- 1Department of Clinical Laboratory, The Third Affiliated Hospital of Wenzhou Medical University, Ruian, Zhejiang, China
- 2Department of Urology, The Third Affiliated Hospital of Wenzhou Medical University, Ruian, Zhejiang, China
- 3Department of Clinical Laboratory, Ruian Traditional Chinese Medicine Hospital, Ruian, Zhejiang, China
- 4Department of Emergency Intensive Care Unit, The Third Affiliated Hospital of Wenzhou Medical University, Ruian, Zhejiang, China
- 5Department of Epidemiology and Biostatistics, Empower U, X&Y Solutions Inc., Boston, MA, United States
Background: Sepsis is defined as a dysregulated host response to infection that results in life-threatening organ dysfunction. The 24-hour urine volume plays a crucial role in assessing the prognosis of septic patients. This study aims to investigate the relationship between 24-hour urine volume and 28-day intensive care unit (ICU) mortality in septic patients and exploring the dose-response relationship between these variables.
Methods: This retrospective cohort study analyzed data from 7,218 sepsis patients in the eICU Collaborative Research Database. Logistic regression models and generalized additive models were used to examine the relationship between 24-hour urine volume and 28-day ICU mortality.
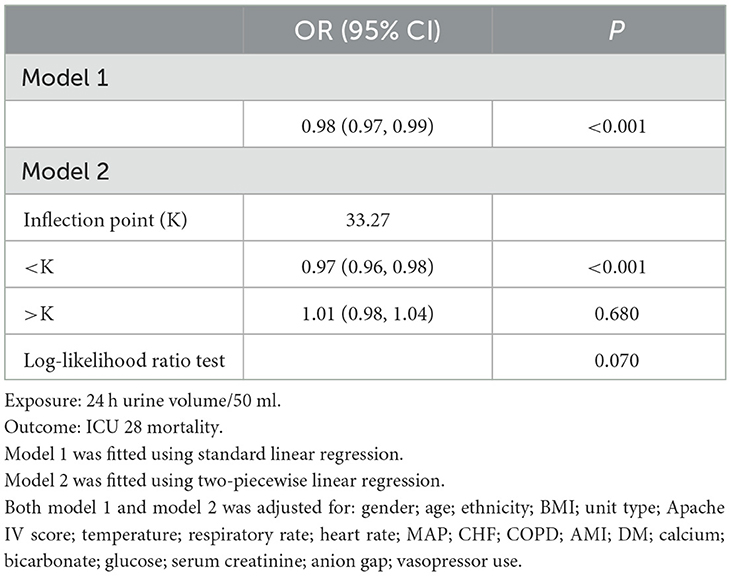
Results: A negative correlation was found between 24-hour urine volume and ICU 28-day mortality. In the fully adjusted model, each 50 mL increase in 24-hour urine volume significantly reduced mortality risk by 1% (OR = 0.99, 95% CI = 0.98–0.99, P < 0.001). A nonlinear dose-response relationship was observed, with an inflection point at ~1,663.5 ml. Below this threshold, increased urine volume was significantly associated with reduced mortality risk (OR = 0.97, 95% CI: 0.96–0.98, P < 0.001), while above this point, the relationship was not statistically significant.
Conclusion: This study demonstrates a non-linear negative correlation between 24-hour urine volume and 28-day ICU mortality in sepsis patients.
Introduction
Sepsis is defined as a dysregulated host response to infection that results in life-threatening organ dysfunction (1). In the United States, the cost of hospitalization due to sepsis is significant, with expenses exceeding $23 billion (6.2%) in 2013 (2). Despite the considerable healthcare resources consumed, the mortality rate from sepsis has not significantly improved in recent years (3).
The literature indicates that the incidence of acute kidney injury (AKI) in sepsis patients ranges from 25 to 75% (4). According to the definition by the Acute Disease and Quality Initiative (ADQI) Group, Sepsis-associated acute kidney injury (SA-AKI) is a heterogeneous syndrome resulting from direct mechanisms related to infection or the host's response to infection, as well as indirect mechanisms caused by adverse effects of sepsis or its treatments (5, 6). SA-AKI has been shown to have a worse prognosis compared to patients with other conditions in the intensive care unit (ICU) (5). It is associated with prolonged ICU and hospital stays, higher mortality rates, increased hospitalization costs, greater long-term disability, and reduced quality of life across different populations (7, 8). Therefore, it is crucial to identify sepsis patients at risk of developing SA-AKI early and provide timely interventions to improve patient outcomes.
The 24-hour urine volume and serum creatinine levels are the most commonly used clinical indicators for assessing AKI and are also key factors in evaluating patient prognosis (7). However, in patients with sepsis, reduced urine volume has greater sensitivity and specificity than serum creatinine levels (9). This is because, in sepsis, decreased muscle perfusion leads to reduced creatine production, slowing the increase in serum creatinine levels and limiting its effectiveness in early AKI assessment (10).
