- General Practice Department, Binzhou Medical University, Yantai, Shandong, China
Objective: To explore the connection between metabolic parameters and the severity of hepatic steatosis determined through ultrasound in elderly individuals with metabolic dysfunction-associated fatty liver disease (MAFLD).
Methods: 4,663 senior individuals who were 65 years of age or older were included in this research. They were examined physically at the Ninghai Street Community Health Service Center in Yantai City between June 7, 2021, and October 15, 2021. There were two categories of individuals identified: the MAFLD group (n = 2,985) and the non-MAFLD group (n = 1,678). Based on liver ultrasonography results, individuals in the MAFLD group were further separated into three groups: mild (n = 2,104), moderate (n = 766), and severe (n = 115). To identify indicators of risk for the severity of hepatic steatosis, metabolic data was contrasted between the groups employing logistic regression.
Results: In comparison to the non-MAFLD group, the MAFLD group showed significantly elevated levels of body mass index (BMI), blood pressure, gender, age, lipid profile, alanine transaminase (ALT), and fasting blood glucose (FBG; p < 0.05). Among individuals with MAFLD, there was a positive correlation between BMI, FBG, ALT, and aspartate transaminase (AST) levels and the severity of hepatic steatosis (p < 0.05). Logistic regression analysis indicated that BMI, female gender, FBG, ALT, triglycerides (TG), and serum uric acid (SUA) constituted risk factors for increased severity of hepatic steatosis in MAFLD.
Conclusion: The severity of hepatic steatosis in elderly MAFLD patients is significantly correlated with female gender, BMI, ALT, FBG, TG, and SUA.
1 Introduction
Metabolic dysfunction-associated fatty liver disease (MAFLD), originally known as non-alcoholic fatty liver disease (NAFLD) (1), has practical and straightforward diagnostic criteria that are superior to NAFLD for determining individuals at elevated risk for liver fibrosis and extrahepatic manifestations, including chronic kidney disease (CKD), type 2 diabetes mellitus (T2DM), and cardiovascular disease (CVD) (2). Currently, its global prevalence has sharply risen to 25% (1), with a prevalence rate as high as 32.3% in China (3). On a worldwide scale MAFLD is an extremely prevalent type of chronic liver disease (4), and among the top causes for mortality from intrahepatic complications (5). The hazards of MAFLD not only include its propensity to cause liver fibrosis and hepatocellular carcinoma but also its association with extrahepatic complications, such as cardiovascular and cerebrovascular diseases, CKD, T2DM, and related malignant tumors, posing significant health risks that cannot be overlooked (6).
However, with the rapid aging population in Asia (7), studies have shown that aging, male gender, and the presence of menopause is a major risk factor for MAFLD (8). A meta-analysis of global MAFLD prevalence has indicated a continuous increase in prevalence across all age groups from 30–39 years to 70–79 years (9). Additionally, individuals aged ≥50 years are more susceptible to MAFLD progression in comparison to younger populations (10). MAFLD typically progresses to advanced stages before manifesting significant symptoms (11). Moreover, given that MAFLD can shorten life expectancy by up to 4 years (12), the need for early detection and screening becomes increasingly important (13).
Liver biopsy are thought to be the most accurate way to diagnose fatty liver disease, however, its invasive nature, risk of bleeding and infection, as well as high costs render it impractical for large-scale screening (14). For determining the presence of fatty liver disease, ultrasound is the most effective and frequently employed technique (15). Moreover, research has demonstrated that ultrasound exhibits an approximate 60% diagnosis accuracy for mild liver steatosis, whereas its accuracy in diagnosing moderate and severe hepatic steatosis is around 90 and 95%, indicating great accuracy in detecting the severity of hepatic fat accumulation (16). Liver steatosis can be detected with excellent specificity and sensitivity using MRI, particularly MRI-PDFF. All grades of steatosis in MAFLD patients can be detected more accurately with MRI-PDFF than with ultrasound because it allows fat mapping of the entire liver (AUROC 0.99) (17). However, MRI-PDFF, typically employed as a research tool, is unlikely to be applied as a first-line screening method in clinical practice due to its logistical complexities, lengthy scan time, and the lack of required expertise at most medical imaging centers.
One important risk factor for metabolic diseases is obesity.Adipocytes in obese people become hypertrophic and dysfunctional, which changes the production of adipokines like adiponectin and leptin.Systemic insulin resistance is exacerbated by adipose tissue malfunction, which fosters ectopic lipid buildup and persistent low-grade inflammation (18).Adipose tissue free fatty acids (FFAs) are released more often in insulin-resistant individuals, and their livers absorb more of them. These FFAs are subsequently converted to hepatic triglycerides, which causes hepatic steatosis (19).However, FFAs also cause hepatic and peripheral insulin resistance, and insulin resistance feeds the vicious cycle by causing more hepatic FFAs to build up (20).It is noteworthy to emphasize that interactions between genes and the environment appear to be essential for hepatic steatosis (20).For instance, there is a high correlation between variations in liver fat content and variations in the patatin-like phospholipase domain-containing 3 (PNPLA3) gene. PNPLA3 is a protein that is expressed in hepatocytes and adipocytes. It is an acyl hydrolase that hydrolyzes triacyl-, diacyl-, and monoacylglycerol (21). Insulin resistance and the buildup of liver fat are linked to the rs738409 polymorphism, which is linked to the loss of the hydrolyzing activity of proteins (22).Moreover, numerous studies have shown that a Mediterranean diet is related with a lower incidence of hepatic steatosis, while a high-fat Western diet is connected to an increased risk of the condition (23).
Referring to previous literature (24, 25), we selected age, gender, BMI, fasting blood glucose (FBG), and blood pressure as potential risk factors based on their critical roles in the pathophysiology of metabolic-associated diseases and fatty liver. Age is a major risk factor for MAFLD, with studies showing that advancing age is associated with significant changes in fat distribution, insulin sensitivity, and metabolic functions, which may increase the risk of hepatic fat accumulation. Older adults, in particular, experience a decline in hepatic insulin sensitivity and associated metabolic disorders, making them more prone to severe fatty liver. Gender also plays an important role in the development of MAFLD, with gender differences potentially linked to hormonal changes. Postmenopausal females, due to declining estrogen levels, may experience reduced hepatic fatty acid oxidation and increased fat accumulation. Additionally, the increased visceral fat ratio in postmenopausal women is thought to contribute to the progression of fatty liver disease. BMI is a core risk factor for MAFLD. Obese individuals, especially those with visceral fat accumulation, are more likely to develop hepatic fat deposition. Studies have shown that for every unit increase in BMI, the risk of MAFLD significantly increases. Furthermore, obesity leads to insulin resistance and chronic low-grade inflammation, further exacerbating hepatic fat accumulation. FBG reflects the state of glucose metabolism, and its elevation is often associated with insulin resistance, a key pathological mechanism of MAFLD. Blood pressure is closely linked to metabolic syndrome. In hypertensive patients, arterial stiffness and endothelial dysfunction may affect hepatic blood supply, further triggering hepatic metabolic abnormalities. Elevated blood pressure may also share common mechanisms with insulin resistance and hepatic fat accumulation (26, 27).
Nevertheless, there is currently insufficient research investigating the connection between metabolic disturbances and hepatic steatosis severity in elderly MAFLD populations. Exploring the connection between metabolic parameters and the severity of hepatic steatosis determined via ultrasonography in elderly MAFLD individuals is the purpose of this research. The objective is to provide a basis for risk stratification of elderly MAFLD populations at the grassroots level and to address the necessity of referral to higher-level hospitals for MAFLD diagnosis and management.
