- 1Cervical Disease Diagnosis and Treatment Health Center, Fujian Maternity and Child Health Hospital College of Clinical Medical for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, China
- 2Department of Obstetrics, Fujian Maternity and Child Health Hospital College of Clinical Medical for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, China
- 3Department of Gynecology, Fujian Maternity and Child Health Hospital College of Clinical Medical for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, China
- 4Department of Pathology, Fujian Maternity and Child Health Hospital College of Clinical Medical for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, China
Objective: This study evaluates the effectiveness of endocervical curettage (ECC) in identifying additional cervical cancer and its precursors in women with atypical glandular cells (AGC) cytology.
Methods: We conducted a retrospective analysis of medical records for women referred to colposcopy with AGC cytology between January 2019 and December 2023. The study included 433 women with AGC cytology who underwent both biopsy and ECC. Clinical characteristics such as demographics, clinical history, cytology, HPV status, colposcopic findings, and pathology were analyzed. Chi-square and Fisher's exact tests were applied to compare the characteristics of ECC-diagnosed cervical precancers or worse (HSIL+) and normal/low-grade squamous intraepithelial lesions (LSIL).
Results: The overall detection rate of HSIL+ in this population was 19.4% (86/443), with ECC alone identifying HSIL+ in 1.3% (6/443) of cases. However, ECC showed greater utility in certain subgroups. The highest additional HSIL+ detection from ECC was observed in women with HPV 16/18 infection (7.2%) and those with AGC-FN cytology (4.4%). ECC's additional yield of HSIL+ was higher in those with normal or LSIL colposcopic impressions compared to those with HSIL+ impressions. Conversely, no additional HSIL+ cases were identified by ECC alone in women under 30 years old, those with negative high-risk HPV results, or those with type 1/2 transformation zones.
Conclusion: For women with AGC cytology, ECC should be performed in patients with AGC-FN cytology, HPV 16/18 infections, type 3 transformation zones, and normal or low-grade colposcopic impressions. This approach enhances the identification of HSIL+ cases by reducing false negatives. However, for women younger than 30 years old and those with type 1/2 transformation zones, ECC offers limited benefit.
1 Introduction
Colposcopy is an important diagnostic procedure for identifying precancerous lesions of the cervix, offering a thorough examination to detect and assess abnormal areas. Nevertheless, the endocervical canal often remains difficult to visualize during colposcopy, which can result in missed significant lesions. Lesions of the cervical glandular epithelium can arise not only from the squamocolumnar junction but also from columnar cells situated further up the cervical canal, frequently involving the endocervical canal. Atypical glandular cells (AGC) are the most common cytological type of cervical glandular epithelial lesions, which include adenocarcinoma in situ (AIS) and adenocarcinoma (1).
Endocervical curettage (ECC) involves scraping cells from the endocervical canal and is used to detect abnormalities in this otherwise challenging-to-access area. The effectiveness of ECC in lowering the miss rate of colposcopy findings remains controversial. The Society of Canadian Colposcopists (2) and the American Society for Colposcopy and Cervical Pathology (ASCCP) (3) advocate for ECC in cases where atypical glandular cells are found in cytology. In contrast, the British Society for Colposcopy and Cervical Pathology (BSCCP) (4) does not support the routine use of ECC during colposcopy, even for glandular lesions.
Research has shown that ECC can effectively detect additional cervical cancer and its precursors that might be overlooked by biopsy alone in women with high-risk cytology, including atypical squamous cells, favor high-grade (ASC-H), high-grade squamous intraepithelial lesions (HSIL), and AGC (5–7). Given its rarity—typically <1% of cervical smear results (8)—AGC has not been extensively examined as a standalone condition. In China, ECC is routinely performed when AGC is identified cytologically, providing an opportunity to evaluate its practical effectiveness.
The Cervical Disease Diagnosis and Treatment Health Center at Fujian Maternity and Child Health Hospital, the largest center for cervical diseases in Fujian province, China, has a robust data collection system that includes histopathology, cytopathology, colposcopic findings, and patient details from all colposcopy exams. This retrospective study utilizes this comprehensive dataset to assess how effectively ECC identifies additional cervical cancer and its precursors in women with AGC cytology.
