- 1Internal Medicine Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 2Vita-Salute San Raffaele University, Milan, Italy
- 3Nephrology and Dialysis Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 4Scientific Technical Secretariat of the Ethics Committee. IRCCS San Raffaele Scientific Institute, Milan, Italy
Background: Frailty, a geriatric syndrome associated with adverse outcomes, lacks a universal definition. No consensus exists on the most effective frailty scale for predicting mortality.
Methods: This prospective observational study followed community-dwelling volunteers for 6 years. Frailty was measured with the Frailty Index (FI) and the Frailty Phenotype (FP). Concordance was assessed using Cohen’s Kappa coefficients. Age-and sex-adjusted Cox regression analyses were conducted to evaluate the association with mortality.
Results: Out of 1,114 participants (median age 72 years, IQR 69–77), 186 were classified as frail by the FI, 13 by the FP and 48 by both definitions. The concordance between the two measures was fair (κ = 0.26). Thirty-nine individuals died during the follow-up period. The FI showed a stronger association with mortality (HR 75.29, 95% CI 8.12–697.68, p < 0.001) compared to the FP (HR 3.3, 95% CI 1.45–7.51, p = 0.004). Individuals classified as frail by both definitions had the highest mortality risk and the highest FI scores (median 0.36).
Conclusion: Definitions of frailty identify different individuals as frail. The FI was more closely related to mortality than the FP. Individuals classified as frail according to both definitions displayed the highest complexity (corresponding also ho higher FI scores) and the greatest mortality. The FI demonstrated a more accurate ability to predict mortality due to its comprehensive nature.
1 Introduction
1.1 Background
The aging population is growing. In 2021, one in ten people worldwide were aged 65 years old or older and it is expected that this age group will be 1 in 6 people globally in 2050 (United Nations) (1). The frail population is growing as well. Frailty is characterized by a decrease of physiological reserves and by a weakened response to stressors (2), but no unique definition still exists. As a consequence, its prevalence among older people varies between 4 and 59.1% depending on the screening tools used (3). This heterogeneity is due to the fact that each frailty definition captures specific aspects of this condition. Two main broad constructs applied in the clinical practice are the frailty phenotype (FP) (4), which describes frailty as a biological syndrome resulting from impairments in at least three out of five categories (global weakness, overall slowness, exhaustion, low physical activity and unintentional weight loss), and the frailty index (FI) (5), which consider frailty as an increased vulnerability to stressors deriving from the accumulation of health deficits in physical, psychological, cognitive and social domains. While the FP is centered on the loss of energy paradigm and on the physical dimension of frailty (6), the FI (5) captures cognitive, affective, social, physical and functional aspects of this geriatric syndrome in addition to comorbidities that can contribute to frailty.
Frail individuals are at an increased risk of poor clinical outcomes, including mortality (7). However, there is no consensus on which frailty definition best captures mortality risk. Few studies have directly compared the FI and the FP (8–13). Except for the study by Xue et al. (9) the FI has generally been found to be a stronger predictor of mortality compared to the FP. Moreover, Hamiduzzaman et al. (8) demonstrated that individuals classified as frail by both definitions had the highest mortality risk.
1.2 Objective
In this prospective observational study, we assessed the concordance between FI and FP and their ability to predict mortality over a six-year follow-up period in a well-characterized group of Italian community-dwelling older adults.
2 Methods
2.1 Study design
The Frailty and Sarcopenia Network (FRASNET) study was a prospective observational cohort study.
2.2 Setting
The study was performed at recreational centers, cultural centers, and retirement homes in the Milan and Monza Brianza regions, as well as at the San Raffaele Scientific Institute in Milan and the Cuggiono Hospital, located near Milan, Italy. The study received approval from the ethical board of the San Raffaele Scientific Institute (24/INT/2017). All participants provided written informed consent prior to their involvement in the study. Recruitment took place between April 1, 2017, and October 16, 2020. In 2023 the follow-up to assess patient mortality was conducted both through telephone interviews and by reviewing medical records.
