- 1Department of Biomedical Science, Noorda College of Osteopathic Medicine, Provo, UT, United States
- 2Federated Department of Biological Science, New Jersey Institute of Technology, Newark, NJ, United States
- 3La Crosse-Mayo Family Medicine Residency Program, Mayo Clinic Health System, La Crosse, WI, United States
Background: Craniosacral osteopathic manipulative medicine—also known as craniosacral therapy (CST)—is a widely taught and used component of osteopathic medicine. This paper seeks to systematically review and conduct a meta-analysis of randomized controlled trials assessing the clinical effectiveness of CST compared to standard care, sham treatment, or no treatment in adults and children.
Methods: A search of Embase, PubMed, and Scopus was conducted on 10/29/2023 and updated on 5/8/2024. There was no restriction placed on the date of publication. A Google Scholar search was conducted to capture grey literature. Backward citation searching was also implemented. All randomized controlled trials employing CST for any clinical outcome were included. Studies not available in English as well as studies that did not report adequate data were excluded. Multiple reviewers were used to assess for inclusions, disagreements were settled by consensus. PRISMA guidelines were followed in the reporting of this meta-analysis. Cochrane’s Risk of Bias 2 tool was used to assess for risk of bias. All data were extracted by multiple independent observers. Effect sizes were calculated using a Hedge’s G value (standardized mean difference) and aggregated using random effects models. The GRADE system was used to assess quality of evidence.
Results: The primary study outcome was the effectiveness of CST for selected outcomes as applied to non-healthy adults or children and measured by standardized mean difference effect size. Twenty-four RCTs were included in the final meta-analysis with a total of 1,613 participants. When subgroup analyses were performed by primary outcome only, no significant effects were found. When secondary outcomes were included in subgroup analyses, results showed that only Neonate health, structure (g = 0.66, 95% CI [0.30; 1.02], Prediction Interval [−0.73; 2.05]) and Pain, chronic somatic (g = 0.34, 95% CI [0.18; 0.50], Prediction Interval [−0.41; 1.09]) show reliable, statistically significant effect. However, these should not be interpreted as positive results as wide prediction intervals, high bias, and statistical limitations temper the real-world implications of this finding.
Conclusions and relevance: CST demonstrated no significant effects in this meta-analysis, indicating a lack of usefulness in patient care for any of the studied indications.
Pre-registration available at https://doi.org/10.17605/OSF.IO/54K6G.
Systematic Review Registration: https://osf.io/54k6g.
1 Background
Craniosacral osteopathic manipulative medicine—also known as craniosacral therapy (CST) or osteopathy in the cranial field—was developed by William G. Sutherland, D.O. in the 1940s (1, 2). He proposed the existence of an inherent movement between the dura, sacrum, and cranial bones known as the cranial rhythmic impulse (CRI). The basis of this theory is predicated on an inherent rhythmic motion of the brain and spinal cord, flow of cerebral spinal fluid, mobility of cranial and spinal membranes, mobility of the cranial bones at their sutures, and physical connection between the sacrum and the bones and membranes of the cranium (2, 3). Practitioners of CST seek to influence the health of patients through the use of manual techniques to affect the CRI. Craniosacral therapy is currently considered a form of complementary medicine by the World Health Organization (4).
The existence of the CRI is in direct conflict with current understanding of anatomy and development. It has been well established by current and past literature that cranial sutures are fused by early adulthood and have minimal clinically significant motion thereafter (5–11). Thus, despite the theories put forth by proponents of CST, no plausible biological mechanism for CST exists. The use of CST in infants may be less impacted by this, however, due to the increased malleability of the cranium before sutures become ossified. It should also be noted that, with the exception of certain neonatal conditions in which the cranial bones may be manipulated, there is no clear link between the proposed mechanisms of CST, or the CRI, and the ailments to which it is commonly applied, including those evaluated in this meta-analysis. Additionally, despite regular application, there is limited indication for manipulation of this type in the pediatric population.
Despite significant controversy over its continued use, CST has gained widespread acceptance in the osteopathic community (12, 13). It is used especially heavily in Europe, where over 90% of osteopaths report using CST (14–18). In the United States, it is more difficult to estimate the number osteopathic physicians actively employing CST. A 2021 study based on American Osteopathic Association survey data found that only about 46% of currently practicing osteopathic physicians use any osteopathic manipulative medicine (OMM) in their practice, though no attempt was made to provide a breakdown by technique (19). Regardless of its use in day-to-day practice, CST is included in the curriculum of all US osteopathic medical schools and is a core component of the COMLEX-USA licensing exams (20).
Studies evaluating the clinical use of CST have not led to a definitive answer regarding its effectiveness, despite relatively strong evidence against its continued use. Prior meta-analyses show significant effects of OMM for the treatment of neck and back pain, but these studies make no attempt to isolate CST modalities (21, 22). Based on existing reviews, other indications for CST are not well supported (23–26). Interrater reliability for palpation of the CRI has been shown to be low (27). Several prior meta-analyses and systematic reviews exploring the efficacy of CST as a standalone treatment disagree on whether there is enough evidence to recommend it for the treatment of any condition (26, 28–32). A more recently published systematic review and meta-analysis by Ceballos-Laita et al. concluded that no significant effect was found in any of several outcome categories (33).
Prior systematic reviews have become outdated by the publication of additional literature (28, 31), are limited to a single indication (26, 30), relied on a few small studies (32), or conducted a more limited meta-analysis than is presented here (33). This review provides an updated meta-analysis of CST and encompasses the breadth of indications for CST that have been evaluated in scientific literature to answer the question: Does CST performed by experts on non-healthy adults or children as compared to standard of care, sham treatment, or no treatment in any setting provide statistically significant benefit to any quantitative outcome? Due to the wide range of available data investigating CST, multiple meta-analyses of general outcome categories have been compiled and presented here.
2 Methods
This study was reported following the guidelines set out in the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) (34). This report was pre-registered with Open Science Framework (OSF) Registries (35) and a preprint is available at Research Square (36).
2.1 Data sources and search
A search of Embase, PubMed, and Scopus was conducted to identify all randomized controlled trials examining the efficacy of craniosacral techniques, published from database inception to October 29th, 2023. An updated search was run on May 8, 2024, to ensure all relevant records were captured prior to publication. The Scopus search was limited to title, abstract, and keyword. A grey literature search using Google Scholar was also conducted using a shortened search term, due to the limitations of the Google Scholar search interface. Additionally, a backward citation search was conducted. The full search term is available via the OSF registration as well as in Supplementary material (Supplementary Table 1). This term was developed with input from a health sciences librarian.
2.2 Eligibility criteria
All randomized controlled trials assessing clinical effectiveness of CST performed by experts on non-healthy adults or children as compared to standard of care, sham treatment, or no treatment in any setting with any measured outcome were included. For the purposes of this study, CST is defined as any manual technique that attempts to influence the cranial sutures, dura mater, sacrum, flow of cerebrospinal fluid, or otherwise subscribes to the theories of biomechanics commonly accepted as cranial manipulative medicine (3). Studies that were not available in English, studies that used animal subjects, studies that did not incorporate craniosacral osteopathic medicine, and studies in which healthy participants were treated with CST without indication were excluded. Additionally, studies that combined craniosacral techniques with other non-osteopathic treatments, and did not separate or distinguish results, were excluded. One study meeting this description was included as the initial trial outcome specified separation but post-hoc analysis was conducted on combined groups due to sample limitations. Studies that did not clearly present trial data were excluded, as were studies that did not present data in such a way as to allow meta-analysis. An attempt was made to contact the authors of any paper lacking data that was deemed otherwise relevant to this report; no additional reports were included following these attempts.
2.3 Data extraction
Search data were retrieved manually and deduplicated using Deduklick (37). Deduplication was manually verified using EndNote. Deduplicated results were then upload to Rayyan for screening (38). Screening was conducted by three independent reviewers (A.A., A.S., and R.P.). All conflicts were resolved via joint discussion prior to final inclusion.
2.4 Quality assessment
Report quality and risk of bias was assessed using the Cochrane Risk of Bias 2 tool (39). The risk of bias was assessed by three independent reviewers. Two reviewers selected from A.S., R.P., A.P., and D.S. were assigned using a random number generator to each included record. A third reviewer (A.A.) conducted an independent review and resolved disputes prior to the final risk of bias assessment.
We assessed the quality of evidence of each outcome category using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system using GRADEpro GDT (40, 41). One reviewer (A.A.) assigned a GRADE rating (high, moderate, low, very low) to each outcome. Each rating was then evaluated independently by two additional authors (R.P., D.S.) and finalized by group discussion. The final ratings were agreed upon by all authors. Quality of evidence was assessed based on the following domains: risk of bias, inconsistency of results, indirectness of evidence, imprecision of results, publication bias. As our review included only RCTs, quality of evidence was initially rated as “high quality” and downgraded by one level per “serious limitation” identified and by two levels per “very serious limitation” identified. Criteria for downgrading are as follows: risk of bias was downgraded one level if between 50 and 75% of papers contributing to an outcome were rated as “High Risk of Bias” and was downgraded two levels if ≥75% of papers contributing to an outcome were rated as “High Risk of Bias.” Inconsistency of results was downgraded by one level if the outcome had an I2 of between 50 and 75% and was downgraded by two levels if I2 was ≥75%. Indirectness of evidence was downgraded based on the presence of confounding factors in the study designs or populations studied, for example, if the studies included in an outcome did not isolate the intervention. Imprecision of results was downgraded by one level if the confidence interval crossed zero. Summary of recommendations tables may be viewed in Supplementary Tables 2, 3.
