- 1Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
- 2Nanjing Hospital of Chinese Medicine Affiliated to Nanjing University of Chinese Medicine, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
- 3College of Rehabilitation Medicine, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
Background: Meniere’s disease (MD) is an idiopathic chronic inner ear disease that seriously impacts patients’ physical and mental health. Medications may be effective for a proportion of patients, and additional effective treatments are still needed. This review aimed to evaluate the efficacy of acupuncture treatment for MD.
Methods: Eight databases were systematically searched from their inception to June 1, 2024, to identify randomized clinical trials on acupuncture treatment for MD. The Cochrane Risk of Bias 2.0 tool was used to assess the risk of bias in included studies, and meta-analysis was conducted by RevMan 5.4 and Stata 16.0 software.
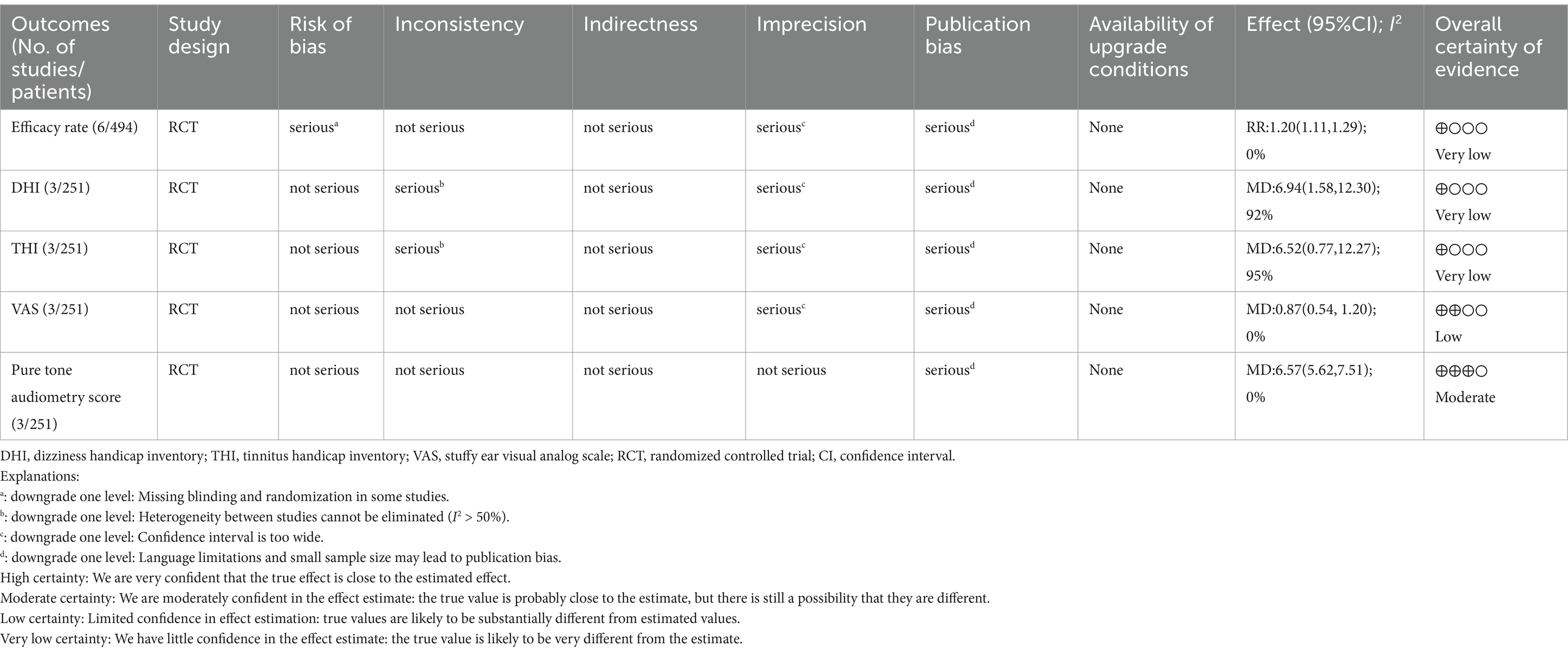
Results: Six studies were included in this review. The treatment group received acupuncture or acupuncture combined with Western medicine, while the control group was treated with Western medicine. The treatment group was superior to the control group in terms of efficacy rate (RR: 1.20; 95% CI: 1.11–1.29; p < 0.0001). The treatment group reduced dizziness handicap inventory (DHI) (MD: 6.94; 95% CI: 1.58–12.30; p = 0.01), tinnitus handicap inventory (THI) (MD: 6.52; 95% CI: 0.77–12.27; p = 0.03), stuffy ear visual analog scale (VAS) (MD: 0.87; 95% CI: 0.54–1.20; p < 0.0001) and pure tone audiometry score (MD: 6.57; 95% CI: 5.62–7.51; p < 0.0001) to a greater degree than those of the control group. There were some methodological shortcomings in the included studies, including failure to implement blinding, inappropriate outcome measures, and heterogeneity of clinical interventions, such as selected acupoints, acupuncture sessions, and therapist techniques.
Conclusion: Acupuncture may improve the symptoms of vertigo, tinnitus, ear fullness and hearing loss in patients with MD. However, due to the lack of literature included in this study and methodological weaknesses like randomization, blinding, and clinical heterogeneity, more well-designed long-term follow-up RCTs are needed to evaluate the efficacy and safety of acupuncture.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/(CRD42024549261).
1 Introduction
Meniere’s disease (MD) is a chronic inner ear disorder characterized by the abnormal accumulation of endolymph within the labyrinth. Clinically, MD manifests as recurrent episodes of vertigo, fluctuating hearing loss, tinnitus, and a sensation of fullness in the affected ear (1). Literature reports show that the prevalence of MD varies widely, ranging from 17 to 513 per 100,000 population, with an increasing trend in Meniere’s disease incidence in the last 10 years (2). It is estimated that approximately 50% of patients diagnosed with MD also experience comorbid depression (3). Additionally, a study found that between 78 and 89% of people with MD experience constraints in their everyday activities, employment, and travel, which significantly affects their general quality of life (4).