2 Materials and methods
2.1 Study design
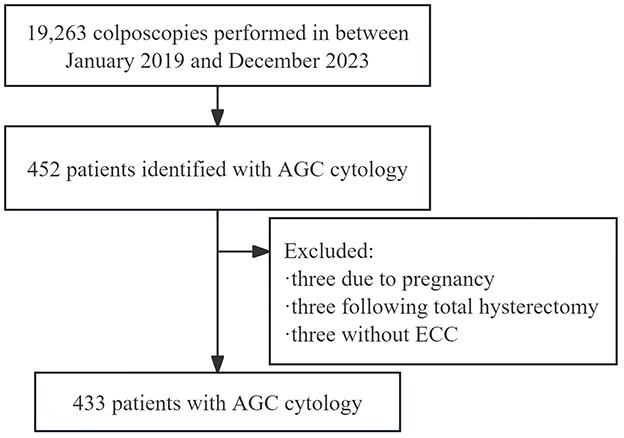
Data were collected from the electronic medical records of women who underwent colposcopic examinations following abnormal cervical screening results at the Cervical Disease Diagnosis and Treatment Health Center of Fujian Maternity and Child Health Hospital. The patient selection process is detailed in Figure 1. Between January 2019 and December 2023, 19,263 colposcopies were performed at the center. Out of the 452 patients identified with AGC cytology, nine were excluded: three due to pregnancy, three following total hysterectomy, and three because of a closed cervical ostium that made endocervical curettage impossible. Therefore, the study included 433 patients with AGC cytology who had both biopsy and ECC. The time between cytology/HPV testing and colposcopy was always <3 months. Collected demographic and clinical information included age, gravidity, parity, menopausal status, cytological results, HPV status, colposcopic impressions, transformation zone type, and pathological findings. This study was approved by the Ethics Committee at Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University (2024KY014). Informed consent was waived due to the retrospective nature of the study. The findings were reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
2.2 Cytology and HPV testing
Cytology was conducted using the liquid-based ThinPrep test. In this procedure, a cell brush is inserted into the external cervical canal to collect cells from the exocervix and endocervix. These cells are then smeared onto a slide and fixed. According to the Bethesda System (9), atypical glandular cells (AGC) can be categorized into AGC-NOS (not otherwise specified, including endocervical and endometrial) and AGC-FN (favor neoplastic).
A total of three HPV testing approaches were utilized: the hybrid capture 2 assay (Qiagen, Hilden, Germany), which detects the DNA of 13 high-risk oncogenic HPV types (including HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68); the polymerase chain reaction-reverse dot blot (PCR-RDB) HPV genotyping method (Yaneng® Limited Corporation, Shenzhen, China), capable of identifying 18 high-risk HPV types (such as HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, and 83) along with 5 low-risk types (6, 11, 42, 43, and 81); and the Aptima assay (Gen-Probe Inc., San Diego, CA), which focuses on the E6/E7 mRNA of 14 high-risk HPV types (including HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68). HPV status is classified as HPV 16/18, non-16/18 high-risk HPV, or negative.
2.3 Colposcopy/ECC and biopsy procedures
All patients with AGC-NOS (endometrial) and negative high-risk HPV results underwent a segmented curettage procedure before colposcopy, with no abnormalities detected in the histopathology. Colposcopic examinations were conducted using a dynamic spectral imaging (DSI) colposcope (Leisegang, Germany). Up to four lesion-directed biopsies were obtained from distinct areas of the epithelium that showed acetowhitening, metaplasia, or other visible abnormalities after the application of 5% acetic acid and Lugol's iodine using Tischler biopsy forceps. If fewer than four directed biopsies were collected, additional biopsies were taken from areas of normal-appearing cervical transformation zone epithelium until a total of four biopsies were achieved. If the cervix appeared entirely normal, random biopsies were performed at the squamocolumnar junction (SCJ) in the 3, 6, 9, or 12 o'clock positions, assuming the SCJ was fully visualized. When the SCJ was not completely visible, biopsies were collected from the external cervical ostium at the corresponding 3, 6, 9, or 12 o'clock positions. Following cervical biopsy, endocervical curettage (ECC) was performed using a Kevorkian curette. Results from ECC and biopsies were categorized as normal, low-grade squamous intraepithelial lesions (LSIL), HSIL, or invasive cancer based on the Lower Anogenital Squamous Terminology system (10). The diagnosis was based on the highest grade lesion identified, with HSIL+ encompassing HSIL, adenocarcinoma in situ (AIS), and invasive cancers, while all other findings were classified as <HSIL. The pathological evaluation of cervical biopsies and ECC samples was carried out independently by two senior pathologists who were blinded to each other's results.
2.4 Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 26.0 (IBM Corp, Armonk, NY). Statistical significance was determined using two-sided tests with a threshold of P < 0.05. Categorical variables were expressed as frequencies and percentages. For analysis, chi-square tests were used, and Fisher's exact test was applied when appropriate.
