- 1Faculty of Health Sciences, Ben Gurion University of the Negev, Beer Sheva, Israel
- 2Clinical Research Unit, Soroka University Medical Center, Beer Sheva, Israel
- 3Pediatric Pulmonary Unit, Soroka University Medical Center, Beer Sheva, Israel
- 4Pediatric Dermatology Service, Soroka University Medical Center, Beer Sheva, Israel
Background: Prior studies demonstrated conflicting results regarding hematologic ratios in acne patients. We sought to further characterize hematologic ratios in acne patients, according to demographics and acne severity.
Methods: National, retrospective cohort study of 122,822 patients using medical records from 2005 to 2024 of patients insured with the largest public healthcare organization in Israel, Clalit Health Maintenance Organization.
Results: Moderate–severe acne patients had higher neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) than mild acne patients at diagnosis and 12–18 months before diagnosis. A multivariable regression confirmed the significance of the correlation of increased NLR and PLR with acne severity. Adults and females had higher NLR and PLR than children and males, respectively, at diagnosis, and 12–18 months before diagnosis.
Conclusion: Acne severity was significantly associated with elevated NLR and PLR. NLR and PLR may also serve as indicators of upcoming acne severity, as they were elevated 12–18 months before diagnosis. These biomarkers may contribute to the diagnosis, management, and follow-up of patients with acne.
1 Introduction
Acne vulgaris is one of the most common skin conditions (1). It affects a staggering 9.4% of people of all ages (1, 2), with 20% of patients suffering from moderate to severe acne (3). Up to 85% of individuals aged 12–24 years suffer from acne (1). The incidence of acne peaks at the age of 15 years and declines throughout late adolescence (4). However, prevalence continues into the second and third decades of life in 64% and 43% of individuals, respectively (1). There is a debate in the literature about whether acne disproportionally affects females or males (4–7), though severe acne may disproportionally affect males (8).
Since the pathophysiology of acne involves activation of the immune system, specifically, neutrophils and helper T cells (9–11), hematologic ratios, specifically neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), may be effective as accessible biomarkers of acne’s inflammation. These ratios have been proposed as useful in the assessment of severity and prognosis of other dermatologic conditions, including psoriasis (12), urticaria (13, 14) atopic dermatitis (15), and vitiligo (16), as well as non-dermatologic conditions (17–20). Notably, they were shown to be higher 12–18 months before the diagnosis of disease in some dermatologic conditions (12, 16). With regards to acne and hematologic ratios, there are very few conflicting studies in the literature, with cohorts ranging from 20 patients to 65 patients (21–24).
To our knowledge, this is the first study to characterize hematologic markers for acne in a large, national cohort that includes patients younger than 16 years old. These ratios may be important considerations in assessing acne severity and prognosis, and thus management.
2 Materials and methods
2.1 Study design
This retrospective, national cohort study included patients of all ages with acne between 2005 and 2024. We extracted data from MDClone, a data-sharing platform with data-synthesizing capabilities, regarding the medical records of patients insured in the Clalit Health Maintenance Organization (HMO). The Clalit HMO is the largest public healthcare organization in Israel, serving around 4,700,000 people, which is about half of the population of Israel, across 27 hospitals and more than 1,500 community-based clinics in Israel. The study was approved by the local Ethics Committee of Soroka University Medical Center (No. 0434-15-SOR).
2.2 Study population and blood count parameters
We included all patients insured by Clalit HMO who were diagnosed with acne according to ICD-10 codes. We compared patients according to age, gender, socioeconomic status, ethnicity, complete blood counts and hematologic ratios. The socioeconomic score was determined by the National Institute for Statistics according to the zip code of the patient. We calculated hematologic ratios from complete blood counts by dividing the relevant variables. For example, NLR was calculated by dividing neutrophils by lymphocytes.
We analyzed patients’ hematologic ratios within 30 days of diagnosis with acne, and 12–18 months before diagnosis. We compared children (ages 0–18 years) vs. adults, females vs. males, and patients with mild vs. moderate–severe acne. We defined patients as having moderate–severe acne if they fulfilled one of the following conditions: (1) If they took systemic medication for acne, including isotretinoin, antibiotics, spironolactone, or oral contraceptive pills. (2) If they were diagnosed with acne fulminans or acne conglobate. (3) If they visited the emergency department for their acne.
Lastly, we excluded patients if they had no blood work done within 30 days of diagnosis with acne or 12–18 months before diagnosis. We excluded patients if they had a history of acute or chronic infections, malignancies, or surgery within the past 30 days of their blood test. We excluded patients if they were diagnosed with a systemic disease that leads to acne, such as polycystic ovary syndrome, Cushing syndrome, Behçet’s disease, androgen secreting tumors, PAPA syndrome, Alpert syndrome, and acromegaly.
2.3 Statistical analysis
We analyzed continuous variables using the Student’s t-test or Mann–Whitney U test, and categorical variables using the Chi-squared test or Fisher’s exact test. We performed a multivariable logistic regression adjusted for age, gender, socioeconomic status, and ethnicity for each hematological ratio independently. We considered p-values < 0.05 statistically significant. All statistical analyses were completed using R software (version 4.0.2).
