- 1Department of Dermatology, The First Affiliated Hospital of Ningbo University, Ningbo, Zhejiang, China
- 2National Clinical Research Center for Dermatologic and Immunologic Diseases, Beijing, China
- 3School of Medicine, Ningbo University, Ningbo, Zhejiang, China
Introduction: Psoriasis, a chronic inflammatory skin disease, is believed to be influenced by both genetic and environmental factors. Despite this understanding, the clinical epidemiological status of psoriasis patients with a family history of the disease remains uncertain.
Methods: In this study, we participated in a multicenter observational epidemiological study involved over 1,000 hospitals and enrolled a total of 5,927 psoriasis patients. These patients were categorized into two groups based on the presence or absence of a family history of psoriasis: family history cases (896) and sporadic cases (5,031). The clinical manifestations of these two groups were analyzed through clinical classification, comorbidities, treatment response, and other relevant factors.
Results: The findings of our study indicate that individuals with a family history of psoriasis predisposition exhibit a notably elevated prevalence of psoriatic arthritis compared to those with sporadic occurrences. Moreover, patients with a family history of psoriasis display a more rapid and efficacious response to secukinumab. Additionally, individuals with moderate to severe psoriasis are at a heightened risk of developing cardiovascular and liver diseases in comparison to those with mild psoriasis, with no discernible impact of familial history on the likelihood of comorbidities.
Discussion: Our study identified the clinical characteristics of individuals with a familial predisposition to psoriasis, offering novel insights into the management and therapeutic approaches for patients with this condition.
Introduction
Psoriasis is a common chronic inflammatory skin disease characterized by erythematous patches covered with silvery scales. The prevalence of psoriasis ranges from approximately 0.51 to 11.43% (1), the 2019 Global Burden of Disease Study shows that over 4620000 incident cases of psoriasis worldwide (2). Extensive research has demonstrated that psoriasis is a systemic disorder, impacting not only the skin but also various organs such as joints, liver, and kidneys et al. Psoriasis has been shown to increase the prevalence of diseases of inflammatory origin. Increased pro-inflammatory cytokines can be transported from the blood to other systems to produce inflammation and subclinical inflammation, thus therapy for psoriasis could potentially be performed to decrease the risk of comorbidities (3). Additionally, individuals with psoriasis often experience comorbidities, including cardiovascular disease, metabolic syndrome, inflammatory bowel disease, depression, hyperlipidemia and so on (4), Buja et al. have shown that at the onset of psoriasis, patients are more likely to be diagnosed with 2–4 comorbidities or over 5 comorbidities (5). Hence, it is imperative for clinicians to adopt a comprehensive approach in the management of psoriasis patients, considering the potential involvement of other physiological systems and comorbidities, rather than solely concentrating on cutaneous manifestations.
Currently, the etiology of psoriasis remains incompletely understood, with the prevailing perspective suggesting that it is an immune-mediated inflammatory disorder influenced by genetic and environmental factors. The initial identification of the HLA-Cw6 gene, encoding MHC class I receptors involved in antigen presentation to T cells, marked a significant milestone in understanding psoriasis susceptibility (6). Subsequent research has uncovered over 70 additional genetic loci associated with psoriasis (7), offering promising avenues for enhancing clinical diagnosis and personalized drug therapies. The treatment approach for patients with psoriasis may vary depending on the disease severity, with options including local or systemic therapies. A variety of biologic agents have been approved for the treatment of psoriasis in China, including TNF-α inhibitors, IL-23 inhibitors, IL-17A inhibitors, IL12/23 inhibitors, etc. The widespread use of biologics in clinical settings has led to notable enhancements in the quality of life for individuals affected by psoriasis (8).
This study seeks to elucidate the influence of familial history on the epidemiological profile of psoriasis patients within the Chinese demographic, investigate potential disparities in clinical presentations and therapeutic outcomes between psoriasis patients with familial predisposition and those without, and offer novel insights for the clinical assessment and management of individuals with psoriasis.
Materials and methods
Questionnaires and patients
A multicenter observational epidemiological study was conducted on psoriasis patients in China, initiated by the National Clinical Research Center for Dermatologic and Immunologic Diseases and involving over 1,000 hospitals. The project has been launched since 2020, and in two years, a total of more than 90,000 patients have been enrolled. As project participants, we applied for part of the data to conduct relevant research. Clinical information was systematically collected using a standardized questionnaire and updated chronologically during each follow-up visit. The questionnaire was structured into baseline and follow-up sections, encompassing fundamental and clinical data, including measurements such as height, weight, age, psoriasis classification, body surface area (BSA) score, psoriasis area and severity index (PASI) score, dermatology life quality index (DLQI) score, comorbidities, previous treatment regimens, and so on. The questionnaire is completed by clinicians in attendance and then uploaded to the system for archival purposes. The comorbidities under investigation encompass cardiovascular disease, diabetes, respiratory disease, liver disease, gastrointestinal disease, rheumato-immune disease, neuropsychiatric disease, tumors, and other skin diseases. A cohort of 7,037 patients with psoriasis was requested for the study, with each patient being diagnosed by two or more dermatologists holding senior professional titles.
