
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med., 22 January 2025
Sec. Pathology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1458606
Epstein–Barr virus-positive mucocutaneous ulcer (EBVMCU) is a rare condition characterized by skin or mucosal lesions resulting from defective immune surveillance of EBV due to immunosuppression, which can be iatrogenic or age-related. It represents a benign lymphoproliferative disorder that clinically may mimic malignant tumors. However, EBVMCU typically progresses slowly and often resolves spontaneously without specific treatment, emphasizing the critical need for differential diagnosis from malignancies. In this study, we report a case of EBVMCU in an elderly patient demonstrating ulcers on the oral mucosa, buccal area, and maxillary mucosa, with associated bone destruction, which was initially suspected to be an oral malignancy but was confirmed as EBVMCU through biopsy. This case underscores the importance of considering EBVMCU in elderly patients with unexplained persistent mucosal ulcers to exclude malignancies. In addition, attention should be given to unhealthy lifestyle habits such as smoking and drinking, as well as poor oral hygiene, which are potential factors that increase the risk of this disease and contribute to worse prognoses.
EBVMCU was described as a rare disease in 2010 (1). In the 5th edition (2022) of the World Health Organization (WHO) classification of hematolymphoid tumors, EBVMCU is classified under lymphoproliferative disorders and lymphomas associated with immunodeficiency and immune dysregulation (2). EBVMCU is considered an indolent tumor with self-limiting clinical features related to EBV infection. It often occurs in the context of drug-induced immunosuppression, age-related immunosenescence, and primary or acquired immunodeficiency, where EBV-infected cells can evade immune surveillance and proliferate, leading to EBV-related lymphoproliferative disorders (3).
EBV, also known as human herpesvirus 4, is a common virus primarily transmitted through saliva (4). It persistently infects B cells in over 95% of adults, resulting in asymptomatic lifelong carriage (5). When EBV disrupts host immune homeostasis, it can lead to a spectrum of Epstein–Barr virus-associated B-cell lymphoproliferative disorders (EBV-B-LPDs), ranging from indolent to lymphomas. These include Burkitt lymphoma (6), Hodgkin lymphoma (7), post-transplant lymphoproliferative disorder (8), and Epstein–Barr virus-positive mucocutaneous ulcer (EBVMCU), among others.
EBVMCU was first characterized as an Epstein–Barr virus-associated B-cell lymphoproliferative disorder in 2010 by Dojcinov et al., who reported a series of cases involving mucosal sites of the oropharynx, skin, and gastrointestinal tract (1). EBVMCU primarily arises due to immunosuppression, which can be age-related immunosenescence and iatrogenic immunosuppression induced by therapies such as methotrexate, cyclosporine, and azathioprine, as well as steroid treatments (9). While it is rarely observed in individuals with primary immunodeficiencies, it is more frequently noted in solid organ or bone marrow transplant recipients, HIV-positive patients, and individuals undergoing treatment for other lymphomas or tumors (10, 11).
EBVMCU presents as well-defined ulcers in the oral cavity, gastrointestinal tract, or skin, characterized by shallow, solitary, and painful ulcers with distinct borders. There are no associated systemic symptoms or involvement of other sites (12). Due to the non-specific nature of ulcerative lesions, diagnosing EBVMCU poses a challenge, making it difficult to distinguish it from other neoplastic conditions. Research indicates that the median age of EBVMCU patients is 71 years, with 27% of reported cases occurring in patients aged 60 years or older, without known immunodeficiency (9). Immunohistochemical studies of ulcer tissues have shown positivity for CD20, CD30, EBER, MUM-1, OCT-2, and PAX-5 in monoclonal B-immunoblasts (13). Therefore, persistent skin ulcers in elderly patients with age-related immunosenescence should raise suspicion for EBVMCU.
In this study, we report a case of EBVMCU in an elderly patient with age-related immunosenescence to improve clinical recognition and reduce the risk of misdiagnosis and missed diagnosis.