3 Results
3.1 Study sample and data features
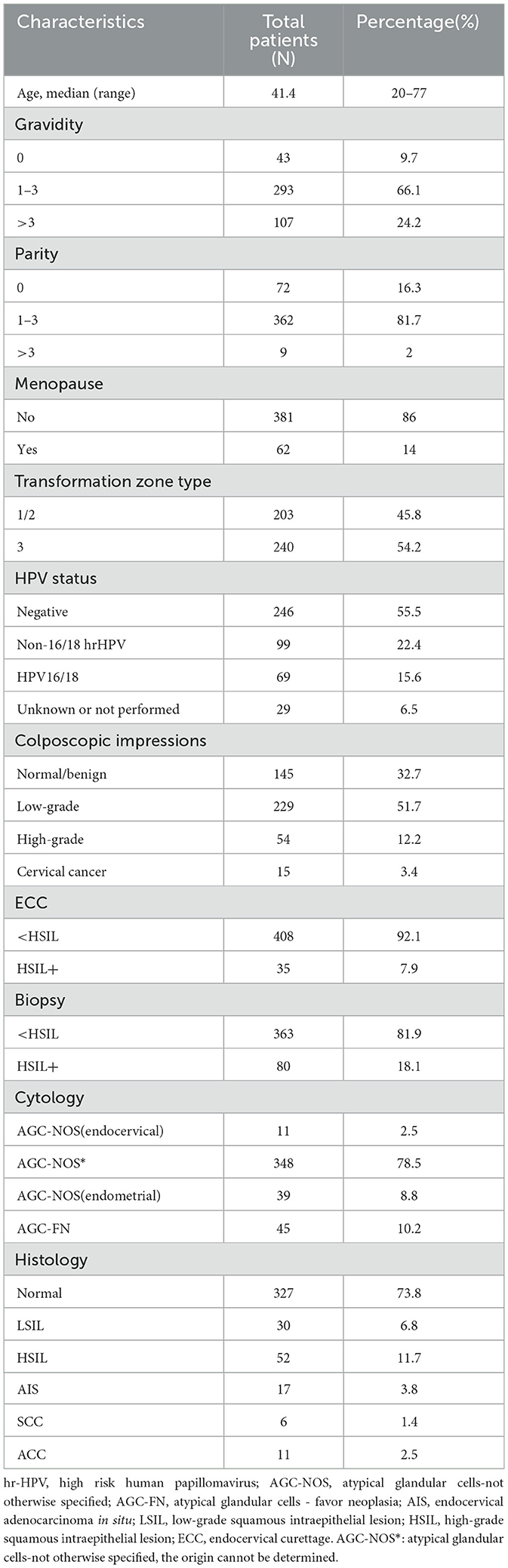
Table 1 provides an overview of the demographic and clinical characteristics of the study participants. The mean age of the patients was 41.4 years, with a range spanning from 20 to 77 years. Among the women, 81.7% (n = 362) had experienced one to three childbirths, and a majority, 86% (n = 381), were premenopausal. Additionally, 54.2% (n = 240) had a transformation zone classified as type 3. The most common cytological result was AGC-NOS, which constituted 78.5% (n = 348) of the cases. HPV 16/18 infections were detected in 15.6% (n = 69) of the participants, whereas 22.4% (n = 99) had infections with other high-risk HPV types. Colposcopic findings were normal in 32.7% (n = 145) of cases, low-grade in 51.7% (n = 229), high-grade in 12.2% (n = 54), and cervical cancer was diagnosed in 3.4% (n = 15). Histopathological examination of biopsies revealed HSIL+ in 18.1% (n = 80) of cases, while ECC histopathology identified HSIL+ in 7.9% (n = 35). Overall, 11.7% (n = 52) of the cases were diagnosed with HSIL, 3.8% (n = 17) with AIS, 1.4% (n = 6) with SCC (cervical squamous carcinoma), and 2.5% (n = 11) with ACC (cervical adenocarcinoma).