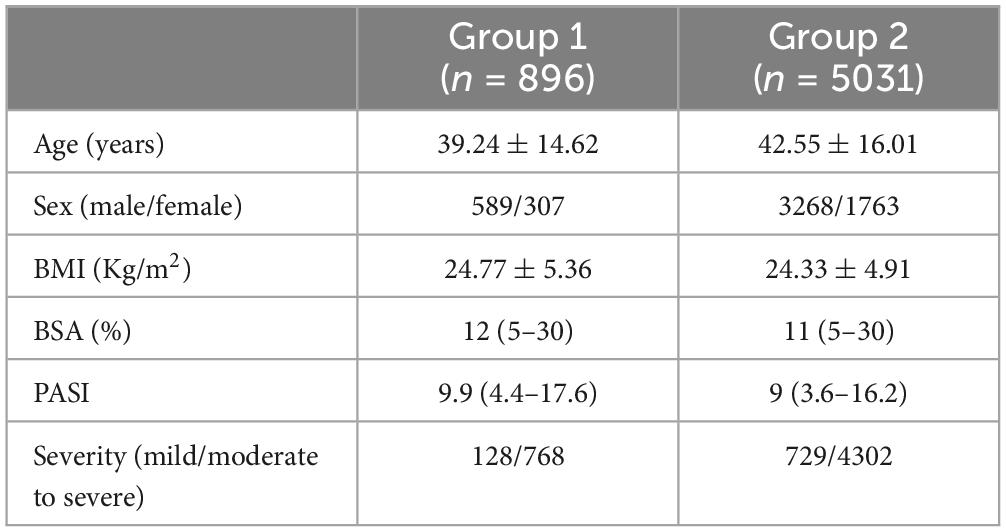
Cases with no follow-up records due to time or various reasons and incomplete information related to the study, such as family history, comorbidity and other blank options were excluded, a total of 5,927 individuals were included in the follow analysis. The study population was stratified into two distinct cohorts based on whether there was presence of a familial history of psoriasis: Group 1 comprised individuals with a documented family history of the condition, while Group 2 consisted of sporadic cases, with sample sizes of 896 and 5,031, respectively. Severity of psoriasis was assessed according to the Chinese guidelines for diagnosis and treatment of psoriasis 2018, categorizing patients with DLQI < 6 or PASI < 3 or BSA < 3% as mild, and those not meeting these criteria as moderate to severe. According to the severity of psoriasis, we divided the patients into mild group, named Group 3, and moderate to severe group, named Group 4, with 857 and 5,070 patients, respectively.
Group 1 consisted of 83 patients treated with biologics, while Group 2 included 422 patients, the specific types of biologics and their usage numbers were detailed in Supplementary Table 1. These patients had not previously received biologic therapy for psoriasis, instead, they had received conventional treatments such as topical medications, oral immunosuppressants, or narrowband ultraviolet B (NB-UVB) phototherapy, which had poor response to the control of psoriatic skin lesions. During the analysis of the efficacy of biological agents, patients who received treatment with secukinumab and had follow-up records after 1 month of treatment were selected as the study subjects, based on a comprehensive consideration of follow-up time, the number of patients using various biological agents, and the frequency of biological agent injections, other samples were excluded. Among them, 48 patients in Group 1 and 152 patients in Group 2 received secukinumab injections and had complete follow-up data.
Statistical analysis
The data analysis was performed using SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). Qualitative and quantitative analyses were conducted on the clinical data. The quantitative data such as age, BMI, BSA, PASI, DLQI, were expressed as mean ± standard deviation, or median (interquartile range) according to whether they fit the normal distribution, while frequency distribution was used in qualitative analysis, like clinical subtypes, sex, and severity. For quantitative data conforming to a normal distribution, t-test was used for analysis. Otherwise rank sum test and chi-square test were used for categorical variables. Rank sum test was used to compare the changes in BSA and PASI scores before and after secukinumab treatment to reflect the effectiveness of biologics. Statistical significance was determined by P < 0.05.