2 Materials and methods
2.1 Study design
A cross-sectional investigation was carried out utilizing the medical records of individuals who had physical examinations at the Ninghai Street Community Health Service Center in Muping District, Yantai City, from June 7, 2021, to October 15, 2021. A total of 4,663 elderly individuals aged ≥65 years were enlisted as participants in the research. Depending on whether MAFLD was present or not, individuals were separated into two groups: the MAFLD group and the non-MAFLD group. The basic characteristics and laboratory parameters of the two groups were compared. The MAFLD group was separated into three subgroups based on the severity of hepatic steatosis: mild, moderate, and severe. The basic characteristics and laboratory parameters among these sub-groups were also compared. Both univariate and multivariate analyses were applied to delineate independent risk variables.
2.2 Data collection
Every registered participant had a physical examination, during which their gender, age, diastolic and systolic blood pressures (DBP and SBP) were measured. The body mass index (BMI) was calculated utilizing the height and weight values. The community health service center’s laboratory department performed various blood tests, such as triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), alanine transaminase (ALT), fasting blood glucose (FBG), and serum uric acid (SUA) et al. Additionally, all participants underwent abdominal ultrasound examinations to evaluate hepatic steatosis.
2.3 Diagnostic criteria
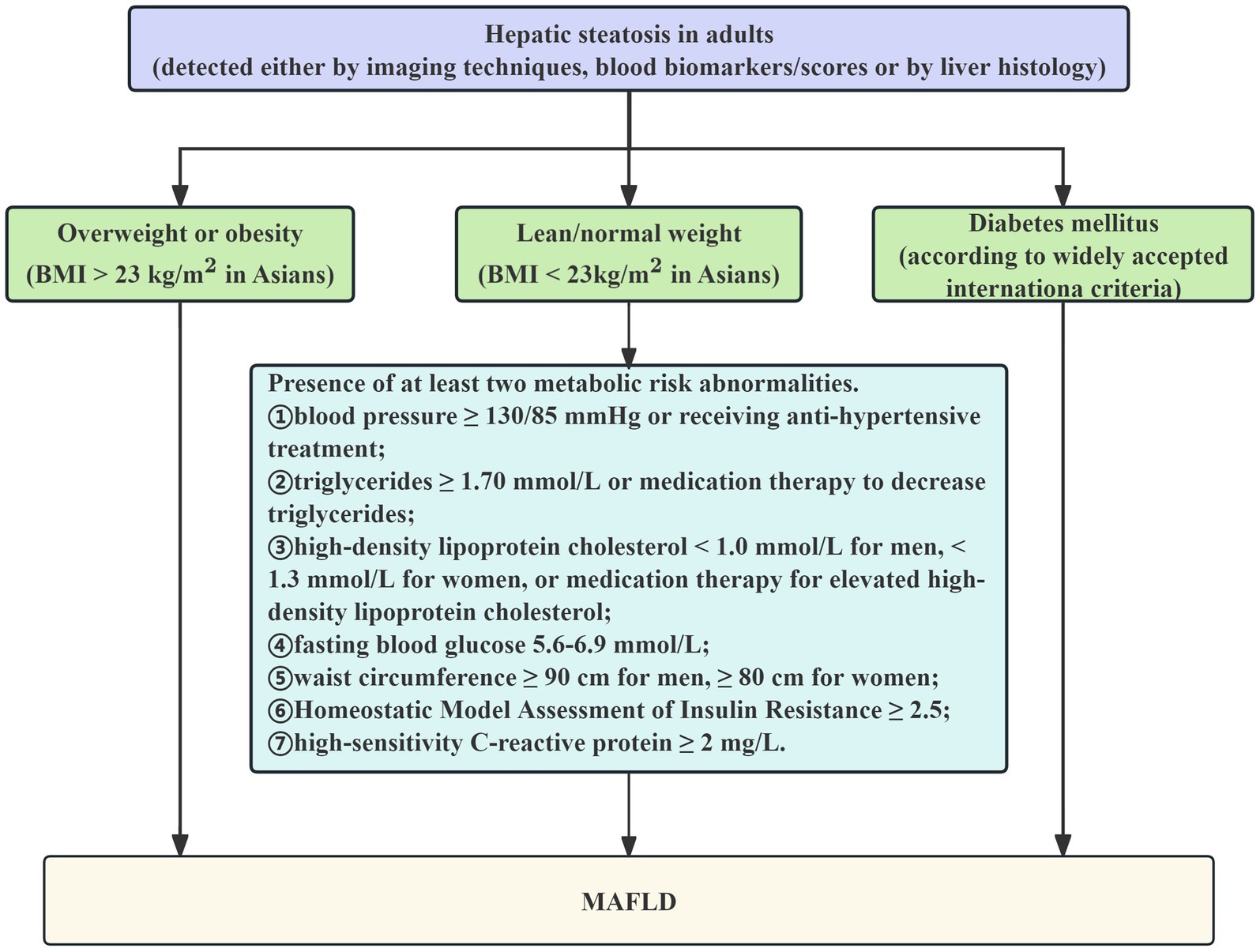
The global consensus among experts statement on the updated definition of MAFLD, which states that the condition is defined as the presence of fatty liver on abdominal ultrasonography together with one or more of the following criteria, served as the basis for the diagnosis of MAFLD, listed in Figure 1 (28): (1) BMI > 23 kg/m2; (2) T2DM; (3)Two or more of the following anomalies related to metabolic risk, including: ① blood pressure ≥ 130/85 mmHg or receiving anti-hypertensive treatment;
② TG ≥ 1.70 mmol/L or medication therapy to decrease TG; ③ HDL-C < 1.0 mmol/L for men, < 1.3 mmol/L for women, or medication therapy for elevated HDL-C; ④ FBG 5.6–6.9 mmol/L; ⑤ waist circumference ≥ 90 cm for men, ≥ 80 cm for women; ⑥ Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) ≥ 2.5; ⑦ high-sensitivity C-reactive protein ≥2 mg/L.
The severity of hepatic steatosis is classified based on liver echogenicity as mild, moderate, and severe (29):
Mild: The liver is of normal size, with a slight diffuse increase in fine echoes; the diaphragm and intrahepatic artery boundaries are clearly visible, and the liver appears brighter than the kidney cortex.
Moderate: The liver size is normal or slightly enlarged, with a moderately distributed rise in fine echoes and a minor impairment in the diaphragm and intrahepatic arteries’ visibility.
Severe: Increased liver volume, which is indicative of a noticeable rise in fine echoes, insufficient penetration of the liver’s posterior right lobe, and little to no visibility of the intrahepatic arteries or diaphragm.
2.4 Statistical analysis
Data analysis was carried out employing IBM SPSS Statistics software (version 26.0). The sample size is sufficient (4,663 individuals), hence the central limit theorem states that the sample is thought to follow a normal distribution. According to previous literature (30, 31), the mean ± standard deviations (±SD) was applied to describe continuous variables, and the t-test was employed to evaluate differences between two groups. The analysis of Variance, followed by least significant difference post-hoc tests were used for comparing continuous variables among multiple groups. The frequencies (percentages) were applied to describe categorical variables, and the chi-square test was employed to assess differences between two or more groups. Referring to previous cross-sectional studies (32, 33), this research employed univariate and multivariate logistic regression analysis to figure out pertinent risk variables for MAFLD at various degrees of hepatic steatosis severity as determined by ultrasonography. A statistically significant result was defined as a two-tailed p value less than 0.05.