2.5 Outcomes
The primary outcome was any statistically significant change in treatment outcomes after treatment with CST. All treatment outcomes presented by the retrieved reports were considered for this study. The conditions treated by CST in the evaluated studies were not sufficiently homogenous to allow for a single meta-analysis, so clinical outcomes were grouped into categories and multiple meta-analyses were conducted (Table 1). All results pertaining to these listed outcomes were retrieved. Outcomes were excluded if all screening authors (A.A., A.S., R.P., A.P., D.S.) agreed that they measured unrelated physiologic changes with no evidence of relationship to either the condition for which treatment was sought or to the treatment itself. No demographic data were compiled from the included reports.
2.6 Data synthesis and analysis
Data extraction was completed by a single author (A.A.) and independently verified by a second author (R.P.). Data analysis was completed by a single author (A.A.) and independently verified by a second author (D.S.). Data were manually extracted from all included trials. All pooling and meta-analysis was conducted with R software utilizing the “metafor,” “dmetar,” “meta,” and “metasens” packages (42–45). General analysis followed the plan laid out in the pre-registration and consisted of effect size calculation, outlier analysis, heterogeneity analysis, publication bias analysis, and risk of bias assessment. Modification was made to the pre-specified plan as data did not allow for analysis without subdivision. No aggregate effect size is presented due to study heterogeneity and outcome variability. Per study effect size has been reported based on the first time point recorded for the primary outcome only. Subgroup analysis based on outcome category was conducted on primary outcomes at the first measured time point. Additionally, primary and secondary outcomes at all time points were included in a separate per-outcome analysis.
Manual effect size calculation was performed if the RCT did not present effect sizes. To account for different scales within outcome measurement, effect sizes were calculated as mean differences using Hedge’s G values. All outcomes were continuous. Outcomes presented as odds ratio were converted to mean differences. An inverse variance random effect model with HKSJ variation correction was used to combine effect sizes in each outcome category as well as for subgroup analysis of primary outcomes. Outcome heterogeneity was assessed using I2 and tau calculated using a Sidik-Jonkman estimator.
Outlier studies were identified by comparing individual aggregate effect size confidence intervals with the pooled effect size confidence interval; non-overlapping confidence intervals were flagged as potential outliers. Outliers were further explored by performing influence analysis and generating a Baujat plot. Per-outcome subgroup analysis was performed including and excluding outliers.
To further explore potential publication bias and p-hacking in significant outcomes, p-curve analysis was conducted following the methodology described by Simonsohn et al. (46). This method attempts to assess publication bias more reliably than a single significant p-value by investigating the plotted curve of all significant p-values. Positive results may be said to have “evidential value,” indicating a true effect.
All code and data used in this meta-analysis are available on GitHub via Zenodo at https://zenodo.org/doi/10.5281/zenodo.10022853.
3 Results
3.1 Study selection
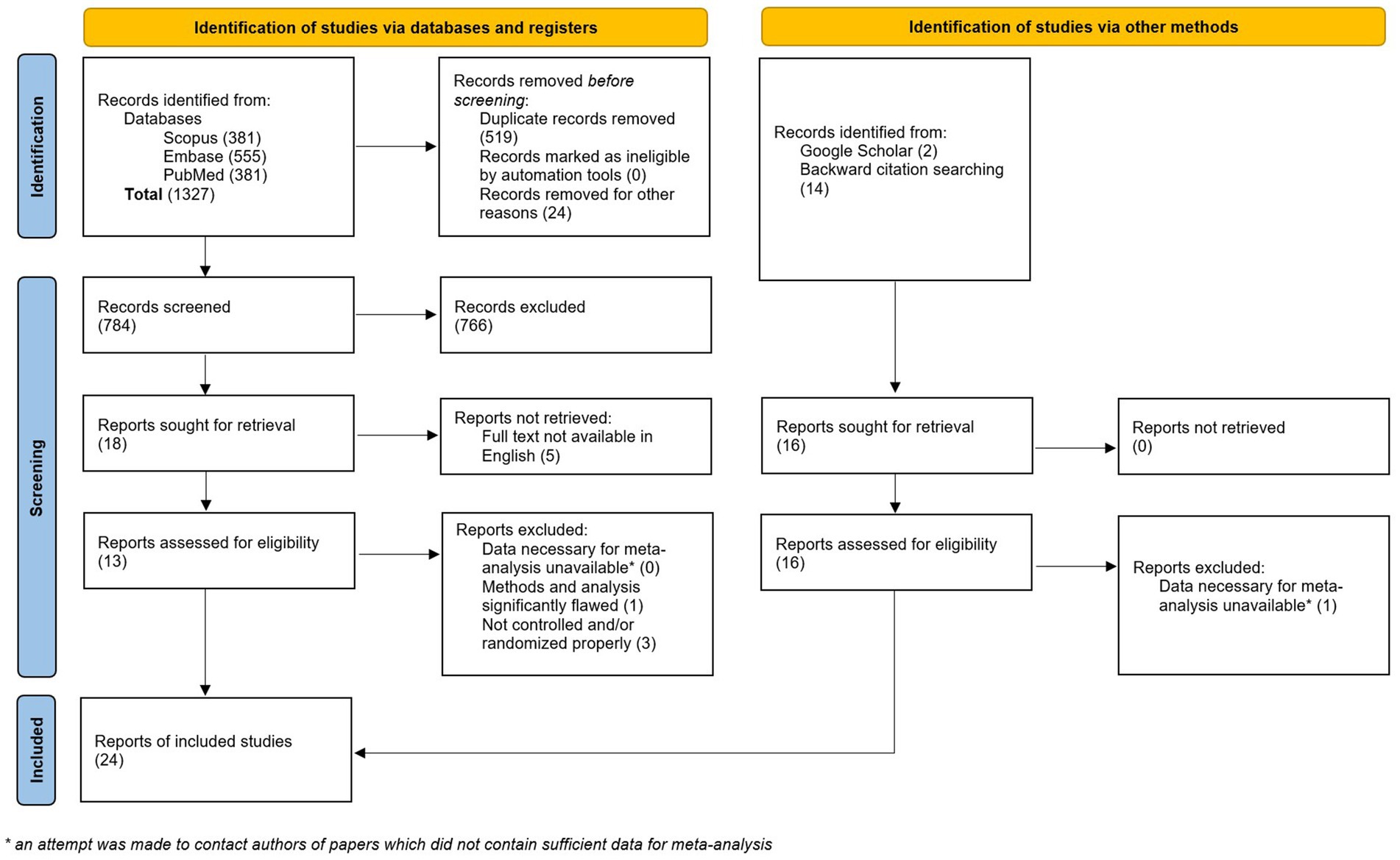
A total of 1,192 studies were retrieved after the initial search. After deduplication, 649 unique studies remained. An additional 16 studies were identified via Google Scholar search and backward citation searching. Of the 665 studies, 34 records were sought for retrieval. Of these, five were unavailable in English (47–51), one did not contain sufficient data for meta-analysis (52), and four were not properly randomized or otherwise contained methodological issues that rendered them unsuitable for inclusion in the meta-analysis (53–56) (Figure 1). Twenty-four studies were included in the final analysis with a total of 1,613 participants (57–80) (Table 2). An updated search on 5/8/2024 retrieved an additional 134 records, none of which met our inclusion criteria.