However, to date, there have been few studies examining the relationship between 24-hour urine volume and mortality. Avila, Maria O. N., and colleagues (11) followed 879 patients with acute kidney injury and used logistic regression analysis to explore the association between 24-hour urine volume and mortality. The study found a negative correlation between urine volume and mortality risk. However, it is worth noting that due to the limitations of generalized linear models, potential non-linear relationships between variables may not be detected. Although various clinical scoring systems, such as the Quick Sequential Organ Failure Assessment (qSOFA) (12) and the New York Sepsis Severity Score (13), as well as serum biomarkers including procalcitonin, erythropoietin, and pentraxin-3, are effective for early prognosis assessment in sepsis patients (14), most primary care hospitals lack the capability to measure these biomarkers. In contrast, 24-hour urine volume is a common and widely applicable indicator. Investigating the relationship between 24-hour urine volume and mortality can help clinicians better understand its clinical significance. Therefore, this study aims to analyze the association between 24-hour urine volume and ICU mortality in sepsis patients using the eICU database and explore any potential dose-response relationship.
Methods
Data source
This study is a multicenter retrospective cohort analysis. The data were sourced from the eICU remote monitoring system developed by Philips Healthcare and established in collaboration with the eICU Research Institute (eRI) and The Laboratory for Computational Physiology at the Massachusetts Institute of Technology. The eICU Collaborative Research Database (eICU-CRD) comprises de-identified health data from over 200,000 ICU admissions of ~140,000 unique patients in the United States between 2014 and 2015 (15). The database includes vital signs measurements, care plan documents, disease severity assessments, and diagnostic and treatment information collected through the Philips eICU system. To ensure patient confidentiality and eliminate the need for additional informed consent, all patient information used in this study has been de-identified. The data were accessed and extracted in accordance with the data usage protocol of the PhysioNet Review Committee, following examination and certification (our record ID: 40859994). Access to this database was granted upon registration with eICU, thus no ethical review or patient consent documents from the author's institution were required.
Patient selection
This study population comprised all patients admitted with a diagnosis of sepsis. Patients were identified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for sepsis, and only those with a primary diagnosis were included. Sepsis was diagnosed based on the presence of a suspected or confirmed infection along with a rapid increase of more than two points in the Sequential Organ Failure Assessment (SOFA) score, which is derived from the Acute Physiology and Chronic Health Evaluation (APACHE) IV scale (16, 17).
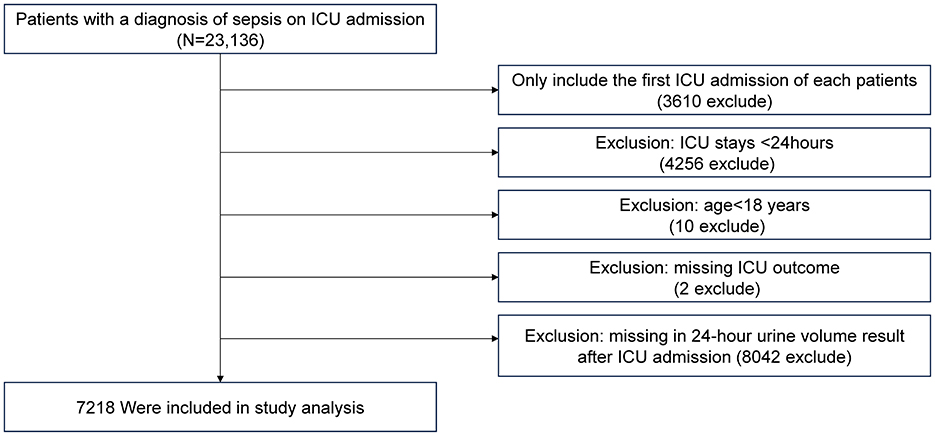
Exclusion criteria were as follows: (1) records of patients not admitted to the ICU for the first time; (2) patients with an ICU stay of <24 h; (3) patients younger than 18 years; (4) patients with indeterminate survival status; (5) patients lacking 24-hour urine volume data after ICU admission.
Study variables
The eICU database encompasses a wide array of patient information, including basic demographic data, physiological monitoring parameters from bedside monitors, diseases diagnosed according to ICD-9-CM codes, and laboratory results collected during routine medical care.
We collected data from the first 24 h after ICU admission. Baseline characteristics, including age, gender, race, and body mass index (BMI). Vital signs data within 24 h of ICU admission included temperature, respiratory rate, heart rate, and mean arterial pressure (MAP). Pre-admission comorbidities included diabetes, liver failure, cirrhosis, and metastatic cancer. The treatment interventions within 24 h of ICU admission included intubation, mechanical ventilation, dialysis, and vasopressor use. The first laboratory measurements within 24 h after ICU admission, including serum albumin, serum creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total cholesterol (TC), were obtained from the relevant laboratory records. Upon ICU admission, a series of severity scores were recorded, including the SOFA score, APACHE IV score, Glasgow Coma Scale (GCS) score, and Acute Physiology Score III.
The exposure variable in this study, 24-hour urine volume, was the total amount of urine produced during the first 24 h after ICU admission. Based on the study by Avila et al. (11), we categorized the population into five groups: anuria (urine volume ≤ 50 mL/24 h), oliguria (urine volume >50 mL/24 h and <400 mL/24 h), and non-oliguric groups further divided into ≥400 mL/24 h and ≤ 1,000 mL/24 h, >1,000 mL/24 h and ≤ 2,000 mL/24 h (Group 4), and >2,000 mL/24 h (Group 5).