3.2 HSIL+ diagnostic yield by biopsy and ECC
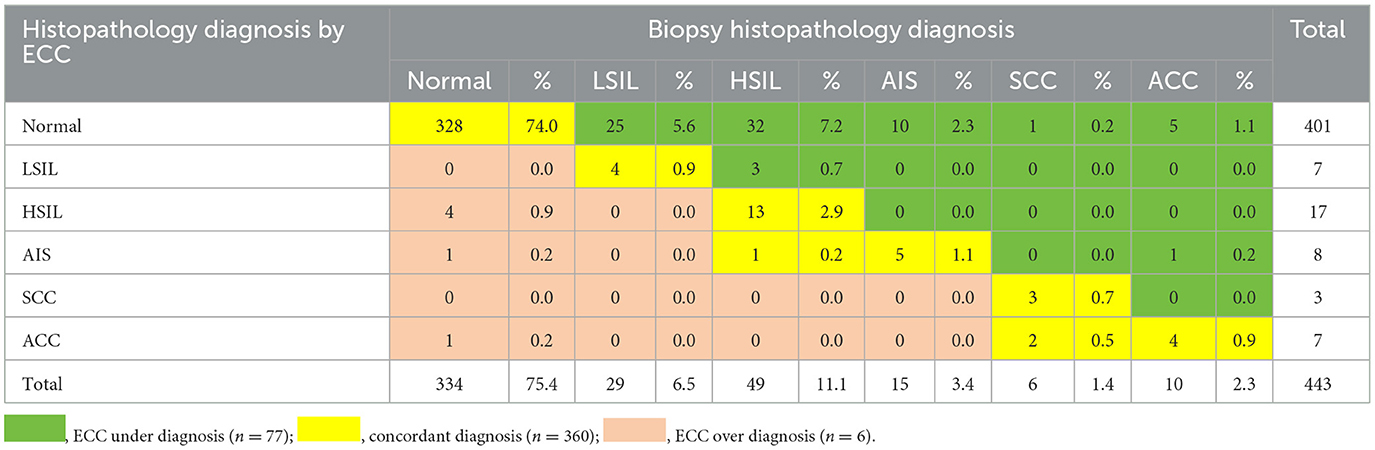
The diagnostic yield for HSIL+ by biopsy and ECC is presented in Table 2. The overall concordance rate between histopathologic results of biopsy and ECC was 81.3% (360/443). In 17.4% (77/443) of cases, the biopsy revealed a higher grade of pathologic results than ECC. Conversely, ECC showed a higher grade of pathologic results in 1.3% (6/443) of cases, including 4 cases of HSIL, 1 case of AIS, and 1 case of ACC, with corresponding biopsy pathology results being normal. Biopsy alone detected 93% (80/86) of HSIL+ cases missed by ECC. Additionally, 7.7% (4/52) of HSIL cases, 5.9% (1/17) of AIS cases, and 9.0% (1/11) of ACC cases were missed by biopsy alone but were detected when biopsy was combined with ECC.
3.3 Clinical characteristics associated with HSIL+ diagnostic yield by ECC
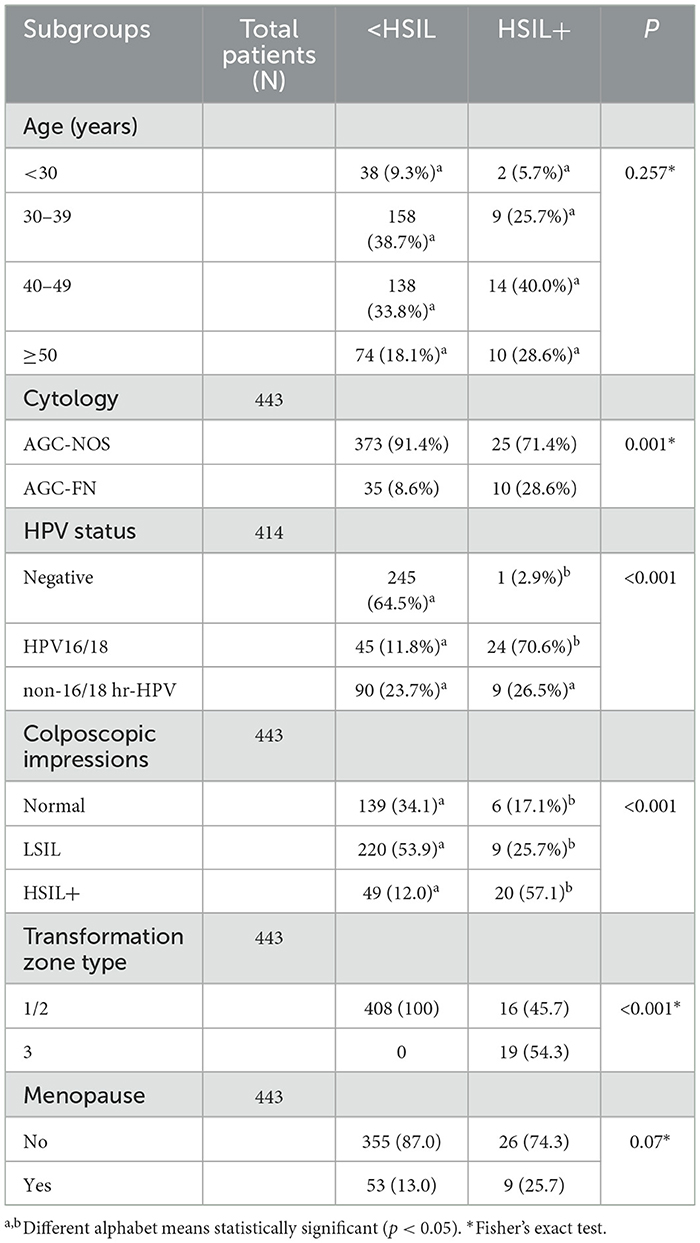
Table 3 presents the clinical features linked to HSIL+ detection via ECC. There was no significant difference between different age groups in terms of ECC-detected normal/LSIL and HSIL+ results (P = 0.257). Women with AGC-FN had a higher likelihood of HSIL+ results on ECC compared to those with AGC-NOS (28.6% vs. 8.6%, P = 0.001). The HPV16/18 positive group exhibited the highest rate of HSIL+ detection by ECC, whereas the high-risk HPV negative group was more likely to present normal or LSIL results (P < 0.001). Notably, HSIL+ was more frequently detected by ECC when colposcopy impressions were normal or LSIL compared to when colposcopy impressions were HSIL+ (P < 0.001). All HSIL+ ECC results were found in type 3 transformation zones. Although menopausal women showed a higher propensity for HSIL+ ECC results (25.7% vs. 13.0%), this finding was not statistically significant (P = 0.07).