3.2 Risk of bias
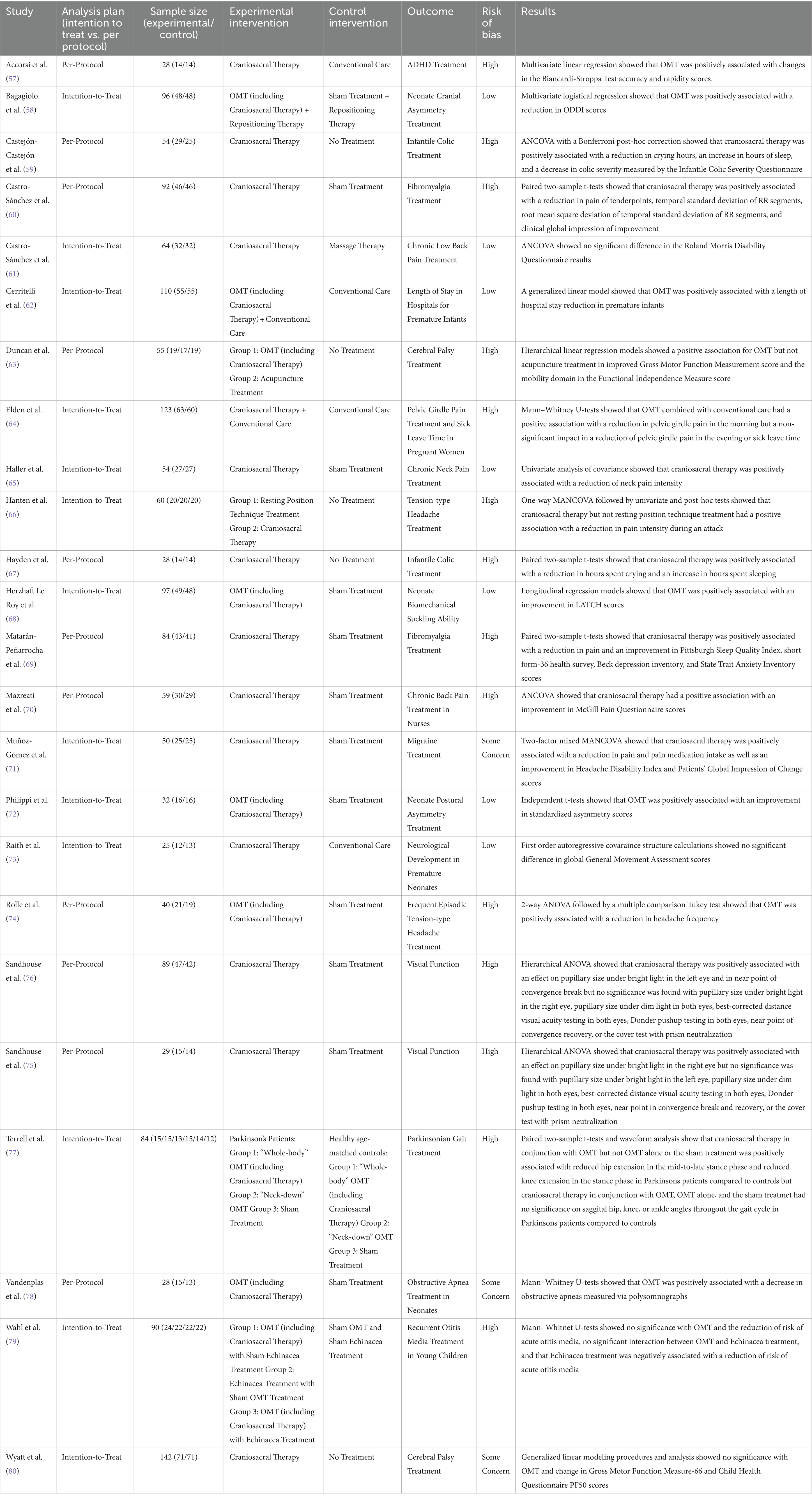
Of the 24 included studies, 14 were rated as “High Risk.” Three additional studies were rated as “Some Concern” (Figure 2). The primary findings that increased bias included following a per-protocol analysis plan, deviations from planned interventions, inconsistent outcome reporting, and statistical analysis that appeared to be post-hoc or otherwise inappropriate given the experimental design. Additionally, small sample sizes in all the studies and lack of power calculations contribute to bias rating. All but two studies were marked either “Some Concern” or “High Risk” in their selection of reported results. Complete and transparent data were provided by 22 of the 24 studies, though this is likely biased by the fact that studies not containing sufficient data were excluded from this meta-analysis.

Figure 2. Risk of bias. Studies have been divided based on stated analysis plan. Seven studies were identified as “Low risk,” three as “Some concerns,” and fourteen as “High risk” for bias.
3.3 Outliers
Three outliers were identified: Castejón-Castejón et al. (59), Mazreati et al. (70), and Terrell et al. (77). All three studies had been marked “High Risk of Bias.” Castejón-Castejón et al. (59) and Mazreati et al. (70) both displayed unusually high effect sizes in the positive direction. To confirm outliers, an influence analysis was conducted and a Baujat plot generated both of which support removal of identified articles (Figures 3, 4). While initial heterogeneity was presumed to be a result of between-study variation, given the significant reduction in heterogeneity when outliers were removed (with outliers I2 = 91.21%; without outliers I2 = 14.8%), the decision was made to partially conduct analysis excluding the most extreme outliers. This resulted in the exclusion of two articles: Castejón-Castejón et al. (59) and Mazreati et al. (70).
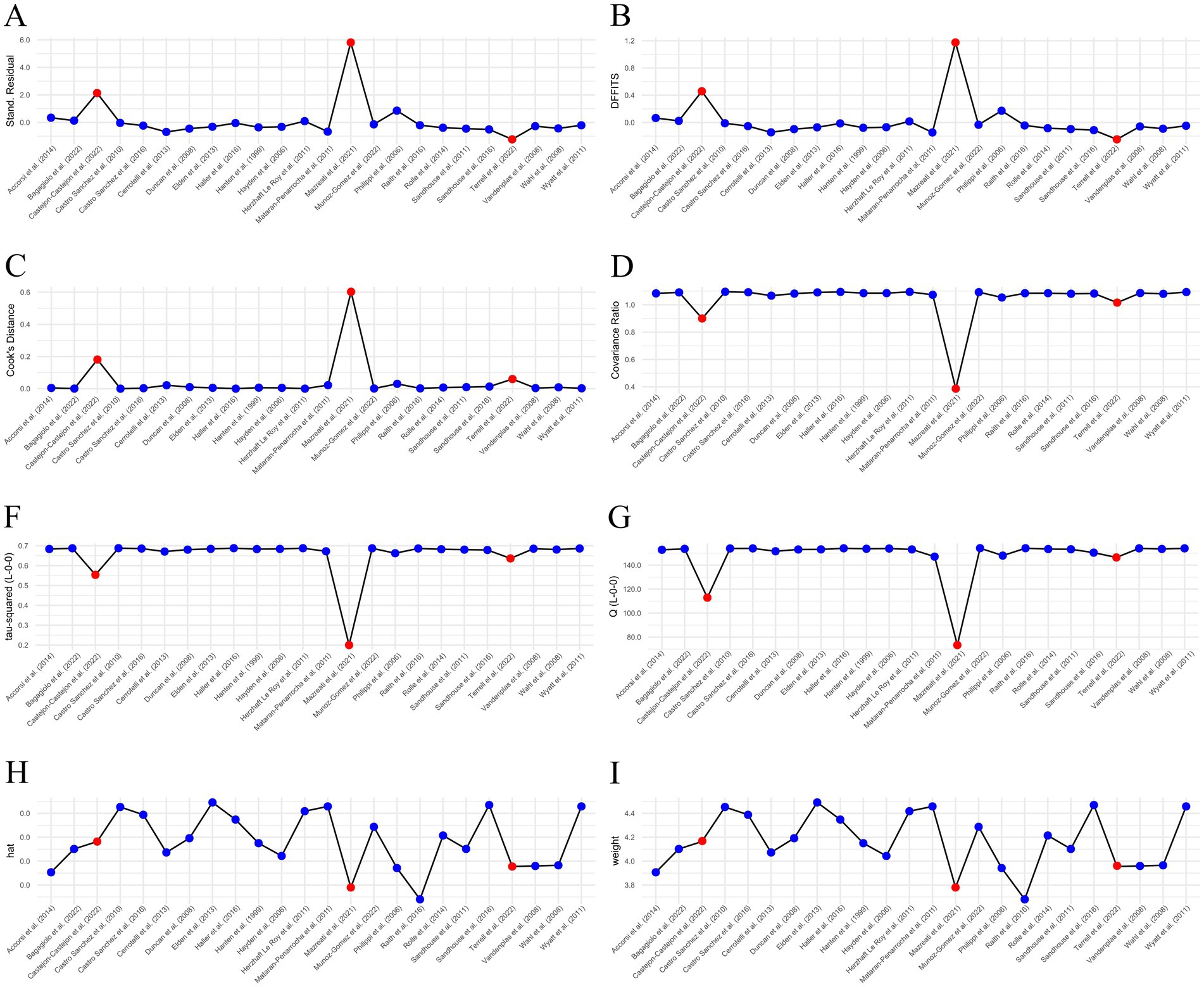
Figure 3. Influence analysis. Both Castejón-Castejón et al. (59) and Mazreati et al. (70) stand out as potential outliers. Terrell et al. (77) is not as convincing. (A) External residuals calculated with a leave-one-out method. (B) Difference in fits (DFFITS). (C) Cook’s distance. (D) Covariance ratio. (F,G) Leave-one-out Tau and Cochrane’s Q. (H) Hat value. (I) Study weights. Potential outliers are highlighted in red.
