- 1Department of Nuclear Medicine, Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 2Department of Pathology, Affiliated Hospital of Zunyi Medical University, Zunyi, China
Inflammatory myofibroblastic tumor (IMT) is an intermediate tumor composed of differentiated myofibroblastic spindle cells with inflammatory cell infiltration. It can occur in all parts of the body, with the lungs being the most common, while the tissues outside the lungs, including the sigmoid colon, are rare. Herein, we present a case of a 10-year-old girl with sigmoid IMT who presented to our hospital with abdominal pain. An abdominal computed tomography (CT) revealed a well-defined, slightly low-density mass in her lower abdomen that was not clearly demarcated from the sigmoid colon. The mass showed significant uneven enhancement on contrast-enhanced CT and increased fluorine-18 fluorodeoxyglucose (18F-FDG) uptake on positron emission tomography (PET). Moreover, a systematic review of the published literature on sigmoid IMT was conducted and its clinical and radiographic features were summarized to increase the understanding of this rare disease.
Introduction
Inflammatory myofibroblastic tumor (IMT) is a rare intermediate mesenchymal tumor, which has previously been described as inflammatory pseudotumor, plasma cell granuloma, and inflammatory myofibrous histiocytic proliferation, consisting of differentiated myofibroblastic spindle cells, often accompanied by extensive lymphocyte and/or plasma cell infiltration (1). The etiology of IMTs is unknown and may be related to certain special bacterial or EB virus infections, chromosomal mutations (2). It can be seen at any age, but is mainly found in children and young adults, with females being more common (3). The tumor can occur in various parts of the body, of which the lung is the most common, accounting for 95%, while the organ tissues outside the lung, including mesentery, omentum, liver, retroperitoneum and limbs, are rare (4). The disease symptoms of patients are related to the disease site, including fever, pain, anemia and mass, without specificity (5). It is precisely because of the rarity of IMTs and the non-specificity of clinical manifestations that it is difficult to make a correct diagnosis before surgery. Here, we present the diagnosis and treatment of a rare patient with sigmoid IMT and review the literature with a view to increasing awareness of this rare disease.
Case presentation
A 10-year-old girl with abdominal pain for 3 days underwent an abdominal ultrasound examination in an outside hospital on November 20, 2023 and found a large mass in her pelvic cavity. She was admitted to our hospital on 23 November 2023 for further diagnosis and treatment. She and her family denied any history of tumors or genetic diseases. Physical examination revealed a large, hard, low-motion mass palpable in her pelvic cavity, while no significant positive signs were found in the rest of her body. The laboratory test results, including serum tumor markers, were all negative. On November 24th, the patient underwent an abdominal CT examination (Figure 1) and the results showed a slightly low-density mass with unclear boundary with the sigmoid colon in her pelvic cavity, which presented significant uneven enhancement on contrast-enhanced CT, suggesting a possible malignant tumor. In order to further evaluate the nature of the tumor and determine the treatment plan, she underwent 18F-FDG PET/CT examination the following day. The results showed obviously increased 18F-FDG uptake in this lesion, and no hot spot lesions were observed in the rest of the body (Figure 2). Based on these imaging findings, the patient was initially suspected to have a malignant lesion. After a series of evaluations, the patient underwent an exploratory laparotomy, radical tumor resection and ileostomy on November 29 under anesthesia. Hematoxylin–eosin staining revealed diffuse spindle shaped tumor cells and scattered inflammatory cells in resected tumor tissue (Figure 3). Immunohistochemical results showed that the tumor cells positively expressed smooth muscle actin (SMA), anaplastic lymphoma kinase (ALK), CD117, vimentin, but negatively expressed cytokeratin (CK), Desmin and Dog-1. Based on these histopathological features, the patient was diagnosed with an inflammatory myofibroblastic tumor. The patient improved after receiving 3 days of anti-inflammatory treatment with ceftriaxone after surgery and was discharged on December 1, 2023. On June 2, 2024, she underwent abdominal ultrasound examination and showed no signs of tumor recurrence. The patient has been following up for 6 months now and has not reported any discomfort.
