- 1Pharmaceutical Care Services, King Abdullah Bin Abdulaziz University Hospital, Riyadh, Saudi Arabia
- 2College of Pharmacy, King Saud Bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
- 3Pharmaceutical Care Services, King Abdulaziz Medical City, Riyadh, Saudi Arabia
- 4King Abdullah International Medical Research Center, Riyadh, Saudi Arabia
- 5Saudi Critical Care Pharmacy Research (SCAPE) Platform, Riyadh, Saudi Arabia
- 6Saudi Society for Multidisciplinary Research Development and Education (SCAPE Society), Riyadh, Saudi Arabia
Vitamin C (Ascorbic acid) has evolved as an emergent co-intervention for sepsis and septic shock patients. Multiple studies discussed the pathophysiological value of vitamin C to reserve endothelial functionality and improve microcirculatory flow in these patients. Nevertheless, most randomized clinical trials failed to show the clinical impact of adding vitamin C to sepsis and septic shock. Pneumonia is the most common infection to induce sepsis and septic shock, which could be an acute respiratory distress syndrome. Preliminary in-vitro data support the role of vitamin C in mitigating the risk of acute respiratory distress syndrome (ARDS) development. This review aims to compare and contrast these trials and explore differences in their patients’ populations, methodologies, and outcomes, emphasizing pneumonia-induced sepsis and septic shock.
Introduction
Sepsis and septic shock are significant public health burdens with 20–35% predicted mortality rate (1, 2). The pathophysiology of sepsis is a complex interaction of infection and a host that causes a misbalance between pro-inflammatory and anti-inflammatory markers (3–5). The concept of defining sepsis and septic shock has recently moved towards organ dysfunction as the landmark signal for this syndrome based on the Sepsis-3 definition (6). Pneumonia-induced sepsis represents up to 50% of these cases, possibly leading to acute respiratory distress syndrome [ARDS; (7, 8)]. Pneumonia is reported to cause ARDS through a direct insult mechanism in 40–60% of the ARDS patient population (9, 10). During weeks 1–2 of ARDS, an acute inflammatory and exudative phase may lead to endothelial injury and permeability loss. This could progress into endothelial cell death and a fibroproliferative phase, possibly resulting in pulmonary fibrosis (11–13). Substantial evidence points to oxidative stress and dysregulated inflammation as playing a significant role in the onset and development of multiorgan dysfunction and injury in sepsis in both experimental animals and human individuals. Vitamin C has been investigated extensively as a potential treatment for sepsis. In-vitro data showed ascorbic acid (or Vitamin C) ability to restore endothelial permeability and improve microcirculatory flow.
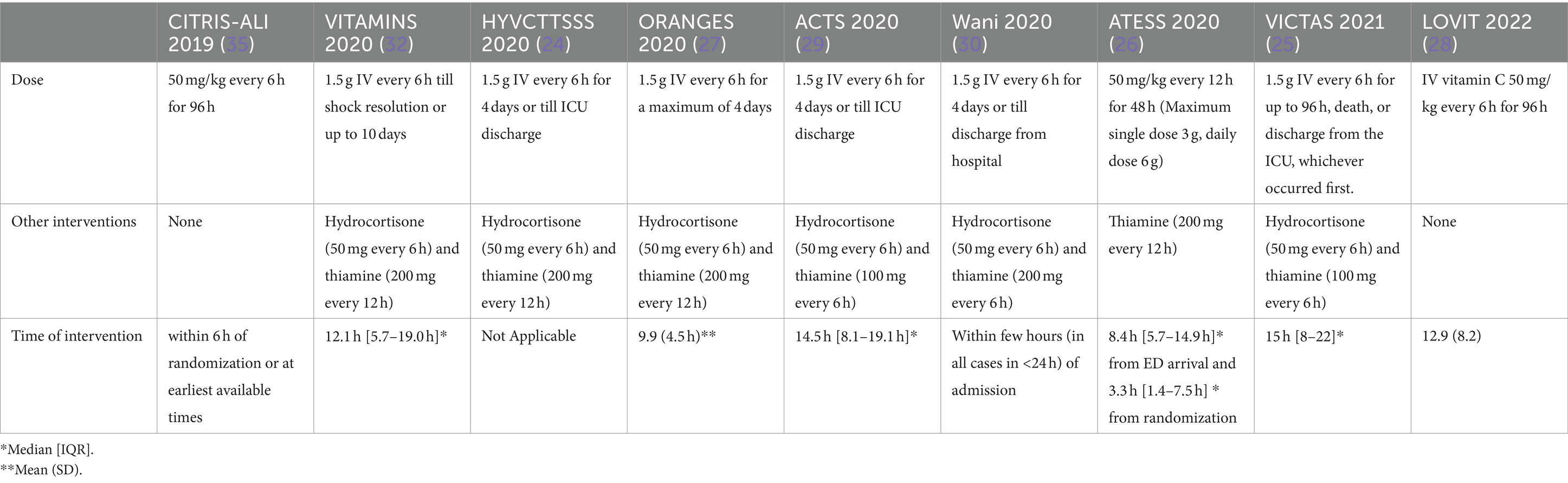
Additionally, ascorbic acid is essential for synthesizing catecholamine and enhances vasopressor sensitivity (14–17). Nathens et al. demonstrated ascorbic acid’s ability when used with alpha-tocopherol to reduce the incidence of ARDS and intensive care unit (ICU) lengths of stay in severely ill surgical patients in a randomized prospective trial which was further investigated in other studies (18–23). In the last few years. Multiple randomized controlled trials (RCTs) examined vitamin C role either as a single intervention or as part of a metabolic resuscitation cocktail besides hydrocortisone and thiamin for sepsis and septic patients with inconsistent findings (18, 24–30, 32). Research has shown that administering vitamin C at a dose of 6 g per day is safe and free of side effects, with even higher doses of 100–150 g being safely given to patients with burns or malignancies (31). Intravenous thiamine (vitamin B1) is often combined with vitamin C to prevent potential renal side effects from high doses of vitamin C, while hydrocortisone is used to boost the body’s endogenous production of catecholamines. A recent retrospective before-and-after study reported a significant reduction in mortality among sepsis patients treated with a combination of high-dose vitamin C, hydrocortisone, and thiamine (20). Our primary aim in this narrative review was to focus on the most updated RCTs pertaining to the role of ascorbic acid in sepsis or septic shock, particularly in cases of pneumonia-induced sepsis and septic shock. While the focus on RCTs was a key aspect of our search strategy, we also aimed to provide a comprehensive overview comparing different RCTs based on their patients’ population, methodologies, and outcomes as presented in Table 1.
