- Department of Ophthalmology, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
Purpose: Diabetic keratopathy has gained increasing attention due to advancements in diagnostic and therapeutic techniques a. This article presents a visual and bibliometric analysis to illustrate the knowledge network, research hotspots, trends, and potential future directions in this field.
Methods: We retrieved articles published since 2000 from the Web of Science and analyzed the authors, institutions, countries, keywords, citations, and co-citations of these articles with VOSviewer and CiteSpace.
Results: A total of 706 highly relevant articles were identified, with the United States, China and England as major contributors; the University of Manchester, Queensland University of Technology and Weill Cornell Medical−Qatar as key institutions; and Malik Rayaz, Efron Nathan and Ferdousi Maryam as prominent authors. High-citation articles have focused mainly on corneal confocal microscopy and diabetic peripheral neuropathy. Keywords form two clusters: one around complications, diabetes and cornea sensitivity, and another around corneal confocal microscopy and peripheral neuropathy.
Conclusion: The identification of diabetic peripheral neuropathy via corneal confocal microscopy has been a major focus of research in this field, but the mechanisms underlying diabetic corneal neuropathy still require further investigation and breakthroughs.
1 Introduction
The increasing global prevalence of diabetes has made it a significant health issue and socioeconomic burden, with more than 600 million individuals projected to be affected by diabetes by 2040 (1). Diabetes-related eye diseases, which manifest early in the course of diabetes, are a leading cause of blindness in both developed and developing countries (2). Initially, diabetic retinopathy and cataracts were the primary focus. However, with advancements in diagnostic techniques, diabetic keratopathy (DK) has garnered increasing attention from clinicians and researchers (3, 4).
DK encompasses a range of corneal alterations caused by diabetes, varying in form and severity. Clinically, DK manifests as superficial punctate keratopathy, delayed epithelial healing, persistent epithelial defects or recurrent erosions, nerve loss, decreased corneal sensitivity, tear film changes, ulcers, and opacities (5, 6). These changes impair patients’ quality of life and vision to varying degrees and are often underestimated in clinical settings, with conventional treatments yielding limited efficacy (7). Understanding the pathogenesis and key factors of DK is crucial for effective treatment.
Bibliometrics, involving the quantitative analysis of scientific publications using mathematical and statistical methods, focuses on citations, publications, co-authorship, journals, keywords, and research trends. Analyzing a large volume of literature provides insights into the structure, development, and dynamics of a field, guiding future research and development (8, 9).
In this study, we employed bibliometric methods to analyze literature on diabetic keratopathy published since the new millennium and presented our findings with visualization software. Our analysis addresses four questions: 1. What are the research trends in diabetic keratopathy? 2. Which countries, institutions, and authors are influential in this field? 3. What are the collaboration networks among leading institutions and authors? 4. What are the current research hotspots, keywords, and potential future directions in this field?
2 Materials and methods
2.1 Literature retrieval
We conducted a literature search using the Web of Science, focusing on the Web of Science Core Collection and the SCIE citation database from 1900 to the present. The search formula was “TS = [(cornea or corneal or keratopathy) and (diabetes or diabetic)] not (retina or retinal)”, with publication dates limited from 2000 to the present (up to May 20, 2024). Document types were restricted to articles or reviews, and the language was limited to English. Two authors independently screened the initial results to exclude studies and articles not primarily focused on ophthalmology or corneal disease. Discrepancies were resolved through discussion. The final selected literature records, which included all the citation information, were exported from the Web of Science.
2.2 Bibliometric analysis
We used VOSviewer and CiteSpace to perform bibliometric analysis and visualization of the exported literature records. The countries, institutions, authors, and keywords of the articles were clustered and visualized using VOSviewer. Journal impact factors and JCR rankings (2022) were obtained from the Web of Science. Time zone view of keywords and burst word analysis was conducted and presented using CiteSpace.
3 Results
3.1 General Information
Our initial search yielded 1595 articles, 706 of which met the inclusion criteria after independent screening by two authors (Figure 1). These articles originated from 57 countries, 731 institutions, and 2670 authors and were published in 172 journals, with 13093 references from 2613 journals. The annual publication volume in this field showed a fluctuating upward trend, with 2013 being a significant turning point. Before 2013, the annual publication volume was between 10 and 20, but after 2013, it surged to between 40 and 50 (Figure 2).
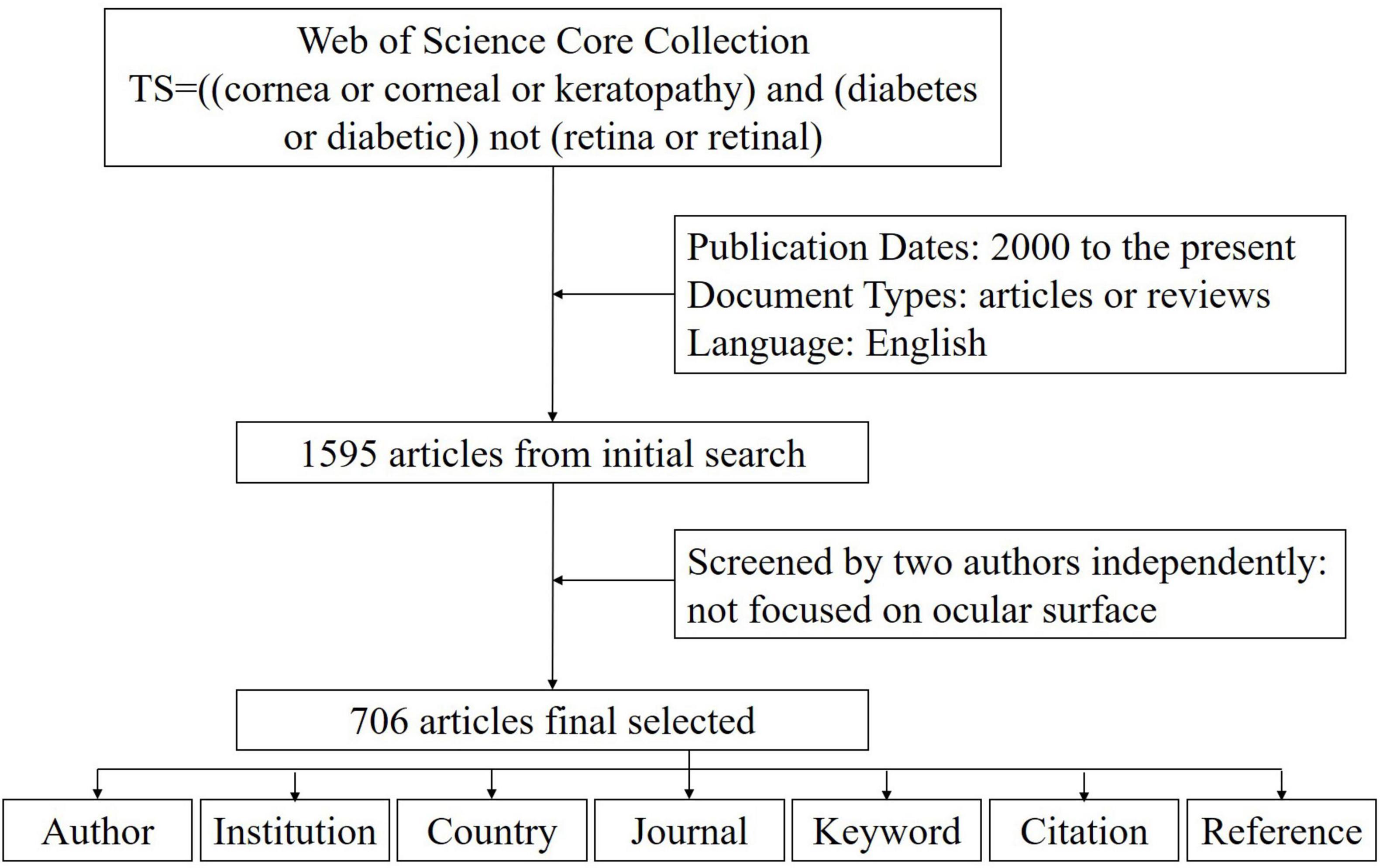
Figure 1. Data screening flow chart and steps of bibliometric analysis. The literature records were exported from the Web of Science.
