
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 09 January 2025
Sec. Hepatobiliary Diseases
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1483744
This article is part of the Research Topic Chronic Hepatitis B Management: Current Status and Future Directions View all 14 articles
Background: Sequential or combined treatment with nucleos(t)ide analogs (NAs) and pegylated interferon alpha-2b (Peg-IFN-α-2b) can improve the clinical cure rate. However, its clinical application is limited due to the adverse reactions associated with IFN.
Methods: A multi-center prospective observational study was conducted involving 59 NAs-treated chronic hepatitis B (CHB) patients who were treated with a combination therapy of NAs and Peg-IFN-α-2b for 48 weeks. Another 327 NAs-treated patients received NAs monotherapy for 48 weeks. At the end of the treatment, patients were classified into either the clinically cured group or the non-clinically cured group based on clinical efficacy. The study aimed to analyze the clinical cure rate and the predictive factors.
Results: After propensity score matching (PSM), a total of 104 patients were included in the exposure and the control groups. After 48 weeks of treatment, 13 patients in the exposed group achieved clinical cure, with a cure rate of 25%. In contrast, in the control group was 1.92%. The clinical cure rate was greater in the population with CHB or compensated cirrhosis treated with sequential or combined Peg-IFN-α-2b and NAs than in the control group (p < 0.001). Patients treated with Peg-IFN-α-2b were divided into a clinical cure group and a non-clinical cure group for single-factor regression and multi-factor binary logistic regression. The results showed that baseline qHBsAg [relative ratio (RR) = 0.997, 95%CI: [0.995, 0.999], p = 0.031] and △TBiL (RR = 0.698, 95%CI: [0.555, 0.879], p = 0.002) were independent influencing factors for achieving clinical cure in patients with CHB or compensated cirrhosis.
Conclusion: A lower baseline qHBsAg and decrease in TBiL at 24 weeks of treatment are independent influencing factors for achieving clinical cure. The lower the baseline qHBsAg and the higher the △TBiL levels after 24 weeks of treatment, the higher the probability of patients achieving clinical cure.
HBV infection is a serious public health issue associated with chronic hepatitis, liver cirrhosis, and HCC (1). According to the World Health Organization (WHO), the global prevalence of HBsAg in the general population is 3.8%, with approximately 296 million chronic carriers. Approximately 820,000 people die each year due to acute or chronic liver failure, cirrhosis, or HCC caused by HBV infection (2). Currently, Entecavir (ETV), tenofovir disoproxil fumarate (TDF), tenofovir alafenamide (TAF), and pegylated interferon-alpha-2b (Peg-IFN-α-2b) are the preferred first-line drugs for antiviral resistance (3, 4). However, the clearance rate of HBsAg after 5 years of ETV treatment in patients with chronic hepatitis B (CHB) is less than 5%, and the rate is also low (less than 7%) in patients receiving IFN monotherapy for more than 1 year (5). The Guidelines for the Prevention and Treatment of Chronic Hepatitis B in China (version 2022) proposed that the treatment goal for certain eligible “preferential populations” is to achieve a clinical cure (6). Compared to patients with persistent surface antigen positivity, those with surface antigen clearance have a lower probability of developing cirrhosis and HCC (7, 8). In recent years, with the increasing number of CHB patients treated with IFN, there has been a growing consensus that a clinical cure can be considered a treatment goal for certain advantaged populations (9–11).
In theory, nucleos(t)ide analogs (NAs) and interferons exert different antiviral effects on HBV, and their rational combination can produce synergistic and complementary effects (12–14). Although IFN is recommended as one of the first-line treatments in all guidelines, its use has been limited due to its subcutaneous injection requirement, poor tolerance, and significant side effects. Additionally, it is contraindicated in patients with decompensated liver function, immunosuppressed states, concomitant severe disease, or pregnancy. Consequently, the use of Peg-IFN-α-2b in the real world remains relatively limited. Moreover, many patients treated with IFN discontinue therapy due to intolerable adverse reactions, and 40–80% of patients still do not achieve surface antigen clearance after treatment completion (5). In summary, identifying accurate predictors of a clinical cure is very important. Currently, there are few studies on the use of interferon in compensated cirrhosis. Previous research has shown that compared to CHB, there is no statistically significant difference in clinical cure rate at the end of 48 weeks of treatment (p > 0.05) (15).
This study included 386 patients with CHB or compensated cirrhosis, analyzed follow-up data and compared clinical cure rates between the exposed and control groups; and analyzed predictive factors for achieving a clinical cure. The aim of this study was to provide additional information for achieving a clinical cure in patients with hepatitis B.
The study included a total of 386 patients with CHB or compensated cirrhosis who sought outpatient care at the Hepatology Department of either our hospital or an external hospital. The exposed group (sequential or add-on group) consisted of 59 patients who used a 1-year sequential or combined treatment with NAs and Peg-IFN-α-2b before September 2023. The control group (NAs group) comprised 327 CHB or compensated cirrhosis patients who sought medical attention at the Hepatology Department of our hospital from March 2023 to October 2023. The inclusion criteria were as follows: (1) diagnosis of CHB and compensated cirrhosis in accordance with the diagnostic criteria outlined in the Chinese guidelines for the prevention and treatment of CHB and cirrhosis; (2) age between 18 and 60 years; (3) CHB or compensated cirrhosis with HBV DNA (−), HBsAg ≤1,500 IU/mL, and HBeAg (−); (4) treatment with NAs for a duration of at least 1 year; (5) no IFN therapy within the past 2 years. (6) For patients receiving IFN in this study, the treatment duration should be a minimum of 1 year. The exclusion criteria were as follows: (1) history of allergy to the active ingredients, IFN-α, or any excipient in the drug; (2) autoimmune hepatitis or history of autoimmune disease, including immunosuppression in transplant recipients; (3) decompensated liver function; (4) uncontrolled thyroid disease; (5) severe mental illness or history of a severe mental disorder, particularly schizophrenia or depression; (6) pregnancy, potential pregnancy (female or a male partner), or lactation; (7) history of allergy to ribavirin or other NAs (such as acyclovir, ganciclovir, adenosine, etc.); (8) severe or unstable history of heart disease, including difficult-to-control cardiac conditions (myocardial infarction, heart failure, arrhythmias, etc.); (9) hemoglobinopathy (such as thalassemia, sickle cell anaemia, etc.); (10) severe renal dysfunction or a creatinine clearance rate < 50 mL/min; (11) white blood cell (WBC) count <3.0 × 109/L, absolute neutrophil count <1.5 × 109/L, hemoglobin (HGB) < 100 g/L, and platelet (PLT) count <90 × 109/L; or (12) another condition, such as coinfection with HIV or cytomegalovirus, hepatitis C, liver cancer, severe fatty liver, etc. The study received ethical approval.