Result
Clinical manifestations of psoriasis patients with or without family history
The 5927 samples examined in this study were stratified into two cohorts based on the presence or absence of a familial history of psoriasis. Group 1 consisted of 896 samples with a family history, while Group 2 comprised 5031 samples without a familial history. Detailed clinical characteristics of both groups, including age, BMI, BSA, and PASI, are presented in Table 1. During the analysis, it was observed that there were numerous deficiencies in the assessment of DLQI values. Therefore, statistical analysis of this parameter was not conducted.
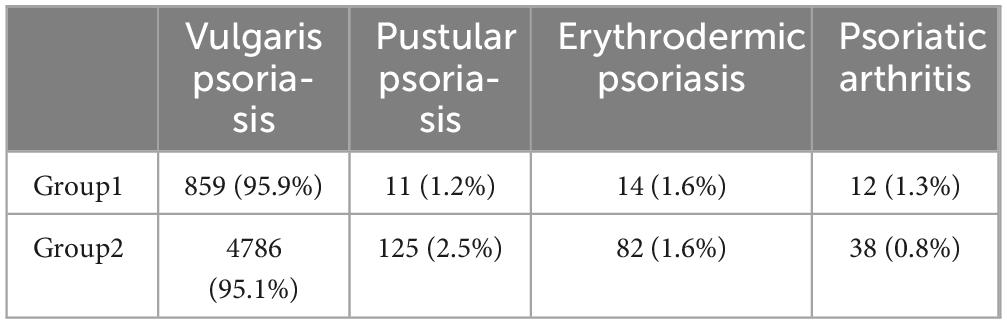
Chi-square test was performed to analyze the severity and classification of disease in Group 1 and Group 2. The results, presented in Tables 1, 2, indicated no significant difference in disease severity distribution between the two groups. However, a statistically significant difference was observed in disease classification (p = 0.039). Patients with a family history of psoriasis were more inclined to exhibit psoriatic arthritis, whereas sporadic patients were more likely to present with pustular psoriasis. The distribution of erythrodermic psoriasis and vulgaris psoriasis was found to be similar in both groups.
Efficacy of biologics
The comparison of BSA and PASI values before and after treatment (Baseline and Week 4 data) revealed that the decline in BSA and PASI in Group 1 was significantly greater than that in Group 2, as indicated by the rank sum test for the difference values. Specifically, the mean declines in BSA and PASI in Group 1 were 22.29 and 11.21, respectively, compared to 14.10 and 7.67 in Group 2 (Pbsa = 0.029, Ppasi = 0.044). These results suggest that the efficacy of secukinumab treatment for psoriasis patients with a family history is more pronounced.
Comorbidities with psoriasis
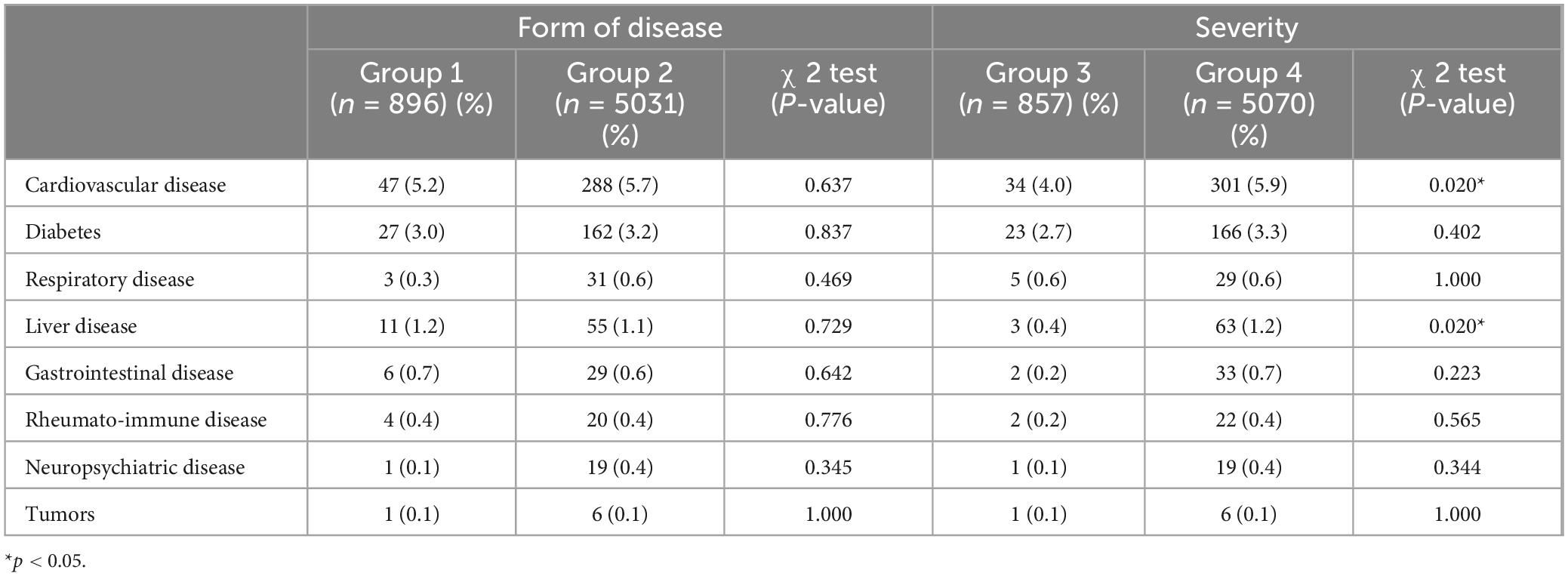
The study examined the presence of comorbidities among patients diagnosed with psoriasis in Group1 and Group 2, the specific types and frequencies of comorbidities are detailed in Table 3 and Figure 1, with cardiovascular diseases being the most prevalent comorbidity in both Group 1 (5.2%) and Group 2 (5.7%). A Chi-square test was conducted to compare the distribution of comorbidities between the two groups, indicating no statistically significant difference. Thus, it can be concluded that family history did not contribute to an increased predisposition to comorbidity prevalence.
