- 1Department of Family and Community Medicine, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
- 2College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
Background: The COVID-19 pandemic has significantly raised public health concerns and efforts to limit its spread, impacting societies and health systems worldwide. As challenges persist, the emergence of Long COVID (LC) marks a turning point in understanding the pandemic’s long-term effects.
Aim: This study aimed to determine the prevalence of LC in the Eastern Province of the Kingdom of Saudi Arabia (KSA) and explore factors contributing to its persistence.
Methods: This descriptive, cross-sectional, questionnaire-based study was carried out between December 1, 2023, and March 1, 2024, involving 1,355 patients who recovered from COVID-19. Participants were conveniently chosen and information was gathered through in-person interviews in public settings after obtaining consent.
Results: A majority of the patients (N = 1,355; 47.5% female; 93.8% Saudis; mean Age ± SD 33.13 ± 12.60 years) had received three COVID-19 vaccine doses (89.5%). Women experienced 17.4% more LC symptoms than men (p < 0.001). The risk of having a higher symptom count increased by 42.5% 12 months after acute COVID-19 infection compared with baseline (<3 months, p < 0.001). A higher body mass index (BMI) was associated with more symptoms (1.1% increase per unit, p = 0.004). More acute-phase symptoms correlated with more LC symptoms (p < 0.001). Higher educational attainment reduced LC risk by 33% (p < 0.001). Finally, age and vaccination status had no effect on LC symptoms count (p > 0.05).
Conclusion: Sociodemographic and clinical factors contribute differently to the chances of having LC and the count of symptoms. Awareness of such factors could provide insight into improving management, leading to better health outcomes.
Introduction
Amidst the global upheaval triggered by the coronavirus disease (COVID-19), an extremely contagious respiratory disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus identified first in Wuhan, China in December 2019, the virus rapidly spread worldwide, prompting the World Health Organization (WHO) declared it a worldwide pandemic on March 11, 2020 (1). The toll has been staggering, with over 704 million cases and 7 million deaths recorded worldwide (2). In the Kingdom of Saudi Arabia (KSA) alone, over 841 thousand confirmed cases and 9,640 deaths were attributed to the virus (2). This rapid escalation raised public health concerns and sparked efforts to limit its spread. The COVID-19 pandemic has profoundly affected societies worldwide, exposing and exacerbating social issues such as income inequality, health disparities, and the strain on public health systems. It has also severely disrupted mental health, education, and social interactions. Moreover, the pandemic highlighted significant inequities in access to healthcare, reshaping perceptions of health, resilience, and societal vulnerabilities (3, 4). In investigating COVID-19, researchers have identified varying degrees of illness severity. The majority of individuals experience mild to moderate illness and recover without the need for hospitalization. For instance, a 2022 cohort study involving 162 COVID-19 patients reported that 22.9% were asymptomatic, 74.6% experienced mild to moderate symptoms that did not require hospitalization, and only 2.5% of patients required hospital care (5). The WHO has outlined COVID-19 symptoms, ranging from common signs such as fever, cough, and loss of taste/smell to less frequent symptoms such as sore throat, headache, and skin rash, and serious symptoms such as chest pain and difficulty in breathing (6).
As the world struggles with the ongoing challenges posed by the virus, the advent of Long COVID (LC) marks a significant chapter in our understanding of the enduring impact of this pandemic. The Centers for Disease Control and Prevention (CDC) and the National Institute for Health and Care Excellence (NICE) have undertaken a dynamic process of refining guidelines, defining LC/post-COVID-19 syndrome (PCS) as signs and symptoms develop during or after COVID-19 infection, persist for 12 weeks or more, and cannot be explained by an alternate diagnosis, while ongoing symptomatic COVID-19 as signs and symptoms persist for less than 12 weeks after the initial infection (7, 8). Despite rigorous investigations of the factors contributing to the persistent development of post-COVID-19 complications in some patients, the precise pathophysiological mechanisms underlying LC remain unclear (9, 10). Some leading hypotheses include autoimmunity, immune dysregulation, microembolization, and endothelial activation or dysfunction (9, 10).
Although LC presentations vary, common symptoms include fatigue, respiratory symptoms, hair loss, muscle and joint pain, attention deficits, and headache (11, 12). However, more serious symptoms include renal failure, pulmonary fibrosis, myocarditis, arrhythmia, and more (12). A detailed list of the most common LC symptoms by affected body system can be found in Supplementary Table S1. This broad spectrum of symptoms contributes to variations in the reported prevalence across global populations. A UK study published in February 2023 estimated that approximately 2 million individuals reported experiencing LC symptoms (13). Continuous analyses by the CDC in the US found that during March–April 2024, approximately 18% of adults had persistent COVID-19 symptoms beyond acute presentation (14). A large observational meta-analysis of 1.2 million people reported that 6.2% of patients with symptomatic COVID-19 had LC, which included ongoing respiratory problems (3.7%), persistent fatigue with bodily pain or mood swings (3.2%), and cognitive issues (2.2%) (15). Additionally, among 21,797 patients surveyed in China, 8.89% self-reported experiencing LC symptoms, with 2.92% reporting two or more symptoms. The most commonly reported symptom was Fatigue (3.38%), followed by sleep difficulties (2.20%), hair loss (2.06%), cough (1.74%), and sore throat (1.27%) (11).
Moreover, a meta-analysis and comprehensive review with a sample size of 1,680,003 patients published in November 2022 found that the pooled worldwide prevalence of LC was 0.43. Estimates were 0.54 for hospitalized patients and 0.34 for non-hospitalized individuals (16). The United States of America (0.31%), Europe (0.44%), and Asia (0.51%) were the other regions with high prevalence (16). Additionally, in January 2023, cross-sectional research including 520 Arabic patients residing in the KSA was published; 25% of them had LC and the most common recorded symptoms were cough, anosmia, fatigue, headache, muscle pain, arthritis, and shortness of breath (32, 32, 28, 19, 19, 18, and 17% of LC patients, respectively) (17). However, data from 504 patients at King Abdulaziz University Hospital in Jeddah revealed a 45% frequency of LC (18).
In light of this, LC presents a significant challenge to patients’ wellbeing, inducing long-lasting physical discomfort, cognitive decline, and emotional stress, ultimately reshaping their quality of life, increasing healthcare utilization, and increasing chronic sickness-related unemployment (19, 20). Several risk variables have also been found to increase the probability of developing LC. These factors include demographic risks, comorbidities, age, and severity of the acute COVID-19 infection, and other factors (11, 21).
Throughout this article, we endeavor to shed light on the LC prevalence in the Eastern Provinces of the KSA and explore the factors contributing to its persistence, including demographic variables, comorbidities, and the severity of the initial infection.
Materials and methods
Study design
This was a descriptive, cross-sectional, questionnaire-based study conducted from December 1, 2023, to March 1, 2024 among 1,350 COVID-19 recovered patients who are currently residing in the Eastern Province of the KSA, who were conveniently selected and whose information was obtained through face-to-face interviews in public community settings.