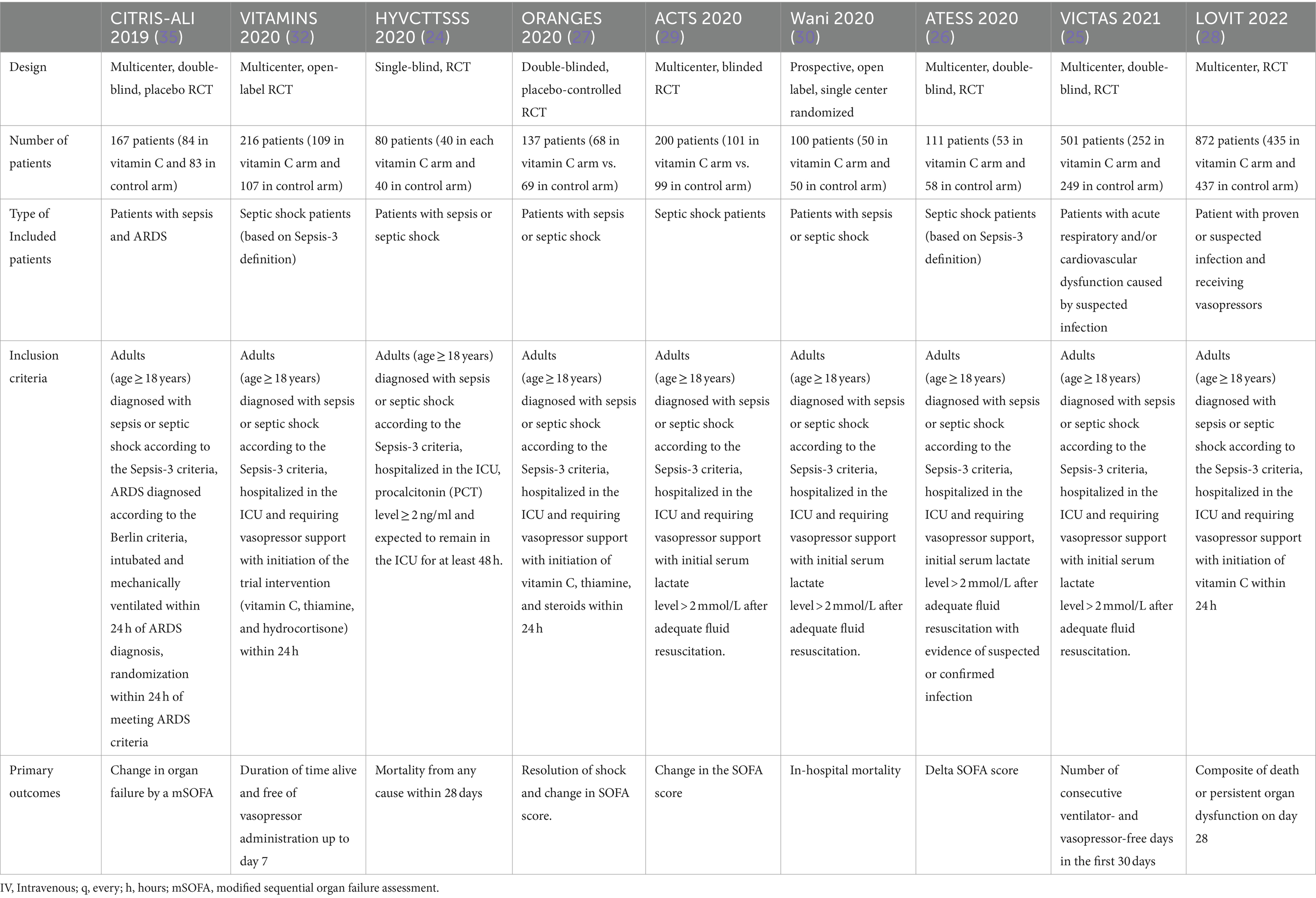
Table 1. Comparison between recent RCTs evaluating the role of vitamin C in sepsis and septic shock patients.
Clinical studies
Multiple clinical studies investigated the clinical advantages of vitamin C in sepsis and septic shock. Using the keywords “Vitamin C” or “Ascorbic acid” AND “Sepsis” or “Septic Shock” in PubMed, the authors discovered a total of 9 recent RCTs assessing the role of vitamin C in sepsis and septic shock patients. Of the recently published RCTs, two looked at patients with sepsis and compared vitamin C with a placebo. The remaining studies were given either cocktail therapy, which includes hydrocortisone, ascorbic acid, and thiamine (HAT), or standard care as a control group. In this opinion paper, we would like to discuss the rational use of vitamin C and evaluate the current evidence related to the time to intervention, its effect on appropriate dosing of vitamin C, organ dysfunction, and mortality rate.