2.3 Participants
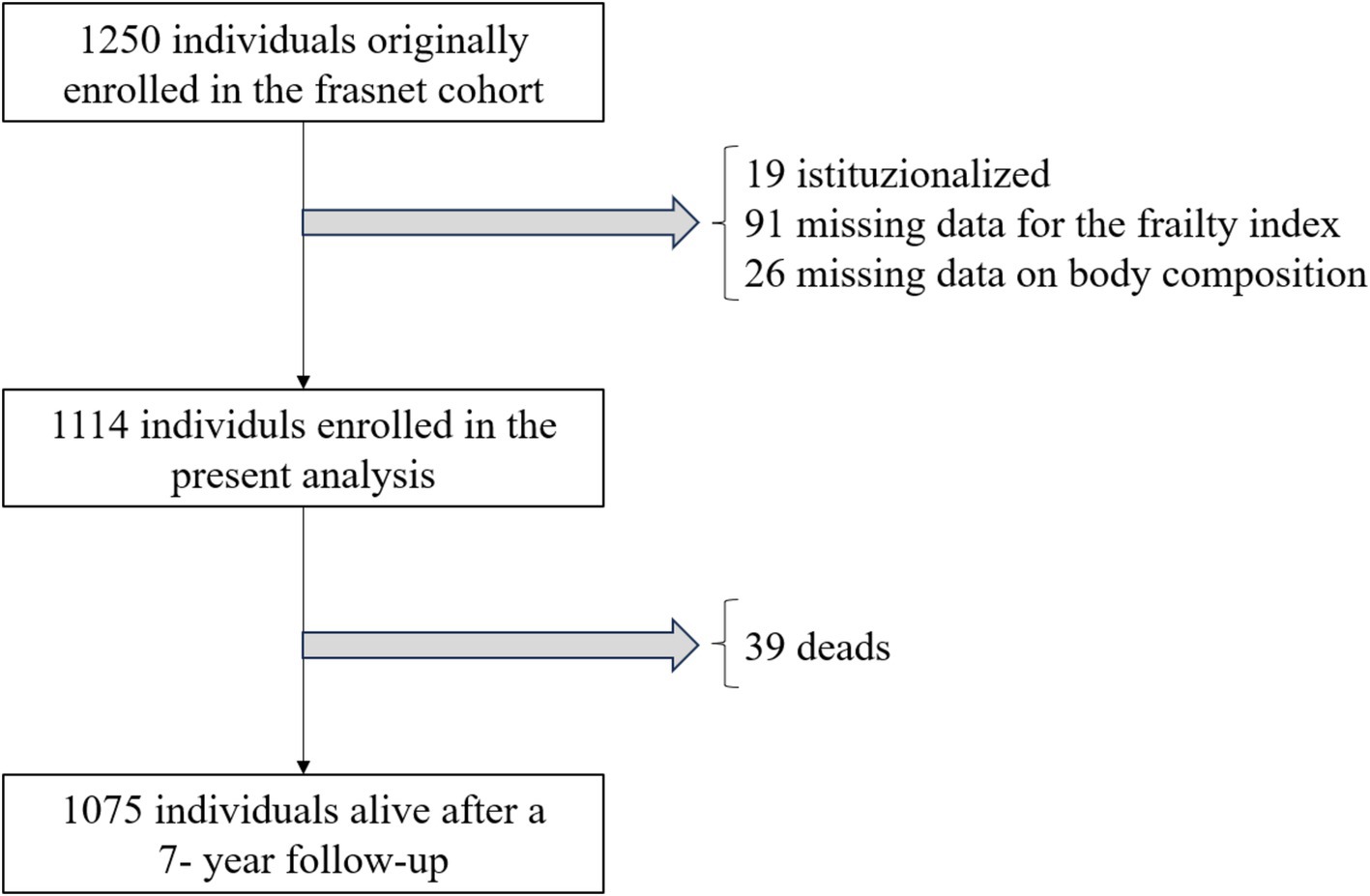
The FRASNET study included both community-dwelling healthy volunteers and institutionalized patients (14). Inclusion criteria were: (i) age 65 years or older, (ii) ability to walk more than 500 meters without assistance, (iii) life expectancy of more than 6 months. Life expectancy was assessed by the enrolling physicians, based on their clinical judgment. Exclusion criteria were: (i) a cognitive impairment indicated by a Mini-Mental State Examination (MMSE) score < 18/30, (ii) inability to provide written informed consent, (iii) severe health issues (e.g., uncontrolled hypertension, recent fractures, myocardial infarction within the past year). Patients originally recruited from retirement homes (n = 19) and those missing data for the computation of frailty (n = 91) or for the analysis of body composition (n = 26) were excluded from the current analysis.
2.4 Variables, data sources, and measurements
Participants underwent comprehensive geriatric assessments (Supplementary Table S1), performed by a multidimensional équipe composed by physicians, nurses, physiotherapists and psychologists who received an ad hoc training for performing the scales and the evaluations of the study.
The comprehensive geriatric assessment included collecting demographic and psychosocial data through self-administered questionnaires, assessing comorbidities and medications, recording the number of falls and emergency department visits in the year preceding the evaluations, and taking anthropometric measurements (weight, height, waist circumference, body mass index (BMI)). Cognitive function was evaluated using the MMSE (15), mood with the 15-item Geriatric Depression Scale (GDS) (16), exhaustion with the Fatigue Severity Scale (FSS) (17), quality of life with the Short Form 36 (SF-36) Health Survey (18), and physical activity level with the Physical Activity Scale for the Elderly (PASE) questionnaire (19). Body composition was assessed using the Full Body Sensor Body Composition Monitor and Scale (OMRON), which employs bioelectrical impedance to estimate body composition. Muscle performance was evaluated using the Short Physical Performance Battery (SPPB) (20), with the chair-stand subtest serving as a measure of muscle strength (21).
Sarcopenia was defined according to the criteria of the European Working Group on Sarcopenia in Older People 2 (22); sarcopenic obesity according to the ESPEN and EASO criteria for (23). Specifically, sarcopenia was defined as a reduction in both muscle strength (chair test >15 s) and muscle mass (muscle mass < 32.9% in males and < 23.9% in females) (22), while obesity was identified by the presence of a fat mass ≥ 30% in men and ≥ 42% in women (23). In 2023 mortality was verified retrieved from the analysis of participants’ electronic health records and confirmed by a direct follow-up.
Frailty was measured though the FP and the FI. Since we often had no information on the weight in the year/months before the evaluations, we used a modified version of the FP which considered low BMI instead of unintentional weight loss as a criterium (24) (Supplementary Table S2). A 49-items FI was created by using the criteria defined by Theou et al. (25) (Supplementary Table S3). The 49 variables used to calculate the FI were obtained from the comprehensive geriatric assessment. Each deficit included in the FI was scored 0 for absence and 1 for presence. In cases of missing data, the FI was calculated using a reduced denominator, excluding missing items. Participants with more than 20% missing variables were excluded from FI computation. A cut-off of ≥0.25 was used to define frail individuals.