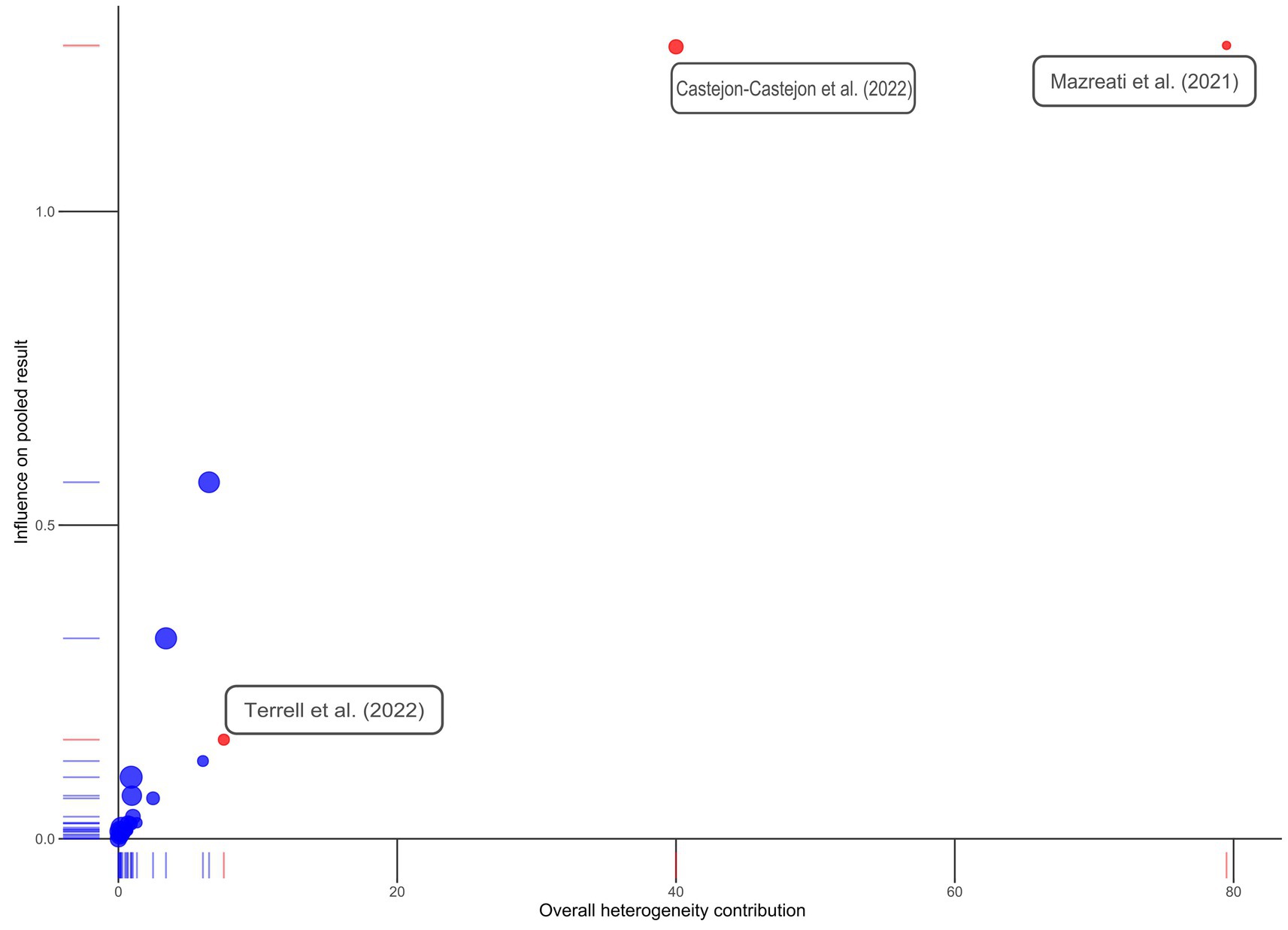
Figure 4. Baujat plot. Studies initially identified as possible outliers are colored in red and labeled. Note that Terrell et al. (77) does not appear to be a true outlier.
3.4 Effect size estimation
The included studies yielded 249 individual effect sizes. When effect sizes of primary outcomes were aggregated on a per-study basis, 17 of 24 (71%) of confidence intervals crossed zero, indicating a non-significant effect (Figure 5). Thirteen of the included studies reported discrete secondary outcomes, 11 of which (85%) were non-significant (Figure 6). Subgroup analysis performed on primary outcomes of all included studies indicated that no significant effect existed in any category (Figure 7A). When effect sizes were aggregated by outcome, including secondary outcomes and multiple time points, non-outlier studies support a significant effect in Disability; Neonate health, structure; and Pain, chronic somatic (Figure 7B). Prediction intervals for all significant per-outcome subgroup analyses substantially cross zero. Expanded results of this analysis, including analysis with outliers included, is available in Supplementary 1.

Figure 5. Per-study effect sizes of primary outcomes. Effect sizes were calculated using the first timepoint of the primary outcome. Effect sizes are displayed as g including a 95% confidence interval, standard error, and risk of bias. Potential outlier studies have been highlighted in red. Positive effect size favors treatment, negative effect size favors comparison.
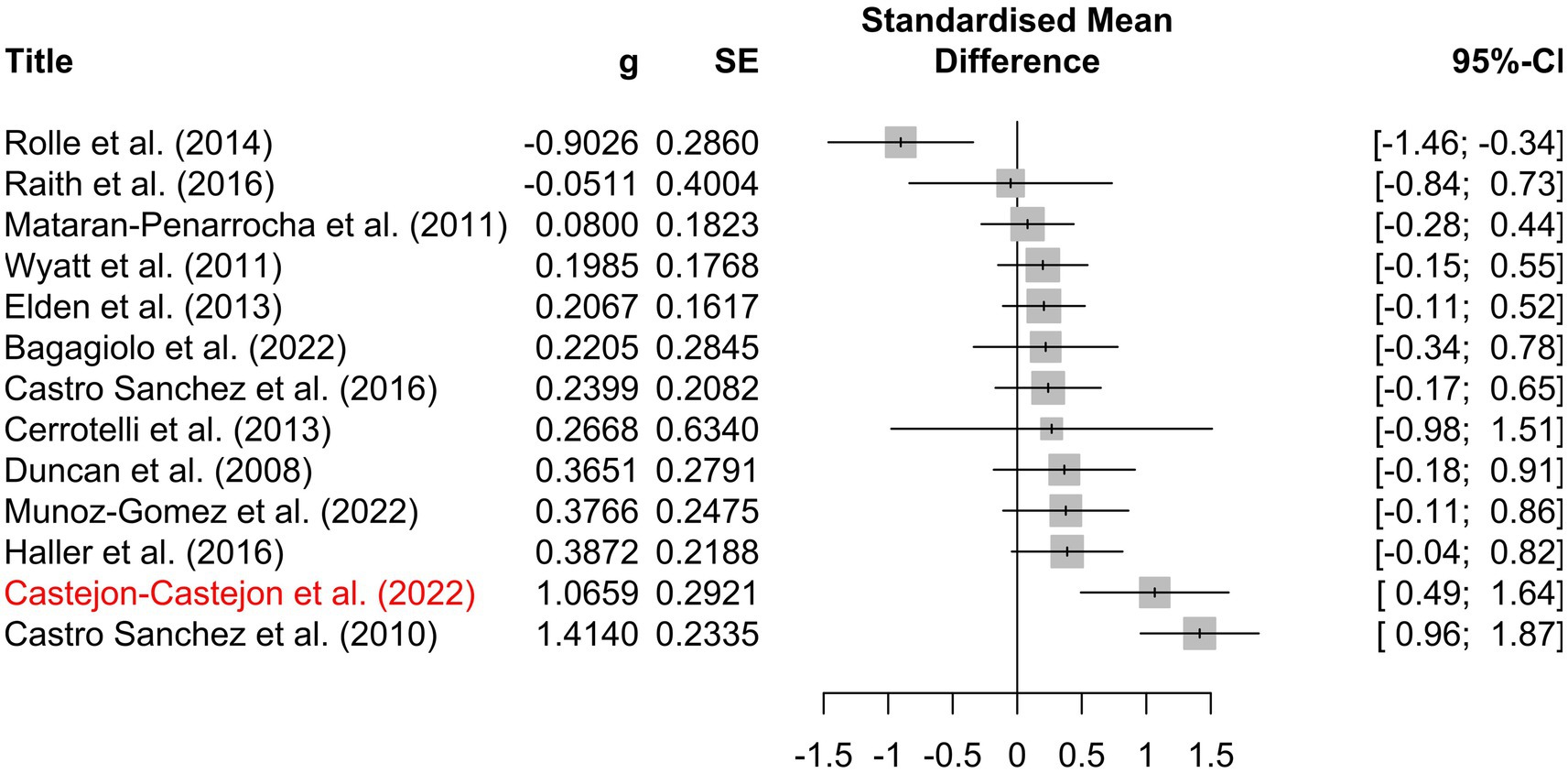
Figure 6. Per-study effect sizes of secondary outcomes. Effect sizes were calculated using the first timepoint of the secondary outcome. Effect sizes are displayed as g including a 95% confidence interval, standard error, and risk of bias. Potential outlier studies have been highlighted in red. Positive effect size favors treatment, negative effect size favors comparison.
