
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 30 July 2024
Sec. Dermatology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1450666
Acne fulminans (AF), a severe acne variant primarily evident in adolescent males, is characterized by the sudden onset of severe and often ulcerating acne with fever and polyarthritis. A case of a 14-year-old initially treated with clindamycin and surgical debridement, highlights the complexity of AF, including challenges in diagnosis, treatment, and the importance of early dermatological consultation. Successful management was achieved through systemic therapy with retinoids and corticosteroids, resulting in significant improvement. This case underscores the necessity of a coordinated effort among dermatologists, endocrinologists, and rheumatologists for effective AF treatment, illustrating the critical role of timely diagnosis and comprehensive care in managing this rare and challenging condition.
Acne fulminans (AF) represents a rare extreme manifestation of acne primarily observed in adolescent males, characterized by the abrupt onset of severe inflammatory nodular acne with systemic symptoms and abnormal laboratory findings. Typically, afflicted individuals have a history of mild to moderate acne before experiencing a sudden onset of severe, hemorrhagic, ulcerative lesions affecting the back, chest, and face, accompanied by fever, elevated white blood cell count, anemia, hepatosplenomegaly, myalgia, and arthralgia (1, 2). Roentgenographic studies may document osteolytic bone changes this condition manifests as intensely painful, hemorrhagic nodules and plaques that progress to severe necrotic ulcers, often resulting in significant scarring (3).
It is crucial to distinguish AF from acne conglobata and acne vulgaris; unlike acne vulgaris, it lacks polyporous comedones, and compared to acne conglobata, it exhibits a broader spectrum of systemic symptoms (4). Effective management of patients with this complex acne syndrome necessitates a collaborative approach involving dermatologists, endocrinologists, and rheumatologists. Additionally, AF may occur concurrently with other rare conditions such as SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, osteitis), PAPA syndrome (pyogenic arthritis, pyoderma gangrenosum, acne), PASH syndrome (pyoderma gangrenosum, acne, suppurative hidradenitis) syndrome, and CAH (congenital adrenal hyperplasia), highlighting the importance of comprehensive clinical evaluation (5).
This case report aims to provide a comprehensive overview of the clinical course, treatment strategy, and outcomes in a patient diagnosed with AF. By doing so, we aim to enhance understanding of this rare condition and emphasize the significance of timely diagnosis and multidisciplinary management.
A 14-year-old boy, suffering for about a year from acne (Figure 1) treated with tetracyclines and rupatadine, presented due to intensiving ankle and knee joint pain persisting of 2 weeks duration. There was no report of fever. Clinical examination revealed significant inflammatory changes on the face and milder changes on the upper back, characterized by warmth and tenderness on palpation. The worsening of acne symptoms was accompanied by an escalation in joint pain. Ultrasound examination of the facial lesions did not detect any abscesses. Sonographic assessment of the knee joints showed no signs of inflammation. However, both the right and left ankle joints exhibited swelling of the fatty tissue around the lateral malleolus, without increased fluid within the joint. Orthopedic causes of joint pain were ruled out after consultation.