2.5 Bias
The prospective study could be affective by information bias related to modified frailty criteria. However, since the modified version of the frailty phenotype has been already applied in other studies (24) we thought that the risk of bias would have been greater by using an unreliable reported weight loss. An attrition bias could have been related to participants lost to follow-up. However, we checked though electronic medical records information related to mortality also for patients lost to follow-up.
2.6 Study size
The sample size of the FRASNET study of at least 1,198 participants has been calculated with a two-sided t-test with an alpha of 5% and a power of 80% by assuming that the mutated allele for the Klotho gene between the frail and non-frail group was (0.12 in frail individuals and 0.19 in non-frail people).
2.7 Quantitative variables and statistical methods
Descriptive statistics were used to show the baseline characteristics of the study population. Continuous variables were presented as mean and standard deviations (SD), when normally distributed, or with median and interquartile range (IQR), when data had a skewed distribution. Dichotomous variables were presented as number (N) and percentage (%). Kappa Cohen coefficients were used to assess the concordance among the different frailty definitions. The differences of distribution of continuous and categorical variables among the different frailty categories were computed with the Kruskal-Wallis’s and the Chi-squared tests, respectively. The Chi-squared test was also used to assess the difference in mortality between frail and non-frail individuals. Cox regression models adjusted for age and sex were used to assess the association between frailty and mortality. Analyses were performed first considering FI as a continue variable and the FP as a 6-level ordinal variable (ranging from 0 to 5). Then we applied dichotomous categorical formats of frailty classifying individuals as frail according to a FI ≥ 0.25 and FP ≥ 3 and non-frail when they had FI < 0.25 and FP < 3.
All statistical analyses were performed with SPSS version 25.0 (SPSS Inc., Chicago, IL, United States).
3 Results
3.1 Participants and descriptive data
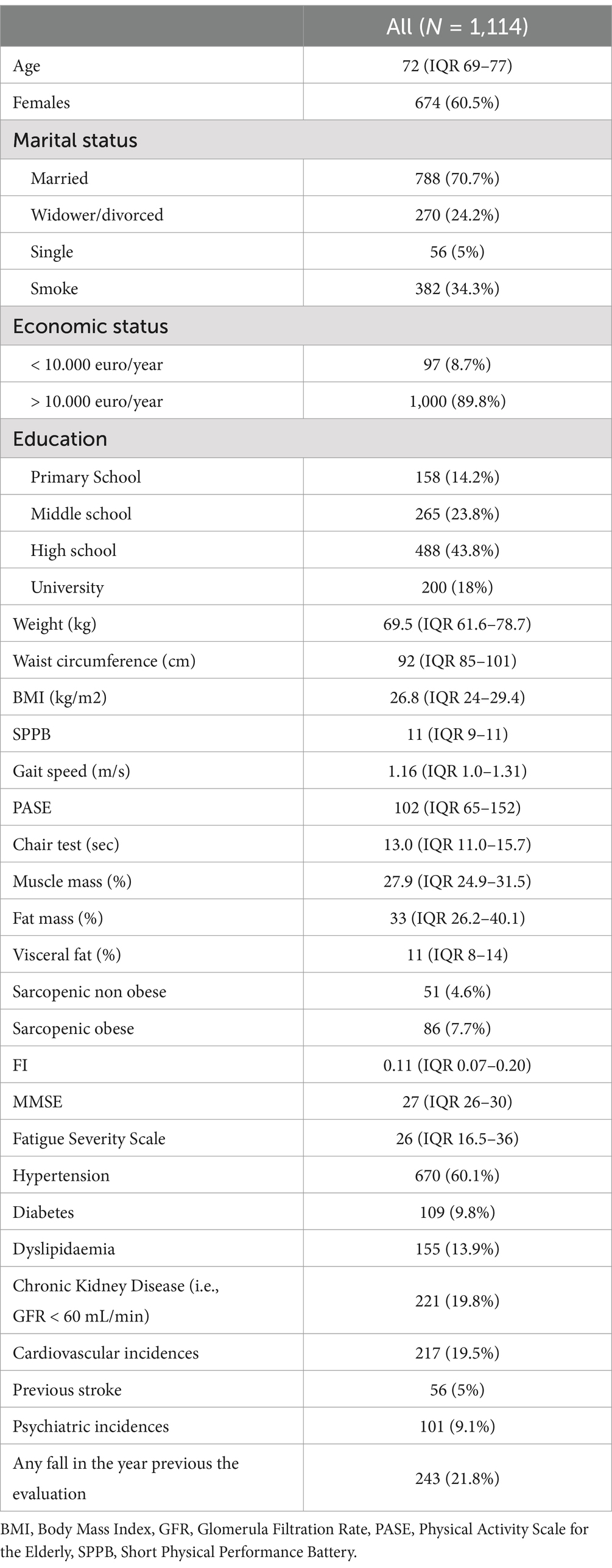
Out of the 1,250 participants enrolled in the FRASNET study, 1,114 were considered for this analysis. Exclusions comprised 19 institutionalized patients, 91 individuals with more than 20% missing data for the Frailty Index calculation, and 26 participants with missing body composition data (Figure 1). The study sample had a median age of 72 years (lowest age 65 years, highest age 93 years) and was composed for the 60.5% by females. Table 1 illustrates participant main characteristics and Supplementary Table S4 the main characteristics according to their frailty status.