Outcome
The outcome of this study was 28-day mortality after ICU admission.
Statistical analysis
Continuous variables were described using the mean ± SD or median with interquartile range (IQR). Categorical variables were presented as counts and percentages. One-way analysis of variance was used to assess differences in normally distributed continuous variables among different urine volume groups. The Kruskal-Wallis test was used to assess differences in non-normally distributed continuous variables among these groups. Chi-square tests or Fisher's exact tests were employed to evaluate differences in categorical variables. A multivariate logistic regression model was employed to estimate the correlation between 24-hour urine volume and 28-day mortality. The results are expressed as OR with their 95% confidence intervals (95% CI). Following the STROBE guidelines, we presented the results of multivariate regression analyses, including unadjusted, minimally adjusted, and fully adjusted models (18). Covariate adjustment was guided by clinical significance, with adjustments made if the matched odds ratio (OR) changed by at least 10% (19).
A generalized additive model (GAM) with curve fitting was used to explore the dose-response relationship between 24-hour urine volume and 28-day mortality. Subsequently, a two-piecewise linear regression model was employed to investigate the threshold effect of 24-hour urine volume on mortality. When a clear non-linear relationship between 24-hour urine volume and 28-day mortality was observed on the smoothed curve, the software automatically calculated the inflection point using a recursive method after adjusting for potential confounders. Furthermore, a log-likelihood ratio test was conducted to compare the generalized linear model with the two-part linear model. The statistical analyses of this study were conducted using the R software, version 4.2.0 (R Foundation), and the EmpowerStats software (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). The level of statistical significance was set at P < 0.05.
Results
Baseline demographic and clinical characteristics
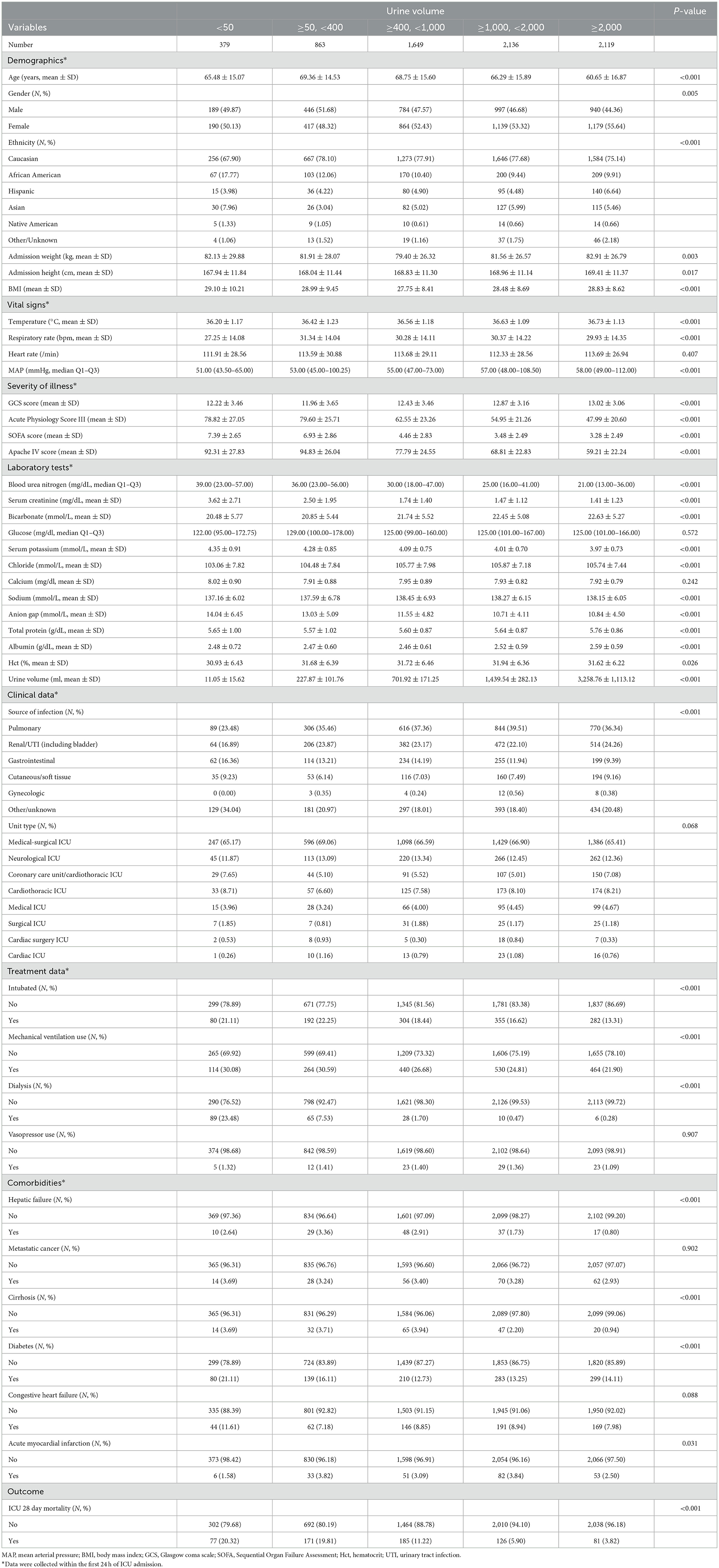
The inclusion and exclusion criteria for the study population are illustrated in Figure 1. A total of 7,218 patients were included in the study, with baseline characteristics summarized in Table 1. The data show that patients with lower 24-hour urine volume tend to be older, have a lower proportion of females (P = 0.005), and have lower body temperature and mean arterial pressure (P < 0.001). These patients also had higher severity scores (APACHE IV, SOFA, Acute Physiology Score III), worse lab results such as kidney function (elevated BUN, creatinine, and anion gap), and electrolyte imbalances (potassium, chloride, sodium). The 28-day ICU mortality rate was 20.32% in the <50 ml/24 h urine group, compared to 3.82% in the highest urine volume group. Patients with lower urine volume required more interventions, including mechanical ventilation, intubation, and dialysis. Comorbidities such as liver failure, cirrhosis, acute myocardial infarction, and diabetes were more common in the low urine volume group. There were no statistically significant differences in ICU type among the groups (P = 0.068).