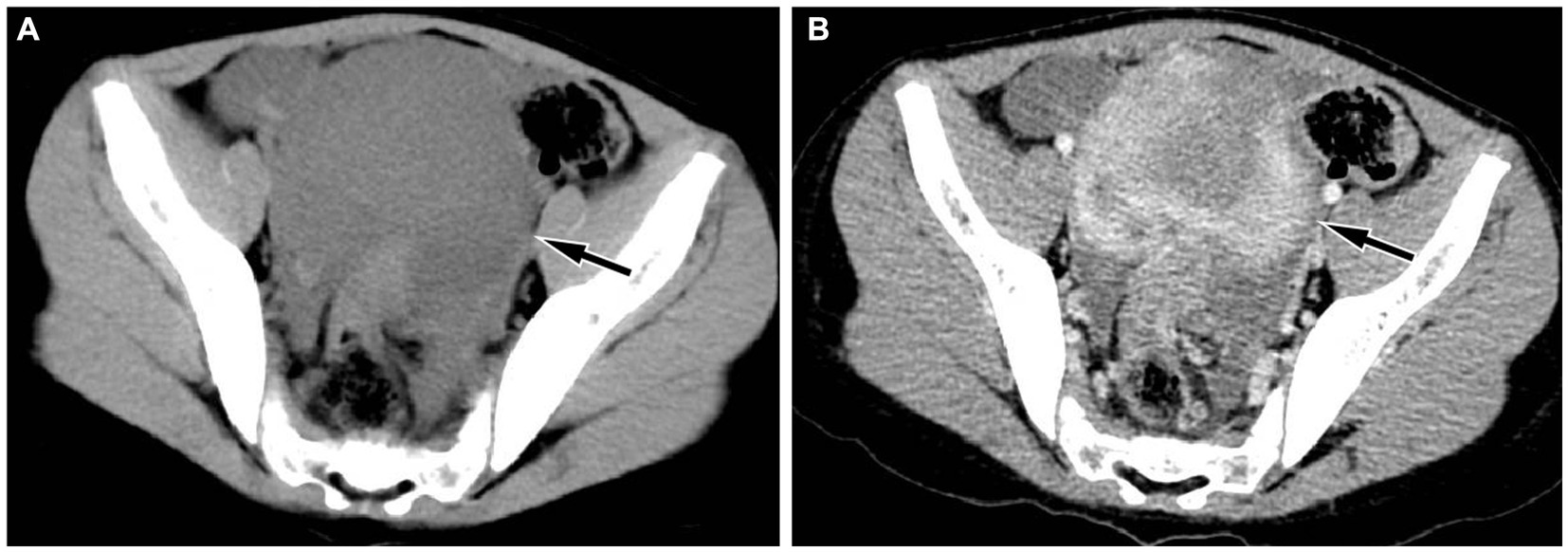
Figure 1. Abdominal CT revealed a regular low-density mass (A, arrow) in the pelvic cavity, about 9.3 cm × 9.0 cm × 6.7 cm in size; which presented significant uneven enhancement on contrast-enhanced CT (B, arrow).
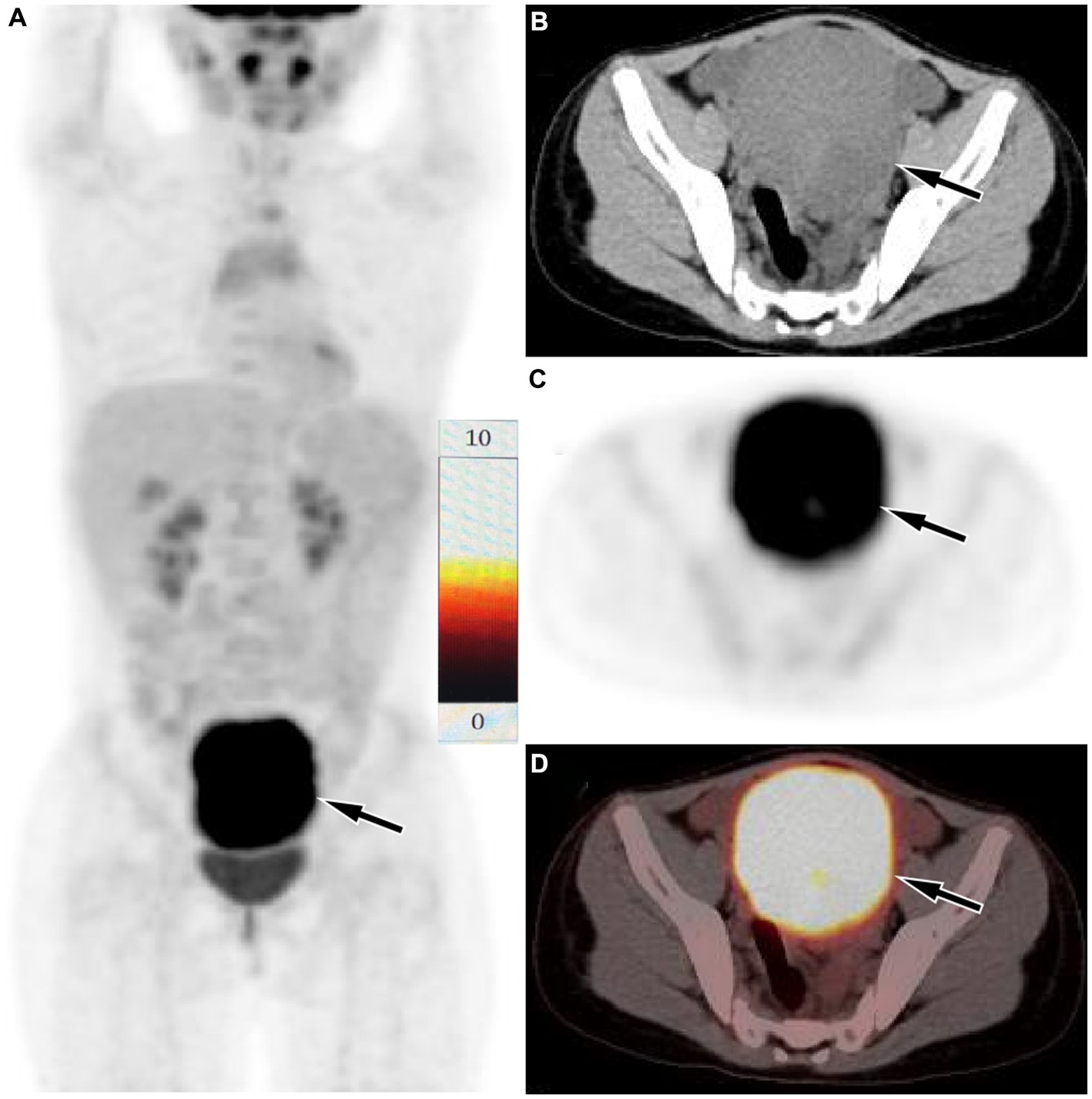
Figure 2. Fluorine-18 fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT imaging of the patient. The maximum intensity projection (MIP, A) showed a significantly increased 18F-FDG uptake in the lower abdominal area (arrow). Axial CT (B), PET (C) and PET/CT fusion (D) showed that the lesion with significantly increased 18F-FDG