3.2 Outcome data
The median FI score was 0.11 (IQR 0.07–0.20). According to the FI, 234 individuals (21%) were classified as frail, whereas 61 individuals (5.5%) were classified as frail according to the FP. Among these, 48 individuals met both frailty definitions, while 186 were considered frail only by the FI definition, and 13 were considered frail only by the FP definition (Figure 2). Individuals classified as frail according to both frailty definitions were mainly females (77.1%) and showed the lowest levels of education (45.8% primary school), income (19.6% < 10.000 euros/year), physical (median SPPB 7, median chair test 19.2 s) and cognitive performance (median MMSE 26). They also had the highest prevalence of fatigue (median FSS 48.5), sarcopenia (8.3%), sarcopenic obesity (41.7%), polypharmacy (54.2%), Emergency Department visits (34%), and falls (45.7%) in the year preceding the study evaluations (Table 1). Participants classified as frail according to the FI were older compared to robust people (median age 76 vs. 71, p < 0.001), with a higher percentage of females (69.9% vs. 57.7%, p < 0.001), sarcopenic obesity (41.7% vs. 5.5%, p < 0.001) polypharmacy (50% vs. 18%, p < 0.001), fatigue (median FSS 33 vs. 22, p < 0.001), falls (27.7% vs. 29.9%, p < 0.001) and emergency department accesses (28.8% vs. 19.1%, p < 0.001) in the year preceding the evaluation and a lower level of education (primary school 24.7% vs. 9.6%, p < 0.001) and physical activity (median PASE 71 vs. 112, p < 0.001) (Table 1). Also individuals classified as frail according to the FP were older compared to robust ones (median age 76 vs. 71 years, p < 0.001), with a lower level of education (primary school 53.8% vs. 9.6%, p < 0.001), physical activity (median PASE 39 vs. 112, p < 0.001) and physical performance (median SPPB 9 vs. 11, p < 0.001; median chair test 16.5 vs. 12.5, p < 0.001), a higher BMI (29 vs. 26.6, p < 0.001), fatigue (median FSS 39 vs. 22, p < 0.001) and prevalence of sarcopenia (30.8% vs. 3.9%, p < 0.001) and sarcopenic obesity (38.5 vs. 5.5%, p < 0.001), more falls (30.8% vs. 19.1%, p < 0.001) and ED accesses (30.8% vs. 19.9%, p < 0.001) in the year preceding the evaluations (Table 1). The concordance between the FI and FP (k = 0.26, p < 0.001) was only fair.

Figure 2. Overlap between the frailty index (FI) ≥ 0.25 and the frailty phenotype definitions in the FRASNET cohort. Dark gray circle = 13 individuals frail according to the frailty phenotype. Light gray circle = 48 individuals frail according to both definitions. White circle = 186 individuals frail according to the frailty index cut off ≥ 0.25.
3.3 Main results
Between the enrolment in 2017 and the 2023 follow-up, 39 individuals (3.5%) had died. People who died were older and frailer (median age 78, IQR 73–84; median FI 0.23, IQR 0.11–0.34) than survivals (median age 72, IQR 69–76; median FI 0.11, IQR 0.07–0.20). Mortality was 2.1% in robust individuals and 8.5% in frail individuals (p < 0.001). The age- and sex-adjusted Cox regression models confirmed that people who were frail had a significantly higher mortality compared to robust individuals (Figure 3). FI had a stronger association with mortality (HR 75.29, 95% C.I. 8.12–697.68, p < 0.001) compared to the FP (HR 1.89, 95% C.I. 1.40–2.55, p < 0.001) when these variables were considered as continuous and ordinal, respectively. Individuals who were frail according to both frailty definitions displayed the highest mortality risk (HR 5.26, 95% C.I. 1.94–14.22, p = 0.001) compared to individual classified frail according to the FI (HR 2.83, 95% C.I. 1.32–6.05, p = 0.007) and the FP (HR 4.71, 95% C.I. 1.05–21.13, p = 0.043) displayed the highest median frailty index score (Table 1).