The patient is an 84-year-old man from China who presented with persistent ulcerations of the gums, buccal mucosa, and maxillary mucosa for 3 months. He was admitted to the Department of Infectious Diseases at the First Affiliated Hospital of Ningbo University. Three months ago, the patient developed ulcers on the maxillary gums and mucosa, accompanied by local purulent plaques (Figure 1). Sequential treatments with intravenous cefmetazole (2.0 g q12h) and oral cefdinir capsules (0.1 g q8h) at previous hospitals showed no improvement, prompting admission to our hospital. The patient had a history of hypertension for over 10 years, well-controlled with oral indapamide 1.5 mg daily. He had a smoking history of over 20 years, averaging 10 cigarettes per day, and an alcohol consumption history of over 30 years, averaging 50 g of liquor daily.
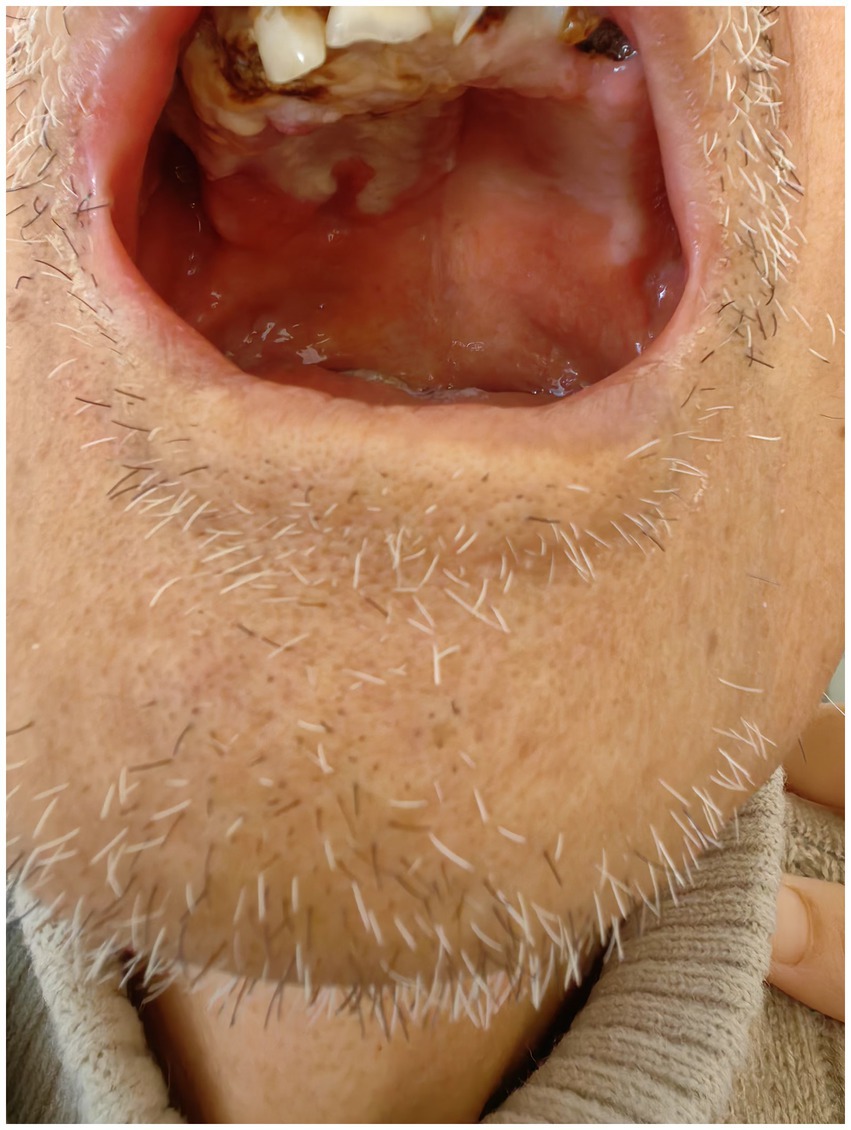
Figure 1. During the initial visit, the patient presented with extensive purulent deposits on the tooth surfaces, accompanied by multiple residual roots. In addition, significant ulceration was noted on the mucosa of the cheeks and upper palate.