The exact cause of MD is still unknown. Unbalances in endolymphatic homeostasis may arise from the dysregulation of hormone secretion, including that of aquaporins, ion channels, adrenaline, and anti-diuretic hormone, according to recent study on etiological causes and pathological processes (5). To date, there is still no gold standard for treatment guidelines for MD. Currently, the main treatments for MD include dietary therapy, betahistine, diuretics, transtympanic corticosteroid injection, transtympanic gentamicin, tympanic low-pressure pulsation therapy, surgery, and vestibular rehabilitation (6). It should be noted that not all patients respond positively to the drugs. Despite taking these drugs, some people may not acquire adequate control, and other medications, like betahistine, can have negative side effects, such as gastrointestinal responses and abnormalities of the neurological system (mostly headaches) (7, 8). Endolymphatic sac decompression is one of the most frequently utilized surgical techniques for the management of vertigo. However, due to the inherent limitations of this approach and the findings of the Cochrane Systematic Review, which suggested that endolymphatic sac surgery may not be a highly efficacious intervention for MD, there is a necessity to reassess its role in clinical practice (9). There is a necessity for the development of more effective and safer complementary and alternative therapies for the treatment of MD. Complementary and alternative therapies refer to a wide range of non-conventional medical practices used in conjunction with, or instead of, conventional medicine, such as western medicine and surgery (10, 11). These practices may include acupuncture, herbal medicine, dietary supplements, and other non-traditional methods.
Acupuncture, as an important component of complementary and alternative therapies, has been widely used in the clinical treatment of MD (12, 13). A combination of scalp clustering acupuncture and ear acupressure has been demonstrated to alleviate the most significant symptoms associated with MD to a certain extent, including vertigo and tinnitus (14). Acupuncture of local points may provide relief of vertigo symptoms by increasing the blood supply to the vertebrobasilar system and promoting blood circulation to the brain, or by improving vestibular function and promoting the recovery of inner ear function (15). Therefore, we conducted this systematic review of randomized controlled trials (RCTs) to further assess the effect of acupuncture for MD.
2 Materials and methods
2.1 Registration
The protocol for this systematic review and meta-analysis was registered in PROSPERO (CRD42024549261) and was in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (16).
2.2 Study design
We only included randomized controlled trials (RCTs). We did not place any restrictions on language.
2.3 Research subjects
The patients met the diagnostic criteria for Meniere’s disease in accordance with the European Academy of Otology and Neurotology, and American Academy of Otolaryngology-head and neck surgery guidelines (17). We do not have any restrictions on age or gender.
2.4 Interventions
The treatment group was treated with acupuncture-related therapy including manual acupuncture, electroacupuncture, auricular point sticking, warm needling, auricular acupuncture, plum blossom needle, bloodletting therapy, or acupuncture combined with Western medicine. The control group received Western medicine such as betahistine and mecobalamin.
2.5 Outcome indicators
The primary outcome indicator was the efficacy rate. The secondary outcome indicators were the dizziness handicap inventory (DHI), tinnitus handicap inventory (THI), stuffy ear visual analog scale (VAS), and pure tone audiometry score.
2.6 Exclusion criteria
We excluded studies based on the following characteristics: (1) studies with other Chinese medicine treatments in any group, such as Chinese herbal medicine, Chinese Patent Medicine, and massage; (2) studies with obvious errors in the literature data, research methodology, design methodology, statistical methodology or the data records are not well documented; (3) lack of outcome indicators; and (4) duplicate studies.
2.7 Literature search strategy
We searched eight databases from their inception to June 1, 2024. Two researchers (TMJ and LYH) independently searched PubMed, EMBASE, Web of Science, Cochrane Library, Chinese National Knowledge Infrastructure (CNKI), Wanfang Database, Chinese Science and Technique Journals Database (VIP) and SinoMed. The search terms were “Meniere’s disease” “Meniere’s Syndrome” “Meniere disease” “acupuncture therapy” “acupuncture” “acupoint” “electroacupuncture” “randomized controlled trial” and related terms. Detailed search strategies are shown in Supplementary material 1.
2.8 Literature screening and data extraction
The literature was imported into Endnote X9 software from various databases. After automatic weight removal, two researchers (TMJ and LYH) conducted preliminary screening by reading all the titles and abstracts. Subsequently, the researchers carefully read the full text of the screened literature and selected the qualified literature based on the inclusion criteria. During the full-text screening process, relevant information will be extracted, the researchers extracted information and entered it into the “information extraction form.” This form included authors, year of publication, sample size, demographic baseline, intervention, duration of treatment, outcomes, and adverse events. Any uncertainties or disputes were resolved through discussion or consultation with a third researcher (LM).
We extracted the treatment details given the Revised Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) (18). The STRICTA checklist contains six main items: (1) Acupuncture rationale, (2) Details of acupuncture, (3) Treatment plan, (4) Other interventions, (5) Therapist’s background, and (6) Control or comparator intervention.
2.9 Risk of bias assessment
The methodological quality of the included studies was independently assessed by two researchers (GYH and ZTC) using the Cochrane Risk of Bias Tool 2.0 (RoB 2.0) (19). It contains six aspects: generation of randomization sequences, deviations from the intended interventions, missing outcome data, measurement of the outcome, selective reporting of outcomes, and overall bias. The assessment was classified into three groups: “low,” “high,” and “some concerns.” Any disagreements or disputes were resolved through discussion with a third researcher (LM).