3.4 Stratification of HSIL+ diagnostic yield based solely on ECC
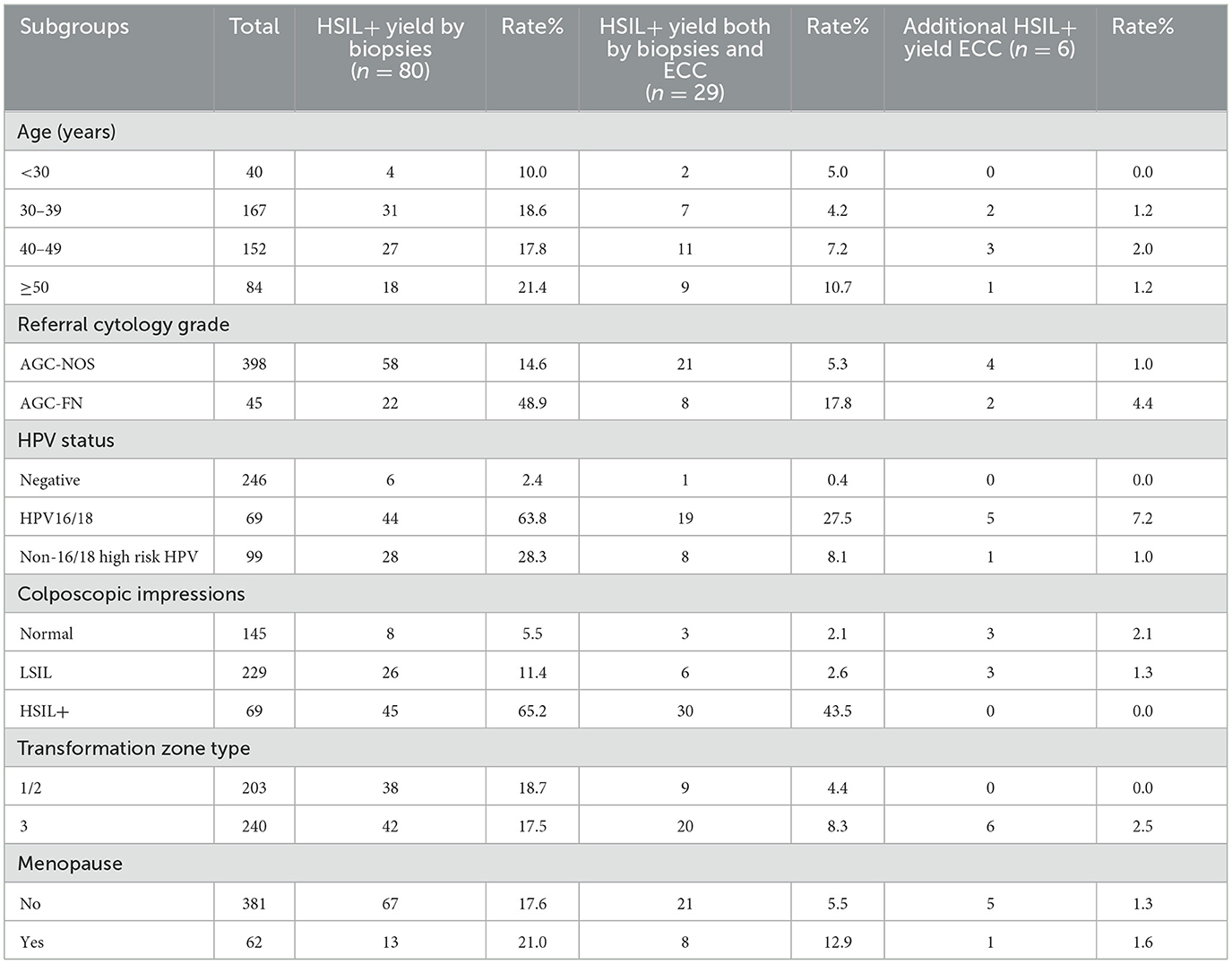
Table 4 presents the rates of HSIL+ detection via ECC and biopsy, organized by age group, cytology, HPV infection, colposcopic impression, transformation zone type, and menstrual status. The additional diagnostic yield of HSIL+ by ECC varied from 0.0% to 7.2% across different risk categories. The highest yield of HSIL+ was observed in women with HPV16/18 infection, reaching 7.2% (5/69). ECC identified an additional 4.4% (2/45) of HSIL+ cases in women with AGC-FN cytology. The most notable increase in detection rate from ECC was in patients aged 40–49, at 2.0% (3/152). For women with normal or LSIL colposcopic impressions, the HSIL+ diagnostic yield by ECC was 3.4% (6/374), whereas in the type 3 transformation zone group, ECC detected 2.5% (6/240) more HSIL+ cases. ECC also identified HSIL+ in 1.3% (5/381) of premenopausal women and 1.6% (1/62) of postmenopausal women, cases that were missed by biopsy alone. In contrast, ECC did not detect any additional HSIL+ cases in patients under 30 years, those with negative high-risk HPV infection, HSIL+ colposcopic impressions, or type 1/2 transformation zones.