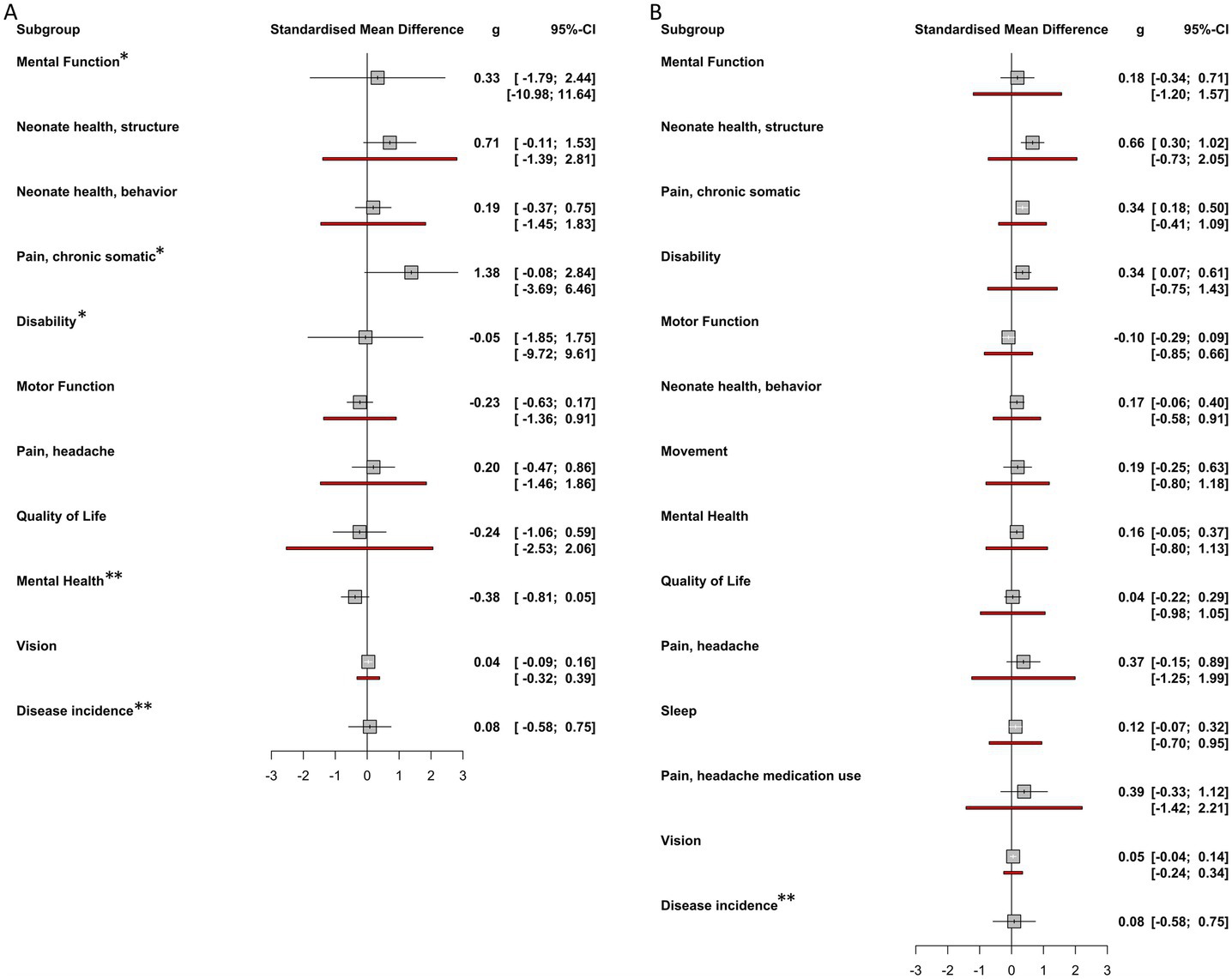
Figure 7. Per-outcome subgroup analysis effect sizes. (A) Overall effect sizes for each primary outcome subgroup calculated using random effects models. No significant effects were found for any outcome subgroup. (B) Overall effect sizes for each outcome subgroup calculated using random effects models. These subgroups include both primary and secondary outcomes. Neonate health, structure, Pain, chronic somatic, and Disability were found to have significant effect sizes. Positive affect sizes favor treatment, negative effect sizes favor comparison group. Red lines indicate prediction intervals (PI). Note that some outcome groups do not include both primary and secondary outcomes and so do not appear in both forest plots. * Indicates that prediction interval extends past the bounds of the forest plot. ** Indicates that insufficient observations were available to calculate prediction interval.
3.5 Publication bias
P curve analysis conducted on significant outcomes suggests evidential value for Neonatal health, structure and pain, chronic somatic, implying that the positive findings are not likely due to publication bias. However, Disability does not show evidential value (Figure 8).
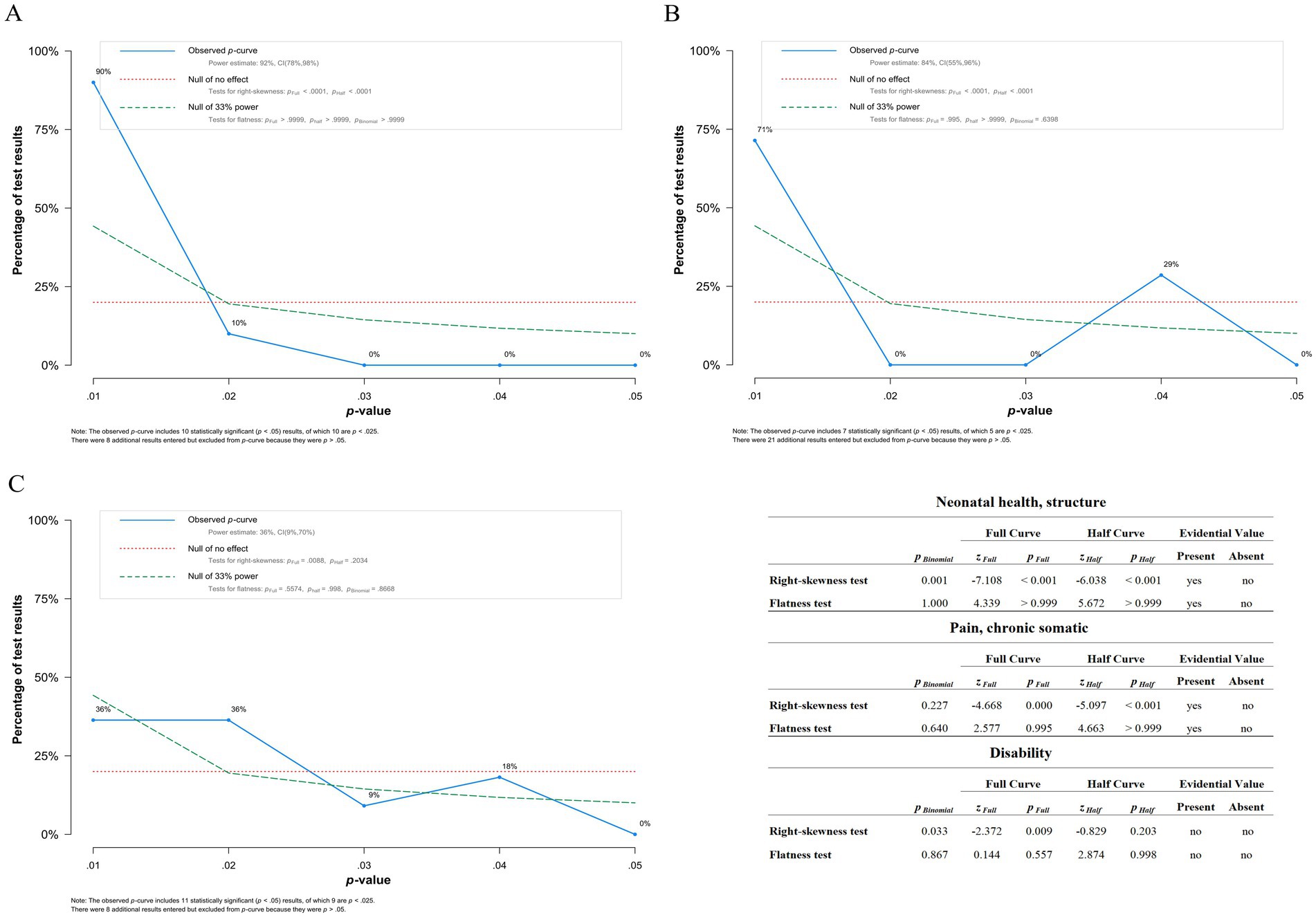
Figure 8. Results of p curve analysis. (A) p-curve of Neonatal health, structure showing evidential value. (B) p-curve of Pain, chronic somatic suggesting evidential value. Though the peak at p = 0.04 is concerning, more studies data is necessary to determine if it is simply an aberration due to low n. (C) p-curve of Disability suggesting no evidential value, however there is not enough evidence to determine definitively. The included table presents results of flatness and right-skewedness tests used to determine if evidential value is present and/or absent.
4 Discussion
The existing models describing CST contradict our modern understanding of health and disease, diminishing its plausibility for clinical effectiveness. To establish CST as a recommended practice in clinical settings, compelling results from prospective studies with adequate sample sizes at low risk of bias are necessary. The findings from this meta-analysis indicate that the current body of evidence surrounding CST does not reach this standard. This is in line with conclusions found in previous reviews and meta-analyses (23, 24, 26, 28, 29, 31, 33). A meta-analysis by Haller et al. (30) found a significant effect of CST on pain and disability. However, our subgroup analysis of primary outcomes indicates no significant effects of CST. Only when secondary outcomes are included—arguably a suboptimal approach due to confounding statistical factors—are significant effects identified. Upon close inspection of the meta-analysis by Haller et al., their claims may be based on over-inflated effects likely found due to low quality studies, small sample sizes, under-assessment of bias, and inappropriate grouping of outcomes. Of note, only six of ten studies included in Haller et al. met inclusion criteria and were reanalyzed in this current report. The only other identified positive evidence, presented in a meta-analysis by Jiang et al. (32), found a significant effect of CST on short hamstring syndrome. This indication was not assessed in this report as no related trials meeting inclusion criteria were identified.