On admission examination, the patient presents with normal facial symmetry and a mouth opening to three fingers’ width with a normal opening type. However, the patient has extremely poor oral hygiene, characterized by numerous purulent plaques, soft calculus deposits on teeth surfaces, and multiple residual roots in the oral cavity. Additionally, there is a yellow pseudomembrane covering various surfaces, along with extensive irregularly shaped erosions and ulcers with unclear borders and tenderness. The upper palate mucosa shows concave surfaces.
Following admission, comprehensive investigations were performed, and detailed laboratory results are shown in Table 1. A plain computed tomography scan of the maxillofacial region indicated local bone destruction with surrounding soft tissue swelling on the right upper palate, prompting clinical correlation and biopsy to exclude neoplasms (Figure 2). The patient was treated with meropenem (1 g q8h) for anti-infective therapy and received oral hygiene measures, with inadequate response. A subsequent positron emission tomography/computed tomography scan revealed bone destruction of the maxilla with surrounding soft tissue shadows, as well as soft tissue shadows in the left nasal cavity. Increased FDG metabolism was noted in these lesions, suggesting the presence of malignant tumors. Lymphoma should be ruled out, and a soft tissue biopsy was recommended for further evaluation. Following a tissue biopsy, the mucosal ulcers and significant lymphoid tissue hyperplasia were evaluated. Immunohistochemical results (Figures 3, 4) indicated CD79a (B cells+), CD20 (B cells+), Mum-1 (plasma cells+), Ki-67 (ulcer area+, 20%), EBER (+), PAX-5 (cells+), C-myc (<5%), and CD30 (+), suggesting EBV-positive mucocutaneous ulcers. Subsequently, we discontinued antibiotic therapy and advised the patient to maintain oral hygiene, cease smoking and alcohol consumption, and undergo follow-up observation. After a 3-month follow-up, it was found that the patient’s disease continued to progress. The persistent oral ulcer caused difficulty in eating, which led the patient to discontinue treatment, and eventually the patient passed away.
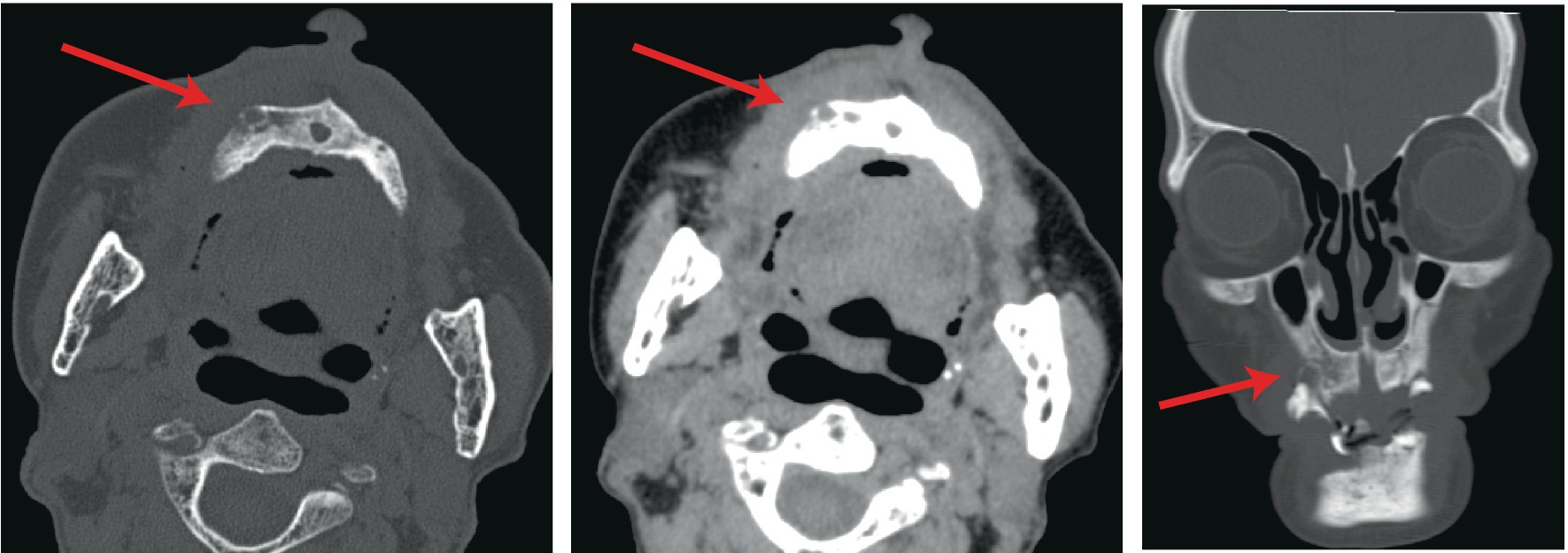
Figure 2. A plain computed tomography scan of the maxillofacial region indicated local bone destruction with surrounding soft tissue swelling on the right upper palate.