3 Results
3.1 Children vs. adults
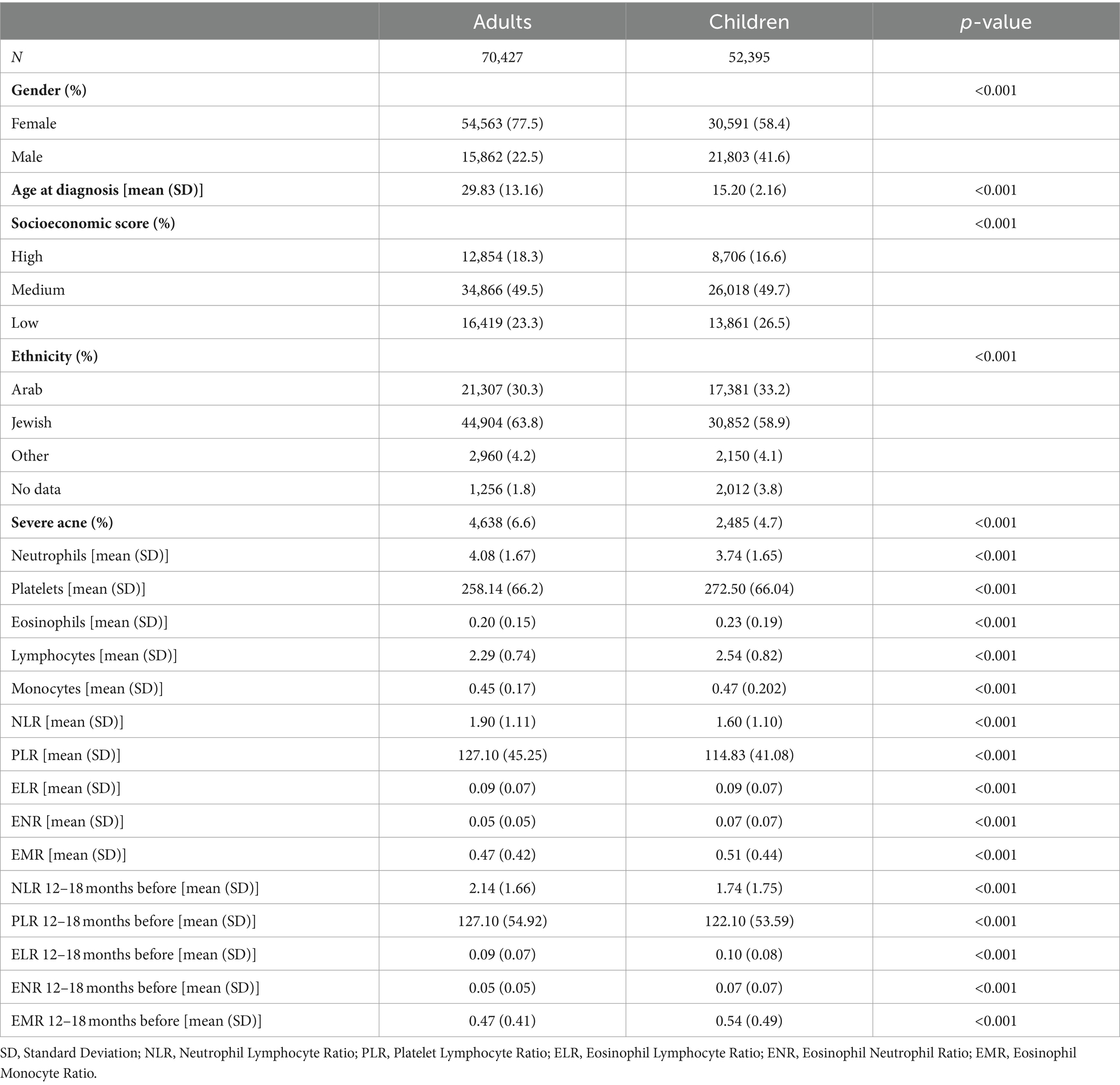
The study included 70,427 adults and 52,395 children aged 0–18 years with acne (Table 1). The proportion of females was significantly higher in the adults compared to the children (mean: 77.5% vs. 58.4%, p < 0.001). Adults with acne had a mean age at diagnosis of 29.83 years, while children were diagnosed at a mean age of 15.2 years (p < 0.001). Notably, adults with acne were predominantly from a higher socioeconomic status than children, and of Jewish ethnicity (p < 0.001 for both). Importantly, adult patients exhibited a significantly higher prevalence of moderate–severe acne (mean: 6.6% of adults compared to 4.7% of children, p < 0.001).
Compared to children, adults had higher NLR (mean: 1.90 vs. 1.60, p < 0.001) and PLR (mean: 121.70 vs. 114.83, p < 0.001). Adults had higher levels of neutrophils, and lower levels of lymphocytes and platelets, compared to children. Furthermore, adults had higher NLR (mean: 2.14 vs. 1.74, p < 0.001) and PLR (mean: 127.10 vs. 122.10, p < 0.001) compared to the children 12–18 months before diagnosis. Eosinophils, monocytes, eosinophils to lymphocyte ratio (ELR), eosinophil-to-neutrophil ratio (ENR), and eosinophil-to-monocyte ratio (EMR) showed statistically significant results of limited clinical significance.