While significant findings were reported by most individual studies evaluated here, per-study effect sizes calculated indicate that few significant effects existed. This was true of both individual effect sizes as well as per-study aggregate effect sizes. One possible explanation for this discrepancy is the occurrence of post-hoc statistical analysis and post-hoc outcome selection in the articles—two common concerns identified during the risk of bias assessment. Another contributing factor may be that few studies calculated an effect size or other standardized measure of efficacy. Many of the included studies also did not correct for multiple outcome measures or repeated measures despite high numbers of outcomes and statistical tests.
Notably, our subgroup analysis of primary outcomes shows evidence that there is no significant effect of CST. This result is unsurprising as there is no prior plausibility for a measurable effect on outcomes such as vision, mental health, mental function, quality of life, sleep, movement, or motor function. It is more reasonable that an effect may be observed in subjective measures of pain and disability through the mechanism of the positive effects of touch, which have been shown convincingly in the literature (81). There may also be a plausible mechanism for manipulation of unfused cranial sutures in infants. Even in these populations, however, our evidence suggests no benefit.
Only when all outcomes and timepoints were considered, potential effects on pain, disability, and neonatal structure were observed. Extreme caution should be used when interpreting this as a positive result. While interesting as a statistical exercise, this observation is tempered by various limitations. These limitations include a high level of bias, multiple measures, multiple time points, wide prediction intervals in calculated effect sizes, inadequate blinding and evidence of post-hoc data analysis. Additionally, p-curve analysis of disability-related outcomes does not support true evidential value, though there is not enough evidence to definitively state that none exists. Consequently, caution is advised in interpreting any positive effects identified as the likelihood of significant positive bias affecting the outcomes is considerable.
Despite significant effect sizes and support from p-curve analysis, outcomes related to neonatal structure and somatic pain displayed prediction intervals that broadly crossed zero. Such a wide prediction interval suggests substantial uncertainty regarding the treatment’s potential effectiveness in practical application or in subsequent studies. Thus, these may not represent true positive outcomes and rather reflect statistical aberrations resulting from study limitations. Perhaps even more importantly, these outcome categories are not significant when only primary outcomes are considered. While more research may help clarify whether there is any benefit from the use of CST for these populations and indications, the findings presented here, as well as in prior reviews, suggest a total absence of strong evidence supporting the efficacy of CST.
4.1 Limitations
Several important limitations to this analysis exist. The results of the outcome-based subgroup meta-analysis were affected by the decision to exclude outliers. Initially, an algorithm flagged studies in which the effect size fell outside the confidence interval of the group effect size. Influence analysis was conducted in order to confirm these results, which agreed with the initial detection. This process is limited by the variation within and between studies, differing outcomes, and multiple indications. The Supplementary material contains results that include outliers, demonstrating only one significant difference in Neonate health, behavior (Supplementary 2C). However, considering the large contribution to heterogeneity and the high bias of the outlier studies, the calculated effect sizes excluding outliers are likely closer to reality.
This analysis is also limited by problems inherent to the included studies. From a statistical perspective, many papers reporting a positive effect failed to account for repeated measures and multiple time point measures. Our own efforts to correct for this based on recommendations by Morris et al. (82) still led to a violation of the assumption of independence, meaning confounding factors may be present. Additionally, a number of the included studies did not provide detailed descriptions of the techniques used and many studies mixed CST with other osteopathic treatments. Rather, precise treatment plans were left to the discretion of the practitioner providing care. Both these factors may have impacted the calculated effect sizes, though likely in the positive direction. What’s more, many included papers suffered from poor blinding, poor randomization, and incomplete result reporting. Given the overall negative outcome, this did not result in reinterpretation of the findings, though care should be taken when interpreting the three positive effects identified.
The limitations in the available literature warrant reservation in considering CST as part of evidence-based treatment plans until substantially higher-quality evidence emerges. Thus, the role and scope of CST should be reevaluated in modern osteopathic medicine. While the findings from this meta-analysis have the potential to inform future research directions, it becomes challenging to advocate for continued exploration of CST considering the predominantly negative outcomes which persist despite various methodological concerns producing probable positive bias. Further research exploring the physiologic mechanisms behind CST could help resolve some of the controversy surrounding its use, though it is unlikely that a plausible biological mechanism will be identified and thus efforts should be directed towards other more promising treatment modalities.
5 Conclusion
Craniosacral therapy did not demonstrate broad significance in this meta-analysis, suggesting limited or no usefulness in patient care for a wide range of indications including attention deficit hyperactivity disorder, cranial asymmetry, infant colic, fibromyalgia, low back pain, cerebral palsy, pelvic girdle pain, neck pain, tension-type headache, infant suckling, vision, obstructive sleep apnea, recurrent otitis media, migraine headache, parkinsonian gait, length of stay of premature infants, or neurodevelopment of premature infants. Subgroup analysis of primary outcomes indicates no significant effect when these indications are treated with CST. When secondary outcomes are included, analysis supports possible clinical utility in reducing disability scores, neonatal structural disease, and chronic somatic pain, though methodological limitations substantially reduce the strength of this result. Taken in whole, the currently available literature shows that CST is not effective as a treatment for any investigated condition in either adults or infants. Given the overwhelming evidence that there is no benefit from CST, we do not recommend further exploration of this topic.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found here: Github via Zenodo—https://doi.org/10.5281/zenodo.10022854.
Author contributions
AA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. AS: Data curation, Investigation, Writing – original draft, Writing – review & editing. RP: Data curation, Investigation, Validation, Writing – original draft, Writing – review & editing. AP: Data curation, Investigation, Validation, Writing – original draft, Writing – review & editing. SS: Conceptualization, Investigation, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. DS: Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Thank you, Darrell Schmick, for providing your expertise and advice when developing our search term.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1452465/full#supplementary-material
Abbreviations
CST, Craniosacral therapy; CRI, Cranial rhythmic impulse; GRADE, Grading of recommendations assessment, development and evaluation; OMM, Osteopathic manipulative medicine; OSF, Open science framework; PRISMA, Preferred reporting items for systematic reviews and meta-analyses; RCT, Randomized controlled trial.
References
1. Bordoni, B, and Zanier, E. Sutherland's legacy in the new millennium: the osteopathic cranial model and modern osteopathy. Adv Mind Body Med. (2015) 29:15–21.
4. Organization, W.H (2019). WHO global report on traditional and complementary medicine 2019. World Health Organization.