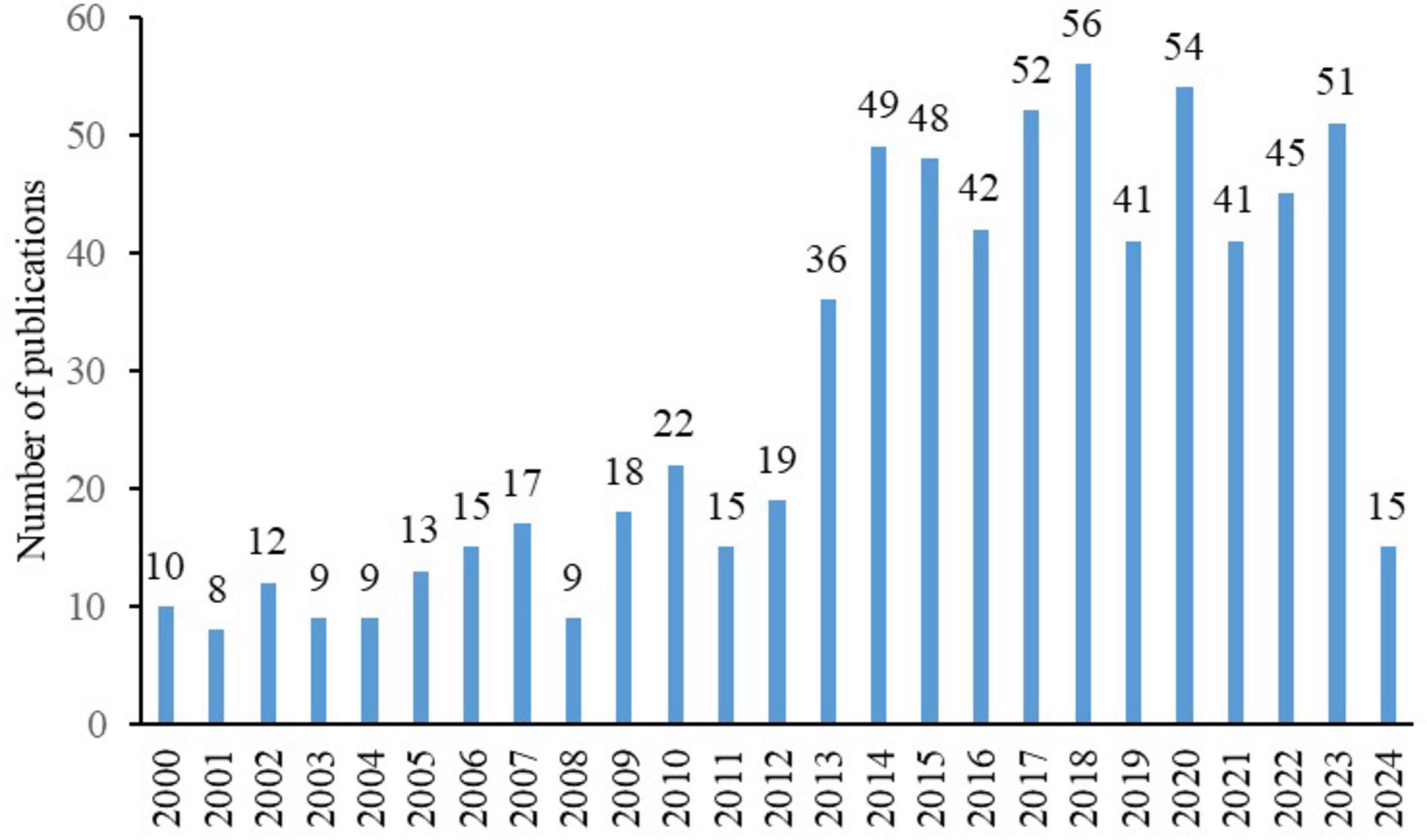
Figure 2. Annual publication volume of diabetic keratopathy research (2000–2024), highlighting the significant increase after 2013.
3.2 Countries
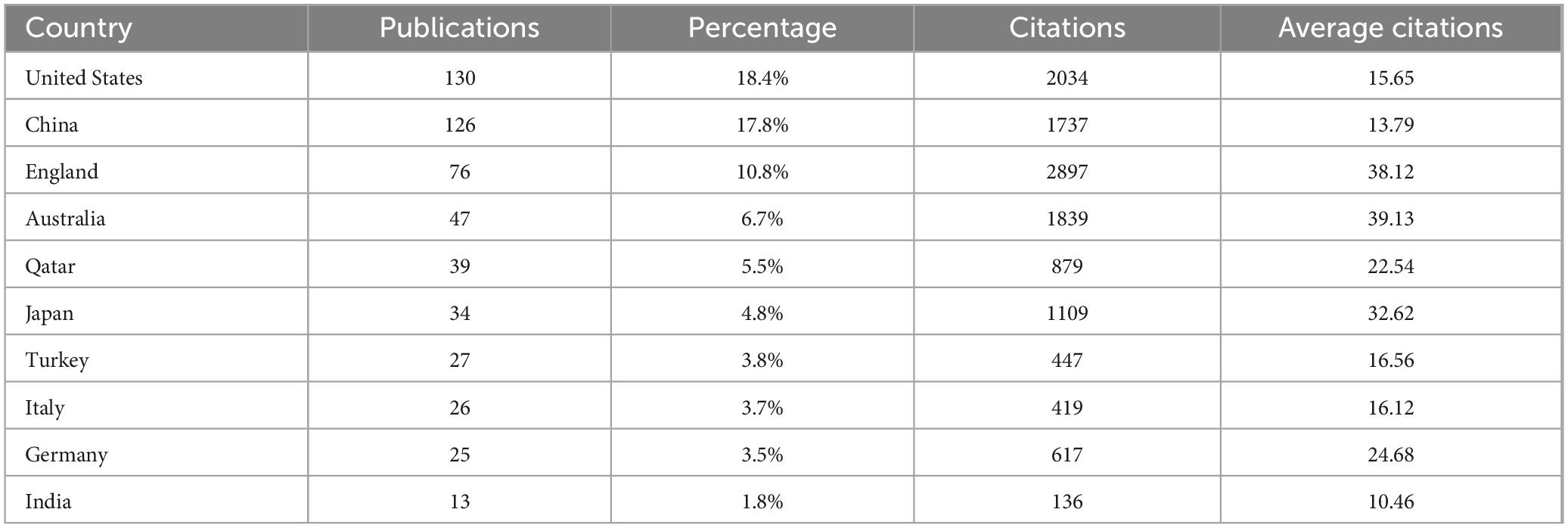
Among the 57 countries publishing in this research field, 29 had more than five articles. Table 1 lists the top ten countries by publication volume, with the United States (average citations 15.65), China (average citations 13.79) and England (average citations 38.12) leading. Analysis of collaboration among countries with more than eight publications revealed two major cooperation clusters: one including the United States and China, and another including England, Australia and Qatar (Figures 3a, b).
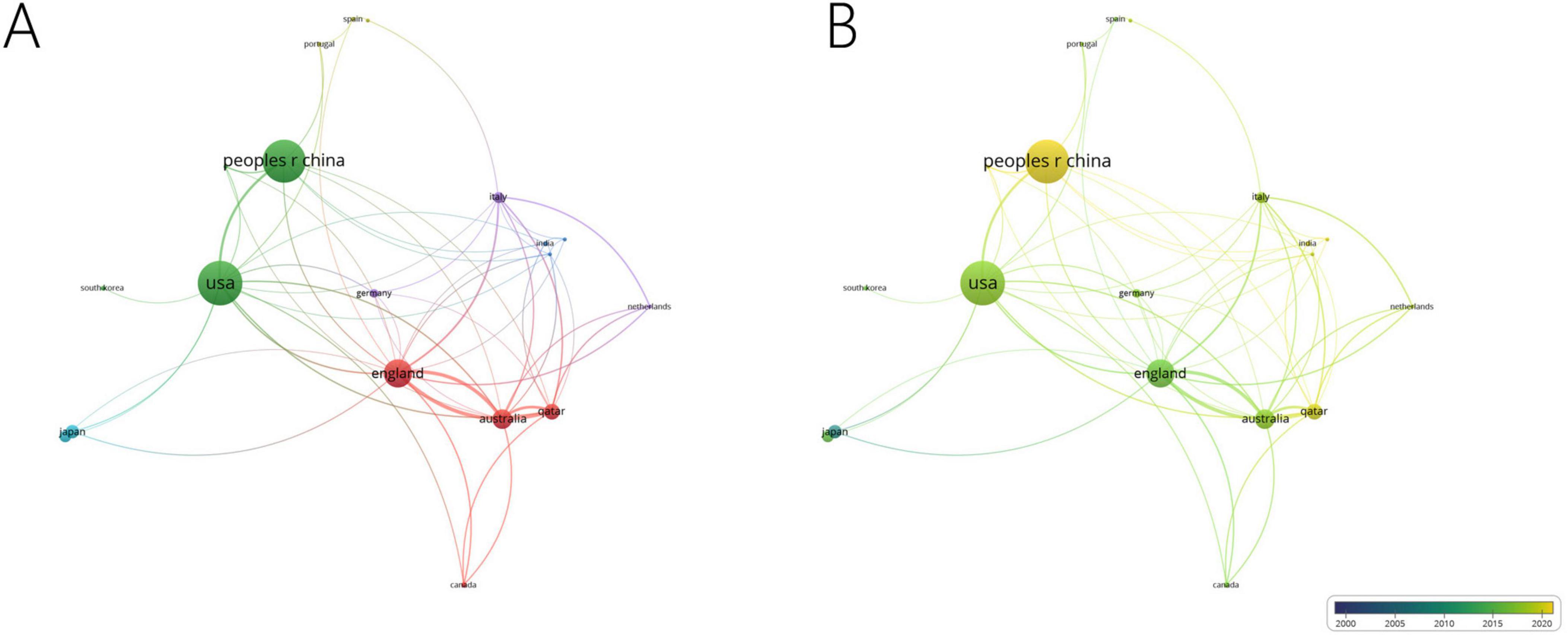
Figure 3. Collaboration network of leading countries. (A) The cooperative network visualization map of countries working on DK. Node size indicates the number of publications, the thickness of the link positively correlates with cooperation strength, and different colors represent different cooperative clusters. (B) The cooperative network overlay visualization map of countries working on DK. Blue represents the early research phase, and yellow represents the recent period.