2.10 Statistical analysis
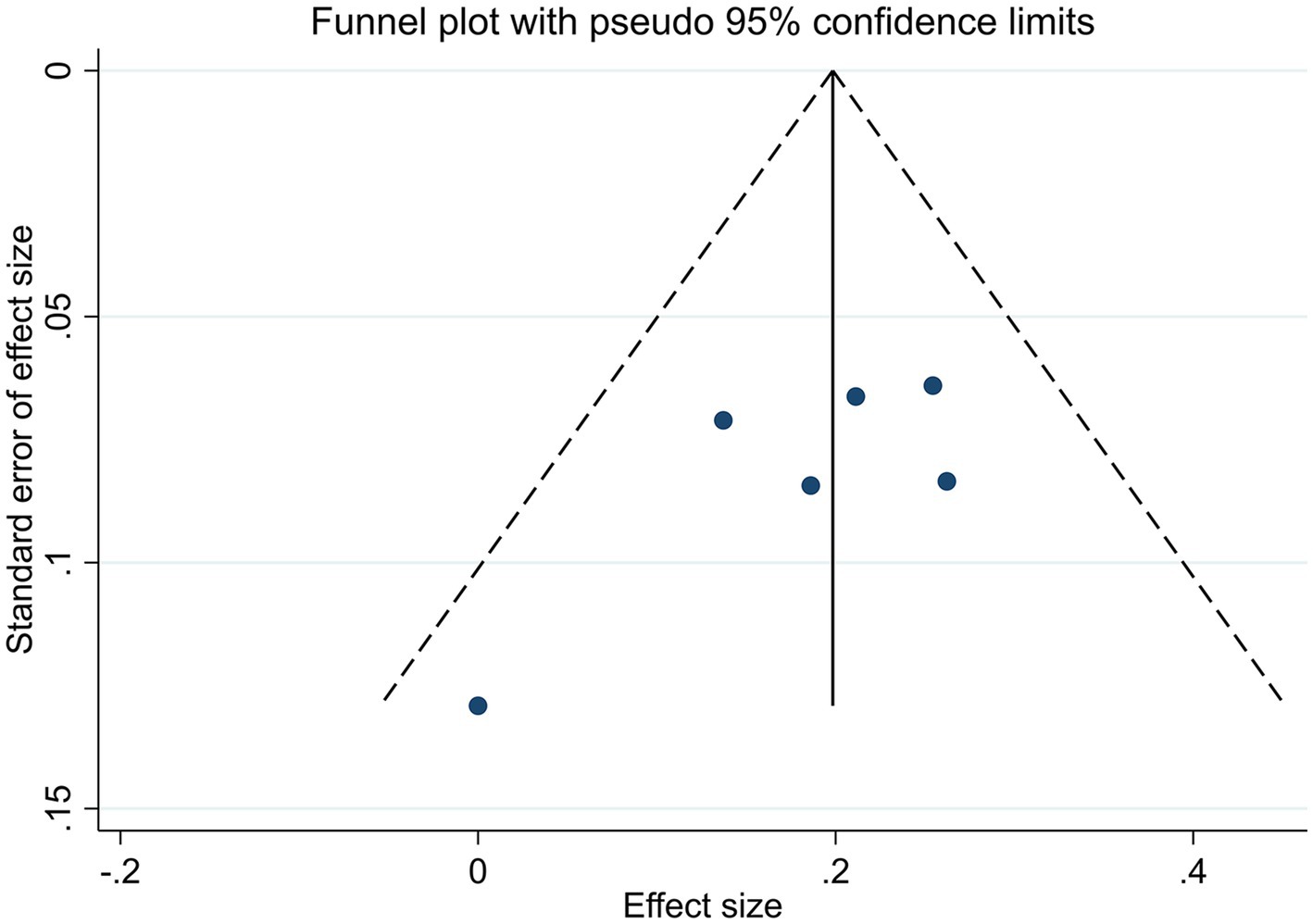
We used Review Manager 5.4 and Stata 16.0 software for statistical analysis. If I2 ≤ 50%, and p ≥ 0.1, it indicates low heterogeneity between studies. If I2>50%, and p < 0.1, it indicates substantial heterogeneity between studies, which may be due to clinical heterogeneity or subgroup analysis. Otherwise, the source of heterogeneity was further analyzed. For continuous variables, the mean difference (MD) was employed as the effect indicator when described by the same scale, whereas the standardized mean difference (SMD) was utilized as the effect indicator when described by different scales. For dichotomous variables, we used the risk ratio (RR). Efficacy rate was calculated as RR, which measures the treatment effect by calculating the ratio of event rates between the treatment group and the control group. We calculated the confidence intervals (CI) at 95%. Funnel plots and Egger’s test were employed to assess the potential for publication bias. The asymmetric funnel plot or a probability value (p < 0.05) of Egger’s test indicated that there was significant publication bias.
2.11 Quality of evidence
The quality of evidence was evaluated using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system (20) for each outcome. It contains five aspects: risk of bias, imprecision, indirectness, inconsistency, and publication bias.
3 Results
3.1 Literature retrieval results
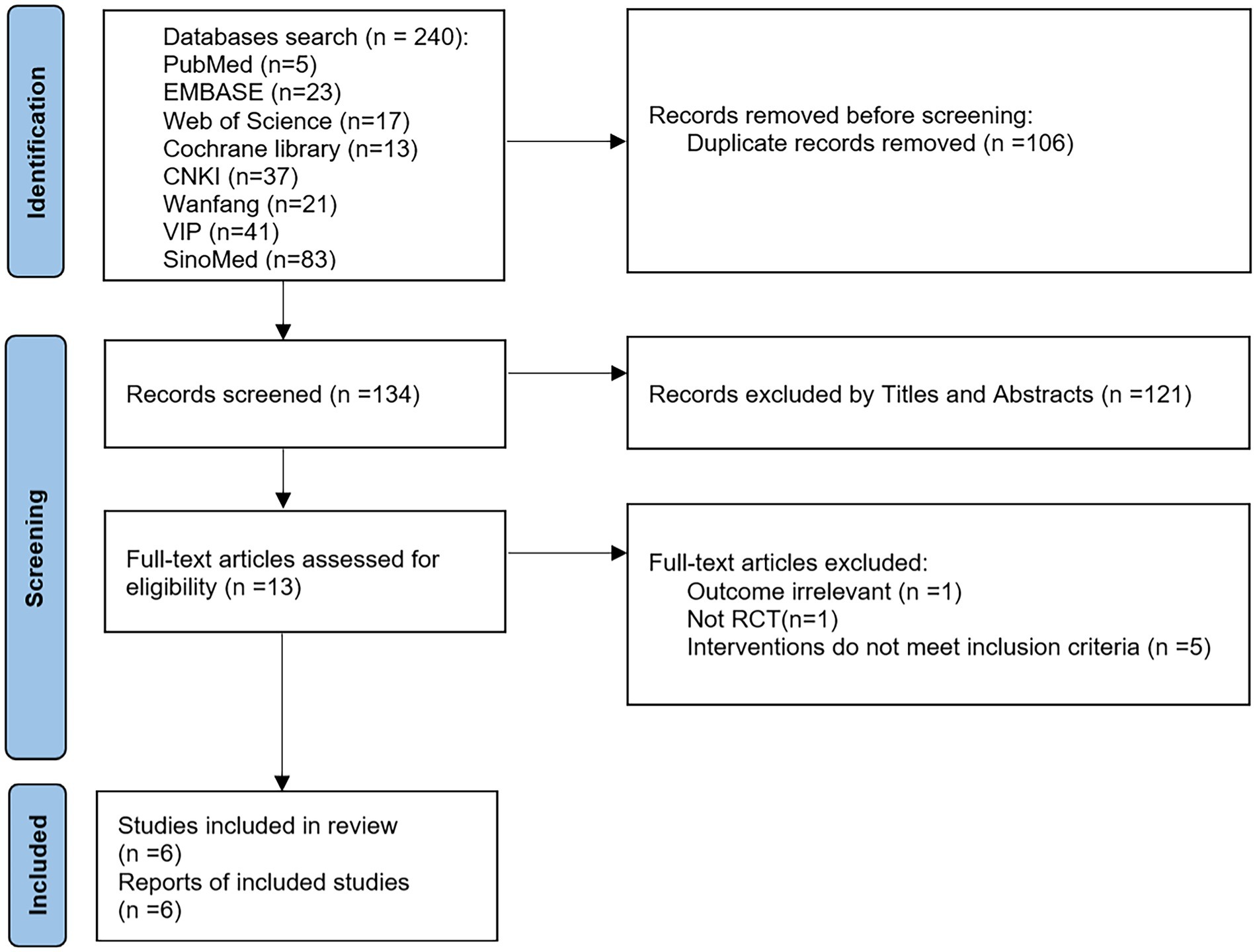
A total of 240 articles were retrieved using the search strategy and then 106 duplicates were excluded. Then 121 articles were excluded by reading the title/abstract and 7 articles by reading the full text (1 with irrelevant outcomes, 1 non-RCT, and 5 with discrepancies in interventions). Finally, 6 studies (21–26) were included. The screening process is shown in Figure 1.