3.2 Females vs. males
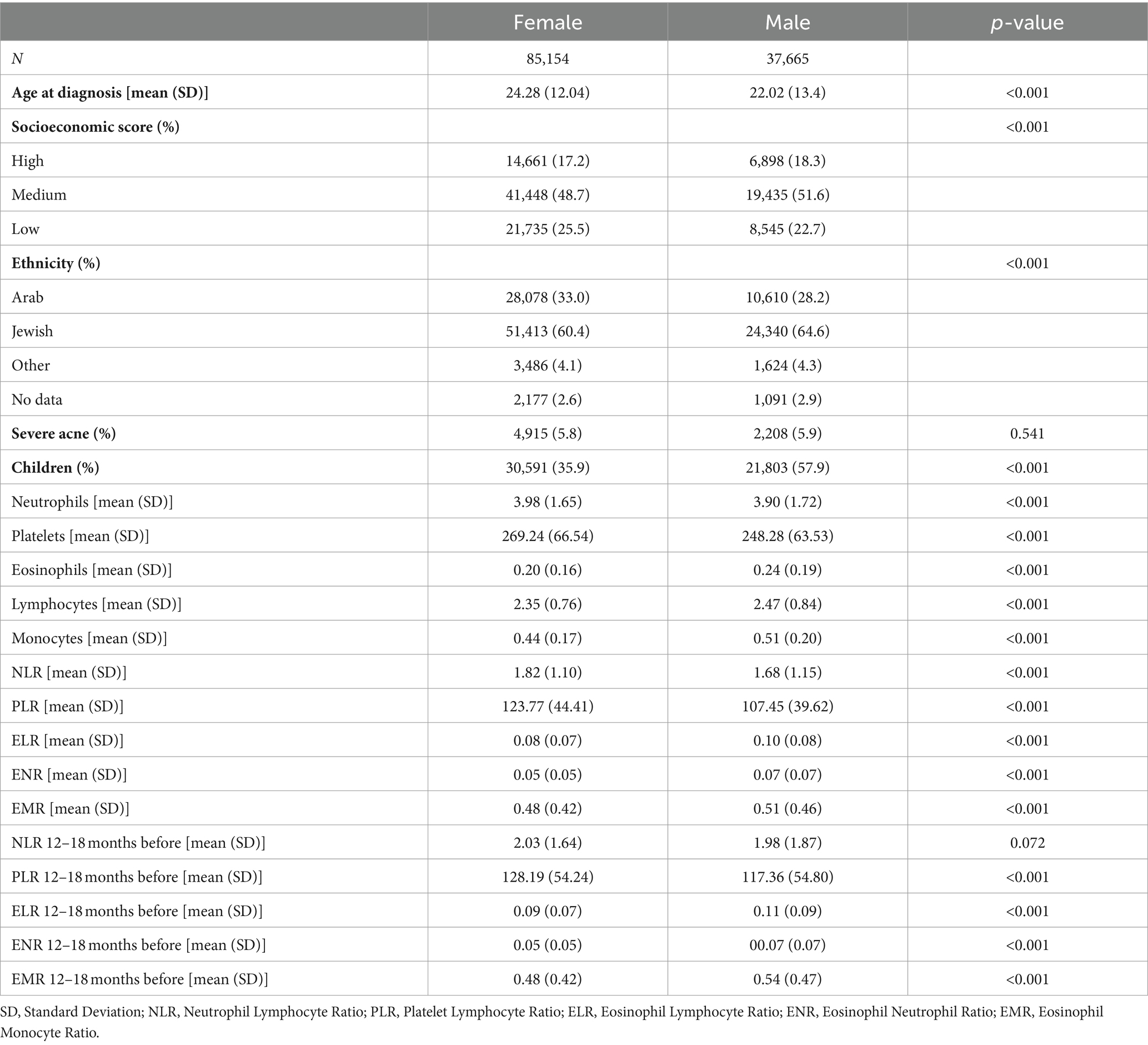
This analysis included 85,154 females with acne and 37,665 males with acne (Table 2). Females were older at diagnosis than males (mean: 24.28 years vs. 22.02 years, p < 0.001), and the proportion of children was lower among females (mean: 35.9% vs. 57.9%, p < 0.001). Socioeconomic score and ethnicity were both significantly different between the females and males, as females tended to be from a lower socioeconomic status, and of Arab ethnicity (p < 0.001 for both). The severity of acne did not differ between males and females (p = 0.541).
Females with acne had higher NLR (mean: 1.82 vs. 1.68, p < 0.001) and PLR (mean: 123.77 vs. 107.45, p < 0.001) compared to males with acne. Females with acne had higher levels of neutrophils and platelets, and lower levels of lymphocytes than males with acne. Moreover, 12–18 months before diagnosis, PLR was higher in females than males (mean: 128.19 vs. 117.36, p < 0.001), but NLR was not significantly different between the genders (p = 0.072).