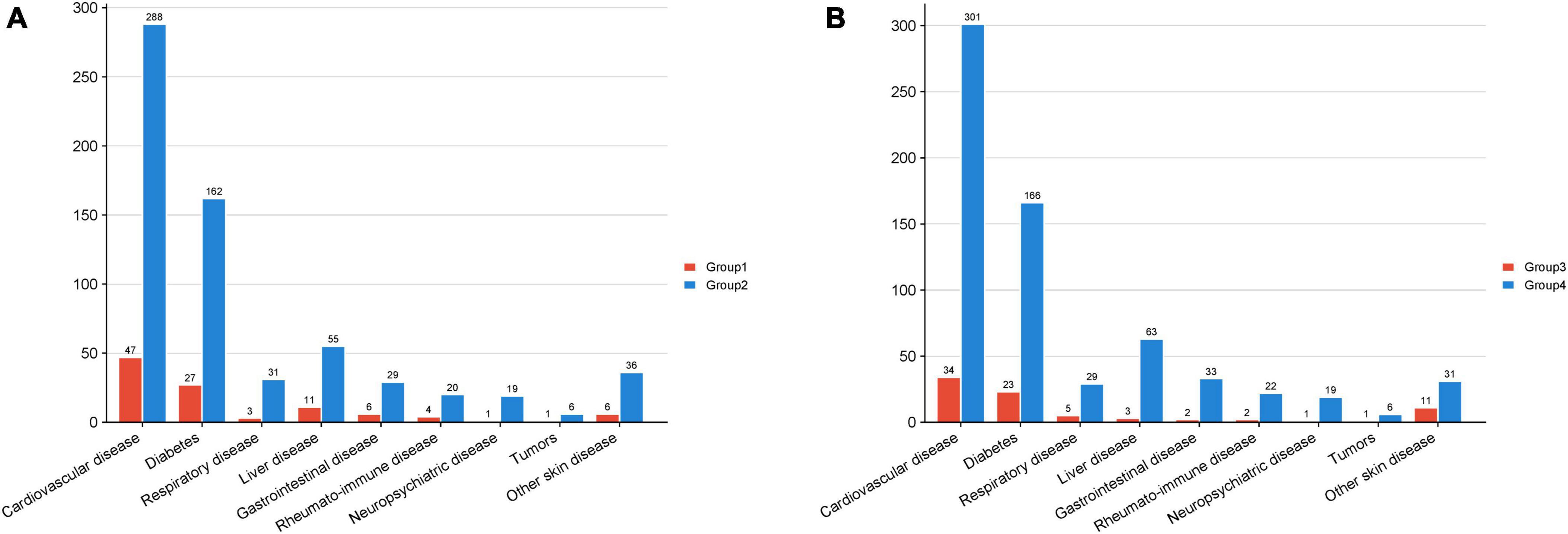
Figure 1. The amount of comorbidity cases in each group. The horizontal axis denotes the various comorbidities, while the vertical axis indicates the quantity of patients. (A) Group 1 pertains to patients with a familial history of psoriasis, Group 2 pertains to patients with sporadic psoriasis. (B) Group 3 pertains to patients with mild psoriasis, and Group 4 pertains to patients with moderate to severe psoriasis.
The samples were reclassified according to the severity of psoriasis, resulting in 857 individuals classified as mild patients and 5,070 individuals classified as moderate to severe patients, designated as Group3 and Group4, respectively. A Chi-square test was performed to analyze the prevalence of comorbidities among the patients, revealing that the proportion of moderate to severe patients with cardiovascular disease and liver disease was significantly higher compared to mild patients (P = 0.020 and P = 0.020, respectively). This suggests that moderate and severe psoriasis patients are more susceptible to cardiovascular disease and liver disease than those with mild psoriasis, with no similar predisposition observed for other conditions.