3 Results
3.1 Comparison of general characteristics between MAFLD and non-MAFLD groups
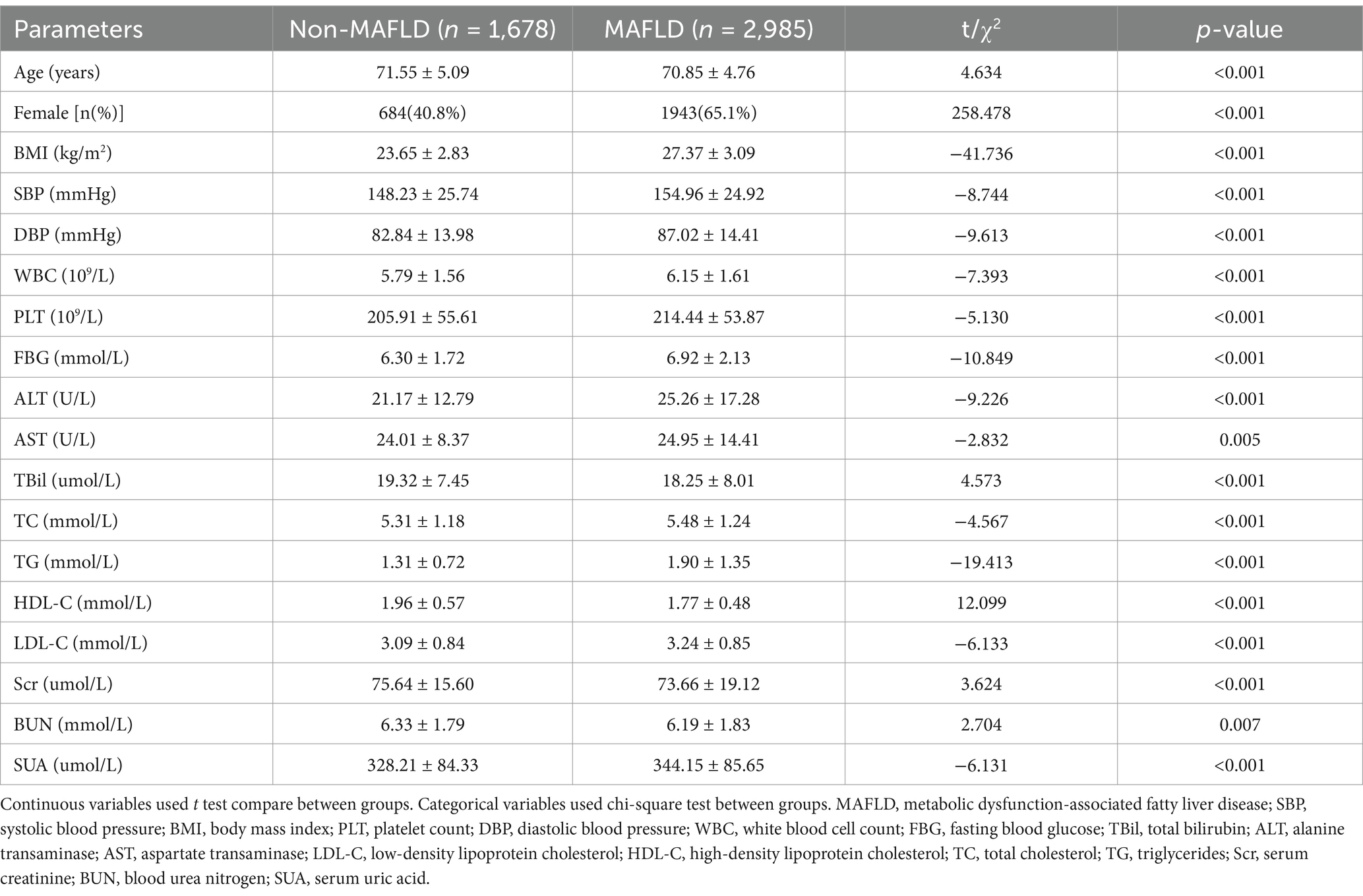
Table 1 compares the general characteristics between MAFLD and non-MAFLD groups. In terms of the category of basic information, the MAFLD group exhibited significantly more indicators of age, female gender, BMI, DBP and SBP than the non-MAFLD group (p < 0.05). Regarding the blood routine index category, the MAFLD group had significantly elevated amounts of white blood cell count (WBC) and platelet count (PLT) in contrast to the non-MAFLD group (p < 0.05). In regard to glucose and lipid metabolism indices, the MAFLD group exhibited substantially greater levels of TG, low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), and FBG, instead a lower amount of HDL-C than the non-MAFLD group (p < 0.05). In the liver function index category,the MAFLD group demonstrated substantially greater levels of ALT and AST and lower levels of total bilirubin (TBil) relative to the non-MAFLD group (p < 0.05). Regarding renal function indices, The MAFLD group demonstrated significantly greater amounts of SUA and significantly less amount of serum creatinine (Scr) and blood urea nitrogen (BUN) contrasted to the non-MAFLD group (p < 0.05).
3.2 Comparison of general characteristics among different grades of hepatic steatosis
As illustrated in Table 2, in the basic information category, the moderate group exhibited considerably greater levels of female gender than the mild group (p < 0.05). And the moderate and severe groups had significantly greater DBP value than the mild group (p < 0.05). All three groups’ BMI values had a positive correlation with the severity of fatty liver, with statistically significant differences (p < 0.05). Conversely, the variations in age and SBP across the three groups were not statistically significant (p > 0.05). Regarding the glucose and lipid metabolism index, FBG values demonstrated a positive correlation with the severity of fatty liver in three groups, with significant differences (p < 0.05). The moderate and severe groups had significantly greater amounts of TG than the mild group (p < 0.05). Nevertheless, LDL-C, HDL-C, and TC values were not substantially different across the groups (p > 0.05). In terms of the liver function index, ALT and AST values were positively correlated with fatty liver severity across the groups, with statistically significant differences (p < 0.05). Whereas the mild and moderate groups had significantly greater TBil values than the severe group (p < 0.05). Regarding renal function indices, the moderate and severe groups exhibited greater SUA values than the mild group, and these differences were statistically significant (p < 0.05). There were no statistically significant variations in Scr and BUN values between the three groups (p > 0.05).
