Abstract
Introduction:
Sepsis is a substantial global health challenge with a considerable disease burden. Despite advancements in sepsis research, the mortality rates associated with this condition remain high. The relationship between the serum albumin-to-creatinine ratio (sACR) and mortality in patients with sepsis remains unclear. Therefore, this study aimed to investigate the association between the sACR and 28-day mortality in intensive care unit (ICU) patients with sepsis.
Methods:
In this retrospective cohort study, we used data sourced from the eICU Collaborative Research Database. The primary exposure variable was sACR, and the primary outcome measure was mortality within 28 days after ICU admission. Statistical analyses included univariate and multivariate logistic regression models, generalized additive models, and two-piecewise linear regression models, which were employed to explore non-linear relationships and threshold effects between sACR and mortality.
Results:
The study cohort comprised 9,690 ICU patients with sepsis, with a 28-day mortality rate of 9.99%. The results of the multivariate logistic regression model indicated that elevated sACR levels were significantly associated with a reduced risk of mortality (odds ratio = 0.78, 95% confidence interval: 0.71–0.87, p < 0.001), even after adjusting for potential confounding variables. Curve fitting revealed a non-linear relationship between sACR and 28-day mortality, with an inflection point of 4.79.
Discussion:
This study demonstrated that sACR is an independent risk factor for 28-day mortality in ICU patients with sepsis, exhibiting a non-linear negative dose–response relationship and a threshold effect. These findings may serve as early warning indicators in high-risk populations.
1 Introduction
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection (1), and its global incidence is increasing. It is estimated that more than 30 million cases of sepsis occur worldwide each year, accounting for approximately one-quarter of all in-hospital deaths (2–4). Despite medical advancements, the hospital mortality rate for sepsis remains unacceptably high at 20%, escalating to more than 40% in cases of septic shock (2, 3, 5). These figures underscore the substantial threat that sepsis poses to global public health and highlight the urgent need for more effective treatments and in-depth research.
Serum albumin levels are closely associated with sepsis severity and prognosis (6, 7). Studies have demonstrated that hypoalbuminemia is associated with illness severity and an increased risk of mortality in patients with sepsis (8). Furthermore, the prevalence of acute kidney injury is high among patients with sepsis, and creatinine, a crucial indicator for assessing kidney function, plays a pivotal role in diagnosing sepsis-associated acute kidney injury (9). A multicenter observational study indicated that elevated creatinine levels were closely related to poor prognosis in patients with sepsis (10). In particular, kidney function affects serum albumin levels (11).
In light of the intricate interrelationships among sepsis, hypoalbuminemia, and kidney dysfunction, the incorporation of serum albumin and creatinine into sACR may offer a more comprehensive evaluation of alterations in sepsis. However, studies on sACR are limited. The limited findings that are currently available suggest that sACR less than the threshold of 3.75 is independently associated with an increased 1-year all-cause mortality risk. However, it should be noted that the study population was limited to intensive care unit (ICU) patients with heart failure (11). In addition, in a study of patients with acute myocardial infarction (AMI), Liu et al. found that sACR at admission was an independent predictor of all-cause and cardiac mortality, further emphasizing the value of sACR in the prognostic assessment of cardiac diseases (12). However, the relationship between sACR and mortality in ICU patients with sepsis remains unclear and requires further investigation. To address this research gap, we conducted a study using the U.S. eICU Collaborative Research Database (eICU-CRD), with the aim of exploring the association between sACR and 28-day mortality in ICU patients with sepsis.
2 Methods
2.1 Data source and ethics statements
In this retrospective cohort study, the data were sourced from eICU-CRD, an online international database in the United States (13). The data were accessed and extracted in compliance with the data usage protocol of the PhysioNet review committee, following examination and certification (record ID: 40859994). The database, jointly established by the eICU Institute and Massachusetts Institute of Technology’s Laboratory for Computational Physiology, is a vast public data resource encompassing more than 200,000 ICU patient records from 208 medical institutions in the United States between 2014 and 2015.
To ensure the protection of patient information and eliminate the need for additional informed consent from patients, all patient information involved in the study was de-identified. This study was conducted in accordance with the principles of the Declaration of Helsinki (14).
2.2 Study population
The study population comprised all patients hospitalized for sepsis. Sepsis was diagnosed based on the presence of a suspected or confirmed infection in conjunction with a rapid increase of more than 2 points in the Sequential Organ Failure Assessment (SOFA) score, derived from the Acute Physiology and Chronic Health Evaluation (APACHE) IV scale (4, 15). Infection identification was based on the International Classification of Diseases, Ninth Edition (ICD-9) codes recorded in the eICU-CRD. The following exclusion criteria were applied during the patient selection process: (1) patients not entering the ICU for the first time, (2) patients with ICU stays of less than 24 h, (3) patients younger than 18 years of age, (4) patients lacking ICU treatment outcome information, and (5) patients lacking sACR data or system recording errors.
2.3 Main study variables
The eICU database encompasses a wide array of patient information, including basic demographic data; physiological monitoring parameters from bedside monitors; diseases diagnosed according to the ICD-9, Clinical Modification (ICD-9-CM) codes; and other laboratory results collected during routine medical care. The research team concentrated their efforts on gathering data from the initial 24-h period following the patient’s admission. Vital sign data, including body temperature, respiratory rate, heart rate, and mean arterial pressure (MAP), were extracted from the Apache Aps Var table. Baseline patient characteristics, including age, sex, race, and body mass index (BMI), were collected from the patient and patient results tables. Comorbidities, including diabetes, liver failure, cirrhosis, and metastatic cancer, were identified using the APACHE IV scoring system. Relevant information was extracted from diagnostic tables. Laboratory indicators, including serum albumin, serum creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total cholesterol, were extracted from the laboratory table using SQL codes. We used the time of measurement (lab result offset) to select the first recorded values within 24 h of ICU admission as baseline indicators for analysis. The severity of the patient’s condition at admission was assessed using a range of scoring systems, including the SOFA, APACHE IV, Glasgow Coma Scale (GCS), and Acute Physiology Score III.
2.4 Outcome
The study outcome was the mortality rate within 28 days of ICU admission.