uptake, with a SUVmax of 9.3 (arrows), which was located in the serosal surface of the sigmoid colon, and the adjacent intestinal tube is compressed, the proximal intestinal tube is dilated, and liquid exudation shadow is seen around the mass, and a small amount of fluid is seen in the pelvic cavity.
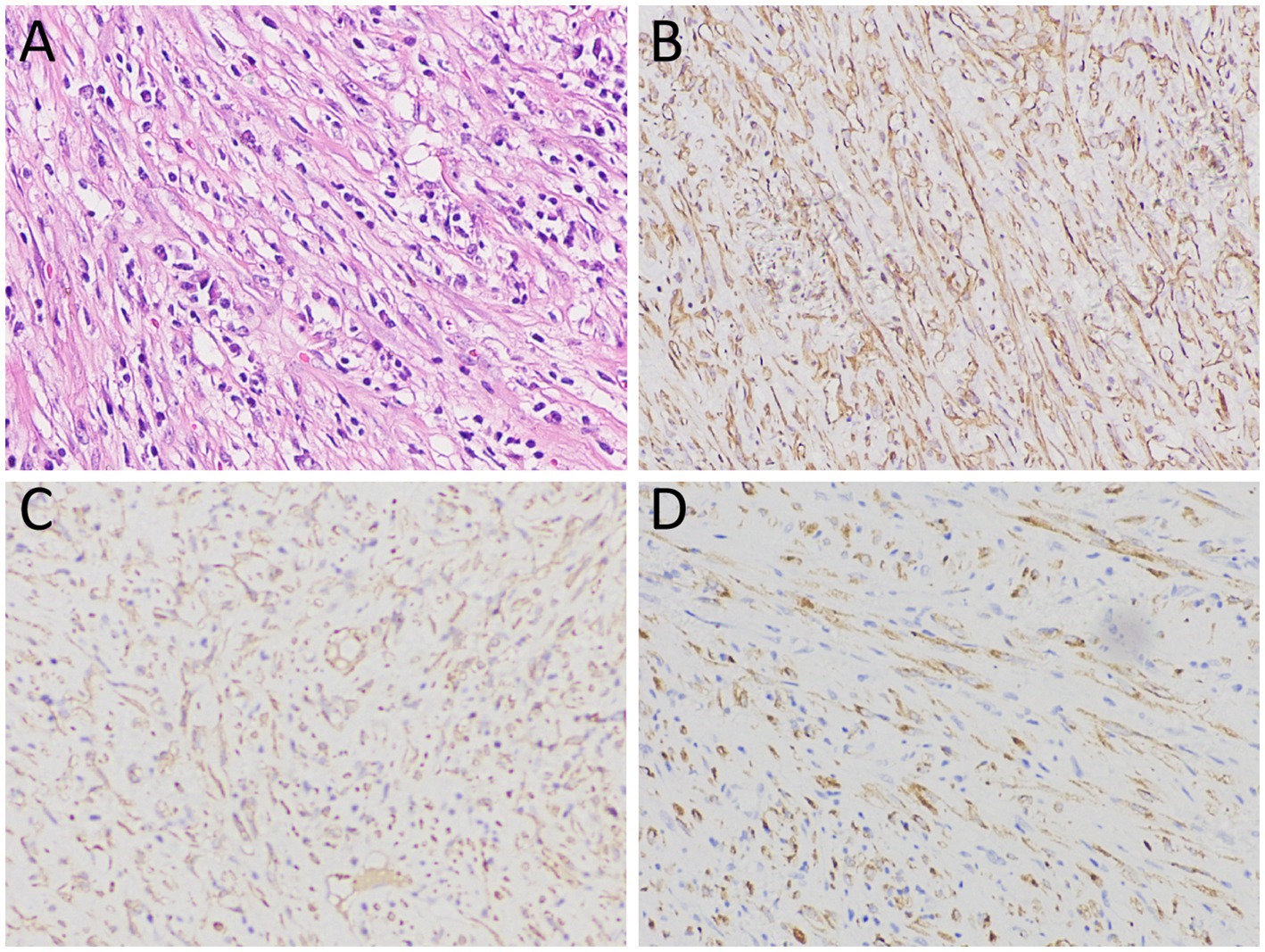
Figure 3. (A) Hematoxylin–eosin staining revealed diffuse spindle shaped tumor cells and scattered inflammatory cells within the lesion; Immunohistochemical results showed that the tumor cells positively expressed SMA (B), ALK (C) and vimentin (D). Notes: SMA, smooth muscle actin; ALK, anaplastic lymphoma kinase.
Literature review
The PubMed and Web of Science databases were searched for case reports and series of sigmoid colon IMT as of June 10, 2024, with language limitations in English. The search strategy was as follows: (“inflammatory myofibroblastic tumor” OR “inflammatory pseudotumor” OR “plasma cell granuloma” OR “inflammatory myofibrous histiocytic proliferation”) AND “sigmoid colon.” For each enrolled case, the first author, publication year, as well as the patient’s gender, age, clinical symptoms, imaging findings including CT and PET, and follow-up results were recorded (Table 1).