Figure 3. The tissue biopsy results revealed diffuse proliferation of atypical lymphocytes of varying sizes. Immunohistochemical results indicated PAX-5 (cells+), Mum-1 (plasma cells+), and Ki-67 (ulcer area+, 20%).
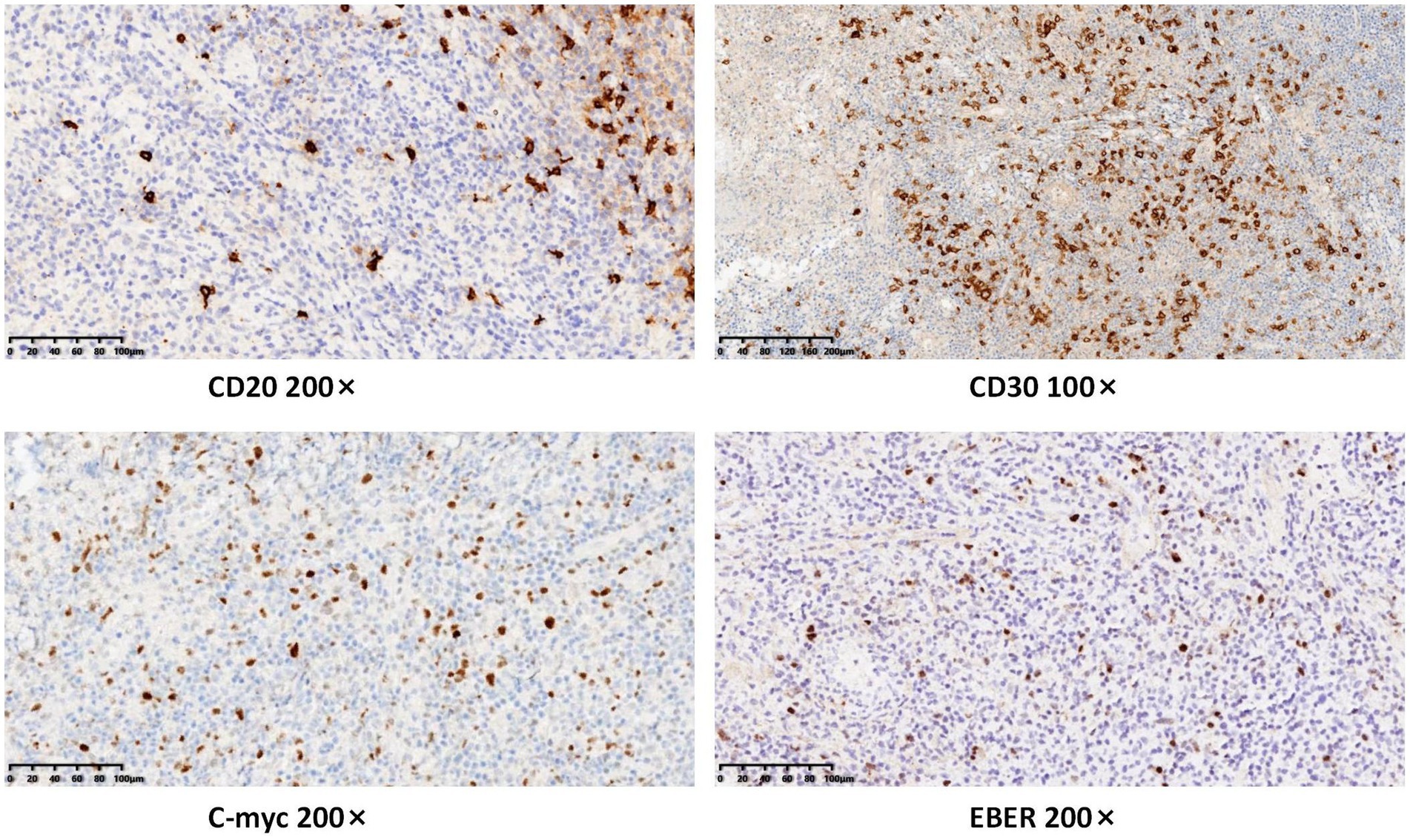
Figure 4. Immunohistochemical results indicated CD20 (B cells+), CD30 (+), C-myc (<5%), and EBER (+).
