- 1Fujian Maternity and Child Health Hospital, College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, China
- 2Fujian Clinical Research Center for Maternal-Fetal Medicine, Fuzhou, China
- 3National Key Obstetric Clinical Specialty Construction Institution of China, Fuzhou, China
Aim: The aim of this study was to explore the association between maternal pre-pregnancy body mass index (BMI) and neonatal birth weight in pregnancies with gestational diabetes mellitus (GDM).
Methods: This was a retrospective cohort study conducted between January 2019 and June 2020 at a university hospital in Fuzhou, China.
Results: Pre-pregnancy BMI was used to categorize 791 pregnant women as underweight (3.03%), normal weight (51.71%), overweight (32.74%), and obese (12.52%). Among the 791 babies, 11.63% were small for gestational age (SGA), 77.37% were normal weight, and 11.00% were large for gestational age (LGA). The rate of the SGA babies increased with higher pre-pregnancy BMI. The percentage of LGA babies was higher in women who were overweight or obese compared to those of normal weight. Neonatal birth weight displayed a significantly increasing trend with increasing maternal pre-pregnancy BMI when maternal pre-pregnancy BMI was less than 27.78 kg/m2 [β = 0.03, 95% CI (0.01, 0.04); p = 0.0052 < 0.05] when maternal pre-pregnancy BMI was greater than 27.78 kg/m2, neonatal birth weight decreased as maternal pre-pregnancy BMI increased [β = −0.01, 95% CI (−0.04, 0.01); p = 0.3555].
Conclusion: The incidence of SGA and LGA babies was higher in the women with GDM who were overweight or obese before pregnancy. The data suggest that different management strategies should be implemented for pregnant women with a pre-pregnancy BMI below 27.78 kg/m2 and above 27.78 kg/m2, particularly in cases of GDM. These findings highlight the importance of providing information, offering preconception counseling, and delivering health education on weight management to ensure healthy pregnancies.
1 Introduction
Gestational diabetes mellitus (GDM) is a metabolic disorder that occurs during pregnancy and can lead to adverse fetal outcomes, as well as maternal and offspring complications (1). The hyperglycemia and adverse pregnancy outcome study has confirmed a linear association between pregnancy complications and maternal glycemia (2, 3). The global prevalence of gestational diabetes is estimated to be 14.0% according to the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) criteria (4). In China, the prevalence of GDM has sharply increased over the past decade from 4% in 2010 to 21% in 2020 (5). Adverse maternal or offspring outcomes are common in women with GDM and can vary based on the underlying metabolic conditions (1). Identifying strategies to mitigate pregnancy risks in this group of women is essential.
A common and potentially modifiable risk factor associated with adverse fetal and neonatal outcomes during pregnancy is maternal obesity. The incidence of obesity has markedly increased worldwide, with global prevalence rates of 23.0% for overweight and 16.3% for obesity, respectively (6). Obese women of reproductive age represent a clinically important subpopulation. It is well known that obesity is linked to complications commonly associated with GDM, such as hypertensive disorders of pregnancy (HDP), gestational proteinuria, postpartum hemorrhage, preterm delivery, fetal malformation or stillbirth, neonatal asphyxia, large for gestational age (LGA), shoulder dystocia, and higher rates of cesarean section (7). Regarding the relationship between maternal pre-pregnancy body mass index (BMI) and neonatal birth weight, recent studies have presented different perspectives. Some researchers have reported a positive correlation, while others have argued that there is no correlation (8, 9).
The relationship between maternal pre-pregnancy BMI and neonatal birth weight remains debatable, especially in cases of GDM. Understanding the impact of maternal pre-pregnancy weight on neonatal outcomes in cases of GDM may enhance pre-conception counseling and help develop strategies to improve adverse outcomes. Therefore, we sought to examine the specific relationship between maternal pre-pregnancy weight and neonatal birth weight in cases of GDM.
2 Methods
2.1 Data sources
This study adhered to the principles outlined in the Declaration of Helsinki and was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital, Fuzhou, Fujian, China (2023KY046). A retrospective cohort study was conducted at the Department of Obstetrics at Fujian Maternity and Child Health Hospital, Fuzhou, China from January 2019 to June 2020. A total of 797 pregnant women with GDM were enrolled in this study. Data were collected from the medical history of the participants. In the present study, subjects were selected based on the GDM screening criteria of the World Health Organization (WHO). All pregnant women between 24 and 28 gestational weeks underwent an oral glucose tolerance test (OGTT) with a 75 g glucose load. Pregnancy with any of the glucose values at or above the specific thresholds (≥5.1 mmol/L for fasting, ≥10 mmol/L at 1 h, ≥8.5 mmol/L at 2 h) was defined as GDM (10). Pregnant women with pre-existing diabetes mellitus or multiple pregnancies were excluded. A total of 791 matched pairs of maternal pre-pregnancy BMI and neonatal birth weight were analyzed, due to information missing for 6 pairs.