3.3 Mild vs. moderate–severe acne patients
Stratification by severity revealed significant differences in demographics (Table 3). This analysis included 115,699 patients with mild acne and 7,123 patients with moderate–severe acne. Socioeconomic status and ethnicity were significantly different between the moderate–severe and mild acne groups, as moderate–severe acne patients had a higher proportion of people from high socioeconomic class and Jewish ethnicity (p < 0.001 and p = 0.024, respectively). The proportion of children was lower among the moderate–severe acne patients (34.9% vs. 43.1%, p < 0.001), and patients with moderate–severe acne were diagnosed at an older age compared to patients with mild acne (mean: 28.26 vs. 23.30 years, p < 0.001). There was no gender disparity between the patients with mild and moderate–severe acne.
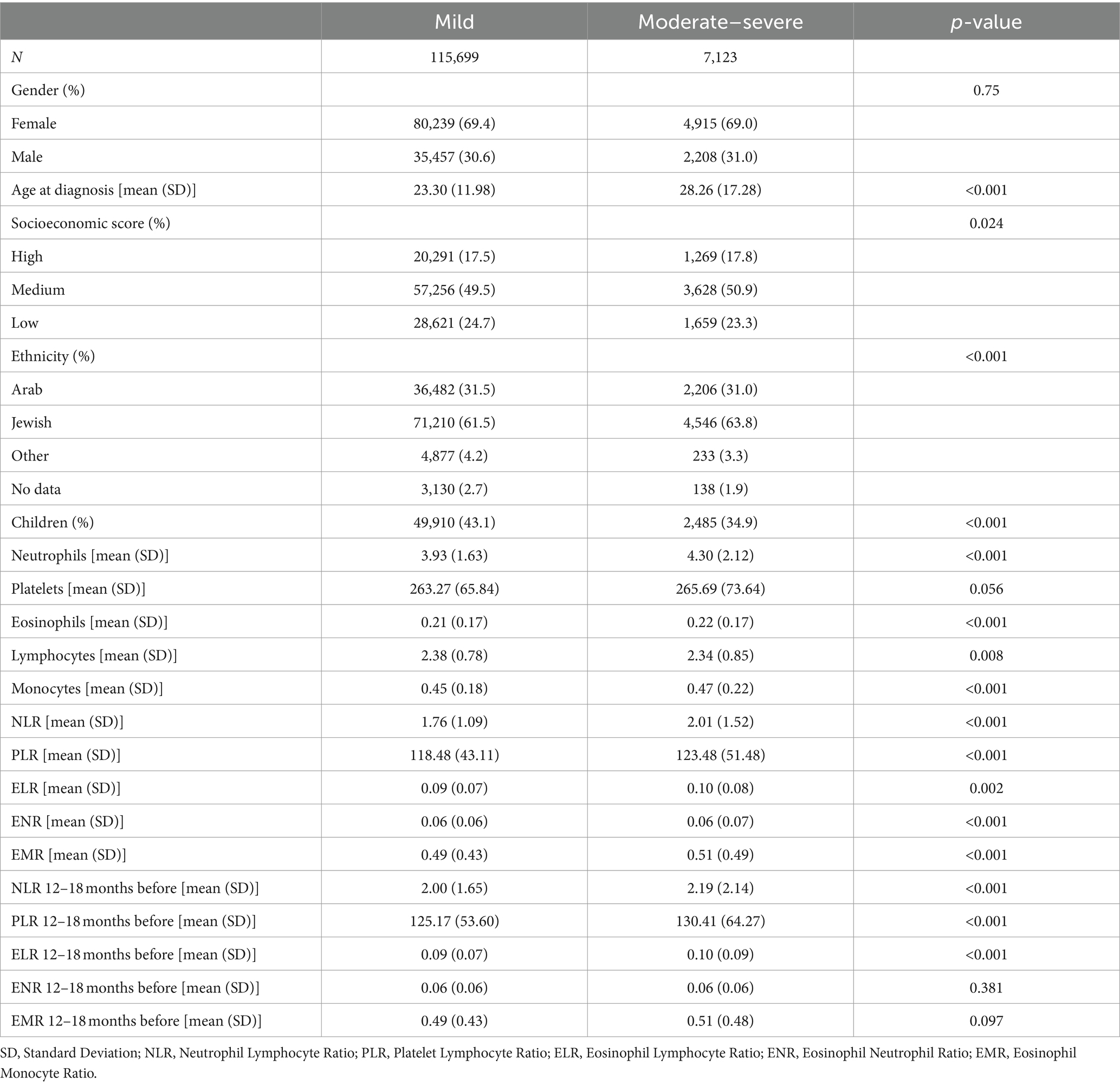
Table 3. Clinical, demographic, and laboratory characteristics of patients with mild and moderate–severe acne.