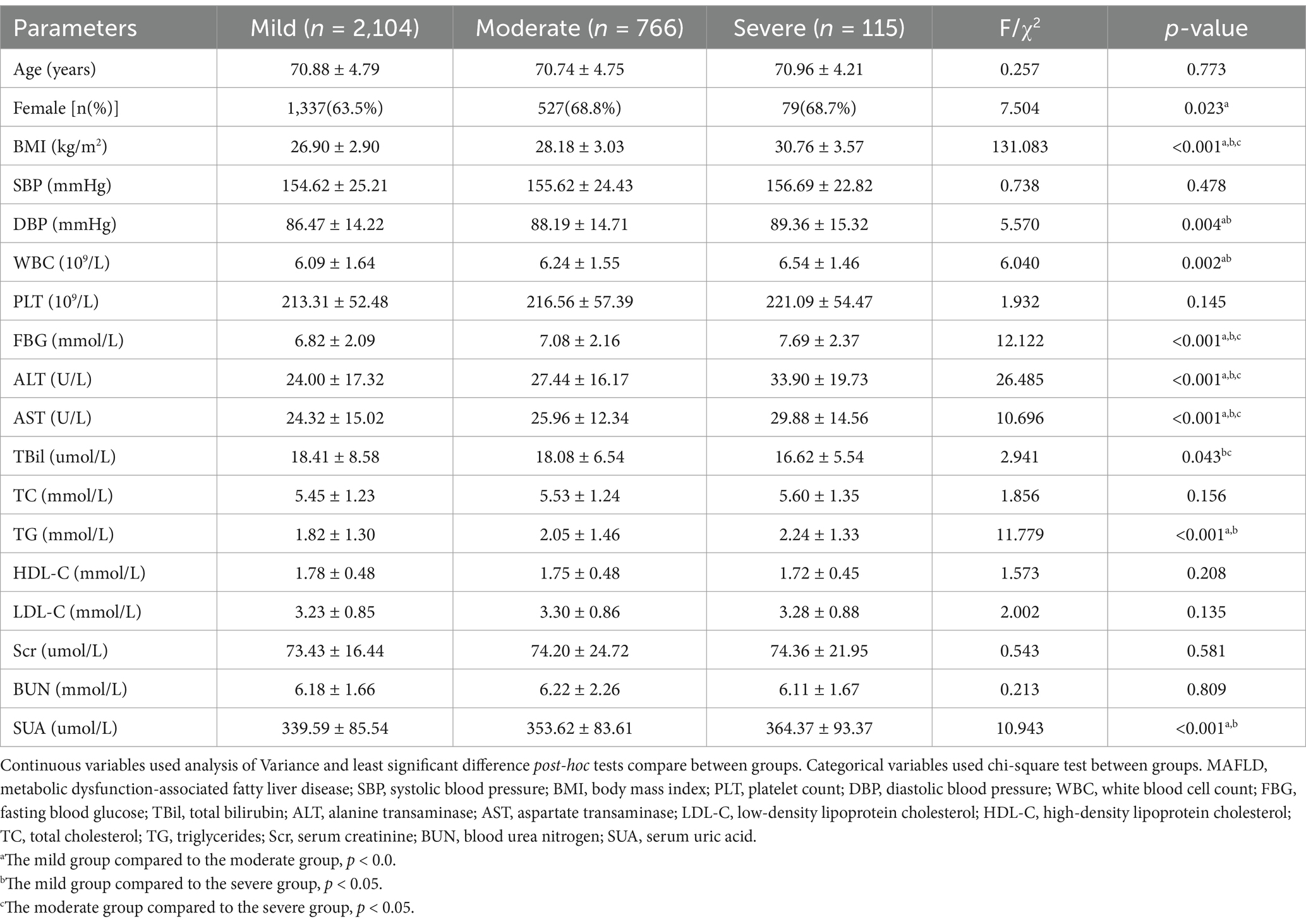
Table 2. Comparison of general characteristics among different grades of hepatic steatosis in MAFLD.
3.3 Analysis of risk factors associated with the severity of hepatic steatosis in MAFLD
In this investigation, the severity of hepatic steatosis in MAFLD, based on ultrasound grading, was considered as the dependent variable. Univariate logistic regression analysis considered factors such as age, gender, BMI, FBG and blood pressure as independent variables. The results indicated that female gender, BMI, WBC, DBP, ALT, FBG, AST, SUA and TG were all risk factors for the severity of hepatic steatosis in MAFLD (p < 0.05; refer to Figure 2). Furthermore, the multivariate logistic regression analysis revealed that female gender, BMI, ALT, FBG, TG, and SUA remained significant after adjusting for all other variables in the model (p < 0.05; refer to Figure 3). And female gender (OR 1.345, 95%CI: 1.116 ~ 1.623) and BMI (OR 1.193, 95%CI: 1.162 ~ 1.225) had the greatest effect on hepatic steatosis in MAFLD.
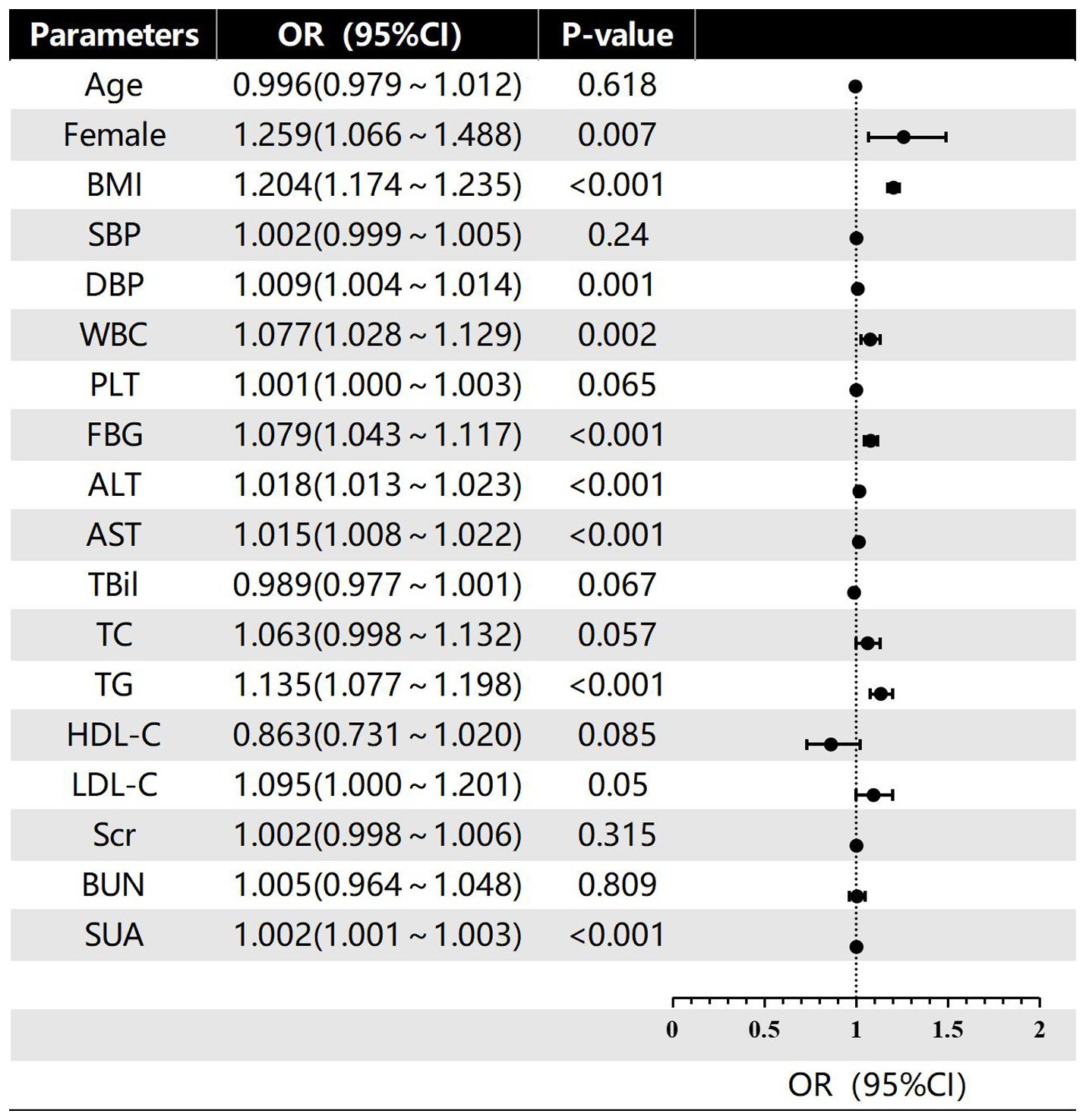
Figure 2. Univariate logistic regression of risk factors for different grades of hepatic steatosis in MAFLD.