4 Discussion
This study evaluated the effectiveness of ECC in identifying HSIL+ among women referred for colposcopy due to AGC cytology. The results revealed an overall HSIL+ detection rate of 19.4% (86/443) in this population, with ECC alone detecting HSIL+ in 1.3% (6/443) of cases. This suggests that approximately 100 women would need to endure the extended discomfort of ECC procedures to identify one additional HSIL+ case not captured by biopsy alone. Nonetheless, ECC proved more beneficial in specific subgroups. The rate of HSIL+ detection by ECC alone increased to 2.0% (8/631) among women aged 40–49 years. The highest additional HSIL+ detection from ECC was observed in women infected with HPV 16/18, at 7.2%, and in those with AGC-FN cytology, where the additional detection rate was 4.4%. ECC's additional yield of HSIL+ was higher in the group with normal or LSIL colposcopic impressions compared to the HSIL+ colposcopic impression group. Conversely, no additional HSIL+ cases were missed by biopsy alone in women under 30 years of age, those with negative high-risk HPV results, or those with type 1/2 transformation zones.
AGC represent cytological abnormalities in which glandular cells show changes but do not meet the criteria for adenocarcinoma in situ or invasive adenocarcinoma of the cervix uteri. These abnormalities can range from benign changes and cervical precursor lesions of glandular or squamous origins to invasive cervical cancer and other gynecological malignancies (8). This study found that 19.4% of women with AGC had cervical intraepithelial neoplasia grade two or worse, including adenocarcinoma in situ (CIN2+/AIS+), a figure comparable to a meta-analysis (11) reporting that 19.8% of women with AGC will have high-grade squamous intraepithelial lesions (HSIL+). Although AIS and ACC often originate within the endocervical canal, this study demonstrated that all AIS cases were detectable by biopsy alone. Of the 11 ACC cases, only one with a type 3 transformation zone would have been missed without the use of ECC.
The varying detection rates of HSIL+ through ECC across different subgroups underscore the heterogeneity of AGC presentations. The additional detection of HSIL+ relies on two key factors: the risk of HSIL+ and whether the colposcopy comprehensively visualizes the transformation zone where nearly all cervical cancers develop. HPV genotype is a significant risk factor. In women with AGC cytology results, HPV 16/18 infection poses a higher risk compared to other high-risk HPV infections and HPV-negative women (12–14). Numerous studies have indicated that ECC has a higher additional detection rate for HSIL+ in patients positive for HPV 16/18 (6, 15–18). Our findings align with these studies. When AGC is identified cytologically, the cumulative incidence risk of cervical cancer is moderately lower than that of HSIL but considerably higher than that of LSIL (1), thus colposcopy is recommended for all patients regardless of HPV status (19). If colposcopy impression is normal or LSIL, HSIL+ may potentially missed by biopsy alone. Women with AGC-FN cytology have a higher risk of HSIL+, especially AIS+, compared to those with AGC-NOS (20). The type 3 transformation zone poses significant challenges for adequate visualization and sampling during colposcopy, making ECC a valuable adjunctive procedure. Conversely, the absence of missed HSIL+ cases in younger women, those with type 1/2 transformation zones, and those negative for high-risk HPV suggests that ECC may have limited utility in these subgroups.
Our findings concur with previous research indicating a relatively low yield of ECC in detecting additional HSIL+ cases in certain populations. For instance, Solomon et al. (6, 21) reported similar low detection rates of HSIL+ by ECC in women younger than 30 years old. This can be attributed to the lower incidence of cervical cancer in women younger than 30 years old (22) and the predominance of type 1/2 transformation zones in this demographic (23).