Univariate regression analysis of baseline variables and 28-day ICU mortality
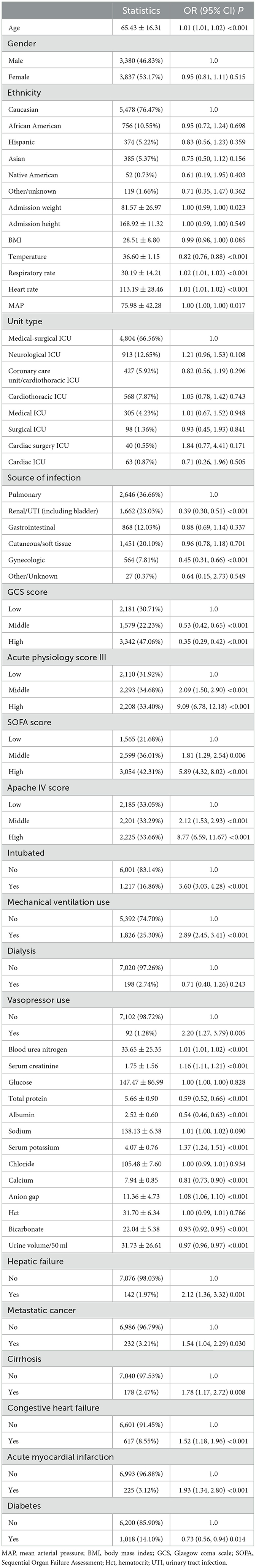
The results of the univariate regression analysis are presented in Table 2. Increased age, elevated respiratory rate, elevated heart rate, higher disease severity scores (APACHE IV, SOFA, Acute Physiology Score III), intubation, mechanical ventilation, use of vasopressors, elevated blood urea nitrogen, serum creatinine, serum potassium, and anion gap were all positively correlated with increased mortality (all P < 0.001). Additionally, comorbidities at admission, such as liver failure, metastatic cancer, cirrhosis, congestive heart failure, and acute myocardial infarction, were also positively associated with mortality risk (all P < 0.05). In contrast, body temperature, GCS score, urine volume, calcium, bicarbonate, total protein, albumin levels, and a history of diabetes were negatively correlated with mortality (all P < 0.001). Compared to pulmonary infections, mortality was lower in patients with renal/urinary tract infections and gynecological infections (P < 0.001).
Results of 24-hour urine volume and ICU hospitalization death in different regression models
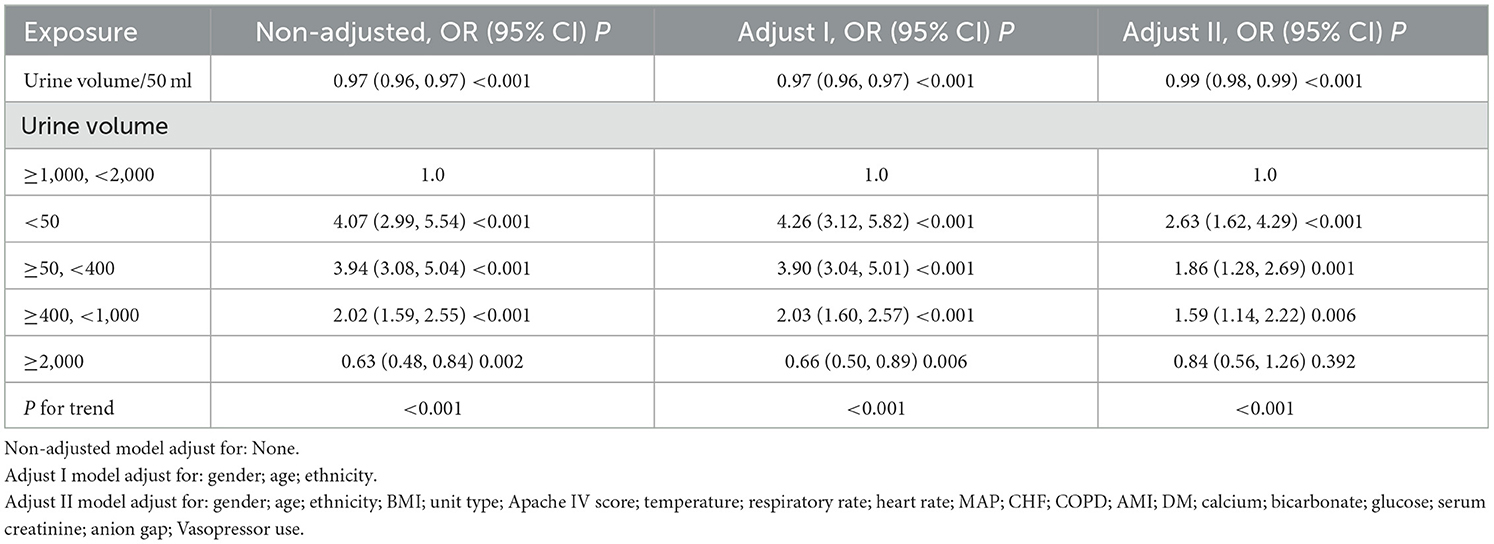
The relationship between 24-hour urine volume and ICU mortality across different adjustment strategies in the logistic regression models is presented in Table 3. The regression analysis results indicate that urine volume is negatively associated with mortality risk across all models: unadjusted, minimally adjusted, and fully adjusted. In the fully adjusted model (adjusted for age; ethnicity; BMI; unit type; Apache IV score; temperature; respiratory rate; heart rate; MAP; CHF; COPD; AMI; DM; calcium; bicarbonate; glucose; serum creatinine; anion gap; Vasopressor use), each 50 mL increase in 24-hour urine volume significantly reduced mortality risk by 1% (OR = 0.99, 95% CI = 0.98–0.99, P < 0.001). Compared to the reference group with normal urine volume (1,000–2,000 mL), the group with urine volume <50 mL had the highest mortality risk (OR = 2.63, 95% CI: 1.62–4.29, P < 0.001), while the group with urine volume ≥2,000 mL had a lower mortality risk (OR = 0.84, 95% CI: 0.56–1.26). A significant trend was observed in the fully adjusted model (P < 0.001).
The dose-response relationship between 24-hour urine volume and 28-day mortality