This was a prospective cohort study in which clinical data were collected from patient follow-ups. To address the two research objectives, the study was grouped twice. The first grouping was based on antiviral treatment methods, categorizing patients into either sequential or combination treatment with NAs and interferon, or monotherapy with NAs. The second grouping was based on whether patients achieved clinical cure at 48 weeks of treatment, categorizing patients into either clinical cure group or non-clinical cure group. Sequential treatment was defined as discontinuing the original oral antiviral medication and switching to subcutaneous injections of Peg-IFN-α-2b at a dose of 180 μg per week. The combination treatment consisted of oral antiviral medication and subcutaneous injections of Peg-IFN-α-2b at a dose of 180 μg/week (Peginterferon alfa-2b Injection, Xiamen Tebao Company). The study endpoint was the clinical cure of patients with CHB or compensated cirrhosis who completed 48 weeks of treatment in two groups. The patients in the control group continued oral NA treatment until the completion of 48 weeks, and their clinical cure rate was assessed. The protocol for the research project had been approved by the constituted Ethics Committee of our hospital and is in line with the Declaration of Helsinki. All persons gave their informed consent prior to their inclusion in the study.
The general information collected included age, sex, type of oral NA, and other concurrent diseases. Laboratory tests, including routine blood tests, liver function tests, quantitative HBsAg, HBV DNA, five hepatitis B markers (HBsAg, HBsAb, HBeAg, HBeAb, and HBcAb), thyroid function tests, renal function tests, blood glucose levels, lipid profiles, creatine kinase levels, and isoenzymes, were conducted at baseline and after 24 and 48 weeks of treatment. The examinations, performed at baseline, 24 weeks, and 48 weeks after treatment, included abdominal ultrasound and electrocardiography. Samples were subsequently sent for testing of routine blood markers, liver, thyroid, and renal function, blood glucose, lipids, and creatine kinase and its isoenzymes to the clinical laboratory of our hospital. Samples for testing of five markers of hepatitis B, HBV DNA, HBsAg, and hepatitis B, were sent to the Hepatology Department Laboratory.
Quantitative variables are expressed as the mean (±SD) or median (IQR). Group comparisons were conducted using the t-test or Wilcoxon–Mann–Whitney test. Categorical variables are represented as rates or proportions, and group comparisons were performed using the chi-square test or Fisher’s exact probability test. Propensity score matching (PSM) was applied to match patients in the exposed and control groups in a 1:1 ratio with a matching tolerance of 0.02. The chi-squared test was used to validate whether there was a difference in the overall clinical cure rate between the two groups. Simple random sampling was used to extract 100 patients from the control group of 370 patients to generate a new dataset. Univariate analysis was also conducted to examine the relationships between all the independent variables and the dependent variable. Potentially meaningless variables were filtered out (to avoid overlooking important factors, p values may have been appropriately relaxed). The selected independent variables were included in a binary logistic regression model to calculate the relative ratio (RR) and 95% CI for each variable. Predictive factors related to clinical cure were identified. Moreover, we constructed a receiver operating characteristic (ROC) curve and calculated the AUC to assess the predictive value of the signature. The statistical analysis was conducted using SPSS software 25, and p < 0.05 was considered to indicate statistical significance.
The exposed group data showed no more than 10% missing values. Out of the 59 patients, only 5 had missing data, and the data for 54 patients were complete. In the control group, 3 variables had more than 10% missing values, 13 variables had missing values, and 8 variables were complete. Multiple imputation was applied to fill in the missing values.
This study included a total of 386 patients, and the baseline characteristics are presented in Table 1. In the exposed group, 54 patients received combination therapy, and 5 received sequential therapy. The predominant antiviral medications in the exposed group were ETV, TDF, and TAF, which accounted for 64.53, 15.60, and 14.98% of the patients, respectively (with 13.56% missing data). The proportions of patients with CHB and compensated cirrhosis were 93.23 and 6.77%, respectively. The study indicated that different types of oral antiviral drugs used in sequential or combination treatment regimens had no impact on treatment outcomes (16).