Dosing of vitamin C
Most RCTs, including VITAMINS, HYVCTTSSS, ORANGES, ACTS, Wani et al., and VICTAS, adopted the fixed-dose strategy reported by Marik et al. study with 1.5 g of IV ascorbic acid every 6 h for 4 days (20, 24, 25, 27, 29, 30, 32). While other trials, including the most recent one, LOVIT, used weight-based dosing of vitamin C with 50 mg/kg (18, 26, 28). Also, the frequency and duration of ascorbic acid administration varied between the trials that used weight-based dosing. For example, in CITRIS-ALI and LOVIT, vitamin C was given every 6 h for 96 h; in ATESS, the dosing interval was every 12 h for 48 h (18, 26, 28). These vitamin C dosage regimen doses may not be optimal for preventing the pathophysiological processes underlying sepsis. In a meta-analysis, patients with sepsis who received a high dose of vitamin C, defined as more than 50 mg/kg/day, significantly reduced overall mortality (33). However, this positive outcome is contrary to the finding of the most recent and significant RCT, the LOVIT trail, which used a high weight-based dosing of vitamin C and did not report any mortality benefit (28). Surprisingly, the composite outcomes of the LOVIT trial found an increased risk of mortality or persistent organ failure among patients who received vitamin C. Multiple meta-analyses suggest a similar lack of benefit, but several studies remain in progress. The PETAL (Prevention and Early Treatment of Acute Lung Injury) network, supported by funding from the National Heart, Lung, and Blood Institute (NHLBI) made the decision to halt the ASTER (Acetaminophen in Sepsis: Targeted Therapy to Enhance Recovery) study following a series of negative outcomes encountered in multiple studies investigating the role of vitamin C in ARDS. Table 2 summarizes the dosing of vitamin C in sepsis randomized clinical trials.
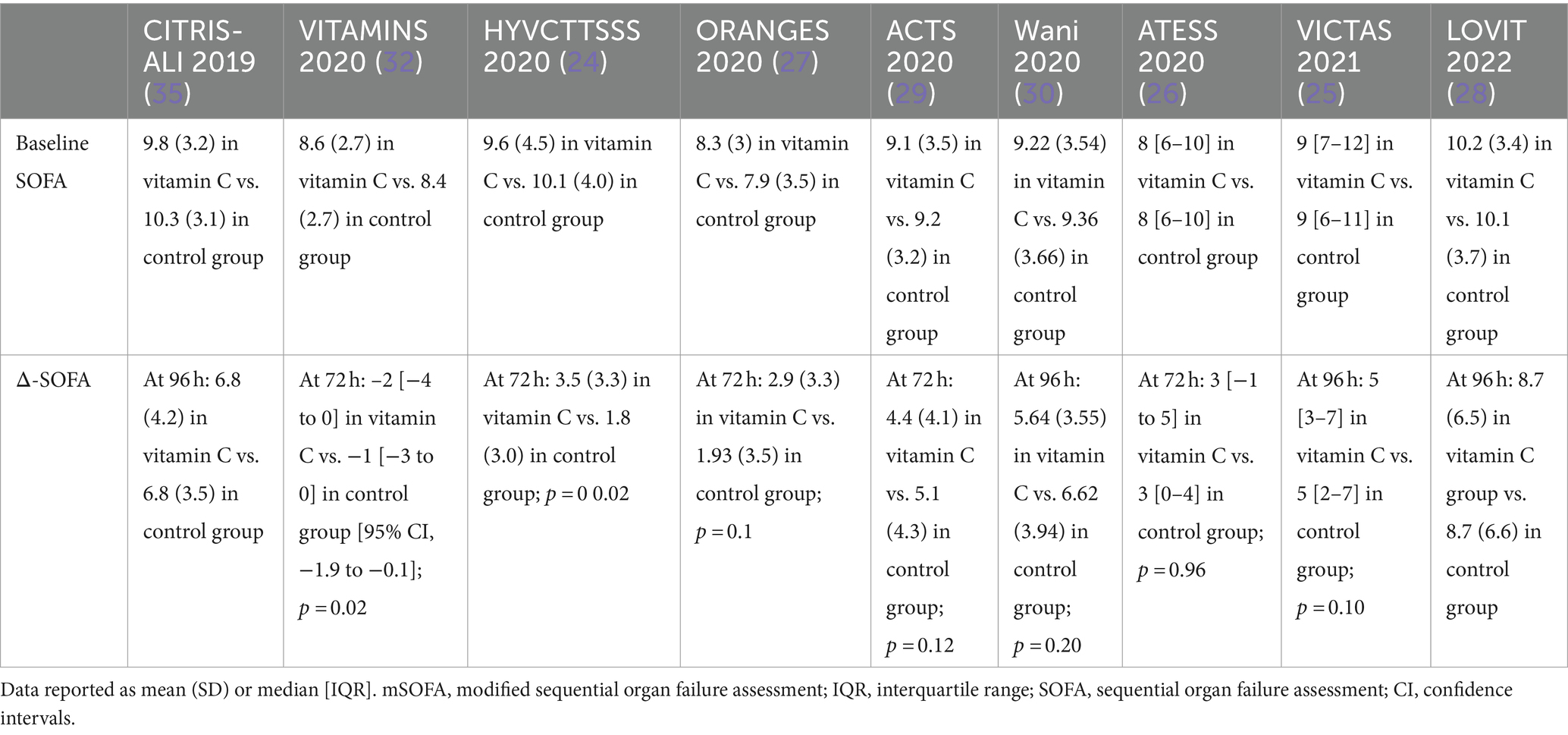
Effect of vitamin C on organ dysfunction
The severity of organ dysfunction can be quantified using a SOFA score based on six different systems (34). CITRIS-ALI and LOVIT trials compared vitamin C with a placebo in patients with sepsis and ARDS. Their patients had more advanced organ dysfunction as measured by SOFA score compared with other trials (28, 35). No significant difference was seen in organ dysfunction as a single outcome among patients who received vitamin C compared to the control group from baseline up to 96 h (28, 35). Studies using vitamin C in combination therapy with HAT were inconsistent with their findings. VITAMINS and HYVCTTSS trials reported more significant composite change in SOFA at 72 h (24, 32). In contrast, none of the other RCTs reported a positive impact of the vitamin C intervention on organ dysfunction from baseline up to 96 h (25–30). This suggests that the observed improvement in SOFA scores might be a result independent of the effect of vitamin C. Table 3 summarizes baseline SOFA and change in this score in sepsis RCTs.