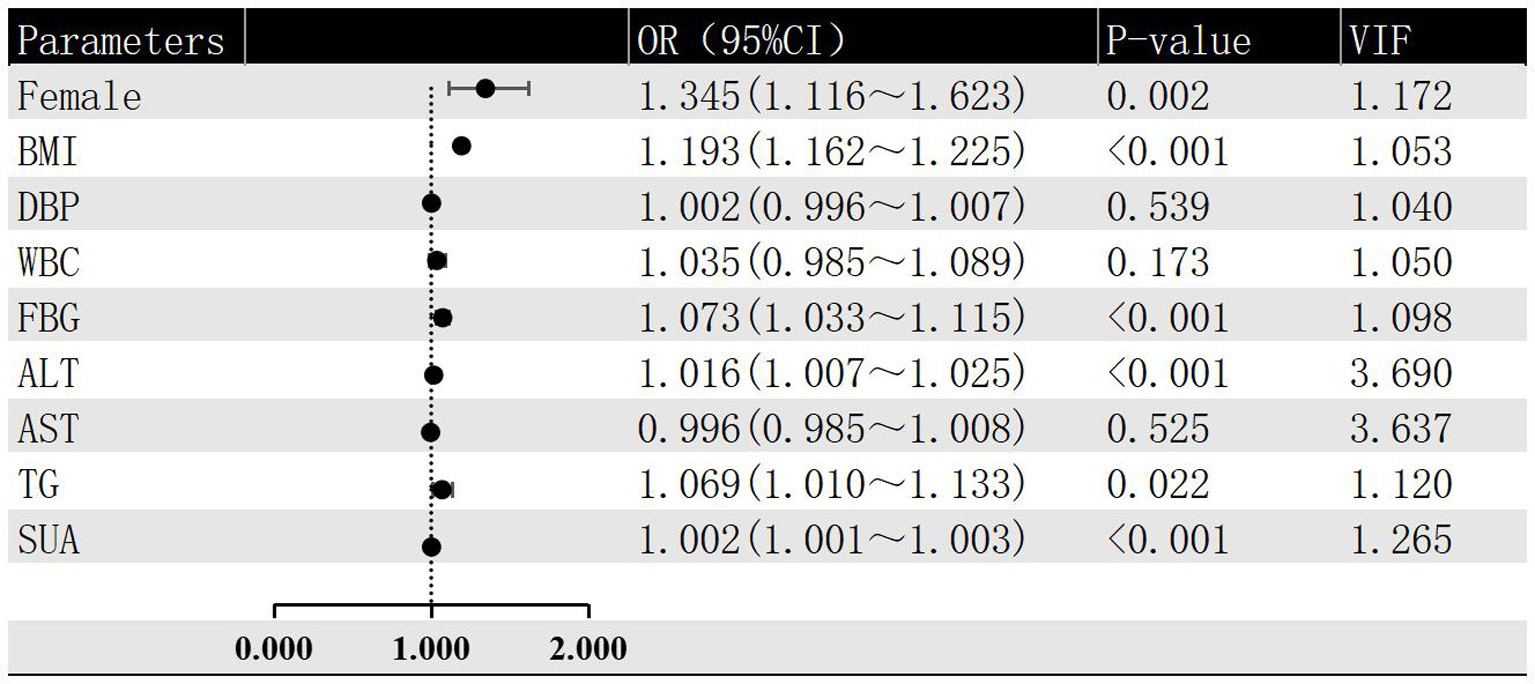
Figure 3. Multivariate logistic regression of risk factors for different grades of hepatic steatosis in MAFLD.
4 Discussion
Over the past 4 years, the nomenclature for NAFLD in scientific literature has evolved, introducing terms such as MAFLD and metabolic dysfunction-associated steatotic liver disease (MASLD). Regardless of whether the term used is MAFLD or MASLD, the strong association with metabolic disorders and cardiovascular risk factors has been globally recognized (34). Most patients with MAFLD present with only hepatic steatosis, however, in a subset of patients, the disease may progress to steatohepatitis. A critical event in the pathophysiology of this condition is the immune system is activated. Various factors are associated with the progression of MAFLD, including hypoxia, endoplasmic reticulum stress, insulin resistance, and dyslipidemia, significantly increasing the risk of cirrhosis and hepatocellular carcinoma (35). MAFLD is now diagnosed independently of other liver diseases, allowing clinicians to identify and manage all liver conditions comprehensively within individual patients (36). Community health services play an essential part in identifying and managing MAFLD through clinical assessment and appropriate referrals to specialized care (37). However, in community settings, individuals with advanced fibrosis and cirrhosis caused by MAFLD are frequently disregarded (38). Early diagnosis, particularly in primary healthcare institutions, is essential to address this gap and improve patient oucomes (39).
In our research, values of ALT, FBG, BMI and TG were positively correlated with the severity of hepatic steatosis, consistent with findings by Tutunchi et al. (40). ALT, a key indicator for liver disease diagnosis, effectively reflects the severity of liver damage (41). Serum transaminase values that are mildly to moderately elevated in MAFLD patients may be an indicator of inflammation or liver damage. Liver damage appears to be progressive as there is a positive connection between the developed liver enzyme levels and the degree of hepatic steatosis (42). This might be because MAFLD individuals’ liver cells contain greater concentrations of free fatty acids. Resulting in oxidative stress or increased liver inflammation, which in turn promotes hepatocyte necrosis and degeneration and increases serum ALT levels (43). Additionally, in chronic liver disease, ALT levels are more commonly elevated than AST, except in alcoholic liver disease, where elevated AST levels also indicate hepatocellular injury (44). While AST is often used in MAFLD studies to predict advanced liver fibrosis (45), our multivariable analysis did not indicate a significant role for AST in the progression of steatosis in our cohort.
T2DM has been associated to both liver fibrosis and MAFLD in several reports (46, 47). Severe intrahepatic lipid buildup was strongly correlated with elevated blood triglyceride levels in patients with MAFLD. 188 outpatients were included in a cross-sectional study measuring liver fat content, which found that the primary cause of ApoB elevation was severe intrahepatic lipid accumulation. Based on their connection to cardiovascular diseases and mortality, these anomalies have been demonstrated to be significant indicators for a dismal prognosis (48). Perhaps the explanation for this is that hepatic triglyceride synthesis rises significantly in persons with diabetes, a condition that can be rectified with diabetes remission (49).
A meta-analysis on BMI and MAFLD demonstrated a 3.5-fold increase in MAFLD risk in subjects with higher BMI, indicating a significant dose–response relationship between BMI levels and MAFLD risk (50). Additionally, Cuenza’s research demonstrated an advantageous connection between body mass index and the severity of hepatic steatosis, which is in line with our findings (51). Interestingly, our research demonstrated that people with MAFLD had less TBil values than individuals without MAFLD, and that individuals who had severe hepatic steatosis had less TBil values than subjects with mild and moderate hepatic steatosis. A rise in bilirubin levels has been adversely correlated with the occurrence of MAFLD, while some evidence indicated that low TBil values were linked to a higher risk of MAFLD (52). Exogenous bilirubin supplementation to increase bilirubin levels might be a promising therapeutic strategy for MAFLD (53).
Previous investigations have revealed that MAFLD is particularly prevalent in men, and male gender is regarded as a separate risk indicator for MAFLD (54). However, our study yielded completely different results, likely attributed to our sample consisting solely of elderly women aged 65 and above. The disappearance of gender differences, possibly due to decreased estrogen levels after menopause, could be an important contributing factor to this phenomenon. MAFLD is more common in postmenopausal women, according to epidemiological statistics (55). For instance, in certain clinical studies, the prevalence of MAFLD was significantly greater in postmenopausal women than in men of the same age (19.4% vs. 14.9%), but lower in premenopausal women than in men (12.7% vs. 26%). Postmenopausal women have approximately a 2.4-fold greater likelihood of MAFLD than premenopausal women, revealed to a meta-analysis (56). The decline in estrogen levels leads to hepatic steatosis by reducing fatty acid oxidation and increasing intrahepatic fat synthesis (57). Furthermore, postmenopausal women tend to accumulate more visceral fat compared to premenopausal women, rendering them more susceptible to metabolic disturbances and hepatic steatosis (58). Intestinal flora imbalance, oxidation and antioxidant system imbalance, IR, glucose metabolic problems, and accelerated blood lipids may all be caused by estrogen insufficiency. The advantages of estrogen replacement treatment (ERT), which lowers the prevalence of postmenopausal MAFLD, have been documented in multiple investigations (59). Age has been connected in certain research to an elevated likelihood of MAFLD, encompassing subjects from various age groups (60). However, our study found no significant relationship between age and MAFLD severity in the elderly population, suggesting that age may not be connected to hepatic steatosis severity in this demographic. Van et al.’s prospective cohort analysis indicated that in those 65 years of age and older, fatty liver is not related to all-cause mortality (61).