In light of the contrasting guidelines regarding ECC, our findings highlight the importance of context and patient-specific factors when considering its use. While the ASCCP recommends ECC for patients with atypical glandular cells to enhance detection rates, the hesitancy of the BSCCP to endorse routine ECC underscores the need for a more nuanced approach. Our results suggest that ECC may be particularly beneficial for women with AGC-FN cytology and specific risk factors like HPV 16/18 infections, yet show limited advantages for younger women and those with type 1/2 transformation zones. This emphasizes the necessity for tailored decision-making in clinical practice, aligning with the evolving discourse on the effectiveness of ECC. This approach could reduce unnecessary procedures in low-risk populations while ensuring high-risk cases are adequately identified and treated.
Several limitations of this study should be acknowledged. First, the retrospective design may introduce selection bias. Second, the sample size, while sufficient for detecting significant differences, may limit the generalization of our findings to broader populations. Additionally, variations in colposcopy and biopsy techniques among practitioners could influence the detection rates of HSIL+.
Future research should focus on prospective studies to validate these findings in larger and more diverse populations. Investigating the molecular and histopathological characteristics of AGC subtypes may provide deeper insights into their malignant potential and guide more precise management strategies. Additionally, exploring the cost-effectiveness of targeted ECC in high-risk subgroups could support evidence-based guidelines for colposcopy referral and follow-up.
5 Conclusion
This study aimed to improve the detection rate of HSIL+ in women with AGC while minimizing unnecessary discomfort. We identified high-risk groups warranting ECC: those with a normal/low-grade colposcopic impression, AGC-FN cytology, type 3 transformation zones, and HPV 16/18 infection. Conversely, ECC offers no benefits for women under 30 years old with type 1/2 transformation zones. These findings may reduce missed occult HSIL+ cases and contribute to the evidence base regarding the clinical use of ECC.
Data availability statement
The datasets generated and/or analysed during the current study are not publicly available due personal information protection, patient privacy regulation, and medical institutional data regulatory policies, etc., but are available from the corresponding author on reasonable request.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because an exemption of informed consent was obtained due to the retrospective nature of the study.
Author contributions
YC: Funding acquisition, Writing – review & editing, Writing – original draft. FW: Writing – original draft, Methodology. JC: Writing – original draft, Project administration, Methodology, Data curation. HX: Writing – original draft, Project administration, Formal analysis, Conceptualization. XZ: Writing – review & editing, Resources, Formal analysis, Conceptualization. DP: Writing – original draft, Project administration, Funding acquisition, Formal analysis, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from Startup Fund for scientific research, the Fujian Medical University (Grant Number: 2021QH1177) and the Fujian Provincial Natural Science Foundation of China (Grant Number: 2022J011030).
Acknowledgments
The authors thank Liyu Dai for supporting in the clinical management and Dafeng Huang for encouragement.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang J, Andrae B, Sundström K, Ström P, Ploner A, Elfström KM, et al. Risk of invasive cervical cancer after atypical glandular cells in cervical screening: nationwide cohort study. BMJ. (2016) 352:i276. doi: 10.1136/bmj.i276
2. Willows K, Selk A, Auclair MH, Jim B, Jumah N, Nation J, et al. 2023 Canadian colposcopy guideline: a risk-based approach to management and surveillance of cervical dysplasia. Curr. Oncol. (2023) 30, 5738–5768. doi: 10.3390/curroncol30060431
3. Massad LS, Perkins RB, Naresh A, Nelson EL, Spiryda L, Gecsi KS, et al. Colposcopy standards: guidelines for endocervical curettage at colposcopy. J Low Genit Tract Dis. (2023) 27:97–101. doi: 10.1097/LGT.0000000000000710
4. Public Health England. Guidance 4. Colposcopic Diagnosis, Treatment and Follow Up. Available at: https://www.gov.uk/government/publications/cervical-screening-programme-and-colposcopy-management/3-colposcopic-diagnosis-treatment-and-follow-up (accessed September 27, 2024).