3.2 Characteristics of the literature
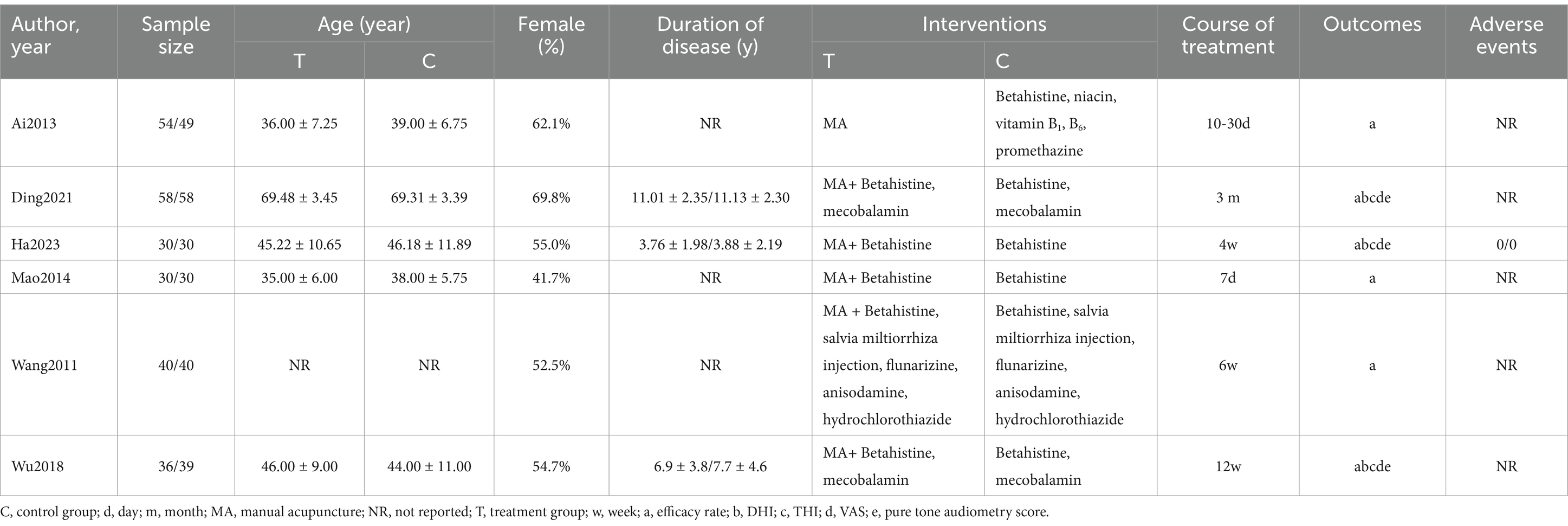
The total sample size of patients included in the 6 studies (21–26) was 494 cases, with 248 in the treatment group and 246 in the control group. All six studies (21–26) were conducted in China and published in Chinese. Six studies (21–26) reported the efficacy rate, and two (23, 26) of them compared and analyzed the efficacy of the two groups in terms of dizziness, hearing, and mobility, and we took the average of these three aspects to calculate the total efficacy rate. Three studies (22, 23, 26) reported DHI, THI, VAS, and pure tone audiometry score. A total of five RCTs (22–26) employed MA in conjunction with Western medicine, in comparison to Western medicine alone. Conversely, only one RCT (21) compared MA with Western medicine. The characteristics of each included study in Table 1. The completeness of reporting based on STRICTA guidelines and therapeutic acupoints for each study are shown in Supplementary materials 2, 3. Details of needling are presented in Supplementary material 4.