2.2 Outcome measures
Maternal characteristics extracted from medical records included age, nationality, education level, history of any abnormal pregnancy, pre-pregnancy BMI, and neonatal birth weight. Pre-pregnancy BMI was calculated by dividing weight in kilograms by the square of height in meters (kg/m2). According to the guidelines issued by the Institute of Medicine (IOM) in 2009, participants were divided into four groups based on their pre-pregnancy BMI: underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5 to 24.9 kg/m2), overweight (BMI 25 to 29.9 kg/m2), and obese (BMI ≥30 kg/m2) (11).
Large-for-gestational-age (LGA) infants are defined as those with a birth weight ≥the 90th percentile for gestational age, typically weighing ≥4 kg. Small-for-gestational-age [SGA, also known as fetal growth restriction (FGR)] infants are defined as those with a birth weight below two standard deviations from the mean weight for the same gestational age or below the 10th percentile of normal weight for the same gestational age (12).
2.3 Statistical analysis
Univariate and multivariate piecewise linear regression analyses were performed using the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). Column graphs were created using GraphPad Prism 5 (GraphPad Software). Continuous variables with a normal distribution were expressed as mean (standard deviation). Categorical variables were expressed as frequencies or percentages. A univariate analysis model was used to determine the significance of the association between maternal pre-pregnancy BMI and neonatal birth weight, along with other independent variables. A multivariate regression model was further used to examine the independent association between maternal pre-pregnancy BMI and neonatal birth weight. The relationship between maternal pre-pregnancy BMI and neonatal birth weight was explored using smooth curve fitting after adjusting for potential confounders. Statistical significance (p < 0.05) was determined using the t-test.
3 Results
3.1 Baseline characteristics of all participants
