- 1Faculty of Medicine, University of Belgrade, Belgrade, Serbia
- 2Clinic for Orthopedic Surgery and Traumatology, University Clinical Center of Serbia, Belgrade, Serbia
Hand infection caused by atypical mycobacteria is an uncommon condition. We present a case of hand infection caused by Mycobacterium chelonae in a patient who had undergone acupuncture. The clinical features, treatment, and outcome are described. Biopsy and cultures are essential for the diagnosis because Mycobacterium chelonae is a rare cause of human infection and is difficult to diagnose unless suspected. The patient was successfully treated through a combination of surgical excision, debridement, and antimicrobial therapy. We also reviewed the available literature to summarize the experience related to this infectious entity.
Introduction
Acupuncture is a form of alternative medicine that treats patients by inserting thin needles into the body. This practice involves piercing the skin with solid, hair-thin needles that vary in length from 15 to 50 mm. The earliest written record of acupuncture dates back to approximately 200 BC (1). In the concept of traditional Chinese medicine, it is believed that acupuncture promotes general health, relieves pain, and prevents disease. There is general agreement that acupuncture is safe when administered by well-trained practitioners using sterile needles (2). However, it is an invasive procedure because the needles penetrate the skin and, therefore, is not without risk. The majority of reported adverse effects are minor, including hematoma (2.2%), minor hemorrhage (2.9%), and dizziness (1%) (2). Since 1970, 50 cases of bacterial infections and more than 80 cases of hepatitis B have been reported in the literature (3). Other risks include nerve injury, kidney damage, hemopericardium, and even brain damage (4).
Non-tuberculous (atypical) mycobacteria are ubiquitous organisms, commonly found in the environment (soil, water, and dust) and animal reservoirs (5).
Other organisms of the Mycobacterium genus that are present in the environment are classified as saprophytes and have been referred to as “atypical mycobacteria” by Pinner (6).
Runyon distinguished between rapidly growing and slowly growing non-tuberculous mycobacteria (NTM), further subdividing them according to their pigment-forming properties in culture. Mycobacterium chelonae (M. chelonae) is an atypical rapidly growing mycobacterium (RGM) belonging to Runyon group IV. M. chelonae was originally isolated from a turtle (7), and it is widely found in soil, freshwater, and dust particles.
M. chelonae infection in the hand is rare (8). Here, we present a case of M. chelonae infection in an immunocompetent patient following multiple acupuncture treatments. We reviewed the available literature to summarize the experience related to this infection.
Case report
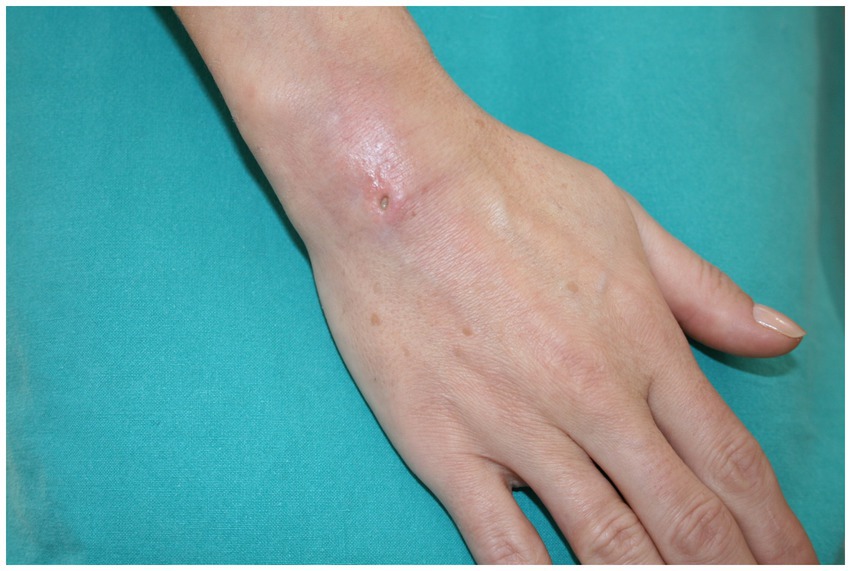
We present the case of a 45-year-old right-handed woman with a punched-out ulceration on her right hand (Figure 1). She presented to her general practitioner in February 2023 with a tender, red, suppurating subcutaneous nodule on the skin of the dorsum of her hand. 3 weeks before the examination, the patient underwent acupuncture treatment for chronic lumbar and shoulder pain. She was initially treated with amoxicillin (500 mg, three times a day, orally), followed by cefazolin (500 mg, twice a day, intravenously), but with no improvement. The routine cultures were negative, while mycobacterial cultures were not requested. The patient initially complained of a prickling sensation around the lesion, accompanied by itchiness. No systemic symptoms, such as fever or myalgia, were reported.