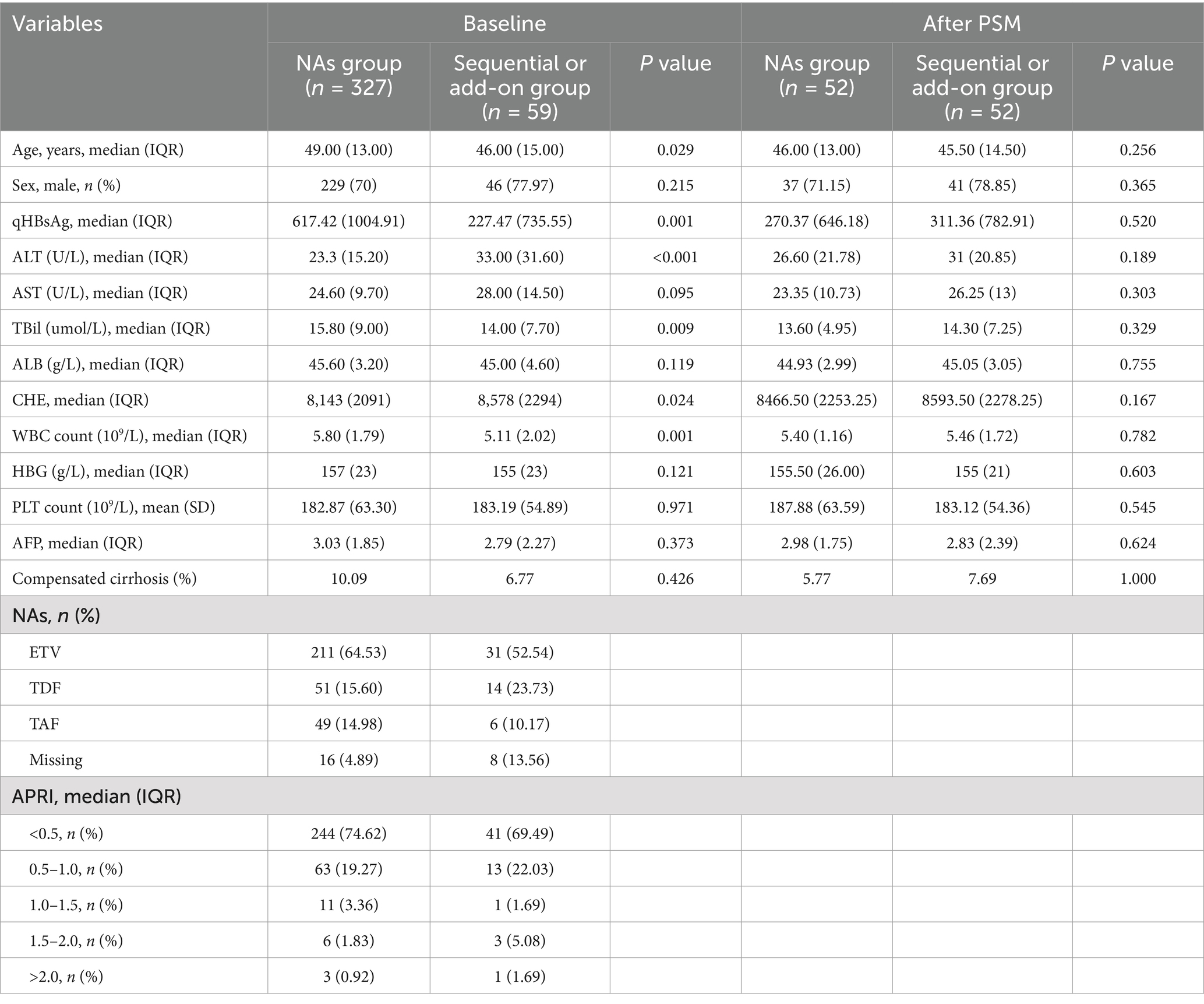
Table 1. Baseline characteristics of the patients in the exposed and control groups before and after PSM.
In the control group, the main oral antiviral drugs used were also ETV, TDF, and TAF, which accounted for 52.54, 23.73, and 10.17% of the patients, respectively (with 4.89% missing data). There were 294 CHB patients and 33 cirrhotic patients in the control group. Statistically significant differences were observed in age; qHBsAg, alanine transaminase (ALT), total bilirubin (TBil), cholinesterase (CHE), and WBC count between the two groups, so PSM was performed at a 1:1 ratio. After PSM, 52 patients in each group were successfully matched, for a total of 104 patients. There were no statistically significant differences in variables between the two groups after PSM, as shown in Table 1.
After PSM, 52 patients were included in both the exposed and control groups. In the exposed group, 13 patients achieved a clinical cure, resulting in a clinical cure rate of 25%. In the NA monotherapy group, only one patient achieved a clinical cure, yielding a clinical cure rate of 1.92%. For the “advantaged population” among patients with CHB or compensated cirrhosis, the clinical cure rate achieved with sequential or combined treatment with NAs and Peg-IFN-α-2b was greater than that of NA monotherapy (p < 0.001). The 13 patients in the exposed group who achieved a clinical cure had an average age of 45.17 (8.25) years, with male patients accounting for 78.85%. The median of baseline qHBsAg level was 311.36 (782.91) IU/ml.
At 24 weeks of treatment, the group receiving sequential or combined therapy with Peg-IFN-α-2b showed significant elevation in transaminases, along with decreases in WBC, HBG, and PLT. The changes in transaminases, WBC, HBG, and PLT in the sequential or combined therapy group compared to baseline were statistically significant (p < 0.001, Table 2; Figure 1), indicating that Peg-IFN-α-2b treatment can modulate the body’s immune function, leading to increased inflammation, thereby exerting antiviral effects. However, it also results in adverse reactions such as bone marrow suppression.