The baseline characteristics of participants are described in Table 1. A total of 797 patients with GDM were included in the present study. Their mean age was 31.50 ± 5.30 years. Of the 797 patients, 790 (99.12%) were ethnic Han. The frequencies of GDM with a family history of diabetes, hypertension, and abnormal pregnancy history were 14.81, 2.63, and 18.70%, respectively. Male infants accounted for 52.57%. Approximately 77.42% of newborns were born by cesarean section.
In 791 pregnancies with GDM, 12.52% of the pregnancies occurred in women with obesity, 32.74% in overweight women, 51.71% in women with normal weight, and 3.03% in underweight women (Figure 1A). As shown in Figure 1B, the weight of 791 babies was analyzed. In the present study, 77.37% of the babies had normal weight, 11.63% were classified as SGA, and 11.00% as LGA. The number of SGA babies rose steadily as pre-pregnancy BMI increased (Figure 2A). In addition, the percentage of LGA babies was higher in the women who were overweight and obese compared to normal-weight women (Figure 2B).
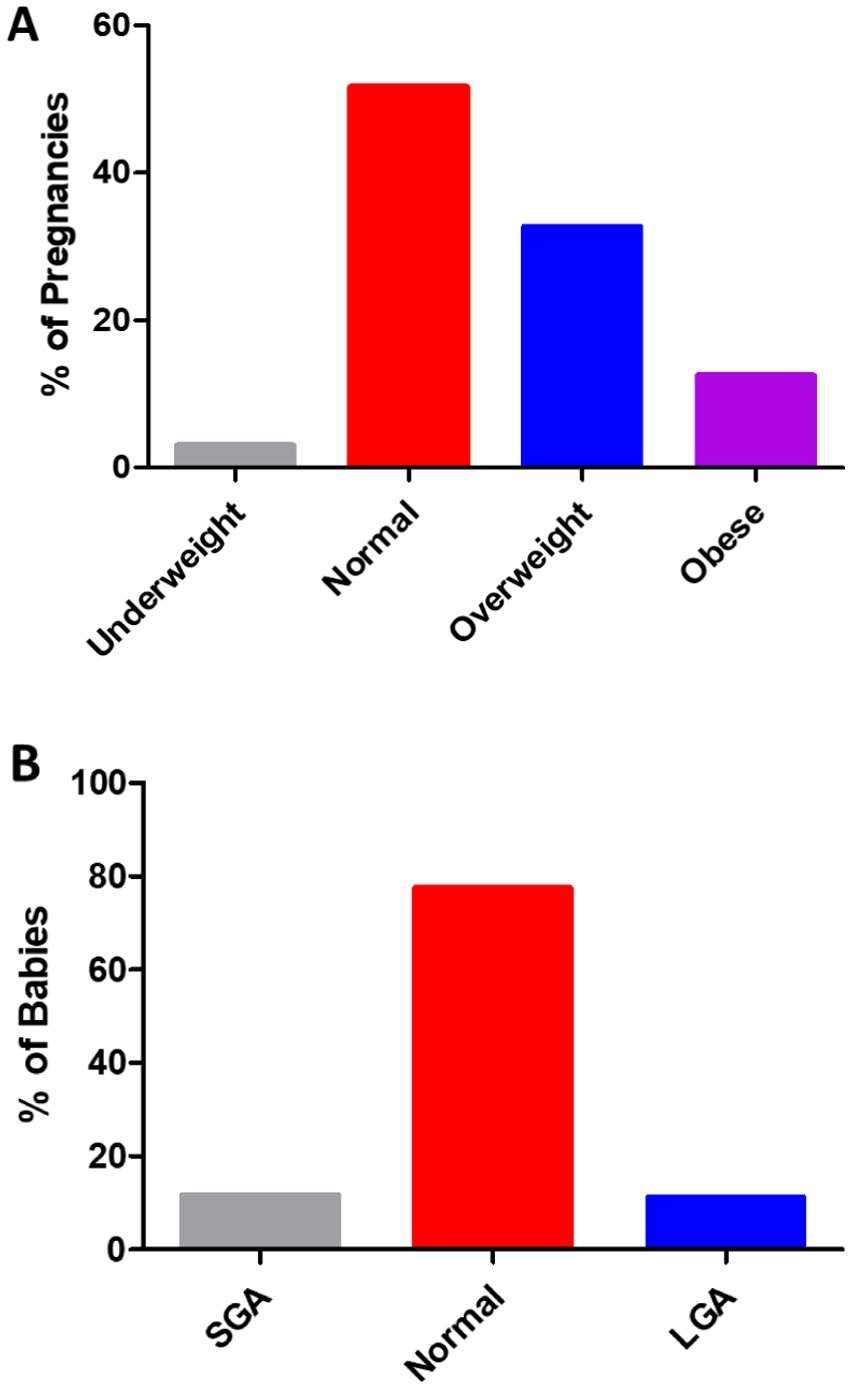
Figure 1. (A) Of the 791 pregnancies in the women with GDM, 3.03% occurred in those who were underweight (BMI <18.5 kg/m2), 51.71% in those with normal weight (BMI 18.5 to 24.9 kg/m2), 32.74% in those who were overweight (BMI 25 to 29.9 kg/m2), and 12.52% in those with obesity (BMI ≥30 kg/m2). (B) The distribution of birth weight in the total of 791 babies from the GDM pregnancies. A total of 11.63% were SGA (<2.5 kg), 77.37% were normal weight (≥2.5, <4.0 kg), and 11.00% were LGA (≥4.0 kg).
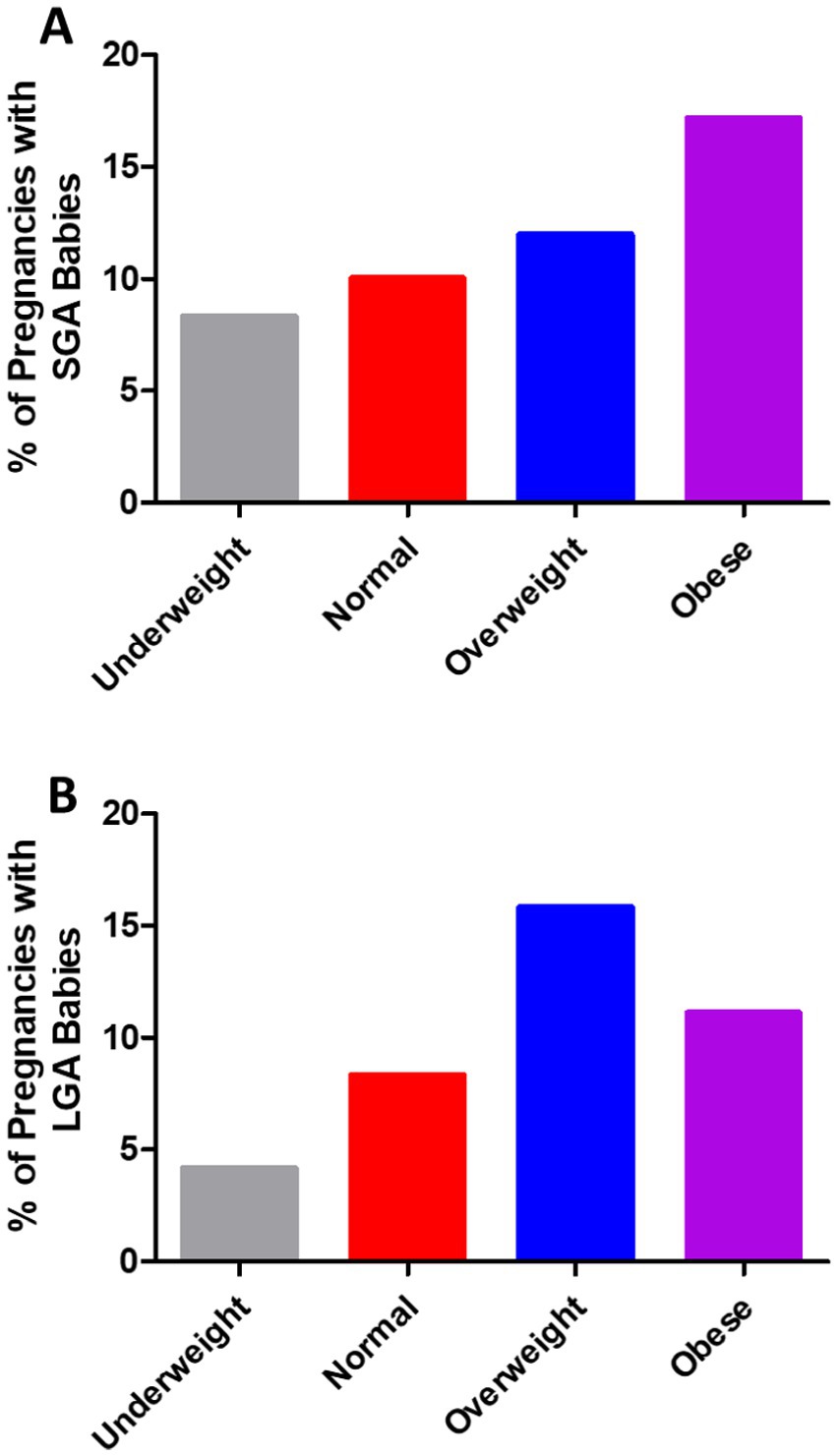
Figure 2. (A) Small for gestational age (SGA) and (B) large for gestational age (LGA) babies were more common in the pregnancies of the women who were overweight or obese compared with those who were underweight or of normal weight in the cases of GDM.
3.2 Factors associated with neonatal birth weight