In May 2023, the patient was referred to a dermatologist due to moderate swelling and a tender erythematous nodule with purulent discharge on the dorsum of the right hand and wrist. The induration was located at the site of the acupuncture point that lies along the triple heater meridian (meridian X). Based on the clinical findings, the patient was treated with ciprofloxacin (500 mg, twice a day, orally) for 10 days. Despite completing the full course of this treatment, discharge from the wound continued. The hepatic and renal function tests were normal. The blood tests showed the following: erythrocyte sedimentation rate = 4 mm/h (reference range: 3–20 mm/h), C-reactive protein = 3 mg/L (reference range: 0.1–8 mg/L), and mean WBC = 3.8 × 109/l (reference range: 3.4–9.7 × 109/l). The rheumatoid factor, antinuclear antibodies, and serological tests for Chlamydia were negative. The Widal, Weil–Felix, and HIV A1 and A2 tests were also negative. The blood culture was sterile.
The hand X-rays showed swelling of the soft tissue on the dorsum of the hand, without any joint or bone erosions.
The patient’s chest X-ray was normal, and the Mantoux test was also negative. The samples from the wound swabs were inoculated onto appropriate nutrient media, including nutrient agar (blood agar, Endo agar, mannitol salt agar (Chapman agar), MacConkey agar, Sabouraud agar, crystal violet blood agar, and Pseudosel agar), as well as nutrient broth (brain heart infusion broth, thioglycollate broth, and Robertson’s Cooked Meat (RCM) medium). All cultures yielded no growth after an incubation period of 16–24 h at a temperature of 35–37°C.
The patient was afebrile and in good overall health. There was no indication of immunodeficiency. The laboratory tests revealed no fungal elements. The patient had no history of systemic immunosuppressive therapy.
The Ziehl–Neelsen stain for acid-fast bacilli was positive. The culture on Löwenstein–Jensen medium showed non-pigmented colonies on the fourth day of the inoculation, which were preliminarily identified as a rapidly growing non-tuberculous mycobacterium. The definitive identification of the pathogen as M. chelonae was based on targeting the mycobacterial 360-bp region of the rpoB gene, which is relatively polymorphic, while it was amplified from the DNA by PCR-restriction fragment length polymorphism analysis (9, 10).
Treatment with clarithromycin (500 mg twice a day, orally) and tobramycin (3 mg/kg/day, intravenously) was initiated for 2 weeks, and there was a positive clinical response to this treatment. The patient did not experience any gastrointestinal side effects. Clarithromycin monotherapy was continued for the remaining of her planned 6-month treatment course. The lesion was excised, and tenosynovectomy of the extensor tendons (Figure 2) was performed. The biopsy sample taken from the soft tissue was sent for microbiological and pathological analyses. Histologically, the biopsy specimens revealed signs of acute inflammation superimposed on chronic dermal and subcutaneous inflammatory lesions. Polymorphonuclear leukocyte infiltration was considerable and associated with signs of granulomatous inflammation. Foreign-body giant cells, Langerhans cells, and histiocytes were present in the biopsy sample.
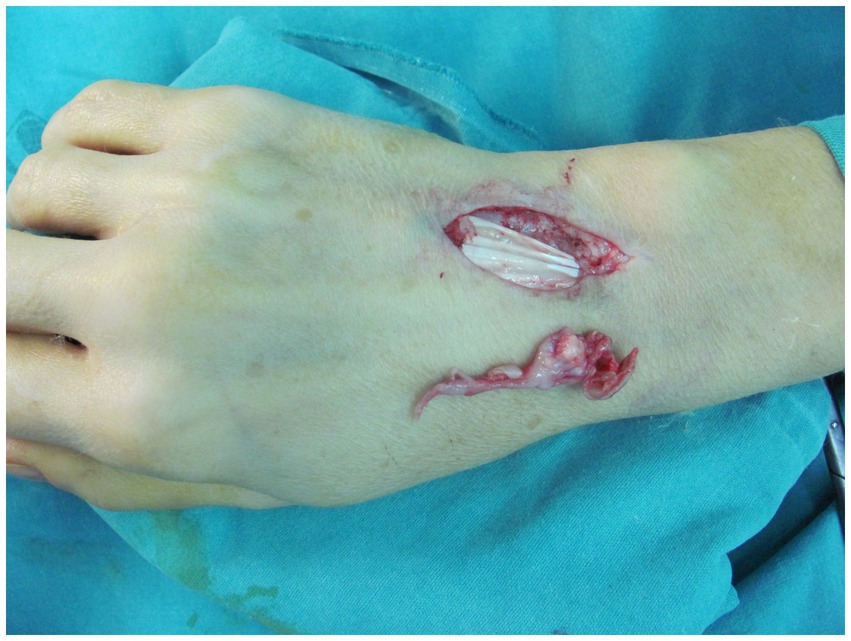
Figure 2. Intraoperative view showing excision of the lesion and tenosynovectomy of the extensor tendons.