Study sample
This study included all COVID-19 diagnosed patients who were at least 18 years old. Patients who refused to provide consent to participate or all requested information were excluded. The Epi Info software (version 7.0) was used to calculate the sample size for a target population of 162,176 patients who recovered from COVID-19, and an expected frequency of 50% for LC. Given a 5% margin of error and a 95% confidence level, 251 participants were the minimum calculated sample size.
Data collection
Participants’ responses were collected by trained volunteers who administered the surveys using tablet devices. The 25-question survey was structured using questionnaires from previously published literature (16, 22–24). Family physicians reviewed the wording of the survey to ensure accuracy. Moreover, the questionnaire included questions regarding sociodemographic data such as age, sex, and occupation; medical history related to COVID-19 infection, including a history of medical illness, hospital, or intensive care unit admission; history of smoking; and lastly, questions about COVID-19 lingering manifestations and questions about the LC. The survey model is provided in Supplementary Document S1 for reference.
Acute COVID-19 was defined as the signs and symptoms of COVID-19 that lasted for up to 4 weeks after the acute infection. The LC/PCS is defined as signs and symptoms that develop during or after a COVID-19 infection, persist for at least 12 weeks, and cannot be explained by an alternative diagnosis, while ongoing symptomatic COVID-19 is defined as signs and symptoms that persist for less than 12 weeks after the initial infection (7, 8).
Statistical analysis
The mean and standard deviation were used to describe continuous variables. The Kolmogorov–Smirnov test of statistical normality was used to assess the statistical normality assumption for the metric variables. The metric variables with statistical Normality assumption violations such as skewness were described using median and interquartile range (IQR) scores. Moreover, the categorically measured variables were described with frequencies and percentages, and multiple response dichotomies analysis was used to describe the variables measured with more than one option, such as COVID-19 symptoms. Generalized estimating equation gamma regression analysis was applied to the reported number (i.e., count) of LC symptoms across time. The data had to be restructured into longitudinal data to account for the effects of time on the GEE analysis. The association between the independent predictor variables in the multivariate analysis and the analyzed outcome variables was expressed as exponentiated beta coefficients (Risk Rates) with their associated 95% confidence intervals. The commercially available SPSS IBM statistical analysis program (version 21) was used for statistical data analysis. The statistical significance level was set at p < 0.05.
Ethical considerations
All participants were informed of their enrolment in the study and participant’s informed written consent was obtained before participation. The Declaration of Helsinki’s ethical standards were followed during data collection, handling, and storage, and all precautions were taken to ensure participant confidentiality. The Institutional Review Board (IRB) of Imam Abdulrahman Bin Faisal University in the Eastern Province of Saudi Arabia gave their approval to the study protocol (IRB Number: IRB-2023-01-320).
Results
Sociodemographic characteristics
One thousand three hundred and fifty-five people residing in the KSA were participated, and interview-based questionnaires were completed by those who consented to participate in the study.
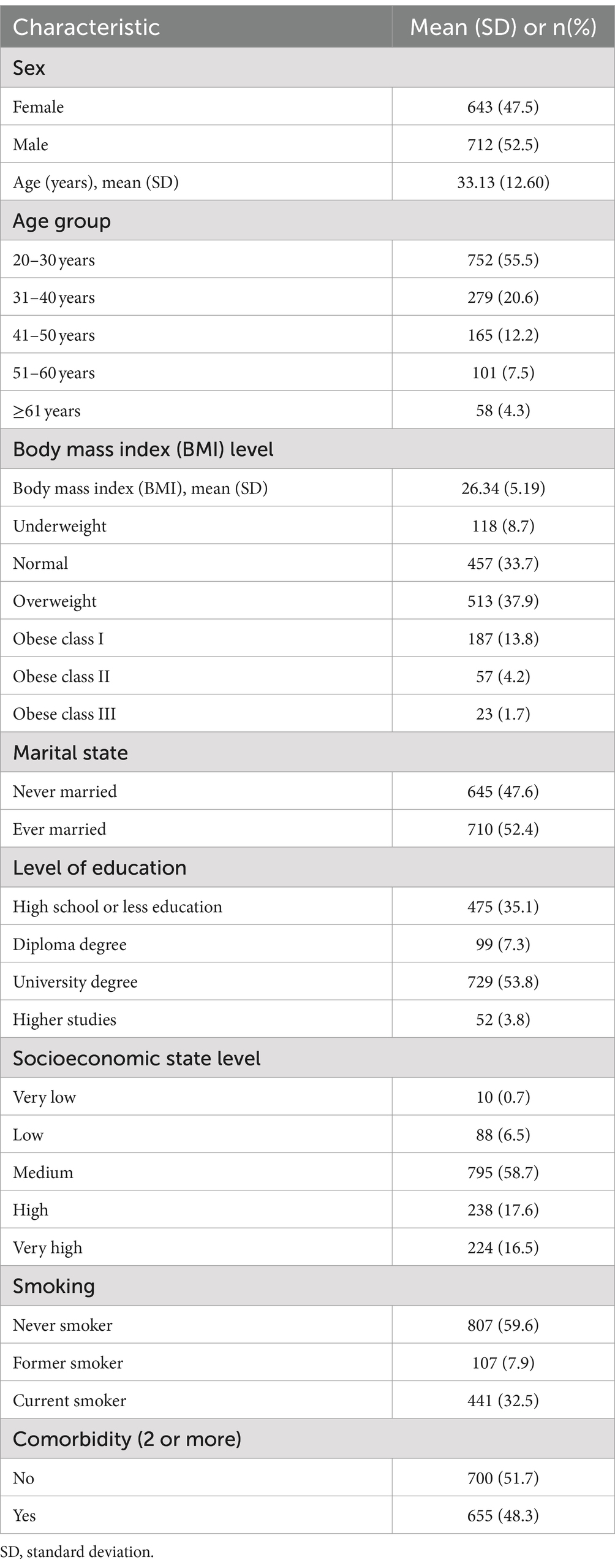
Most of the participants (93.8%) were Saudi citizens, and 6.2% were expatriates living and working within the Kingdom; 47.5% were women and the remainder (52.5%) were men. The mean ± SD age for the sample was 33.13 ± 12.60 years. The mean body mass index (BMI) score was measured at 26.34 ± 5.19%. Participants were also asked to indicate their smoking habit status; the findings showed that 7.9% were ex-smokers and 32.5% were current smokers, while most of the sample (59.6%) were never smokers. Finally, 48.3% of the participants reported having two or more comorbidities (Table 1).