Univariate linear regression analysis was performed to determine the relationship between the clinical parameters and neonatal birth weight. As shown in Table 2, in the unadjusted model, we observed no significant association between maternal pre-pregnancy BMI and neonatal birth weight (p > 0.05). Moreover, no significant association was observed between neonatal birth weight and a family history of diabetes or mode of delivery (p > 0.05). Notably, there was a significant negative relationship between neonatal birth weight and maternal age [β = −0.01, 95% CI (−0.02, −0.00); p = 0.045], nationality [β = −0.88, 95% CI (−1.39, −0.37); p = 0.001], pre-pregnancy hypertension [β = −0.89, 95% CI (−1.18, −0.60); p < 0.000], infant sex [β = −0.11, 95% CI (−0.20, −0.01); p = 0.029], and abnormal pregnancy history [β = −0.19, 95% CI (−0.31, −0.07); p = 0.003].
3.3 Relationship between maternal pre-pregnancy BMI and neonatal birth weight
As shown in Figure 3, smooth curve fitting was performed after adjusting for possible factors, including maternal age, status, nationality, a family history of diabetes, abnormal pregnancy history, infant sex, and mode of delivery. Maternal pre-pregnancy BMI exhibited a non-linear relationship with neonatal birth weight, and the resulting curve exhibited a two-stage change with a breakpoint at 27.78 kg/m2. When the maternal pre-pregnancy BMI value was below the breakpoint, there was a positive relationship between maternal pre-pregnancy BMI and neonatal birth weight. However, when the value exceeded the breakpoint, there was an inverse relationship between maternal pre-pregnancy BMI and neonatal birth weight. Table 3 shows that the threshold effect was further analyzed based on curve fitting. Specifically, neonatal birth weight displayed a significantly increasing trend with increasing maternal pre-pregnancy BMI when maternal pre-pregnancy BMI was less than 27.78 kg/m2 [β = 0.03, 95%CI (0.01, 0.04); p = 0.0052 < 0.05]. However, when maternal pre-pregnancy BMI was greater than 27.78 kg/m2, neonatal birth weight decreased as maternal pre-pregnancy BMI increased [β = −0.01, 95% CI (−0.04, 0.01); p = 0.3555].