3.3 Institutions
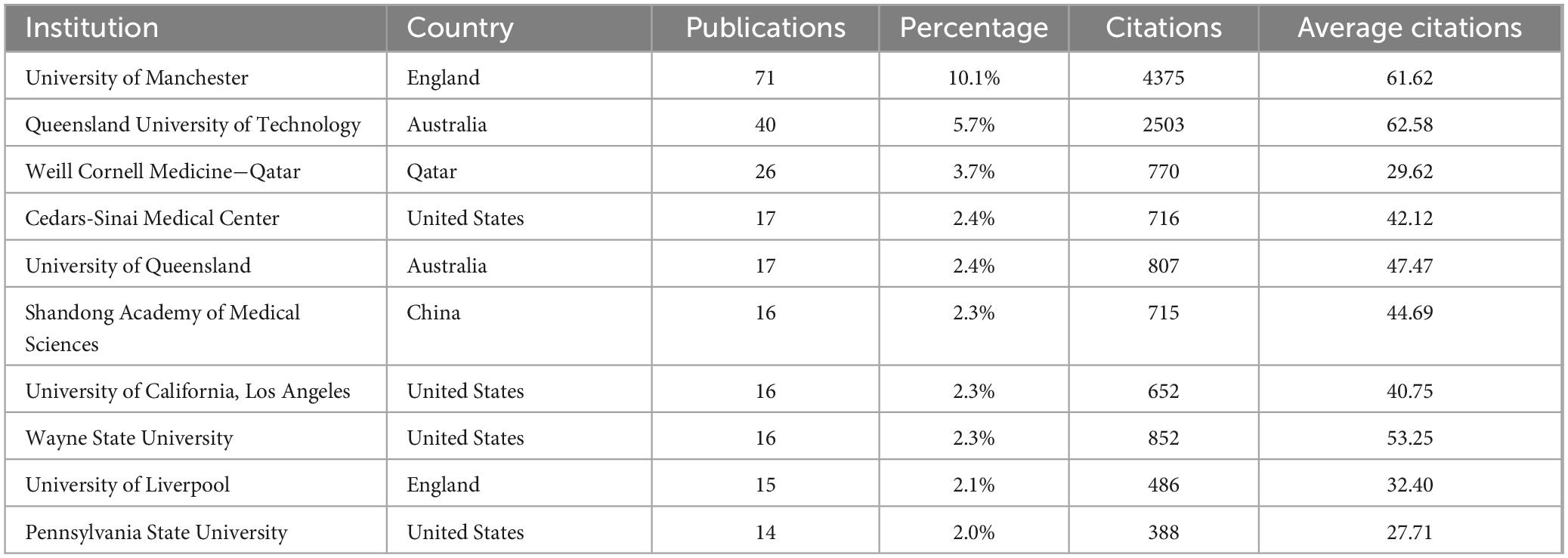
Of the 731 institutions publishing in this area, 64 had more than five publications. Table 2 lists the top ten institutions, with the University of Manchester far leading (71 publications, average citations 61.62), followed by Queensland University of Technology (40 publications, average citations 62.58) and Weill Cornell Medicine – Qatar (26 publications, average citations 29.62). Analysis of collaboration among institutions with over seven publications revealed two major collaboration centers: the University of Manchester and Weill Cornell Medicine - Qatar (Figures 4a, b).
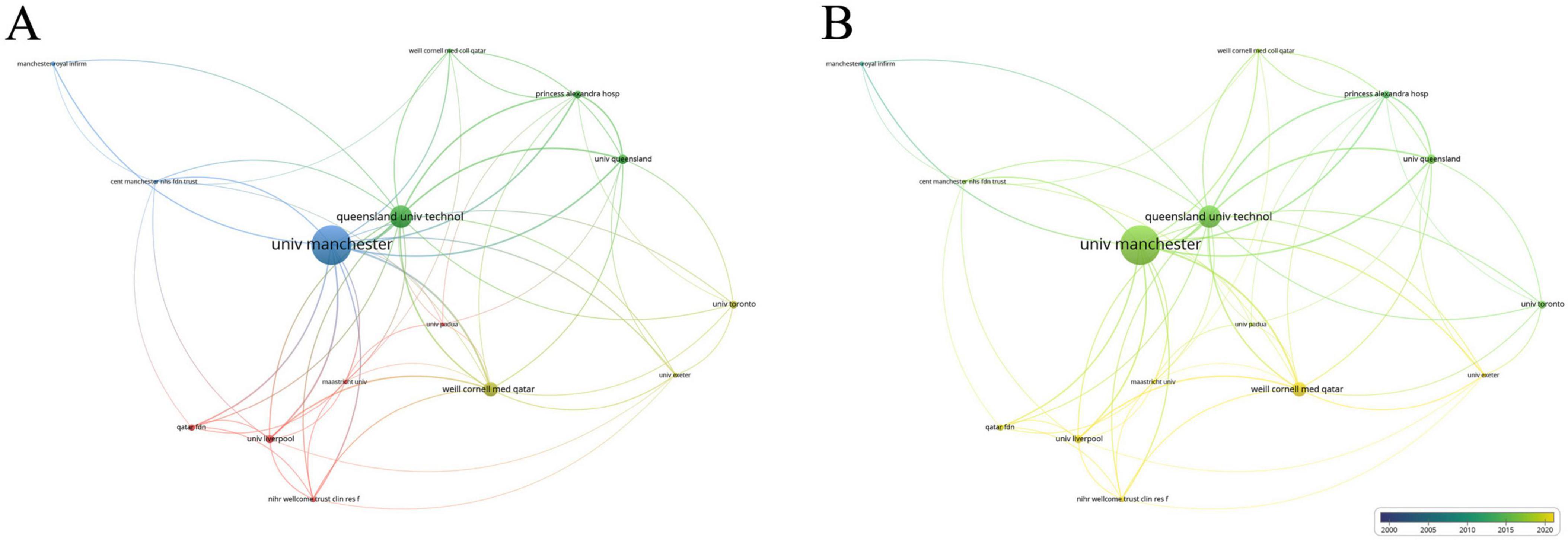
Figure 4. Collaboration network of leading institutions. (A) The cooperative network visualization map of institutions working on DK. Node size indicates the number of publications, the thickness of the link positively correlates with cooperation strength, and different colors represent different cooperative clusters. (B) The cooperative network overlay visualization map of institutions working on DK. Blue represents the early research phase, and yellow represents the recent period.