Prevalence of comorbidities
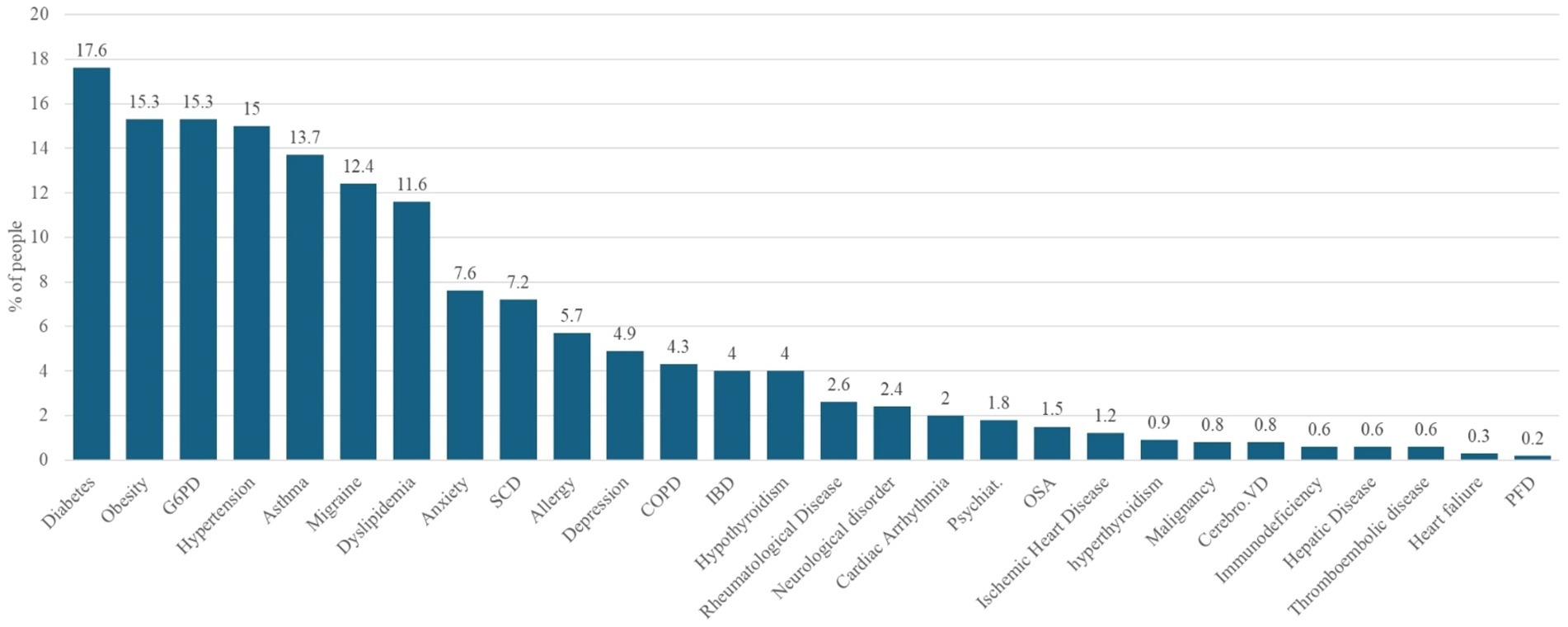
Figure 1 shows the prevalence of comorbidities among patients with COVID-19. A notable proportion of the patients exhibited coexisting medical conditions. The most prevalent comorbidities exceeding the 10% threshold were diabetes (17.6%), obesity (15.3%), G6PD deficiency (15.3%), hypertension (15.0%), and asthma (13.7%). These conditions were followed by migraine, which was reported in 12.4% of patients, and dyslipidemia, which affected 11.6% of the study cohort.
Acute COVID-19 manifestations and clinical characteristics
The patients were asked to state the number of PCR-confirmed COVID-19 infections they had experienced, and the findings showed that 75.9% of them had at least one PCR-confirmed infection. Moreover, the results revealed that the majority (89.5%) had received three COVID-19 vaccine doses. Upon presentation to the hospital, 11.4% of patients had positive evidence of pneumonia. Regarding the need for healthcare, 19% of the patients had no need for any healthcare services, whereas 60.8% needed some form of healthcare that could be managed at home. On the other hand, about 15% of our patients required Emergency Room/Outpatient services, and the remainder, 5.2% of the patients, needed hospital admission (Table 2).
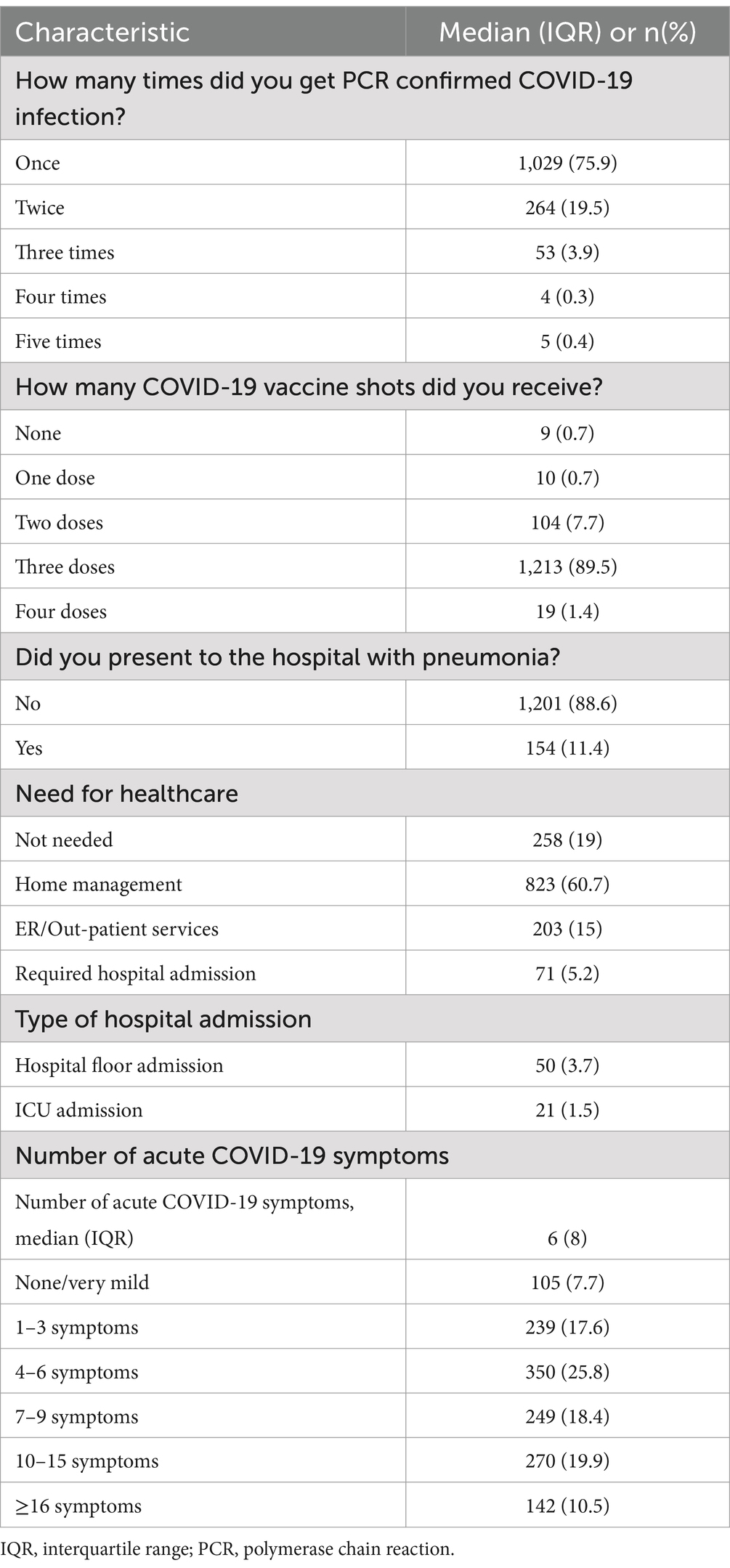
Table 2. Acute COVID-19 manifestations and clinical characteristics of study participants (n = 1,355).