2.5 Statistical analysis
The patients were divided into four distinct cohorts, with each cohort corresponding to a quartile of sACR. Subsequently, a comprehensive description of the characteristics of each cohort was provided. Continuous variables that exhibited a normal distribution are expressed as mean ± standard deviation. By contrast, continuous variables that did not adhere to a normal distribution are expressed as median with interquartile range. One-way analysis of variance was used to compare normally distributed continuous variables across groups, whereas the non-parametric Kruskal–Wallis test was used for continuous variables that were not normally distributed. Numerical representations of categorical data are presented as count (N) and relative frequency (percentage). Statistical analyses of group disparities were conducted using the chi-square test.
Univariate and multivariate logistic regression models were used to estimate the correlation between sACR and 28-day mortality. The results are expressed as odds ratio (OR) with a 95% confidence interval (95% CI). The principle of adjusting covariates is determined based on clinical significance and change in the matched OR by at least 10% (16). The following covariates were adjusted for age, sex, BMI, ethnicity, temperature, respiratory rate, heart rate, MAP, blood urea nitrogen level, calcium level, AST level, ALT level, platelet count, hemoglobin level, white blood cell count, GCS score, SOFA score, chronic obstructive pulmonary disease, congestive heart failure, acute myocardial infarction, diabetes, pneumonia, hepatic failure, metastatic cancer, intubation status, mechanical ventilation use, and dialysis.
A generalized additive model (GAM) was used to investigate the dose–response relationship between sACR and 28-day mortality. Subsequently, a two-piecewise linear regression model was employed to investigate the threshold effect of sACR on mortality. When a clear relationship between sACR and 28-day mortality was observed on the smooth curve, software automatically calculated the inflection point using a recursive method after adjusting for potential confounders. Furthermore, a log-likelihood ratio test was conducted to compare univariate and two-piece linear regression models. Considering the heterogeneity of the population, we employed stratified linear regression models for subgroup analyses across different baseline indicators and presented the results as part of our sensitivity analysis. Statistical analyses were conducted using R software (version 4.2.0; R Foundation) and EmpowerStats software1 (X&Y Solutions, Inc., Boston, MA), and the level of statistical significance was set at a p-value of <0.05.
3 Results
3.1 Participants’ characteristics
A flowchart illustrating the study’s inclusion and exclusion criteria is shown in Figure 1. Table 1 presents a comprehensive account of the baseline characteristics of the 9,690 participants, including demographic data, vital signs, laboratory results, infection sites, comorbidities, and disease severity. The participants were divided into quartiles based on their sACR levels: Q1 (0.08–0.96), Q2 (0.96–1.74), Q3 (1.74–2.84), and Q4 (2.84–7.71). The group with lower sACR values exhibited a higher proportion of female patients (Q1: 1,355, 55.97%; Q2: 1,277, 52.73%; Q3: 1,306, 53.94%; Q4: 1,036, 42.72%) and patients with higher BMI (29.98 ± 9.26 kg/m2), along with significant increases in indicators of liver and kidney function impairment, including serum creatinine (Q1: 3.83, 2.93–5.41), ALT (Q1: 29.00, 17.00–70.00), and AST levels (Q1: 45.00, 23.00–119.25). Furthermore, these patients demonstrated markedly elevated Acute Physiology Score III, SOFA score, and APACHE IV score. The 28-day ICU mortality rates differed significantly among the groups (p < 0.001), with the number of deaths in each group as follows: Q1, 391 (16.15%); Q2, 283 (11.68%); Q3, 190 (7.85%); and Q4, 104 (4.29%).
Figure 1

Flow chart of the subject inclusion.
Table 1
| Characteristic | sACR (quartiles) | ||||
|---|---|---|---|---|---|
| Q1(0.08-0.96) | Q2(0.96-1.74) | Q3(1.74-2.84) | Q4(2.84-7.71) | p-value | |
| No. of participants | 2421 | 2423 | 2421 | 2425 | |
| Demographics | |||||
| Age (years) | 65.51 ± 14.69 | 68.76 ± 14.67 | 67.24 ± 15.52 | 61.25 ± 17.23 | <0.001 |
| Gender (N, %) | <0.001 | ||||
| Male | 1066 (44.03) | 1145 (47.27) | 1115 (46.06) | 1389 (57.28) | |
| Female | 1355 (55.97) | 1277 (52.73) | 1306 (53.94) | 1036 (42.72) | |
| Ethnicity (N, %) | <0.001 | ||||
| Caucasian | 1728 (71.73) | 1920 (79.77) | 1947 (81.06) | 1890 (78.59) | |
| African American | 353 (14.65) | 226 (9.39) | 193 (8.03) | 178 (7.40) | |
| Hispanic | 135 (5.60) | 106 (4.40) | 122 (5.08) | 162 (6.74) | |
| Asian | 108 (4.48) | 93 (3.86) | 80 (3.33) | 86 (3.58) | |
| Native American | 36 (1.49) | 15 (0.62) | 22 (0.92) | 31 (1.29) | |
| Other/Unknown | 49 (2.03) | 47 (1.95) | 38 (1.58) | 58 (2.41) | |
| BMI (kg/m2) | 29.98 ± 9.26 | 29.60 ± 9.34 | 28.58 ± 8.44 | 27.54 ± 8.16 | <0.001 |
| Vital signs | |||||
| Temperature (°C) | 36.35 ± 1.31 | 36.52 ± 1.35 | 36.67 ± 1.29 | 36.68 ± 1.12 | <0.001 |
| Respiratory rate (bpm) | 29.07 ± 14.34 | 30.64 ± 14.21 | 30.74 ± 14.15 | 31.11 ± 14.53 | <0.001 |
| Heart rate (/min) | 110.83 ± 29.08 | 111.27 ± 30.10 | 113.79 ± 28.72 | 114.09 ± 27.57 | <0.001 |