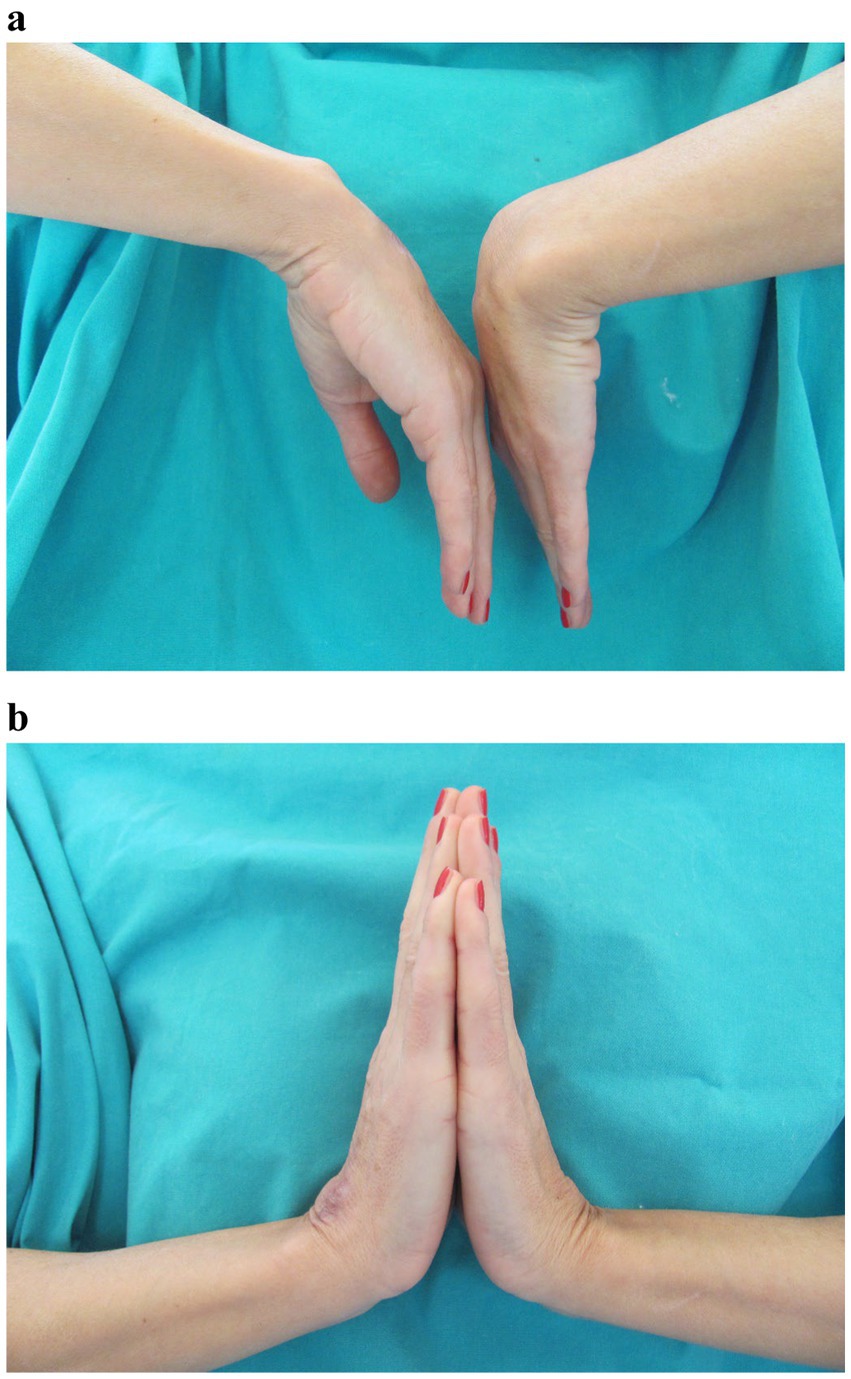
After 4 months of the treatment, the edema resolved, discharge from the wound ceased, and the nodules disappeared. After 2 months, the treatment was discontinued. The incision site healed with no signs of infection. To prevent adhesions, occupational hand therapy consisting of a wide range of motion exercises was carried out. The final assessment was condcuted after 6 months. There were no signs of infection. The wound completely healed without any further discharge from the wound site. The patient developed residual scarring and hyperpigmentation (Figure 3). The grip strength was measured using the Jamar Hydraulic Hand Dynamometer, with the elbow in 90° flexion and the wrist in a neutral position. The measurement showed that the patient regained 90% of the grip strength of the contralateral hand. She also regained a satisfactory range of motion, with almost full dorsiflexion and palmar flexion, reaching 75% of the contralateral hand (Figure 4). The Disabilities of the Arm, Shoulder, and Hand (DASH) score was 1.7.