Prevalence of COVID-19 symptoms across acute, ongoing, and LC/PCS phases
Table 3 displays a head-to-head description of the most prevalent COVID-19 symptoms during the acute phase (<1 month) versus the ongoing symptomatic COVID-19 phase (1–3 months) and the LC/PCS phase (≥3 months). Comparing the prevalence of symptoms across the different phases, fever dropped significantly from 74.2% in the acute phase to 2.07% in the LC/PCS phase. Similarly, dyspnea, anosmia, and headache significantly decreased but remained among the most reported symptoms in the LC/PCS phase. Cough, which was reported by 74.2% of patients in the acute phase, witnessed a significant drop in the ongoing symptomatic COVID-19 phase (7%), while still being the most reported symptom. However, in the LC/PCS phase, cough increased in prevalence and was the second most reported symptom (14.24%). Finally, fatigue, which was not the most prevalent symptom in the acute phase, was among the top reported symptoms in the ongoing symptomatic COVID-19 phase (4.1%), and then spiked to rank first in the LC/PCS phase (17.49%).
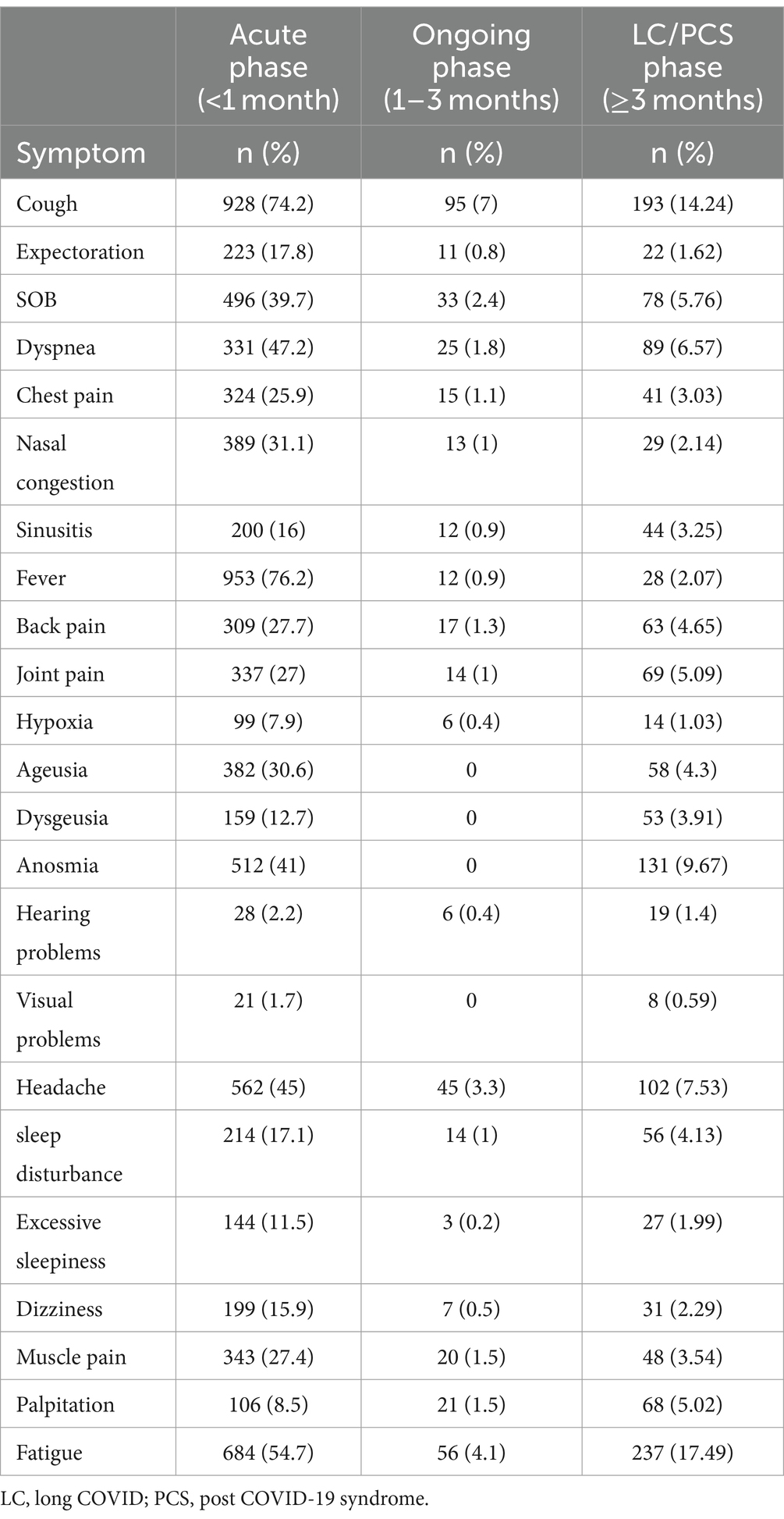
Table 3. Prevalence of COVID-19 symptoms across acute, ongoing, and LC/PCS phases among study participants (n = 1,355).
Longitudinal trends in prevalence of top reported long COVID symptoms
Analysis of the top-reported symptoms among COVID-19 patients over different time periods (1–3 months, 3–6 months, 6–12 months, and > 12 months) revealed distinct trends (Figure 2). Symptoms such as hair loss, memory loss/impairment problems, concentration problems, low mood, joint pain, insomnia, and low performance increased over time, peaking at more than 12 months post-infection. Conversely, the prevalence of smell loss decreased after its initial peaks at 1–3 months. Several symptoms, including headache, shortness of breath, palpitations, vertigo, and muscle pain, exhibited a U-shaped trend, with initial peaks in the early months (1–3 months), a decrease at 3–6 and 6–12 months, and a subsequent increase at >12 months. Fatigue showed a relatively consistently high prevalence over time with a slight increase during the 3–6-month period but no significant long-term increase or decrease. Other symptoms, such as exertional dyspnea, orthostatic hypotension, back pain, and sleep disturbances, declined steadily after their initial peaks of 1–3 months, before re-emerging in prevalence after 12 months. These findings suggest a diverse range of symptom trajectories, some indicating long-term persistence, others resolving over time, and others showing fluctuating patterns.