| MAP (mmHg) | 74.63 ± 45.73 | 76.14 ± 44.72 | 75.24 ± 40.52 | 80.67 ± 40.89 | <0.001 |
| Laboratory data | |||||
| Lactate (mmol/l) | 2.10 (1.30-3.70) | 2.20 (1.40-3.60) | 1.80 (1.20-2.80) | 1.50 (1.00-2.30) | <0.001* |
| Triglycerides (mg/dl) | 155.00 (109.00-219.00) | 124.00 (86.00-192.00) | 114.00 (75.00-170.00) | 99.50 (65.75-144.00) | <0.001* |
| Total cholesterol (mg/dl) | 98.50 (80.25-121.75) | 102.00 (85.00-136.00) | 103.50 (81.00-137.00) | 112.00 (91.00-151.00) | 0.009* |
| Albumin (g/dl) | 2.28 ± 0.62 | 2.42 ± 0.57 | 2.52 ± 0.56 | 2.75 ± 0.57 | <0.001 |
| Serum creatinine (mg/dl) | 3.83 (2.93-5.41) | 1.90 (1.54-2.30) | 1.17 (0.90-1.40) | 0.69 (0.54-0.82) | <0.001* |
| Sodium (mmol/l) | 137.16 ± 6.93 | 138.45 ± 6.59 | 138.80 ± 5.93 | 138.20 ± 5.75 | <0.001 |
| Calcium (mg/dl) | 7.80 ± 1.00 | 7.87 ± 0.89 | 7.94 ± 0.80 | 8.07 ± 0.78 | <0.001 |
| Troponin I (ng/ml) | 0.18 (0.07-0.81) | 0.16 (0.06-0.69) | 0.14 (0.04-0.71) | 0.10 (0.04-0.39) | <0.001* |
| BNP (pg/ml) | 1010.60 (372.00-3730.50) | 828.00 (388.00-2148.50) | 559.00 (215.00-1917.00) | 332.00 (144.00-1055.00) | <0.001* |
| Blood urea nitrogen (mg/dl) | 54.00 (39.00-76.00) | 37.00 (27.00-50.00) | 24.00 (17.00-33.00) | 15.00 (10.00-21.00) | <0.001* |
| PT – INR | 1.60 (1.30-2.30) | 1.53 (1.30-2.20) | 1.40 (1.20-2.00) | 1.30 (1.10-1.70) | <0.001* |
| Platelets (x 109/l) | 168.00 (106.00-246.00) | 170.00 (111.00-248.00) | 176.00 (120.00-243.00) | 199.00 (142.00-264.00) | <0.001* |
| Hemoglobin (g/dl) | 9.92 ± 2.08 | 10.24 ± 2.15 | 10.52 ± 2.11 | 10.80 ± 2.16 | <0.001 |
| White blood cell count (×109/l) | 14.50 (9.30-21.60) | 14.07 (9.00-20.54) | 12.80 (8.50-18.40) | 12.20 (8.20-17.50) | <0.001* |
| ESR (mm/hr) | 55.50 (30.00-89.25) | 53.00 (19.00-83.50) | 51.50 (27.50-89.75) | 34.00 (17.00-63.50) | 0.017* |
| CRP (mg/dl) | 21.10 (10.40-31.10) | 23.35 (13.54-280.70) | 18.30 (9.60-30.25) | 17.16 (8.93-263.40) | 0.149* |
| ALT, U/L | 29.00 (17.00-70.00) | 30.00 (17.00-64.00) | 27.00 (17.00-50.00) | 25.00 (15.50-46.00) | <0.001* |
| AST, U/L | 45.00 (23.00-119.25) | 42.00 (23.00-99.00) | 34.00 (21.00-65.00) | 29.00 (18.00-53.00) | <0.001* |
| Severity of illness | |||||
| Acute Physiology Score III | 68.00 (55.00-88.00) | 62.00 (49.00-78.00) | 51.00 (40.00-66.00) | 44.00 (34.00-59.00) | <0.001* |
| SOFA score | 7.00 (5.00-9.00) | 5.00 (3.00-7.00) | 4.00 (2.00-6.00) | 3.00 (1.00-4.00) | <0.001* |
| Apache IV score | 82.00 (68.00-102.00) | 78.00 (64.00-94.00) | 66.00 (53.00-83.00) | 57.00 (44.00-72.00) | <0.001* |
| GCS score | 12.11 ± 3.67 | 12.34 ± 3.51 | 12.54 ± 3.42 | 12.60 ± 3.34 | <0.001 |
| Sepsis source of infection (N, %) | <0.001 | ||||
| Pulmonary | 670 (27.67) | 818 (33.76) | 1021 (42.17) | 1133 (46.72) | |
| Renal/UTI (including bladder) | 582 (24.04) | 650 (26.83) | 501 (20.69) | 448 (18.47) | |
| Gastrointestinal | 408 (16.85) | 346 (14.28) | 337 (13.92) | 283 (11.67) | |
| Cutaneous/Soft Tissue | 230 (9.50) | 196 (8.09) | 160 (6.61) | 172 (7.09) | |
| Gynecologic | 5 (0.21) | 6 (0.25) | 6 (0.25) | 12 (0.49) | |
| Unknown/Other | 526 (21.73) | 407 (16.80) | 396 (16.36) | 377 (15.55) | |
| Comorbidities | |||||
| Hepatic failure (N, %) | 0.001 | ||||
| No | 2321 (96.71) | 2331 (96.76) | 2337 (97.70) | 2349 (98.24) | |
| Yes | 79 (3.29) | 78 (3.24) | 55 (2.30) | 42 (1.76) | |
| Metastatic cancer (N, %) | 0.137 | ||||
| No | 2332 (97.17) | 2311 (95.93) | 2308 (96.49) | 2305 (96.40) | |
| Yes | 68 (2.83) | 98 (4.07) | 84 (3.51) | 86 (3.60) | |
| Cirrhosis (N, %) | <0.001 | ||||
| No | 2289 (95.38) | 2304 (95.64) | 2315 (96.78) | 2337 (97.74) | |
| Yes | 111 (4.62) | 105 (4.36) | 77 (3.22) | 54 (2.26) | |
| Diabetes (N, %) | <0.001 | ||||
| No | 1602 (66.75) | 1746 (72.48) | 1820 (76.09) | 1947 (81.43) | |
| Yes | 798 (33.25) | 663 (27.52) | 572 (23.91) | 444 (18.57) | |
| COPD (N, %) | <0.001 | ||||
| No | 2284 (94.34) | 2233 (92.16) | 2217 (91.57) | 2211 (91.18) | |
| Yes | 137 (5.66) | 190 (7.84) | 204 (8.43) | 214 (8.82) | |
| CHF (N, %) | <0.001 | ||||
| No | 2227 (91.99) | 2167 (89.43) | 2211 (91.33) | 2286 (94.27) | |
| Yes | 194 (8.01) | 256 (10.57) | 210 (8.67) | 139 (5.73) | |
| AMI (N, %) | 0.070 | ||||
| No | 2328 (96.16) | 2332 (96.24) | 2347 (96.94) | 2360 (97.32) | |
| Yes | 93 (3.84) | 91 (3.76) | 74 (3.06) | 65 (2.68) | |
| Pneumonia (N, %) | <0.001 | ||||
| No | 1813 (74.89) | 1699 (70.12) | 1539 (63.57) | 1489 (61.40) | |
| Yes | 608 (25.11) | 724 (29.88) | 882 (36.43) | 936 (38.60) | |
| Acute renal failure (N, %) | <0.001 | ||||
| No | 1609 (66.46) | 1755 (72.43) | 2123 (87.69) | 2362 (97.40) | |
| Yes | 812 (33.54) | 668 (27.57) | 298 (12.31) | 63 (2.60) | |
| Others | |||||
| Intubated (N, %) | <0.001 | ||||
| No | 1855 (77.29) | 1927 (79.99) | 1911 (79.89) | 1989 (83.19) | |
| Yes | 545 (22.71) | 482 (20.01) | 481 (20.11) | 402 (16.81) | |
| Mechanical ventilation use (N, %) | 0.348 | ||||
| No | 1675 (69.79) | 1680 (69.74) | 1669 (69.77) | 1715 (71.73) | |
| Yes | 725 (30.21) | 729 (30.26) | 723 (30.23) | 676 (28.27) | |
| Dialysis (N, %) | <0.001 | ||||
| No | 1933 (80.54) | 2349 (97.51) | 2382 (99.58) | 2386 (99.79) | |
| Yes | 467 (19.46) | 60 (2.49) | 10 (0.42) | 5 (0.21) | |
| ICU 28-day mortality (N, %) | <0.001 | ||||
| No | 2030 (83.85) | 2140 (88.32) | 2231 (92.15) | 2321 (95.71) | |
| Yes | 391 (16.15) | 283 (11.68) | 190 (7.85) | 104 (4.29) | |
Baseline Characteristics of participants (N =9690).