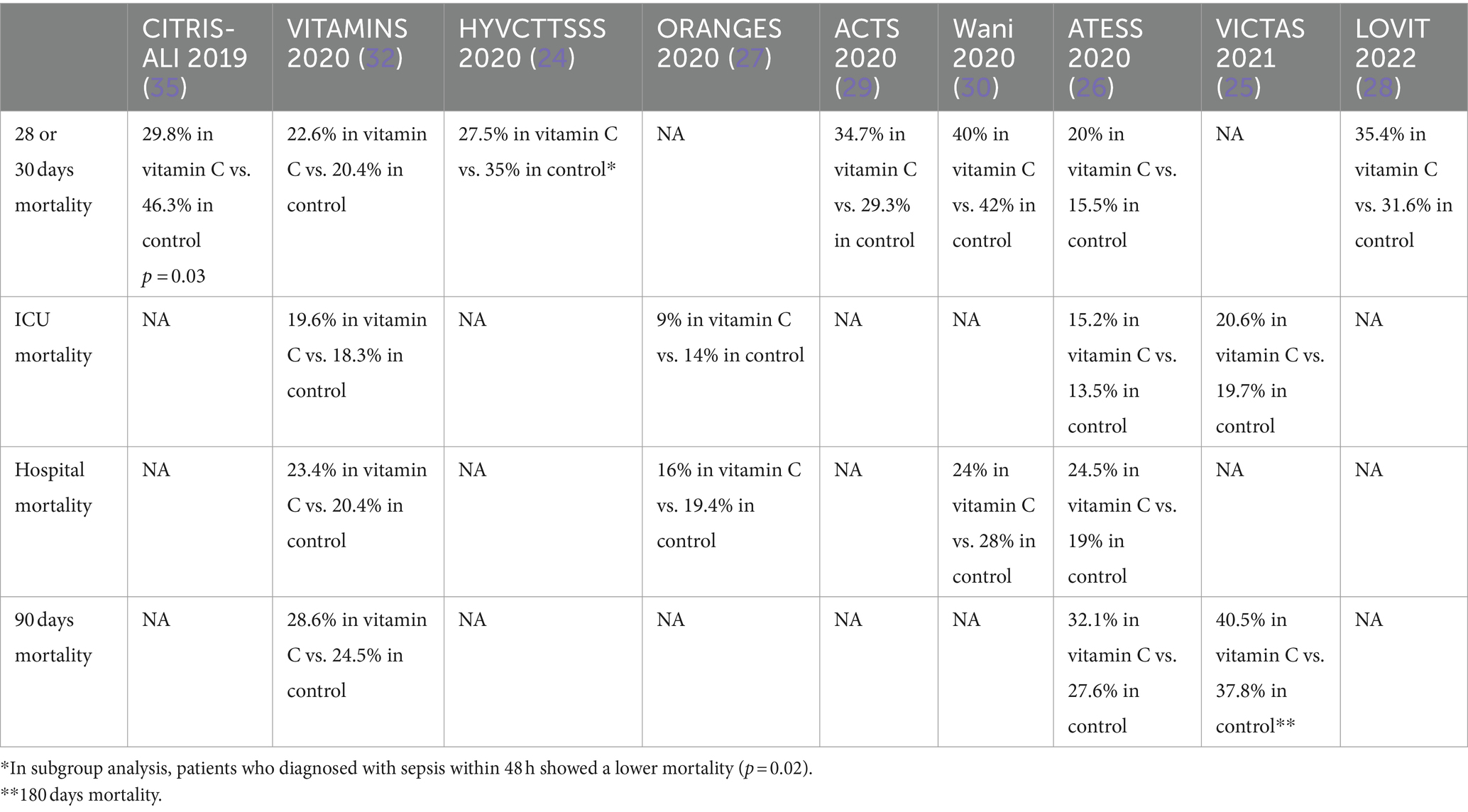
Effect of vitamin C on mortality
Mortality is a commonly used endpoint in RCTs among critically ill patients (36). In all RCTs that investigated the role of vitamin C, mortality was reported either as primary or secondary outcomes with different duration (37). Out of the RCTs considered, the CITRIS-ALI trial was the only trial that showed a mortality benefit with vitamin C intervention (35). In this trial, 25/84 (29.8%) of vitamin C died by day 28 compared with 38/82 (46.3%) in the control group. However, this finding must be interpreted with caution since the analysis was exploratory and did not account for multiple comparisons. In other RCTs, including HYVCTTSSS, ORANGES, and Wanni et al., a numerically lower mortality percentage was observed among the intervention arm (24, 27, 30). Interestingly, in a subgroup analysis of the ORANGES trial, only patients who were diagnosed within the first 48 h with sepsis showed a significant mortality benefit in the vitamin C group. On the other hand, a numerically more mortality percentage was seen in the vitamin C group in VITAMINS, ACTS, ATESS, VICTAS, and LOVIT trials (25, 26, 28–30, 32). A possible explanation of this could be attributed to the trials’ inclusion criteria, which were mainly septic shock patients or patients with sepsis-induced cardiovascular dysfunction. Additionally, the negative finding of LOVIT trial may be affected by fact that they reported the primary outcome as composite, including death or persistent of orang dysfunction (28). The results of the LOVIT trial showed an implicit lack of benefit and an increase in mortality risks associated with the administration of large dosages of vitamin C. The effect of vitamin C on mortality among sepsis RCTs are summarized in Table 4.