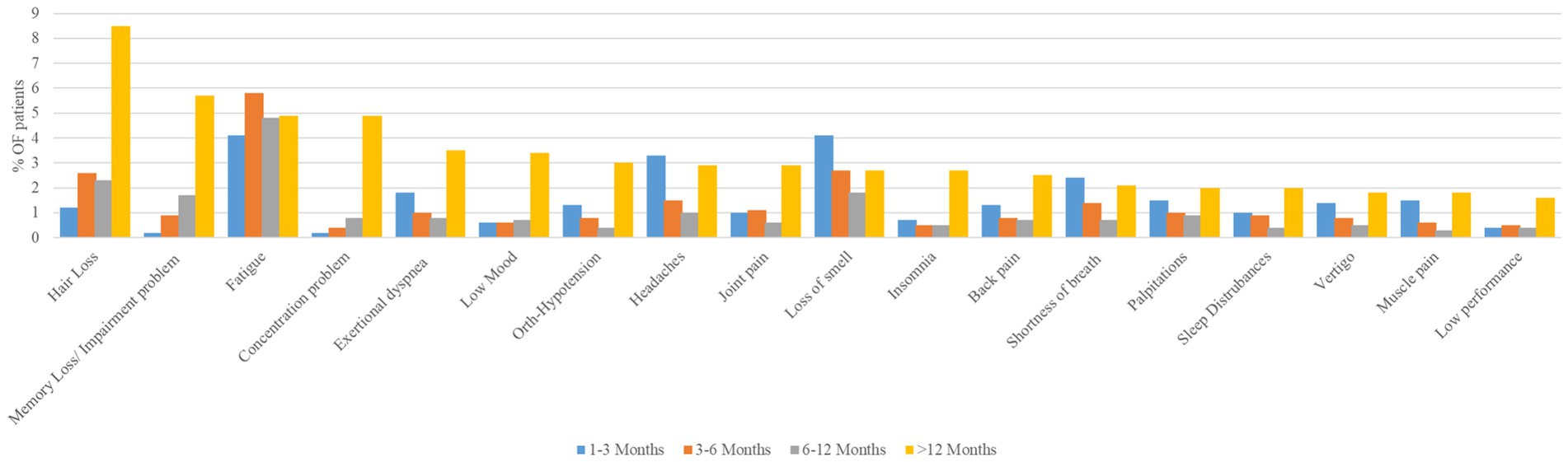
Figure 2. Longitudinal trends in prevalence of top reported long COVID symptoms among study participants across different time periods (n = 1,355).
GEE multivariable gamma regression analysis results of risk factors for long COVID symptom count
Notably, women had a significantly higher mean LC symptom rate (17.4% more) compared to men (p < 0.001). However, the patients’ age did not converge significantly on their symptom rate across time (p = 0.228). Moreover, the patients’ measured count of symptoms was significantly higher (42.5% more) after 12 months on average compared to their baseline (<3 months) (p < 0.001), but the patients’ mean measured symptom rate at 6–12 months and during 3–6 months may not differ significantly compared to the 1–3 months ongoing symptomatic COVID-19 time (p > 0.050) (Table 4).
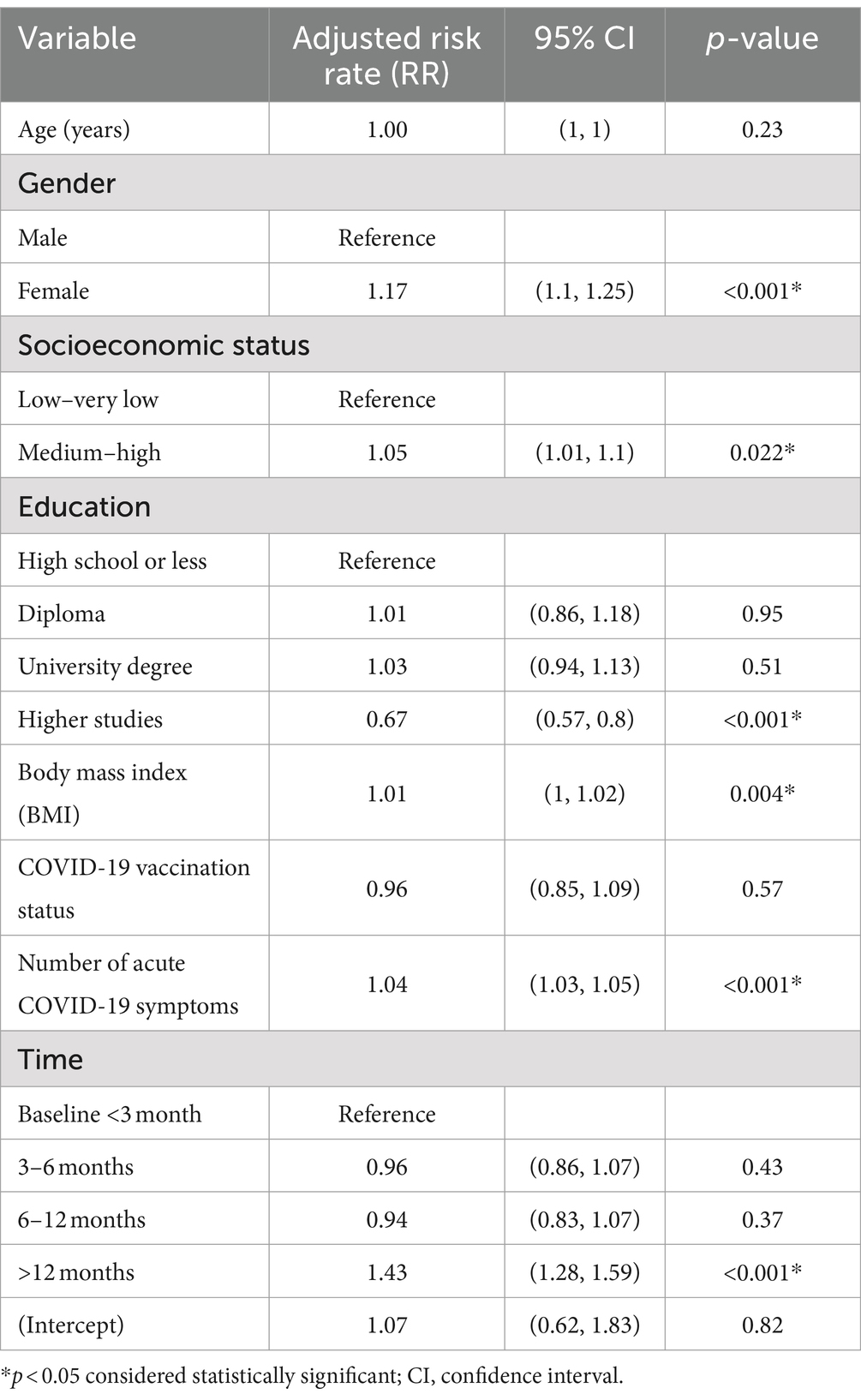
Table 4. GEE multivariable gamma regression analysis results of risk factors for long COVID symptom count among study participants (n = 1,355).
