- 1Institute of Dermatology and Venereal Diseases, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, China
- 2Department of Dermatology and Cosmetology, Dongguan Maternity and Child Healthcare Hospital, Dongguan, Guangdong, China
- 3Institute of Dermatology, Chinese Academy of Medical Sciences & Peking Union Medical College, Nanjing, Jiangsu, China
Background: Erythrodermic psoriasis (EP) is a rare but life-threatening variant of psoriasis less responsive to conventional systemic therapies (CST). Limited research exists on the management of EP with secukinumab.
Objectives: To compare the effectiveness, quality-of-life effects and safety of secukinumab versus CST in patients with EP.
Methods: EP patients treated with either secukinumab or CST between August 2020 and October 2022 were identified using the National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID) database encompassing 962 healthcare organizations. Propensity score matching (PSM) was performed to balance the cohorts based on demographic and clinical characteristics. The primary outcomes assessed were Psoriasis Area and Severity Index (PASI), Body Surface Area (BSA), and Dermatology Life Quality Index (DLQI) scales at 4 weeks, 10–14 weeks, and 22–24 weeks.
Results: The study included 311 patients (160 receiving secukinumab and 151 receiving CST), among them, 101 matched pairs were generated by propensity score matching (PSM). Secukinumab recipients displayed a notably accelerated response compared to those receiving CST, evidenced by significantly higher rates of achieving PASI50 (before PSM: 73.8% vs. 61.6%, after PSM: 76.2% vs. 63.4%), PASI90 (before PSM: 36.9% vs. 25.8%, after PSM: 40.6% vs. 25.7%), and BSA50 (before PSM: 64.4% vs. 50.3%, after PSM: 68.3% vs. 51.5%) at week 4 (p < 0.05). However, before PSM, secukinumab showed significantly higher DLQI0/1 rates at weeks 4 (41.3% vs. 29.8%) and 12 (63.8% vs. 44.8%). After PSM, statistically significant differences were observed at week 12 for PASI and BSA scores, and at week 4 for DLQI scores (p < 0.05). Similar efficacy trends were observed in other outcomes at week 0 up to week 24, but no statistical differences were noted.
Conclusion: Compared to the CST, secukinumab tend to offer a more rapid response and achieve greater improvements in clinical symptoms and quality of life for EP patients.
Introduction
Erythrodermic psoriasis (EP) is a rare and life-threatening subtype of psoriasis, representing approximately 1 to 2.25% of psoriasis cases, with a mortality rate reaching 9% (1, 2). Clinically, EP is a condition triggered by certain stimulations, characterized by widespread erythema, scaling, and infiltration that rapidly affects more than 75% of the body surface area (BSA) (3). Urgent management is required to prevent complications, including electrolyte imbalances, malabsorption, anemia, cardiac failure, and sepsis (4, 5). Due to its rarity and complexity, treating EP is challenging and the conventional systemic therapy (hereinafter referred to as CST) was commonly employed including retinoid, cyclosporine, methotrexate. Acitretin and methotrexate suit less acute cases due to slower onset (6, 7). By contrast, cyclosporine monotherapy elicits faster effects compared to acitretin and methotrexate, yet high doses may lead to hypertension and nephrotoxicity (1, 8). Furthermore, CST is often accompanied by treatment failure and the potential risks of blanket immunosuppression, intolerance, visceral toxicity, and opportunistic infections (9). Alternative and innovative options are urgently needed for this fatal psoriatic entity.
Biological therapy (BT) represents an emerging and highly effective therapy in EP, including TNF (tumor necrosis factor)-α inhibitors, interleukin (IL)-17 inhibitors, and IL-12/23 inhibitors. A recent systematic review of EP patients treated with various biologics hitherto reported that, out of 122 patients who underwent BTs and provided Psoriasis Area and Severity Index (PASI) scores, 91.0% reached PASI50, indicating the high effectiveness of BT for EP (3). The latest National Psoriasis Foundation Consensus Guidelines recommend infliximab as the only first-line biologic due to limited experience with other biologic drugs at the time (10).
IL-17A is considered a major ‘driver’ of pro-inflammatory cytokines in the pathogenesis of psoriasis, as it can activate keratinocytes and promote their hyper-proliferation (11–13). As the first biologic targeting IL-17 approved for the management of psoriasis, secukinumab has been proven to provide safe, rapid, and sustained improvements in patients with plaque psoriasis, psoriatic arthritis (PsA), and ankylosing spondylitis. Successful management of EP with secukinumab has been described in several case series and reports (10, 14, 15). It may play a positive role in the therapy of EP. As the rarity and severity of EP, which complicates the execution of clinical randomized controlled trials (RCTs), there is insufficient high-level evidence to conclude that secukinumab is a superior substitute for CST in acute and unstable EP patients. Post-marketing registries facilitate the collection of large samples of real-world data from diverse patient populations. As a supplement to clinical trials, comparative cohort studies based on these data can provide evidence to evaluate the efficacy and safety of drugs and offer insights into drug performance in real-life settings (16). The current study utilized the national database of the Chinese Psoriasis Group network and employed the propensity score-based matching method, and aimed to compare the effectiveness of secukinumab versus CST in patients with EP. The study protocol was reviewed and approved by the Ethics Committee of the Affiliated Hospital of Guangdong Medical University (No. KT2023-011-01).