Data are given as mean ± SD, median (interquartile range), or number (percentage).
P*P*: Kruskal Wallis test was used to compare non-normally distributed continuous variables between groups.
BMI, body mass index; bmp, beats per minute; MAP, mean arterial pressure; BNP, brain natriuretic peptide; PT, prothrombin time; INR, international normalized ratio; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GCS, Glasgow Coma Scale; SOFA, Sequential Organ Failure Assessment; UTI, urinary tract infection; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; AMI, acute myocardial infarction.
Table 2
| Covariate | Statistics | OR (95%CI) p-value |
|---|---|---|
| sACR | 2.03 ± 1.41 | 0.65 (0.61, 0.70) <0.0001 |
| Demographics | ||
| Gender | ||
| Male | 4,784 (48.88%) | Ref. |
| Female | 5,003 (51.12%) | 1.02 (0.89, 1.16) 0.78 |
| Age (years) | 28.87 ± 8.86 | 0.99 (0.98, 1.00) 0.005 |
| BMI (kg/m2) | 24.74 ± 3.12 | 0.98 (0.94, 1.02) 0.335 |
| Ethnicity | ||
| Caucasian | 7,549 (77.66%) | Ref. |
| African American | 964 (9.92%) | 1.00 (0.80, 1.26) 0.97 |
| Hispanic | 535 (5.50%) | 1.01 (0.75, 1.35) 0.95 |
| Asian | 372 (3.83%) | 0.92 (0.64, 1.33) 0.67 |
| Native American | 105 (1.08%) | 1.19 (0.65, 2.17) 0.58 |
| Other/Unknown | 196 (2.02%) | 1.34 (0.88, 2.06) 0.18 |
| Vital signs | ||
| Temperature (°C) | 36.56 ± 1.28 | 0.83 (0.79, 0.87) <0.0001 |
| Respiratory rate (bpm) | 30.41 ± 14.35 | 1.02 (1.01, 1.02) <0.0001 |
| Heart rate (/min) | 112.56 ± 28.90 | 1.01 (1.01, 1.01) <0.0001 |
| MAP (mmHg) | 76.73 ± 43.10 | 1.00 (1.00, 1.00) 0.09 |
| Laboratory data | ||
| Lactate (mmol/l) | 2.63 ± 2.44 | 1.26 (1.23, 1.29) <0.0001 |
| Triglycerides (mg/dl) | 175.32 ± 308.86 | 1.00 (1.00, 1.00) 0.66 |
| Total cholesterol (mg/dl) | 116.25 ± 53.62 | 0.99 (0.98, 1.00) 0.21 |
| Albumin (g/dl) | 2.50 ± 0.61 | 0.51 (0.46, 0.57) <0.0001 |
| Serum creatinine (mg/dl) | 2.09 ± 1.95 | 1.11 (1.08, 1.15) <0.0001 |
| Sodium (mmol/l) | 138.15 ± 6.35 | 1.01 (1.00, 1.02) 0.30 |
| Calcium (mg/dl) | 7.93 ± 0.88 | 0.85 (0.78, 0.92) <0.0001 |
| Troponin I (ng/ml) | 1.61 ± 6.36 | 1.01 (1.00, 1.03) 0.07 |
| BNP (pg/ml) | 3191.37 ± 9343.04 | 1.00 (1.00, 1.00) 0.09 |
| CRP (mg/dl) | 378.83 ± 791.60 | 1.00 (1.00, 1.00) 0.63 |
| Blood urea nitrogen (mg/dl) | 36.07 ± 26.41 | 1.01 (1.01, 1.01) <0.0001 |
| PT - INR | 1.94 ± 1.42 | 1.18 (1.13, 1.24) <0.0001 |
| Platelets (x 109/l) | 196.27 ± 113.23 | 1.00 (1.00, 1.00) <0.0001 |
| Hemoglobin (g/dl) | 10.37 ± 2.15 | 0.97 (0.94, 1.00) 0.08 |
| White blood cell count (×109/l) | 15.49 ± 12.19 | 1.01 (1.01, 1.01) <0.0001 |
| ALT, U/L | 115.52 ± 443.93 | 1.00 (1.00, 1.00) <0.0001 |
| AST, U/L | 186.44 ± 827.06 | 1.00 (1.00, 1.00) <0.0001 |
| Severity of illness | ||
| Acute physiology score III tertile | ||
| 4–46 | 2,710 (31.39%) | Ref. |
| 47–66 | 2,994 (34.68%) | 2.30 (1.77, 3.00) < 0.0001 |
| 67–177 | 2,930 (33.94%) | 8.77 (6.90, 11.14) < 0.0001 |
| SOFA score tertile | 678 (61.41) | |
| 0–2 | 2,554 (26.09%) | Ref. |
| 3–5 | 3,599 (36.77%) | 1.68 (1.32, 2.14) < 0.0001 |
| 6–21 | 3,635 (37.14%) | 5.17 (4.16, 6.42) < 0.0001 |
| Apache IV score tertile | ||
| 13–60 | 2,819 (32.65%) | Ref. |
| 61–81 | 2,875 (33.30%) | 2.70 (2.05, 3.55) < 0.0001 |
| 82–193 | 2,940 (34.05%) | 9.99 (7.81, 12.80) < 0.0001 |
| GCS score tertile | ||
| 3–11 | 2,812 (29.37%) | Ref. |
| 12–14 | 2,591 (27.06%) | 0.62 (0.53, 0.73) < 0.0001 |
| 15–15 | 4,173 (43.58%) | 0.40 (0.34, 0.47) < 0.0001 |
| Sepsis source of infection | ||
| Pulmonary | 3,692 (37.72%) | Ref. |
| Renal/UTI (including bladder) | 2,206 (22.54%) | 0.46 (0.37, 0.56) <0.0001 |
| GI | 1,380 (14.10%) | 1.10 (0.91, 1.33) 0.32 |