Discussion
Our study found a significant correlation between the presence of a family genetic history and the type of psoriasis. Specifically, the proportion of patients with a family history of psoriasis exhibiting psoriatic arthritis was notably higher than that of sporadic cases, while the prevalence of pustular psoriasis was higher among sporadic cases compared to those with a family history. Psoriasis vulgaris was the most common type in both groups. Studies in the Japanese population have also found that psoriatic arthritis was significantly more common in patients with a family history (9), psoriatic arthritis usually involves the peripheral joints, axial skeleton, and periarticular (10). Several previous studies have also investigated the clinical manifestations of psoriasis in patients with a family history, research from Canada and Turkey had shown that people with a family history of psoriasis or psoriatic arthritis were more likely to be women, had an earlier age of onset, and had more frequent nail involvement, joint deformities, and presence of enthesitis. Furthermore, patients with a family history of psoriatic arthritis had lower risk for plaque psoriasis (11). Additionally, several literatures had also documented a higher proportion of nail involvement in patients with a positive family history (12, 13). Therefore, it is important to focus on whether there are joint and nail involvement in the clinical diagnosis and treatment of patients with a family history of psoriasis. Duffin et al. demonstrated that family history of psoriasis was a predisposing factor for guttate psoriasis, however we did not disaggregate statistics for psoriasis vulgaris, it is not clear whether the same association exists in the Chinese population (14).
Given the chronic and recurrent nature of psoriasis, it remains a challenging condition for both healthcare providers and patients (15). The use of biologic has enabled the effective treatment of lesions in individuals with moderate to severe psoriasis, with several biologics receiving approval from the FDA, including IL-23 inhibitors, IL-17A inhibitors, and IL-12/23 inhibitors. Among these, secukinumab, a fully human IL-17A monoclonal antibody, has been sanctioned for the management of plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, among other conditions. Our research findings indicate that patients with a familial predisposition to psoriasis exhibit a more robust response to secukinumab.
In light of this conclusion, we have posited two hypotheses. The first hypothesis suggests that individuals with psoriasis who have a familial predisposition may exhibit shared genetic characteristics that facilitate a more expedited and effective response to secukinumab, thereby enabling the categorization of the disease based on molecularly informed taxonomy (16), a methodology frequently employed in the study of respiratory ailments (17). Recent research has indicated that the efficacy of secukinumab treatment is not influenced by common genetic variants, HLA alleles, or other genetic factors (18). As a multifaceted condition, the genetic underpinnings of psoriasis extend beyond the polygenic risk scores of identified susceptibility genes (19). The reliability of these scores for non-European populations remains uncertain, as does the utility of group identification in this context. Furthermore, the effectiveness of secukinumab appears consistent across diverse genetic backgrounds and does not equate to a subset of the population responding more quickly or efficiently. Additionally, the enhanced or expedited response to secukinumab may be linked to psoriatic subtypes. Our study revealed that a greater percentage of individuals with a familial predisposition to psoriasis were categorized as having psoriatic arthritis in comparison to those with sporadic cases. Secuchiumab has been shown to inhibit the progression of psoriatic arthritis at the radiographic level, and is recommended as a treatment for psoriatic arthritis in multiple international and domestic guidelines. Moreover, IL-17A inhibitors demonstrated a more favorable efficacy in treating psoriatic arthritis than pustular psoriasis (20). Therefore, patients with a family history of psoriasis show a stronger response to secuchiumab, however, whether this reaction is specific to secuchiumab or to all biologics needs further study. In 2020, a dichotomous definition was developed by the International Psoriasis Council, that is, based on BSA size, whether special sites are involved and whether local treatment is effective to define psoriasis severity (21). Since early selection of appropriate treatments can provide both immediate and long-term benefits (22), based on the clinical characteristics of psoriasis patients with a family history and the effective response to secuchiumab were found in this study, we suggest that patients with a family history of psoriasis may try to initiate biologics as early as possible.