5. Franklin, D, and Flavel, A. Brief communication: timing of spheno-occipital closure in modern Western Australians. Am J Phys Anthropol. (2014) 153:132–8. doi: 10.1002/ajpa.22399
6. Shirley, NR, and Jantz, RL. Spheno-occipital synchondrosis fusion in modern Americans. J Forensic Sci. (2011) 56:580–5. doi: 10.1111/j.1556-4029.2011.01705.x
7. Ingervall, B, and Thilander, B. The human spheno-occipital synchondrosis. I. The time of closure appraised macroscopically. Acta Odontol Scand. (1972) 30:349–56. doi: 10.3109/00016357209004602
8. Thilander, B, and Ingervall, B. The human spheno-occipital synchondrosis. II. A histological and microradiographic study of its growth. Acta Odontol Scand. (1973) 31:323–34. doi: 10.3109/00016357309002520
9. Krishan, K, and Kanchan, T. Evaluation of spheno-occipital synchondrosis: a review of literature and considerations from forensic anthropologic point of view. J Forensic Dent Sci. (2013) 5:72–6. doi: 10.4103/0975-1475.119764
10. Bassed, RB, Briggs, C, and Drummer, OH. Analysis of time of closure of the spheno-occipital synchondrosis using computed tomography. Forensic Sci Int. (2010) 200:161–4. doi: 10.1016/j.forsciint.2010.04.009
11. di Ieva, A, Bruner, E, Davidson, J, Pisano, P, Haider, T, Stone, SS, et al. Cranial sutures: a multidisciplinary review. Childs Nerv Syst. (2013) 29:893–905. doi: 10.1007/s00381-013-2061-4
12. Rogers, JS, and Witt, PL. The controversy of cranial bone motion. J Orthop Sports Phys Ther. (1997) 26:95–103. doi: 10.2519/jospt.1997.26.2.95
13. Hartman, SE. Cranial osteopathy: its fate seems clear. Chiropr Osteopat. (2006) 14:10. doi: 10.1186/1746-1340-14-10
14. Alvarez, G, Roura, S, Cerritelli, F, Esteves, JE, Verbeeck, J, and van Dun, PLS. The Spanish osteopathic practitioners estimates and RAtes (OPERA) study: a cross-sectional survey. PLoS One. (2020) 15:e0234713. doi: 10.1371/journal.pone.0234713
15. Santiago, RJ, Nunes, A, Esteves, JE, Cerritelli, F, Verbeeck, J, Lopes, S, et al. The Portuguese osteopathic practitioners estimates and RAtes (OPERA): a cross-sectional survey. Int J Osteop Med. (2022) 43:23–30. doi: 10.1016/j.ijosm.2022.04.002
16. van Dun, PLS, Arcuri, L, Verbeeck, J, Esteves, JE, and Cerritelli, F. The Austrian osteopathic practitioners estimates and RAtes (OPERA): a cross-sectional survey. PLoS One. (2022) 17:e0278041. doi: 10.1371/journal.pone.0278041
17. van Dun, PLS, Verbeeck, J, Arcuri, L, Esteves, JE, and Cerritelli, F. The profile of Belgian osteopaths: a cross-sectional survey. Healthcare. (2022) 10:2136. doi: 10.3390/healthcare10112136
18. Wagner, A, Ménard, M, Jacquot, E, Marangelli, G, Merdy, O, Clouzeau, C, et al. The profile of French osteopaths: a cross-sectional survey. Int J Osteop Med. (2023) 49:100672. doi: 10.1016/j.ijosm.2023.100672
19. Healy, CJ, Brockway, MD, and Wilde, BB. Osteopathic manipulative treatment (OMT) use among osteopathic physicians in the United States. J Osteopath Med. (2021) 121:57–61. doi: 10.1515/jom-2020-0013
21. Dal Farra, F, Risio, RG, Vismara, L, and Bergna, A. Effectiveness of osteopathic interventions in chronic non-specific low back pain: a systematic review and meta-analysis. Complement Ther Med. (2021) 56:102616. doi: 10.1016/j.ctim.2020.102616
22. Dal Farra, F, Buffone, F, Risio, RG, Tarantino, AG, Vismara, L, and Bergna, A. Effectiveness of osteopathic interventions in patients with non-specific neck pain: a systematic review and meta-analysis. Complement Ther Clin Pract. (2022) 49:101655. doi: 10.1016/j.ctcp.2022.101655
23. Buffone, F, Monacis, D, Tarantino, AG, Dal Farra, F, Bergna, A, Agosti, M, et al. Osteopathic treatment for gastrointestinal disorders in term and preterm infants: a systematic review and meta-analysis. Healthcare. (2022) 10:1525. doi: 10.3390/healthcare10081525
24. DeMarsh, S, Huntzinger, A, Gehred, A, Stanek, JR, Kemper, KJ, and Belsky, JA. Pediatric osteopathic manipulative medicine: a scoping review. Pediatrics. (2021) 147:e2020016162. doi: 10.1542/peds.2020-016162
25. Posadzki, P, Kyaw, BM, Dziedzic, A, and Ernst, E. Osteopathic manipulative treatment for pediatric conditions: an update of systematic review and Meta-analysis. Journal of. Clin Med. (2022) 11:4455. doi: 10.3390/jcm11154455
26. Carrasco-Uribarren, A, Mamud-Meroni, L, Tarcaya, GE, Jiménez-del-Barrio, S, Cabanillas-Barea, S, and Ceballos-Laita, L. Clinical effectiveness of craniosacral therapy in patients with headache disorders: a systematic review and meta-analysis. Pain Manag Nurs. (2023) 25:e21–8. doi: 10.1016/j.pmn.2023.07.009
27. Hartman, SE, and Norton, JM. Interexaminer reliability and cranial osteopathy. Sci Rev Altern Med. (2002) 6:23–34.
28. Green, C., Martin, CW, Bassett, K, and Kazanjian, A. (1999). A systematic review and critical appraisal of the scientific evidence on craniosacral therapy. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet].
29. Jäkel, A, and von Hauenschild, P. Therapeutic effects of cranial osteopathic manipulative medicine: a systematic review. J Am Osteopath Assoc. (2011) 111:685–93.
30. Haller, H, Lauche, R, Sundberg, T, Dobos, G, and Cramer, H. Craniosacral therapy for chronic pain: a systematic review and meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. (2019) 21:1. doi: 10.1186/s12891-019-3017-y
31. Green, C, Martin, CW, Bassett, K, and Kazanjian, A. A systematic review of craniosacral therapy: biological plausibility, assessment reliability and clinical effectiveness. Complement Ther Med. (1999) 7:201–7. doi: 10.1016/S0965-2299(99)80002-8
32. Jiang, WB, Samuel, OC, Li, Z, Chen, W, and Sui, HJ. Effectiveness of craniosacral therapy in the human suboccipital region on hamstring muscle: a meta-analysis based on current evidence. Medicine (Baltimore). (2023) 102:e32744. doi: 10.1097/MD.0000000000032744
33. Ceballos-Laita, L, Ernst, E, Carrasco-Uribarren, A, Cabanillas-Barea, S, Esteban-Pérez, J, and Jiménez-del-Barrio, S. Is craniosacral therapy effective? A systematic review and meta-analysis. Healthcare (Basel). (2024) 12:679. doi: 10.3390/healthcare12060679
34. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
35. Amendolara, A., Sheppert, A., and Sant, D., (2023). Efficacy of osteopathic craniosacral techniques: a systematic review with meta-analysis. OSF.
36. Amendolara, A, Sheppert, A, Powers, R, Payne, A, Stacey, S, and Sant, D. (2024). Effectiveness of osteopathic craniosacral techniques: a meta-analysis. Research Square. PREPRINT.
37. Borissov, N, Haas, Q, Minder, B, Kopp-Heim, D, von Gernler, M, Janka, H, et al. Reducing systematic review burden using Deduklick: a novel, automated, reliable, and explainable deduplication algorithm to foster medical research. Syst Rev. (2022) 11:172. doi: 10.1186/s13643-022-02045-9
38. Ouzzani, M, Hammady, H, Fedorowicz, Z, and Elmagarmid, A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
39. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
40. Schünemann, H, Guyatt, G, and Oxman, A, ed. (2013). GRADE handbook for grading quality of evidence and strength of recommendationsGrading quality of evidence and strength of recommendations. GRADE Working Group.
41. GRADEpro GDT (2024). GRADEpro Guideline Development Tool 2024, McMaster University and Evidence Prime.
42. Viechtbauer, W. Conducting Meta-analyses in R with the metafor package. J Stat Softw. (2010) 36:1–48. doi: 10.18637/jss.v036.i03
43. Harrer, M., Cuijpers, P, Furukawa, T, and Ebert, DD. (2019). dmetar: Companion R Package For The Guide 'Doing Meta-Analysis in R'.
44. Balduzzi, S, Rücker, G, and Schwarzer, G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117
46. Simonsohn, U, Nelson, LD, and Simmons, JP. P-curve and effect size: correcting for publication bias using only significant results. Perspect Psychol Sci. (2014) 9:666–81. doi: 10.1177/1745691614553988
47. Bychkova, EY, Sandakov, AV, and Kuzmina, YO. Long-term results of combined use of speech therapy and osteopathic correction in 5–6 year old children with dysarthria. Rossijskij Osteopaticeskij Zurnal. (2022) 2022:35–48. doi: 10.32885/2220-0975-2022-1-35-48
48. Geldschläger, S. Osteopathic versus orthopedic treatments for chronic epicondylopathia humeri radialis: a randomized controlled trial. Forsch Komplementarmed Klass Naturheilkd. (2004) 11:93–7. doi: 10.1159/000078230
49. Kudryashova, VG, and Chetverikova, NA. Study of the combined use of osteopathic correction and EEG-biofeedback training in children with attention deficit hyperactivity disorder. Rossijskij Osteopaticeskij Zurnal. (2022) 2022:60–8. doi: 10.32885/2220-0975-2022-1-60-68
50. Quesada, BM. Effects of osteopathic techniques of balancing SEB and separating the front-ethmoid suture in children with narrow palate. Rossijskij Osteopaticeskij Zurnal. (2022) 2022:121–33. doi: 10.32885/2220-0975-2022-1-121-133
51. Belash, VO, Gritsay, EO, and Musina, TS. The use of osteopathic correction in the complex rehabilitation of patients with acute cerebrovascular accident. Rossijskij Osteopaticeskij Zurnal. (2022) 2022:27–39. doi: 10.32885/2220-0975-2022-2-27-39
52. Mehl-Madrona, L, Kligler, B, Silverman, S, Lynton, H, and Merrell, W. The impact of acupuncture and craniosacral therapy interventions on clinical outcomes in adults with asthma. Explore. (2007) 3:28–36. doi: 10.1016/j.explore.2006.10.003
53. Malak, R, Kozłowska, Z, Owsiańska, Z, Sikorska, D, Andrusiewicz, M, Szymankiewicz -Bręborowicz, M, et al. Cerebral tissue oxygenation during cranial osteopathic CV4 procedure in newborns. Adv Clin Exp Med. (2020) 29:1187–91. doi: 10.17219/acem/126048
54. Müller, T, and Pietsch, A. Comparison of gait training versus cranial osteopathy in patients with Parkinson's disease: a pilot study. NeuroRehabilitation. (2013) 32:135–40. doi: 10.3233/NRE-130830
55. Demers, K, Grenier, E, Wolfe, D, and DeMont, R. Cranial bone and upper cervical vertebrae mobility restrictions in adults with post-concussion syndrome. Adv Integ Med. (2019) 6:S14. doi: 10.1016/j.aimed.2019.03.037
56. Bendixen, K, Beinlich, A, Beck, B, Hashmi, N, and Craig, A. Pilot study assessing the effect of osteopathic manipulative treatment (OMT) on length of stay in neonates after therapeutic hypothermia. J Osteopath Med. (2021) 121:97–104. doi: 10.1515/jom-2020-0005
57. Accorsi, A, Lucci, C, di Mattia, L, Granchelli, C, Barlafante, G, Fini, F, et al. Effect of osteopathic manipulative therapy in the attentive performance of children with attention-deficit/hyperactivity disorder. J Osteop Med. (2014) 114:374–81. doi: 10.7556/jaoa.2014.074
58. Bagagiolo, D, Priolo, CG, Favre, EM, Pangallo, A, Didio, A, Sbarbaro, M, et al. A randomized controlled trial of osteopathic manipulative therapy to reduce cranial asymmetries in young infants with nonsynostotic plagiocephaly. Am J Perinatol. (2022) 39:S52–62. doi: 10.1055/s-0042-1758723
59. Castejón-Castejón, M, Murcia-González, MA, Todri, J, Lena, O, and Chillón-Martínez, R. Treatment of infant colic with craniosacral therapy. A randomized controlled trial. Complement Ther Med. (2022) 71:102885. doi: 10.1016/j.ctim.2022.102885
60. Castro-Sanchez, AM, Matarán-Peñarrocha, GA, Sánchez-Labraca, N, Quesada-Rubio, JM, Granero-Molina, J, and Moreno-Lorenzo, C. A randomized controlled trial investigating the effects of craniosacral therapy on pain and heart rate variability in fibromyalgia patients. Clin Rehabil. (2011) 25:25–35. doi: 10.1177/0269215510375909
61. Castro-Sánchez, AM, Lara-Palomo, IC, Matarán-Peñarrocha, GA, Saavedra-Hernández, M, Pérez-Mármol, JM, and Aguilar-Ferrándiz, ME. Benefits of craniosacral therapy in patients with chronic low Back pain: a randomized controlled trial. J Altern Complement Med. (2016) 22:650–7. doi: 10.1089/acm.2016.0068
62. Cerritelli, F, Pizzolorusso, G, Ciardelli, F, la Mola, E, Cozzolino, V, Renzetti, C, et al. Effect of osteopathic manipulative treatment on length of stay in a population of preterm infants: a randomized controlled trial. BMC Pediatr. (2013) 13:1–8. doi: 10.1186/1471-2431-13-65
63. Duncan, B, McDonough-Means, S, Worden, K, Schnyer, R, Andrews, J, Meaney, FJ, et al. Effectiveness of osteopathy in the cranial field and myofascial release versus acupuncture as complementary treatment for children with spastic cerebral palsy: a pilot study. J Osteop Med. (2008) 108:559–70.