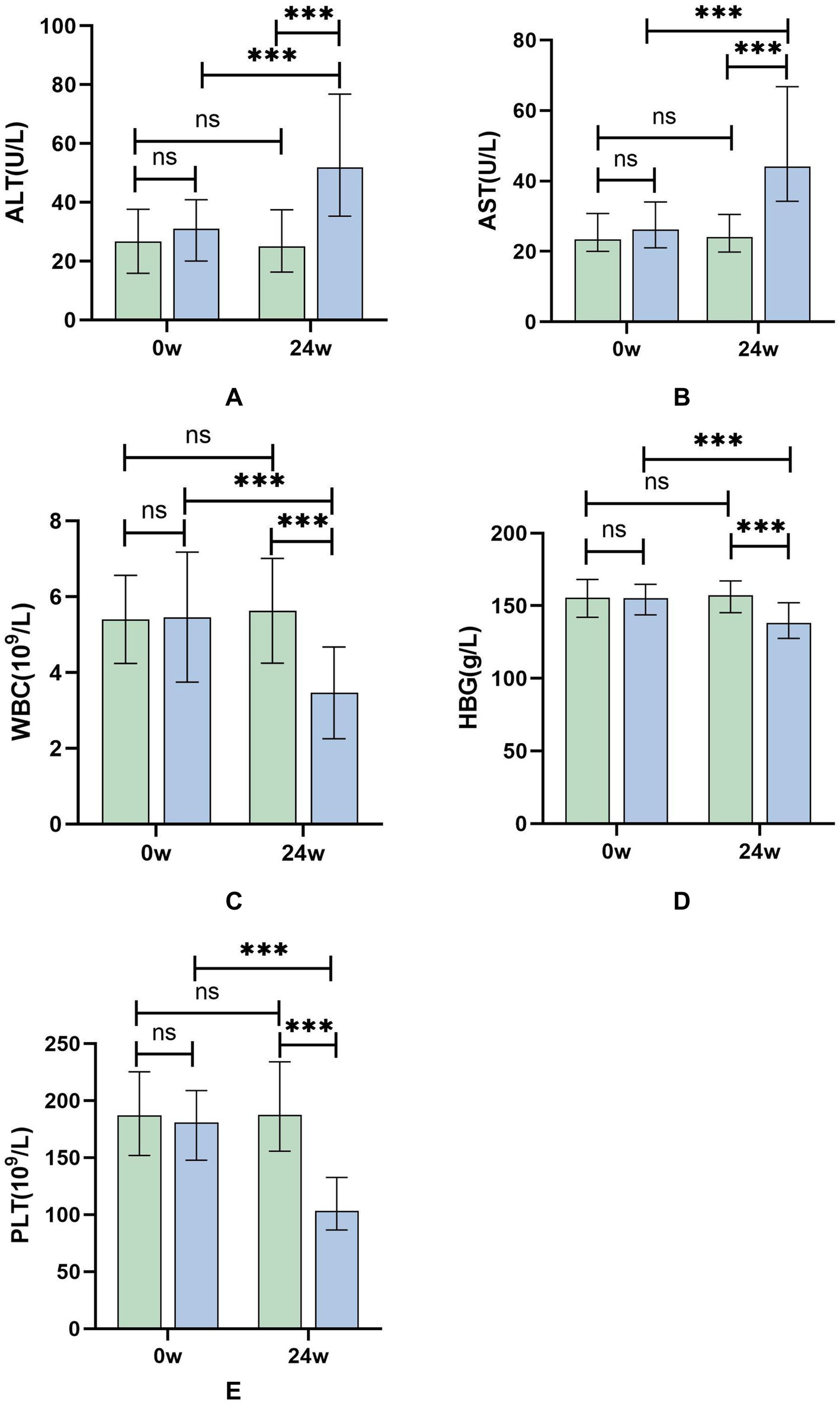
Figure 1. (A–E) Differential test results of ALT, AST, WBC, HBG, and PLT at 24 weeks of treatment for patients in the Peg-IFN-α-2b sequential or combined therapy group and the NAs group. Green: NAs group; Blue: sequential or combined therapy group. *p < 0.05, ***p < 0.001; ns, not significant.
The 386 patients were divided into a case group (cured group, 16 patients) and a control group (uncured group, 370 patients). First, to ensure more reliable regression results, a random sample of 100 patients was selected from the 370 patients in the control group. Univariate analysis was subsequently conducted to examine the relationships between all the independent variables and the dependent variable. Among the 23 variables included in the univariate analysis, 7 variables showed statistical significance: qHBsAg (p < 0.001), ΔTBil (p = 0.001), ΔALB (p = 0.026), ΔWBC (p < 0.001), ΔHGB (p < 0.001), and ΔPLT (p < 0.001), sequential or add-on treatment (p < 0.001). ΔTBil, ΔALB, ΔWBC, ΔHGB, and ΔPLT represent the changes in variables after 24 weeks of treatment. The results of the univariate analysis are shown in Table 3.
These 7 variables were subsequently entered into a binary logistic regression model, and the logistic regression model exhibited overall significance (X2 = 60.888, p < 0.001). In this study, the tolerance is much greater than 0.1, and the variance inflation factor (VIF) is less than 10, indicating the absence of multicollinearity. The sensitivity of the model was 75%, and the specificity was 96%. The results showed that baseline qHBsAg (p = 0.023), ΔHGB (p = 0.042) and sequential or add-on treatment (p < 0.001) were found to be independent factors influencing the clinical cure rate in patients with CHB or compensated cirrhosis, while ΔTBil, ΔALB, ΔWBC, and ΔPLT showed no statistical significance. Because changes in blood routine are an adverse effect of interferon therapy, they were not considered as predictors. The results of the multivariate logistic regression are presented in Table 3.