| Cutaneous/soft tissue | 767 (7.84%) | 0.54 (0.40, 0.72) <0.0001 |
| Other | 616 (6.29%) | 0.74 (0.55, 0.99) 0.04 |
| Gynecologic | 29 (0.30%) | 0.56 (0.13, 2.35) 0.43 |
| Unknown | 1,098 (11.22%) | 1.00 (0.81, 1.24) 0.99 |
| Comorbidities | ||
| Hepatic failure | ||
| No | 9,435 (97.37%) | Ref. |
| Yes | 255 (2.63%) | 2.75 (2.04, 3.72) <0.0001 |
| Metastatic cancer | ||
| No | 9,354 (96.53%) | Ref. |
| Yes | 336 (3.47%) | 1.81 (1.34, 2.43) <0.0001 |
| Cirrhosis | ||
| No | 9,341 (96.40%) | Ref. |
| Yes | 349 (3.60%) | 2.73 (2.10, 3.54) <0.0001 |
| Diabetes | ||
| No | 7,197 (74.27%) | Ref. |
| Yes | 2,493 (25.73%) | 0.78 (0.66, 0.92) 0.002 |
| COPD | ||
| No | 9,037 (92.33%) | Ref. |
| Yes | 751 (7.67%) | 0.91 (0.70, 1.18) 0.48 |
| CHF | ||
| No | 8,987 (91.82%) | Ref. |
| Yes | 801 (8.18%) | 1.26 (1.01, 1.58) 0.04 |
| AMI | ||
| No | 9,462 (96.67%) | Ref. |
| Yes | 326 (3.33%) | 1.32 (0.94, 1.84) 0.11 |
| Pneumonia | ||
| No | 6,592 (67.35%) | Ref. |
| Yes | 3,196 (32.65%) | 1.29 (1.12, 1.48) 0.0003 |
| Others | ||
| Intubated | ||
| No | 7,755 (80.03%) | Ref. |
| Yes | 1935 (19.97%) | 2.96 (2.57, 3.40) <0.0001 |
| Mechanical ventilation use | ||
| No | 6,794 (70.11%) | Ref. |
| Yes | 2,896 (29.89%) | 2.36 (2.07, 2.71) <0.0001 |
| Dialysis | ||
| No | 9,148 (94.41%) | Ref. |
| Yes | 542 (5.59%) | 1.13 (0.86, 1.49) 0.39 |
Univariate analysis for 28-day mortality.
Data are given as mean ± SD, median (interquartile range), or percentage.
CI, confidence interval; OR, odds ratio.
3.2 Relationship between sACR and 28-day mortality in the different models
Table 2 presents the various factors associated with sACR and 28-day mortality using a univariate logistic model. The analysis indicated that sACR levels were negatively correlated with the risk of 28-day mortality (OR = 0.65, 95% CI: 0.61–0.70, p < 0.0001). Regarding disease severity scores, an increase in the Acute Physiology Score III and SOFA score was significantly associated with an elevated risk of mortality. It is noteworthy that the group with a SOFA score of 6–21 points exhibited a significantly elevated risk of mortality compared with the lowest-scoring group (0–2 points) (OR = 5.17, 95% CI: 4.16–6.42, p < 0.0001). Furthermore, an increase in the APACHE IV score was significantly correlated with a marked increase in the risk of death. The highest-scoring group (82–193 points) exhibited a markedly elevated risk of mortality compared with the lowest-scoring group (13–60 points). The OR was 9.99 (95% CI: 7.81–12.80, p < 0.0001).
Table 3
| Exposure | Non-adjusted OR (95%CI) p-value | Model I OR (95%CI) p-value | Model II OR (95%CI) p-value |
|---|---|---|---|
| sACR | 0.65 (0.61, 0.70) <0.0001 | 0.65 (0.61, 0.69) <0.0001 | 0.78 (0.71, 0.87) <0.0001 |
| sACR (quartiles) | |||
| Q1 | Ref. | Ref. | Ref. |
| Q2 | 0.68 (0.58, 0.80) <0.0001 | 0.66 (0.56, 0.79) <0.0001 | 0.81 (0.64, 1.02) 0.07 |
| Q3 | 0.43 (0.36, 0.52) <0.0001 | 0.42 (0.34, 0.50) <0.0001 | 0.63 (0.48, 0.84) 0.002 |
| Q4 | 0.23 (0.19, 0.29) <0.0001 | 0.23 (0.18, 0.29) <0.0001 | 0.44 (0.31, 0.63) <0.0001 |
| p for trend | <0.0001 | <0.0001 | <0.0001 |
Relationship between sACR and 28-day mortality.
Non-adjusted model adjust for: None. Model I adjusted for age, gender, BMI, ethnicity.
Model II adjusted for age, gender, BMI, ethnicity, temperature, respiratory rate, heart rate, MAP, blood urea nitrogen, calcium, AST, ALT, platelets, hemoglobin, white blood cell count, GCS score, SOFA score, COPD, CHF, AMI, diabetes, pneumonia, hepatic failure, metastatic cancer, intubated, mechanical ventilation use, dialysis.