Our research findings indicate that individuals with moderate to severe psoriasis exhibit notably elevated incidences of cardiovascular and liver diseases compared to those with mild psoriasis. Specifically, the prevalence of cardiovascular disease is most pronounced in cases of hypertension and coronary heart disease. Existing literature has established a link between chronic systemic inflammation and the pathogenesis of cardiovascular disease (23–25). Individuals diagnosed with psoriasis demonstrated an odds ratio of 2.18 for developing atherosclerosis and a 6% elevation in the Framingham risk score (26). The adipose tissue of psoriasis patients harbors a significant quantity of immune cells linked to cardiometabolic function, including T cells, B cells, dendritic cells, mast cells, and adipose tissue macrophages. These cells play a role in the pathogenesis of obesity and insulin resistance, producing pro-inflammatory cytokines and adipose tissue dysfunction, which are implicated in the advancement of atherosclerotic vascular disease (27). In addition, there are many links between immune cell dysfunction in psoriasis patients and the progression of cardiovascular disease (28).
Previous research has shown a positive correlation between the severity of psoriasis and the occurrence of cardiovascular events. The analysis of computed tomography scans of individuals with psoriasis, showed a notable increase in both the rate and severity of coronary artery calcification among these patients, with a direct correlation to the severity of their psoriasis (29). Additionally, research has demonstrated that patients with psoriasis who do not have cardiovascular disease exhibit a diminished Coronary flow reserve (CFR), suggesting the presence of coronary microvascular dysfunction. This finding is associated with the severity and advancement of the disease (30). The TH1/TH17 axis is widely recognized for its significant involvement in the pathogenesis of psoriasis, with the progression of atherosclerosis being closely linked to the secretion of IL-17A by TH17 cells. Consequently, the susceptibility of individuals with psoriasis to cardiovascular events may be intricately tied to the underlying pathophysiology of the disease (31). Furthermore, Su et al. demonstrated that psoriasis and atherosclerosis exhibit a shared differential expression of up to 16 genes, offering a novel insight into the investigation of the mutual pathogenesis of these two conditions (32).
As for the other comorbidities associated with severity, liver disease, non-alcoholic fatty liver disease (NAFLD) is the predominant chronic liver condition, encompassing non-alcoholic fatty liver, non-alcoholic steatohepatitis, cirrhosis, and other related conditions. It is prevalent in approximately 30% of adults residing in Western nations (33). Prior research has established a correlation between non-alcoholic fatty liver disease and psoriasis (4, 34), with psoriasis patients exhibiting a 1.5–3 times higher incidence of NAFLD compared to other populations (35). This association is believed to be linked to metabolic syndrome and systemic inflammation (36), and psoriasis patients with NAFLD tend to experience higher disease activity and PASI scores (37).
Interestingly, studies have also shown an inextricable link between cardiovascular disease and liver disease. Studies have indicated that NAFLD is a significant risk factor for the development of atherosclerosis. Originally believed to be a manifestation of metabolic syndrome in the liver, NAFLD is now understood to contribute to the progression of atherosclerosis through various mechanisms, including the secretion of pro-inflammatory cytokines such as TNF-α and IL-6 (38). For instance, TNF-α facilitates the proliferation of keratinocytes in psoriasis, enhances the uptake of low-density lipoprotein in endothelial cells leading to the deposition of blood vessel walls in cardiovascular disease, and concurrently disrupts insulin metabolism, a factor closely associated with the development of non-alcoholic fatty liver disease (39–41).
As research progresses, mounting evidence indicates that NAFLD is an autonomous risk factor for cardiovascular disease (CVD). NAFLD is implicated in oxidative stress, chronic inflammation, dyslipidemia, and other physiological processes (42–44). Hence, clinicians should consider the potential presence of comorbidities in psoriasis patients, particularly those with moderate to severe psoriasis, and prioritize enhanced screening and prevention measures for cardiovascular and liver diseases.
Conclusion
Our research delineates the clinical features of individuals with a familial predisposition to psoriasis and demonstrates that those with a familial history exhibit enhanced responsiveness to secuchiumab treatment. Additionally, individuals with moderate to severe psoriasis are more needed to undergo screening for cardiovascular and hepatic risks. These findings offer novel insights for the diagnostic and therapeutic management of psoriasis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Clinical Research Ethics Committee of National Clinical Research Center for Dermatologic and Immunologic Disease. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
LC: Writing−original draft. LL: Writing−review and editing. YY: Writing−review and editing. HZ: Writing−review and editing. BL: Supervision, Writing−review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of the article. This work was supported by the Medical Health Science and Technology Project of Zhejiang Province (Grant No. 2022PY022) and the Key Medical Discipline of Ningbo City, Rheumatology and Autoimmunology (Grant No. 2022-F08).
Acknowledgments
Thank you to all patients for their participation in our study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1455953/full#supplementary-material
References
1. Michalek I, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. (2017) 31:205–12.
2. Damiani G, Bragazzi N, Karimkhani Aksut C, Wu D, Alicandro G, McGonagle D, et al. The global, regional, and national burden of psoriasis: results and insights from the global burden of disease 2019 Study. Front Med. (2021) 8:743180.
