with resectable pancreatic ductal adenocarcinoma after curative intent surgical resection. Hepatobiliary Pancreat Dis Int. (2018) 17:450–5. doi: 10.1016/j.hbpd.2018.09.003
55. Vasan, N, Baselga, J, and Hyman, DM. A view on drug resistance in cancer. Nature. (2019) 575:299–309. doi: 10.1038/s41586-019-1730-1
56. Groot, V, Gemenetzis, G, Blair, A, Ding, D, Javed, AA, Burkhart, RA, et al. Implications of the Pattern of Disease Recurrence on Survival Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol. (2018) 25:2475–83. doi: 10.1245/s10434-018-6558-7
57. Groot, V, Blair, A, Gemenetzis, G, Ding, D, Burkhart, RA, Yu, J, et al. Recurrence after neoadjuvant therapy and resection of borderline resectable and locally advanced pancreatic cancer. Eur J Surg Oncol. (2019) 45:1674–83. doi: 10.1016/j.ejso.2019.04.007
58. Zhao, J, Zhang, W, Zhang, J, Chen, YT, Ma, WJ, Liu, SY, et al. Independent Risk Factors of Early Recurrence After Curative Resection for Perihilar Cholangiocarcinoma: Adjuvant Chemotherapy May Be Beneficial in Early Recurrence Subgroup. Cancer Manag Res. (2020) 12:13111–23. doi: 10.2147/cmar.S289094
59. Pulvirenti, A, Javed, AA, Michelakos, T, Sekigami, Y, Zheng, J, Kalvin, HL, et al. Recurring Pancreatic Neuroendocrine Tumor: Timing and Pattern of Recurrence and Current Treatment. Ann Surg. (2023) 278:e1063–7. doi: 10.1097/SLA.0000000000005809
60. Honselmann, K, Pergolini, I, Castillo, C, Deshpande, V, Ting, D, Taylor, MS, et al. Timing But Not Patterns of Recurrence Is Different Between Node-negative and Node-positive Resected Pancreatic Cancer. Ann Surg. (2020) 272:357–65. doi: 10.1097/sla.0000000000003123
Keywords: nomogram, recurrence, chemotherapy, pancreatic adenocarcinoma, post-progression-free survival
Citation: Qin D, Xi P, Huang K, Jiang L, Yao Z, Wei R and Li S (2024) Nomogram for predicting post-progression-free survival in patients with recurrent pancreatic ductal adenocarcinoma after radical surgery: a retrospective analysis. Front. Med. 11:1486750. doi: 10.3389/fmed.2024.1486750
Edited by:
Hua Zhong, University of Hawaii at Manoa, United StatesReviewed by:
Peng Wang, Coriell Institute for Medical Research, United StatesShunian Xiang, Admera Health, United States
Ling Xiao, Harvard Medical School, United States
Copyright © 2024 Qin, Xi, Huang, Jiang, Yao, Wei and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ran Wei, d2VpcmFuQHN5c3VjYy5vcmcuY24=; Shengping Li, bGlzaGVuZ3BAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work
 Dailei Qin
Dailei Qin Pu Xi
Pu Xi Kewei Huang
Kewei Huang Lingmin Jiang
Lingmin Jiang Zehui Yao
Zehui Yao Ran Wei
Ran Wei Shengping Li
Shengping Li