
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 23 January 2025
Sec. Hepatobiliary Diseases
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1458586
This article is part of the Research Topic Case Reports in Hepatobiliary Diseases View all 16 articles
 Ekaterina Bondarenko1
Ekaterina Bondarenko1 Dmitriy Kalinin2
Dmitriy Kalinin2 Liliya Urusova1
Liliya Urusova1 Dariya Pastukhova1
Dariya Pastukhova1 Rustam Salimkhanov3*
Rustam Salimkhanov3* Natalia Mokrysheva4
Natalia Mokrysheva4Intrahepatic cholangiocarcinoma is a highly malignant tumor with a poor prognosis. Radical surgical resection remains the “gold standard” for improving patient outcomes; however, only a minority of patients qualify for this approach. Intrahepatic cholangiocarcinoma is primarily classified into two major histologic types: small and large ductal cholangiocarcinomas. Nevertheless, rare subtypes with unique diagnostic and prognostic characteristics are increasingly reported. These subtypes often exhibit features such as slow growth, a histologic architecture resembling thyroid tissue, or ductal ectasia, and are associated with a more favorable prognosis. We present the case of a 61-year-old patient with a solitary liver mass initially identified as a hemangioma through imaging studies. Histopathologic examination of the postoperative specimen revealed a thyroid-like structural pattern. Immunohistochemical analysis showed positive staining for CK7 and CK19, confirming the diagnosis of intrahepatic cholangiocarcinoma with a thyroid-like structure. The tumor was completely resected with clear margins, and no evidence of metastasis was found. Consequently, the patient was managed without adjuvant chemotherapy. At 14 months of follow-up, there were no signs of recurrence or metastasis. This clinical case underscores the importance of recognizing novel subtypes of cholangiocarcinoma and exercising vigilance in the management of patients with presumed benign hepatic lesions.
Cholangiocarcinoma is the second most common primary liver malignancy after hepatocellular carcinoma, accounting for approximately 15% of all primary liver tumors and 3% of all gastrointestinal cancers (1, 2). Despite its high morbidity and mortality rates, the clinical course of cholangiocarcinoma is highly variable (3). The tumor is classified into two main histologic types based on duct size: small-duct and large-duct cholangiocarcinomas (2, 4). Differentiating these types requires histopathologic, clinicopathologic, and molecular analyses. The most common subtypes include sclerosing, nodular, and papillary (polypoid) forms (5).
A particularly rare variant of intrahepatic cholangiocarcinoma exhibits histologic features resembling thyroid carcinoma. Fewer than 10 cases of this subtype have been reported globally. This rarity, coupled with its slow growth and histologic resemblance to thyroid tumors or ectopia, poses significant diagnostic challenges. Accurate diagnosis requires a thorough immunohistochemical (IHC) study and the exclusion of thyroid pathology.
A 61-year-old male patient presented with a 3-year history of right subcostal heaviness and belching. Based on clinical manifestations, a diagnosis of gastroesophageal reflux disease (GERD) with reflux esophagitis was established. Conservative therapy following standard protocols was initially effective; however, symptoms recurred after discontinuing the treatment.
In September 2021, hepatobiliary ultrasound (US) revealed a 54 × 30 mm mass in the 5th segment of the liver’s right lobe. Continued monitoring of the mass was recommended. By September 2022, contrast-enhanced magnetic resonance imaging (MRI) of the abdomen showed tumor growth to 70 × 42 × 50 mm, now occupying the 5th and 6th liver segments. The imaging characteristics suggested a vascular origin, such as hemangioma (Figure 1).