3.4 Authors
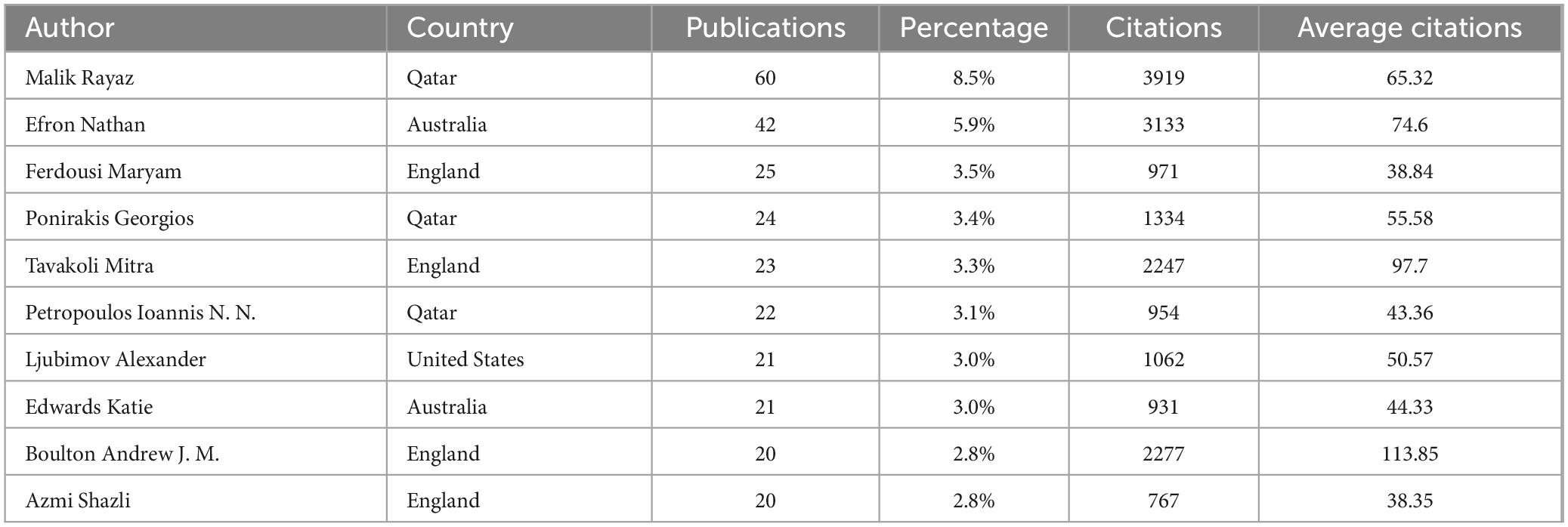
Among the 2670 authors who have published research results in this field, 91 authors have published more than 5 papers. Table 3 lists the top ten authors, with Malik Rayaz (60 publications, average citations 65.32), Efron Nathan (42 publications, average citations 74.6) and Ferdousi Maryam (25 publications, average citations 38.84) leading. Analysis of collaboration among authors with over 12 publications revealed two core networks: one led by Malik Rayaz and Efron Nathan, and another including Ponirakis Georgios, Ferdousi Maryam, Marshail Andrew, Petropoulos Ioannis N. and so on (Figures 5a, b).
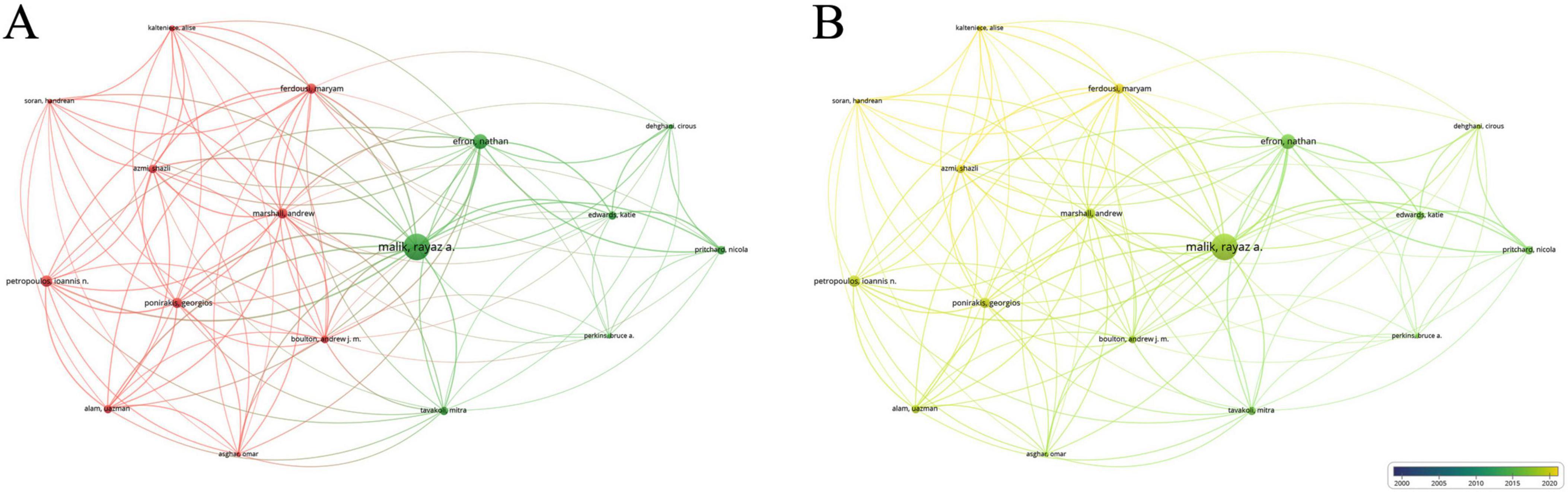
Figure 5. Collaboration network of leading authors. (A) The cooperative network visualization map of authors working on DK. Node size indicates the number of publications, and the thickness of the link positively correlates with cooperation strength, and different colors represent different cooperative clusters. (B) The cooperative network overlay visualization map of authors working on DK. Blue represents the early research phase, and yellow represents the recent period.
3.5 Journals
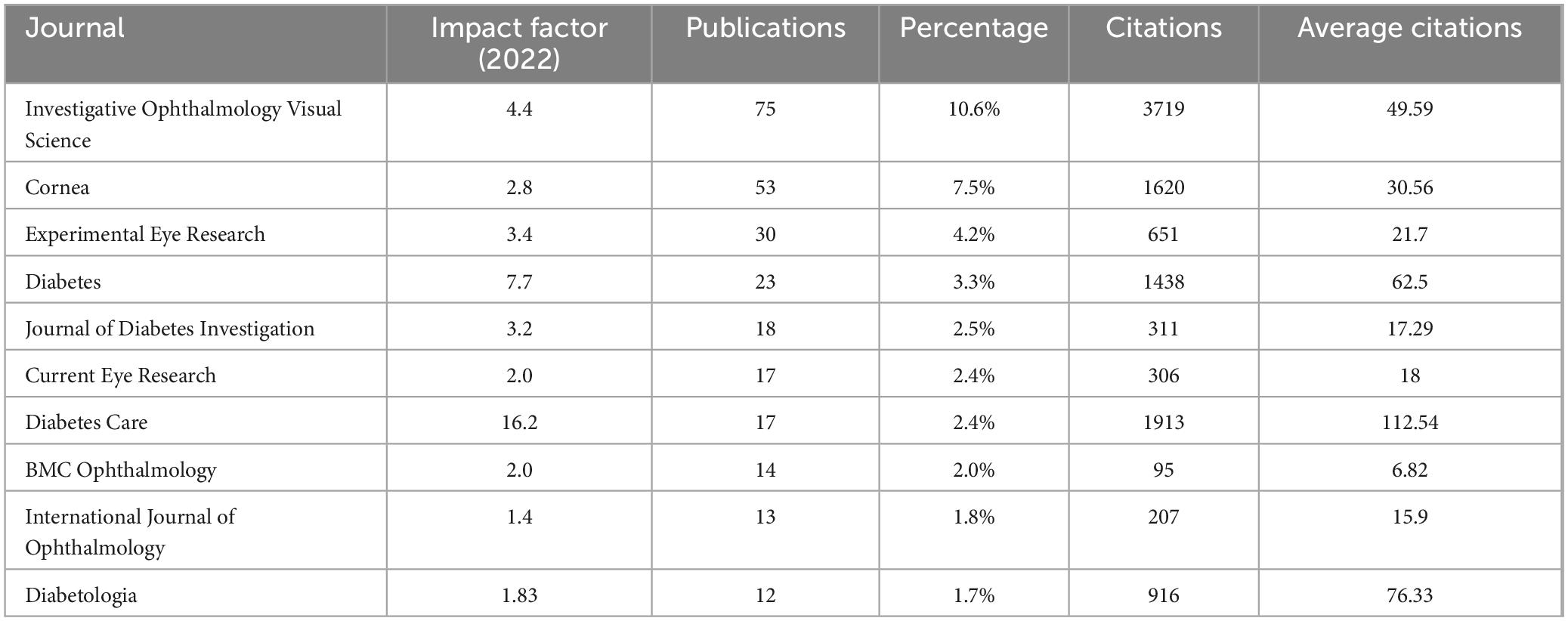
A total of 172 journals published research in this field, with 36 having more than five publications. Table 4 lists the top ten journals, primarily from the ophthalmology and diabetes fields. The leading journals included Investigative Ophthalmology Visual Science (impact factor 4.4, 75 publications, average citations 49.59), JCR Ophthalmology Category Q1, Cornea (impact factor 2.8, 53 publications, average citations 30.56), JCR Ophthalmology Category Q2, and Experimental Eye Research (impact factor 3.4, 30 publications, average citations 21.7), JCR Ophthalmology Category Q2.