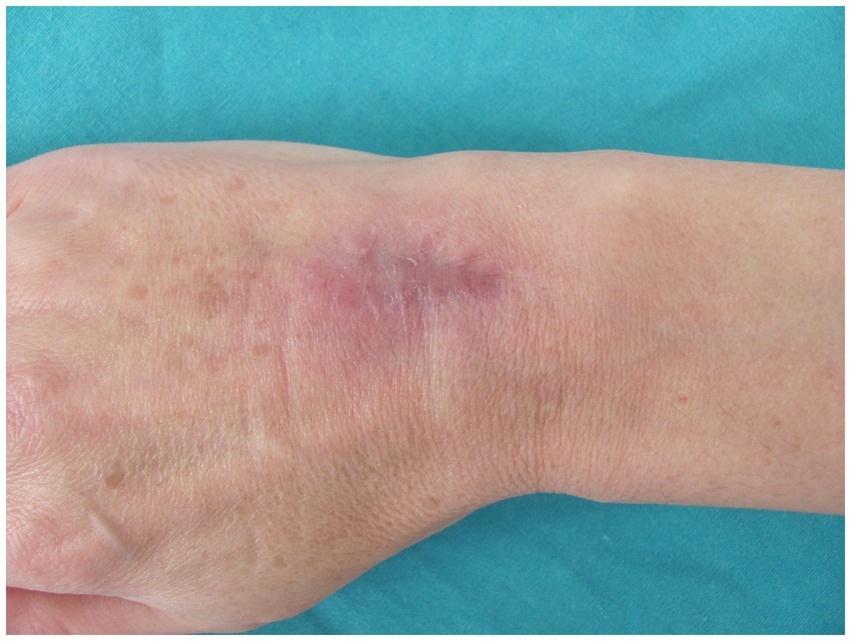
Figure 3. Wound completely healed without any further discharge from the site, with residual scarring and hyperpigmentation.
Discussion
Rapidly growing mycobacteria (RGM) are commonly found in soil, dust, and water. RGM are known to cause respiratory infections, lymphadenitis, localized cutaneous infections, chronic granulomatous infections of the bursae, joints, tendon sheaths, and bones, and, rarely, disseminated systemic illness (11). Depending on the host’s immune status, cutaneous disease caused by M. chelonae follows two different patterns (12). In an immunocompetent host, a traumatic injury, injection, or surgical wound is followed by the development of localized cellulitis or abscess formation. Disseminated disease is usually observed only in immunocompromised hosts, such as those with immunosuppression, AIDS, or those who use corticosteroids.
Cases or outbreaks of M. chelonae infection have been reported following liposuction (13), intramuscular injection (14), vaccination (15), laparoscopy (16, 17), mesotherapy (18, 19), catheter infections (12), breast augmentation (20), silicon arthroplasty of the metacarpophalangeal joints (21), at the site of tattoos (22), open fractures (23), and carpal tunnel release procedures (24). A history of trauma preceded the appearance of lesions in 44% of patients (25).
Terry et al. reported an M. chelonae ulcerous lesion on the leg of a patient with a history of diabetes mellitus, following a scratch from a cat (26). Other authors have reported mycobacterium tenosynovitis of the hand and wrist without a recognized penetrating injury (8, 24, 27). Chung et al. reported bilateral cutaneous M. chelonae infection on both lower legs with no history of trauma, while Sari-Kouzel et al. reported pustules on a finger associated with Raynaud’s phenomenon (28, 29). Uslu et al. reported five cases of cutaneous and/or soft tissue infections due to M. chelonae, where two of the five patients were receiving immunosuppressive treatment (30).
We found several reports of cases and outbreaks of skin and soft tissue infections caused by M. abscessus following acupuncture in the literature (31–35), as well as two articles reporting on M. chelonae infection following acupuncture treatment: induration of the leg in a 79-year-old woman and disseminated subcutaneous nodules on the skin of the abdomen of a 58-year-old woman (36, 37). To the best of our knowledge, this is the first reported case of M. chelonae infection in the hand following acupuncture treatment in the literature. The exact source of the infection was not determined. However, there are several possible explanations. First, the acupuncture needle itself could have been the source of the infection due to improper sterilization. A second possibility is skin contamination before or after acupuncture by an object in the environment, such as disposable needles, towels, alcohol bottles, or water from the water heater. This was demonstrated in the case presented by Chroneou et al., where a positive culture of M. chelonae was found in an automated bronchoscope washer and the water supply (38).
In the majority of postinjection abscesses caused by M. chelonae and M. abscessus, the contaminated source was not identified. However, in general, non-sterile water used to dissolve different substances has been suggested as the source of the pathogen (37, 39). In an effort to identify the source of M. abscessus infection following acupuncture treatment, Koh et al. investigated an outbreak among patients who had visited an oriental medical clinic (34). Fifty environmental samples were collected from the clinic for mycobacterium culture inoculation. These samples were taken from all devices and materials that could have come into contact with the patients’ skin before, during, and after the acupuncture sessions. However, none of the cited studies have elucidated the source of the infection or the mode of transmission. In two articles, the authors reported that the source of M. chelonae infection after mesotherapy was the tap water system in the treating physician’s office, which was used to rinse the injector (18, 19).