3. Damiani G, Radaeli A, Olivini A, Calvara-Pinton P, Malerba M. Increased airway inflammation in patients with psoriasis. Br J Dermatol. (2016) 175:797–9.
4. Elmets C, Leonardi C, Davis D, Gelfand J, Lichten J, Mehta N, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. (2019) 80:1073–113. doi: 10.1016/j.jaad.2018.11.058
5. Buja A, Miatton A, Cozzolino C, Brazzale A, Lo Bue R, Mercuri S, et al. The prevalent comorbidome at the onset of psoriasis diagnosis. Dermatol Therapy. (2023) 13:2093–105. doi: 10.1007/s13555-023-00986-0
6. Membrive Jiménez C, Pérez Ramírez C, Sánchez Martín A, Vieira Maroun S, Arias Santiago S, Ramírez Tortosa M, et al. Influence of Genetic Polymorphisms on Response to Biologics in Moderate-to-Severe Psoriasis. J Pers Med. (2021) 11:4.
8. Albaghdadi A. Current and under development treatment modalities of psoriasis: a review. Endoc Metab Immune Disord Drug Targets. (2017) 17:189–99.
9. Ohata C, Anezaki H, Kaneko S, Okazaki F, Ito K, Matsuzaka Y, et al. Clinical characteristics of patients with psoriasis with family history: A multicenter observational study. J Dermatol. (2023) 50:746–52.
10. Calabresi E, Monti S, Terenzi R, Zanframundo G, Perniola S, Carli L. One year in review 2019: psoriatic arthritis. Clin Exp Rheumatol. (2020) 38:1046–55.
11. Solmaz D, Bakirci S, Kimyon G, Gunal E, Dogru A, Bayindir O, et al. Impact of having family history of psoriasis or psoriatic arthritis on psoriatic disease. Arthr Care Res. (2020) 72:63–8.
12. Darjani A, Nafezi R, Moladoust H, Eftekhari H, Nejad K, Rafiei R, et al. Nail involvements as an indicator of skin lesion severity in psoriatic patients. Acta Dermatovenerol Croat. (2018) 26:307–13.
13. Truong B, Rich-Garg N, Ehst B, Deodhar A, Ku J, Vakil-Gilani K, et al. Demographics, clinical disease characteristics, and quality of life in a large cohort of psoriasis patients with and without psoriatic arthritis. Clin Cosm Investig Dermatol. (2015) 8:563–9. doi: 10.2147/CCID.S90270
14. Duffin K, Hwang S, Krueger J. Advances and Controversies in Our Understanding of Guttate and Plaque Psoriasis. J Rheumatol. (2023) 50(Suppl 2):4–7. doi: 10.3899/jrheum.2023-0500
16. National Research Council Committee on AFfDaNToD. The National Academies Collection: Reports funded by National Institutes of Health. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. Washington, DC: National Academies Press (2011).
17. Kaur R, Chupp G. Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J Allergy Clin Immunol. (2019) 144:1–12.
18. Zhang C, Shestopaloff K, Hollis B, Kwok C, Hon C, Hartmann N, et al. Response to anti-IL17 therapy in inflammatory disease is not strongly impacted by genetic background. Am J Hum Genetics. (2023) 110:1817–24.
19. Lewis C, Vassos E. Polygenic risk scores: from research tools to clinical instruments. Genome Med. (2020) 12:44.
20. Mahmood A, Ali A, Haider H, Zulfiqar B. Long-term efficacy and safety of secukinumab in patients with psoriatic arthritis. Euro J Intern Med. (2023) 118:152–4.
21. Strober B, Ryan C, van de Kerkhof P, van der Walt J, Kimball A, Barker J, et al. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J Am Acad Dermatol. (2020) 82:117–22. doi: 10.1016/j.jaad.2019.08.026
22. Armstrong A, Read C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. Jama. (2020) 323:1945–60.
23. Gelfand J, Neimann A, Shin D, Wang X, Margolis D, Troxel A. Risk of myocardial infarction in patients with psoriasis. JAMA. (2006) 296:1735–41.
24. Brauchli Y, Jick S, Miret M, Meier C. Psoriasis and risk of incident myocardial infarction, stroke or transient ischaemic attack: an inception cohort study with a nested case-control analysis. Br J Dermatol.. (2009) 160:1048–56.
25. Mehta N, Azfar R, Shin D, Neimann A, Troxel A, Gelfand J. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. (2010) 31:1000–6.
26. Frieder J, Ryan C. Psoriasis and cardiovascular disorders. Giornale Ital Dermatol Venereol. (2016) 151:678–93.
27. Rose S, Stansky E, Dagur P, Samsel L, Weiner E, Jahanshad A, et al. Characterization of immune cells in psoriatic adipose tissue. J Transl Med. (2014) 12:258.