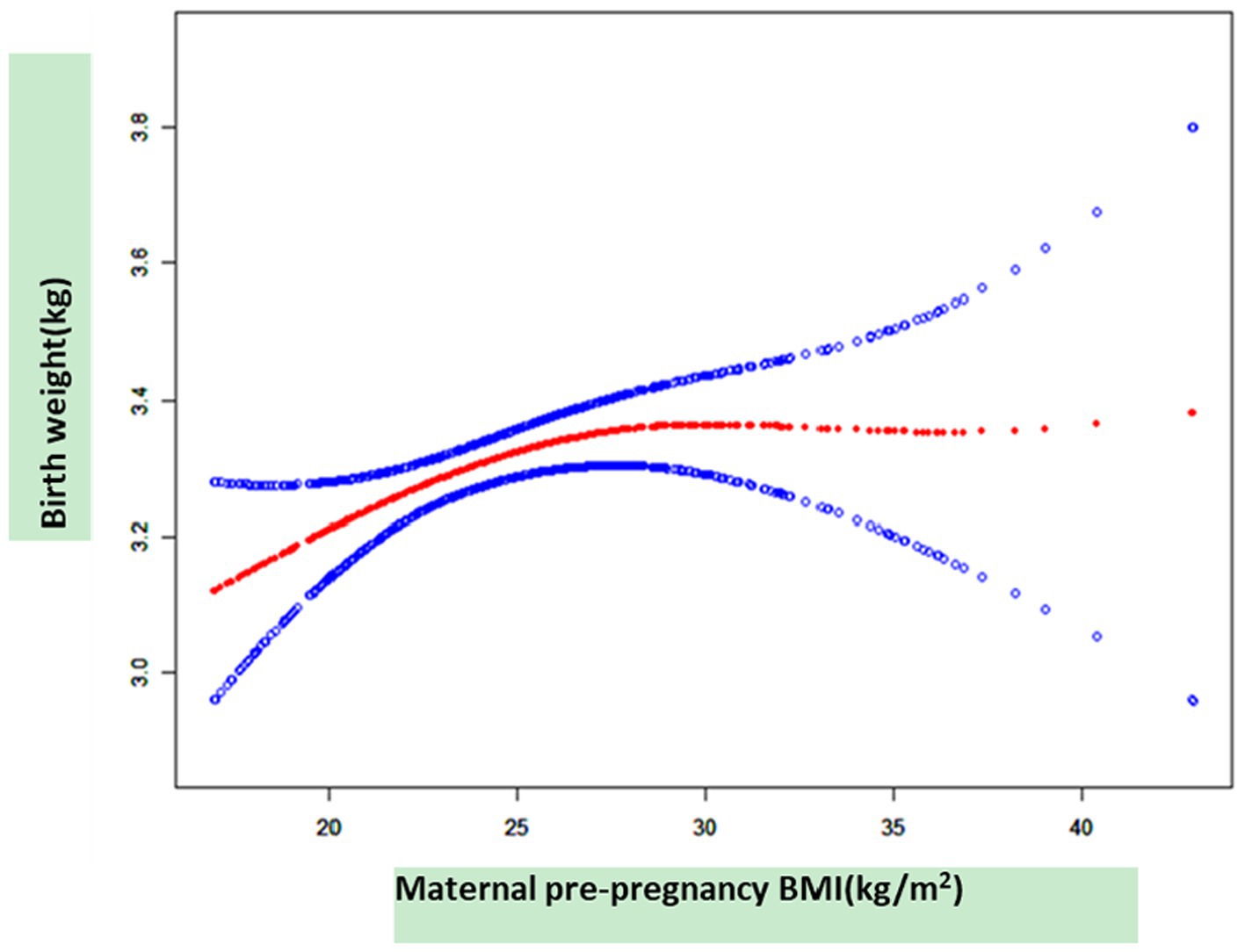
Figure 3. The relationship between maternal pre-pregnancy BMI and neonatal birth weight, as determined by smooth curve fitting.
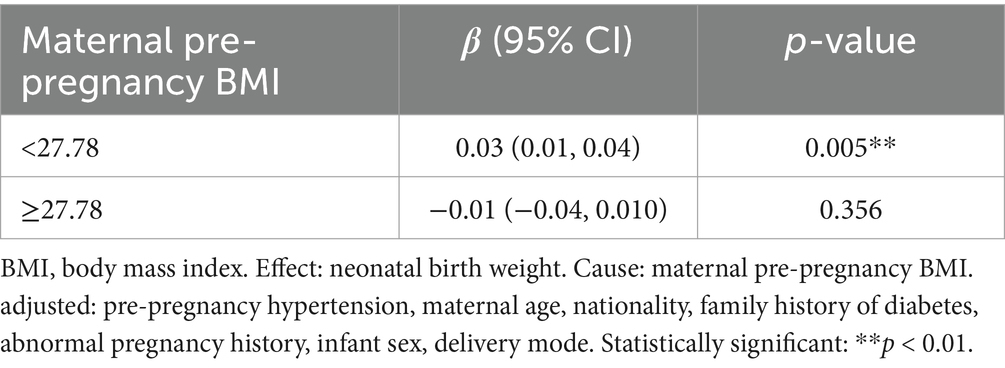
Table 3. The independent association between maternal pre-pregnancy BMI (kg/m2) and neonatal birth weight (kg) by multivariate piecewise linear regression.