Interestingly, the patients’ mean BMI score was positively associated with the mean number of LC symptoms. For each additional unit in the patients’ BMI, the mean predicted symptom rate tended to increase by a factor of 1.1% on average (p = 0.004), and heavier people reported a greater number of symptoms in general. The patients’ COVID-19 vaccination status had no significant influence on the mean number of reported persistent COVID-19 symptoms, (p = 0.568). In significant ways, the number of acute COVID-19 symptoms was positively associated with the mean number of LC symptoms (p < 0.001). For each additional symptom in the acute phase, the mean number of LC symptoms increased by an average of 4% (Table 4). Lastly, patients with higher education had a 33% lower risk of developing LC symptoms than those with a high school degree or lower (p < 0.001).
Discussion
When looking at the results of our analysis of the responses of the 1,355 participants, it is evident that women had considerable odds of having a higher count of persistent symptomatology from their acute COVID-19 infection. Every unit increase in BMI in our study increased the risk of having higher symptoms count by 1%. Regarding economic capacity and education, there were elevated chances of persistent symptoms, only with an increase in the former (5.4%). Moreover, after looking at a timeline trend, it appeared that the number of LC symptoms was on average higher 12 months than before, and the number of acute COVID-19 symptoms was directly correlated with the number of lingering symptoms.
Research has demonstrated a 26% higher relative risk for individuals with COVID-19 to develop at least one of the LC symptoms. Several factors have been identified as contributing to this increased risk, including female gender, low socio-economic status, smoking, high BMI, and comorbidities (25). Among individuals with a proven history of COVID-19 infection, many risk factors were linked to the reporting of symptoms ≥12 weeks post-infection. Previous studies have consistently shown that women are more susceptible to experiencing long-lasting symptoms (26). In our analysis, the multivariate regression model revealed that women had a 17% chance (RR = 1.174) of having a higher count of persistent symptoms. This phenomenon is consistent with the findings of previous studies. While the literature offers many hypotheses on the underlying mechanisms that explain why women are at a higher risk of LC, among the most cited are immunological variations, such as reduced pro-inflammatory interleukin-6 (IL-6) production following viral infection in women, which would explain their more lasting symptoms (27). Additional variables, such as heightened psychological stress, isolation effects, and inactivity, may have also contributed to their higher risk (28).
Regarding BMI, larger target population studies found that a higher BMI is associated with more persisting symptoms, especially >30 kg/m2 as there is around a 10% relative increase in comparison to those with a BMI between 18.5–25 kg/m2 (28). Another study labeled BMI as the third strongest predictor of LC after increasing age and female sex (29). Notably, there was a positive correlation between the patients’ mean BMI score and the average number of LC symptoms. Every one-unit increase in BMI tended to increase LC symptoms by a factor of 1.1% on average. This relationship was statistically significant (p = 0.004). Age was not found to be a significant predictor in our study. However, the majority of other studies, including a 2023 meta-analysis of over 40 studies, suggested that older age was a significant contributing factor to LC (30). According to another study, this issue is primarily a liability for people who are already frail when infected (31).
Most research examining health disparities has utilized singular outlooks, focusing on individual factors such as sex, race, or deprivation, without adequately exploring the combined impact of intersecting inequalities on population health (32). For example, a study conducted in Brazil highlighted how the cumulative effects of poor health coverage, community disengagement, and low-income households are determinants that may play a significant role in the burden of COVID-19 disease and its complications (33). Their increased vulnerability to the virus may be linked to weakened immune systems owing to relatively higher stress levels (34). To complement this, a recent study inferred that individuals belonging to the most socioeconomically disadvantaged populations face the greatest susceptibility to LC, with an 11% higher risk than thriving individuals, and this disparity persists regardless of variations in the risk of initial infection (34). Our results however, showed that the scales minutely tip in favor of higher socioeconomic status correlated with persistent symptoms. However, this could be explained by the nature of our study population, as more than 90% had medium to high socioeconomic status. In the aforementioned study conducted in Brazil, researchers examined regions with comparatively broad healthcare coverage and observed an increase in the likelihood of identifying new cases only because their symptoms were more reportable and had better accessibility to healthcare facilities, which is also known as a detection bias (33). This was also the main takeaway message from a 2022 Swiss study that found that public health surveillance that determines epidemic severity depending on the number of positive testing cases alone was rather precarious, as it was highly limited to the availability of testing methods at certain locations (35). A higher education level was found to be a protective factor against LC in our data. This was consistent with a Spanish study that found that individuals with tertiary education were not only less likely to be affected by LC but also recovered faster if affected (36).
The regression table illustrates that the measured number of symptoms was significantly higher (42.5%) for >12 months than for the baseline phase (< 3 months). Correspondingly, this study shows how French patients’ COVID-related health conditions started to intensify 6 months after onset (37). Another study from South America showed that approximately 64% patients had at least one symptom reported 12 months after infection. The main risk factor is the mean number of symptoms observed during the acute phase (38). In our data, it was found that every symptom increases in acute presentation raised the risk of more persistent symptoms by approximately 4%. This is not surprising, as we know that the number of acute-phase symptoms correlates with disease severity, which tends to significantly increase the occurrence odds of LC, according to another UK study (39). Figure 2 shows that among the 18 top reported symptoms, 14 were reported at >12 months more than in the acute/ongoing phase.
Strengths and limitations
As for the strengths, the questionnaire used was conducted via face-to-face interviews rather than online, prompting more genuine responses and immediate clarification by volunteers if any question was slightly confusing for the participants. Second, the study assessed syndrome prevalence 2 years post-COVID, a research area that is understudied. Moreover, this patient pool was evident because of its large sample size. It is also notable that a significant subgroup of the patients only needed at-home management, which is interesting as much of the literature regarding the topic always tends to target hospitalized patients or outpatient visitors.