Our patient presented with persistent gingival and palatal mucosal ulceration lasting 3 months. After conventional anti-infective treatment proved ineffective, a maxillofacial CT scan revealed localized bone destruction. The primary differential diagnosis focused on excluding oral cancer. Oral cancer often manifests as persistent ulcers lasting more than 3 weeks, typically accompanied by indurated edges, erythroleukoplakia (red and white patches), nodules, and bone destruction. These features are frequently associated with risk factors such as smoking, alcohol consumption, and poor oral hygiene (14, 15). Unlike the typical characteristics of EBVMCU, our patient’s clinical presentation was more suggestive of oral cancer, especially given the presence of high-risk factors. However, pathological examination ultimately indicated lymphoid hyperplasia, and immunohistochemistry confirmed features consistent with EBVMCU. As a result, we excluded the diagnosis of oral cancer.
In over 90% of EBVMCU cases, there is an underlying primary or secondary immunodeficiency (1). The literature suggests that unhealthy habits, such as long-term smoking and alcohol consumption, may weaken the immune system, impairing the body’s ability to control EBV and potentially increasing the risk of EBV-related diseases (16). The primary cause of age-related EBVMCU is thought to be the gradual decline in immune system function with aging. This decline includes thymic atrophy, reduced production of naïve T cells, accumulation of activated memory T cells, decreased lymphocyte cytokine secretion, impaired antigen-presenting cell function, and chronic inflammation. These changes lead to diminished T-cell activity in response to antigen stimulation (16, 17). A direct consequence of this immunosenescence is the potential reactivation of EBV, which can result in EBVMCU (3). EBVMCU lesions can occur at various body sites but are most commonly found in the oropharyngeal mucosa (10, 18). This distribution is attributed to the high concentration of EBV-infected B cells in Waldeyer’s ring and the oral cavity’s propensity for microtrauma to mucosal surfaces due to food debris and dentures (19). Our patient, an 84-year-old man, had no history of immunosuppressant use or immunodeficiency-related diseases, making this a clear case of age-related EBVMCU. In addition, the patient exhibited extremely poor oral hygiene and a long history of smoking and alcohol consumption. Coupled with age-related immune decline, these factors likely created a susceptible environment for the development of EBVMCU.
EBVMCU is typically described as an indolent, self-limiting disease with a benign clinical course, often requiring no treatment (20). For age-related EBVMCU, current treatment and prognosis recommendations prioritize follow-up monitoring. It is advised to observe for 2–8 weeks, with chemotherapy, radiotherapy, or surgical intervention recommended only if the lesions remain unchanged or progress during follow-up (11). Regarding prognosis, studies report that 80% of EBVMCU cases achieve remission, with only 1% resulting in mortality due to disease progression (9). In a follow-up study of 34 EBVMCU patients, all demonstrated favorable outcomes after either immunosuppressant reduction or chemotherapy (21). In addition, a systematic review of 186 EBVMCU cases found that during a follow-up period (ranging from 1 to 180 months), 8 cases showed spontaneous ulcer resolution, and 126 achieved complete or partial remission after stopping or reducing immunosuppressants, chemotherapy, or radiotherapy (10). However, other studies suggest different approaches. Roberts et al. argue that although many EBVMCU cases exhibit a self-limiting course, the disease may also present as a debilitating, persistent condition requiring active treatment to prevent progression (22). In our case, the patient initially opted for close monitoring. Unfortunately, due to the progressive nature of the oral ulcerations, the patient missed the optimal treatment window and ultimately succumbed to the condition after forgoing further treatment. Currently, no evidence-based guidelines or expert consensus exists for EBVMCU treatment. However, based on the unfavorable outcome of this case, we suggest that early intervention may be warranted for age-related EBVMCU. A comprehensive assessment of the patient’s overall health, immune status, and lifestyle factors may help prevent disease progression and avoid missed opportunities for effective treatment.