Using a fully adjusted model, we applied GAM and curve fitting to examine the non-linear relationship between 24-hour urine volume upon ICU admission and 28-day mortality in the ICU (Figure 2). As shown in the figure, the 28-day mortality rate in the ICU decreased with increasing 24-hour urine volume. However, this negative relationship weakened after reaching a certain level. We then used a two-piecewise linear regression model to analyze the potential dose-response relationship between the two variables (Table 4). In Model II, the calculated inflection point was 1,663.5 (33.27*50). On the left side of this inflection point, there was a significant association between 24-hour urine volume and reduced mortality risk (OR = 0.97, 95% CI 0.96–0.98, P < 0.001). On the right side of the inflection point, the relationship between the two variables was not statistically significant (OR = 1.01, 95% CI 0.98–1.04, P = 0.680).
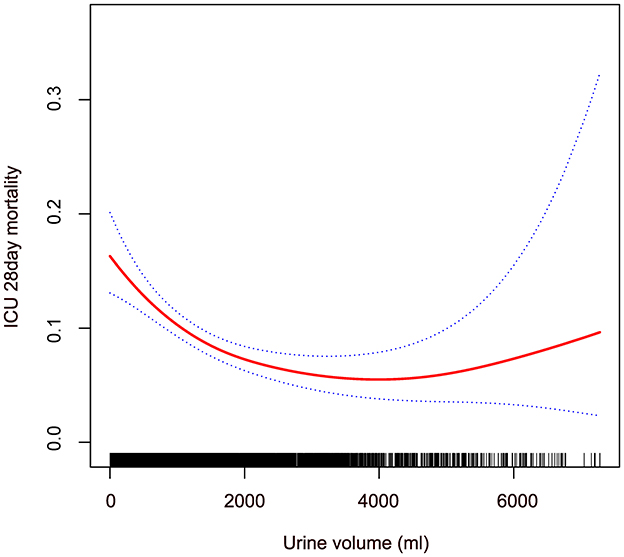
Figure 2. Non-linear relationship between 24-hour urine volume and 28-day mortality in patients with sepsis in ICU. The red line shows the smooth curve between the variables. The blue line represents the 95% confidence interval of the fit. Adjusting gender; age; ethnicity; BMI; unit type; Apache IV score; temperature; respiratory rate; heart rate; MAP; CHF; COPD; AMI; DM; calcium; bicarbonate; glucose; serum creatinine; anion gap; vasopressor use.
Discussion
One advantage of this study is the use of a multicenter cohort from a publicly available database, which includes data from 7,218 ICU sepsis patients. Our findings indicate a negative correlation between urine output in the first 24 h after ICU admission and the 28-day mortality rate, with a non-linear relationship observed between the two. Patients with low urine volume typically exhibit more severe clinical characteristics, including older age, lower body temperature and mean arterial pressure, and higher disease severity scores (APACHE IV, SOFA, and Acute Physiology Score III). The GAM revealed a non-linear relationship between urine volume and mortality. Using a piecewise linear regression model, we found that before reaching an approximate urine volume of 1,663.5 ml (33.27*50 ml), increased urine volume was significantly associated with reduced mortality risk. However, beyond this threshold, the relationship was no longer significant.