Patients with moderate–severe acne had higher NLR (mean: 2.01 vs. 1.76, p < 0.001) and PLR (mean: 123.48 vs. 118.48, p < 0.001) than patients with mild acne. Patients with moderate–severe acne had higher levels of neutrophils and lower levels of lymphocytes than patients with mild acne. Platelets were not significantly different between the mild and moderate–severe acne patients. 12–18 months before diagnosis, NLR (mean: 2.19 vs. 2.00, p < 0.001) and PLR (mean: 130.41 vs. 125.17, p < 0.001), were higher in moderate–severe acne patients than mild acne patients 12–18 months before diagnosis.
3.4 Multivariable logistic regression analysis for acne severity
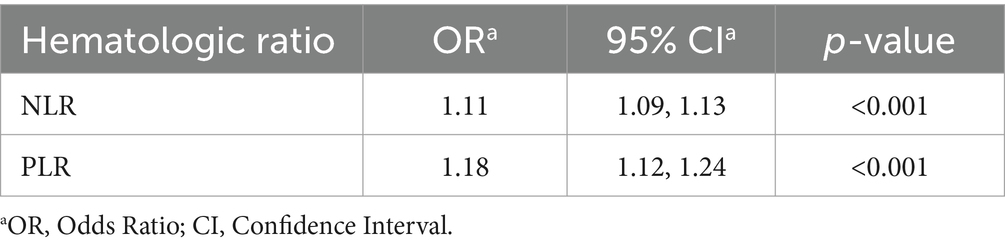
We chose to conduct a multivariable logistic regression analysis for acne severity with NLR and PLR, as they showed the most clinically relevant statistical differences (Table 4). When considering patients with moderate–severe acne, there were statistically significant increases in NLR and PLR, corresponding with increased severity. NLR (odds ratio (OR) 1.11, confidence interval (CI) 1.13) and PLR (OR 1.18, CI 1.24), demonstrated statistically significant associations with acne severity (both p < 0.001).
4 Discussion
In this study, we found that elevated NLR and PLR were significantly associated with severity of acne. Previous studies about hematologic ratios and acne severity showed conflicting results, with smaller sample sizes. One study of 76 patients aged 16–35 years showed that NLR was not elevated in patients with severe-very severe acne compared to patients with mild–moderate acne (21). Another study showed no differences in any complete blood count value or hematologic ratio, including NLR and PLR, between 10 severe acne patients, 10 mild acne patients, and 10 healthy controls (22). By contrast, other studies, with cohorts of 65 or fewer patients, showed that NLR and neutrophils were elevated in acne patients compared to healthy controls (23, 24). The elevated NLR and PLR may be related to the presence of Cutibacterium acnes, which has been implicated in the pathogenesis of inflammatory acne (25). Cutibacterium acnes leads to T-cell mitogenic activity (25). These lymphocytes enter the skin lesions of patients with acne, and thus may be decreased in the peripheral blood. Furthermore, Cutibacterium acne is known to increase neutrophil activity and chemotaxis (26). Since acne is an inflammatory disease (10), the slightly elevated platelets may be related to platelets’ role in immunothrombosis and chronic inflammation (26). Overall, our study expanded on previous studies about hematologic ratios and acne and utilized a much larger sample size of more than a hundred thousand patients.
In our study, we noted that moderate–severe patients had an increase in NLR and PLR 12–18 months before acne diagnosis. This contributes to the notion that acne is an inflammatory disease before the development of clinical lesions (27). Inflammatory changes have been seen in uninvolved skin of acne patients before the formation of lesions. For instance, both T lymphocytes and the pro-inflammatory cytokine IL-1 have been shown to infiltrate healthy skin of patients with acne prior to the formation of lesions (10, 28). Since IL1 is associated with leukocyte chemotaxis (29), it is possible that these lymphocytes infiltrate the site of the acne lesion as it forms, and thus decrease relatively in the peripheral blood, corresponding with elevated NLR and PLR. Overall, these hematological changes may imply that PLR and NLR should be considered as indicators for acne prognosis and severity, much before the development of clinical lesions.
In our study, we saw adults with acne had higher NLR and PLR than children with acne. Post adolescence acne is more likely to consist of inflammatory lesions (30). In studies examining biopsies of inflammatory acne lesions, IL-8, a pro-inflammatory cytokine crucial for the chemotaxis of neutrophils toward the acne unit, has been shown to be upregulated, compared to biopsies of uninvolved skin from the same patients (31, 32). Neutrophils, in particular, have been shown to be elevated inside 24-h acne pustules, with an even higher elevation shown in 72-h acne lesions (33). Hence, NLR may be higher in adults due to their pre-disposition for inflammatory, neutrophil-rich acne.
Lastly, in our study, females had higher NLR and PLR, despite no significant differences in the severity of their acne. The literature is inconclusive about whether, in the general population, healthy females and males have naturally different hematologic ratio profiles (34), though it is known that hematologic ratios can vary with age and race (34–36). In particular, NLR has been shown to increase with older age, potentially due to a greater disposition to inflammation and disease as age increases (35, 37). A study of Chinese females and males showed a trend of increasing NLR and PLR up to age 40–59, with a subsequent decrease, in females, while there were little to no age specific differences in males (34). Thus, the differences between the female and male populations of our study may be explained by the higher proportion of adults in the female population of our study.