In conclusion, we present a unique case of EBVMCU involving the buccal mucosa and upper palate with bone destruction, initially misdiagnosed as oral malignancy but confirmed through biopsy. Unlike the majority of EBVMCU cases, which exhibit spontaneous remission, our patient experienced progressive ulceration and eventually succumbed to the disease. This case highlights the potential impact of lifestyle factors, such as oral hygiene, smoking, and alcohol consumption, on disease progression. It also raises questions about the optimal management strategy for age-related EBVMCU, as current evidence suggests that the majority of cases are resolved with close monitoring. Further large-scale studies are needed to clarify treatment protocols for age-related EBVMCU and assess the influence of lifestyle factors on prognosis. Close collaboration between clinicians and pathologists is essential to prevent misdiagnosis and ensure timely intervention.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Ningbo University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JY: Conceptualization, Data curation, Writing – original draft, Writing – review & editing, Formal analysis. SJ: Data curation, Formal analysis, Writing – original draft. ZW: Data curation, Formal analysis, Writing – original draft. GM: Data curation, Formal analysis, Writing – original draft. JC: Data curation, Formal analysis, Writing – original draft. SW: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. YX: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We appreciate Shengqiang Ji’s valuable contributions to the pathological diagnosis and immunohistochemistry of the patients. We also thank the patient and their family for their selfless assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dojcinov, SD, Venkataraman, G, Raffeld, M, Pittaluga, S, and Jaffe, ES. EBV positive mucocutaneous ulcer--a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. (2010) 34:405–17. doi: 10.1097/PAS.0b013e3181cf8622
2. Alaggio, R, Amador, C, Anagnostopoulos, I, Attygalle, AD, Araujo, IBO, Berti, E, et al. The 5th edition of the World Health Organization classification of Haematolymphoid Tumours: lymphoid neoplasms. Leukemia. (2022) 36:1720–48. doi: 10.1038/s41375-022-01620-2
3. Fafi-Kremer, S, Morand, P, Brion, JP, Pavese, P, Baccard, M, Germi, R, et al. Long-term shedding of infectious epstein-barr virus after infectious mononucleosis. J Infect Dis. (2005) 191:985–9. doi: 10.1086/428097
4. Taylor, GS, Long, HM, Brooks, JM, Rickinson, AB, and Hislop, AD. The immunology of Epstein-Barr virus-induced disease. Annu Rev Immunol. (2015) 33:787–821. doi: 10.1146/annurev-immunol-032414-112326
5. Thorley-Lawson, DA, and Gross, A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. (2004) 350:1328–37. doi: 10.1056/NEJMra032015
6. De-Thé, A, G, Geser, NED, Tukei, PM, Williams, EH, Beri, DP, Smith, PG, et al. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt's lymphoma from Ugandan prospective study. Nature. (1978) 274:756–61. doi: 10.1038/274756a0
7. Weiss, LM, Movahed, LA, Warnke, RA, and Sklar, J. Detection of Epstein-Barr viral genomes in reed-Sternberg cells of Hodgkin's disease. N Engl J Med. (1989) 320:502–6. doi: 10.1056/nejm198902233200806
8. Brink, AA, Dukers, DF, van den Brule, AJ, Oudejans, JJ, Middeldorp, JM, Meijer, CJ, et al. Presence of Epstein-Barr virus latency type III at the single cell level in post-transplantation lymphoproliferative disorders and AIDS related lymphomas. J Clin Pathol. (1997) 50:911–8. doi: 10.1136/jcp.50.11.911