SUA was determined to be a distinct risk indicator for MAFLD in Tao et al.’s trial (62). This is consistent with our findings. Previous research has indicated that elevated amounts of SUA may cause insulin resistance, which in turn contributes to the accumulation of visceral fat (63). Conversely, lowering SUA levels through hyperuricemia treatment can enhance overall metabolic status and reduce visceral fat deposition (64). Computed tomography studies have independently associated hyperuricemia with hepatic and visceral fat tissue accumulation (65). Liu et al.’s prospective analysis discovered that higher SUA levels significantly increase the risk of MAFLD, with a 17% increase in MAFLD risk for every 60 mg/dL increase in SUA levels (95% CI 9–24%). SUA levels also serve as predictive markers for long-term mortality in MAFLD patients (66).
Our research has an assortment of limitations. Firstly, as a cross-sectional study of elderly MAFLD patients from the Eastern China region, the unique demographic, geographic, and environmental characteristics of this cohort may limit the generalizability of the findings to broader populations or other ethnic groups. Without controlling for potential confounding factors, such as dietary habits, exercise, and smoking, which could impact the accuracy and interpretability of the results. Moreover, the specific genetic predispositions and lifestyle factors prevalent in this region may not be representative of those found in other populations, further restricting the applicability of our results. Therefore, caution should be exercised when applying these findings to other populations, as they may not accurately reflect the distinct characteristics and risk factors present in different regions or ethnic groups. Additionally, the data source from community health service centers might not fully represent the broader elderly population, especially those with poorer health who did not undergo physical examinations. It is necessary to compare the findings with those of other regions or larger-scale studies to comprehensively validate the conclusions. And the cross-sectional design is suitable for identifying associations between variables but cannot establish causal relationships, it is need for future longitudinal studies to confirm causality. Secondly, since fasting serum insulin and C-reactive protein levels were not assessed, both of which are components of metabolic dysregulation that define MAFLD, the diagnosis of MAFLD in certain individuals with normal BMI may be ignored. Lastly, although ultrasound is widely used to detect hepatic steatosis, its sensitivity is lower compared to methods such as MRI or liver biopsy, particularly in cases of mild steatosis. Its limitations include difficulty in accurately quantifying fat infiltration and distinguishing simple steatosis from more severe conditions. This reduced sensitivity may lead to underdiagnosis or misdiagnosis. The diagnostic accuracy of ultrasound significantly depends on the operator’s expertise and experience, potentially leading to variability in results. There is no universally accepted standardization for ultrasound-based diagnostic criteria for severity of fatty liver, which may result in inconsistent diagnoses across studies and clinical practices.
In conclusion, this research examined the metabolic profiles of elderly MAFLD patients with varying severity of hepatic steatosis as determined by ultrasound. The severity of hepatic steatosis in elderly MAFLD patients was significantly connected with female gender, BMI, FBG, ALT, TG and SUA levels. Since diet and exercise are the cornerstones of therapy for MAFLD, no particular drugs are currently licensed for its treatment. Therefore, early diagnosis and prevention are essential, and the aforementioned indicators can serve as valuable references for early MAFLD screening and diagnosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Binzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable data included in this article because this is just retrospective study analyzing existing medical data, informed consent from the patients was not required. No additional interventions were performed and the analysis relied only on these previously recorded clinical variables. Patient identity remained anonymized throughout the study. The study procedures were reviewed and approved by the Institutional Review Board of Binzhou Medical University, who determined that individual informed consent was not necessary for this type of retrospective chart review that posed minimal risk to patients. This approach aligns with ethical guidelines for medical research involving human subjects when utilizing already collected data where consent would be impractical to obtain. By waiving the informed consent requirement, we were able to conduct this research efficiently while upholding patient privacy and confidentiality.
Author contributions
ZL: Software, Writing – original draft, Writing – review & editing. RH: Writing – review & editing. LZ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Eslam, M, Sarin, SK, Wong, VW, Fan, J, Kawaguchi, T, Ahn, SH, et al. The asian pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. (2020) 14:889–919. doi: 10.1007/s12072-020-10094-2
2. Eslam, M, Ratziu, V, and George, J. Yet more evidence that mafld is more than a name change. J Hepatol. (2021) 74:977–9. doi: 10.1016/j.jhep.2020.12.025
3. Wang, Z, Zhao, X, Chen, S, Wang, Y, Cao, L, Liao, W, et al. Associations between nonalcoholic fatty liver disease and cancers in a large cohort in China. Clin Gastroenterol Hepatol: Official Clin Prac J American Gastroenterolog Assoc. (2021) 19:788–796.e4. doi: 10.1016/j.cgh.2020.05.009
4. Chalasani, N, Younossi, Z, Lavine, JE, Charlton, M, Cusi, K, Rinella, M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the american association for the study of liver diseases. Hepatology (Baltimore, Md). (2018) 67:328–57. doi: 10.1002/hep.29367
5. Wong, GL, and Wong, VW. How many deaths are caused by non-alcoholic fatty liver disease in the asia-pacific region? Lancet Gastroenterol Hepatol. (2020) 5:103–5. doi: 10.1016/S2468-1253(19)30338-3
6. Pelusi, S, Cespiati, A, Rametta, R, Pennisi, G, Mannisto, V, Rosso, C, et al. Prevalence and risk factors of significant fibrosis in patients with nonalcoholic fatty liver without steatohepatitis. Clin Gastroenterol Hepatol: Official Clin Prac J American Gastroenterolog Assoc. (2019) 17:2310–2319.e6. doi: 10.1016/j.cgh.2019.01.027
7. Barber, SL, and Rosenberg, M. Aging and universal health coverage: implications for the asia pacific region. Health systems and reform. (2017) 3:154–8. doi: 10.1080/23288604.2017.1348320
8. Kim, Y, Chang, Y, Ryu, S, Wild, SH, and Byrne, CD. Nafld improves risk prediction of type 2 diabetes: with effect modification by sex and menopausal status. Hepatology (Baltimore, Md). (2022) 76:1755–65. doi: 10.1002/hep.32560
9. Younossi, ZM, Koenig, AB, Abdelatif, D, Fazel, Y, Henry, L, and Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md). (2016) 64:73–84. doi: 10.1002/hep.28431