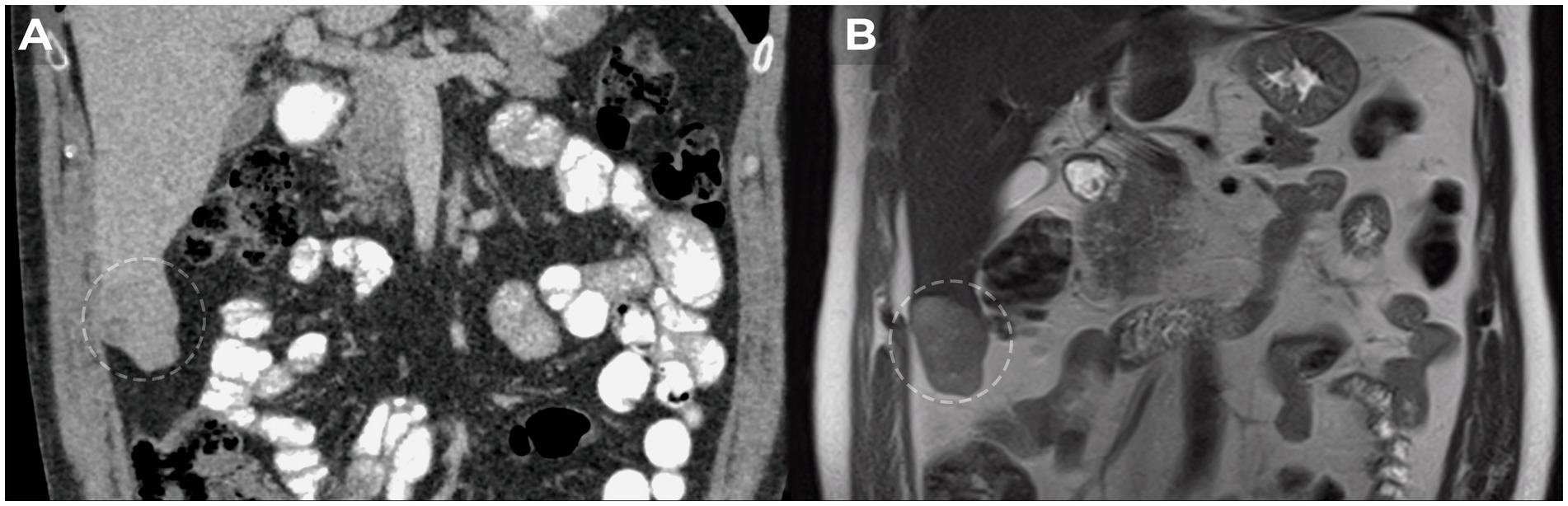
Figure 1. MRI dynamics of liver tumor growth. (A) Heterogeneous mass with clear irregular contours of 5×3 cm in the lower part of the 6th liver segment detected in 2021. (B) Growth dynamics of the liver tumor up to 7×5 cm in 2022.
Due to progressive growth, surgical resection of the 5th and 6th liver segments was performed in January 2023. The postoperative period was uneventful.
Macroscopic examination of the resected specimen revealed a well-circumscribed subcapsular nodule measuring 7.5 × 3.0 × 4.0 cm, composed of dense, gray cystic tissue filled with transparent yellow colloid.
Histologic evaluation confirmed that the tumor consisted of well-differentiated tubular and glandular structures with focal cystic expansion and an abundance of eosinophilic, amorphous material within the lumen. Tumor cells appeared relatively uniform, cubic or cylindrical, with narrow cytoplasm and rounded nuclei. The tumor structures were set within a weakly hyalinized, edematous, desmoplastic stroma in certain areas. There was no evidence of invasive growth into the serosa or vascular invasion.
Given the unique architectural features, a secondary malignant neoplasm of the liver was initially suspected, raising the possibility of metastasis from thyroid carcinoma or renal carcinoma. However, thyroid ultrasonography revealed no abnormalities.
Multistep IHC demonstrated moderate focal membrane-cytoplasmic expression of cytokeratin 19 (CK19, clone A53-B1/A2.26, Cell Marque) and diffuse, prominent membrane-cytoplasmic expression of cytokeratin 7 (CK7, clone OV-TL 12/30, Cell Marque). The proliferative index (Ki-67, clone MIB-1, DAKO) was 13.2% (75 of 569 cells). No expression was detected for thyroid transcription factor-1 (TTF1, clone 8G7G3/1, Cell Marque), inhibin alpha (clone R1, Cell Marque), CDX2 (clone DAK-CDX2, DAKO), CD56 (clone 123C3.D5, Cell Marque), chromogranin A (clone DAK-A3, DAKO), synaptophysin (clone MRQ-40, Cell Marque), or PAX-8 (polyclonal, Cell Marque) (Figure 2).