Table 3 presents the findings of the multivariate logistic regression model for sACR and 28-day mortality. In Model I, after adjusting for age, sex, BMI, and race, the inverse correlation between sACR and 28-day mortality remained significant (OR = 0.65, 95% CI: 0.61–0.69, p < 0.0001). Furthermore, in Model II, when controlling for various potential confounding factors, including vital signs, laboratory data, disease severity scores, comorbidities, and treatment modalities, the correlation between sACR and 28-day mortality was somewhat attenuated but remained statistically significant (OR = 0.78, 95% CI: 0.71–0.87, p < 0.0001). Moreover, quartile analysis of sACR demonstrated that, in comparison with the lowest quartile (Q1), the risk of death was significantly reduced in the other three quartiles (Q2, Q3, and Q4) in the unadjusted model. After adjustment for Models I and II, the mortality risk increased in Q2, Q3, and Q4 but remained significantly lower than that in Q1. The trend test yielded a statistically significant result in all models (p < 0.0001), indicating a significant dose–response relationship between sACR and 28-day mortality Table 4.
Table 4
| Per 1-unit increase | |
|---|---|
| Models | OR (95%CI) p-value |
| Model I | |
| One line effect | 0.78 (0.71, 0.87) <0.0001 |
| Model II | |
| Inflection point (K) | 4.79 |
| sACR < K | 0.75 (0.67, 0.84) <0.0001 |
| sACR > K | 1.19 (0.72, 1.96) 0.49 |
| p value for log likelihood ratio test | 0.13 |
Threshold effect analysis of sACR and 28-day mortality using a two- piecewise regression model.
Adjusted: age, gender, BMI, ethnicity, temperature, respiratory rate, heart rate, MAP, blood urea nitrogen, calcium, AST, ALT, platelets, hemoglobin, white blood cell count, GCS score, SOFA score, COPD, CHF, AMI, diabetes, pneumonia, hepatic failure, metastatic cancer, intubated, mechanical ventilation use, dialysis.
3.3 Results of the stratified analysis
Considering the heterogeneity of the population, we conducted stratified regression analyses for all baseline indicators; the results are presented in Supplementary Table S1. The results indicated that the direction of the effect size was consistent across various subgroups, including sex, age, presence of intubation, mechanical ventilation, diabetes, and renal failure.
3.4 Analysis of the non-linear relationship between sACR and 28-day mortality
We investigated the potential non-linear correlation between sACR and 28-day mortality in the entire cohort using GAM and smooth curve fitting while accounting for variables such as age, sex, BMI, race, vital signs, and disease severity scores (Figure 2). As illustrated, there was a decreasing trend in 28-day mortality in the ICU with an increase in sACR, indicating a non-linear correlation.
Figure 2
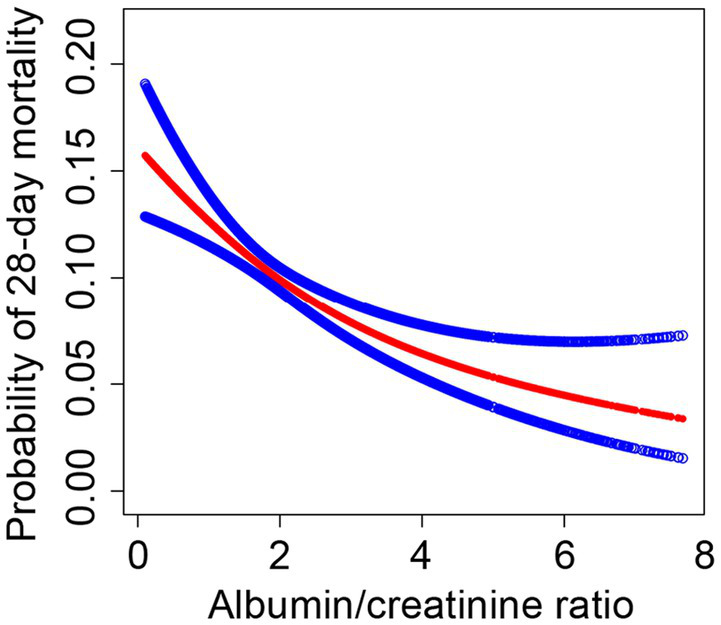
The association between the SACR and 28-day mortality in eICU patients with sepsis. A threshold, nonlinear association between SACR and 28-day mortality was found in a generalized additive model (GAM). Red line represents the smooth curve fit between variables. Blue lines represent the 95% of confidence interval from the fit. Adjusted for age, gender, BMI, ethnicity, temperature, respiratory rate, heart rate, MAP, blood urea nitrogen, calcium, AST, ALT, platelets, hemoglobin white blood cell count, GCS score, SOFA score, COPD, CHF, AMI, diabetes, pneumonia, hepatic failure, metastatic cancer, intubated, mechanical ventilation use, dialysis.
The threshold effect of sACR on 28-day mortality was analyzed using a two-piecewise linear regression model. Model I demonstrates that for each unit increase in sACR, the OR for 28-day mortality was 0.78 (95% CI: 0.71–0.87), with a p-value <0.0001, indicating a significant association between sACR and the reduced risk of death. Model II demonstrated a threshold effect when the sACR index was less than 4.79, wherein a significant association was observed between sACR and a reduced risk of death (OR = 0.75, 95% CI: 0.67–0.84, p < 0.0001). Conversely, above this threshold, the observed association was not statistically significant (OR = 1.19, 95% CI: 0.72–1.96, p = 0.49). Several covariates, including age, sex, and BMI, were adjusted for in the analysis. The results demonstrated a non-linear relationship between sACR and 28-day mortality rate, with a particularly pronounced association below a specific threshold.