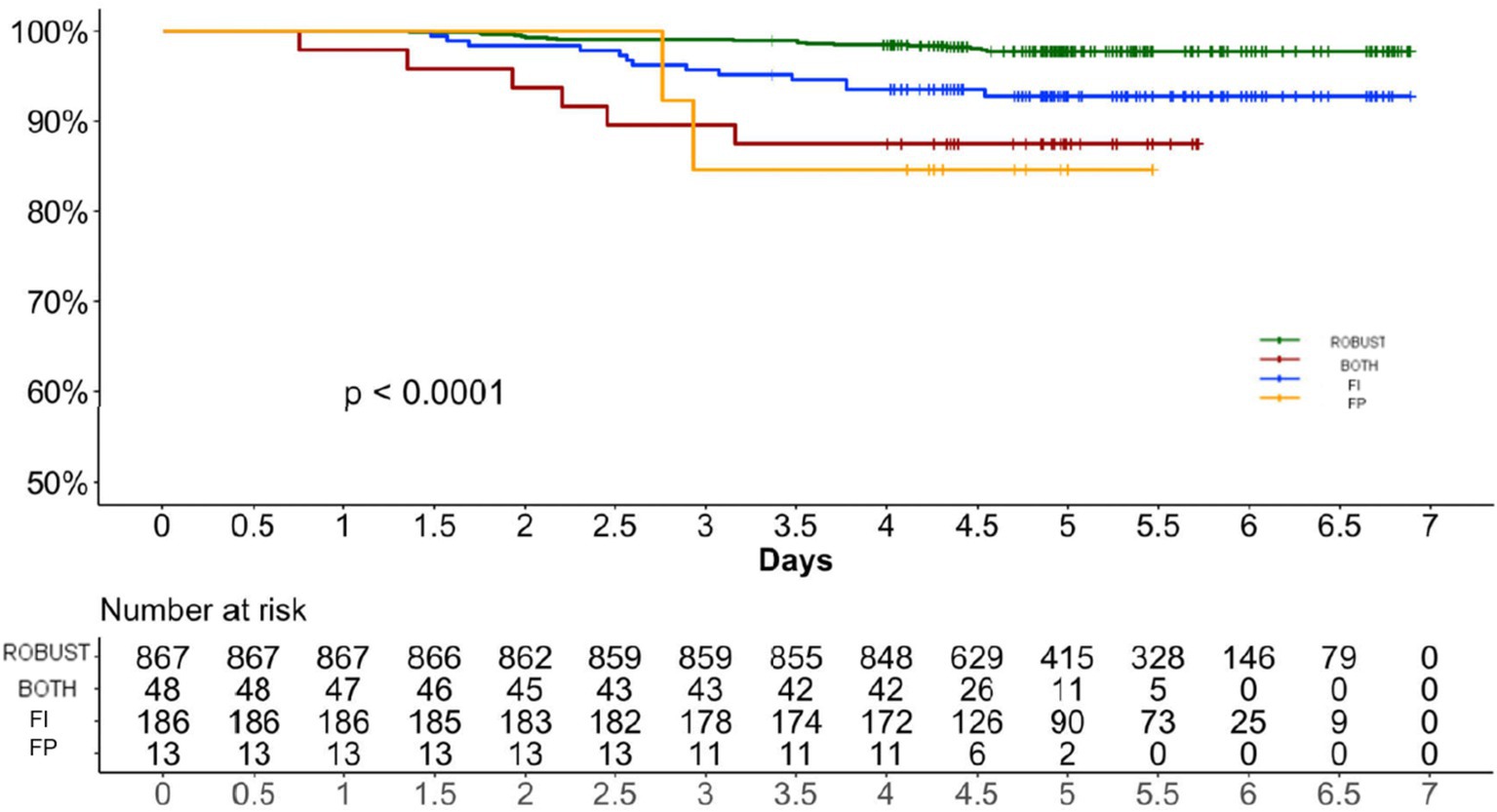
Figure 3. Age and sex-adjusted cox regressions showing the association between frailty and mortality. FI Frailty Index. FP Frailty Phenotype.
4 Discussion
4.1 Key results
In this observational study we found that the prevalence of frailty in a sample of geriatric Italian community dwelling volunteers was higher when computed with FI than with the FP. The concordance between FI and FP was only fair. Both FI and FP predicted mortality, but FI displayed a stronger association with mortality than the FP. People who were frail according to both definitions had the highest mortality, but had also the highest median FI score.
4.2 Interpretation
Our findings regarding the prevalence of frailty, with rates of 21% using the FI and 5.5% using the FP, align closely with those reported in a European population study (SHARE) which aimed at assessing the effects of health, social, economic and environmental policies over the life-course (frailty in the SHARE study: 21.6% with the FI and 11% with the FP) (26). Our data were also in line with the results of an American population (NHANES) study designed to assess the health and nutritional status United States citizens (frailty in NHANES 22% with the FI and 6% with the FP) (27). Finally, our detections on frailty in ambulatory patients closely align with those of a recent meta-analysis on frailty prevalence in community-dwelling older individuals worldwide (frailty prevalence in Europe: 22% with the FI and 8% with the FP) (28). The poor concordance (k = 0.26) between the FI and FP reflects the different nature of these instruments (29). It would be inappropriate to view the FP and the FI as interchangeable or equivalent tools. These two instruments serve different purposes and should be regarded as complementary. The FP is based on a predefined set of criteria that assess the presence or absence of specific signs or symptoms. Therefore, it can be utilized during the initial interaction with a subject and does not require prior clinical evaluation, making it useful as a screening tool for initial risk stratification across various profiles. Since the FP focuses on broad signs or symptoms, it primarily serves as a warning for potential issues. In contrast, the FI cannot be easily applied during the first contact with a patient, as it is derived from a comprehensive geriatric assessment. Anyway, once this assessment is complete, the FI provides valuable information for ongoing monitoring, and it is more sensitive to changes than the categorical frailty phenotype. Furthermore, because the FI is largely based on clinically classified conditions, it reflects a risk profile that may align more closely with the clinician’s assessment, potentially identifying vulnerabilities that differ from those indicated by the frailty phenotype (29).
Our finding on the poor concordance between the FP and FI was also previously highlighted in the NHANES study (k = 0.166) (27). The FP is a screening instrument that is easily applicable in clinical evaluations. However, it focuses solely on the physical dimension of frailty and may not capture the cognitive, social, and psychological aspects of frailty, nor the related comorbidity burden. This limitation could explain the weaker association of FP with mortality compared to the FI.
Frailty has been extensively associated with mortality (11, 30, 31). Prior systematic reviews indicated that the FI tool might be more effective than FP in predicting overall mortality (32–35) and that using continuous and ordinal formats instead of categorical ones in either tool improved their capacity to forecast overall mortality (13). Our findings are in line with the literature and in our work the FI considered as continuous variable displayed the strongest association with mortality.