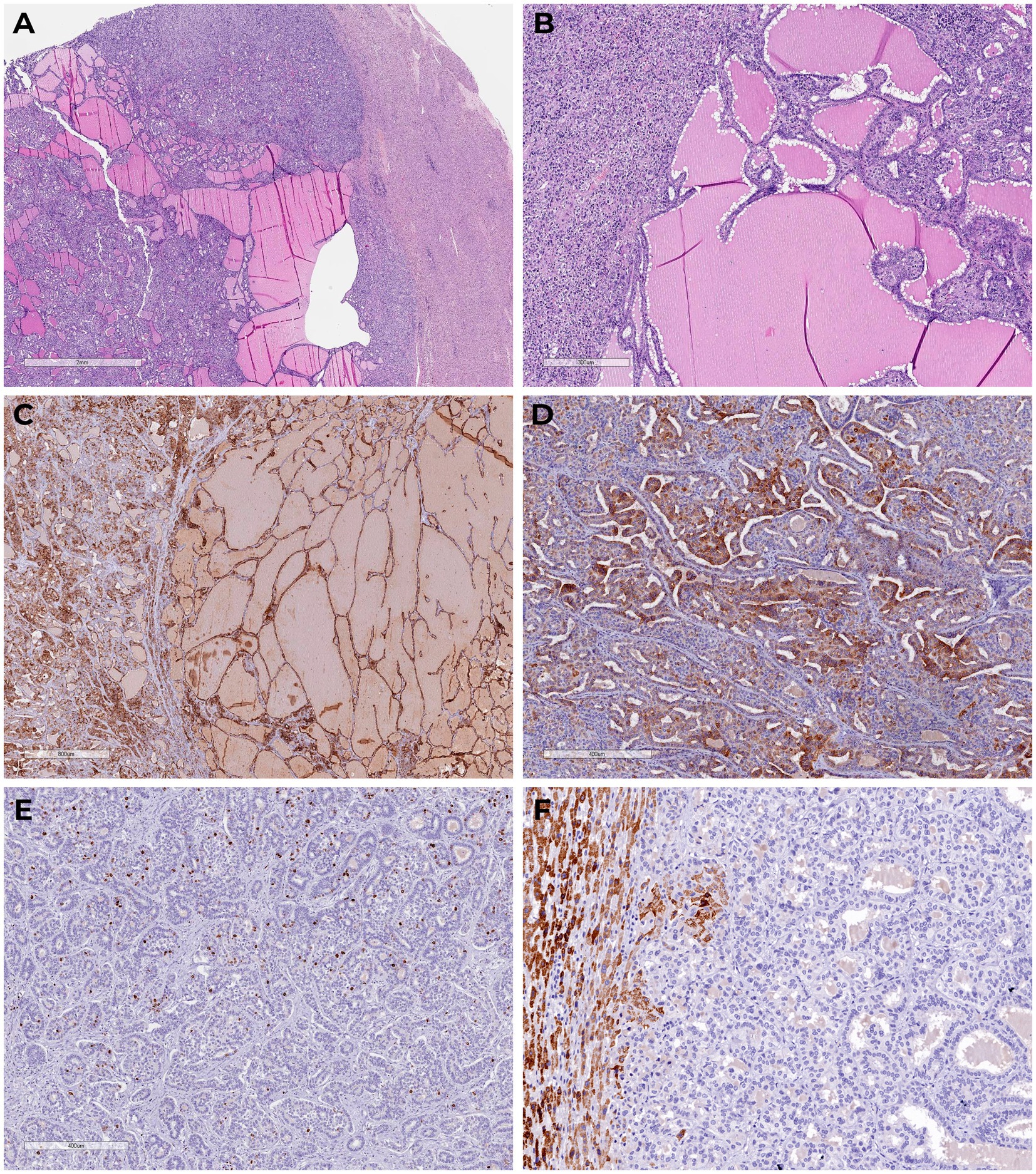
Figure 2. Tumor sections with glandular structures, cystic expansion, abundance of eosinophilic amorphous material in the lumen (H&E). (A,B) There is diffuse marked membranous cytoplasmic expression of cytokeratin 7 (C) and moderate focal membranous cytoplasmic expression of cytokeratin 19 (D) in the tumor cells; proliferative expression index by Ki-67 (E); we detected granular cytoplasmic expression of TTF1 in the surrounding normal liver tissue, in the absence of primary thyroid cancer-specific nuclear expression in the tumor (F).