To date, there is limited research on the correlation between urine volume and mortality. A study by Avila et al. (11) found a significant negative correlation between urine volume and mortality in patients with acute kidney injury, which is consistent with our findings. Additionally, in their study, multivariate regression results show that patients with anuria and oliguria have increased mortality risk by 95% (OR = 1.95, 95% CI: 1.06–3.60, P = 0.032) and 76% (OR = 1.76, 95% CI: 1.02–3.03, P = 0.042), respectively. Patients with urine volume >1,000 mL/24 h have a 50–70% reduced mortality risk, and every 100 mL increase in urine volume is associated with a 7.2% decrease in mortality risk (P < 0.001). Hakemi et al. (20) investigated the relationship between baseline urine volume and mortality in 1,472 outpatient peritoneal dialysis patients. Using a Cox proportional hazards regression model, this study also found that patients with a baseline urine volume ≥1,000 mL/day had higher survival rates compared to those with <250 mL/day. Shafi et al. (21) conducted a multicenter cohort study including 734 hemodialysis patients from 81 clinics, examining the relationship between residual kidney function and mortality. This study also used a Cox proportional hazards regression model and found that hemodialysis patients with urine volume (defined as the ability to produce at least 250 mL of urine per day) had a 30% lower risk of all-cause mortality (HR = 0.70, 95% CI: 0.52–0.93). The results of these studies confirm that reduced urine volume is a risk factor for poor outcomes. The advantage of our study is that it extends these findings by identifying a non-linear (L-shaped curve) relationship between 24-hour urine volume and ICU mortality in septic patients, with an inflection point at 1,663.5. Our findings can help clinicians better understand the relationship between 24-hour urine volume and mortality in septic patients, based on existing theoretical knowledge.
Currently, there are two known mechanisms leading to poor prognosis in septic patients: inflammation (22) and microcirculatory dysfunction (23). In septic patients, dysregulation of the inflammatory response leads to organ dysfunction and poor prognosis. Inflammatory mediators are released into the vascular lumen, where they bind to membrane receptors on immune cells, initiating downstream signaling cascades that stimulate the synthesis and release of pro-inflammatory factors, exacerbating the patient's condition. Similar membrane-bound receptors are present in renal tubular epithelial cells (TECs), primarily TLR2, and TLR4. When inflammatory mediators pass through the glomeruli or adjacent peritubular capillaries, proximal TECs also exhibit increased oxidative stress, reactive oxygen species production, and mitochondrial damage (24, 25). Additionally, microthrombi formation and capillary blockage caused by endothelial damage, autonomic nervous system responses, glycocalyx shedding, and activation of the coagulation cascade lead to microcirculatory dysfunction, prolonging TEC exposure to activated circulating inflammatory mediators (24, 26). TECs can also inactivate adjacent cells by initiating paracrine signaling (27), and there is increased infiltration of inflammatory cells in the glomerular and peritubular regions (28). These factors lead to progressive deterioration of renal function and consequently reduced urine volume.
It is well-known that the volume of urine produced by the kidneys is closely related to the patient's systemic circulation, metabolism, and prognosis (29). Research has shown that in hemodialysis patients, those with residual urinary function can effectively avoid fluid overload and its complications, including left ventricular hypertrophy and uncontrolled hypertension (30). Considering the variability in baseline kidney function, we used serum creatinine levels at ICU admission as a proxy for baseline kidney function and adjusted for this variable in the multivariate regression model to account for differences in kidney function. Additionally, studies have demonstrated that vasopressor medications can significantly improve survival rates in septic patients and reduce the likelihood of renal replacement therapy (31, 32). Therefore, we also adjusted for the use of vasopressors as a covariate in the regression analysis.
This study has several limitations. First, our study focuses on the association between urine volume during the first 24 h of ICU admission and 28-day ICU mortality in septic patients. However, the evidence provided by urine volume on the 1st day is still limited. More data are needed to supplement the assessment of how changes in urine volume relate to disease prognosis. For example, considering urine volume on the 2nd day of admission or the relationship between urine volume changes and 28-day mortality would be beneficial. Additionally, due to limitations of the database, we could only extract the ICU 28-day survival outcomes for septic patients. Therefore, our conclusions are applicable only within this time frame, and further research is needed to extend the applicability of these findings. Furthermore, as an observational study, this research is subject to baseline confounding factors. Therefore, we adjusted for various confounding variables to ensure the consistency of the results. For instance, the use of vasopressors may impact disease prognosis, so we included their use as a covariate and found consistent results before and after adjustment. This indicates that after controlling for this confounding factor, the core findings of our study remain unchanged. Our study population consists of patients diagnosed with sepsis upon ICU admission, which limits the generalizability of our findings to this specific group. Future research should explore the relationship between 24-hour urine volume and 28-day ICU mortality in different populations to expand the evidence base.
Conclusion
This study found a negative correlation between 24-hour urine volume and 28-day mortality in septic patients, with a non-linear dose-response relationship.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://physionet.org/content/eicu-crd/2.0/.