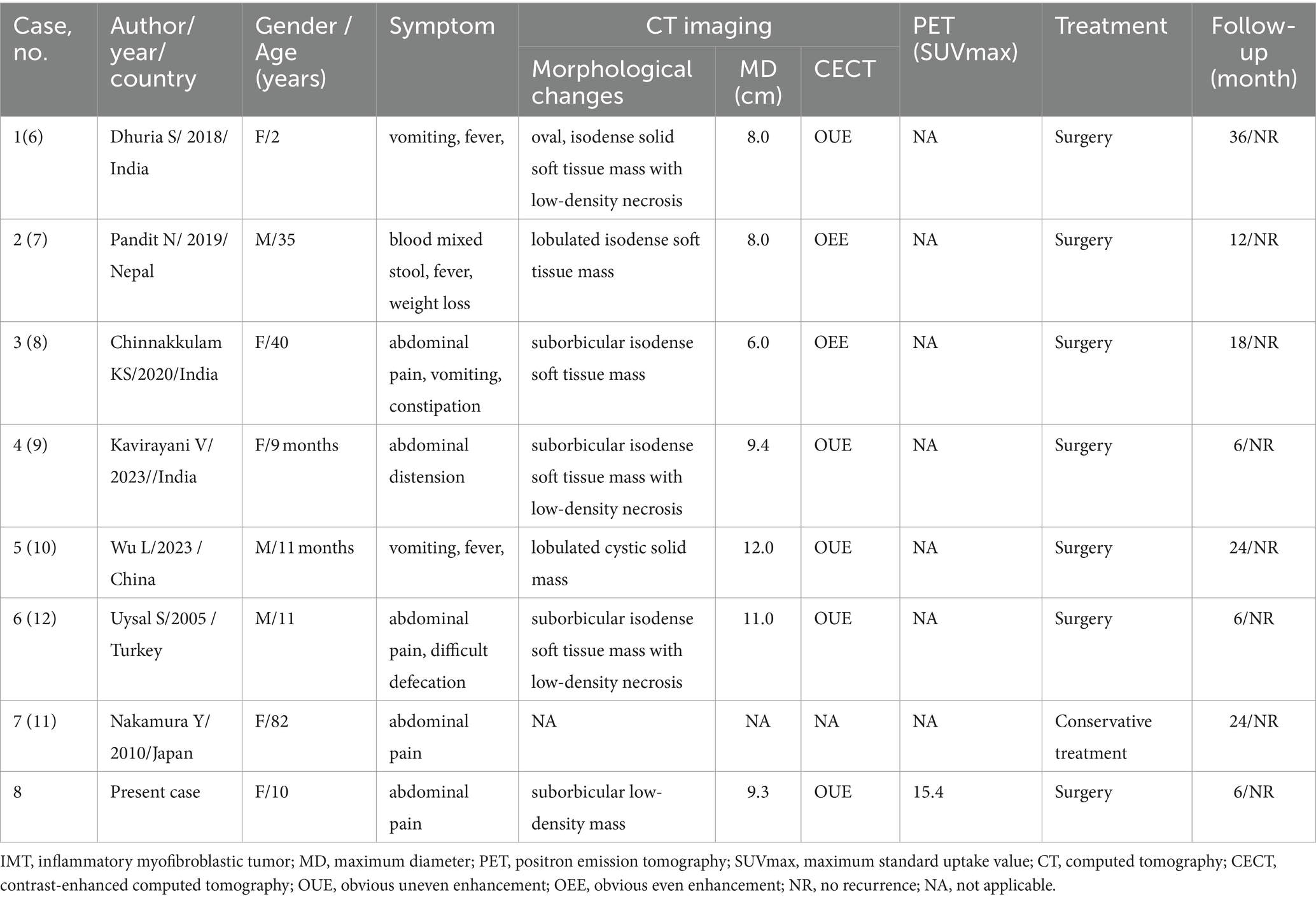
Table 1. Clinical and imaging features of the cases of sigmoid colon sigmoid colon IMT from the literature review and current case.
After a systematic search and careful reading of the full text of the preliminary screening, it was finally determined that there were seven cases of sigmoid IMT published before our case (6–12). Including the current patient that we reported, a total of eight sigmoid IMT cases, consisting of three male (3/8) and five female (5/8) patients, with a median age of 10.5 years (range, 9 months-81 years old), were included in the analysis. Common clinical symptoms include abdominal pain, vomiting, fever, and abdominal discomfort. IMTs are generally large in size, with a mean maximum diameter of 9.1 cm. Most of the patients (7/8) underwent surgery, and only one patient received conservative treatment due to poor lung function. The prognosis of IMT was good, and no significant signs of tumor recurrence were found in all patients who underwent surgical resection of the mass during the follow-up period.
Discussion
IMT in the sigmoid colon is rare. Our current study presents a case of a child diagnosed with sigmoid IMT who complained of abdominal pain. To further understand the characteristics of this disease, we conducted a systematic review of relevant literature, and the results showed that most IMT patients in the sigmoid colon were children and young adults, with more females than males. This is consistent with the epidemiology of IMT patients occurring in other parts of the body (13). Most patients seek medical help due to vomiting, fever, abdominal pain, and abdominal discomfort. The literature reported that the etiology of IMT may be related to chronic infections, autoimmune diseases, and trauma (2). However, our case and previous literature on IMT in the sigmoid colon have not reported any such medical history, so this viewpoint may need to be confirmed in the future.