Diagnosing cutaneous infections caused by rapidly growing mycobacteria is difficult for several reasons. First, the clinical symptoms of these infections are often non-specific, such as abscesses or subcutaneous nodules, without systemic features (19). The morphology of skin lesions is highly heterogeneous. Some lesions remain in the form of papules, whereas other lesions progress to nodules, abscesses, ulcers, and even confluent plaques filled with pus (25, 31). In some cases, patients were found to delay seeking medical advice because the symptoms were relatively mild (36). Second, the incubation period can vary. In our case, the onset of symptoms occurred 3 weeks after the acupuncture treatment. The literature reports a wide range of incubation periods: a median incubation period of 9 days or 9.5 weeks (range: 1–29 weeks) after mesotherapy (18, 19) and approximately 3–5 months after multiple acupuncture treatments (33, 37, 40). In some cases, patients failed to associate the acupuncture procedures with cutaneous lesions because of the long incubation period (36). The average delay between the onset of symptoms and the correct diagnosis has been found to be 1 year (41). A clinical suspicion of cutaneous non-tuberculous infections, such as M. chelonae infection, is essential for diagnosis. The symptoms typically include prolonged or failed wound healing and a lack of response to broad-spectrum antibiotics in cases of suspected bacterial infection.
These pathogens are difficult to treat once the infection is diagnosed. Mortality typically occurs only in cases of extensive pulmonary or disseminated disease. No physical examination findings are pathognomonic for M. chelonae infection. In the differential diagnosis, conditions such as wound infection, actinomycosis, blastomycosis, sporotrichosis, histoplasmosis, fungal infection, and other mycobacterial infections must be considered (42–44).
After establishing the diagnosis, susceptibility testing should be performed. M. chelonae is usually susceptible to tobramycin, clarithromycin, and linezolid (19). Some studies have reported a 100% susceptibility to ciprofloxacin and clarithromycin (25).
The type and duration of antimicrobial therapy for M. chelonae infection vary considerably in the literature. Although numerous reports have documented cases of successful monotherapy (e.g., clarithromycin, 1 g a day or 500 mg twice a day, orally) (21, 22, 26, 28, 37), antibiotic therapy with two or three drugs is preferable in most patients (14, 29, 36). The duration of antibiotic treatment may vary from as little as 5 weeks (29) to as long as 3 months (28, 37), 5 months (17), or even 9 months (8). The majority of authors have recommended 6 months (19, 21, 23, 36, 39, 45) or a total of 12 months of treatment (46).
Another important consideration is whether surgical procedures are necessary in such cases. Some authors consider that a prompt diagnosis of atypical mycobacteria infections, along with appropriate antimicrobial treatment, may help avoid the need for surgical debridement (8). In two cases of M. chelonae infection following acupuncture treatment, antibiotics alone were sufficient to completely clear the lesions (36, 37). In other articles, we found that the standard guidelines typically include both antibiotic treatment and surgical management. This management involves debridement of affected skin and tissues, irrigation, removal of existing implants, and drainage (21, 26, 28, 29, 39, 45, 47, 48).
M. chelonae is a rapidly growing mycobacterium responsible for a variety of generally chronic infections (44, 49, 50). The infections sometimes develop after surgery or trauma and affect the skin and soft tissues. Localized infections without a history of trauma are uncommon.
Sterilization and the use of disinfectants play a crucial role in the proper control of NTM, especially in healthcare settings (51). Glutaraldehyde, peracetic acid, povidone-iodine, alcohol, and chlorine are still used for the sterilization of medical instruments, and common methods of water disinfection include chlorine, monochloramine, and ozone. NTM are resistant to chlorine and other disinfectants used in drinking water systems; however, most disinfectants have some sterilizing effects against NTM (52, 53). Lee et al. (53) showed that glutaraldehyde has the greatest sterilizing effect on non-tuberculous mycobacteria, while only alkyldiaminoethylglycine hydrochloride has a reduced effect on NTM. Glutaraldehyde-resistant strains of M. chelonae have been isolated from environmental samples. An alternative disinfectant, such as glucoprotamin, often shows good efficiency against the majority of the different NTM isolates (e.g., M. smegmatis, M. avium, M. kansasii, M. terrae, and M. xenopi), except for M. chelonae (51, 54).
Biopsy and cultures are essential for the diagnosis. The optimal treatment for cutaneous infections caused by M. chelonae is yet to be established. Effective treatment of these infections is therefore challenging, and the identification of antimicrobial susceptibility is essential. Clarithromycin has been reported as the most effective drug for treating M. chelonae infection (55, 56). Awareness of the spectrum of clinical presentations of this infection can help prevent delays in diagnosis and facilitate the timely application of appropriate antibiotic and surgical therapies.