Ethics statement
Ethical approval was not required for the studies involving humans because, the data were sourced from the eICU remote monitoring system developed by Philips Healthcare and established in collaboration with the eICU Research Institute (eRI) and the Laboratory for Computational Physiology at the Massachusetts Institute of Technology (LCP-MIT). Access to this database was granted upon registration with eICU, thus no ethical review or patient consent documents from the author's institution were required. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements because, to ensure patient confidentiality and eliminate the need for additional informed consent, all patient information used in this study has been de-identified.
Author contributions
YL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. WL: Conceptualization, Data curation, Formal analysis, Writing – review & editing. CF: Conceptualization, Data curation, Formal analysis, Writing – review & editing. RS: Conceptualization, Data curation, Formal analysis, Writing – review & editing. WH: Conceptualization, Investigation, Software, Supervision, Writing – review & editing. XC: Formal analysis, Investigation, Methodology, Project administration, Writing – review & editing. SY: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank the patients for participating in this study.
Conflict of interest
XC was employed by X&Y Solutions Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ICU, intensive care unit; AKI, acute kidney injury; eICU-CRD, eICU Collaborative Research Database; ADQI, acute disease and quality initiative; SA-AKI, sepsis-associated acute kidney injury; ICD-9-CM, the international classification of diseases, ninth revision, clinical modification; SOFA, sequential organ failure assessment; APACHE IV, acute physiology and chronic health evaluation IV; BMI, body mass index; MAP, mean arterial pressure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TC, total cholesterol; GCS, glasgow coma scale; SD, standard deviation; IQR, interquartile range; OR, odds ratios; CI, confidence intervals; GAM, generalized additive model; BUN, blood urea nitrogen; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; AMI, acute myocardial infarction; DM, diabetes mellitus; TEC, tubular epithelial cell.
References
1. Cajander S, Kox M, Scicluna BP, Weigand MA, Mora RA, Flohé SB, et al. Profiling the dysregulated immune response in sepsis: overcoming challenges to achieve the goal of precision medicine. Lancet Respir Med. (2024) 12:305–22. doi: 10.1016/S2213-2600(23)00330-2
2. Torio Cm Fau-Moore BJ, Moore BJ. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer. BTI—Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US) (2013).
3. Luhr R, Cao Y, Söderquist B, Cajander S. Trends in sepsis mortality over time in randomised sepsis trials: a systematic literature review and meta-analysis of mortality in the control arm, 2002-2016. Crit Care. (2019) 23:241. doi: 10.1186/s13054-019-2528-0
4. Pais T, Jorge S, Lopes JA. Acute kidney injury in sepsis. Int J Mol Sci. (2024) 25:115924. doi: 10.3390/ijms25115924
5. Zarbock A, Nadim MK, Pickkers P, Gomez H, Bell S, Joannidis M, et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat Rev Nephrol. (2023) 19:401–17. doi: 10.1038/s41581-023-00683-3
6. Kounatidis D, Vallianou NG, Psallida S, Panagopoulos F, Margellou E, Tsilingiris D, et al. Sepsis-associated acute kidney injury: where are we now? Medicina. (2024) 60:30434. doi: 10.3390/medicina60030434
7. Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. (2019) 96:1083–99. doi: 10.1016/j.kint.2019.05.026
8. Stanski NL, Cvijanovich NZ, Fitzgerald JC, Bigham MT, Wong HR. Severe acute kidney injury is independently associated with mortality in children with septic shock. Intensive Care Med. (2020) 46:1050–1. doi: 10.1007/s00134-020-05940-8
9. Leedahl DD, Frazee EN, Schramm GE, Dierkhising RA, Bergstralh EJ, Chawla LS, et al. Derivation of urine output thresholds that identify a very high risk of AKI in patients with septic shock. Clin J Am Soc Nephrol. (2014) 9:1168–74. doi: 10.2215/CJN.09360913
10. Li J, Zhu M, Yan L. Predictive models of sepsis-associated acute kidney injury based on machine learning: a scoping review. Ren Fail. (2024) 46:2380748. doi: 10.1080/0886022X.2024.2380748
11. Avila MON, Zanetta DMT, Abdulkader RCRM, Yu L, Burdmann EA. Urine volume in acute kidney injury: how much is enough? Ren Fail. (2009) 31:884–90. doi: 10.3109/08860220903216089
12. Fernando SM, Tran A, Taljaard M, Cheng W, Rochwerg B, Seely AJE, et al. Prognostic accuracy of the quick sequential organ failure assessment for mortality in patients with suspected infection: a systematic review and meta-analysis. Ann Intern Med. (2018) 168:266–75. doi: 10.7326/M17-2820