Patients with CHB or compensated cirrhosis treated with Peg-IFN-α-2b were divided into a clinical cure group (14 patients) and a non-clinical cure group (45 patients). Firstly, univariate analysis was conducted to examine the relationship between all independent variables and the dependent variable. 22 variables were included in the univariate analysis, with 3 variables showing statistical significance: qHBsAg (p = 0.024), TBil (p = 0.001), and ΔTBil (p = 0.001). ΔTBil represents the change in variable values at 24 weeks of treatment. The results of the univariate analysis are shown in Table 4. The 3 variables were included in a binary logistic regression model. The results indicated that baseline qHBsAg (RR = 0.997, 95% CI [0.995, 0.999], p = 0.031) and ΔTBiL (RR = 0.698, 95% CI [0.555, 0.879], p = 0.002) were independent predictors for achieving clinical cure in patients with CHB or compensated cirrhosis, while TBiL did not show statistical significance (Table 5). The β coefficient for Baseline qHBsAg is-0.003, meaning that for each one-unit increase in qHBsAg, the likelihood of the outcome occurring is 0.997 times. The β coefficient for △TBiL is-0.359, meaning that for each one-unit increase in △TBiL, the likelihood of the outcome occurring is 0.698 times.
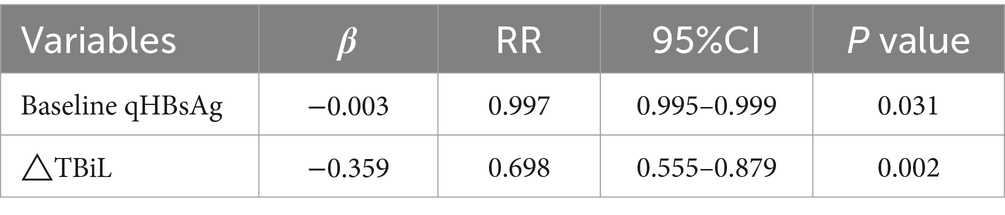
Table 5. Multivariate logistic regression analysis of patients with CHB and compensated cirrhosis using Peg-IFN-α-2b.
To visualize the results of the logistic regression analysis, a forest plot (Figure 2) was created for the test results of the 3 variables. The results indicated that among the included variables, qHBsAg (p = 0.031) and ΔTBiL (p = 0.002) were independent factors influencing the clinical cure rate in patients with CHB or compensated cirrhosis treated with sequential or combined Peg-IFN-α-2b. A ROC curve was plotted (Figure 3), with an AUC of 0.884 and a Youden index of 0.621.
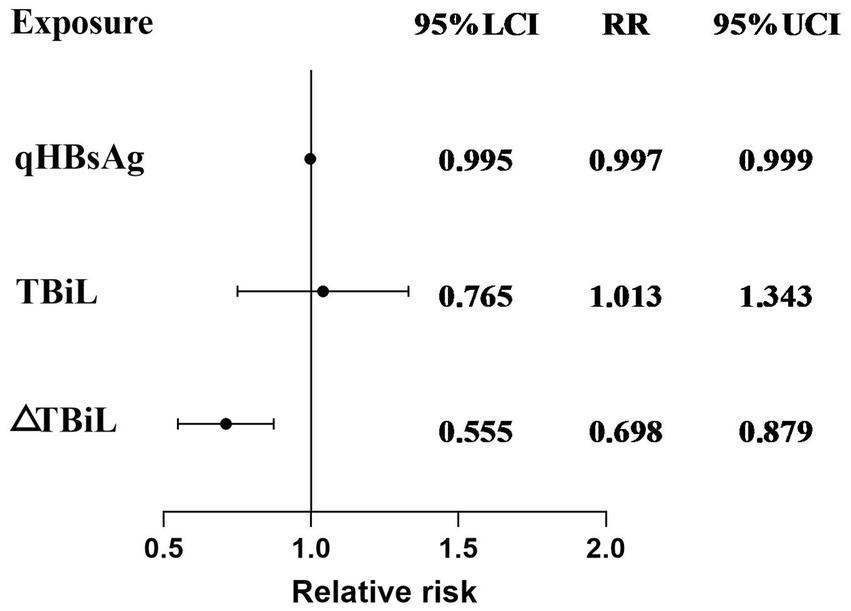
Figure 2. Multivariate logistic regression model forest plot results for achieving clinical cure in patients with CHB or compensated cirrhosis treated with sequential or combined Peg-IFN-α-2b therapy.
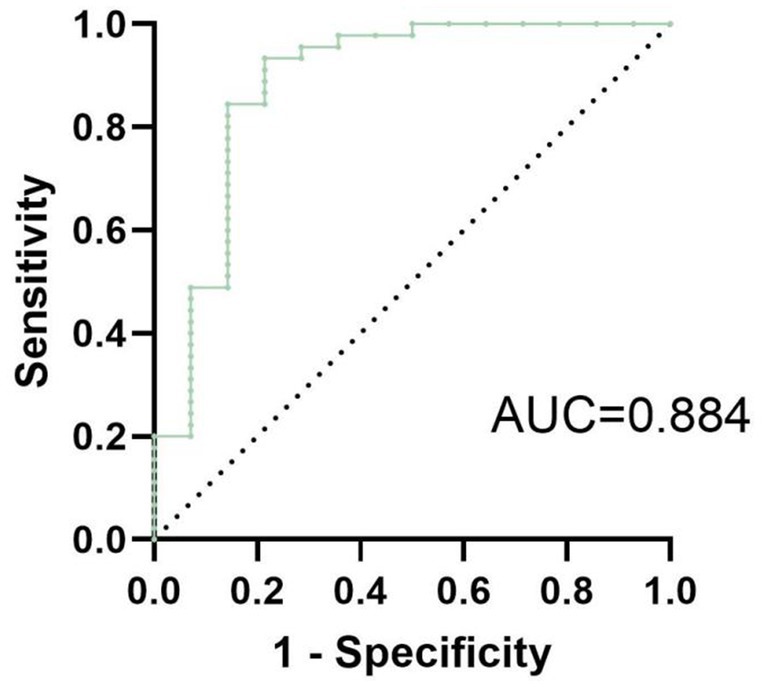
Figure 3. The ROC curve of the multivariate logistic regression model for achieving clinical cure in patients with CHB or compensated cirrhosis treated with sequential or combined Peg-IFN-α-2b therapy has an AUC of 0.884, with a Youden index of approximately 0.621.