Conclusion
Non-tuberculous mycobacteria should be recognized as a preventable cause of acupuncture-associated infections. Atypical mycobacterial infections should be considered when skin lesions develop following any procedure that involves the subcutaneous tissue. Proper handling of equipment, appropriate skin preparation, and strict adherence to sterilization procedures can help prevent future cases and outbreaks of M. chelonae infection. Efforts should focus on educating acupuncture practitioners about hygienic practices based on established guidelines, especially those related to invasive procedures in non-hospital settings. Moreover, future epidemiological studies are needed to identify the exact source of the infection.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SM: Writing – original draft, Writing – review & editing, Conceptualization, Validation. VT: Investigation, Writing – review & editing. SZ: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Ernst, G, Strzyz, H, and Hagmeister, H. Incidence of adverse effects during acupuncture therapy- a multicentre survey. Complement Ther Med. (2003) 11:93–7. doi: 10.1016/S0965-2299(03)00004-9
3. Woo, PC, Lin, AW, Lau, SK, and Yuen, KY. Acupuncture transmitted infections. BMJ. (2010) 340:c1268. doi: 10.1136/bmj.c1268
4. Yekeler, E, Tunaci, M, Tunaci, A, Dursun, M, and Acunas, G. Frequency of sternal variations and anomalies evaluated by MDCT. AJR Am J Roentgenol. (2006) 186:956–60. doi: 10.2214/AJR.04.1779
5. Wallace, RJ, Glassroth, J, Griffith, DE, Olivier, KN, Cook, JL, and Gordon, F. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med. (1997) 156:S1–S25.
7. Grange, JM. Mycobacterium chelonei. Tubercle. (1981) 62:273–6. doi: 10.1016/S0041-3879(81)80007-4
8. Mateo, L, Rufi, G, Nolla, JM, and Alcaide, F. Mycobacterium chelonae tenosynovitis of the hand. Semin Arthritis Rheum. (2004) 34:617–22. doi: 10.1016/j.semarthrit.2004.06.002
9. Hyeyoung, L, Hee-Jung, P, Sang-Nae, C, Gill-Han, B, and Sang-Jae, K. Species identification of mycobacteria by PCR-restriction fragment length polymorphism of the rpoB gene. J Clinic Mycrobiol. (2000) 38:2966–71. doi: 10.1128/jcm.38.8.2966-2971.2000
10. Järvinen, AK, Laakso, S, Piiparinen, P, Aittakorpi, A, Lindfors, M, Huopaniemi, L, et al. Rapid identification of bacterial pathogens using a PCR- and microarray-based assay. BMC Microbiol. (2009) 9:161. doi: 10.1186/1471-2180-9-161
11. Petitijean, G, Fluckiger, U, Scharen, S, and Laifer, G. Vertebral ostemyelitis caused by non-tuberculous mycobactera. Clin Microbiol Infect. (2004) 10:951–3. doi: 10.1111/j.1469-0691.2004.00949.x
12. Wallace, RJ, Brown, BA, and Only, GO. Skin, soft tissue and bone infections due to Mycobacterium chelonaechelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance to oral antimicrobials other than claritomycin. J Infect Dis. (1992) 166:405–12. doi: 10.1093/infdis/166.2.405
13. Kim, MJ, and Mascola, L. Mycobacterium chelonae wound infection after liposuction. Emerge Infect Dis. (2010) 16:1173–5. doi: 10.3201/eid1607.090156
14. Devi, DRG, Indumathi, VA, Indira, S, Babu, PRS, Sridharan, D, and Belwadi, MRS. Injection site abscess due to mycobacterium fortuitum: a case report. Indian J of Med Microb. (2003) 21:133–4. doi: 10.1016/S0255-0857(21)03139-X
15. Borghans, JGA, and Stanford, JL. Mycobacterium chelonei in abscesses after injection of diphtheria-pertussis-tetanus-polio vaccine. Am Rev Respir Dis. (1973) 107:1–8.
16. Sethi, S, Sharma, M, Ray, P, Singh, M, and Gupta, A. Mycobacterium fortuitum wound infection following laparoscopy. Indian J Med Res. (2001) 113:83–4.
17. Devi, DRG, Sridaran, D, Indumathi, VA, Babu, PRS, Belwadi, MRS, and Swamy, ACV. Isolation of mycobacterium chelonae from wound infection following laparoscopy: a case report. Indian J Tuberc. (2004) 51:149–51.
18. Carbonne, A, Brossier, F, Arnaud, I, Bougmiza, I, Caumes, E, Meningaud, JP, et al. Outbreak of nontuberculous mycobacterial subcutaneous infections related to multiple mesotherapy injections. J Clin Microbiol. (2009) 47:1961–4. doi: 10.1128/JCM.00196-09
19. Regnier, S, Cambau, E, Meningaud, JP, Guihot, A, Deforges, L, Carbonne, A, et al. Clinical management of rapidly growing mycobaterial cutaneous infections in patients after mesotherapy. Clin Infect Dis. (2009) 49:1358–64. doi: 10.1086/606050
20. Macadam, SA, Mehling, BM, Fanning, A, Dufton, JA, Kowalewska-Grochowska, KT, Lennox, P, et al. Nontuberculous mycobacterial breast implant infections. Plast Reconstr Surg. (2007) 119:337–44. doi: 10.1097/01.prs.0000244924.61968.d2
21. Iordache, SD, Daneman, N, and Axelrod, TS. Mycobacterium chelonae infection following silicone arthroplasty of the metacarpophalangeal joints: a case report. Hand. (2009) 4:129–33. doi: 10.1007/s11552-008-9138-7
22. Rodriguez-Blanco, I, Fernandez, LC, Penaranda, JMS, del Molino, MLP, Esteban, J, and Almagro, M. Mycobacterium chelonae infection associated with tattoos. Acta Derm Venerol. (2011) 91:61–2. doi: 10.2340/00015555-1034
23. Kwan, K, and Ho, ST. Mycobacterium chelonae and mycobacterium fortuitum infection following open fracture: a case report and review of the literature. Indian J Med Microbiol. (2010) 28:248–50. doi: 10.4103/0255-0857.66488
24. Yoon, HJ, Kwon, JW, Yoon, YC, and Choi, SH. Nontuberculous mycobacterial tenosynovitis in the hand: two case reports with the MR imaging findings. Korean J Radiol. (2011) 12:745–9. doi: 10.3348/kjr.2011.12.6.745
25. Dodiuk-Gad, R, Dyachenko, P, Ziv, M, Shani-Adir, A, Oren, Y, Mendelovici, S, et al. Nontuberculous mycobacterial infections of the skin: a retrospective study of 25 cases. J Am Acad Dermatol. (2007) 57:413–20. doi: 10.1016/j.jaad.2007.01.042
26. Terry, S, Timothy, NH, Zurlo, JJ, and Manders, EK. Mycobacterium chelonae: nonhealing leg ulcers treated successfully with an oral antibiotics. J Am Board Fam Pract. (2001) 14:457–61.