28. Sajja A, Joshi A, Teague H, Dey A, Mehta N. Potential Immunological Links Between Psoriasis and Cardiovascular Disease. Front Immunol. (2018) 9:1234. doi: 10.3389/fimmu.2018.01234
29. Ludwig R, Herzog C, Rostock A, Ochsendorf F, Zollner T, Thaci D, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. (2007) 156:271–6.
30. Osto E, Piaserico S, Maddalozzo A, Forchetti G, Montisci R, Famoso G, et al. Impaired coronary flow reserve in young patients affected by severe psoriasis. Atherosclerosis. (2012) 221:113–7. doi: 10.1016/j.atherosclerosis.2011.12.015
31. von Stebut E, Boehncke W, Ghoreschi K, Gori T, Kaya Z, Thaci D, et al. IL-17A in Psoriasis and Beyond: Cardiovascular and Metabolic Implications. Front Immunol. (2019) 10:3096. doi: 10.3389/fimmu.2019.03096
32. Su W, Zhao Y, Wei Y, Zhang X, Ji J, Yang S. Exploring the Pathogenesis of Psoriasis Complicated With Atherosclerosis via Microarray Data Analysis. Front Immunol. (2021) 12:667690. doi: 10.3389/fimmu.2021.667690
33. Targher G. What’s past is prologue: history of nonalcoholic fatty liver disease. Metabolites. (2020) 10:10. doi: 10.3390/metabo10100397
34. Lønnberg A, Skov L. Co-morbidity in psoriasis: mechanisms and implications for treatment. Exp Rev Clin Immunol. (2017) 13:27–34.
35. Balato N, Megna M, Palmisano F, Patruno C, Napolitano M, Scalvenzi M, et al. Psoriasis and sport: a new ally? J Eur Acad Dermatol Venereol. (2015) 29:515–20. doi: 10.1111/jdv.12607
36. Prussick R, Miele L. Nonalcoholic fatty liver disease in patients with psoriasis: a consequence of systemic inflammatory burden? Br J Dermatol. (2018) 179:16–29. doi: 10.1111/bjd.16239
37. Abedini R, Salehi M, Lajevardi V, Beygi S. Patients with psoriasis are at a higher risk of developing nonalcoholic fatty liver disease. Clin Exp Dermatol. (2015) 40:722–7. doi: 10.1111/ced.12672
38. Adams L, Angulo P. Recent concepts in non-alcoholic fatty liver disease. Diabetic Med. (2005) 22:1129–33.
39. Hui E, Xu A, Bo Yang H, Lam K. Obesity as the common soil of non-alcoholic fatty liver disease and diabetes: Role of adipokines. Journal of diabetes investigation. (2013) 4:413–25. doi: 10.1111/jdi.12093
40. Zhang Y, Yang X, Bian F, Wu P, Xing S, Xu G, et al. TNF-α promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: crosstalk between NF-κB and PPAR-γ. J Mol Cell Cardiol. (2014) 72:85–94.
41. Gottlieb A, Dann F, Menter A. Psoriasis and the metabolic syndrome. J Drugs Dermatol. (2008) 7:563–72.
42. Leach N, Dronca E, Vesa S, Sampelean D, Craciun E, Lupsor M, et al. Serum homocysteine levels, oxidative stress and cardiovascular risk in non-alcoholic steatohepatitis. Eur J Intern Med. (2014) 25:762–7.
43. Maurantonio M, Ballestri S, Odoardi M, Lonardo A, Loria P. Treatment of atherogenic liver based on the pathogenesis of nonalcoholic fatty liver disease: a novel approach to reduce cardiovascular risk? Arch Med Res. (2011) 42:337–53. doi: 10.1016/j.arcmed.2011.08.004
Keywords: psoriasis, family history, biologics, comorbidities, characteristics
Citation: Cao L, Lu L, Yu Y, Zhou H and Lin B (2024) Clinical characteristics of patients with a family history of psoriasis: an observational epidemiological study in Chinese Han population. Front. Med. 11:1455953. doi: 10.3389/fmed.2024.1455953
Received: 27 June 2024; Accepted: 01 August 2024;
Published: 16 August 2024.
Edited by:
Guangtong Deng, Central South University, ChinaReviewed by:
Giovanni Damiani, University of Milan, ItalyShengxiu Liu, Anhui Medical University, China
Copyright © 2024 Cao, Lu, Yu, Zhou and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bingjiang Lin, bGluYmluZ2pAMTYzLmNvbQ==
 Lu Cao
Lu Cao Lingyi Lu1,2
Lingyi Lu1,2 Bingjiang Lin
Bingjiang Lin