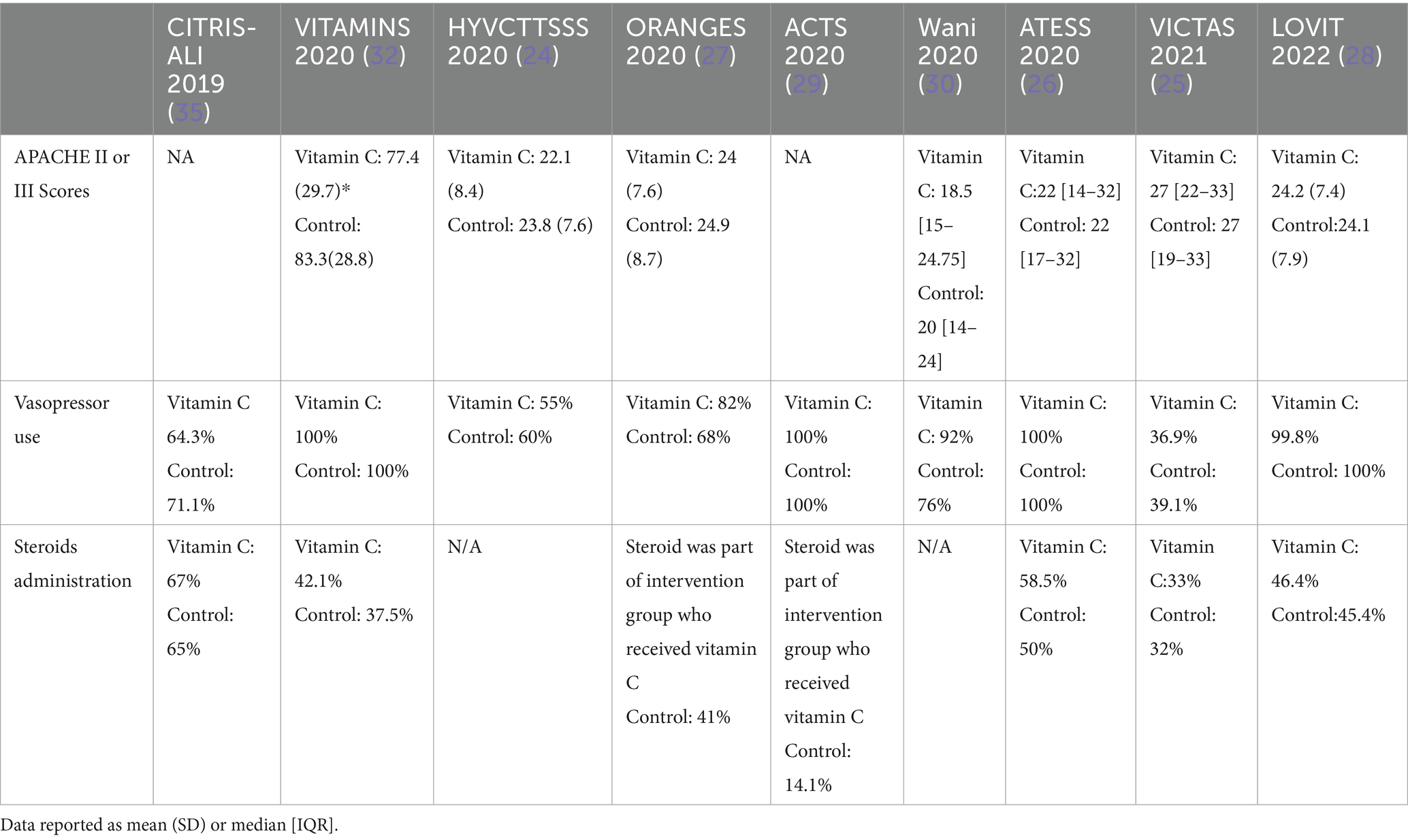
Effect of vitamin C on mortality in sepsis and septic shock
The severity of sepsis and advanced organ dysfunction could affect mortality (6, 36). Among different sepsis RCTs, the percentage of septic shock patients varied between studies and ranging from 55 to 100% (18, 24–30, 32). Some trials, including HYVCTTSSS and CITRIS ALI, included patients with sepsis and septic shock, while other trials like ORANGES and LOVIT have targeted septic shock patients as inclusion criteria (18, 24, 27, 28). Regarding the reduction of vasopressors duration, the same conflicting findings were observed among the published RCT. In ORANGES and Wani et al. trial, the use of vitamin C has led to a significant reduction in vasopressor duration (27, 30). Also, the ACTS trial showed more shock-free days among patients assigned to vitamin C group (29). These data could point towards a potential benefit for vitamin C in patients with early signs of sepsis. Table 5 summarized the factors that may affect mortality.