27. Noguchi, M, Taniwaki, Y, and Tani, T. Atypical mycobacterium infections of the upper extremity. Arch Orthop Trauma Surg. (2005) 125:475–8. doi: 10.1007/s00402-005-0042-0
28. Chung, WK, Kim, MS, Kim, CH, Lee, MW, Choi, JH, Moon, KC, et al. Cutaneous mycobacterium chelonae infection presenting as symmetrical plaques on both shins in an immunocompetent patient. Acta Derm Venerol. (2009) 89:663–4. doi: 10.2340/00015555-0693
29. Sari-Kouzel, H, Chadwick, PR, Muir, LTSW, Herrick, AL, and Denning, DW. Mycobacterium chelonae finger infection associated with Raynaud's phenomenon. Ann Rheum Dis. (2004) 63:1178–9. doi: 10.1136/ard.2003.009506
30. Uslu, U, Böhm, O, Heppt, F, and Sticherling, M. Skin and soft tissue infections caused by Mycobacterium chelonae: more common than expected? Acta Derm Venereol. (2019) 99:889–93. doi: 10.2340/00015555-3230
31. Ryu, HJ, Kim, WJ, Oh, CH, and Song, HJ. Iatrogenic mycobacterium abscessus infection associated with acupuncture: clinical manifestations and its treatment. Int J Dermatol. (2005) 44:846–50. doi: 10.1111/j.1365-4632.2005.02241.x
32. Tang, P, Murray, C, Alterman, C, Varia, M, Broukhanski, G, Chedore, P, et al. Outbreak of acupuncture-associated cutaneous mycobacterium abscessus infections. J Cutan Med Surg. (2006) 10:166–9. doi: 10.2310/7750.2006.00041
33. Cho, HJ, Lee, DY, Lee, JH, Yang, JM, and Lee, ES. A case of mycobacterium abscessus skin infection caused by multiple acupuncture. Clin Experimental Dermatol. (2009) 35:440–50.
34. Koh, SJ, Song, T, Kang, YA, Choi, JW, Chang, KJ, Chui, CS, et al. An outbreak of skin and soft tissue infection cause by mycobacterium abscessus following acupuncture. Clin Microbiol Infect. (2010) 16:895–901. doi: 10.1111/j.1469-0691.2009.03026.x
35. Choi, WS, Kim, MJ, Park, DW, Son, SW, Yoon, YK, Song, T, et al. Clarithromycin and amikacin vs. clarithromycin and moxifloxacin for the treatment of post-acupuncture cutaneous infections due to mycobacterium abscessus: a prospective observational study. Clin Microbiol Infect. (2011) 17:1084–90. doi: 10.1111/j.1469-0691.2010.03395.x
36. Woo, PCY, Li, JHC, Tang, WM, and Yuen, KJ. Acupuncture mycrobacteriosis. N Engl J Med. (2001) 345:842–3. doi: 10.1056/NEJM200109133451119
37. Ara, M, de Santamaria, CS, Zaballos, P, Yus, C, and Lezcano, M. Mycobacterium Chelonae infection with multiple cutaneous lesions after treatment with acupuncture. Int J Dermatol. (2003) 42:642–4. doi: 10.1046/j.1365-4362.2003.01639_3.x
38. Chroneou, A, Zimmerman, SK, Cook, S, Willey, S, Eyre-Kelly, J, Zias, N, et al. Molecular typing of Mycobacterium chelonae isolates from a pseudo-outbreak involving an automated bronchoscope washer. Infect Control Hosp Epidemiol. (2008) 29:1088–90. doi: 10.1086/591451
39. Cho, SY, Peck, KR, Kim, J, Ha, YE, Kang, CI, Chung, DR, et al. Mycobacterium chelonae infections associated with bee venom acupuncture. Clin Infect Dis. (2014) 58:e110–3. doi: 10.1093/cid/cit753
40. Song, JY, Sohn, JW, Jeong, HW, Cheong, HJ, Kim, WJ, and Kim, MJ. An outbreak of post-acupuncture cutaneous infection due to Mycobacterium abscessus. BMC Infect Dis. (2006) 6:6. doi: 10.1186/1471-2334-6-6
41. Kozin, SH, and Bishop, AT. Atypical Mycobacterium infections of the upper extremity. J Hand Surg Am. (1994) 19:480–7. doi: 10.1016/0363-5023(94)90067-1
42. Khan, S, Khan, B, Batool, W, Khan, M, and Khan, AH. Primary cutaneous Actinomycosis: a diagnostic enigma. Cureus. (2023) 15:e37261. doi: 10.7759/cureus.37261
43. Haytoglu, NSK, Gurel, MS, Erdemir, VA, Leblebici, C, and Haytoglu, TG. An unusual case of sporotrichoid nodules: metastatic cutaneous squamous cell carcinoma. Dermatol Online J. (2013) 19:5. doi: 10.5070/D3195018174
44. Akram, SA, Rathish, B, and Saleh, D. Mycobacterium chelonae infection. Treasure Island (FL): StatPearls Publishing (2023).