64. Elden, H, Östgaard, HC, Glantz, A, Marciniak, P, Linnér, AC, and Olsén, MF. Effects of craniosacral therapy as adjunct to standard treatment for pelvic girdle pain in pregnant women: a multicenter, single blind, randomized controlled trial. Acta Obstet Gynecol Scand. (2013) 92:775–82. doi: 10.1111/aogs.12096
65. Haller, H, Lauche, R, Cramer, H, Rampp, T, Saha, FJ, Ostermann, T, et al. Craniosacral therapy for the treatment of chronic neck pain: a randomized sham-controlled trial. Clin J Pain. (2016) 32:441–9. doi: 10.1097/AJP.0000000000000290
66. Hanten, WP, Olson, SL, Hodson, JL, Imler, VL, Knab, VM, and Magee, JL. The effectiveness of CV-4 and resting position techniques on subjects with tension-type headaches. J Manual Manipul Therapy. (1999) 7:64–70. doi: 10.1179/106698199790811816
67. Hayden, C, and Mullinger, B. A preliminary assessment of the impact of cranial osteopathy for the relief of infantile colic. Complement Ther Clin Pract. (2006) 12:83–90. doi: 10.1016/j.ctcp.2005.12.005
68. Herzhaft-Le Roy, J, Xhignesse, M, and Gaboury, I. Efficacy of an osteopathic treatment coupled with lactation consultations for infants’ biomechanical sucking difficulties: a randomized controlled trial. J Hum Lact. (2017) 33:165–72. doi: 10.1177/0890334416679620
69. Matarán-Peñarrocha, GA, Castro-Sánchez, AM, García, GC, Moreno-Lorenzo, C, Carreño, TP, and Zafra, MDO. Influence of craniosacral therapy on anxiety, depression and quality of life in patients with fibromyalgia. Evid Based Complement Alternat Med. (2011) 2011:178769. doi: 10.1093/ecam/nep125
70. Mazreati, N, Rahemi, Z, Aghajani, M, Ajorpaz, NM, and Mianehsaz, E. Effect of craniosacral therapy on the intensity of chronic back pain of nurses: a randomized controlled trial. Nurs Pract Today. (2021) 8:313–321. doi: 10.18502/npt.v8i4.6707
71. Muñoz-Gómez, E, Inglés, M, Aguilar-Rodríguez, M, Mollà-Casanova, S, Sempere-Rubio, N, Serra-Añó, P, et al. Effect of a craniosacral therapy protocol in people with migraine: a randomized controlled trial. J Clin Med. (2022) 11:759. doi: 10.3390/jcm11030759
72. Philippi, H, Faldum, A, Schleupen, A, Pabst, B, Jung, T, Bergmann, H, et al. Infantile postural asymmetry and osteopathic treatment: a randomized therapeutic trial. Dev Med Child Neurol. (2006) 48:5–9. doi: 10.1017/S001216220600003X
73. Raith, W, Marschik, PB, Sommer, C, Maurer-Fellbaum, U, Amhofer, C, Avian, A, et al. General movements in preterm infants undergoing craniosacral therapy: a randomised controlled pilot-trial. BMC Complement Altern Med. (2016) 16:12. doi: 10.1186/s12906-016-0984-5
74. Rolle, G, Tremolizzo, L, Somalvico, F, Ferrarese, C, and Bressan, LC. Pilot trial of osteopathic manipulative therapy for patients with frequent episodic tension-type headache. J Osteop Med. (2014) 114:678–85. doi: 10.7556/jaoa.2014.136
75. Sandhouse, ME, Shechtman, D, Sorkin, R, Drowos, JL, Caban-Martinez III, AJ, Patterson, MM, et al. Effect of osteopathy in the cranial field on visual function—a pilot study. J Osteop Med. (2010) 110:239–43.
76. Sandhouse, ME, Shechtman, D, Fecho, G, and Timoshkin, EM. Effect of osteopathic cranial manipulative medicine on visual function. J Am Osteopath Assoc. (2016) 116:706–14. doi: 10.7556/jaoa.2016.141
77. Terrell, ZT, Moudy, SC, Hensel, KL, and Patterson, RM. Effects of osteopathic manipulative treatment vs. osteopathic cranial manipulative medicine on parkinsonian gait. J Osteop Med. (2022) 122:243–51. doi: 10.1515/jom-2021-0203
78. Vandenplas, Y, Denayer, E, Vandenbossche, T, Vermet, L, Hauser, B, DeSchepper, J, et al. Osteopathy may decrease obstructive apnea in infants: a pilot study. Osteop Med Prim Care. (2008) 2:8–4. doi: 10.1186/1750-4732-2-8
79. Wahl, RA, Aldous, MB, Worden, KA, and Grant, KL. Echinacea purpurea and osteopathic manipulative treatment in children with recurrent otitis media: a randomized controlled trial. BMC Complement Altern Med. (2008) 8:1–9. doi: 10.1186/1472-6882-8-56
80. Wyatt, K, Edwards, V, Franck, L, Britten, N, Creanor, S, Maddick, A, et al. Cranial osteopathy for children with cerebral palsy: a randomised controlled trial. Arch Dis Child. (2011) 96:505–12. doi: 10.1136/adc.2010.199877
81. Packheiser, J, Hartmann, H, Fredriksen, K, Gazzola, V, Keysers, C, and Michon, F. A systematic review and multivariate meta-analysis of the physical and mental health benefits of touch interventions. Nat Hum Behav. (2024) 8:1088–107. doi: 10.1038/s41562-024-01841-8
Keywords: osteopathic medicine, craniosacral, osteopathic manipulation (osteopathic manipulative treatment/osteopathic manual medicine), manual therapy, alternative and complementary medicine
Citation: Amendolara A, Sheppert A, Powers R, Payne A, Stacey S and Sant D (2024) Effectiveness of osteopathic craniosacral techniques: a meta-analysis. Front. Med. 11:1452465. doi: 10.3389/fmed.2024.1452465
Edited by:
Ching-Mao Chang, Taipei Veterans General Hospital, TaiwanReviewed by:
Tiancheng Xu, Nanjing University of Chinese Medicine, ChinaLuis Ceballos, University of Valladolid, Spain
Copyright © 2024 Amendolara, Sheppert, Powers, Payne, Stacey and Sant. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alfred Amendolara, ZG8yNS5hYmFtZW5kb2xhcmFAbm9vcmRhY29tLm9yZw==
 Alfred Amendolara
Alfred Amendolara Alexander Sheppert1
Alexander Sheppert1 David Sant
David Sant