3.3 Risk of bias
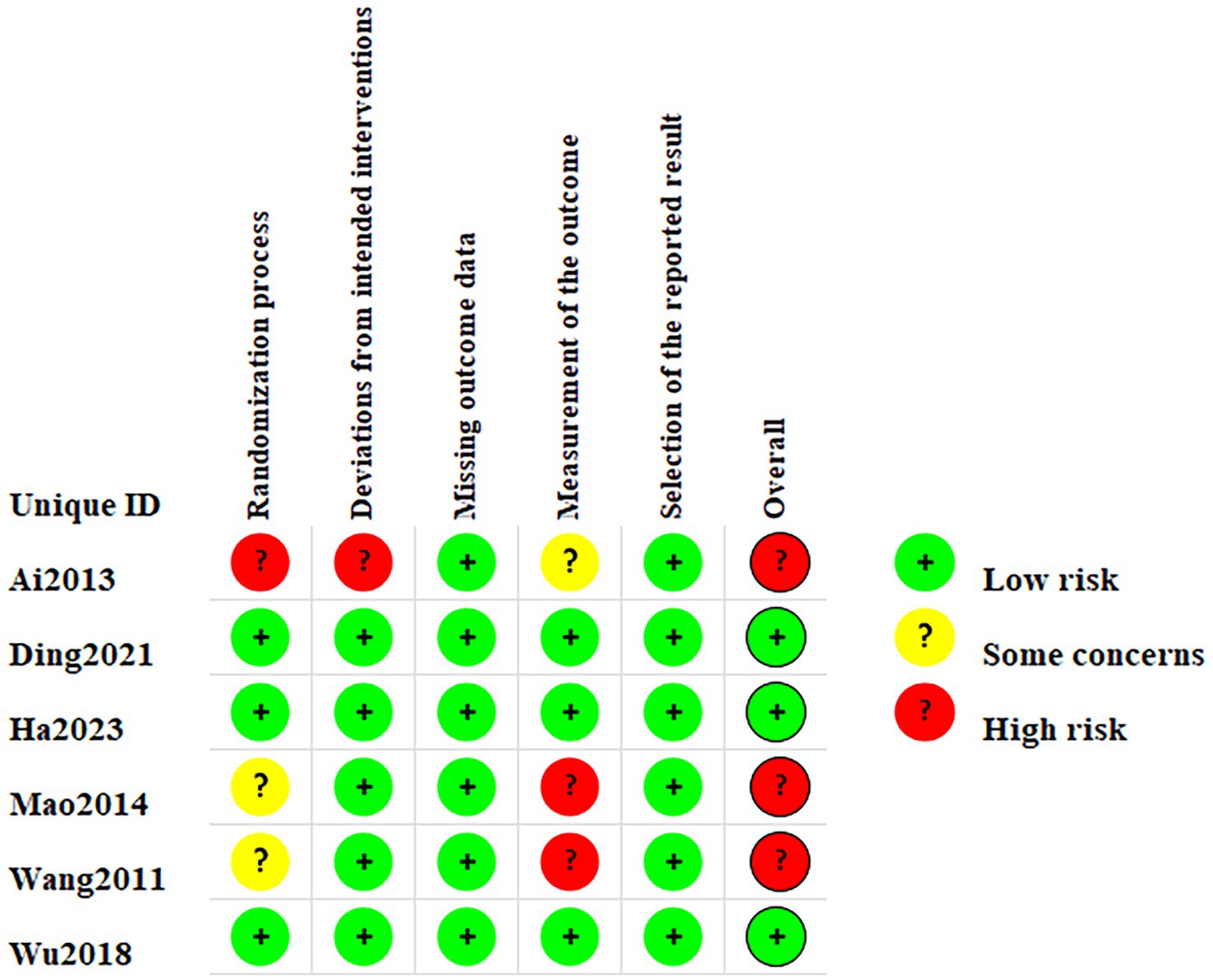
Two RCTs (22, 26) used the random number table method and one RCT (23) used the single-blind method thus leading to low risk during randomization. The remaining two RCTs (24, 25) did not provide any further information regarding the randomization methods employed leading to unclear risk of bias, and one RCT (21) without randomization grouping led to high risk of bias. One RCT (21) may have deviations from established intervention leading to high risk of bias. Two RCTs (24, 25) may have inappropriate outcome measures and were therefore classified as high risk of bias. Measurements from the RCT (21) may receive influences from the person who measured them and be classified as unclear risk of bias. Figure 2 summarizes the risk of bias in the 6 articles.
3.4 Certainty of evidence (GRADE)
Results of the GRADE evidence quality rating from very low to moderate are shown in Table 2.
3.5 Main findings
3.5.1 Efficacy rate
Six studies (21–26) compared the efficacy rate between groups. There was no significant heterogeneity among the studies in the efficacy rate (p = 0.60, I2 = 0%), so the fixed-effect model was used for the meta-analysis. The results showed that the treatment group was superior to the control group in terms of efficacy rate (RR: 1.20; 95% CI: 1.11–1.29; p < 0.0001) (Figure 3A).
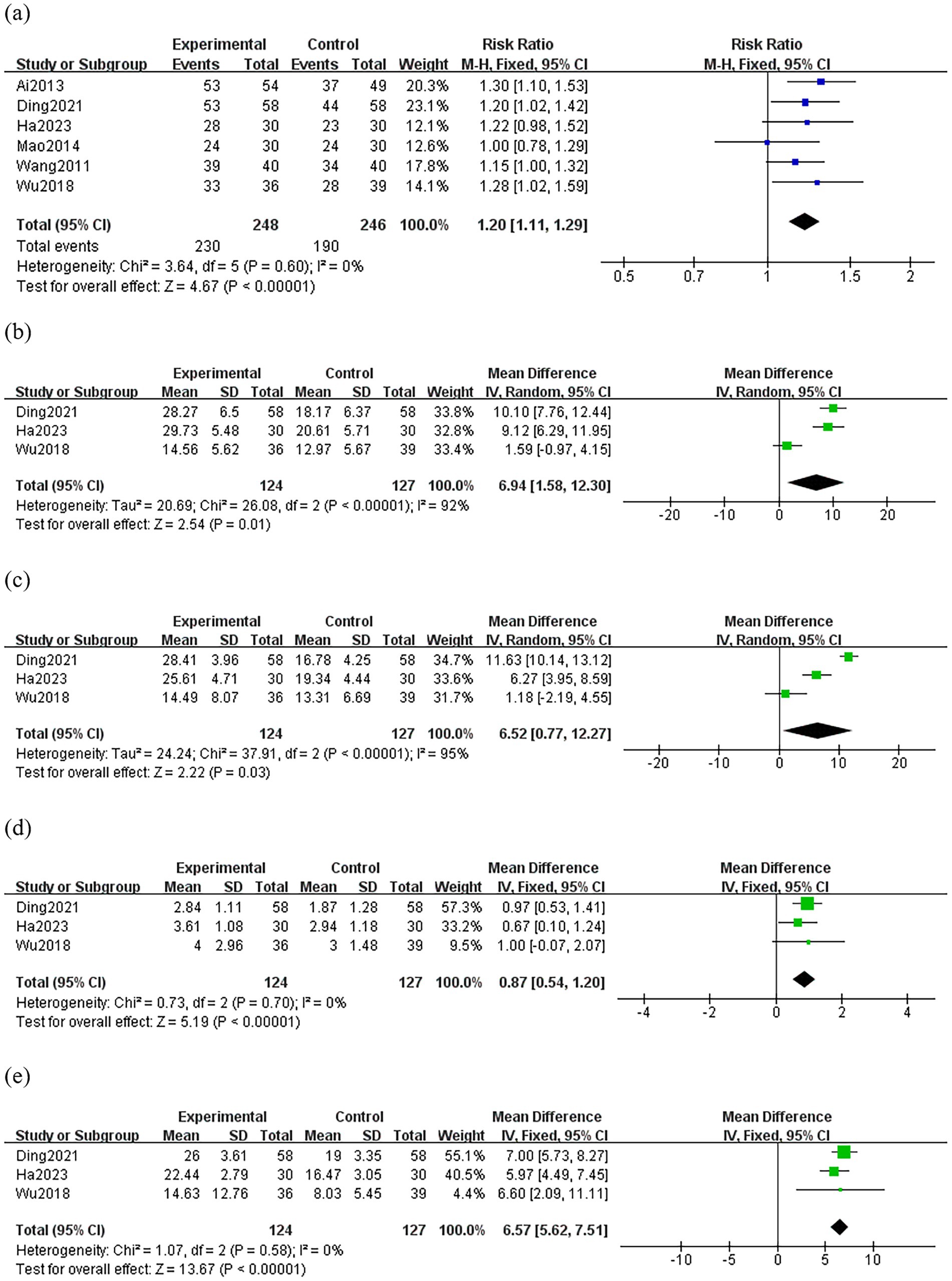
Figure 3. Forest plot comparing the treatment efficacy between the treatment group and the control group. Experimental group: treatment group; (A) efficacy rate (B) DHI (C) THI (D) VAS (E) pure tone audiometry score.