Methods
Study design and materials
This is an exploratory analysis of treatment outcomes with secukinumab versus CST. The sample was obtained from the Chinese Psoriasis Group network database of the National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID)1 between the establishment (August 2020) of the database to October 2022. NCRC-DID is a national health research network that provides access to psoriasis registration data from 962 healthcare organizations in China.
Inclusion and exclusion criteria
Eligible patients satisfied the following inclusion criteria simultaneously: (i) a diagnosis of EP, characterized by diffuse erythema and desquamation affecting more than 75% of BSA. While the traditional definition of erythroderma involves more than 90% of BSA and is widely cited in dermatology, a 75% BSA threshold is sometimes used in EP to identify severe cases requiring prompt intervention (1, 17), thereby ensuring timely and appropriate systemic treatment for patients with significant disease; (ii) having a documented history of diagnosed psoriasis, displaying prototypical psoriatic lesion characteristics clinically, or undergoing histopathological examination confirming psoriasis while excluding other primary differential diagnoses for erythroderma; (iii) undergoing assessments utilizing the PASI, BSA, and Dermatology Life Quality Index (DLQI) scale after receiving treatment at 4 weeks, 10–14 weeks, and 22–24 weeks. Exclusion criteria included incomplete key data and receiving small-molecule inhibitors, PDE4 inhibitors, and combination treatments with both CST and secukinumab. To maximize external validity, comorbid conditions and concomitant topical therapies were not excluded.
Primary outcomes
According to treatment methods, the patients were divided into secukinumab or CST cohorts (receiving systemic immunosuppressants, retinoid derivatives, and any other conventional medications). The following information of included patients was recorded for analysis: (i) demographic and clinical characteristics, including gender, body mass index (BMI), smoking situation (yes/no/quit), duration since psoriasis diagnosis, family history of psoriasis, and age at treatment initiation; (ii) comorbidities associated with psoriasis, such as cardiovascular disease, diabetes, and rheumatism; (iii) association with PsA, diagnosed by a rheumatologist; (iv) baseline and follow-up (W4, W12, W24) BSA and PASI scores, and the number of patients achieving BSA50/75 and PASI50/75/90, which are defined as a reduction of at least 50%/75% in body surface area involvement and an improvement of at least 50%/75%/90% in the PASI score from baseline, respectively; (v) DLQI score and the count of cases with DLQI0/1 (obtained a DLQI score of ≤1, no effect of psoriasis on QoL) at different time points; (vi) Observed adverse events (AEs).
Statistical analysis
Continuous variables for baseline characteristics were compared using the paired t test or the Mann–Whitney U test, and categorical variables were analyzed using chi-square test or Fisher exact test. Propensity score matching (PSM) was performed to balance the cohorts. The variables gender, age, BMI, marital status, education level, smoking history, family history, psoriasis duration, previous systemic treatment, PsA, comorbidity and baseline PASI, BSA, and DLQI score were included in the logistic regression model for calculating the propensity scores, with treatment type as the dependent variable. Patients treated with secukinumab were matched 1:1 with patients treated with CST using nearest neighbor matching with a caliper of 0.1 without replacement. All analysis were performed using SPSS 26.0 software (SPSS Inc., Chicago, IL, USA).
Results
Study population
A total of 311 patients (160 received secukinumab and 151 received CST) were identified with EP who were recorded with complete data. The pre-PSM baseline characteristics of the two cohorts revealed statistically significant differences in marital status, education level, family history of psoriasis, and PsA (p < 0.05), while gender was not as significant but was still important (p = 0.061) (Table 1). Post PSM, no statistical differences were observed in any features in each pairwise comparison, confirming the comparability in two cohorts. An additional 109 patients were excluded due to non-overlapping propensity scores, leaving a total of 202 patients for the final analysis. The rest of the baseline characteristics of the population are summarized in Table 1. Because of some cases lost to follow-up at W12 and W24, a complete case analysis (CCA) was utilized for the per-protocol estimate (i.e., analysis of participants according to the completion of the assigned treatment).