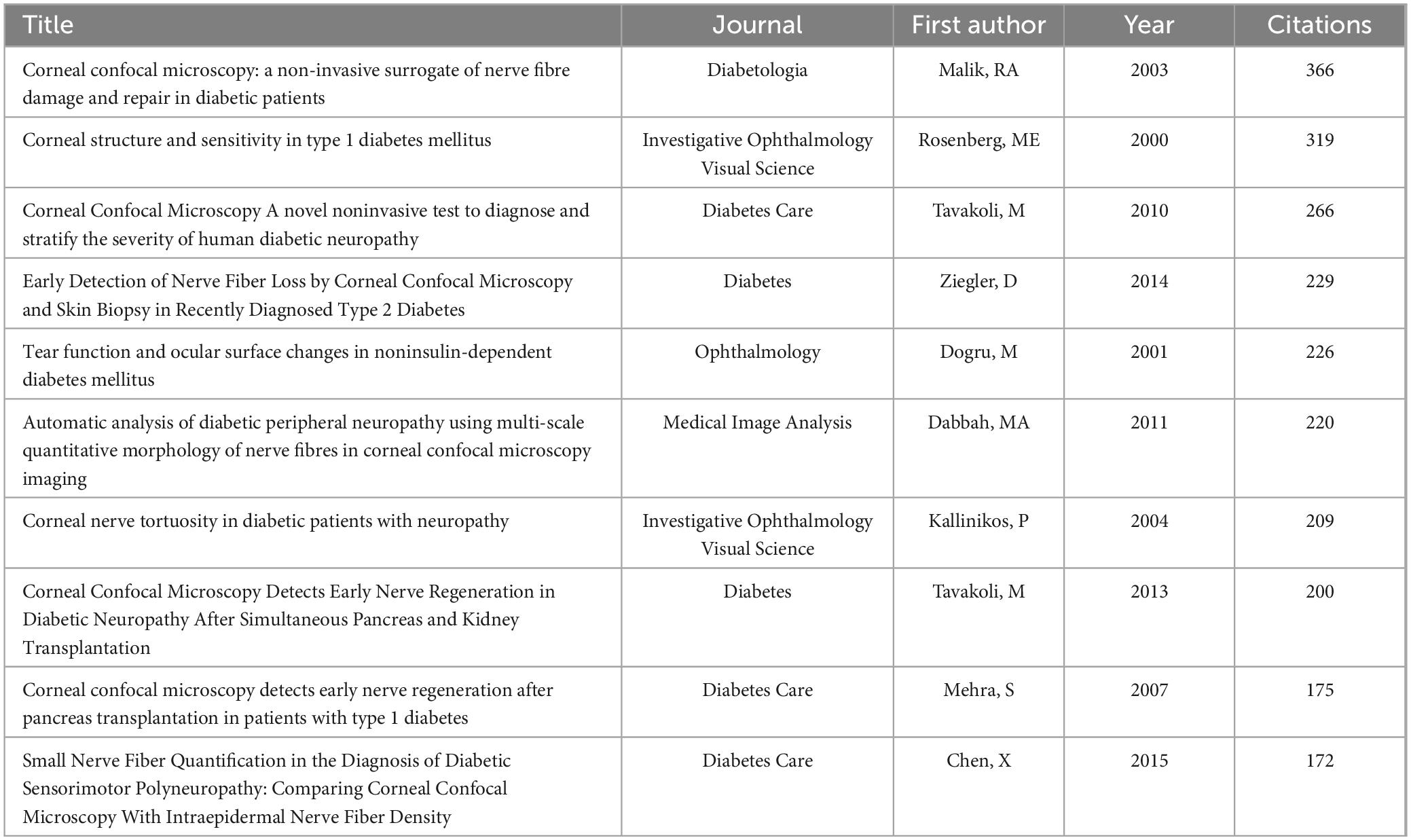
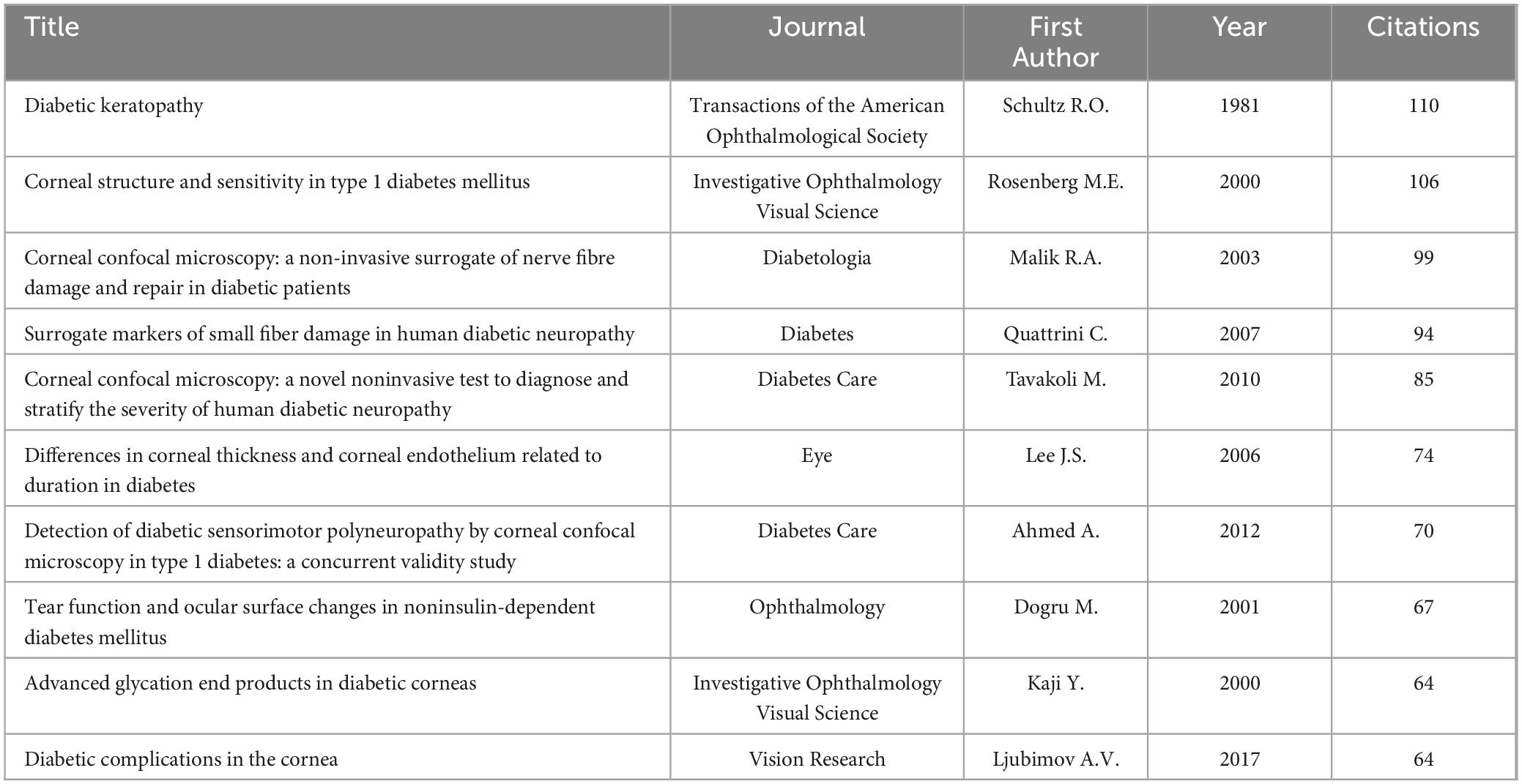
3.6 Highly cited articles
Studying highly cited articles in this field helps us understand the research hotspots in the area. Table 5 lists the top ten most cited articles, predominantly focusing on corneal confocal microscopy and diabetic peripheral neuropathy. Table 6 lists the top ten co-cited articles, highlighting keywords like corneal confocal microscopy, diabetic complications, ocular surface structure and so on. Clustering of references with over 40 co-citations revealed two major clusters (Figure 6). The red cluster is mainly about diabetic complications, cornea structure and so on, while the green cluster is focusing on corneal confocal microscopy.