This study has its limitations. First, it was conducted approximately 18 months after the peak of COVID-19 infections in the KSA (according to the WHO), so its retrospective nature may have led to a recall bias of acute and lingering symptoms. Second, the raw data were dependent on face-to-face interviews using questionnaires in public places, potentially leading to selection bias where individuals with LC symptoms may have been more motivated to participate; however, individuals with more severe symptoms may not have been equally represented due to difficulty in participating. Furthermore, the use of convenience sampling may limit the generalizability of the results to the broader population. The symptom ratings in the questionnaire could introduce a degree of subjectivity, and the lack of a control group consisting of non-COVID individuals complicates comparison. However, given the nature of a pandemic, it is challenging, if not impossible, to find individuals who have not been infected to act as a control group, which presents a methodological challenge. Additionally, the absence of objective clinical measures or biomarkers reduces the accuracy and precision of symptom assessment.
Due to these limitations, this study might not accurately reflect the experiences of the entire LC population. Our sample exhibited a significantly higher level of immunity and vaccination compared to other locations, where the majority of individuals may have received only one or two doses, or even none. This factor could possibly explain the distinct findings in our sample and may further limit the generalizability of the results to less vaccinated populations.
We recommend that the data presented be interpreted within the parameters of this study, and caution should be taken when generalizing the findings to all individuals with the condition.
Conclusion
Potential factors linked to a higher number of LC manifestations included female sex, lower socioeconomic status, higher BMI, timing >12 months since COVID-19 infection, and a higher number of acute COVID-19 symptoms. Conversely, higher education offers a greater likelihood of protection against lingering symptoms. Thus, prospective health policy recommendations should integrate several elements of inequality, including sex, occupation, education, and socioeconomic disadvantages, when addressing the approach to and management of LC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The Institutional Review Board (IRB) of Imam Abdulrahman Bin Faisal University in the Eastern Province of Saudi Arabia gave their approval to the study protocol (IRB Number: IRB-2023-01-320). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AFA: Writing – review & editing, Writing – original draft, Visualization, Resources, Conceptualization. MB: Writing – review & editing, Software, Methodology, Formal analysis, Data curation. NA: Writing – original draft, Investigation. AAln: Writing – original draft, Methodology. JA: Writing – original draft, Project administration. AAlm: Writing – review & editing, Supervision. KA: Writing – review & editing, Validation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1459583/full#supplementary-material
References
1. Lai, CC, Shih, TP, Ko, WC, Tang, HJ, and Hsueh, PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. (2020) 55:105924. doi: 10.1016/j.ijantimicag.2020.105924
2. Worldometer. Coronavirus cases. (2024). Available from: https://www.worldometers.info/coronavirus/ (Accessed April 13, 2024).
3. Aspinall, M, and Ruark, L. A pandemic not Just of infection but of inequality: the Social impact of COVID-19. In: SC Schachter, WE Bolton, editors. Accelerating diagnostics in a time of crisis: the response to COVID-19 and a roadmap for future pandemics. (Cambridge, England: Cambridge University Press) (2024) p. 250–62.
4. Alizadeh, H, Sharifi, A, Damanbagh, S, Nazarnia, H, and Nazarnia, M. Impacts of the COVID-19 pandemic on the social sphere and lessons for crisis management: a literature review. Nat Hazards. (2023) 117:2139–64. doi: 10.1007/s11069-023-05959-2
5. Møller, M, Abelsen, T, Sørensen, AIV, Andersson, M, Friis-Hansen, L, Dilling-Hansen, C, et al. Exploring the dynamics of COVID-19 in a Greenlandic cohort: mild acute illness and moderate risk of long COVID. IJID Regions. (2024) 11:100366. doi: 10.1016/j.ijregi.2024.100366
6. World Health Organization. Coronavirus disease (COVID-19). (2024). Available at:https://www.who.int/news-room/fact-sheets/detail/coronavirus-disease-(covid-19) (Accessed August 9, 2023).
7. National Institutes for Health and Care Excellence. COVID-19 rapid guideline: managing the long-term effects of COVID-19. (2020). Available at:https://www.nice.org.uk/guidance/ng188 (Accessed December 18, 2020).
8. Centers for Disease Control and Prevention. Long COVID Basics. (2024). Available at:https://www.cdc.gov/covid/long-term-effects/index.html (Accessed July 11, 2024).
9. Castanares-Zapatero, D, Chalon, P, Kohn, L, Dauvrin, M, Detollenaere, J, Maertens de Noordhout, C, et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med. (2022) 54:1473–87. doi: 10.1080/07853890.2022.2076901
10. Liu, Y, Gu, X, Li, H, Zhang, H, and Xu, J. Mechanisms of long COVID: an updated review. Chin Med J Pulm Crit Care Med. (2023) 1:231–40. doi: 10.1016/j.pccm.2023.10.003
11. Cai, J, Lin, K, Zhang, H, Xue, Q, Zhu, K, Yuan, G, et al. A one-year follow-up study of systematic impact of long COVID symptoms among patients post SARS-CoV-2 omicron variants infection in Shanghai, China. Emerg Microbes Infect. (2023) 12:2220578. doi: 10.1080/22221751.2023.2220578
12. Kamal, M, Abo Omirah, M, Hussein, A, and Saeed, H. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract. (2021) 75:e13746. doi: 10.1111/ijcp.13746
13. Office for National Statistics.Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. (2023). Available at:https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/30march2023 (Accessed March 30, 2023).
14. National Center for Health Statistics. U.S. Census Bureau, Household Pulse Survey, 2022–2024. Long COVID. Generated interactively. (2024). Available at:https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm (Accessed June 17, 2024).
15. Wulf Hanson, S, Abbafati, C, Aerts, JG, Al-Aly, Z, Ashbaugh, C, Ballouz, T, et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. (2022) 328:1604–15. doi: 10.1001/jama.2022.18931
16. Chen, C, Haupert, SR, Zimmermann, L, Shi, X, Fritsche, LG, and Mukherjee, B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. (2022) 226:1593–607. doi: 10.1093/infdis/jiac136
17. Mahmoud, N, Radwan, N, Alkattan, A, Hassanien, M, Elkajam, E, Alqahtani, S, et al. Post-COVID-19 syndrome: nature of symptoms and associated factors. J Public Health. (2024) 32:207–12. doi: 10.1007/s10389-022-01802-3
18. Alghamdi, SA, Alfares, MA, Alsulami, RA, Alghamdi, AF, Almalawi, AM, Alghamdi, MS, et al. Post-COVID-19 syndrome: incidence, risk factor, and the most common persisting symptoms. Cureus. (2022) 14:e32058. doi: 10.7759/cureus.32058
19. Líška, D, Liptaková, E, Babičová, A, Batalik, L, Baňárová, PS, and Dobrodenková, S. What is the quality of life in patients with long COVID compared to a healthy control group? Front Public Health. (2022) 10:975992. doi: 10.3389/fpubh.2022.975992
20. O’ Mahony, L, Buwalda, T, Blair, M, Forde, B, Lunjani, N, Ambikan, A, et al. Impact of long COVID on health and quality of life. HRB Open Res. (2022) 5:31. doi: 10.12688/hrbopenres.13516.1
21. Office for National Statistics. Self-reported long COVID after infection with the Omicron variant in the UK (2022). Available at:https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/selfreportedlongcovidafterinfectionwiththeomicronvariant/18july2022 (Accessed July 18, 2022).