Several studies suggested a potential benefit for vitamin C to prevent or treat pneumonia (19, 37, 38). These patients may express superimposed inflammatory responses, which ascorbic acid may be able to mitigate. In HYVCTTSSS, CITRIS-ALI, and Wani et al., pulmonary infections were the most predominant types of infections ranging at least 72–82% of patients included in these trials, followed by urinary infection gastrointestinal, respectively (18, 30, 32). Both of HYVCTTSSS, and CITRIS-ALI reported a lower mortality with the administration of vitamin C (18, 24). In VITAMINS, ACTS, and LOVIT trials, the primary infection site, in patients who received vitamin C, was almost equally shared between pulmonary and gastrointestinal infections, which could have diluted the potential effect of vitamin C (29, 32). Moreover, unlike the previous trials, the suspected source of sepsis was an intra-abdominal infection in 50.9% of cases in the ATESS trial followed by respiratory and urinary tract infections, which did not have beneficial clinical outcomes with the administration of vitamin C (26). It is hard to correlate this mortality benefit to the subset of patients who developed pneumonia-induced sepsis or septic shock; however, this observation may necessitate further investigation. In the subgroup analysis of LOVIT trial, a trend towards benefits of vitamin C was observed among patients with severe acute respiratory syndrome coronavirus 2 (COVID-19) although it was not clinically significant (28). In most of the aforementioned sepsis randomized clinical trials, the administration of vitamin C was not associated with mortality benefit (24–30, 32). Sepsis and septic shock mortality could be attributed to multiple interventions such as the timing of antibiotics from disease onset, appropriate selection of antibiotics, appropriate volume resuscitation and de-resuscitation, vasopressor administration, and corticosteroids administration (39–43). Mostly vitamin C administration was combined with hydrocortisone 50 mg every 6 h in the intervention arm as part of hydrocortisone, ascorbic acid, thiamine, or (HAT) protocol (20, 25, 32). For instance, 67% received corticosteroids in the CITRIS-ALI trial, and 58.5% received corticosteroids in the ATESS trial (26, 32). Among all RCTs, the resolution of shock was observed in two trials only (27, 30). In the ORANGES trial, the time of shock reversal was 27 ± 22 h in vitamin C arm compared with 53 ± 38 h in the control arm (27). Additionally, in Wani et al. trial, the duration of vasopressors was 75.2 ± 30.2 h in the vitamin C group compared with 96.1 ± 40.5 in the control group (30). Likewise, the use of vitamin C was associated with significant shock-free days compared with placebo in ACTS trial; however, this finding was not replicated in VITAMINS nor HYVCTTSSS trials (24, 27, 30). Moreover, recently a systematic review and meta-analysis included 18 RCTs that showed the use of IV vitamin C in septic shock patients was associated with better delta SOFA scores but did not show a decrease in short-term mortality (44).
Precision medicine
Sepsis is a complex syndrome with a broad spectrum of conditions, severities, and clinical presentations. Standardized treatments often fall short of addressing the diverse needs of all patients, underscoring the necessity for targeted, individualized therapies. The heterogeneity of sepsis—shaped by factors such as patient age, causative microorganisms, infection type, and pre-existing conditions—highlights the need for precision medicine approaches that tailor treatment to the unique clinical and biological characteristics of each patient.
Recent research has increasingly explored the potential benefits of high-dose vitamin C, often administered in combination with hydrocortisone and thiamine, commonly referred to as a “sepsis cocktail.” This combination has been proposed to reduce mortality, decrease reliance on vasopressors, and mitigate organ damage in septic patients. However, RCTs have produced inconsistent results on the efficacy of vitamin C in improving clinical outcomes. For example, the CITRIS-ALI trial suggested a potential mortality benefit, while other studies reported no benefit and even showed potential harm, such as increased risk of mortality or persistent organ failure with high-dose vitamin C. Although some trials have shown improvements in SOFA scores with vitamin C, these benefits were not uniformly seen, suggesting that other factors may also contribute to organ function recovery. It is important to note that the SOFA score may not capture all dimensions of treatment outcomes and might overlook certain effects of interventions. The variability in dosing strategies and outcomes across different RCTs further emphasizes the need for personalized treatment approaches. While some studies have shown benefits with fixed or higher doses of vitamin C, others, like the LOVIT trial, found no mortality benefit and even suggested potential harm with high-dose vitamin C.
These mixed results from RCTs highlight the limitations of a one-size-fits-all approach in sepsis management. Precision medicine, which emphasizes individualized treatment strategies, offers a promising pathway to improving outcomes for critically ill patients. By focusing on early identification and targeted intervention, precision medicine aims to enhance the effectiveness of therapies like vitamin C, especially for patient subgroups most likely to benefit. Advancing our understanding of patient-specific factors and refining treatment protocols could play a pivotal role in improving care for patients with sepsis and septic shock.
Conclusion
The co-administration of vitamin C with sepsis and septic shock patients did not consistently demonstrate a mortality benefit. However, our review suggests a potential mortality advantage of vitamin C co-administration, especially in patients with advanced disease severity when initiated early in sepsis or septic shock therapy. A subgroup analysis highlights the possibility of a beneficial effect in critically ill patients developing sepsis or septic shock secondary to pneumonia, indicating a potential area for further research. It’s noteworthy that the observed improvement in SOFA scores raises questions about whether this improvement is independent of the effect of vitamin C. Although several well-designed randomized clinical trials have investigated the role of vitamin C in sepsis, further data and research are warranted to confirm and elucidate these findings.
Author contributions
AAli: Conceptualization, Formal analysis, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Data curation, Funding acquisition, Investigation, Project administration, Resources, Software. MA: Conceptualization, Formal analysis, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AAls: Writing – original draft, Writing – review & editing. KA: Writing – original draft, Writing – review & editing. SA: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ARDS, Acute respiratory distress syndrome; ICU, intensive care unit; RCT, Randomized clinical trials; CITRIS-ALI, Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial; VITAMINS, Effect of Vitamin C, hydrocortisone, and thiamine vs. hydrocortisone alone on time alive and free of vasopressor support among patients with septic Shock: the VITAMINS randomized clinical trial; HYVCTTSSS, Combined treatment with hydrocortisone, vitamin c, and thiamine for sepsis and septic shock: a randomized controlled trial; ORANGES, Outcomes of metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in the early treatment of sepsis: the ORANGES trial; ACTS, Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: the ACTS randomized clinical trial; VICTAS, The vitamin C, thiamine and steroids in sepsis (VICTAS) protocol: a prospective, multi-center, double-blind, adaptive sample size, randomized, placebo-controlled, clinical trial; LOVIT, Lessening organ dysfunction with vitamin C (LOVIT): protocol for a randomized controlled trial.