Our study has the merit of having compared the risk of mortality in mutually exclusively categories of frailty (i.e., frail according only to the FI, frail according only to the FP and frail according to both definitions) in community dwelling individuals. Consistent with the findings of Hamiduzzaman et al. (8), we showed that individuals classified as frail according to both frailty definitions exhibited the highest mortality risk. However, key differences exist in the populations studied and our findings are novel. Hamiduzzaman’s research focused on American dialysis-dependent patients, comparing the Veterans Affairs Frailty Index (VAFI) with the FP. They found poor concordance between the two tools but noted that frailty, regardless of the measure, predicted higher mortality within this specific population. Notably, individuals classified as frail by both definitions showed the highest mortality risk compared to those deemed robust. However, their study did not directly compare mortality risk across mutually exclusive categories of frailty—those who were frail according to FI alone, FP alone, or both—something our study does. Moreover, our research centered on a relatively healthy, community-dwelling cohort, rather than dialysis patients. Additionally, our study provides novel insights into the interaction between sarcopenia, sarcopenic obesity, and frailty in relation to mortality, especially among individuals classified as frail by both FI and FP. This subgroup was the most compromised in the study, characterized by the highest FI scores, the lowest levels of education, income, physical and cognitive performance. They also had the highest prevalence of fatigue, polypharmacy, Emergency Department visits, and falls in the year preceding the study evaluations. It is important to underline that sarcopenia and sarcopenic obesity, which were highly prevalent in this subgroup (8.3 and 41.7%, respectively), may have contributed to the increased mortality. Both conditions have been associated with an elevated risk of mortality (36). It is interestingly to underline that in our sample mortality was particularly low (only 3.5%), even considering the intercurrent COVID-19 pandemics, when all cause excess deaths reached nearly the 60% in the North regions of Italy (37). Indeed, the study population was constituted mainly by robust and active individuals and could be seen as a model of healthy ageing.
To sum up, our study confirmed that the FI is a superior tool for predicting mortality in community dwelling older adults when compared to the FP. This finding is consistent with previous studies that have shown the FI’s greater predictive power for mortality due to its ability to capture a wider range of health deficits, including physical, cognitive, and social factors, as well as comorbidities (33, 34) which can contribute to mortality risk.
4.3 Limitations
Anyway, there are some limitations that should be considered in our study. We assessed two out of numerous indices currently used to evaluate frailty the FP and FI selected based on the strong predictive capabilities demonstrated in other studies (38, 39). Inclusion of other frailty scales would have given a more comprehensive evaluation of the association between frailty and mortality. Additionally, while we analyzed a substantial prospective cohort of community-dwelling older adults, our study was regionally focused, and the generalizability of our findings should be validated through future multicenter studies that include populations beyond just ambulatory patients.
Self-reported data for psychosocial variables and physical activity may be prone to recall bias or social desirability bias, potentially affecting the accuracy of some assessments.
4.4 Generalisability
Increasing awareness of the prevalence of frailty and its associated mortality risk is a crucial first step in promoting the adoption of preventive and therapeutic measures to mitigate the negative consequences of frailty.
5 Conclusion
Frailty is a common geriatric condition found in up to one-fifth of Italian community dwelling older adults. Frailty is closely linked to mortality. Various definitions of frailty identify different individuals as frail. The FI, due to its comprehensive nature showed a stronger association with mortality. Individuals classified as frail by both frailty definitions were the most compromised, displayed the highest FI score and the faced the highest mortality risk.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ospedale San Raffaele Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SD: Methodology, Writing – original draft, Writing – review & editing, Conceptualization, Formal analysis. RL: Formal analysis, Writing – original draft, Writing – review & editing. LC: Investigation, Writing – original draft, Writing – review & editing. LZ: Investigation, Writing – original draft, Writing – review & editing. EB: Investigation, Writing – original draft, Writing – review & editing. CM: Investigation, Writing – original draft, Writing – review & editing. MS: Investigation, Writing – original draft, Writing – review & editing. MR: Investigation, Writing – original draft, Writing – review & editing. SS: Investigation, Writing – original draft, Writing – review & editing. ES: Investigation, Writing – original draft, Writing – review & editing. MM: Investigation, Writing – original draft, Writing – review & editing. FF: Investigation, Writing – original draft, Writing – review & editing. CF: Investigation, Writing – original draft, Writing – review & editing. GV: Conceptualization, Data curation, Supervision, Visualization, Writing – original draft, Writing – review & editing. PM: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. AM: Data curation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. CL: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. PR-Q: Conceptualization, Data curation, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Fondazione CARIPLO, Milano grant to P.M. [grant number 2016-0980] and P8–Project Age-It: “Ageing Well in an Ageing Society.” This resource was co-financed by the Next Generation EU [DM 1557 11.10.2022].
Acknowledgments
We acknowledge co-funding from Next Generation EU, in the context of the National Recovery and Resilience Plan, Investment PE8 – Project Age-It: “Ageing Well in an Ageing Society.” This resource was co-financed by the Next Generation EU [DM 1557 11.10.2022]. We also acknowledge the nurses of our outpatient clinic Barbara Banti and Rosalba Scrimieri.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views and opinions expressed are only those of the authors and do not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible for them.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1465066/full#supplementary-material
References
1. United Nations International Day of Older Persons. Available at: https://www.un.org/en/observances/older-persons-day (Accessed May 28, 2024).