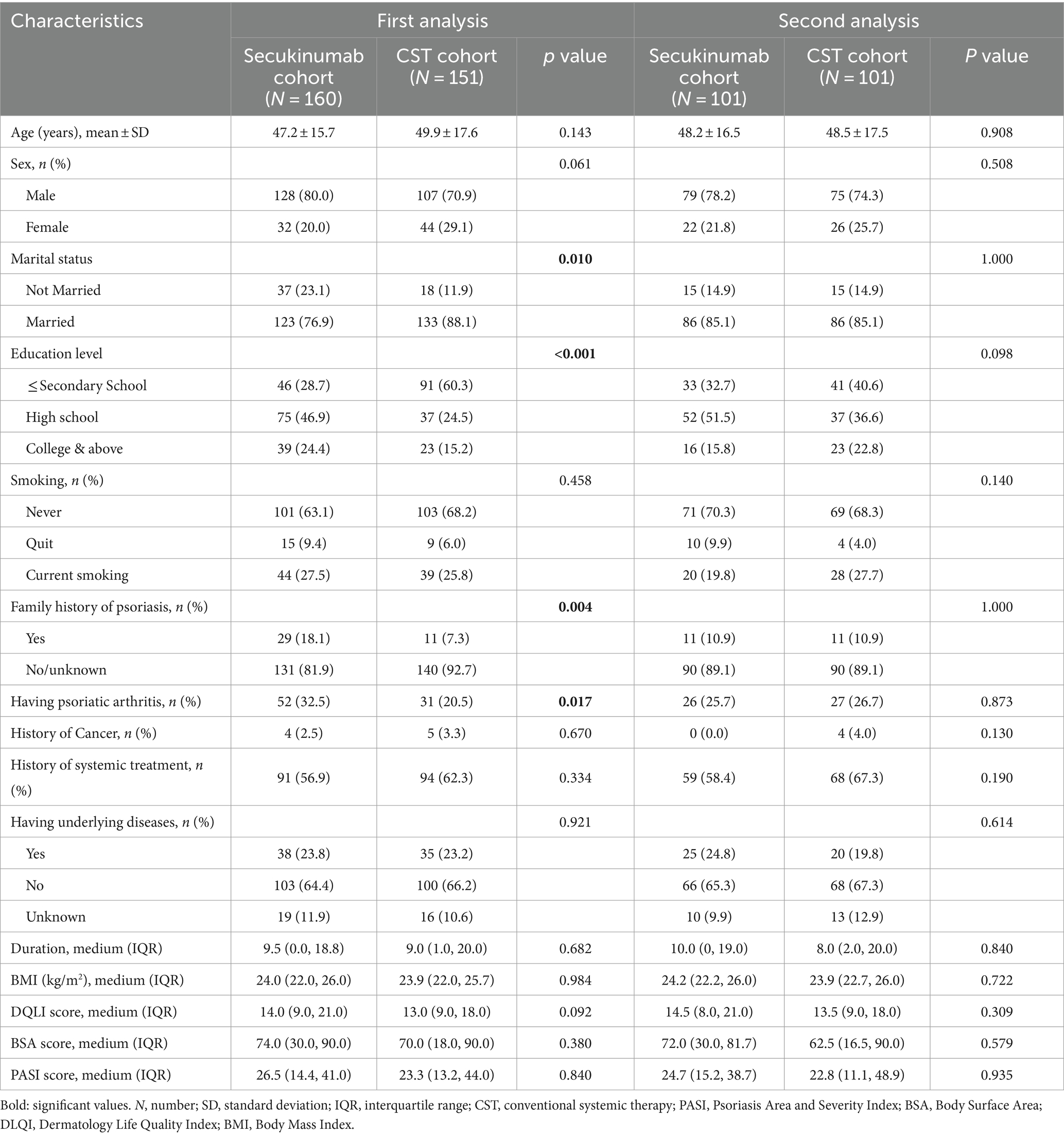
Table 1. Baseline characteristics of erythrodermic psoriasis patients identified in the study (N = 311).
Efficacy and quality of life
Before and after PSM, secukinumab recipients demonstrated a faster response compared to CSTs, with significantly higher PASI50 (before: p = 0.022, after: p = 0.046), PASI90 (before: p = 0.036, after: p = 0.025), and BSA50 (before: p = 0.012, after: p = 0.015) rates achieved at week 4. However, before matching, the secukinumab cohort had significantly higher DLQI0/1 rates than the CST cohort at W4 (p = 0.035) and W12 (p = 0.04). Additionally, similar changing patterns trends were also observed for most efficacy indicators over time up to W24 in all recipients, but with no statistical differences. The magnitude of improvement over time was mostly greater in patients treated with secukinumab compared to CST; however, after PSM, statistically significant differences were observed only at W12 for PASI scores (p = 0.012), at W12 for BSA scores (p = 0.008), and at W4 for DLQI scores (p = 0.046). The comparative results of key efficacy and QoL outcomes at each time point, before and after matching, are detailed in Table 2 Comparisons of PASI, BSA, and DLQI scores at each time point after PSM are outlined in Table 3.