References
1. Kaukonen, KM, Bailey, M, Suzuki, S, Pilcher, D, and Bellomo, R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. (2014) 311:1308–16. doi: 10.1001/jama.2014.2637
2. Stevenson, EK, Rubenstein, AR, Radin, GT, Wiener, RS, and Walkey, AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med. (2014) 42:625–31. doi: 10.1097/CCM.0000000000000026
3. Angus, DC, and van der Poll, T. Severe sepsis and septic shock. N Engl J Med. (2013) 369:840–51. doi: 10.1056/NEJMra1208623
4. Boomer, JS, To, K, Chang, KC, Takasu, O, Osborne, DF, Walton, AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. (2011) 306:2594–605. doi: 10.1001/jama.2011.1829
5. Van der Poll, T, and Opal, SM. Host-pathogen interactions in sepsis. Lancet Infect Dis. (2008) 8:32–43. doi: 10.1016/S1473-3099(07)70265-7
6. Singer, M, Deutschman, CS, Seymour, CW, Shankar-Hari, M, Annane, D, Bauer, M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
7. Bauer, TT, Ewig, S, Rodloff, AC, and Müller, EE. Acute respiratory distress syndrome and pneumonia: a comprehensive review of clinical data. Clin Infect Dis. (2006) 43:748–56. doi: 10.1086/506430
8. De Freitas, CN, Gaudet, A, Portier, L, Tsicopoulos, A, Mathieu, D, and Lassalle, P. Endocan, sepsis, pneumonia, and acute respiratory distress syndrome. Crit Care. (2018) 22:280. doi: 10.1186/s13054-018-2222-7
9. Frat, JP, Thille, AW, Mercat, A, Girault, C, Ragot, S, Perbet, S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. (2015) 372:2185–96. doi: 10.1056/NEJMoa1503326
10. Petty, TL, and Ashbaugh, DG. The adult respiratory distress syndrome. Clinical features, factors influencing prognosis and principles of management. Chest. (1971) 60:233–9. doi: 10.1378/chest.60.3.233
11. Ranieri, VM, Rubenfeld, GD, Thompson, BT, Ferguson, ND, Caldwell, E, Fan, E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. (2012) 307:2526–33. doi: 10.1001/jama.2012.5669
12. Gattinoni, L, Pelosi, P, Suter, PM, Pedoto, A, Vercesi, P, and Lissoni, A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med. (1998) 158:3–11. doi: 10.1164/ajrccm.158.1.9708031
13. Griffiths, MJD, McAuley, DF, Perkins, GD, Barrett, N, Blackwood, B, Boyle, A, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir Res. (2019) 6:e000420. doi: 10.1136/bmjresp-2019-000420
14. Dillon, PF, Root-Bernstein, RS, and Lieder, CM. Antioxidant-independent ascorbate enhancement of catecholamine-induced contractions of vascular smooth muscle. Am J Physiol Heart Circ Physiol. (2004) 286:H2353–60. doi: 10.1152/ajpheart.00968.2003
15. Jackson, TS, Xu, A, Vita, JA, and Keaney, JF Jr. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res. (1998) 83:916–22. doi: 10.1161/01.RES.83.9.916
16. Kuzkaya, N, Weissmann, N, Harrison, DG, and Dikalov, S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. (2003) 278:22546–54. doi: 10.1074/jbc.M302227200
17. Tyml, K . Vitamin C and microvascular dysfunction in systemic inflammation. Antioxidants (Basel). (2017) 6:49. doi: 10.3390/antiox6030049
18. Fowler, AA 3rd, Syed, AA, Knowlson, S, Sculthorpe, R, Farthing, D, DeWilde, C, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. (2014) 12:32. doi: 10.1186/1479-5876-12-32
19. Hunt, C, Chakravorty, NK, Annan, G, Habibzadeh, N, and Schorah, CJ. The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. Int J Vitam Nutr Res. (1994) 64:212–9.