4 Discussion
This retrospective cohort study identified a non-linear correlation between sACR and 28-day ICU mortality, with a threshold effect, within the eICU-CRD database comprising 208 different ICUs across the United States between 2014 and 2015. Thus, this study’s findings indicate that sACR is an independent predictor of 28-day mortality. To our best knowledge, this is the first study to report an association between sACR and 28-day mortality in ICU patients with sepsis.
sACR has also been identified as a novel biomarker (11). Prior research has demonstrated a strong correlation between sACR and unfavorable outcomes in cardiovascular diseases, with an increasing body of evidence emerging for its role in other diseases. For example, Li et al. reported that a lower sACR was significantly correlated with a higher risk of death in patients with heart failure (17). This finding remained significant in the multivariate analysis, indicating that sACR was an independent risk factor for mortality. Türkyılmaz et al. found that a decrease in sACR was associated with the deterioration of short-term clinical outcomes, including 30-day mortality and other cardiac events, in patients with ST-segment elevation myocardial infarction (18). Kong et al. further elucidated the clinical significance of sACR, demonstrating that in patients with ST-elevation myocardial infarction undergoing percutaneous coronary intervention, a low sACR was significantly associated with an increased risk of pulmonary infection and major adverse cardiovascular events during hospitalization. In addition, they established that sACR was an independent predictor of long-term all-cause mortality (19). These findings highlight the potential role of sACR in assessing the risk of complications after cardiac surgery. Lastly, Nseir et al. demonstrated that sACR values were predictive factors for 30-day all-cause mortality in patients with Clostridium difficile-associated diarrhea, indicating that sACR may also have potential applications in the prognostic assessment of non-cardiac diseases (20). On the basis of these findings, we postulated that sACR might also have important clinical value in the assessment of other diseases. To our best knowledge, no previous study has explored the sACR values that are associated with the lowest mortality risk in patients with sepsis. Our findings lend further support to the existing view as set forth in the literature.
Our investigation revealed a negative correlation between sACR and 28-day mortality rate in ICU patients with sepsis. In addition, we conducted stratified regression analyses on the basis of various baseline indicators to assess the effect of population heterogeneity on this relationship and found that the negative correlation remained stable. However, the relationship was not linear. The principal finding was that sACR exhibited a more pronounced correlation with 28-day mortality below the threshold of 4.79; however, the correlation was no longer statistically significant at levels exceeding this threshold. The insights derived from this study contribute a novel dimension to the existing literature, enhancing our understanding of the dose–response relationship between sACR and survival rates of patients with sepsis. They can provide clinicians with more precise guidance when assessing patient risks.
The precise mechanism underlying this study’s findings remains unclear. In light of previous clinical research, we postulated that the impact of sACR levels on the 28-day ICU mortality rate in patients with sepsis may be mediated through the following pathways. First, low sACR may indicate malnutrition, which can affect immune function and lead to multiorgan dysfunction, increasing the risk of death (21). Second, low sACR may indicate an inflammatory response that can hinder albumin synthesis and accelerate its metabolism, resulting in a decrease in plasma colloid osmotic pressure and insufficient blood circulation, which affects organ perfusion and function (22). Third, serum albumin plays a role in the protection of vascular endothelial cells through its antioxidant effects, which are exerted through the capture of reactive oxygen and nitrogen species by its thiol moiety as well as through the binding of metal ions and fatty acids. Consequently, oxidative stress-related vascular injuries may be exacerbated in patients with serum albumin deficiency (23, 24). Fourth, serum albumin deficiency may result in a reduction in the anticoagulant capacity of blood, thereby increasing the likelihood of clot formation. This hypercoagulable state may be associated with an elevated risk of mortality, particularly in critically ill patients (25, 26). Fifth, increased creatinine levels are markers of kidney injury, and the pathophysiological mechanisms of sepsis-associated acute kidney injury, including microvascular dysfunction, inflammation, and metabolic reprogramming, may lead to decreased kidney function and increased mortality (27). Further research is required to fully elucidate the pathophysiological importance of sACR.
The present study also has the following limitations. (1) As a retrospective cohort study, it was only able to establish associations rather than causality. Given the observational nature of our study, other unmeasured confounding factors may have influenced the results. Therefore, large-scale prospective studies should be conducted in the future to verify the findings presented here. (2) Serum albumin and creatinine levels were determined within the initial 24-h period following ICU admission. It is possible that these initial values do not fully reflect the changes in renal function or albumin levels that may occur during the ICU stay. (3) It is challenging to determine whether sepsis is the primary cause of ICU admission. However, it should be noted that ICU admissions are typically driven by critical conditions associated with multiple diseases, including sepsis. (4) The present study used data from the U.S. eICU database, which may limit the generalizability of its findings to other populations.
5 Conclusion
This study established that low sACR values are independently associated with an increased 28-day mortality rate in ICU patients with sepsis, exhibiting a non-linear dose–response relationship and a threshold effect. These findings may serve as early warning indicators in high-risk populations.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://physionet.org/content/eicu-crd/2.0/.
Ethics statement
Ethical approval was not required for the studies involving humans because the data was sourced from the eICU-CRD, an online international database in the United States. The data was accessed and extracted in compliance with the data usage protocol of the PhysioNet Review Committee, following examination and certification (our record ID: 40859994). So the study didn't need an ethical review. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements because in order to ensure the protection of patient information and to eliminate the need for additional informed consent from patients, all patient information involved in the study has been de-identified. So no informed consent is required for this study.
Author contributions
WL: Conceptualization, Data curation, Formal analysis, Validation, Writing – original draft, Writing – review & editing. CF: Conceptualization, Data curation, Supervision, Validation, Writing – review & editing. JM: Conceptualization, Data curation, Formal analysis, Writing – review & editing. WH: Conceptualization, Data curation, Formal analysis, Writing – review & editing. XC: Conceptualization, Data curation, Formal analysis, Writing – review & editing. SY: Conceptualization, Data curation, Project administration, Resources, Writing – review & editing. YL: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank the patients for their participation in this study.
Conflict of interest
XC was employed by X&Y Solutions Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1484370/full#supplementary-material
Footnotes
References
1.
EvansLRhodesAAlhazzaniWAntonelliMCoopersmithCMFrenchCet al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. (2021) 49:e1063–143. doi: 10.1097/CCM.0000000000005337
2.
LiuDRenBTianYChangZZouT. Association of the tyg index with prognosis in surgical intensive care patients: data from the mimic-iv. Cardiovasc Diabetol. (2024) 23:193. doi: 10.1186/s12933-024-02293-0
3.
LiangMXuYRenXHuangDJinMQiuZ. The U-shaped association between serum osmolality and 28-day mortality in patients with sepsis: a retrospective cohort study. Infection. (2024) 2024:2256. doi: 10.1007/s15010-024-02256-3
4.
SingerMDeutschmanCSSeymourCWShankar-HariMAnnaneDBauerMet al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
5.
FleischmannCScheragAAdhikariNKJHartogCSTsaganosTSchlattmannPet al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. (2016) 193:259–72. doi: 10.1164/rccm.201504-0781OC
6.