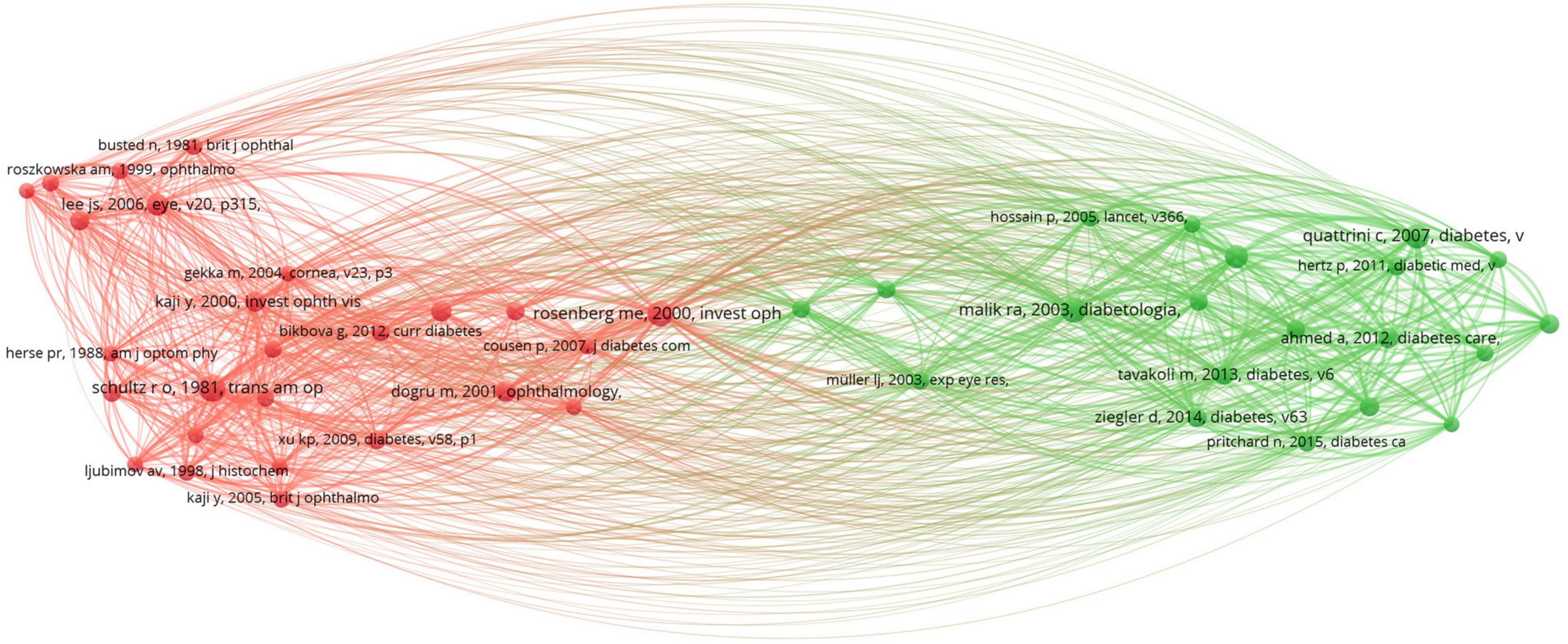
Figure 6. The visualization network of highly co-citated references. Node size indicates the frequency of co-citations, and different colors represent different co-citation clusters.
3.7 Keywords
Keywords reflect the main research content and direction of an article. Analyzing the high-frequency keywords in this field can help us understand the research focus and trends over time. Analysis of keywords with over 30 occurrences using VOSviewer revealed two major clusters: one around “complications”, “diabetes mellitus”, “cornea” and “sensitivity” and another around “corneal confocal microscopy” and “peripheral neuropathy” (Figure 7a). Keywords such as “complications”, “cornea” and “sensitivity” appeared earlier, while “corneal confocal microscopy” and “peripheral neuropathy” appeared later (Figure 7b). The time zone view of keywords analyzed by CiteSpace effectively reveals the changes in research directions. It can be observed that the keywords have evolved from “complications”, “diabetes mellitus” and “cornea”, to “corneal confocal microscopy” and “peripheral neuropathy”, and more recently to “ocular surface” (Figure 7c). The top burst words from 2000 to 2024 are shown in Figure 7d, with “surrogate” having the highest burst strength (11.73) from 2011 to 2016. Other keywords with high burst intensity mainly included “complications”, “sensitivity” and “diabetic neuropathy”.
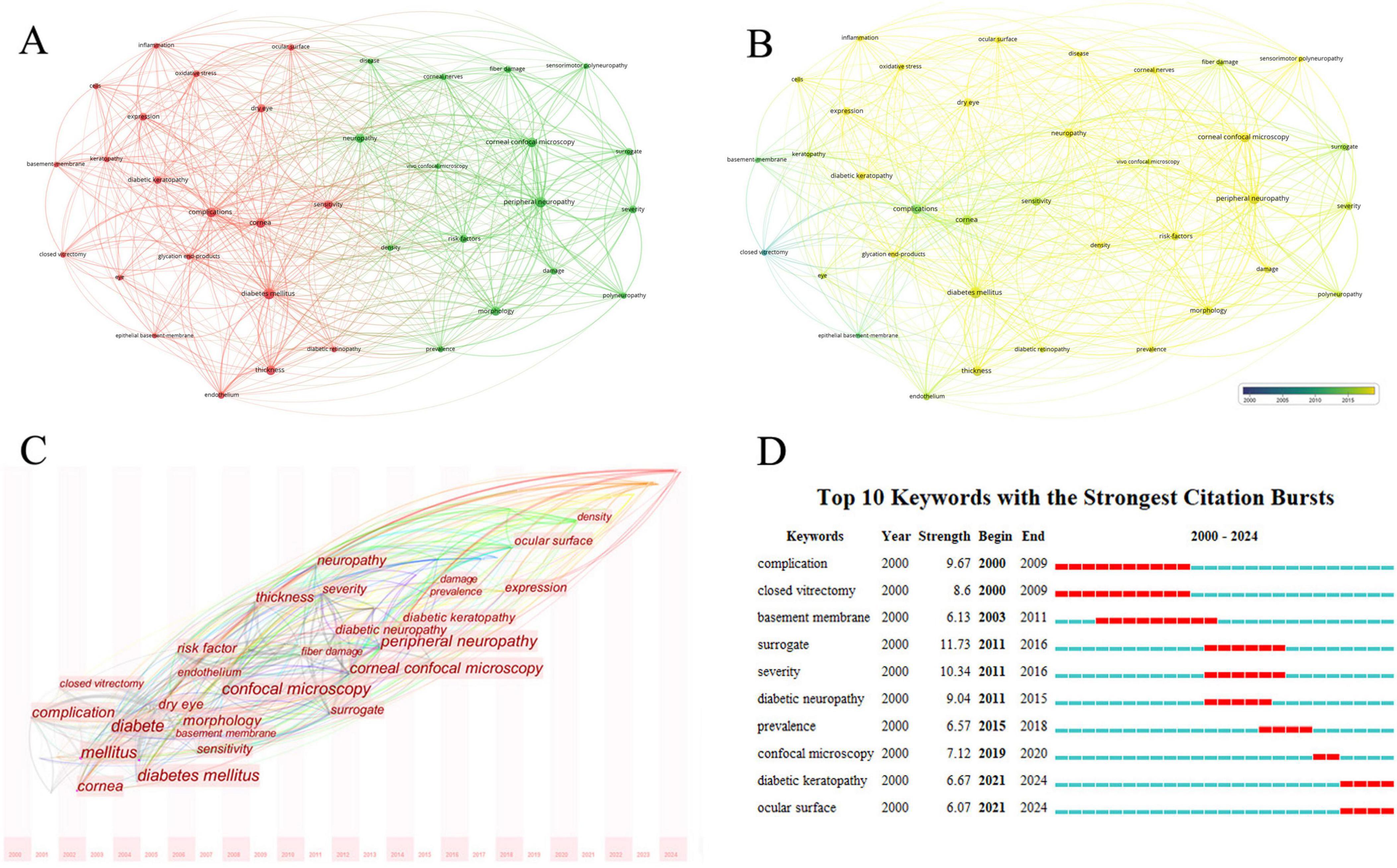
Figure 7. Analysis of keywords related to DK. (A) The co-occurrence network visualization map of keywords related to DK. The node size indicates the frequency of keywords, and different colors represent different co-occurrence clusters. (B) The co-occurrence network overlay visualization map of keywords related to DK. Blue represents the early research phase, and yellow represents the recent period. (C) The time zone view of keywords related to DK. The horizontal axis represents time, and the font size indicates the frequency of keywords. (D) The top 10 keywords with the strongest citation bursts related to DK from 2000 to 2024. The red segment of the blue line denotes the burst duration of a keyword.