9. Sinit, RB, Horan, KL, Dorer, RK, and Aboulafia, DM. Epstein-Barr virus-positive Mucocutaneous ulcer: case report and review of the first 100 published cases. Clin Lymphoma Myeloma Leuk. (2019) 19:e81–92. doi: 10.1016/j.clml.2018.10.003
10. Ikeda, T, Gion, Y, Nishimura, Y, Nishimura, MF, Yoshino, T, and Sato, Y. Epstein-Barr virus-positive Mucocutaneous ulcer: A unique and curious disease entity. Int J Mol Sci. (2021) 22:53. doi: 10.3390/ijms22031053
11. Ikeda, T, Gion, Y, Yoshino, T, and Sato, Y. A review of EBV-positive mucocutaneous ulcers focusing on clinical and pathological aspects. J Clin Exp Hematop. (2019) 59:64–71. doi: 10.3960/jslrt.18039
12. Donzel, M, Neyrand, S, Skowron, F, Balme, B, and Traverse-Glehen, A. An EBV-positive mucocutaneous ulcer in an unusual location with a surprising evolution. Eur J Dermatol. (2021) 31:121–5. doi: 10.1684/ejd.2021.3982
13. Swerdlow, SH, Campo, E, Pileri, SA, Harris, NL, Stein, H, Siebert, R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. (2016) 127:2375–90. doi: 10.1182/blood-2016-01-643569
14. Ellington, TD, Henley, SJ, Senkomago, V, O'Neil, ME, Wilson, RJ, Singh, S, et al. Trends in incidence of cancers of the Oral cavity and pharynx - United States 2007-2016. MMWR Morb Mortal Wkly Rep. (2020) 69:433–8. doi: 10.15585/mmwr.mm6915a1
15. Shield, KD, Ferlay, J, Jemal, A, Sankaranarayanan, R, Chaturvedi, AK, Bray, F, et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. (2017) 67:51–64. doi: 10.3322/caac.21384
16. Hislop, AD, Taylor, GS, Sauce, D, and Rickinson, AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. (2007) 25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553
17. Barber, DL, Wherry, EJ, Masopust, D, Zhu, B, Allison, JP, Sharpe, AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. (2006) 439:682–7. doi: 10.1038/nature04444
18. Plaza, JA, Gru, AA, Sangueza, OP, Lourenco, SV, Puccio, FB, Sanches, JA, et al. An update on viral-induced cutaneous lymphoproliferative disorders. CME part I. J Am Acad Dermatol. (2023) 88:965–80. doi: 10.1016/j.jaad.2021.11.068
19. Laichalk, LL, Hochberg, D, Babcock, GJ, Freeman, RB, and Thorley-Lawson, DA. The dispersal of mucosal memory B cells: evidence from persistent EBV infection. Immunity. (2002) 16:745–54. doi: 10.1016/s1074-7613(02)00318-7
20. Ohata, Y, Tatsuzawa, A, Ohyama, Y, Ichikawa, A, Mochizuki, Y, Ishibashi, S, et al. A distinctive subgroup of oral EBV+ B-cell neoplasm with polymorphous features is potentially identical to EBV+ mucocutaneous ulcer. Hum Pathol. (2017) 69:129–39. doi: 10.1016/j.humpath.2017.09.013
21. Ikeda, T, Gion, Y, Sakamoto, M, Tachibana, T, Nishikori, A, Nishimura, MF, et al. Clinicopathological analysis of 34 Japanese patients with EBV-positive mucocutaneous ulcer. Mod Pathol. (2020) 33:2437–48. doi: 10.1038/s41379-020-0599-8
Keywords: Epstein–Barr virus, Epstein–Barr virus-positive mucocutaneous ulcer, age-related immunosuppression, mandibular necrosis, case report
Citation: Ye J, Ji S, Wu Z, Ma G, Chen J, Wu S and Xie Y (2025) Age-related immunosenescence and Epstein–Barr virus-positive mucocutaneous ulcer presenting as oral cancer: a case report. Front. Med. 11:1458606. doi: 10.3389/fmed.2024.1458606
Received: 02 July 2024; Accepted: 31 December 2024;
Published: 22 January 2025.
Edited by:
Maria Contaldo, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Wenjun Meng, Sichuan University, ChinaCopyright © 2025 Ye, Ji, Wu, Ma, Chen, Wu and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shimin Wu, OTk3ODA3OEBxcS5jb20=; Yilian Xie, c3VnZXI4NDIwMDNAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.