To the best of our knowledge, this study is the first study to analyze NLR, PLR, ELR, ENR, and EMR in a national cohort of acne patients, and in a cohort that includes children younger than 16 years, nevertheless, we must acknowledge some limitations. First, as this study is a retrospective study, there may be potential biases pertaining to data collection and especially acne severity, since there is still debate in the literature regarding the classification of acne and treatment of acne based on classification (38–40). This is especially relevant in our cohort because we included patients who were diagnosed with acne by primary care physicians and board-certified dermatologists, who may employ different acne diagnosis criteria, leading to a lower prevalence of moderate–severe acne patients within our cohort than expected. As a result, the use of ICD-10 codes in our study, though practical, may not fully reflect the clinical complexity of the condition. To mitigate this limitation, we have used clear criteria for acne severity, as detailed in the methods section. Second, complete blood counts are not part of the routine diagnosis of acne. While lab work may be monitored prior to and during initiation of treatment with isotretinoin, this practice remains controversial (41, 42), and complete blood count analysis is not typically part of the diagnostic workup of mild acne. Therefore, it is possible that the acne patients had an underlying condition warranting a complete blood count analysis. It is known that many conditions may lead to changes in hematologic ratios (12–17). Additionally, in patients with moderate–severe acne, bloodwork may be part of the workup for systemic diseases that involve acne. To minimize this bias, we excluded patients with histories of acute or chronic infections, malignancies, or surgery, and systemic diseases that lead to acne, such as polycystic ovary syndrome and Cushing syndrome. Despite this, it is still possible that we have included patients with other conditions that may affect blood count values. Thirdly, there are other confounding factors, including stress, diet, and use of certain medications, which may affect NLR and PLR, and were not accounted for in this current study. These variables are likely to be present in both the mild and moderate–severe acne patients, though due to the retrospective nature of the study, it is impossible to extrapolate to which degree. Future, prospective, longitudinal studies of hematologic ratios may account for these factors with questionnaires regarding participants’ stress levels and diets. Lastly, while the lab values were taken 30 days before and after the diagnosis of acne according to ICD-10 codes, it could not be determined whether the disease was present at that time.
5 Conclusion
In this national retrospective cohort study, we saw significant elevations of NLR and PLR in patients with moderate–severe acne compared to patients with mild acne, both at diagnosis and 12–18 months before diagnosis. According to our multivariable regression model, acne severity was significantly associated with higher NLR and PLR. These biomarkers may contribute to the diagnosis, management, and follow-up of patients with acne. Future studies regarding the mechanism of the changes in these hematologic ratios are warranted, with longitudinal design and additional populations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the local Ethics Committee of Soroka University Medical Center (No. 0434-15-SOR). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
VW: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. SW: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Writing – original draft, Writing – review & editing. BC: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. IG-T: Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. AH: Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NLR, Neutrophil-to-lymphocyte ratio; PLR, Platelet-to-lymphocyte ratio; ELR, Eosinophil-to-lymphocyte ratio; ENR, Eosinophil-to-neutrophil ratio; EMR, Eosinophil-to-monocyte ratio; HMO, Health Maintenance Organization.
References
1. Bhate, K, and Williams, HC. Epidemiology of acne vulgaris. Br J Dermatol. (2013) 168:474–85. doi: 10.1111/bjd.12149
2. Vos, T, Flaxman, AD, Naghavi, M, Lozano, R, Michaud, C, Ezzati, M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. (2012) 380:2163–96. doi: 10.1016/S0140-6736(12)61729-2
3. Dréno, B. Recent data on epidemiology of acne. Ann Dermatol Venereol. (2010) 137:3–5. doi: 10.1016/S0151-9638(10)70045-4
4. Lynn, DD, Umari, T, Dunnick, CA, and Dellavalle, RP. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. (2016) 7:13–25. doi: 10.2147/AHMT.S55832
5. Heng, AHS, and Chew, FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. (2020) 10:5754. doi: 10.1038/s41598-020-62715-3
6. Hogewoning, AA, Koelemij, I, Amoah, AS, Bouwes Bavinck, JN, Aryeetey, Y, Hartgers, F, et al. Prevalence and risk factors of inflammatory acne vulgaris in rural and urban Ghanaian schoolchildren. Br J Dermatol. (2009) 161:475–7. doi: 10.1111/j.1365-2133.2009.09259.x