13. Sathaporn N, Khwannimit B. Validation the performance of New York Sepsis Severity Score compared with sepsis severity score in predicting hospital mortality among sepsis patients. J Crit Care. (2019) 53:155–61. doi: 10.1016/j.jcrc.2019.06.017
14. Li Y, She Y, Fu L, Zhou R, Xiang W, Luo L. Association between red cell distribution width and hospital mortality in patients with sepsis. J Int Med Res. (2021) 49:3000605211004221. doi: 10.1177/03000605211004221
15. Pollard T, Johnson A, Raffa J, Celi L, Mark R, Badawi OJSd. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Nature. (2018) 5:180178. doi: 10.1038/sdata.2018.178
16. Singer M, Deutschman C, Seymour C, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock. J. Am. Med. Assoc. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
17. Zimmerman J, Kramer A, McNair D, Malila FJC-cm. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med. (2006) 34:1297–310. doi: 10.1097/01.CCM.0000215112.84523.F0
18. Liu Y, Zhao P, Cheng M, Yu L, Cheng Z, Fan L, et al. AST to ALT ratio and arterial stiffness in non-fatty liver Japanese population: a secondary analysis based on a cross-sectional study. Lipids Health Dis. (2018) 17:275. doi: 10.1186/s12944-018-0920-4
19. Lei CY, Xie JY, Ran QB, Zhang MX. Non-linear relationship between age and subfoveal choroidal thickness in Chinese patients with proliferative diabetic retinopathy. World J Diabetes. (2024) 15:1903–15. doi: 10.4239/wjd.v15.i9.1903
20. Hakemi MS, Najafi I, Nassiri AA, Alatab S, Saddadi F, Soleymanian T, et al. Association of overtime urine volume and ultrafiltration changes with patient survival in continuous ambulatory peritoneal dialysis patients. Ren Fail. (2012) 34:1223–8. doi: 10.3109/0886022X.2012.723552
21. Shafi T, Jaar BG, Plantinga LC, Fink NE, Sadler JH, Parekh RS, et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kid Dis. (2010) 56:348–58. doi: 10.1053/j.ajkd.2010.03.020
22. Moskowitz A, Berg KM, Grossestreuer AV, Balaji L, Liu X, Cocchi MN, et al. Thiamine for Renal Protection in Septic Shock (TRPSS): a randomized, placebo-controlled, clinical trial. Am J Respir Crit Care Med. (2023) 208:570–8. doi: 10.1164/rccm.202301-0034OC
23. De Backer D, Hajjar L, Monnet X. Vasoconstriction in septic shock. Intensive Care Med. (2024) 50:459–62. doi: 10.1007/s00134-024-07332-8
24. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. (2003) 348:138–50. doi: 10.1056/NEJMra021333
25. Tang F, Zhao XL, Xu LY, Zhang JN, Ao H, Peng C. Endothelial dysfunction: pathophysiology and therapeutic targets for sepsis-induced multiple organ dysfunction syndrome. Biomed Pharmacother. (2024) 178:117180. doi: 10.1016/j.biopha.2024.117180
26. Raia L, Zafrani L. Endothelial activation and microcirculatory disorders in sepsis. Front Med. (2022) 9:907992. doi: 10.3389/fmed.2022.907992
27. Lerolle N, Nochy D, Guérot E, Bruneval P, Fagon JY, Diehl JL, et al. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med. (2010) 36:471–8. doi: 10.1007/s00134-009-1723-x
28. Aslan A, van den Heuvel MC, Stegeman CA, Popa ER, Leliveld AM, Molema G, et al. Kidney histopathology in lethal human sepsis. Crit Care. (2018) 22:359. doi: 10.1186/s13054-018-2287-3
29. Lengton R, van der Willik EM, de Rooij ENM, Meuleman Y, Le Cessie S, Michels WM, et al. Effect of residual kidney function and dialysis adequacy on chronic pruritus in dialysis patients. Nephrol Dial Transplant. (2023) 38:1508–18. doi: 10.1093/ndt/gfac341
30. Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. (2009) 119:671–9. doi: 10.1161/CIRCULATIONAHA.108.807362
31. Gordon AC, Mason AJ, Thirunavukkarasu N, Perkins GD, Cecconi M, Cepkova M, et al. Effect of early vasopressin vs. norepinephrine on kidney failure in patients with septic shock: the VANISH randomized clinical trial. J Am Med Assoc. (2016) 316:509–18. doi: 10.1001/jama.2016.10485
Keywords: dose-response relationship, intensive care unit, mortality, sepsis, urine volume
Citation: Lin Y, Lin W, Fu C, Sun R, Hong W, Chen X and Yan S (2024) Association between 24-hour urine volume and 28-day intensive care unit mortality in sepsis patients: a multi-center retrospective cohort study. Front. Med. 11:1486232. doi: 10.3389/fmed.2024.1486232
Received: 25 August 2024; Accepted: 11 November 2024;
Published: 26 November 2024.
Edited by:
Marcos Ferreira Minicucci, São Paulo State University, BrazilReviewed by:
Dimitris Kounatidis, Laiko General Hospital of Athens, GreeceGeorgia Damoraki, National and Kapodistrian University of Athens, Greece
Copyright © 2024 Lin, Lin, Fu, Sun, Hong, Chen and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuzhan Lin, MTAxNjA5OTA0N0BxcS5jb20=; Shaorong Yan, NTU0MzQ2MTA3QHFxLmNvbQ==
†These authors have contributed equally to this work
 Yuzhan Lin
Yuzhan Lin Weiguo Lin
Weiguo Lin Cheng Fu3
Cheng Fu3 Xinglin Chen
Xinglin Chen