2. Rodríguez-Mañas, L, Féart, C, Mann, G, Viña, J, Chatterji, S, Chodzko-Zajko, W, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement. The frailty operative definition-consensus conference project. J Gerontol. (2013) 68:62–7. doi: 10.1093/gerona/gls119
3. Collard, RM, Boter, H, Schoevers, RA, and Oude Voshaar, RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. (2012) 60:1487–92. doi: 10.1111/J.1532-5415.2012.04054.X
4. Fried, LP, Tangen, CM, Walston, J, Newman, AB, Hirsch, C, Gottdiener, J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56:M146–57. doi: 10.1093/gerona/56.3.M146
5. Rockwood, K, and Mitnitski, A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. (2007) 62:722–7. doi: 10.1093/GERONA/62.7.722
6. Ferrucci, L, Guralnik, JM, Studenski, S, Fried, LP, Cutler, GB, and Walston, JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. (2004) 52:625–34. doi: 10.1111/J.1532-5415.2004.52174.X
7. Clegg, A, Young, J, Iliffe, S, Rikkert, MO, and Rockwood, K. Frailty in elderly people. Lancet. (2013) 381:752–62. doi: 10.1016/S0140-6736(12)62167-9
8. Hamiduzzaman, A, Wu, R, Murray, V, Kalantar-Zadeh, K, Streja, E, and Sy, J. Comparing the Fried frailty phenotype versus the veterans affairs frailty index among dialysis dependent patients. Hemodial Int. (2023) 27:444–53. doi: 10.1111/hdi.13101
9. Xue, QL, JingTian, WJD, Chaves, PHM, Newman, AB, and Bandeen-Roche, K. Discrepancy in frailty identification: move beyond predictive validity. J Gerontol. (2020) 75:387–93. doi: 10.1093/gerona/glz052
10. Qin, F, Guo, Y, Ruan, Y, Huang, Z, Sun, S, Gao, S, et al. Frailty and risk of adverse outcomes among community-dwelling older adults in China: a comparison of four different frailty scales. Front. Public Health. (2023) 11:809. doi: 10.3389/fpubh.2023.1154809
11. Shi, GP, Ma, T, Zhu, YS, Wang, ZD, Chu, XF, Wang, Y, et al. Frailty phenotype, frailty index and risk of mortality in Chinese elderly population- Rugao longevity and ageing study. Arch Gerontol Geriatr. (2019) 80:115–9. doi: 10.1016/J.ARCHGER.2018.11.001
12. Oviedo-Briones, M, Rodríguez-Laso, Á, Carnicero, JA, Gryglewska, B, Sinclair, AJ, Landi, F, et al. The ability of eight frailty instruments to identify adverse outcomes across different settings: the FRAILTOOLS project. J Cachexia Sarcopenia Muscle. (2022) 13:1487–501. doi: 10.1002/jcsm.12990
13. Kim, DJ, Massa, MS, Potter, CM, Clarke, R, and Bennett, DA. Systematic review of the utility of the frailty index and frailty phenotype to predict all-cause mortality in older people. Syst Rev. (2022) 11:187. doi: 10.1186/S13643-022-02052-W
14. Delli Zotti, GB, Citterio, L, Farinone, S, Concas, MP, Brioni, E, Zagato, L, et al. Association between perceived health-related quality of life and depression with frailty in the FRASNET study. Int J Environ Res Public Health. (2022) 19:776. doi: 10.3390/IJERPH192416776
15. Folstein, MF, Folstein, SE, and McHugh, PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
16. Burke, WJ, Roccaforte, WH, and Wengel, SP. The short form of the geriatric depression scale: a comparison with the 30-item form. J Geriatr Psychiatry Neurol. (1991) 4:173–8. doi: 10.1177/089198879100400310
17. Krupp, LB, Larocca, NG, Muir Nash, J, and Steinberg, AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. (1989) 46:1121–3. doi: 10.1001/ARCHNEUR.1989.00520460115022
18. Apolone, G, and Mosconi, P. The Italian SF-36 health survey: translation, validation and norming. J Clin Epidemiol. (1998) 51:1025–36. doi: 10.1016/S0895-4356(98)00094-8
19. Washburn, RA, Smith, KW, Jette, AM, and Janney, CA. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. (1993) 46:153–62. doi: 10.1016/0895-4356(93)90053-4
20. Guralnik, JM, Ferrucci, L, Simonsick, EM, Salive, ME, and Wallace, RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. (1995) 332:556–62. doi: 10.1056/NEJM199503023320902
21. Cesari, M, Kritchevsky, SB, Newman, AB, Simonsick, EM, Harris, TB, Penninx, BW, et al. Added value of physical performance measures in predicting adverse health-related events: results from the health, aging and body composition study. J Am Geriatr Soc. (2009) 57:251–9. doi: 10.1111/J.1532-5415.2008.02126.X
22. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/AGEING/AFY169
23. Donini, LM, Busetto, L, Bischoff, SC, Cederholm, T, Ballesteros-Pomar, MD, Batsis, JA, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin Nutr. (2022) 41:990–1000. doi: 10.1016/j.clnu.2021.11.014