Imaging examinations play a significant role in the diagnosis of IMT, and the imaging of IMT in the sigmoid colon has certain characteristics. On CT, it usually presents as a large isodense soft tissue mass with smooth edges, and there may be low-density cystic necrosis area within the mass. On contrast-enhanced CT, the mass showed obvious uniform or uneven enhancement (6–10). Unlike previous literature reports, the current case showed a uniform low-density mass on CT, but still showed significant enhancement on contrast-enhanced CT. At present, research on the 18F-FDG/glucose metabolism of IMT is relatively rare and is mostly seen in case reports. Most IMTs present significantly increased 18F-FDG uptake on PET and are often misdiagnosed as malignant tumors in the corresponding area (14–16). The mechanism of 18F-FDG uptake by IMT may be correlated with tumor cellularity, inflammatory cell infiltration and Ki-67 expression. The higher tumor cellularity, more composition of inflammatory cell and higher Ki67 expression, the greater SUVmax (17). The patient we reported presented with low density on CT, with exudative shadows around the mass and obvious uptake of 18F-FDG on PET, which may be related to the strong inflammatory cell infiltration of the mass. To our knowledge, our case study is the first to report PET findings of sigmoid colon IMT, which, like IMT occurring in other organ tissues, also showed significantly increased 18F-FDG uptake.
According to the imaging findings of IMT, IMT originating from the sigmoid colon needs to be differentiated from gastrointestinal stromal tumors (GISTs), lymphoma and sigmoid colon cancer. GISTs also presented as large, circular or lobulated soft tissue masses on CT, with cystic necrosis at the center of the mass and uneven delayed enhancement on contrast-enhanced CT (18). On PET, GISTs with different risk levels show varying levels of increased 18F-FDG uptake, and high-risk GISTs presenting a higher SUVmax than medium-to low-risk GISTs (19). Moreover, due to the fact that GISTs typically grow outside the intestinal lumen, GISTs located in the lower abdomen may exhibit migration of the mass over time on dual-time point PET/CT, which is a relatively specific sign (20). Lymphomas that occur in the intestine are mostly B-cell non-Hodgkin lymphomas. Like IMT, it presents significantly increased 18F-FDG uptake on PET (21). However, intestinal lymphoma usually infiltrates along the intestinal wall, presenting as a circular thickening of the intestinal wall, and rarely causing proximal intestinal duct dilation and obstruction (22). Adenocarcinoma is the most common tumor in the sigmoid colon, and it also shows significantly increased 18FDG uptake on PET. However, sigmoid colon cancer often grows infiltratively along the intestinal wall and has an irregular shape on CT (23), which is significantly different from IMT.
Pathological examination is currently the gold standard for diagnosing IMT. Microscopically, the characteristic fusiform myofibroblast proliferation was observed, accompanied by abundant infiltration of chronic inflammatory cells such as plasma cells, T lymphocytes, neutrophils, and eosinophils (24). Immunohistochemistry showed that tumor cells positively expressed vimentin, SMA, and Desmin usually, and CK, CD68, CD30 and ALK were partially expressed positively, while CD117 and CD34 were usually negative expressed (25). In the tumor tissue of the patient we reported, diffuse fusiform tumor cells and scattered inflammatory cells were found in the lesion under microscope. Immunohistochemical results showed that the tumor cells positively expressed vimentin, SMA, and ALK, which was consistent with the pathological diagnosis of IMT.
At present, radical surgical resection of tumor tissue is the preferred treatment for IMT. Only when the tumor cannot be surgically removed, other treatment options including chemotherapy, immunomodulatory therapy, corticosteroids, radiotherapy, nonsteroidal anti-inflammatory drugs and so on should be considered (26, 27). Since IMT is an intermediate tumor with the possibility of recurrence and metastasis, close and careful follow-up after complete surgical resection of the tumor tissue is crucial to improve the prognosis of patients (28). The patient we reported did not show any signs of tumor recurrence or metastasis during follow-up after surgical removal of the tumor.