7. Chen, H, Zhang, TC, Yin, XL, Man, JY, Yang, XR, and Lu, M. Magnitude and temporal trend of acne vulgaris burden in 204 countries and territories from 1990 to 2019: an analysis from the global burden of disease study 2019*. Br J Dermatol. (2022) 186:673–83. doi: 10.1111/bjd.20882
8. Say, YH, Heng, AHS, Reginald, K, Wong, YR, Teh, KF, Rawanan Shah, SM, et al. Modifiable and non-modifiable epidemiological risk factors for acne, acne severity and acne scarring among Malaysian Chinese: a cross-sectional study. BMC Public Health. (2021) 21:601. doi: 10.1186/s12889-021-10681-4
9. Dréno, B. What is new in the pathophysiology of acne, an overview. J Eur Acad Dermatol Venereol. (2017) 31:8–12. doi: 10.1111/jdv.14374
10. Jeremy, AHT, Holland, DB, Roberts, SG, Thomson, KF, and Cunliffe, WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. (2003) 121:20–7. doi: 10.1046/j.1523-1747.2003.12321.x
11. Moreno-Arrones, OM, and Boixeda, P. The importance of innate immunity in acne. Actas Dermosifiliogr. (2016) 107:801–5. doi: 10.1016/j.ad.2016.07.005
12. Weissmann, S, Babyev, AS, Gordon, M, Golan-Tripto, I, and Horev, A. Association of hematological ratios with psoriasis: a nationwide retrospective cohort study. Int J Dermatol. (2024) 240:597–605. doi: 10.1159/000539365
13. Weissmann, S, Burrack, N, Babyev, AS, Gordon, M, Golan-Tripto, I, and Horev, A. Eosinophil-lymphocyte ratio, eosinophil-neutrophil ratio, and eosinophil-monocyte ratio in chronic and severe Urticaria. Am J Clin Dermatol. (2023) 24:669–71. doi: 10.1007/s40257-023-00781-9
14. Weissmann, S, Burrack, N, Golan-Tripto, I, and Horev, A. Increased neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in chronic and severe Urticaria. Acta Derm Venereol. (2024) 104:adv23932. doi: 10.2340/actadv.v103.23932
15. Weissmann, S, Babyev, AS, Gordon, M, Golan-Tripto, I, and Horev, A. Hematological Markers in Children and Adults with Atopic Dermatitis: A Retrospective Cohort Study. Dermatology. (2024).
16. Weissmann, S, Burrack, N, Golan-Tripto, I, and Horev, A. Determination of elevated eosinophil to lymphocyte ratio, eosinophil to neutrophil ratio, eosinophil to monocyte ratio and its association with severe vitiligo: a retrospective cohort study. PLoS One. (2024) 19:e0296626. doi: 10.1371/journal.pone.0296626
17. Burrack, N, Adar, A, Goldbart, A, Weissmann, S, Cohen, B, Hazan, I, et al. Monocyte and neutrophil to lymphocyte ratios in hospitalized children with RSV bronchiolitis. Pediatr Pulmonol. (2023) 58:3530–41. doi: 10.1002/ppul.26687
18. Celikbilek, M, Dogan, S, Ozbakır, O, Zararsız, G, Kücük, H, Gürsoy, S, et al. Neutrophil–lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J Clin Lab Anal. (2013) 27:72–6. doi: 10.1002/jcla.21564
19. Alan, S, Tuna, S, and Türkoğlu, EB. The relation of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume with the presence and severity of Behçet’s syndrome. Kaohsiung J Med Sci. (2015) 31:626–31. doi: 10.1016/j.kjms.2015.10.010
20. Arwas, N, Shvartzman, SU, Goldbart, A, Bari, R, Hazan, I, Horev, A, et al. Elevated neutrophil-to-lymphocyte ratio is associated with severe asthma exacerbation in children. JCM. (2023) 12:3312. doi: 10.3390/jcm12093312
21. Daye, M, Temiz, SA, Ozturk, ENY, and Isik, B. Assessment of ELR, NLR, MPV, and CRP levels in patients with acne vulgaris. Cyprus J Med Sci. (2022) 7:241–4. doi: 10.4274/cjms.2021.2306
22. Chen, T, Chen, Y, Shao, X, Chen, J, Liu, L, Li, Y, et al. Hematological parameters in patients with acnes. J Cosmet Dermatol. (2023) 22:2099–104. doi: 10.1111/jocd.15676
23. Turkmen, D, Altunisik, N, and Sener, S. Investigation of monocyte HDL ratio as an indicator of inflammation and complete blood count parameters in patients with acne vulgaris. Int J Clin Pract. (2020) 74:e13639. doi: 10.1111/ijcp.13639
24. Duman, N, Uzunali, E, and Manav, V. A comparative study on hematological inflammation markers in acne vulgaris. Inte Blood Res Rev. (2015) 4:1–5. doi: 10.9734/IBRR/2015/21905
25. Jappe, U, Ingham, E, Henwood, J, and Holland, KT. Propionibacterium acnes and inflammation in acne; P. acnes has T-cell mitogenic activity. Br J Dermatol. (2002) 146:202–9. doi: 10.1046/j.1365-2133.2002.04602.x
26. Akamatsu, H, Horio, T, and Hattori, K. Increased hydrogen peroxide generation by neutrophils from patients with acne inflammation. Int J Dermatol. (2003) 42:366–9. doi: 10.1046/j.1365-4362.2003.01540.x
27. Rocha, MA, Costa, CS, and Bagatin, E. Acne vulgaris: an inflammatory disease even before the onset of clinical lesions. Inflamm Allergy Drug Targets (Discontinued). (2014) 13:162–7. doi: 10.2174/1871528113666140606110024
28. Ingham, E, Eady, EA, Goodwin, CE, Cove, JH, and Cunliffe, WJ. Pro-inflammatory levels of interleukin-1 alpha-like bioactivity are present in the majority of open comedones in acne vulgaris. J Invest Dermatol. (1992) 98:895–901. doi: 10.1111/1523-1747.ep12460324
29. Hunninghake, GW, Glazier, AJ, Monick, MM, and Dinarello, CA. Interleukin-1 is a chemotactic factor for human T-lymphocytes. Am Rev Respir Dis. (1987) 135:66–71.