4 Discussion
In this retrospective cohort study, approximately half of the women with GDM were overweight or obese before pregnancy. In contrast, very few women were underweight. The women who were overweight or obese before pregnancy were more likely to have SGA or LGA births, especially in the cases of GDM. Furthermore, we observed a non-linear relationship between maternal pre-pregnancy BMI and neonatal birth weight in cases of GDM, with the turning point of maternal pre-pregnancy BMI at 27.78 kg/m2. When the maternal pre-pregnancy BMI value was below the breakpoint, there was a positive relationship between maternal pre-pregnancy BMI and neonatal birth weight. However, when the value exceeded the breakpoint, the relationship reversed. The data suggest that different management strategies should be implemented for pregnant women with a pre-pregnancy BMI value below 27.78 kg/m2 and above 27.78 kg/m2, particularly in cases of GDM. These findings highlight the importance of providing information, preconception counseling, and health education on weight management for healthy pregnancies.
Previous studies have shown that the fetuses of pregnant women with GDM may be macrosomic, SGA, or of normal birth weight, depending on the severity of diabetes and the degree of diabetes control (2, 13). Better control of diabetes normalizes fetal growth, while severe diabetes often results in SGA fetuses (14). Although there are different causes for FGR or LGA, paradoxically, both are related to the “metabolic syndrome” (15). Therefore, we are working to find a way to improve maternal-fetal outcomes before pregnancy.
In a previous study, it was confirmed that there is a direct relationship between maternal BMI and neonatal birth weight (16). Another study supported the view that pre-pregnancy BMI is positively associated with the birth weight of neonates in cases of GDM (17). However, some researchers have pointed out that pre-gestational BMI is not significantly associated with macrosomia (18). Such discrepancies may be attributed to other factors, such as gestational diabetes treatment during pregnancy, pathological obesity, and ethnic differences.
In previous studies, maternal age, family history of diabetes, education, week of gestation at diagnosis, and neonatal sex were the adjusted variables that affected the relationship between maternal pre-pregnancy BMI and neonatal birth weight (17, 19). In our study, we enrolled women who were diagnosed with GDM between 24 and 28 gestational weeks, and we excluded participants with multiple pregnancies and those with pre-pregnancy diabetes mellitus. In addition, we adjusted for maternal family history of diabetes and abnormal pregnancy history. The information obtained from our study showed that maternal pre-pregnancy overweight/obesity evidently increased the rates of SGA or LGA births. It is worth noting that a non-linear relationship was observed between maternal pre-pregnancy BMI and neonatal birth weight. When the maternal pre-pregnancy BMI value was ≤27.78 kg/m2, the relationship was positive. However, when the maternal pre-pregnancy BMI value was ≥27.78 kg/m2, the relationship was reversed.
Pre-conception counseling should be offered to all women with GDM. The pre-conception assessment should be performed by an endocrinologist or obstetrician with expertise in diabetes and pregnancy. This evaluation should address pregnancy risks, medication safety, and the necessary interventions to optimize pre-pregnancy health. For women who are overweight or obese, it is recommended to review lifestyle modifications for weight reduction, provide guidance on appropriate weight gain during pregnancy, and screen for comorbidities. Medical nutritional therapy and exercise have been proven to be effective in lowering LGA and macrosomia rates without increasing SGA rates (20). A meta-analysis of randomized trials focusing on lifestyle interventions during pregnancy showed that improving BMI before pregnancy, rather than during pregnancy, may be more effective in the prevention of pregnancy or offspring complications (21). Based on this study, women should also be educated about the association between maternal pre-pregnancy weight and adverse newborn outcomes.
Neonatal birth weight and pre-pregnancy BMI values were obtained from the hospital’s medical record system, which reduced potential bias in the study. Moreover, the data included specific maternal information, which was helpful in adjusting for potential confounding factors. In addition, information on maternal age at conception, mode of delivery, and fetal sex was available and helped us to control for confounding factors.
Inevitably, our study has several limitations. First, this was a retrospective study, and thus, the collected data may have introduced bias into the results of this study. Second, the specimens were obtained from only one district. Therefore, the results of the study are not nationally representative and may not apply to all women in China or to populations beyond this region. Finally, this study lacked a control group. Setting a control parameter would allow us to visualize fetal birth weight changes with pre-pregnancy BMI changes in cases without gestational diabetes.