24. Wilhelm-Leen, ER, Hall, YN, Tamura, MK, and Chertow, GM. Frailty and chronic kidney disease: the third National Health and nutrition evaluation survey. Am J Med. (2009) 122:664–671.e2. doi: 10.1016/J.AMJMED.2009.01.026
25. Theou, O, Haviva, C, Wallace, L, Searle, SD, and Rockwood, K. How to construct a frailty index from an existing dataset in 10 steps. Age Ageing. (2023) 52:221. doi: 10.1093/AGEING/AFAD221
26. Theou, O, Brothers, TD, Mitnitski, A, and Rockwood, K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. (2013) 61:1537–51. doi: 10.1111/JGS.12420
27. Blodgett, J, Theou, O, Kirkland, S, Andreou, P, and Rockwood, K. Frailty in NHANES: comparing the frailty index and phenotype. Arch Gerontol Geriatr. (2015) 60:464–70. doi: 10.1016/J.ARCHGER.2015.01.016
28. O’Caoimh, R, Sezgin, D, O’Donovan, MR, William Molloy, D, Clegg, A, Rockwood, K, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. (2021) 50:96–104. doi: 10.1093/AGEING/AFAA219
29. Cesari, M, Gambassi, G, van Kan, GA, and Vellas, B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. (2014) 43:10–2. doi: 10.1093/ageing/aft160
30. Ekram, ARMS, Woods, RL, Britt, C, Espinoza, S, Ernst, ME, and Ryan, J. The association between frailty and all-cause mortality in community-dwelling older individuals: an umbrella review. J Frailty Aging. (2021) 10:320–6. doi: 10.14283/JFA.2021.20
31. Kojima, G, Iliffe, S, and Walters, K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. (2018) 47:193–200. doi: 10.1093/AGEING/AFX162
32. de Vries, NM, Staal, JB, van Ravensberg, CD, Hobbelen, JSM, Olde Rikkert, MGM, and Nijhuis-van der Sanden, MWG. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. (2011) 10:104–14. doi: 10.1016/J.ARR.2010.09.001
33. Bouillon, K, Kivimaki, M, Hamer, M, Sabia, S, Fransson, EI, Singh-Manoux, A, et al. Measures of frailty in population-based studies: an overview. BMC Geriatr. (2013) 13:64. doi: 10.1186/1471-2318-13-64
34. Dent, E, Kowal, P, and Hoogendijk, EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. (2016) 31:3–10. doi: 10.1016/J.EJIM.2016.03.007
35. Sutton, JL, Gould, RL, Daley, S, Coulson, MC, Ward, EV, Butler, AM, et al. Psychometric properties of multicomponent tools designed to assess frailty in older adults: a systematic review. BMC Geriatr. (2016) 16:55. doi: 10.1186/S12877-016-0225-2
36. Benz, E, Pinel, A, Guillet, C, Capel, F, Pereira, B, De Antonio, M, et al. Sarcopenia and Sarcopenic obesity and mortality among older people. JAMA Netw Open. (2024) 7:E243604. doi: 10.1001/JAMANETWORKOPEN.2024.3604
37. Dorrucci, M, Minelli, G, Boros, S, Manno, V, Prati, S, Battaglini, M, et al. Excess mortality in Italy during the COVID-19 pandemic: assessing the differences between the first and the second wave. Front Public Health. (2020) 9:669209. doi: 10.3389/FPUBH.2021.669209
38. Hoogendijk, EO, Afilalo, J, Ensrud, KE, Kowal, P, Onder, G, and Fried, LP. Frailty: implications for clinical practice and public health. Lancet. (2019) 394:1365–75. doi: 10.1016/S0140-6736(19)31786-6
Keywords: frailty index, frailty phenotype, mortality, community dwelling older people, older people
Citation: Damanti S, De Lorenzo R, Citterio L, Zagato L, Brioni E, Magnaghi C, Simonini M, Ruggiero MP, Santoro S, Senini E, Messina M, Farina F, Festorazzi C, Vitali G, Manunta P, Manfredi AA, Lanzani C and Rovere-Querini P (2025) Frailty index, frailty phenotype and 6-year mortality trends in the FRASNET cohort. Front. Med. 11:1465066. doi: 10.3389/fmed.2024.1465066
Edited by:
Alessandra Coin, Unità di Geriatria, Azienda Ospedaliera Universitaria di Padova, ItalyReviewed by:
Maria Teresa Caetano Tomás, Escola Superior de Tecnologia da Saúde de Lisboa (ESTeSL), PortugalKornanong Yuenyongchaiwat, Thammasat University, Thailand
Copyright © 2025 Damanti, De Lorenzo, Citterio, Zagato, Brioni, Magnaghi, Simonini, Ruggiero, Santoro, Senini, Messina, Farina, Festorazzi, Vitali, Manunta, Manfredi, Lanzani and Rovere-Querini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah Damanti, ZGFtYW50aS5zYXJhaEBoc3IuaXQ=
 Sarah Damanti
Sarah Damanti Rebecca De Lorenzo
Rebecca De Lorenzo Lorena Citterio
Lorena Citterio Laura Zagato
Laura Zagato Elena Brioni
Elena Brioni Cristiano Magnaghi
Cristiano Magnaghi Marco Simonini3
Marco Simonini3 Simona Santoro
Simona Santoro Marco Messina
Marco Messina Francesca Farina
Francesca Farina Costanza Festorazzi
Costanza Festorazzi Paolo Manunta
Paolo Manunta Angelo Andrea Manfredi
Angelo Andrea Manfredi Chiara Lanzani
Chiara Lanzani Patrizia Rovere-Querini
Patrizia Rovere-Querini