NAs act on HBV DNA polymerase and reverse transcriptase, blocking the replication of HBV. DNA polymerase tends to cause errors during virus replication, and prolonged use of such drugs can lead to the accumulation of resistance, increasing potential side effects. Additionally, once the administration of these drugs is stopped, the virus is prone to rebound (7). IFNs enhance the function of immune cells, promote the expression of cytokines, induce the production of IFN-stimulated genes (ISGs) through the IFN signaling pathway, and encode various antiviral proteins that act on crucial biological processes such as HBV replication and transcription (17). This dual action of IFN plays a role in immune regulation and antiviral defence (3). IFN therapy can trigger an antiviral state in liver cells by regulating gene expression and protein translation, affecting multiple stages of the HBV lifecycle (18). Moreover, IFNs can inhibit HBV transcription and reduce the expression of viral proteins such as HBsAg by enhancing the degradation of pregenomic RNA (pgRNA) and core particles or through epigenetic modifications of covalently closed circular DNA (cccDNA) (7, 19–21).
Although sequential or combined treatment with NAs and Peg-IFN-α-2b can increase the clinical cure rate in patients with CHB, in practical application, many patients may not complete the 48-week course due to difficulties in tolerating the adverse reactions associated with IFN, such as extreme fatigue, hair loss, muscle pain, bone marrow suppression, depressive symptoms, and decreased gonadal function. Considering the clinical reality, when treating CHB clinically, it is first necessary to select the “advantaged population” suitable for IFN treatment. Second, accurate predictive factors need to be determined. Assessment before starting IFN therapy is recommended for patients with a lower likelihood of achieving a clinical cure and those unsuitable for IFN therapy.
The patients included in this study were recruited from two centers, and all patients were strictly screened based on the inclusion and exclusion criteria. In actual clinical treatment, patients with compensated cirrhosis also receive IFN therapy, which can reduce liver fibrosis. Therefore, patients with compensated cirrhosis were included in this study. After PSM, 52 patients were included in both the exposed and control groups, with a clinical cure rate of 25% in the exposed group. Sequential or combined Peg-IFN-α-2b treatment significantly improved the clinical cure rate of patients with CHB or compensated cirrhosis.
The AST-to-platelet ratio index (APRI), calculated as APRI = (AST × 100)/(ULNAST × PLT), is a simple and non-invasive method for predicting liver fibrosis. Many studies have assessed the diagnostic value of the APRI. The APRI was calculated for all enrolled patients (Table 1). In the control group, the proportion of patients with an APRI >2 was 0.92%, which was lower than the actual proportion of patients with fibrosis (10.09%). In the exposed group, the proportion of patients with an APRI >2 was 1.69%, which was lower than the actual proportion of patients with fibrosis (6.77%). In this study, the diagnostic value of this indicator for liver fibrosis and cirrhosis was not high. A study by Behnaz Amernia et al. (22) suggested that the APRI is a suitable alternative for detecting significant fibrosis in NAFLD patients using FibroScan. Another study (23) indicated that the APRI has less diagnostic value than the Fibrosis-4 index (FIB-4) and NAFLD fibrosis score (NFS). However, further research is needed on non-invasive indicators and models for detecting liver fibrosis.
The 386 patients were divided into a cure group and a control group. Logistic regression analysis was performed on a randomly sampled subset of 100 patients from the control group. The results indicated that baseline qHBsAg and sequential or add-on treatment are independent influencing factors for achieving a clinical cure in patients with CHB or compensated cirrhosis. Among the 59 patients treated with Peg-IFN-α-2b, they were divided into a clinical cured group and a non-clinical cured group. Logistic regression analysis indicated that qHBsAg and ΔTBiL are independent influencing factors for achieving clinical cure in CHB or compensated cirrhosis patients treated with interferon. The baseline qHBsAg had a p-value less than 0.05, with a 95% CI for the RR value of 0.995–0.999, indicating that changes in baseline qHBsAg have a minor impact on achieving clinical cure at 48 weeks of treatment. In this study, none of the patients with compensated cirrhosis who were treated with Peg-IFN-α-2b developed decompensated cirrhosis. In summary, lower baseline qHBsAg and a decrease in total bilirubin levels at 24 weeks of treatment are independent factors for achieving clinical cure. Previous studies have shown that the greater the decrease in qHBsAg at 24 weeks of treatment, the higher the likelihood of achieving clinical cure. However, the data from this study do not support this conclusion. The limited number of patients treated with Peg-IFN-α-2b and those who completed the one-year treatment course may explain the difference in results and the impact on the RR value compared to other studies.
For patients who intend to undergo sequential or combined NA and Peg-IFN therapy for CHB or compensated cirrhosis, combining baseline indicators, reexamined indicators during treatment, and the presence of intolerable adverse reactions can help determine whether it is necessary to persist in completing the course of treatment when adverse reactions occur. A Chinese study showed a surface antigen clearance rate of 46.7% in patients who completed 96 weeks of treatment (24). Another prospective study by Ning Qin et al. also indicated an increased response rate in patients who completed 96 weeks of treatment (25). In this study, two patients did not achieve a clinical cure after 48 weeks but achieved surface antigen clearance after 68 weeks and 56 weeks by extending the treatment duration. These findings suggest that IFN-based treatment plans need to be individualized.