10. Sayiner, M, Koenig, A, Henry, L, and Younossi, ZM. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin Liver Dis. (2016) 20:205–14. doi: 10.1016/j.cld.2015.10.001
11. Pal, SC, and Méndez-Sánchez, N. Screening for mafld: who, when and how? Ther Adv Endocrinol Metab. (2023) 14:1859452366. doi: 10.1177/20420188221145650
12. Allen, AM, Therneau, TM, Larson, JJ, Coward, A, Somers, VK, and Kamath, PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology (Baltimore, Md). (2018) 67:1726–36. doi: 10.1002/hep.29546
13. Cai, X, Aierken, X, Ahmat, A, Cao, Y, Zhu, Q, Wu, T, et al. A nomogram model based on noninvasive bioindicators to predict 3-year risk of nonalcoholic fatty liver in nonobese mainland chinese: a prospective cohort study. Biomed Res Int. (2020) 2020:8852198. doi: 10.1155/2020/8852198
14. Sumida, Y, Nakajima, A, and Itoh, Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. (2014) 20:475–85. doi: 10.3748/wjg.v20.i2.475
15. Hernaez, R, Lazo, M, Bonekamp, S, Kamel, I, Brancati, FL, Guallar, E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology (Baltimore, Md). (2011) 54:1082–90. doi: 10.1002/hep.24452
16. Lee, SS, and Park, SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol. (2014) 20:7392–402. doi: 10.3748/wjg.v20.i23.7392
17. Park, CC, Nguyen, P, Hernandez, C, Bettencourt, R, Ramirez, K, Fortney, L, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. (2017) 152:598–607.e2. doi: 10.1053/j.gastro.2016.10.026
18. Pipitone, RM, Ciccioli, C, Infantino, G, La Mantia, C, Parisi, S, Tulone, A, et al. Mafld: a multisystem disease. Ther Adv Endocrinol Metab. (2023) 14:1859452467. doi: 10.1177/20420188221145549
19. Garibay-Nieto, N, Pedraza-Escudero, K, Omaña-Guzmán, I, Garcés-Hernández, MJ, Villanueva-Ortega, E, Flores-Torres, M, et al. Metabolomic phenotype of hepatic steatosis and fibrosis in mexican children living with obesity. Medicina (Kaunas). (2023) 59:59. doi: 10.3390/medicina59101785
20. Kuchay, MS, Martínez-Montoro, JI, Choudhary, NS, Fernández-García, JC, and Ramos-Molina, B. Non-alcoholic fatty liver disease in lean and non-obese individuals: current and future challenges. Biomedicine. (2021) 9:9. doi: 10.3390/biomedicines9101346
21. Niriella, MA, Kasturiratne, A, Pathmeswaran, A, De Silva, ST, Perera, KR, Subasinghe, SKCE, et al. Lean non-alcoholic fatty liver disease (lean nafld): characteristics, metabolic outcomes and risk factors from a 7-year prospective, community cohort study from Sri Lanka. Hepatol Int. (2019) 13:314–22. doi: 10.1007/s12072-018-9916-4
22. Oniki, K, Saruwatari, J, Izuka, T, Kajiwara, A, Morita, K, Sakata, M, et al. Influence of the pnpla3 rs738409 polymorphism on non-alcoholic fatty liver disease and renal function among normal weight subjects. PLoS One. (2015) 10:e132640. doi: 10.1371/journal.pone.0132640
23. Khalatbari-Soltani, S, Imamura, F, Brage, S, De Lucia, RE, Griffin, SJ, Wareham, NJ, et al. The association between adherence to the mediterranean diet and hepatic steatosis: cross-sectional analysis of two independent studies, the Uk fenland study and the swiss colaus study. BMC Med. (2019) 17:19. doi: 10.1186/s12916-019-1251-7
24. You, Y, Chen, Y, Liu, R, Zhang, Y, Wang, M, Yang, Z, et al. Inverted u-shaped relationship between sleep duration and phenotypic age in us adults: a population-based study. Sci Rep. (2024) 14:6247. doi: 10.1038/s41598-024-56316-7
25. You, Y, Li, J, Zhang, Y, Li, X, Li, X, and Ma, X. Exploring the potential relationship between short sleep risks and cognitive function from the perspective of inflammatory biomarkers and cellular pathways: insights from population-based and mice studies. CNS Neurosci Ther. (2024) 30:e14783. doi: 10.1111/cns.14783
26. Shen, D, Cai, X, Hu, J, Song, S, Zhu, Q, Ma, H, et al. Associating plasma aldosterone concentration with the prevalence of mafld in hypertensive patients: insights from a large-scale cross-sectional study. Front Endocrinol (Lausanne). (2024) 15:1451383. doi: 10.3389/fendo.2024.1451383
27. Hu, J, Cai, X, Zhu, Q, Heizhati, M, Wen, W, Luo, Q, et al. Relationship between plasma aldosterone concentrations and non-alcoholic fatty liver disease diagnosis in patients with hypertension: a retrospective cohort study. Diabetes, metabolic syndrome and obesity: targets and therapy. (2023) 16:1625–36. doi: 10.2147/DMSO.S408722
28. Eslam, M, Newsome, PN, Sarin, SK, Anstee, QM, Targher, G, Romero-Gomez, M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. (2020) 73:202–9. doi: 10.1016/j.jhep.2020.03.039
29. Jang, W, and Song, JS. Non-invasive imaging methods to evaluate non-alcoholic fatty liver disease with fat quantification: a review. Diagnostics (Basel, Switzerland). (2023) 13:13. doi: 10.3390/diagnostics13111852
30. You, Y, Wang, R, Li, J, Cao, F, Zhang, Y, and Ma, X. The role of dietary intake of live microbes in the association between leisure-time physical activity and depressive symptoms: a population-based study. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. (2024) 49:1014–24. doi: 10.1139/apnm-2023-0550
31. You, Y, Liu, J, Li, X, Wang, P, Liu, R, and Ma, X. Relationship between accelerometer-measured sleep duration and stroop performance: a functional near-infrared spectroscopy study among young adults. Peerj. (2024) 12:e17057. doi: 10.7717/peerj.17057
32. You, Y. Accelerometer-measured physical activity and sedentary behaviour are associated with c-reactive protein in us adults who get insufficient sleep: a threshold and isotemporal substitution effect analysis. J Sports Sci. (2024) 42:527–36. doi: 10.1080/02640414.2024.2348906
33. You, Y, Chen, Y, Zhang, Y, Zhang, Q, Yu, Y, and Cao, Q. Mitigation role of physical exercise participation in the relationship between blood cadmium and sleep disturbance: a cross-sectional study. BMC Public Health. (2023) 23:1465. doi: 10.1186/s12889-023-16358-4
34. Portincasa, P, Khalil, M, Mahdi, L, Perniola, V, Idone, V, Graziani, A, et al. Metabolic dysfunction-associated steatotic liver disease: from pathogenesis to current therapeutic options. Int J Mol Sci. (2024) 25:25. doi: 10.3390/ijms25115640
35. Huang, DQ, El-Serag, HB, and Loomba, R. Global epidemiology of nafld-related hcc: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2021) 18:223–38. doi: 10.1038/s41575-020-00381-6
36. Gofton, C, Upendran, Y, Zheng, M, and George, J. Mafld: how is it different from nafld? Clin Mol Hepatol. (2023) 29:S17–31. doi: 10.3350/cmh.2022.0367
37. Balci, IC, Haciagaoglu, N, Oner, C, Cetin, H, and Simsek, EE. Non-invasive screening of metabolic associated fatty liver disease and affecting factors in primary care. J College of Physicians and Surgeons--Pakistan: JCPSP. (2023) 33:390–5. doi: 10.29271/jcpsp.2023.04.390
38. Srivastava, A, Gailer, R, Tanwar, S, Trembling, P, Parkes, J, Rodger, A, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol. (2019) 71:371–8. doi: 10.1016/j.jhep.2019.03.033