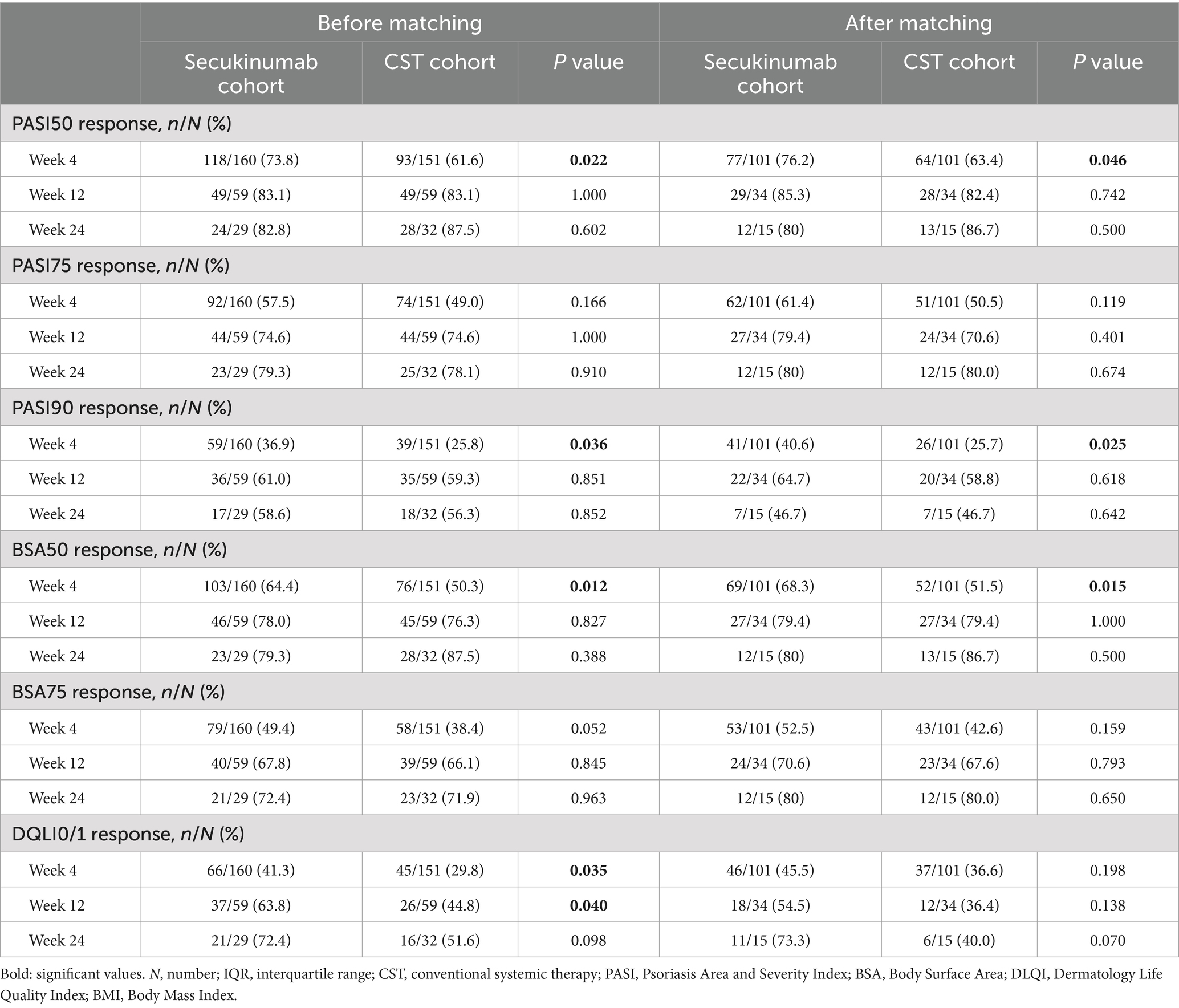
Table 2. Key efficacy and quality-of-life outcomes at follow-up time points in erythrodermic psoriasis patients treated with secukinumab and conventional systemic therapies.
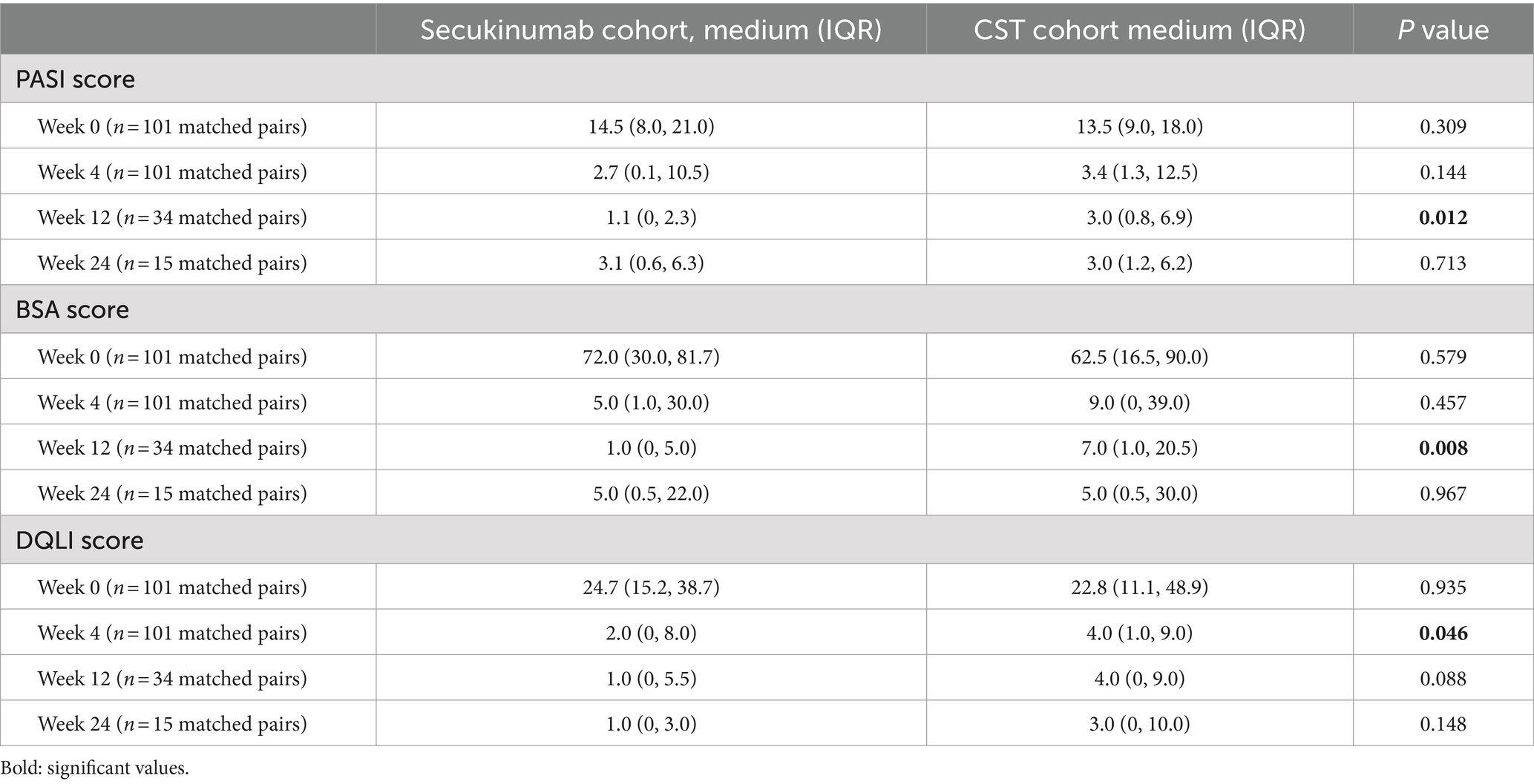
Table 3. Comparison of Psoriasis Area and Severity Index (PASI), Body Surface Area (BSA), and Dermatology Life Quality Index (DLQI) score between Secukinumab recipients and conventional systemic therapies (CSTs) recipients, after propensity-score matching (PASI: score range 0–72, BSA: percentage, DLQI: score range 0–30).
Safety
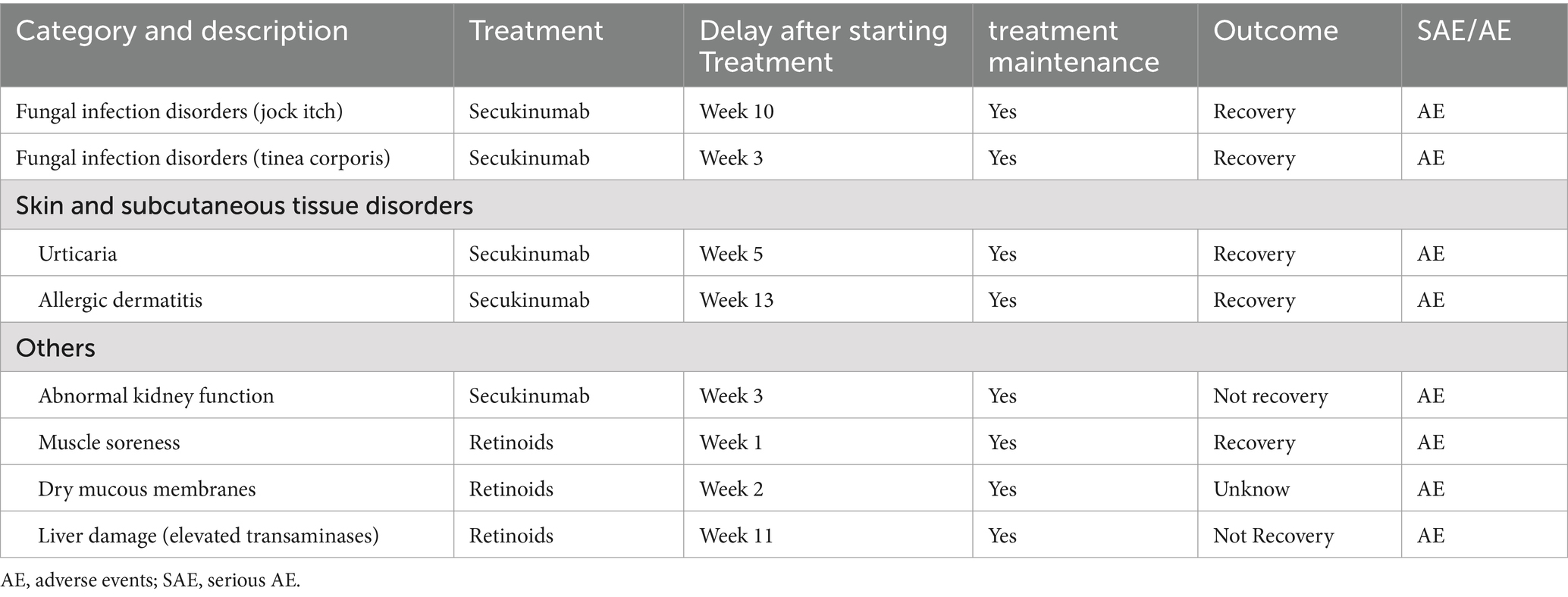
Throughout the 24-week observation period, eight AEs were documented in eight distinct patients (2.57%) of both cohorts. Among them, five were from the secukinumab group (3.12%) and three from the CST group (1.98%). The majority of reported AEs were mild and self-limiting in two groups. No AEs were recorded that required prolonged hospitalization or presented potential life-threatening consequences, thereby avoiding their classification as serious AEs (SAEs). However, two AEs who underwent secukinumab were categorized under the MedDRA high-level term of fungal infection disorders. The result details are listed in Table 4.