Currently, there is limited evidence from fully randomized controlled trials (RCTs) regarding clinical cure in patients with CHB. An RCT conducted by Li Jing et al. showed that IFN therapy combined with TDF treatment can improve the clinical cure rate in HBeAg-positive treatment-naive CHB patients (26). Another study by Kin Seng Liem et al. suggested that IFN therapy combined with ETV treatment in HBeAg-positive CHB patients resulted in a greater response rate (27). Another RCT indicated that lower hepatitis B core-related antigen (HBcrAg) levels and higher HBsAb levels at the treatment endpoint could be used to identify patients who might achieve a sustained functional cure after Peg-IFN-based treatment.
This was an observational cohort study of patients from two centers, including those who met the criteria for inclusion in the “advantaged population” with CHB or compensated cirrhosis. This study has several limitations. First, conducting RCTs is challenging in clinical practice, as treatment plans often follow patient preferences. Second, the inclusion of a small number of compensated cirrhosis patients in this study might have contributed to a lower clinical cure rate than that in other studies, which could have impacted the RRs in the data analysis. The conclusions of this study might also not be applicable to other regions. Additionally, as an observational study, there was a significant proportion of missing data at the 12-week follow-up, preventing an analysis of the relationship between changes in the data at 12 weeks and clinical cure. As RCTs related to clinical treatment are still limited, future research should focus on large-scale, multicenter, RCTs to provide more reliable evidence for the treatment of patients with CHB.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethics Review Committee of the First Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from the results that patients examined at the hospital. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Y-QL: Formal analysis, Investigation, Software, Supervision, Writing – original draft, Writing – review & editing. R-HG: Investigation, Writing – review & editing. K-YL: Investigation, Writing – review & editing. J-JL: Software, Writing – review & editing. H-FJ: Funding acquisition, Project administration, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study was funded by Wu Jieping Medical Foundation (3D4233217428).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AST, Aspartate aminotransferase; ALT, Alanine transaminase; APRI, AST-to-platelet ratio index; ALB, Albumin; AFP, Alpha-fetal protein; CHB, Chronic hepatitis B; CHE, Cholinesterase; ETV, Entecavir; HGB, Hemoglobin; NAs, Nucleos(t)ide analogs; peg-IFN-α-2b, Pegylated interferon alpha-2b; PLT, Platelet; PSM, Propensity score matching; RR, Relative ratio; ROC, Receiver operating characteristic; TAF, Tenofovir alafenamide; TDF, Tenofovir disoproxil fumarate; TBiL, Total bilirubin; WBC, White blood cell.
1. Peng, M-J, Guo, X-Q, Zhang, W-L, Chen, J, Kang, W, Yang, XF, et al. Effect of pegylated interferon-α2b add-on therapy on renal function in chronic hepatitis B patients: a real-world experience. Front Microbiol. (2022) 13:980250. doi: 10.3389/fmicb.2022.980250
2. WHO. (2021). Hepatitis B. Global progress report on HIV, viral hepatitis and sexually transmitted infections. Available online at: https://www.who.int/news-room/factsheets/detail/hepatitis-b.
3. Li, N, Yu, K, Dong, M, Wang, J, Yang, F, Zhu, H, et al. Intrahepatic transcriptomics reveals gene signatures in chronic hepatitis B patients responded to interferon therapy. Emerg Microbes Infect. (2022) 11:1876–89. doi: 10.1080/22221751.2022.2100831
4. Jeng, W-J, Papatheodoridis, GV, and Lok, ASF. Hepatitis B. Lancet. (2023) 401:1039–52. doi: 10.1016/S0140-6736(22)01468-4
5. Wu, F-P, Yang, Y, Li, M, Liu, YX, Li, YP, Wang, WJ, et al. Add-on pegylated interferon augments hepatitis B surface antigen clearance vs continuous nucleos(t)ide analog monotherapy in Chinese patients with chronic hepatitis B and hepatitis B surface antigen ≤ 1500 IU/mL: an observational study. World J Gastroenterol. (2020) 26:1525–39. doi: 10.3748/wjg.v26.i13.1525
6. You, H, Wang, F-S, Li, T-S, Xu, X, Sun, Y, Nan, Y, et al. Guidelines for the prevention and treatment of chronic hepatitis B (version 2022). J Prac Hepatol. (2023) 26:S18–39. doi: 10.3969/j.issn.1672-5069.2023.03.040
7. Yang, X, Zhang, K, Xu, Q, Shu, X, Mo, Z, Xie, D, et al. Interferon add-on therapy increased clinical cure significantly for interferon-experienced chronic hepatitis B patients with low HBsAg. Front Immunol. (2022) 13:997608. doi: 10.3389/fimmu.2022.997608
8. Wang, W-X, Jia, R, Gao, Y-Y, Liu, JY, Luan, JQ, Qiao, F, et al. Quantitative anti-HBc combined with quantitative HBsAg can predict HBsAg clearance in sequential combination therapy with PEG-IFN-α in NA-suppressed chronic hepatitis B patients. Front Immunol. (2022) 13:894410. doi: 10.3389/fimmu.2022.894410
9. Lim, SG, Yang, WL, Ngu, JH, Chang, J, Tan, J, Ahmed, T, et al. Switching to or add-on Peginterferon in patients on Nucleos(t)ide analogues for chronic hepatitis B: the SWAP RCT. Clin Gastroenterol Hepatol. (2022) 20:e228–50. doi: 10.1016/j.cgh.2021.04.031