Overall, the association between pre-pregnancy BMI and fetal birth weight should be further investigated in other populations to confirm our results. Establishing a clear relationship between pre-pregnancy body mass index and fetal birth weight is crucial for developing early prevention strategies for pregnant women with gestational diabetes.
5 Conclusion
We described a non-linear relationship between maternal pre-pregnancy BMI and neonatal birth weight in the women with GDM, after adjusting for potential confounders. The turning point of maternal pre-pregnancy BMI was 27.78 kg/m2. Neonatal birth weight displayed a significantly increasing trend with increasing maternal pre-pregnancy BMI when maternal pre-pregnancy BMI was less than 27.78 kg/m2. However, when maternal pre-pregnancy BMI was greater than 27.78 kg/m2, neonatal birth weight decreased as maternal pre-pregnancy BMI increased. These findings highlight the importance of providing information, preconception counseling, and health education on weight management for healthy pregnancies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Fujian Maternity and Child Health Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
QL: Data curation, Funding acquisition, Investigation, Writing – original draft. TY: Data curation, Investigation, Writing – original draft. JC: Data curation, Investigation, Writing – review & editing. XZ: Data curation, Funding acquisition, Investigation, Writing – review & editing. LZ: Funding acquisition, Supervision, Writing – review & editing. JY: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Innovation Platform Project of Science and Technology, Fujian Province (2021Y2012); National Key Clinical Specialty Construction Program of China (Obstetric); Fujian Provincial Natural Science Foundation of China (2020J01333); Joint Funds for the Innovation of Science and Technology, Fujian Province (2021Y9164); Fujian Provincial Natural Science Foundation of China (2023J011220).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
GDM, Gestational diabetes mellitus; BMI, Body mass index; SGA, Small for gestational age; LGA, Large for gestational age; FGR, Fetal growth restriction; WHO, World Health Organization; OGTT, Oral glucose tolerance test.
References
1. Sweeting, A, Hannah, W, Backman, H, Catalano, P, Feghali, M, Herman, WH, et al. Epidemiology and management of gestational diabetes. Lancet. (2024) 404:175–92. doi: 10.1016/S0140-6736(24)00825-0
2. Perak, AM, Lancki, N, Kuang, A, Labarthe, DR, Allen, NB, Shah, SH, et al. Associations of gestational cardiovascular health with pregnancy outcomes: the hyperglycemia and adverse pregnancy outcome study. Am J Obstet Gynecol. (2021) 224:210.e1–210.e17. doi: 10.1016/j.ajog.2020.07.053
3. Kaul, P, Savu, A, Yeung, RO, and Ryan, EA. Association between maternal glucose and large for gestational outcomes: real-world evidence to support hyperglycaemia and adverse pregnancy outcomes (HAPO) study findings. Diabet Med. (2022) 39:e14786. doi: 10.1111/dme.14786
4. Wang, H, Li, N, Chivese, T, Werfalli, M, Sun, H, Yuen, L, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res Clin Pract. (2022) 183:109050. doi: 10.1016/j.diabres.2021.109050
5. Zhu, H, Zhao, Z, Xu, J, Chen, Y, Zhu, Q, Zhou, L, et al. The prevalence of gestational diabetes mellitus before and after the implementation of the universal two-child policy in China. Front Endocrinol. (2022) 13:960877. doi: 10.3389/fendo.2022.960877
6. Martinez-Hortelano, JA, Cavero-Redondo, I, Alvarez-Bueno, C, Garrido-Miguel, M, Soriano-Cano, A, and Martinez-Vizcaino, V. Monitoring gestational weight gain and prepregnancy BMI using the 2009 IOM guidelines in the global population: a systematic review and meta-analysis. BMC Pregnancy Childbirth. (2020) 20:649. doi: 10.1186/s12884-020-03335-7
7. Song, Z, Cheng, Y, Li, T, Fan, Y, Zhang, Q, and Cheng, H. Effects of obesity indices/GDM on the pregnancy outcomes in Chinese women: a retrospective cohort study. Front Endocrinol. (2022) 13:1029978. doi: 10.3389/fendo.2022.1029978
8. Fayed, A, Wahabi, HA, Esmaeil, S, Elkouny, R, Elmorshedy, H, and Bakhsh, H. Independent effect of gestational weight gain and prepregnancy obesity on pregnancy outcomes among Saudi women: a sub-cohort analysis from Riyadh mother and baby cohort study (RAHMA). PLoS One. (2022) 17:e0262437. doi: 10.1371/journal.pone.0262437