Discussion
Differences in proinflammatory cytokine expression are observed in various clinical subtypes of psoriasis. Literature suggests that the immunopathogenesis of EP involves inflammation with Th1/Th17/TNF-skewing and mild Th2 (IL-4, IL-10, IL-13, and IL-25) upregulation, as well as notable increases in epidermal-development markers (18, 19). Disease severity in EP was strongly correlated with Th17 markers (18). Similar tendencies were observed in immunohistochemical analysis, which revealed that the primary T-cell subset present in EP lesions is Th17. Increased IL-17 mRNA expression and elevated serum IL-17 levels have been demonstrated in patients with EP (12). This leads to the production of antimicrobial peptides, cytokines, and chemokines, which further enhance inflammation (11–13). These results indicate that IL-17A is a crucial pathway in both plaque psoriasis and EP, potentially being the most essential cytokine in EP. As a fully human monoclonal IgG1κ antibody that neutralizes IL-17A, secukinumab has been shown to decrease IL-17 production and modulate gene expression associated with various cytokines and chemokines in chronic plaque psoriasis, including IL-17A, IL-21, IL-22, CCL20, KRT16, and DEFB4 (20). However, in EP patients, the cytokine pathways are not entirely consistent with those in chronic plaque psoriasis, and the molecular regulatory mechanisms of secukinumab require further investigation.
Secukinumab has exploratory applications in EP, providing some clinical experience and anecdotal evidence. The largest case series recently, a multi-center, international, retrospective pilot study that enrolled 13 EP patients receiving secukinumab, reported that PASI90/100 responses were achieved by 5 (38.5%) and 4 (30.8%) patients at week 16 (4). Similarly, a real-life study that enrolled 10 patients reported that PASI75/90 responses were achieved by 5 (50.0%) and 3 (30.0%) patients at week 8 (21). In a head-to-head cohort study involving 15 EP patients, ixekizumab achieved faster PASI90 at 12 weeks compared to secukinumab. However, secukinumab demonstrated statistically superior results at the endpoint, with 82% of patients achieving PASI-90 and 54% achieving PASI-100 at week 48 (22). Additionally, several significant results regarding secukinumab for pediatric EP were obtained from case studies (23, 24). For the low incidence rate of EP and the necessity for immediate intervention, large-sample RCTs remain unavailable, posing challenges in establishing standardized treatment protocols supported by high-level clinical evidence.
It has been reported that the prevalence of EP is higher in Asians and Hispanics compared to Caucasians, this may be due to socioeconomic differences and healthcare accessibility (2). Due to the diversity in incidence rate, NCRC-DID documented a relatively substantial number of Chinese patients with this rare psoriasis subtype. To our knowledge, this study is the first comparative cohort study to investigate the real-world effectiveness of secukinumab compared to traditional systemic therapies for EP, including 311 patients and utilizing the PSM approach. Consistent with prior studies, our findings provide additional evidence supporting the relative superiority of secukinumab compared to other systemic agents in EP. There was a statistically significant improvement in clinical symptoms and signs from baseline in both EP cohorts. The secukinumab cohort demonstrated a faster response compared to CST, with significantly higher PASI50, PASI90, and BSA50 rates achieved at week 4 in both the unadjusted and PSM analysis. Assessment of DLQI0/1 revealed that secukinumab also tended to result in quicker and greater improvement in the quality of life for EP. Moreover, the reduction in PASI, BSA, and DLQI scores of the secukinumab cohort was numerically more than that of the CST cohort at all follow-up timepoints, implying a potentially higher degree of clinical response. The AEs observed in our study were consistent with the established safety profile of the biologics used (10, 14, 25). These AEs did not recur in subsequent treatment cycles.
The retention rate is often used to measure adherence and evaluate the long-term effectiveness, safety, and real-world utility of medications. In this study, the drug survival of secukinumab in patients with erythrodermic psoriasis (EP) at Week 8 and Week 24 was 36.9% (59/160) and 18.1% (29/160), respectively. These rates are lower compared to plaque psoriasis (PP) in similar studies, which reported 12-month survival rates of 83% and 4-year survival rates of 74.7% (26, 27). This discrepancy may be attributed to several factors. The more localized and IL-17-centric nature of PP allows for better outcomes with secukinumab. In contrast, the systemic and multifactorial nature of EP, involving additional inflammatory pathways such as TNF-skewing and Th2 upregulation (18, 19), suggests that EP patients often require combination therapies or alternative biologics to achieve and maintain disease control, which may contribute to higher rates of treatment discontinuation. Furthermore, EP patients are more likely to have a history of biologics failures and comorbidities, further complicating treatment outcomes.