45. Lee, EY, Ip, JW, Fung, BK, and Ted, UE. Mycobacterium chelonae hand infection: a review. Hand Surg. (2009) 14:7–13. doi: 10.1142/S0218810409004219
46. Olesen, JS, Wang, M, and Wejse, C. Mycobacterium chelonae hand infection after steroid injection in a patient with rheumatoid arthritis receiving long-term linezolid therapy. BMJ Case Rep. (2017) 2017:bcr2016217257. doi: 10.1136/bcr-2016-217257
47. Bosman, AG, Beer, J, and Grimwood, K. Mycobacterium chelonae soft-tissue infection in an immunocompetent child. J Paediatr Child Health. (2023) 59:401–3. doi: 10.1111/jpc.16310
48. Zhao, XK, Ding, R, Sun, K, Wang, W, Zhang, Q, and Cheng, XG. Osteomyelitis of the femur caused by Mycobacterium chelonae: a case report. JOS Case Rep. (2023) 3:92–6. doi: 10.1016/j.joscr.2023.12.005
49. Howard, ST, and Byrd, TF. The rapidly growing mycobacteria: saprophytes and parasites. Microbes Infect. (2000) 2:1845–53. doi: 10.1016/S1286-4579(00)01338-1
50. Brown-Elliott, BA, and Philley, JV. Rapidly growing mycobacteria. Microbiol Spectr. (2017) 5:10.1128/microbiolspec.tnmi7-0027-2016. doi: 10.1128/microbiolspec.TNMI7-0027-2016
51. Aranke, M, Moheimani, R, Phuphanich, M, Kaye, AD, Ngo, AL, Viswanath, O, et al. Disinfectants in interventional practices. Curr Pain Headache Rep. (2021) 25:1–10.
52. Norton, GJ, Williams, M, Falkinham, JO 3rd, and Honda, JR. Physical measures to reduce exposure to tap water–associated nontuberculous mycobacteria. Front. Public Health. (2020) 8:190. doi: 10.3389/fpubh.2020.00190
53. Lee, Y-H, Kim, H-K, Kim, M-S, You, H-J, Kim, D-W, and Lee, T-Y. Chemical sterilization of Lipoplasty cannula and nontuberculous mycobacteria disinfection: an experimental study. J Craniofac Surg. (2022) 33:719–22. doi: 10.1097/SCS.0000000000008175
54. Tarashi, S, Siadat, SD, and Fateh, A. Nontuberculous mycobacterial resistance to antibiotics and disinfectants: challenges still ahead. Biomed Res Int. (2022) 2022:1–12. doi: 10.1155/2022/8168750
55. Rapp, RO, McCraney, SA, Goodman, NL, and Shaddick, DJ. New macrolide antibiotics: usefulness in infections caused by mycobacteria other than Mycobacterium tuberculosis. Ann Pharmacother. (1994) 28:1255–63. doi: 10.1177/106002809402801109
Keywords: Mycobacterium chelonae , acupuncture, hand infection, surgical excision, non tubercolous mycobacteria
Citation: Matic S, Teodosic V and Zagorac S (2024) Mycobacterium chelonae hand infection following acupuncture: a case report and literature review. Front. Med. 11:1482236. doi: 10.3389/fmed.2024.1482236
Edited by:
Adwoa Asante-Poku, University of Ghana, GhanaReviewed by:
Joseph Oliver Falkinham, Virginia Tech, United StatesAnna Grzegorzewicz, Colorado State University, United States
Copyright © 2024 Matic, Teodosic and Zagorac. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valerija Teodosic, dmFsZXJpamEudGVvZG9zaWM5OTZAZ21haWwuY29t
 Sladjana Matic1,2
Sladjana Matic1,2 Valerija Teodosic
Valerija Teodosic