9. Xie, YJ, Peng, R, Han, L, Zhou, X, Xiong, Z, Zhang, Y, et al. Associations of neonatal high birth weight with maternal pre-pregnancy body mass index and gestational weight gain: a case-control study in women from Chongqing, China. BMJ Open. (2016) 6:e010935. doi: 10.1136/bmjopen-2015-010935
10. Metzger, BE, Gabbe, SG, Persson, B, Buchanan, TA, Catalano, PA, Damm, P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. (2010) 33:676–82. doi: 10.2337/dc09-1848
11. Gilmore, LA, and Redman, LM. Weight gain in pregnancy and application of the 2009 IOM guidelines: toward a uniform approach. Obesity. (2015) 23:507–11. doi: 10.1002/oby.20951
12. Villar, J, Cheikh Ismail, L, Victora, CG, Ohuma, EO, Bertino, E, Altman, DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. (2014) 384:857–68. doi: 10.1016/S0140-6736(14)60932-6
13. Andrews, C, Monthé-Drèze, C, Sacks, DA, Ma, RCW, Tam, WH, McIntyre, HD, et al. Role of maternal glucose metabolism in the association between maternal BMI and neonatal size and adiposity. Int J Obes. (2021) 45:515–24. doi: 10.1038/s41366-020-00705-1
14. Li, J, Pan, Y, Zheng, Q, Chen, X, Jiang, X, Liu, R, et al. Risk factors and glycaemic control in small-for-gestational-age infants born to mothers with gestational diabetes mellitus: a case-control study using propensity score matching based on a large population. BMJ Open. (2024) 14:e078325. doi: 10.1136/bmjopen-2023-078325
15. Aji, AS, Lipoeto, NI, Yusrawati, Y, Malik, SG, Kusmayanti, NA, Susanto, I, et al. Association between pre-pregnancy body mass index and gestational weight gain on pregnancy outcomes: a cohort study in Indonesian pregnant women. BMC Pregnancy Childbirth. (2022) 22:492. doi: 10.1186/s12884-022-04815-8
16. Gul, R, Iqbal, S, Anwar, Z, Ahdi, SG, Ali, SH, and Pirzada, S. Pre-pregnancy maternal BMI as predictor of neonatal birth weight. PLoS One. (2020) 15:e0240748. doi: 10.1371/journal.pone.0240748
17. Zhao, R, Xu, L, Wu, ML, Huang, SH, and Cao, XJ. Maternal pre-pregnancy body mass index, gestational weight gain influence birth weight. Women Birth. (2018) 31:e20–5. doi: 10.1016/j.wombi.2017.06.003
18. Alberico, S, Montico, M, Barresi, V, Monasta, L, Businelli, C, Soini, V, et al. The role of gestational diabetes, pre-pregnancy body mass index and gestational weight gain on the risk of newborn macrosomia: results from a prospective multicentre study. BMC Pregnancy Childbirth. (2014) 14:23. doi: 10.1186/1471-2393-14-23
19. Kirkegaard, H, Bliddal, M, Støvring, H, Rasmussen, KM, Gunderson, EP, Køber, L, et al. Maternal weight change from prepregnancy to 18 months postpartum and subsequent risk of hypertension and cardiovascular disease in Danish women: a cohort study. PLoS Med. (2021) 18:e1003486. doi: 10.1371/journal.pmed.1003486
20. Kgosidialwa, O, Egan, AM, Carmody, L, Kirwan, B, Gunning, P, and Dunne, FP. Treatment with diet and exercise for women with gestational diabetes mellitus diagnosed using IADPSG criteria. J Clin Endocrinol Metab. (2015) 100:4629–36. doi: 10.1210/jc.2015-3259
21. Voerman, E, Santos, S, Patro Golab, B, Amiano, P, Ballester, F, Barros, H, et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: an individual participant data meta-analysis. PLoS Med. (2019) 16:e1002744. doi: 10.1371/journal.pmed.1002744
Keywords: GDM, neonatal birth weight, pre-pregnancy BMI, small for gestational age, large for gestational age
Citation: Liao Q, Yu T, Chen J, Zheng X, Zheng L and Yan J (2025) Relationship between maternal pre-pregnancy BMI and neonatal birth weight in pregnancies with gestational diabetes mellitus: a retrospective cohort study. Front. Med. 11:1478907. doi: 10.3389/fmed.2024.1478907
Edited by:
Momcilo Jankovic, Fondazione MBBM, ItalyReviewed by:
Xiangyuan Yu, Guilin Medical University, ChinaOluwasegun Akinyemi, Howard University, United States
Copyright © 2025 Liao, Yu, Chen, Zheng, Zheng and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lianghui Zheng, emhlbmdsaWFuZ2h1aTI5MkAxNjMuY29t; Jianying Yan, eWFuamlhbnlpbmc2NkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Qiuping Liao1,2,3†
Qiuping Liao1,2,3† Tiantian Yu
Tiantian Yu Jiajia Chen
Jiajia Chen Jianying Yan
Jianying Yan