The primary limitation pertains to the extent of missing outcome data. Although missing outcome data was evenly distributed across groups, the available information did not provide sufficient clarity to discern whether recipients were lost to follow-up due to clinical improvement, nonresponse, side effects, financial burden, or other factors. Adjusted comparative studies could offer prompt insights and complement the comparative effectiveness gap in treatments for EP. However, this approach has inherent contingency and selection bias and is not a substitute for direct comparison RCTs.
Conclusion
This comparative study using PSM was adjusted for potential confounding factors to reduce bias due to reverse causation and provided preliminary evidence regarding the comparative effectiveness of secukinumab versus CST in EP treatments. Compared to CST, secukinumab recipients tend to experience a more rapid response and achieve greater improvement, which is demonstrated by higher rates of achieving PASI50, PASI75, and DLQI 0/1 at week 4, as well as greater improvements in PASI, BSA, and DLQI scores throughout the follow-up period.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Affiliated Hospital of Guangdong Medical University Clinical Research Program. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YZ: Conceptualization, Resources, Writing – original draft, Writing – review & editing. WC: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. LF: Data curation, Investigation, Validation, Writing – original draft. FQ: Investigation, Validation, Writing – original draft. JW: Data curation, Methodology, Writing – original draft. JL: Methodology, Supervision, Validation, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The work was funded by (1) Guangdong Medical University Affiliated Hospital High-Level Talent Research Start-up Project (GCC2023027) and (2) Affiliated Hospital of Guangdong Medical University Clinical Research Program (LCYJ2021B009).
Acknowledgments
The authors would like to acknowledge the data support provided by the National Key Research and Development Program of China (2023YFC2508100) and the National Clinical Research Center for Skin and Immune Disease.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Reynolds, KA, Pithadia, DJ, Lee, EB, Liao, W, and Wu, JJ. A systematic review of treatment strategies for erythrodermic psoriasis. J Dermatolog Treat. (2021) 32:49–55. doi: 10.1080/09546634.2019.1689228
2. Yan, D, Afifi, L, Jeon, C, Cordoro, KM, and Liao, W. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. (2018) 24:909. doi: 10.5070/D3247040909
3. Carrasquillo, OY, Pabón-Cartagena, G, Falto-Aizpurua, LA, Santiago-Vázquez, M, Cancel-Artau, KJ, Arias-Berrios, G, et al. Treatment of erythrodermic psoriasis with biologics: a systematic review. J Am Acad Dermatol. (2020) 83:151–8. doi: 10.1016/j.jaad.2020.03.073
4. Damiani, G, Pacifico, A, Russo, F, Pigatto, PDM, Bragazzi, NL, Bonifati, C, et al. Use of Secukinumab in a cohort of Erythrodermic psoriatic patients: a pilot study. J Clin Med. (2019) 8:770. doi: 10.3390/jcm8060770
5. Boyd, AS, and Menter, A. Erythrodermic psoriasis. Precipitating factors, course, and prognosis in 50 patients. J Am Acad Dermatol. (1989) 21:985–91. doi: 10.1016/S0190-9622(89)70287-5
6. Ortiz, NE, Nijhawan, RI, and Weinberg, JM. Acitretin. Dermatol Ther. (2013) 26:390–9. doi: 10.1111/dth.12086
7. Haustein, UF, and Rytter, M. Methotrexate in psoriasis: 26 years' experience with low-dose long-term treatment. J Eur Acad Dermatol Venereol. (2000) 14:382–8. doi: 10.1046/j.1468-3083.2000.00058.x
8. Studio Italiano Multicentrico nella Psoriasi (SIMPSO). Management of erythrodermic psoriasis with low-dose cyclosporin. Dermatology. (1993) 187:30–7. doi: 10.1159/000247289
9. Wu, C, Yu, C, Yang, Y, and Jin, H. Heart failure in erythrodermic psoriasis: a retrospective study of 225 patients. Front Cardiovasc Med. (2023) 10:1169474. doi: 10.3389/fcvm.2023.1169474
10. Dogra, S, and Mehta, H. Biological treatment for erythrodermic psoriasis. Expert Opin Biol Ther. (2022) 22:1531–43. doi: 10.1080/14712598.2022.2128669
11. Shao, S, Wang, G, Maverakis, E, and Gudjonsson, JE. Targeted treatment for Erythrodermic psoriasis: rationale and recent advances. Drugs. (2020) 80:525–34. doi: 10.1007/s40265-020-01283-2
12. Xing, X, Liang, Y, Sarkar, MK, Wolterink, L, Swindell, WR, Voorhees, JJ, et al. IL-17 responses are the dominant inflammatory signal linking inverse, Erythrodermic, and chronic plaque psoriasis. J Invest Dermatol. (2016) 136:2498–501. doi: 10.1016/j.jid.2016.07.008