10. Chu, J, Huang, Y, Xie, D, Deng, H, Wei, J, Guan, Y-J, et al. Real-world study on HBSAG loss of combination therapy in HBEAG-NEGATIVE chronic hepatitis B patients. J Viral Hepat. (2022) 29:765–76. doi: 10.1111/jvh.13722
11. Wu, D, Wang, P, Han, M, Chen, Y, Chen, X, Xia, Q, et al. Sequential combination therapy with interferon, interleukin-2 and therapeutic vaccine in entecavir-suppressed chronic hepatitis B patients: the endeavor study. Hepatol Int. (2019) 13:573–86. doi: 10.1007/s12072-019-09956-1
12. Tang, LSY, Covert, E, Wilson, E, and Kottilil, S. Chronic hepatitis B infection: a review. JAMA. (2018) 319:1802. doi: 10.1001/jama.2018.3795
13. Li, M. Dynamic changes of cytokine profiles and Virological markers associated with HBsAg loss during Peginterferon alpha-2a treatment in HBeAg-positive chronic hepatitis B patients. Front Immunol. (2022) 13:13. doi: 10.3389/fimmu.2022.892031
14. Wong, GLH, Gane, E, and Lok, ASF. How to achieve functional cure of HBV: stopping NUCs, adding interferon or new drug development? J Hepatol. (2022) 76:1249–62. doi: 10.1016/j.jhep.2021.11.024
15. Xu, Y, Wang, X, Liu, Z, Zhou, C, Qi, W, Jiao, J, et al. Addition of nucleoside analogues to peg-IFNα-2a enhances virological response in chronic hepatitis B patients without early response to peg-IFNα-2a: a randomized controlled trial. BMC Gastroenterol. (2017) 17:102. doi: 10.1186/s12876-017-0657-y
16. Van Zonneveld, M, Flink, HJ, Verhey, E, Senturk, H, Zeuzem, S, Akarca, US, et al. The safety of pegylated interferon alpha-2b in the treatment of chronic hepatitis B: predictive factors for dose reduction and treatment discontinuation. Aliment Pharmacol Ther. (2005) 21:1163–71. doi: 10.1111/j.1365-2036.2005.02453.x
17. Li, M, Yi, W, Zhang, L, Lu, Y, Lu, H-H, Shen, G, et al. Predictors of sustained functional cure in hepatitis B envelope antigen-negative patients achieving hepatitis B surface antigen seroclearance with interferon-alpha–based therapy. J Viral Hepat. (2019) 26:32–41. doi: 10.1111/jvh.13151
18. BourliÃ, DM. Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen-negative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: a randomised, controlled, open-label trial. Science. (2017) 2:1243462. doi: 10.1016/S2468-1253(16)30189-3
19. Kim, SW, Yoon, JS, Lee, M, and Cho, Y. Toward a complete cure for chronic hepatitis B: novel therapeutic targets for hepatitis B virus. Clin Mol Hepatol. (2022) 28:17–30. doi: 10.3350/cmh.2021.0093
20. Lucifora, J, Xia, Y, Reisinger, F, Zhang, K, Stadler, D, Cheng, X, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. (2014) 343:1221–8. doi: 10.1126/science.1243462
21. Wursthorn, K, Lutgehetmann, M, Dandri, M, Volz, T, Buggisch, P, Zollner, B, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology. (2006) 44:675–84. doi: 10.1002/hep.21282
22. Amernia, B, Moosavy, SH, Banookh, F, and Zoghi, G. FIB-4, APRI, and AST/ALT ratio compared to FibroScan for the assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease in Bandar Abbas, Iran. BMC Gastroenterol. (2021) 21:453. doi: 10.1186/s12876-021-02038-3
23. Lee, J, Vali, Y, Boursier, J, Spijker, R, Anstee, QM, Bossuyt, PM, et al. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: a systematic review. Liver Int. (2021) 41:261–70. doi: 10.1111/liv.14669
24. Li, K, Ning, HB, Jin, HM, Peng, Z, and Shang, J. Effect of pegylated interferon α-2b on serum HBsAg clearance rate in treatment of patients with chronic hepatitis B. J Clin Hepatol. (2023) 39:1819–24. doi: 10.3969/j.issn.1001-5256.2023.08.009
25. Huang, D, Wu, D, Wang, P, Wang, Y, Yuan, W, Hu, D, et al. End-of-treatment HBcrAg and HBsAb levels identify durable functional cure after peg-IFN-based therapy in patients with CHB. J Hepatol. (2022) 77:42–54. doi: 10.1016/j.jhep.2022.01.021
26. Li, J, Qu, L, Sun, X, Liu, Y, Gong, Q, Yu, D, et al. Peg-interferon alpha add-on Tenofovir disoproxil fumarate achieved more HBsAg loss in HBeAg-positive chronic hepatitis B naïve patients. J Viral Hepat. (2021) 28:1381–91. doi: 10.1111/jvh.13571
27. Liem, KS, Van Campenhout, MJH, Xie, Q, Brouwer, WP, Chi, H, Qi, X, et al. Low hepatitis B surface antigen and HBV DNA levels predict response to the addition of pegylated interferon to entecavir in hepatitis B e antigen positive chronic hepatitis B. Aliment Pharmacol Ther. (2019) 49:448–56. doi: 10.1111/apt.15098
Keywords: chronic hepatitis B, compensated cirrhosis, Peg-IFN-α-2b, clinical cure, predictive factors
Citation: Lv Y-Q, Guo R-H, Liu K-Y, Li J-J and Ji H-F (2025) Predictive factors for clinical cure in the treatment of HBeAg(−) chronic hepatitis B or compensated cirrhosis: a prospective observational study. Front. Med. 11:1483744. doi: 10.3389/fmed.2024.1483744
Received: 20 August 2024; Accepted: 27 December 2024;
Published: 09 January 2025.
Edited by:
Krzysztof Tomasiewicz, Medical University of Lublin, PolandReviewed by:
Weiyang Li, Jining Medical University, ChinaCopyright © 2025 Lv, Guo, Liu, Li and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Fan Ji, amloZkBqbHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.