In conclusion, sigmoid IMT is a relatively rare intermediate tumor and should be considered in the differential diagnosis of other sigmoid malignancies such as GISTs and cancers. It appears as a large, smooth edge, or low-density mass on CT, with obvious uniform or uneven enhancement on contrast-enhanced CT, which showed significantly increased 18F-FDG uptake on PET. These imaging findings contribute to the diagnosis of IMT.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing – original draft. WZ: Investigation, Methodology, Project administration, Writing – original draft. RY: Conceptualization, Methodology, Validation, Writing – original draft. PW: Investigation, Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Guizhou Province Science and Technology Plan Project (grant numbers: Qiankehe-ZK[2024]-329) and Zunyi Science and Technology Joint Fund (grant number: HZ-2023-284).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pire, A, Orbach, D, Galmiche, L, Berrebi, D, Irtan, S, Boudjemaa, S, et al. Clinical, pathologic, and molecular features of inflammatory myofibroblastic tumors in children and adolescents. Pediatr Blood Cancer. (2022) 69:e29460. doi: 10.1002/pbc.29460
2. Lai, LM, McCarville, MB, and Kirby, P. Shedding light on inflammatory pseudotumor in children: spotlight on inflammatory myofibroblastic tumor. Pediatr Radiol. (2015) 45:1738–52. doi: 10.1007/s00247-015-3360-6
3. Alhumaid, H, Bukhari, M, Rikabi, A, Farahat, M, Mesallam, TA, Malki, KH, et al. Laryngeal myofibroblastic tumor: case series and literature review. Int J Health Sci (Qassim). (2011) 5:187–95.
4. Bennett, JA, Nardi, V, Rouzbahman, M, Morales-Oyarvide, V, Nielsen, GP, and Oliva, E. Inflammatory myofibroblastic tumor of the uterus: a clinicopathological, immunohistochemical, and molecular analysis of 13 cases highlighting their broad morphologic spectrum. Mod Pathol. (2017) 30:1489–503. doi: 10.1038/modpathol.2017.69
5. Parra-Herran, C, Quick, CM, Howitt, BE, Dal Cin, P, Quade, BJ, and Nucci, MR. Inflammatory myofibroblastic tumor of the uterus: clinical and pathologic review of 10 cases including a subset with aggressive clinical course. Am J Surg Pathol. (2015) 39:157–68. doi: 10.1097/PAS.0000000000000330
6. Dhuria, S, Kwatra, KS, and Ghosh, DN. Inflammatory myofibroblastic tumour of sigmoid mesocolon in a child. ANZ J Surg. (2018) 88:E674–6. doi: 10.1111/ans.13625
7. Pandit, N, Yadav, TN, Shrestha, D, Adhikari, P, and Awale, L. IMFT of the sigmoid colon: a case report. J Surg Case Rep. (2019) 2019:rjz334. doi: 10.1093/jscr/rjz334
8. Chinnakkulam Kandhasamy, S, Sundaramurthi, S, Vijayakumar, C, Goneppanavar, M, and Nelamangala Ramakrishnaiah, VP. Inflammatory Myofibroblastic tumor of sigmoid Colon: unusual cause of intestinal obstruction. Cureus. (2020) 12:e11809. doi: 10.7759/cureus.11809
9. Kavirayani, V, Pai, NG, Nayal, B, and Prabhu, S. Infantile inflammatory myofibroblastic tumour of the sigmoid colon: a diagnostic dilemma. BMJ Case Rep. (2023) 16:e256505. doi: 10.1136/bcr-2023-256505
10. Wu, L, Meng, X, Wang, J, Wang, Q, Sun, X, Zhu, T, et al. Inflammatory Myofibroblastic tumor of the sigmoid Colon in an infant: a case report and literature review. Fetal Pediatr Pathol. (2023) 42:123–30. doi: 10.1080/15513815.2022.2062500
11. Nakamura, Y, Kayano, H, Shimada, T, Ito, Y, and Bessho, M. Plasma cell granuloma of the sigmoid colon associated with diverticular disease and accompanying IgM-type monoclonal gammopathy. Intern Med. (2010) 49:227–30. doi: 10.2169/internalmedicine.49.2240
12. Uysal, S, Tunçbilek, I, Unlübay, D, Tiraş, U, Bilaloğlu, P, and Koşar, U. Inflammatory pseudotumor of the sigmoid colon mesentery: US and CT findings (2004:12b). Eur Radiol. (2005) 15:633–5. doi: 10.1007/s00330-004-2535-6