20. Marik, PE, Khangoora, V, Rivera, R, Hooper, MH, and Catravas, J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. (2017) 151:1229–38. doi: 10.1016/j.chest.2016.11.036
21. Nathens, AB, Neff, MJ, Jurkovich, GJ, Klotz, P, Farver, K, Ruzinski, JT, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg. (2002) 236:814–22. doi: 10.1097/00000658-200212000-00014
22. Schorah, CJ, Downing, C, Piripitsi, A, Gallivan, L, Al-Hazaa, AH, Sanderson, MJ, et al. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am J Clin Nutr. (1996) 63:760–3. doi: 10.1093/ajcn/63.5.760
23. Zabet, MH, Mohammadi, M, Ramezani, M, and Khalili, H. Effect of high-dose ascorbic acid on vasopressor's requirement in septic shock. J Res Pharm Pract. (2016) 5:94–100. doi: 10.4103/2279-042X.179569
24. Chang, P, Liao, Y, Guan, J, Guo, Y, Zhao, M, Hu, J, et al. Combined treatment with hydrocortisone, vitamin C, and thiamine for sepsis and septic shock: a randomized controlled trial. Chest. (2020) 158:174–82. doi: 10.1016/j.chest.2020.02.065
25. Hager, DN, Hooper, MH, Bernard, GR, Busse, LW, Ely, EW, Fowler, AA, et al. The vitamin C, thiamine and steroids in Sepsis (VICTAS) protocol: a prospective, multi-center, double-blind, adaptive sample size, randomized, placebo-controlled, clinical trial. Trials. (2019) 20:197. doi: 10.1186/s13063-019-3254-2
26. Hwang, SY, Ryoo, SM, Park, JE, Jo, YH, Jang, DH, Suh, GJ, et al. Combination therapy of vitamin C and thiamine for septic shock: a multi-Centre, double-blinded randomized, controlled study. Intensive Care Med. (2020) 46:2015–25. doi: 10.1007/s00134-020-06191-3
27. Iglesias, J, Vassallo, AV, Patel, VV, Sullivan, JB, Cavanaugh, J, and Elbaga, Y. Outcomes of metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in the early treatment of Sepsis: the ORANGES trial. Chest. (2020) 158:164–73. doi: 10.1016/j.chest.2020.02.049
28. Lamontagne, F, Masse, MH, Menard, J, Sprague, S, Pinto, R, Heyland, DK, et al. Intravenous vitamin C in adults with sepsis in the intensive care unit. N Engl J Med. (2022) 386:2387–98. doi: 10.1056/NEJMoa2200644
29. Moskowitz, A, Huang, DT, Hou, PC, Gong, J, Doshi, PB, Grossestreuer, AV, et al. Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: the ACTS randomized clinical trial. JAMA. (2020) 324:642–50. doi: 10.1001/jama.2020.11946
30. Wani, SJ, Mufti, SA, Jan, RA, Shah, SU, Qadri, SM, Khan, UH, et al. Combination of vitamin C, thiamine and hydrocortisone added to standard treatment in the management of sepsis: results from an open label randomised controlled clinical trial and a review of the literature. Infect Dis (Lond). (2020) 52:271–8. doi: 10.1080/23744235.2020.1718200
31. Wacker, C, Prkno, A, Brunkhorst, FM, and Schlattmann, P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. (2013) 13:426–35. doi: 10.1016/S1473-3099(12)70323-7
32. Fujii, T, Luethi, N, Young, PJ, Frei, DR, Eastwood, GM, French, CJ, et al. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial. JAMA. (2020) 323:423–31. doi: 10.1001/jama.2019.22176
33. Jincan, L, Hua, L, Yan, W, and Minwei, Z. Adjuvant administration of vitamin C improves mortality of patients with sepsis and septic shock: a systems review and meta-analysis. Open J Int Med. (2018) 8:146–59. doi: 10.4236/ojim.2018.82015
34. Lambden, S, Laterre, PF, Levy, MM, and Francois, B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care. (2019) 23:374. doi: 10.1186/s13054-019-2663-7
35. Fowler, AA 3rd, Truwit, JD, Hite, RD, Morris, PE, DeWilde, C, Priday, A, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. (2019) 322:1261–70. doi: 10.1001/jama.2019.11825
36. Hiemstra, B, Eck, RJ, Wiersema, R, Kaufmann, T, Koster, G, TWL, S, et al. Clinical examination for the prediction of mortality in the critically ill: the simple intensive care studies-I. Crit Care Med. (2019) 47:1301–9. doi: 10.1097/CCM.0000000000003897
37. Lee, SI, Lim, CM, Koh, Y, Huh, JW, Lee, JS, and Hong, SB. The effectiveness of vitamin C for patients with severe viral pneumonia in respiratory failure. J Thorac Dis. (2021) 13:632–41. doi: 10.21037/jtd-20-1306
38. Hemilä, H, and Louhiala, P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. (2013) 2013:CD000980. doi: 10.1002/14651858.CD000980.pub4
39. Annane, D, Renault, A, Brun-Buisson, C, Megarbane, B, Quenot, JP, Siami, S, et al. CRICS–TRIGGERSEP network: hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. (2018) 378:809–18. doi: 10.1056/NEJMoa1705716
40. Annane, D, Sébille, V, Charpentier, C, Bollaert, PE, François, B, Korach, JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. (2002) 288:862–71. doi: 10.1001/jama.288.7.862
41. Buchman, TG, Simpson, SQ, Sciarretta, KL, Finne, KP, Sowers, N, Collier, M, et al. Sepsis among Medicare beneficiaries, 1: the burdens of sepsis, 2012-2018. Crit Care Med. (2020) 48:276–88. doi: 10.1097/CCM.0000000000004224
42. Rhee, C, Dantes, R, Epstein, L, Murphy, DJ, Seymour, CW, Iwashyna, TJ, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. (2017) 318:1241–9. doi: 10.1001/jama.2017.13836
43. Rhodes, A, Evans, LE, Alhazzani, W, Levy, MM, Antonelli, M, Ferrer, R, et al. Surviving Sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. (2017) 45:486–552. doi: 10.1097/CCM.0000000000002255
Keywords: vitamin C, ascorbic acid, sepsis, septic shock, critically ill, pneumonia
Citation: Alissa A, Alrashed MA, Alshaya AI, Al Sulaiman K and Alharbi S (2024) Reevaluating vitamin C in sepsis and septic shock: a potential benefit in severe cases? Front. Med. 11:1476242. doi: 10.3389/fmed.2024.1476242
Edited by:
Claude E. Guérin, Hospices Civils de Lyon, FranceReviewed by:
Luis Chiscano-Camón, Vall d’Hebron University Hospital, SpainFrancesco Forfori, University of Pisa, Italy
Copyright © 2024 Alissa, Alrashed, Alshaya, Al Sulaiman and Alharbi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed A. Alrashed, cnhlZG1vQGdtYWlsLmNvbQ==
 Abdulrahman Alissa1
Abdulrahman Alissa1 Mohammed A. Alrashed
Mohammed A. Alrashed Abdulrahman I. Alshaya
Abdulrahman I. Alshaya Khalid Al Sulaiman
Khalid Al Sulaiman