3.5.2 DHI
Three studies (22, 23, 26) compared the DHI between groups, and the random-effects model was used because of the high heterogeneity among the studies (p < 0.0001, I2 = 92%). As shown in Figure 3B, the treatment group resulted in a greater reduction in DHI than the control group (MD: 6.94; 95% CI: 1.58–12.30; p = 0.01). Sensitivity analysis revealed that heterogeneity decreased significantly (p = 0.60, I2 = 0%) after removing Wu’s study (26). It is possible that inconsistencies in the assessors’ criteria for judgment led to variability in their baseline and outcome levels thus resulting in clinical heterogeneity.
3.5.3 THI
Three studies (22, 23, 26) compared the THI between groups, and the random-effects model was used because of the high heterogeneity among the studies (p < 0.0001, I2 = 95%). The result showed a greater reduction in THI of the treatment group than those of the control group (MD: 6.52; 95% CI: 0.77–12.27; p = 0.03) (Figure 3C). However, through sensitivity analysis, we were unable to find the source of heterogeneity, thus this result is unreliable.
3.5.4 VAS
Three studies (22, 23, 26) compared VAS between groups. There was no significant heterogeneity among the studies in the change of VAS. (p = 0.70, I2 = 0%). The result showed a greater reduction in VAS of the treatment group than those of the control group (MD: 0.87; 95% CI: 0.54–1.20; p < 0.0001) (Figure 3D).
3.5.5 Pure tone audiometry score
Three studies (22, 23, 26) compared pure tone audiometry score between groups. There was no significant heterogeneity among the studies in the change of pure tone audiometry score (p = 0.58, I2 = 0%). As shown in Figure 3E, the treatment group resulted in a greater reduction in pure tone audiometry score than the control group (MD: 6.57; 95% CI: 5.62–7.51; p < 0.0001).
3.5.6 Adverse event
Only one RCT (23) reported adverse effects, and its two groups were safe and effective, none of the enrolled cases had adverse effects after treatment, and there was no obvious subcutaneous hemorrhage and local soft tissue injury.
3.6 Publication bias
In all studies that included efficacy rate, the funnel plots were symmetric (Figure 4). Egger’s test yielded a p-value of 0.144, indicating the absence of discernible publication bias (Supplementary material 5). Accordingly, the meta-analysis demonstrated that acupuncture was less susceptible to publication bias.
4 Discussion
A total of 6 RCTs with 494 (248/246) patients were included in our study to systematically evaluate the efficacy of acupuncture for MD. We found that the treatment group was superior to the control group with regard to efficacy rate. The treatment group resulted in a greater reduction in DHI, THI, VAS and pure tone audiometry score than the control group. However, due to the inability to identify the cause of heterogeneity, the results on THI should be considered unreliable.
A summary of the acupuncture protocols included in the study revealed that the most common acupoints were Baihui (GV20), Fengchi (GB20), Tinggong (SI19), Hegu (LI4), Quchi (LI11), Zusanli (ST36), Fenglong (ST40), Taichong (LR3), and Taixi (KI3). All trials used the uniform reinforcing-reducing needling method.
The pathology of MD is based on endolymphatic hydrops, which may be affected by various causes of excessive lymphatic fluid production or impaired absorption in the inner ear. In guinea pigs with endolymphatic hydrops, electroacupuncture was found to be able to increase the expression level of aquaporin 1 (AQP1) while also having a benign effect on serum ion concentration. This suggests that the improvement of endolymphatic hydrops by electroacupuncture may be related to the up-regulation of cochlear AQP1 expression and affected by the change in ion concentration (27). It was found that in the guinea pig model of AVP-induced endolymphatic hydrops, plasma AVP concentration was increased, cochlear V2R expression was downregulated, and cyclic adenosine monophosphate (cAMP) and aquaporin 2(AQP2) expression were upregulated. Electroacupuncture treatment reduced cochlear hydrops and reversed the expression of plasma arginine vasopressin (AVP), vasopressin type 2 receptor (V2R), cAMP, and AQP2 (28). Acupuncture at Baihui (GV20) was observed to reduce the degree of cochlear hydrops in the animal model of AVP-induced endolymphatic hydrops, accompanied by a down-regulation of cochlear AQP2 expression (29). Moxibustion at Tinggong (SI19) has been demonstrated to down-regulate plasma AVP, cochlear AQP2, and aquaporin 7(AQP7) expression in the animal model with endolymphatic hydrops, thereby achieving the effect of intervening in endolymphatic hydrops (30). The aforementioned findings supported the theory that acupuncture-mediated relief of endolymphatic hydrops and amelioration of clinical symptoms linked to MD may target the altered expression of inner ear water channel proteins in the model of hydrops. Our study has the following advantages. First, we used the updated and objective DHI, THI, VAS, and pure tone audiometry score to assess the severity of dizziness, tinnitus, hearing, and aural fullness in patients with MD. Secondly, three studies (22, 23, 26) had a low RoB, thus increasing the credibility of the findings. Finally, we restricted the type of control intervention used to reduce heterogeneity in the included studies and excluded Chinese herbs and Chinese Patent Medicine as co-treatment.