4 Discussion
Past research on diabetic ocular complications primarily focused on diabetic retinopathy due to its severe impact on vision (10–12). However, DK is gaining increasing attention from researchers worldwide for two main reasons (13, 14). First, the prevalence of DK continues to increase with the increasing number of diabetes patients and the prolonged duration of the disease. Second, with the application of confocal microscopy, observing the morphology and quantity of corneal nerves has become an important surrogate marker for evaluating peripheral neuropathy in diabetic patients (15–17).
The increasing number of publications also confirms that DK is receiving more attention. The annual publication volume in this field shows a year-on-year increase, with explosive growth, especially after 2013. This surge is mainly due to the application of confocal microscopy, which allows for more intuitive observation of nerve damage in patients with DK. Additionally, damage to corneal nerve tissue serves as an important indicator for evaluating peripheral neuropathy in diabetic patients (17–19). The publication volume has not shown any decline in recent years, indicating that research in this field remains a hotspot.
Analyzing publications by country, institution, and author reveals the global distribution and characteristics of research in this field. Although the United States and China lead in terms of publication volume, their citation rates are not very high, and they share the same collaboration cluster. In contrast, England, Australia and Qatar have high citation rates, with the England-Australia-Qatar collaboration cluster being a key research focus. The University of Manchester in the UK, which ranks first in both the number of publications and citations, is especially noteworthy. The University of Queensland in Australia is also a research hub in this field for the country, ranking second in publication volume and having a high number of citations. Malik Rayaz from Qatar is the most prolific researcher in this field, making Qatar a leading research frontier, on par with traditionally developed countries such as the UK and Australia. Nathan Efron from Australia ranks second in publication volume and collaborates closely with Malik Rayaz. It is believed that core researchers like Malik Rayaz and Nathan Efron have made the UK-Australia-Qatar research collaboration circle the current global core in this field.
Research outcomes in this field are primarily published in ophthalmology and diabetes journals. Although more articles are published in ophthalmology journals, those in diabetology journals have higher impact factors and receive more citations. The main reason is that diabetes and its peripheral neuropathy constitute a larger research area, leading to a greater impact factor and more frequent citations for related articles (20, 21). Despite the lower impact factors of ophthalmology journals, those such as IOVS and Cornea are very influential within the ophthalmology field and merit attention from researchers (22, 23). Moreover, the research focus of articles published in ophthalmology journals differs from those in diabetes journals. Articles published in the diabetes journals primarily focus on corneal nerve damage detected by confocal microscopy, which serves as an indicator of diabetic peripheral neuropathy. In contrast, articles published in ophthalmology journals cover various aspects of diabetic keratopathy.
High-citation articles and main keyword analysis indicated that past research focused on observing diabetic corneal neuropathy and structural changes via cornea confocal microscopy. Studies have shown that diabetic patients have reduced corneal nerve fiber density, nerve branch density, and nerve fiber length (17, 18, 24). Damage to the corneal nerves in diabetic patients not only leads to decreased corneal sensitivity, making them more susceptible to external injuries, but also results in a reduction of neurotrophic substances in the corneal tissue (25–27). This lack of nutrients impairs the function of corneal stromal cells and epithelial cells. Additionally, corneal nerve fibers are crucial for maintaining healthy corneal epithelium and promoting wound healing after ocular trauma (28, 29). The loss of these nerve fibers leads to corneal epithelial damage and persistent nonhealing wounds. Moreover, confocal microscopy revealed a decrease in corneal epithelial cell density in diabetic patients, along with reduced activation and migration of these cells postinjury (30–32). Similarly, corneal stromal cells and endothelial cells in diabetic patients are also affected to varying degrees, showing a decrease in numbers (33, 34).
From the analysis of keyword clustering over time, time zone maps, and burst word analysis, we find that terms like “ocular surface”, “tear film” and “dry eye” have become hotspots in recent years. This shift indicates a broader interest beyond corneal pathology to the entire ocular surface, including the conjunctiva and tear film. Diabetic patients exhibit decreased tear film stability, increased osmolarity, and elevated levels of advanced glycation end products and inflammatory factors, all of which are detrimental to corneal epithelial repair (35–37). Immune cell activation, proteomic changes, and reduced mucin secretion in the conjunctiva also impact diabetic keratopathy to varying degrees (38, 39).
Keyword analysis also revealed that research on the mechanisms of DK and its associated neural damage is still limited. Previous studies have suggested that oxidative stress, neurotrophic deficits and inflammatory mechanisms play crucial roles in the pathogenesis of diabetic corneal neuropathy (40–42). Corneal epithelial damage is mainly related to the accumulation of advanced glycation end products on the ocular surface due to hyperglycemia (43, 44). Corneal stromal damage is linked to hyperglycemia-induced disruption of collagen subtypes and proteoglycans, affecting the structural integrity of the cornea (45, 46). These mechanisms are not prominent in the keyword network, suggesting that these areas have not yet become central to research in this field. Researchers need to further explore the key mechanisms underlying DK, or perhaps the pathogenesis of DK is multifactorial, lacking a single core mechanism.
5 Conclusion
This study systematically analyzed the literature on diabetic keratopathy published since 2000. We found that this field is gaining increasing attention and research interest. Visualization analysis provides a clear and rapid understanding of the current research status, collaboration networks, and research hotspots. Analyzing these hotspots suggests that future research may focus on the mechanisms of diabetic corneal neuropathy.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
WZ: Writing – review and editing, Supervision, Methodology, Investigation. GJ: Writing – review and editing, Methodology, Data curation. WT: Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 81700796).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J, et al. Epidemiology of type 2 diabetes - global burden of disease and Forecasted trends. J Epidemiol Glob Health. (2020) 10:107–11. doi: 10.2991/jegh.k.191028.001
2. Nentwich MM, Ulbig MW. Diabetic retinopathy - ocular complications of diabetes mellitus. World J Diabetes. (2015) 6:489–99. doi: 10.4239/wjd.v6.i3.489
3. Bikbova G, Oshitari T, Tawada A, Yamamoto S. Corneal changes in diabetes mellitus. Curr Diabetes Rev. (2012) 8:294–302. doi: 10.2174/157339912800564016
4. Shah R, Amador C, Tormanen K, Ghiam S, Saghizadeh M, et al. Systemic diseases and the cornea. Exp Eye Res. (2021) 204:108455. doi: 10.1016/j.exer.2021.108455
5. Abdelaziz MH, Abdelwahab SF, Wan J, Cai W, Huixuan W, et al. Alternatively activated macrophages; a double-edged sword in allergic asthma. J Transl Med. (2020) 18:58. doi: 10.1186/s12967-020-02243-5
6. Priyadarsini S, Whelchel A, Nicholas S, Sharif R, Riaz K, et al. Diabetic keratopathy: Insights and challenges. Surv Ophthalmol. (2020) 65:513–29. doi: 10.1016/j.survophthal.2020.02.003
7. Barsegian A, Lee J, Salifu MO, McFarlane SI. Corneal neuropathy: An underrated manifestation of diabetes mellitus. J Clin Endocrinol Diabetes. (2018) 2:JCED–111. doi: 10.1002/j.2161-0673.2018.tb01334.x
8. Bornmann L, Daniel H-D. What do we know about the h index? J Am Soc Inf Sci Technol. (2008) 58:1381–5. doi: 10.1002/asi.20609
9. van Raan AFJ. The use of bibliometric analysis in research performance assessment and monitoring of interdisciplinary scientific developments. Technol Assess Theory Pract. (2003) 1:20–9.
10. Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. (2012) 366:1227–39. doi: 10.1056/NEJMra1005073
11. Yau JWY, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. (2012) 35:556–64. doi: 10.2337/dc11-1909
12. Zhang X, Saaddine JB. The increasing burden of diabetes-related ocular complications. Am J Ophthalmol. (2020) 210:59–65. doi: 10.1016/j.ajo.2019.10.026
13. Herse P. A review of manifestations of diabetes mellitus in the anterior eye and cornea. Am J Optom Physiol Opt. (2005) 82:209–19. doi: 10.1016/j.optom.2004.09.010
14. Ozawa Y, Kurihara T. Diabetic keratopathy: Clinical features, pathogenesis, and management. Diabetes Metab Res Rev. (2018) 34:e2983. doi: 10.1002/dmrr.2983
15. Malik RA, Tesfaye S. The role of the cornea in the assessment of diabetic neuropathy. Diabetes Care. (2013) 36:2120–7. doi: 10.2337/dc12-2235
16. Zhivov A, Guthoff R. Confocal microscopy of corneal nerve fibers in diabetes: Early diagnosis of neuropathy. Curr Diab Rep. (2013) 13:488–92. doi: 10.1007/s11892-013-0398-3
17. Edwards K, Pritchard N, Vagenas D, Russell AW. Utility of corneal confocal microscopy for the diagnosis of diabetic peripheral neuropathy. Clin Exp Optom. (2012) 95:257–62. doi: 10.1111/j.1444-0938.2012.00701.x
18. Tavakoli M, Quattrini C, Abbott C, Kallinikos P, Marshall A, Finnigan J, et al. Corneal confocal microscopy: A novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. (2010) 33:1792–7. doi: 10.2337/dc10-0265
19. Petropoulos IN, Ponirakis G, Khan A, Gad H, Akhtar N, Dehghani C, et al. Corneal confocal microscopy: An imaging endpoint for axonal degeneration in diabetic neuropathy. Invest Ophthalmol Vis Sci. (2014) 55:5872–80. doi: 10.1167/iovs.14-14442
20. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. (2014) 103:137–49. doi: 10.1016/j.diabres.2013.11.002
21. Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, Shaw J, et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. (2010) 87:293–301. doi: 10.1016/j.diabres.2010.01.026
22. Foster CS, Azar DT, Dohlman CH. The influence of “IOVS” and “Cornea” in advancing corneal research: A retrospective analysis. Invest Ophthalmol Vis Sci. (2002) 43:1380–5.
23. Rosenthal AR, Harocopos GJ. Cornea: A unique journal at the forefront of corneal research. Cornea. (2009) 28:117–20. doi: 10.1097/ICO.0b013e31818c25d3
24. Quattrini C, Tavakoli M, Jeziorska M, Begum P, Smith G, Tesfaye S, et al. Corneal nerve fiber pathology and risk factors for diabetic neuropathy in type 1 diabetes patients. Diabetologia. (2007) 50:822–9. doi: 10.1007/s00125-007-0617-6
25. Bitirgen G, Ozkagnici A, Malik RA, Ozkagnici S. Corneal nerve fibre damage precedes diabetic retinopathy in patients with type 2 diabetes mellitus. Diabet Med. (2019) 36:673–9. doi: 10.1111/dme.13908
26. Ferdousi M, Kalteniece A, Azmi S, Petropoulos IN, Ponirakis G, Alam U, et al. Corneal confocal microscopy detects small nerve fibre damage in patients with painful diabetic neuropathy. Sci Rep. (2020) 10:1–9. doi: 10.1038/s41598-020-62709-3
27. Khan A, Petropoulos IN, Ponirakis G, Malik RA. Visualizing corneal nerve damage to understand peripheral neuropathy in diabetes. Eye Vis. (2021) 8:1–13. doi: 10.1186/s40662-020-00239-3
28. Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: Membrane nanotubes in vivo: A feature of MHC class II+ cells in the mouse cornea. J Immunol. (2017) 198:2225–31. doi: 10.4049/jimmunol.1601978
29. Labbe A, Liang H, Martin C. Corneal nerve structure and function in diabetic patients: An in vivo confocal microscopy study. Eye Vis. (2020) 7:1–9. doi: 10.1186/s40662-020-00203-1
30. Luo L, Li J, Chen C, Zhang X. Corneal epithelial cell changes in patients with diabetes mellitus: A confocal microscopy study. BMC Ophthalmol. (2021) 21:1–9. doi: 10.1186/s12886-020-01754-5
31. Zhang Y, Liu Y, Zhang H, Wang C, Zhang L, Liu X. Corneal nerve and epithelial cell changes in type 2 diabetes mellitus: An in vivo confocal microscopy study. Eye Vis. (2022) 9:1–9. doi: 10.1186/s40662-021-00256-2
32. Pritchard N, Edwards K, Shahidi AM, Sampson GP, Russell AW, Malik RA, et al. Corneal markers of diabetic neuropathy. Ocul Surf. (2019) 17:31–44. doi: 10.1016/j.jtos.2018.10.001
33. Roszkowska AM, Tringali CG, Colosi P, Squeri CA, Meduri A, Aragona P. Corneal endothelium evaluation in type I and II diabetes mellitus. Ophthalmologica. (2016) 235:24–9. doi: 10.1159/000438948
34. Kheirkhah A, Saboo US, Abud TB, Dohlman TH, Arnoldner MA, et al. Reduced corneal endothelial cell density in patients with diabetes mellitus. Am J Ophthalmol. (2018) 194:46–52. doi: 10.1016/j.ajo.2018.06.003
35. Kheirkhah A, Zheng L, Li Q, Dana R, Yang C. Corneal epithelial immune dendritic cell alterations in dry eye disease: A systematic review. Ocul Surf. (2015) 13:95–108. doi: 10.1016/j.jtos.2014.12.002
36. Dogru M, Takano Y, Tsubota K. Tear osmolarity and dry eye symptoms in diabetic patients. Am J Ophthalmol. (2020) 123:191–6. doi: 10.1016/j.ajo.2020.05.002
37. Li Z, Zhang Y, Yan X, Song Z, Zhu Z, Bi H, et al. Advanced glycation end products induce the synthesis of inflammatory mediators in corneal epithelial cells via the NF-κB pathway. J Cell Mol Med. (2021) 25:1584–95. doi: 10.1111/jcmm.16165
38. Zhang X, Zhao L, Deng S, Sun X, Wang N, Yang X. Proteomic analysis of human tears from diabetic patients with dry eye syndrome. Invest Ophthalmol Vis Sci. (2019) 50:856–60. doi: 10.1167/iovs.09-3877
39. Lan W, Petznick A, Hoon T. Decreased mucin 5AC expression in the conjunctiva and tear film of patients with type 2 diabetes mellitus. Am J Ophthalmol. (2021) 150:656–62. doi: 10.1016/j.ajo.2021.07.008
40. Sridharan M, Laycock H, Pritchard N, Efron N. Alterations in corneal nerve morphology and tear film oxidative stress markers in diabetes. Br J Ophthalmol. (2018) 102:611–7. doi: 10.1136/bjophthalmol-2017-310469
41. Shetty R, Sethu S, Deshmukh R, Ghosh A, Jayadev C, Glaser T. Corneal dendritic cell density and ocular surface changes in the diabetic eye: A real-time in vivo confocal microscopy study. Am J Ophthalmol. (2020) 213:167–76. doi: 10.1016/j.ajo.2020.02.019
42. Calder VL, Pearce E, Rolando M. Cytokine and chemokine levels in tears and corneal sensitivity in diabetic patients. Clin Exp Ophthalmol. (2021) 49:234–45. doi: 10.1111/ceo.13840
43. Yu FS, Yin J, Lee P, Lee BL. The role of AGE/RAGE signaling in diabetic ocular complications. Curr Diab Rep. (2020) 20:1–12. doi: 10.1007/s11892-020-01326-8
44. Kim JH, Kim SJ, Kim TI. Advanced glycation end products in diabetic corneal nerve damage and repair. Cornea. (2021) 40:387–95. doi: 10.1097/ICO.0000000000002545
45. Sanchez PK, Patel S, Maheshwari A. The effects of diabetes on the corneal stroma: Morphometric analysis and proteomic changes. Invest Ophthalmol Vis Sci. (2017) 58:3656–63. doi: 10.1167/iovs.16-21187
Keywords: diabetes, cornea, ocular surface, confocal microscopy, peripheral neuropathy, bibliometrics
Citation: Zhenyu W, Jing G and Tianhong W (2024) Bibliometric and visual analysis of diabetic keratopathy research: trends, collaborations, and future directions. Front. Med. 11:1468402. doi: 10.3389/fmed.2024.1468402
Received: 21 August 2024; Accepted: 17 September 2024;
Published: 18 October 2024.
Edited by:
Yu-Chi Liu, Singapore Eye Research Institute (SERI), SingaporeReviewed by:
Georgios Ponirakis, Weill Cornell Medicine − Qatar, QatarJun Cheng, Shandong Eye Institute, China
Copyright © 2024 Zhenyu, Jing and Tianhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wang Zhenyu, bGxidGlhb0AxMjYuY29t
 Wang Zhenyu
Wang Zhenyu Gao Jing
Gao Jing