KlinkmannGWaterstradtKKlammtSSchnurrKScheweJ-CWasserkortRet al. Exploring albumin functionality assays: a pilot study on sepsis evaluation in intensive care medicine. Int J Mol Sci. (2023) 24:12551. doi: 10.3390/ijms241612551
7.
Arnau-BarrésIGüerri-FernándezRLuqueSSorliLVázquezOMirallesR. Serum albumin is a strong predictor of sepsis outcome in elderly patients. Eur J Clin Microbiol Infect Dis. (2019) 38:743–6. doi: 10.1007/s10096-019-03478-2
8.
QianSYLiuJ. Relationship between serum albumin level and prognosis in children with sepsis, severe sepsis or septic shock. Zhonghua Er Ke Za Zhi. (2012) 50:184–7. doi: 10.3760/cma.j.issn.0578-1310.2012.03.006
9.
BellomoRKellumJARoncoCWaldRMartenssonJMaidenMet al. Acute kidney injury in sepsis. Intensive Care Med. (2017) 43:816–28. doi: 10.1007/s00134-017-4755-7
10.
WhiteKCSerpa-NetoAHurfordRClementPLauplandKBSeeEet al. Sepsis-associated acute kidney injury in the intensive care unit: incidence, patient characteristics, timing, trajectory, treatment, and associated outcomes. A multicenter, observational study. Intensive Care Med. (2023) 49:1079–89. doi: 10.1007/s00134-023-07138-0
11.
WangJLiNMuYWangKFengG. Association between serum albumin creatinine ratio and all-cause mortality in intensive care unit patients with heart failure. Front Cardiovasc Med. (2024) 11:1406294. doi: 10.3389/fcvm.2024.1406294
12.
LiuHZhangJYuJLiDJiaYChengYet al. Prognostic value of serum albumin-to-creatinine ratio in patients with acute myocardial infarction: results from the retrospective evaluation of acute chest pain study. Medicine. (2020) 99:e22049. doi: 10.1097/MD.0000000000022049
13.
PollardTJJohnsonAEWRaffaJDCeliLAMarkRGBadawiO. The Eicu collaborative research database, a freely available multi-center database for critical care research. Sci Data. (2018) 5:180178. doi: 10.1038/sdata.2018.178
14.
World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
15.
ZimmermanJEKramerAAMcNairDSMalilaFM. Acute physiology and chronic health evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients (Apache). Crit Care Med. (2006) 34:1297–310. doi: 10.1097/01.CCM.0000215112.84523.F0
16.
KernanWNViscoliCMBrassLMBroderickJPBrottTFeldmannEet al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. (2000) 343:1826–32. doi: 10.1056/NEJM200012213432501
17.
LiSXieXZengXWangSLanJ. Association between serum albumin to serum creatinine ratio and mortality risk in patients with heart failure. Clin Transl Sci. (2023) 16:2345–55. doi: 10.1111/cts.13636
18.
TurkyilmazEOzkalayciFBirdalOKaragozATanbogaIHTanalpACet al. Serum albumin to creatinine ratio and short-term clinical outcomes in patients with St-elevation myocardial infarction. Angiology. (2022) 73:809–17. doi: 10.1177/00033197221089423
19.
KongSYuSHeWHeYChenWZhangYet al. Serum albumin-to-creatinine ratio: a novel predictor of pulmonary infection in patients with St-segment elevation myocardial infarction undergoing percutaneous coronary intervention. J Atheroscler Thromb. (2024) 18:64717. doi: 10.5551/jat.64717
20.
NseirWNjidatJAmaraAPeretzAKahatibHMariAet al. Serum albumin to creatinine ratio as predictor for 30-day all-cause mortality in patients with Clostridium Di!Cile-associated diarrhea. Ann Clin Lab Sci. (2021) 51:557–61. PMID:
21.
DaudMUllahFUzairMSiddiqASiddiqURiazFBet al. Malnutrition and its influence on sepsis outcomes in elderly patients. Cureus. (2024) 16:e63433. doi: 10.7759/cureus.63433
22.
SheinenzonAShehadehMMichelisRShaoulERonenO. Serum albumin levels and inflammation. Int J Biol Macromol. (2021) 184:857–62. doi: 10.1016/j.ijbiomac.2021.06.140
23.
AnrakuMChuangVTGMaruyamaTOtagiriM. Redox properties of serum albumin. Biochim Biophys Acta. (2013) 1830:5465–72. doi: 10.1016/j.bbagen.2013.04.036
24.
RocheMRondeauPSinghNRTarnusEBourdonE. The antioxidant properties of serum albumin. FEBS Lett. (2008) 582:1783–7. doi: 10.1016/j.febslet.2008.04.057
25.
LamFWCruzMALeungH-CEParikhKSSmithCWRumbautRE. Histone induced platelet aggregation is inhibited by normal albumin. Thromb Res. (2013) 132:69–76. doi: 10.1016/j.thromres.2013.04.018
26.
PaarMRossmannCNussholdCWagnerTSchlagenhaufALeschnikBet al. Anticoagulant action of low, physiologic, and high albumin levels in whole blood. PLoS One. (2017) 12:e0182997. doi: 10.1371/journal.pone.0182997
27.
PeerapornratanaSManrique-CaballeroCLGómezHKellumJA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. (2019) 96:1083–99. doi: 10.1016/j.kint.2019.05.026
Summary
Keywords
sepsis, serum albumin–creatinine ratio, sACR, 28-day mortality, intensive care unit, ICU, retrospective cohort study
Citation
Lin W, Fu C, Miao J, Hong W, Chen X, Yan S and Lin Y (2024) Association between the serum albumin–creatinine ratio and 28-day intensive care unit mortality among patients with sepsis: a multicenter retrospective cohort study. Front. Med. 11:1484370. doi: 10.3389/fmed.2024.1484370
Received
21 August 2024
Accepted
14 October 2024
Published
05 November 2024
Volume
11 - 2024
Edited by
Aruna Sharma, Uppsala University, Sweden
Reviewed by
Vlatka Sotošek, University of Rijeka, Croatia
Feng Shen, Affiliated Hospital of Guizhou Medical University, China
Updates
Copyright
© 2024 Lin, Fu, Miao, Hong, Chen, Yan and Lin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuzhan Lin, 1016099047@qq.com; Shaorong Yan, 554346107@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.