39. Blanco-Grau, A, Gabriel-Medina, P, Rodriguez-Algarra, F, Villena, Y, Lopez-Martínez, R, Augustín, S, et al. Assessing liver fibrosis using the fib4 index in the community setting. Diagnostics (Basel, Switzerland). (2021) 11:11. doi: 10.3390/diagnostics11122236
40. Tutunchi, H, Saghafi-Asl, M, Asghari-Jafarabadi, M, and Ostadrahimi, A. The relationship between severity of liver steatosis and metabolic parameters in a sample of iranian adults. BMC Res Notes. (2020) 13:218. doi: 10.1186/s13104-020-05059-5
41. Fan, N, Peng, L, Xia, Z, Zhang, L, Song, Z, Wang, Y, et al. Triglycerides to high-density lipoprotein cholesterol ratio as a surrogate for nonalcoholic fatty liver disease: a cross-sectional study. Lipids Health Dis. (2019) 18:39. doi: 10.1186/s12944-019-0986-7
42. Khov, N, Sharma, A, and Riley, TR. Bedside ultrasound in the diagnosis of nonalcoholic fatty liver disease. World J Gastroenterol. (2014) 20:6821–5. doi: 10.3748/wjg.v20.i22.6821
43. Huang, X, Yin, M, Zhou, B, Tan, X, Xia, Y, and Qin, C. Impact renaming non-alcoholic fatty liver disease to metabolic associated fatty liver disease in prevalence, characteristics and risk factors. World J Hepatol. (2023) 15:985–1000. doi: 10.4254/wjh.v15.i8.985
44. Kim, WR, Flamm, SL, Di Bisceglie, AM, and Bodenheimer, HC. Serum activity of alanine aminotransferase (alt) as an indicator of health and disease. Hepatology (Baltimore, Md). (2008) 47:1363–70. doi: 10.1002/hep.22109
45. Kani, HT, Demirtas, CO, Keklikkiran, C, Ergenc, I, Mehdiyev, S, Akdeniz, E, et al. Evaluation of the impact of metabolic syndrome on fibrosis in metabolic dysfunction-associated fatty liver disease. Turkish J Gastroenterol: Official J Turkish Society of Gastroenterol. (2021) 32:661–6. doi: 10.5152/tjg.2021.20512
46. Barb, D, Repetto, EM, Stokes, ME, Shankar, SS, and Cusi, K. Type 2 diabetes mellitus increases the risk of hepatic fibrosis in individuals with obesity and nonalcoholic fatty liver disease. Obesity (Silver Spring, Md). (2021) 29:1950–60. doi: 10.1002/oby.23263
47. Lomonaco, R, Godinez Leiva, E, Bril, F, Shrestha, S, Mansour, L, Budd, J, et al. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care. (2021) 44:399–406. doi: 10.2337/dc20-1997
48. Wu, T, Ye, J, Shao, C, Li, F, Lin, Y, Ma, Q, et al. Varied relationship of lipid and lipoprotein profiles to liver fat content in phenotypes of metabolic associated fatty liver disease. Front Endocrinol (Lausanne). (2021) 12:691556. doi: 10.3389/fendo.2021.691556
49. Al-Mrabeh, A, Zhyzhneuskaya, SV, Peters, C, Barnes, AC, Melhem, S, Jesuthasan, A, et al. Hepatic lipoprotein export and remission of human type 2 diabetes after weight loss. Cell Metab. (2020) 31:233–249.e4. doi: 10.1016/j.cmet.2019.11.018
50. Li, L, Liu, D, Yan, H, Wang, Z, Zhao, S, and Wang, B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev. (2016) 17:510–9. doi: 10.1111/obr.12407
51. Cuenza, LR, Razon, TLJ, and Dayrit, JC. Correlation between severity of ultrasonographic nonalcoholic fatty liver disease and cardiometabolic risk among filipino wellness patients. J Cardiovasc Thorac Res. (2017) 9:85–9. doi: 10.15171/jcvtr.2017.14
52. Bellarosa, C, Bedogni, G, Bianco, A, Cicolini, S, Caroli, D, Tiribelli, C, et al. Association of serum bilirubin level with metabolic syndrome and non-alcoholic fatty liver disease: a cross-sectional study of 1672 obese children. J Clin Med. (2021) 10:10. doi: 10.3390/jcm10132812
53. Stec, DE, and Hinds, TDJ. Natural product heme oxygenase inducers as treatment for nonalcoholic fatty liver disease. Int J Mol Sci. (2020) 21:21. doi: 10.3390/ijms21249493
54. Ito, T, Ishigami, M, Zou, B, Tanaka, T, Takahashi, H, Kurosaki, M, et al. The epidemiology of nafld and lean nafld in Japan: a meta-analysis with individual and forecasting analysis, 1995-2040. Hepatol Int. (2021) 15:366–79. doi: 10.1007/s12072-021-10143-4
55. DiStefano, JK. Nafld and Nash in postmenopausal women: implications for diagnosis and treatment. Endocrinology. (2020) 161:161. doi: 10.1210/endocr/bqaa134
56. Jaroenlapnopparat, A, Charoenngam, N, Ponvilawan, B, Mariano, M, Thongpiya, J, and Yingchoncharoen, P. Menopause is associated with increased prevalence of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Menopause. (2023) 30:348–54. doi: 10.1097/GME.0000000000002133
57. Suzuki, A, and Abdelmalek, MF. Nonalcoholic fatty liver disease in women. Women's Health (Lond Engl). (2009) 5:191–203. doi: 10.2217/17455057.5.2.191
58. Shi, H, and Clegg, DJ. Sex differences in the regulation of body weight. Physiol Behav. (2009) 97:199–204. doi: 10.1016/j.physbeh.2009.02.017
59. Polyzos, SA, Lambrinoudaki, I, and Goulis, DG. Menopausal hormone therapy in women with dyslipidemia and nonalcoholic fatty liver disease. Hormones (Athens). (2022) 21:375–81. doi: 10.1007/s42000-022-00369-8
60. Chen, Y, Li, H, Li, S, Xu, Z, Tian, S, Wu, J, et al. Prevalence of and risk factors for metabolic associated fatty liver disease in an urban population in China: a cross-sectional comparative study. BMC Gastroenterol. (2021) 21:212. doi: 10.1186/s12876-021-01782-w
61. van Kleef, LA, Sonneveld, MJ, Kavousi, M, Ikram, MA, de Man, RA, and de Knegt, RJ. Fatty liver disease is not associated with increased mortality in the elderly: a prospective cohort study. Hepatology (Baltimore, Md). (2023) 77:585–93. doi: 10.1002/hep.32635
62. Tao, M, Liu, J, Chen, X, Wang, Q, He, M, Chen, W, et al. Correlation between serum uric acid and body fat distribution in patients with mafld. BMC Endocr Disord. (2023) 23:204. doi: 10.1186/s12902-023-01447-7
63. Fernández-Chirino, L, Antonio-Villa, NE, Fermín-Martínez, CA, Márquez-Salinas, A, Guerra, EC, Vargas-Vázquez, A, et al. Elevated serum uric acid is a facilitating mechanism for insulin resistance mediated accumulation of visceral adipose tissue. Clin Endocrinol. (2022) 96:707–18. doi: 10.1111/cen.14673
64. Chen, J, Ge, J, Zha, M, Miao, J, Sun, Z, and Yu, J. Effects of uric acid-lowering treatment on glycemia: a systematic review and meta-analysis. Front Endocrinol (Lausanne). (2020) 11:577. doi: 10.3389/fendo.2020.00577
65. Yamada, A, Sato, KK, Kinuhata, S, Uehara, S, Endo, G, Hikita, Y, et al. Association of visceral fat and liver fat with hyperuricemia. Arthritis Care Res. (2016) 68:553–61. doi: 10.1002/acr.22729
Keywords: liver fat content, ultrasonography, metabolic-associated fatty disease, elderly, metabolic parameters
Citation: Liang Z, Huang R and Zhang L (2025) Correlation between hepatic steatosis severity diagnosed by ultrasound and metabolic indexes in elderly patients with MAFLD. Front. Med. 11:1467773. doi: 10.3389/fmed.2024.1467773
Edited by:
Xintian Cai, People’s Hospital of Xinjiang Uygur Autonomous Region, ChinaReviewed by:
Jonathan Soldera, University of Caxias do Sul, BrazilYanwei You, Tsinghua University, China
Giovani Schulte Farina, Harvard University, United States
Di Shen, People’s Hospital of Xinjiang Uygur Autonomous Region, China
Copyright © 2025 Liang, Huang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingyun Zhang, Z3B6aGFuZzIwMjJAMTI2LmNvbQ==
 Zhitang Liang
Zhitang Liang Renhao Huang
Renhao Huang