13. Thompson, L . Inflammatory Myofibroblastic tumor. Ear Nose Throat J. (2021) 100:520S–1S. doi: 10.1177/0145561319890165
14. Chong, A, Ha, JM, Hong, R, Park, SG, and Lee, HJ. Inflammatory myofibroblastic tumor mimicking gastric gastrointestinal stromal tumor on 18F-FDG PET/CT. Clin Nucl Med. (2014) 39:725–7. doi: 10.1097/RLU.0000000000000392
15. Raad, RA, Haddad, L, Jabbour, T, and El-Rassi, Z. Inflammatory Myofibroblastic tumor of the liver mimicking metastasis on 18F-FDG PET/CT. Clin Nucl Med. (2021) 46:47–8. doi: 10.1097/RLU.0000000000003356
16. Tokunaga, K, Maeda, C, Horikawa, S, Nakayama, R, and Umeoka, S. 18F-FDG PET/CT imaging of G-CSF-producing inflammatory Myofibroblastic tumor of the pleura. Clin Nucl Med. (2023) 48:e84–6. doi: 10.1097/RLU.0000000000004457
17. Dong, A, Wang, Y, Dong, H, Gong, J, Cheng, C, Zuo, C, et al. Inflammatory myofibroblastic tumor: FDG PET/CT findings with pathologic correlation. Clin Nucl Med. (2014) 39:113–21. doi: 10.1097/RLU.0b013e3182952caa
18. Inoue, A, Ota, S, Yamasaki, M, Batsaikhan, B, Furukawa, A, and Watanabe, Y. Gastrointestinal stromal tumors: a comprehensive radiological review. Jpn J Radiol. (2022) 40:1105–20. doi: 10.1007/s11604-022-01305-x
19. Li, S, Lin, D, Tang, M, Liu, D, Lyu, Q, and Zhang, J. Value of (18)F-FDG PET/CT for differentiating diagnosis between malignant and benign primary gastric gastrointestinal mesenchymal tumors: a single-center retrospective study. J Gastrointest Oncol. (2022) 13:637–46. doi: 10.21037/jgo-22-287
20. Li, C, Li, W, Shang, M, Wang, P, and Hu, X. Case report: detection of multiple sporadic gastrointestinal stromal tumors by dual-time (18) F-FDG PET/CT. Front Oncol. (2024) 14:1321179. doi: 10.3389/fonc.2024.1321179
21. Da, B, Zhang, J, Zhu, F, Wang, Z, and Diao, Y. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue of the ileum in an adult presenting with intussusception: a case report and literature review. Front Oncol. (2024) 14:1395144. doi: 10.3389/fonc.2024.1395144
22. Liu, Y, Peng, Y, Li, J, Zeng, J, Sun, G, and Gao, P. MSCT manifestations with pathologic correlation of abdominal gastrointestinal tract and mesenteric tumor and tumor-like lesions in children: a single center experience. Eur J Radiol. (2010) 75:293–300. doi: 10.1016/j.ejrad.2010.05.036
23. Goh, V, and Glynne-Jones, R. Perfusion CT imaging of colorectal cancer. Br J Radiol. (2014) 87:20130811. doi: 10.1259/bjr.20130811
24. Surabhi, VR, Chua, S, Patel, RP, Takahashi, N, Lalwani, N, and Prasad, SR. Inflammatory Myofibroblastic tumors: current update. Radiol Clin North Am. (2016) 54:553–63. doi: 10.1016/j.rcl.2015.12.005
25. Chmiel, P, SłOWIKOWSKA, A, and Banaszek, Ł. Inflammatory myofibroblastic tumor from molecular diagnostics to current treatment. Oncol Res. (2024) 32:1141–62. doi: 10.32604/or.2024.050350
26. Liu, M, and Zhu, D. Two cases of inflammatory myofibroblastic tumor treated with targeted drugs: a case report. Medicine (Baltimore). (2024) 103:e38136. doi: 10.1097/MD.0000000000038136
27. Soyer, T, Talim, B, Karnak, İ, Ekinci, S, Andiran, F, Çiftçi, AÖ, et al. Surgical treatment of childhood inflammatory Myofibroblastic tumors. Eur J Pediatr Surg. (2017) 27:319–23. doi: 10.1055/s-0036-1593380
Keywords: inflammatory myofibroblastic tumor, sigmoid colon, 18F-FDG, PET/CT, CT
Citation: Hu X, Zhao W, Yu R and Wang P (2024) Imaging findings of inflammatory myofibroblastic tumor of sigmoid colon: literature review and case report. Front. Med. 11:1461205. doi: 10.3389/fmed.2024.1461205
Edited by:
Nataliya Lutay, Skåne University Hospital, SwedenReviewed by:
Ding Chong Yang, Nanjing Medical University, ChinaGuohua Shen, Sichuan University, China
Hubing Wu, Southern Medical University, China
Copyright © 2024 Hu, Zhao, Yu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pan Wang, MTI5ODE3ODgyOEBxcS5jb20=
 Xianwen Hu
Xianwen Hu Wei Zhao2
Wei Zhao2