30. Goulden, V, Clark, SM, and Cunliffe, WJ. Post-adolescent acne: a review of clinical features. Br J Dermatol. (1997) 136:66–70. doi: 10.1111/j.1365-2133.1997.tb08748.x
31. Trivedi, NR, Gilliland, KL, Zhao, W, Liu, W, and Thiboutot, DM. Gene Array expression profiling in acne lesions reveals marked upregulation of genes involved in inflammation and matrix remodeling. J Invest Dermatol. (2006) 126:1071–9. doi: 10.1038/sj.jid.5700213
32. Kelhälä, HL, Palatsi, R, Fyhrquist, N, Lehtimäki, S, Väyrynen, JP, Kallioinen, M, et al. IL-17/Th17 pathway is activated in acne lesions. PLoS One. (2014) 9:E105238. doi: 10.1371/journal.pone.0105238
33. JFB, N, and Cunliffe, WJ. A histological and immunocytochemical study of early acne lesions. Br J Dermatol. (1988) 118:651–9. doi: 10.1111/j.1365-2133.1988.tb02566.x
34. Wu, L, Zou, S, Wang, C, Tan, X, and Yu, M. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in Chinese Han population from Chaoshan region in South China. BMC Cardiovasc Disord. (2019) 19:125. doi: 10.1186/s12872-019-1110-7
35. Azab, B, Camacho-Rivera, M, and Taioli, E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS One. (2014) 9:e112361. doi: 10.1371/journal.pone.0112361
36. Meng, X, Chang, Q, Liu, Y, Chen, L, Wei, G, Yang, J, et al. Determinant roles of gender and age on SII, PLR, NLR, LMR and MLR and their reference intervals defining in Henan, China: a posteriori and big-data-based. J Clin Lab Anal. (2018) 32:e22228. doi: 10.1002/jcla.22228
37. Li, J, Chen, Q, Luo, X, Hong, J, Pan, K, Lin, X, et al. Neutrophil-to-lymphocyte ratio positively correlates to age in healthy population. J Clin Lab Anal. (2014) 29:437–43. doi: 10.1002/jcla.21791
38. López-Estebaranz, JL, Herranz-Pinto, P, and Dréno, B. Consensus-based acne classification system and treatment algorithm for Spain. Actas Dermosifiliogr (Engl Ed). (2017) 108:120–31. doi: 10.1016/j.ad.2016.10.001
39. Layton, AM, Alexis, A, Baldwin, H, Bettoli, V, Del Rosso, J, Dirschka, T, et al. The personalized acne treatment tool — recommendations to facilitate a patient-centered approach to acne management from the personalizing acne: consensus of experts. JAAD Int. (2023) 12:60–9. doi: 10.1016/j.jdin.2023.03.013
40. Prapapan, O, Chatchavarn, CC, Suvanprakorn, P, Neumann, HAM, Knobler, R, Prombandankul, A, et al. Proposal for a 4-type classification of acne: an evidence-based review of the literature. TODJ. (2020) 14:38–43. doi: 10.2174/1874372202014010038
41. Barbieri, JS, Shin, DB, Wang, S, Margolis, DJ, and Takeshita, J. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. (2020) 82:72–9. doi: 10.1016/j.jaad.2019.06.025
Keywords: acne vulgaris, hematologic ratios, NLR, PLR, acne, ELR, ENR
Citation: Wiesel V, Weissmann S, Cohen B, Golan-Tripto I and Horev A (2024) Elevated hematologic ratios are correlated with acne severity: a national, retrospective cohort study. Front. Med. 11:1475117. doi: 10.3389/fmed.2024.1475117
Edited by:
Simone Ribero, University of Turin, ItalyReviewed by:
Nelva Jusuf, Universitas Sumatera Utara, IndonesiaMaria Catorze, Hospital de Egas Moniz, Portugal
Copyright © 2024 Wiesel, Weissmann, Cohen, Golan-Tripto and Horev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amir Horev, YW1pcmhvckBjbGFsaXQub3JnLmls
†These authors share first authorship
‡ORCID: Vered Wiesel, orcid.org/0000-0002-3880-6814
Sarah Weissmann, orcid.org/0000-0001-5284-9805
Inbal Golan-Tripto, orcid.org/0000-0001-6259-405X
Amir Horev, orcid.org/0000-0001-6646-9061
 Vered Wiesel
Vered Wiesel Sarah Weissmann
Sarah Weissmann Bracha Cohen2
Bracha Cohen2 Inbal Golan-Tripto
Inbal Golan-Tripto Amir Horev
Amir Horev