5. van der Marel J, Rodriguez A, Del Pino M, van Baars R, Jenkins D, van de Sandt MM, et al. The value of endocervical curettage in addition to biopsies in women referred to colposcopy. J Low Genit Tract Dis. (2015) 19:282–7. doi: 10.1097/LGT.0000000000000124
6. Liu AH, Walker J, Gage JC, Gold MA, Zuna R, Dunn ST, et al. Diagnosis of cervical precancers by endocervical curettage at colposcopy of women with abnormal cervical cytology. Obstet Gynecol. (2017) 130:1218–25. doi: 10.1097/AOG.0000000000002330
7. Gage JC, Duggan MA, Nation JG, Gao S, Castle PE. Detection of cervical cancer and its precursors by endocervical curettage in 13,115 colposcopically guided biopsy examinations. Am J Obstetr Gynecol. (2010) 203:481.e1–9. doi: 10.1016/j.ajog.2010.06.048
8. Schnatz PF, Guile M, O'Sullivan DM, Sorosky JI. Clinical significance of atypical glandular cells on cervical cytology. Obstet Gynecol. (2006) 107:701–8. doi: 10.1097/01.AOG.0000202401.29145.68
9. Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. Jama. (2002) 287:2114–9. doi: 10.1001/jama.287.16.2114
10. Cree IA, White VA, Indave BI, Lokuhetty D. Revising the WHO classification: female genital tract tumours. Histopathology. (2020) 76:151–6. doi: 10.1111/his.13977
11. Verdoodt F, Jiang X, Williams M, Schnatz PF, Arbyn M. High-risk HPV testing in the management of atypical glandular cells: a systematic review and meta-analysis. Int J Cancer. (2016) 138:303–10. doi: 10.1002/ijc.29424
12. Norman I, Yilmaz E, Hjerpe A, Hortlund M, Elfström KM, Dillner J. Atypical glandular cells and development of cervical cancer: population-based cohort study. Int J Cancer. (2022) 151:2012–9. doi: 10.1002/ijc.34242
13. Schiffman M, Mirabello L, Egemen D, Befano B, Xiao Y, Wentzensen N, et al. The combined finding of HPV 16, 18, or 45 and cytologic Atypical Glandular Cells (AGC) indicates a greatly elevated risk of in situ and invasive cervical adenocarcinoma. Gynecol Oncol. (2023) 174:253–61. doi: 10.1016/j.ygyno.2023.05.011
14. Zhou X, Lin W, Qin Y, Zhang J, Zhang X, Zhang H, et al. Correlation of immediate prevalence of cervical precancers and cancers with HPV genotype and age in women with atypical glandular cells cytology: a retrospective analysis of 369 cases. Cancer Cytopathol. (2024) 132:119–28. doi: 10.1002/cncy.22780
15. Sijing L, Ying J, Jing W, Xiaoge L, Ming L, Zhaoning D. Additional role of ECC in the detection and treatment of cervical HSIL. Front Med. (2023) 10:1206856. doi: 10.3389/fmed.2023.1206856
16. Li Y, Luo H, Zhang X, Chang J, Zhao Y, Li J, et al. Development and validation of a clinical prediction model for endocervical curettage decision-making in cervical lesions. BMC Cancer. (2021) 21:804. doi: 10.1186/s12885-021-08523-y
17. Xue P, Wei B, Seery S, Li Q, Ye Z, Jiang Y, et al. Development and validation of a predictive model for endocervical curettage in patients referred for colposcopy: A multicenter retrospective diagnostic study in China. Chin J Cancer Res. (2022) 34:395–405. doi: 10.21147/j.issn.1000-9604.2022.04.07
18. Lang L, Jia Y, Duan Z, Wu J, Luo M, Tian P. The role of endocervical curettage in detection and treatment of cervical canal lesions. Histol Histopathol. (2022) 37:63–8. doi: 10.14670/HH-18-394
19. Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. (2020) 24:102–31. doi: 10.1097/LGT.0000000000000525
20. Levine L, Lucci JA 3rd, Dinh TV. Atypical glandular cells: new Bethesda Terminology and Management Guidelines. Obstetr Gynecol Surv. (2003) 58:399–406. doi: 10.1097/01.OGX.0000070068.74408.F6
21. Solomon D, Stoler M, Jeronimo J, Khan M, Castle P, Schiffman M. Diagnostic utility of endocervical curettage in women undergoing colposcopy for equivocal or low-grade cytologic abnormalities. Obstet Gynecol. (2007) 110:288–95. doi: 10.1097/01.AOG.0000270154.69879.09
22. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Global health. (2020) 8:e191–203. doi: 10.1016/S2214-109X(19)30482-6
Keywords: cervical curettage, colposcopy, biopsy, cervical lesions, atypical glandular cells
Citation: Chen Y, Wen F, Chen J, Xue H, Zheng X and Pan D (2024) The value of endocervical curettage for diagnosis of cervical precancers or worse at colposcopy of women with atypical glandular cells cytology. Front. Med. 11:1476361. doi: 10.3389/fmed.2024.1476361
Received: 05 August 2024; Accepted: 25 November 2024;
Published: 13 December 2024.
Edited by:
Cristina Secosan, Victor Babes University of Medicine and Pharmacy, RomaniaReviewed by:
Elliot M. Levine, Rosalind Franklin University of Medicine and Science, United StatesTeodor Florin Georgescu, Carol Davila University of Medicine and Pharmacy, Romania
Nicolae Bacalbasa, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2024 Chen, Wen, Chen, Xue, Zheng and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diling Pan, cGRsMjY5QGZqbXUuZWR1LmNu; Xiangqin Zheng, Wmhlbmd4cTEyMTVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yusha Chen
Yusha Chen Fanghong Wen2†
Fanghong Wen2† Xiangqin Zheng
Xiangqin Zheng