13. Moy, AP, Murali, M, Kroshinsky, D, Duncan, LM, and Nazarian, RM. Immunologic overlap of helper T-cell subtypes 17 and 22 in Erythrodermic psoriasis and atopic dermatitis. JAMA Dermatol. (2015) 151:753–60. doi: 10.1001/jamadermatol.2015.2
14. Liu, LC, Jin, XH, Sun, C, and Xia, JX. Two cases of refractory erythrodermic psoriasis effectively treated with secukinumab and a review of the literature. Dermatol Ther. (2021) 34:e14825. doi: 10.1111/dth.14825
15. Mugheddu, C, Atzori, L, Lappi, A, Pau, M, Murgia, S, and Rongioletti, F. Successful Secukinumab treatment of generalized pustular psoriasis and erythrodermic psoriasis. J Eur Acad Dermatol Venereol. (2017) 31:e420–1. doi: 10.1111/jdv.14234
16. Schmitt-Egenolf, M. Psoriasis therapy in real life: the need for registries. Dermatology. (2006) 213:327–30. doi: 10.1159/000096196
17. Rosenbach, M, Hsu, S, Korman, NJ, Lebwohl, MG, Young, M, Bebo, BF Jr, et al. Treatment of erythrodermic psoriasis: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. (2010) 62:655–62. doi: 10.1016/j.jaad.2009.05.048
18. Song, B, Ning, X, Guo, L, Liu, W, and Jin, H. Comparative proteomics analysis reveals distinct molecular phenotype and biomarkers in patients with Erythrodermic atopic dermatitis and Erythrodermic psoriasis. Inflammation. (2024). doi: 10.1007/s10753-024-02078-3
19. Zhang, P, Chen, HX, Duan, YQ, Wang, WZ, Zhang, TZ, Li, JW, et al. Analysis of Th1/Th2 response pattern for erythrodermic psoriasis. J Huazhong Univ Sci Technolog Med Sci. (2014) 34:596–601. doi: 10.1007/s11596-014-1322-0
20. Krueger, JG, Wharton, KA Jr, Schlitt, T, Suprun, M, Torene, RI, Jiang, X, et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol. (2019) 144:750–63. doi: 10.1016/j.jaci.2019.04.029
21. Weng, HJ, Wang, TS, and Tsai, TF. Clinical experience of secukinumab in the treatment of erythrodermic psoriasis: a case series. Br J Dermatol. (2018) 178:1439–40. doi: 10.1111/bjd.16252
22. Avallone, G, Cariti, C, Dapavo, P, Ortoncelli, M, Conforto, L, Mastorino, L, et al. Real-life comparison between secukinumab and ixekizumab in the treatment of pustular and erythrodermic psoriasis. J Eur Acad Dermatol Venereol. (2022) 36:e574–6. doi: 10.1111/jdv.18069
23. Zhao, Z, Zhang, X, Wang, R, Wang, Y, Gong, L, and Li, C. Vaccine-induced erythrodermic psoriasis in a child successfully treated with secukinumab: a case report and brief literature review. Dermatol Ther. (2022) 35:e15684. doi: 10.1111/dth.15684
24. Dogra, S, Bishnoi, A, Narang, T, and Handa, S. Long-term remission induced by secukinumab in a 13-year-old boy having recalcitrant chronic erythrodermic psoriasis. Dermatol Ther. (2018) 31:e12611. doi: 10.1111/dth.12611
25. Viguier, M, Pagès, C, Aubin, F, Delaporte, E, Descamps, V, Lok, C, et al. Efficacy and safety of biologics in erythrodermic psoriasis: a multicentre, retrospective study. Br J Dermatol. (2012) 167:417–23. doi: 10.1111/j.1365-2133.2012.10940.x
26. Gargiulo, L, Ibba, L, Malagoli, P, Balato, A, Bardazzi, F, Burlando, M, et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for moderate-to-severe plaque psoriasis: a retrospective multicenter real-world experience on 5932 treatment courses - IL PSO (Italian landscape psoriasis). Front Immunol. (2023) 14:1341708. doi: 10.3389/fimmu.2023.1341708
Keywords: erythrodermic psoriasis, secukinumab, conventional systemic therapy, propensity score matching, real-world evidence
Citation: Zhou Y, Chen W, Fang L, Qiu F, Wu J and Li J (2024) Effectiveness, quality of life, and safety of secukinumab versus conventional systemic therapy in patients with erythrodermic psoriasis: a comparative study. Front. Med. 11:1473356. doi: 10.3389/fmed.2024.1473356
Edited by:
Paola Savoia, Università degli Studi del Piemonte Orientale, ItalyReviewed by:
Luigi Gargiulo, Humanitas Research Hospital, ItalyLuca Potestio, University of Naples Federico II, Italy
Karolína Vorčáková, Comenius University, Slovakia
Copyright © 2024 Zhou, Chen, Fang, Qiu, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Li, bGlqQG5jc3RkbGMub3Jn
‡Present address: Ying Zhou, The National Clinical Research Register Center for Skin and Immune Diseases, Beijing, China
†These authors have contributed equally to this work and share first authorship
•ORCID: Ying Zhou, https://orcid.org/0000-0003-1427-7652
Weiquan Chen, https://orcid.org/0000-0002-2465-8697
Jing Li, https://orcid.org/0000-0001-8387-2872
 Ying Zhou
Ying Zhou Weiquan Chen
Weiquan Chen Linglu Fang1
Linglu Fang1 Fang Qiu
Fang Qiu Jiayuan Wu
Jiayuan Wu