22. AlRadini, FA, Alamri, F, Aljahany, MS, Almuzaini, Y, Alsofayan, Y, Khan, A, et al. Post-acute COVID-19 condition in Saudi Arabia: a national representative study. J Infect Public Health. (2022) 15:526–32. doi: 10.1016/j.jiph.2022.03.013
23. Khodeir, MM, Shabana, HA, Rasheed, Z, Alkhamiss, AS, Khodeir, M, Alkhowailed, MS, et al. COVID-19: post-recovery long-term symptoms among patients in Saudi Arabia. PLoS One. (2021) 16:e0260259. doi: 10.1371/journal.pone.0260259
24. Alkodaymi, MS, Omrani, OA, Fawzy, NA, Shaar, BA, Almamlouk, R, Riaz, M, et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect. (2022) 28:657–66. doi: 10.1016/j.cmi.2022.01.014
25. Subramanian, A, Nirantharakumar, K, Hughes, S, Myles, P, Williams, T, Gokhale, KM, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. (2022) 28:1706–14. doi: 10.1038/s41591-022-01909-w
26. Morioka, S, Tsuzuki, S, Maruki, T, Terada, M, Miyazato, Y, Kutsuna, S, et al. Epidemiology of post-COVID conditions beyond 1 year: a cross-sectional study. Public Health. (2023) 216:39–44. doi: 10.1016/j.puhe.2023.01.008
27. Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. (2021) 27:28–33. doi: 10.1038/s41591-020-01202-8
28. Fernández-de-las-Peñas, C, Martín-Guerrero, JD, Pellicer-Valero, ÓJ, Navarro-Pardo, E, Gómez-Mayordomo, V, Cuadrado, ML, et al. Female sex is a risk factor associated with long-term post-COVID related-symptoms but not with COVID-19 symptoms: the LONG-COVID-EXP-CM multicenter study. J Clin Med. (2022) 11:413. doi: 10.3390/jcm11020413
29. Shabnam, S, Razieh, C, Dambha-Miller, H, Yates, T, Gillies, C, Chudasama, YV, et al. Socioeconomic inequalities of long COVID: a retrospective population-based cohort study in the United Kingdom. J R Soc Med. (2023) 116:263–73. doi: 10.1177/01410768231168377
30. Tsampasian, V, Elghazaly, H, Chattopadhyay, R, Debski, M, Naing, TKP, Garg, P, et al. Risk factors associated with post−COVID-19 condition. JAMA Intern Med. (2023) 183:566–80. doi: 10.1001/jamainternmed.2023.0750
31. Li, X, Zhang, C, and Bao, Z. Mast cell activation may contribute to adverse health transitions in COVID-19 patients with frailty. Emerg Microbes Infect. (2023) 12:2251589. doi: 10.1080/22221751.2023.2251589
32. Bambra, C. Placing intersectional inequalities in health. Health Place. (2022) 75:102761. doi: 10.1016/j.healthplace.2022.102761
33. Santos, VS, Siqueira, TS, Atienzar, AIC, Santos, MAR, Vieira, SCF, Lopes, ASA, et al. Spatial clusters, social determinants of health and risk of COVID-19 mortality in Brazilian children and adolescents: a nationwide population-based ecological study. Lancet Reg Health Am. (2022) 13:100311. doi: 10.1016/j.lana.2022.100311
34. Khanijahani, A, Iezadi, S, Gholipour, K, Azami-Aghdash, S, and Naghibi, D. A systematic review of racial/ethnic and socioeconomic disparities in COVID-19. Int J Equity Health. (2021) 20:248. doi: 10.1186/s12939-021-01582-4
35. Tancredi, S, Cullati, S, and Chiolero, A. Estimating the magnitude of surveillance bias in COVID-19. Eur J Pub Health. (2022) 32:ckac130.096. doi: 10.1093/eurpub/ckac130.096
36. Mateu, L, Tebe, C, Loste, C, Santos, JR, Lladós, G, López, C, et al. Determinants of the onset and prognosis of the post-COVID-19 condition: a 2-year prospective observational cohort study. Lancet Reg Health Eur. (2023) 33:100724. doi: 10.1016/j.lanepe.2023.100724
37. Tran, VT, Porcher, R, Pane, I, and Ravaud, P. Course of post COVID-19 disease symptoms over time in the ComPaRe long COVID prospective e-cohort. Nat Commun. (2022) 13:1812. doi: 10.1038/s41467-022-29513-z
38. Silva, KM, Freitas, DCA, Medeiros, SS, Miranda, LVA, Carmo, JBM, Silva, RG, et al. Prevalence and predictors of COVID-19 long-term symptoms: a cohort study from the Amazon Basin. Am J Trop Med Hyg. (2023) 109:466–70. doi: 10.4269/ajtmh.22-0362
Keywords: long COVID, post-COVID-19 syndrome, post-COVID condition, ongoing symptomatic COVID-19, COVID-19
Citation: Aldhawyan AF, BuSaad MA, Almaghlouth NE, Alnasser AH, Alnasser JA, Almansour AH and AlHarkan KS (2024) Understanding long COVID: prevalence, characteristics, and risk factors in the Eastern Province of Saudi Arabia. Front. Med. 11:1459583. doi: 10.3389/fmed.2024.1459583
Edited by:
Arch Mainous, University of Florida, United StatesReviewed by:
Esteban Ortiz-Prado, University of the Americas, EcuadorZaki A. Sherif, Howard University, United States
Copyright © 2024 Aldhawyan, BuSaad, Almaghlouth, Alnasser, Alnasser, Almansour and AlHarkan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adam F. Aldhawyan, YWFhbGRoYXd5YW5AaWF1LmVkdS5zYQ==
†ORCID: Adam F. Aldhawyan, orcid.org/0000-0002-5066-2243
Mohammed A. BuSaad, orcid.org/0000-0002-7695-3428
Nawaf E. Almaghlouth, orcid.org/0009-0008-8514-5052
Abdullah H. Alnasser, orcid.org/0009-0001-9668-4851
Jomana A. Alnasser, orcid.org/0009-0002-8231-7812
Abdulelah H. Almansour, orcid.org/0000-0003-3741-6520
Khalid S. AlHarkan, orcid.org/0000-0002-5480-2557
 Adam F. Aldhawyan
Adam F. Aldhawyan Mohammed A. BuSaad
Mohammed A. BuSaad Nawaf E. Almaghlouth
Nawaf E. Almaghlouth Abdullah H. Alnasser2†
Abdullah H. Alnasser2† Jomana A. Alnasser
Jomana A. Alnasser Abdulelah H. Almansour
Abdulelah H. Almansour Khalid S. AlHarkan
Khalid S. AlHarkan