Based on histologic and IHC findings, a diagnosis of intrahepatic cholangiocarcinoma with a thyroid-like variant (pT1b, N0, cM0, Pn0, L0, V0, R0) was established.
Dynamic surveillance without chemotherapy was recommended due to the absence of tumor foci in the resection margins, vascular invasion, and evidence of metastasis. At 14 months after the initial diagnosis, there were no signs of tumor recurrence. The patient reported satisfactory subjective quality of life.
The incidence of malignant neoplasms of the liver and intrahepatic bile ducts has risen significantly over the past decade. Despite advancements in diagnostic techniques and therapeutic approaches, the prognosis remains poor, with rising mortality rates, particularly among men (1, 6, 7). Surgical resection with histologic confirmation of negative margins remains the gold standard for treatment (2, 6). However, surgery often has a palliative intent and is frequently followed by chemotherapy, which is challenging due to the severity of the disease (8). According to the World Health Organization, nearly all intrahepatic bile duct neoplasms are adenocarcinomas, varying in their degree of differentiation (2, 9). Recent years have seen an increasing number of reports on rare variants of cholangiocarcinoma. These include spindle cell carcinomas, Epstein–Barr virus-positive lymphoepithelioma-like carcinomas resembling nasopharyngeal carcinoma (10), and cholangioblastic variants of cholangiocarcinoma (11, 12).
Intrahepatic cholangiocarcinoma resembling thyroid cancer, also known as thyroid-like cholangiocarcinoma, typically presents as a solitary, well-circumscribed nodule without evidence of serosal involvement. This variant is characterized by a relatively low Ki-67 proliferative index, absence of pathologic mitoses, and a desmoplastic stromal reaction, often complicating diagnosis. In some cases, these lesions are initially deemed benign. A detailed clinical history and thorough patient evaluation are crucial for establishing an accurate diagnosis. Most cases of thyroid-like cholangiocarcinoma described in the literature have demonstrated a favorable prognosis with long-term follow-up (Table 1). Tumor sizes reported in the literature range from 3 to 19 cm (Table 1).
Morphologically, thyroid-like cholangiocarcinoma is composed of glandular structures with focal cystic expansion and abundant eosinophilic, amorphous material within the lumen. These features closely resemble metastases from thyroid cancer or ectopic thyroid tissue. While metastatic liver involvement in thyroid cancer is rare, occurring in less than 20% of cases, it is generally associated with widespread systemic disease (13, 14). Similarly, ectopic thyroid tissue in the liver is rare but can mimic a tumor or a primary multifocal neoplasm (15, 16).
Our case represents the first reported instance of intrahepatic cholangiocarcinoma with thyroid-like morphology confirmed through IHC in a 61-year-old male. Over a 14-month follow-up period, there was no evidence of recurrence or metastasis. This case underscores the importance of expanding the classification of cholangiocarcinoma to include novel subtypes with unique morphologic criteria and clinical behavior. Recognizing such subtypes will enable more accurate diagnoses and closer surveillance of patients presenting with benign-appearing hepatic masses identified through imaging studies.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) and legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
EB: Conceptualization, Visualization, Methodology, Writing – original draft. DK: Conceptualization, Writing – review & editing, Visualization. LU: Writing – review & editing, Conceptualization. DP: Writing – review & editing. RS: Conceptualization, Visualization, Writing – review & editing. NM: Conceptualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Ministry of Science and Higher Education of the Russian Federation (agreement no. 075-15-2022-310 from 20 April 2022).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1458586/full#supplementary-material