This systematic review and meta-analysis had several limitations. First, the credibility of our conclusions may have limitations due to the small sample size. Only six RCTs were included and the sample size was small, ranging from 30 to 58 patients, which may affect the precision and stability of the results. Second, three RCTs (21, 24, 25) did not describe the randomization method, and two RCTs (24, 25) had a high risk of bias regarding the measurement of outcome, all of which may affect the scientific reliability and validity of the RCTs. Third, all six studies (21–26) were conducted in China, which had regional and ethnic limitations. The culture, medical system, patient characteristics and other factors of different countries may affect the differences in the efficacy of acupuncture. Therefore, the location of all studies in China may affect the generalizability and external effect of the result. Similar studies should be conducted in other countries in the future. Fourth, the choice of acupuncture points, number, depth, retention time and type of needle suggest potential heterogeneity. Fifthly, only one RCT (22) reported a recurrence rate at 6-month follow-up (the treatment group 5.17% VS the control group18.97%). Due to other incomplete follow-up data, we were unable to compare the long-term effects of acupuncture for MD. Sixthly, only one RCT (23) reported adverse effects, which is an area for improvement in the future. Therefore, caution should be exercised when interpreting the results of this review.
In addition, some outcomes showed high heterogeneity, and sensitivity analyses were carried out to determine the source of heterogeneity. However, there is still high heterogeneity, possibly due to the following reasons. This is possibly related to differences in patient’s baseline levels, differences in interventions (acupoints selected, the manipulation of the performer, Western medicine), and differences in the measurement of outcome indicators by assessors.
Our meta-analysis indicates that acupuncture may have great potential for treating MD and warrants further investigation. The evidence base for acupuncture therapy in the treatment of MD is still limited. It is recommended that more large-scale, multi-center, rigorously designed, and conducted RCTs be carried out to provide the best evidence for clinical decision-making. Specific recommendations are as follows: Firstly, we should further evaluate the optimal acupuncture treatment protocol, acupuncture technique, efficacy criteria, and staging of MD for targeted treatment; Secondly, based on the current study (24), the minimum effective time for acupuncture treatment of MD maybe one week, and we need to further clarify the minimum effective time for acupuncture treatment of MD; Thirdly, we need to keep detailed records of follow-up data to assess the long-term efficacy of acupuncture for MD; Eventually, use high-quality clinical trial designs, such as RoB 2.0 and Consolidated Standards of Reporting Trials (CONSORT) guidelines (31), to optimize study design.
5 Conclusion
To sum up, acupuncture may improve the symptoms of vertigo, tinnitus, ear fullness and hearing loss in patients with MD, suggesting that acupuncture is a potential treatment for MD. However, due to the lack of literature included in this study and methodological weaknesses like randomization, blinding, and clinical heterogeneity, such as selected acupoints, acupuncture sessions, and therapist techniques more well-designed long-term follow-up RCTs are needed to evaluate the efficacy and safety of acupuncture.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MT: Writing – original draft. YL: Writing – original draft. ML: Writing – original draft. TZ: Writing – original draft. YG: Writing – original draft. JH: Writing – original draft. JT: Writing – review & editing. ZC: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1463821/full#supplementary-material
References
1. Basura, GJ, Adams, ME, Monfared, A, Schwartz, SR, Antonelli, PJ, Burkard, R, et al. Clinical practice guideline: Ménière’s disease executive summary. Otolaryngol Head Neck Surg. (2020) 162:415–34. doi: 10.1177/0194599820909439
2. Kim, MH, and Cheon, C. Epidemiology and seasonal variation of Ménière’s disease: data from a population-based study. Audiol Neurootol. (2020) 25:224–30. doi: 10.1159/000506921
3. Patel, JJ, Levy, DA, Nguyen, SA, Rizk, HG, and Meyer, TA. Depression in Ménière’s disease: a systematic review and meta-analysis. J Laryngol Otol. (2020) 134:293–301. doi: 10.1017/S002221512000081X
4. Ward, B, Wettstein, V, Golding, J, Corallo, G, Nuti, D, Trabalzini, F, et al. Patient perceptions of effectiveness in treatments for Menière’s disease: a National Survey in Italy. J Int Adv Otol. (2019) 15:112–7. doi: 10.5152/iao.2019.5758
5. Oberman, BS, Patel, VA, Cureoglu, S, and Isildak, H. The aetiopathologies of Ménière’s disease: a contemporary review. Acta Otorhinolaryngol Ital. (2017) 37:250–63. doi: 10.14639/0392-100X-793
6. Magnan, J, Özgirgin, ON, Trabalzini, F, Lacour, M, Escamez, AL, Magnusson, M, et al. European position statement on diagnosis, and treatment of Meniere’s disease. J Int Adv Otol. (2018) 14:317–21. doi: 10.5152/iao.2018.140818
7. Adrion, C, Fischer, CS, Wagner, J, Gürkov, R, Mansmann, U, and Strupp, M. Efficacy and safety of betahistine treatment in patients with Meniere’s disease: primary results of a long term, multicentre, double blind, randomised, placebo controlled, dose defining trial (BEMED trial). BMJ. (2016) 352:h6816. doi: 10.1136/bmj.h6816
8. Băjenaru, O, Roceanu, AM, Albu, S, Zainea, V, Pascu, A, Georgescu, MG, et al. Effects and tolerability of betahistine in patients with vestibular vertigo: results from the Romanian contingent of the OSVaLD study. Int J Gen Med. (2014) 7:531–8. doi: 10.2147/IJGM.S71015
9. Kitahara, T. Evidence of surgical treatments for intractable Meniere’s disease. Auris Nasus Larynx. (2018) 45:393–8. doi: 10.1016/j.anl.2017.07.016
10. Nam, J, Lee, H, Lee, S, and Park, H. Literature review of complementary and alternative therapies: using text mining and analysis of trends in nursing research. BMC Nurs. (2024) 23:526. doi: 10.1186/s12912-024-02172-9
11. Fuggle, NR, Cooper, C, Oreffo, ROC, Price, AJ, Kaux, JF, Maheu, E, et al. Alternative and complementary therapies in osteoarthritis and cartilage repair. Aging Clin Exp Res. (2020) 32:547–60. doi: 10.1007/s40520-020-01515-1
12. Djaali, W, Simadibrata, CL, Nareswari, I, and Djaali, NA. Acupuncture therapy for peripheral vestibular Vertigo (with suspected Ménière’s disease). Med Acupunct. (2023) 35:89–93. doi: 10.1089/acu.2022.0012
13. He, J, Jiang, L, Peng, T, Xia, M, and Chen, H. Acupuncture points stimulation for Meniere’s disease/syndrome: a promising therapeutic approach. Evid Based Complement Alternat Med. (2016) 2016:6404197. doi: 10.1155/2016/6404197
14. Zhang, YN, Xue, YP, Guo, X, Wang, J, Yin, XY, and Jie, WJ. Clinical observation of scalp clustering acupuncture combined with ear acupressure in treatment of Meniere’s syndrome. Information TCM. (2022) 39:62–5. doi: 10.19656/j.cnki.1002-2406.20220412
15. Liao, YR, Dou, D, Xie, TT, and Xie, H. Needling “Niwan Bazhen” to treat residual symptoms of peripheral vestibular Vertigo. J Sichuan Traditi Chinese Med. (2017) 35:198–201.
16. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
17. Lopez-Escamez, JA, Carey, J, Chung, WH, Goebel, JA, Magnusson, M, Mandalà, M, et al. Diagnostic criteria for Menière’s disease. J Vestib Res. (2015) 25:1–7. doi: 10.3233/VES-150549
18. MacPherson, H, Altman, DG, Hammerschlag, R, Youping, L, Taixiang, W, White, A, et al. Revised STandards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. PLoS Med. (2010) 7:e1000261. doi: 10.1371/journal.pmed.1000261
19. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
20. Guyatt, GH, Oxman, AD, Kunz, R, Vist, GE, Falck-Ytter, Y, and Schünemann, HJ. What is “quality of evidence” and why is it important to clinicians? BMJ. (2008) 336:995–8. doi: 10.1136/bmj.39490.551019.BE
21. Ai, X, and G, Q. Acupuncture treatment of Meniere’s syndrome in 103 cases. Shaanxi J Taditi Chinese Med. (2013) 34:71–2.
22. Ding, D, and DengY, WSF. Intervention of acupuncture combined with mecobalamin and betahistine on the degree and recurrence of vertigo disorders in elderly patients with Meniere’s disease. Chin J Gerontol. (2021) 41:3731–4.
23. Ha, J, Song, XJ, Liu, ZH, and Li, GH. Clinical study of acupuncture in the treatment of sputum cloudy upper Meniere’s disease. J Ningxia Med Univ. (2023) 45:844–8. doi: 10.16050/j.cnki.issn1674-6309.2023.08.015
24. Mao, LY, Lu, HJ, Shen, XH, and Shen, ZF. Observations on the efficacy of acupuncture in the treatment of Meniere’s disease. Shanghai J Acupunc Moxibustion. (2014) 33:575. doi: 10.13460/j.issn.1005-0957.2014.06.0575
25. Wang, X, Xia, DK, Cui, YL, and Gao, WB. Clinical observation on the treatment of Ménière’s disease by acupuncture and medicine cooperation. J Clin Acupunc Moxibustion. (2011) 27:32–3.
26. Wu, D, Liu, B, Wang, HC, Rong, PJ, Chen, LQ, and Duan, JP. Acupuncture combined with oral western medication for Meniere’s disease: a randomized controlled trial. Zhongguo Zhen Jiu. (2018) 38:1047–52. doi: 10.13703/j.0255-2930.2018.10.005
27. Jiang, LY, Wang, CJ, Ni, FY, and Chen, HD. Effects of electroacupuncture on cochlea morphology and expression of aquaporins in guinea pigs with endolymphatichy drops. Zhongguo Zhen Jiu. (2015) 35:579–84. doi: 10.13703/j.0255-2930.2015.06.016
28. Jiang, LY, He, JJ, Chen, XX, Sun, XJ, Wang, XZ, Zhong, S, et al. Arginine vasopressin-Aquaporin-2 pathway-mediated dehydration effects of Electroacupuncture in Guinea pig model of AVP-induced Eendolymphatic Hydrops. Chin J Integr Med. (2019) 25:763–9. doi: 10.1007/s11655-017-2411-2
29. Jiang, LY, Wang, XZ, Zhong, S, and Chen, HD. Effects of needling Baihui (GV20) on the morphology of cochlea and the AQP2 expression in rats with endolymphatic hydrops. China J Trandit Chinese Med Pharm. (2016) 31:5281–4.
30. Jiang, LY, Sun, XJ, Chen, XX, and Chen, HD. Effects of Moxibustion at Tinggong (SI19) on rats endolymphatic Hydrops. Chinese Arch J Taditi Chinese Med. (2017) 35:2625–9. doi: 10.13193/j.issn.1673-7717.2017.10.040
Keywords: acupuncture, systematic review, meta-analysis, Meniere’s disease, vertigo
Citation: Tang M, Li Y, Lu M, Zhang T, Ge Y, Han J, Tang J and Chen Z (2024) Efficacy and safety of acupuncture in the treatment of Meniere’s disease: a systematic review and meta-analysis. Front. Med. 11:1463821. doi: 10.3389/fmed.2024.1463821
Edited by:
Antonio Sarría-Santamera, Nazarbayev University, KazakhstanReviewed by:
Pedro Marques, University of Porto, PortugalCarlos Sanchez-Piedra, Carlos III Health Institute (ISCIII), Spain
Copyright © 2024 Tang, Li, Lu, Zhang, Ge, Han, Tang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaoming Chen, Y3ptNjcwNEAxNjMuY29t
 Mingjie Tang
Mingjie Tang Yinghong Li1
Yinghong Li1 Man Lu
Man Lu