1. Sarcognato, S, Sacchi, D, Fassan, M, Fabris, L, Cadamuro, M, Zanus, G, et al. Cholangiocarcinoma. Pathologica. (2021) 113:158–69. doi: 10.32074/1591-951X-252
2. Nagtegaal, ID, Odze, RD, Klimstra, D, Paradis, V, Rugge, M, Schirmacher, P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. (2020) 76:182–8. doi: 10.1111/HIS.13975
3. Swed, B, Gandarilla, O, Chiu, K, Halazun, KH, Samstein, B, Yantiss, R, et al. Rare histological variants of liver Cancer and their management: a single-institution experience. Case Rep Hepatol. (2021) 2021:1–7. doi: 10.1155/2021/6654229
4. Kendall, T, Verheij, J, Gaudio, E, Evert, M, Guido, M, Goeppert, B, et al. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. (2019) 39:7–18. doi: 10.1111/LIV.14093
5. Breder, VV, Bazin, IS, Balakhnin, PV, Virshke, ER, Kosyrev, VY, Ledin, EV, et al. Practical recommendations for drug treatment of patients with malignant tumors of the liver and biliary system. Malignant Tumors. (2022) 12:467–529. doi: 10.18027/2224-5057-2022-12-3s2-467-529
6. Bridgewater, J, Galle, PR, Khan, SA, Llovet, JM, Park, JW, Patel, T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. (2014) 60:1268–89. doi: 10.1016/J.JHEP.2014.01.021
7. Cronin, KA, Scott, S, Firth, AU, Sung, H, Henley, SJ, Sherman, RL, et al. Annual report to the nation on the status of cancer, part 1: national cancer statistics. Cancer. (2022) 128:4251–84. doi: 10.1002/CNCR.34479
8. Hissong, E, Chiu, K, Park, H, Solomon, J, Song, W, and Jessurun, J. Thyroid-like Cholangiocarcinoma: histopathological, Immunohistochemical, in-situ hybridization and molecular studies on an uncommon emerging entity. Int J Surg Pathol. (2021) 29:920–5. doi: 10.1177/10668969211013906
9. Zen, Y. Intrahepatic cholangiocarcinoma: typical features, uncommon variants, and controversial related entities. Hum Pathol. (2023) 132:197–207. doi: 10.1016/J.HUMPATH.2022.06.001
10. Fornelli, A, Maggiore, O, Pizzardi Di Bologna, CA, Bondi, A, Ospedale, EJ, Carlo, M, et al. Intrahepatic cholangiocarcinoma resembling a thyroid follicular neoplasm. Virchows Archiv. (2010) 456:339–42. doi: 10.1007/s00428-009-0874-z
11. Verhoeff, K, Bacani, J, Fung, C, and Canterbury, LA. A Cholangioblastic variant of Cholangiocarcinoma. ACG Case Rep J. (2022) 9:e00746. doi: 10.14309/CRJ.0000000000000746
12. Ayvazyan, KA, Gurmikov, BN, Anvari, RO, Stepanova, YA, Kalinin, DV, Glotov, AV, et al. Cholangioblastic liver carcinoma. Annaly khirurgicheskoy gepatologii. Ann HPB Surg. (2022) 27:125–34. doi: 10.16931/1995-5464.2022-4-125-134
13. Vuong, HG, Le, MK, Hassell, L, Kondo, T, and Kakudo, K. The differences in distant metastatic patterns and their corresponding survival between thyroid cancer subtypes. Head Neck. (2022) 44:926–32. doi: 10.1002/HED.26987
14. Ren, H, Ke, N, Tan, C, Wang, X, Cao, W, and Liu, X. Unusual metastasis of papillary thyroid cancer to the pancreas, liver, and diaphragm: a case report with review of literature. BMC Surg. (2020) 20:1–4. doi: 10.1186/S12893-020-00748-1/FIGURES/4
15. Chen, M, Hu, J, and Cai, X. Ectopic thyroid gland tissue in the liver. Clin Gastroenterol Hepatol. (2020) 18:e157. doi: 10.1016/j.cgh.2019.09.042
Keywords: cholangiocarcinoma, liver, thyroid-like structure, immunohistochemistry, case report
Citation: Bondarenko E, Kalinin D, Urusova L, Pastukhova D, Salimkhanov R and Mokrysheva N (2025) Case report: Rare observation of thyroid-like cholangiocarcinoma. Front. Med. 11:1458586. doi: 10.3389/fmed.2024.1458586
Received: 02 July 2024; Accepted: 27 December 2024;
Published: 23 January 2025.
Edited by:
Yingcheng Charles Wu, Fudan University, ChinaReviewed by:
Jian Kong, Capital Medical University, ChinaCopyright © 2025 Bondarenko, Kalinin, Urusova, Pastukhova, Salimkhanov and Mokrysheva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rustam Salimkhanov, cnVzdGFtLnNhbGlta2hhbm92QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.