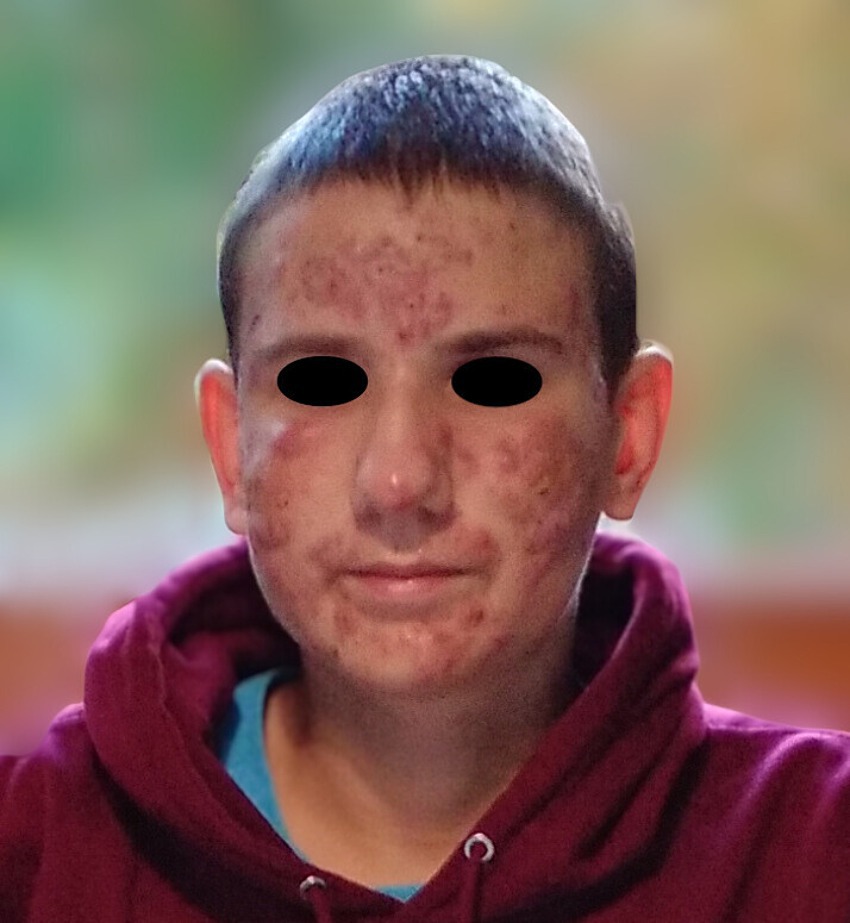
Figure 1. Patient’s face during treatment with tetracyclines and rupatadine, showing multiple acne lesions characterized by redness and nodules particularly on the cheeks and forehead.
Laboratory tests upon admission revealed abnormal white blood cell counts (15.42 10^9/L with 16.5% lymphocytes 1.26% monocytes, 73.9% neutrophils, 0.8% eosinophils), hemoglobin (11.7 g/dL), serum ferritin level (235.5 ng/mL), hematocrit (36.1%), creatinine (0.96 mg/dL), and fibrinogen (5.54 g/L). Elevated C-reactive protein levels were noted (69.4–17.9 mg/L).
During the patient’s stay in the surgery department, extensive necrosis prompted surgical debridement of the facial lesions on two occasions (due to healing complications), resulting in skin loss on both cheeks (Figure 2). Bacteroides fragilis was cultured from swabs of the excised lesions, and antibiotic therapy with clindamycin was initiated based on the antibiogram.
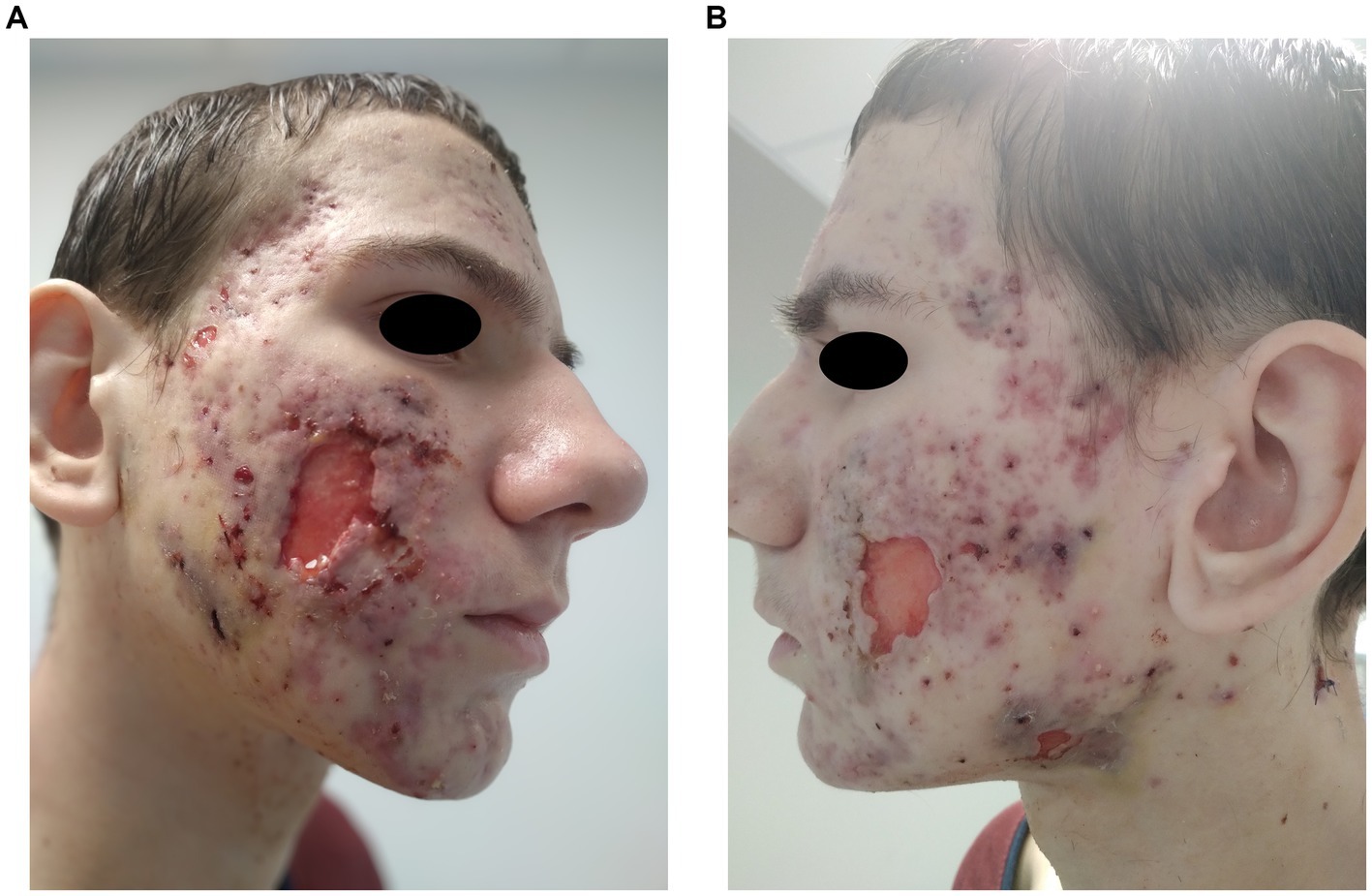
Figure 2. (A,B) Patient’s face post-surgical procedures, depicting significant skin loss on both cheeks.
Following approximately a month of hospitalization in the surgical department, a dermatological consultation was sought, leading to the initiation of treatment with methylprednisolone 0.5 mg/kg/day for 4 weeks then tapered to 5 mg/day. After 2 weeks of methylprednisolone treatment 0.3 mg/kg/day oral isotretinoin therapy was introduced for a month, then escalated to 0.5 mg/kg/day.
A month after isotretinoine introduction, the joint symptoms resolved. After a year of isotretinoin therapy significant improvement in acne lesions was observed (Figure 3). Subsequently, the patient was referred to a nephrologist due to confirmed proteinuria during follow-up tests (exceeding 150 mg/dL) without leukocyturia, nitrituria, or hematuria, and in the absence of concurrent infection. Discontinuation of isotretinoin was considered, suspecting its impact on the occurrence of proteinuria (6). However, imaging studies showed the compression of the left renal vein between the abdominal aorta and superior mesenteric artery (nutcracker syndrome), which was deemed more likely to contribute to the proteinuria (7).
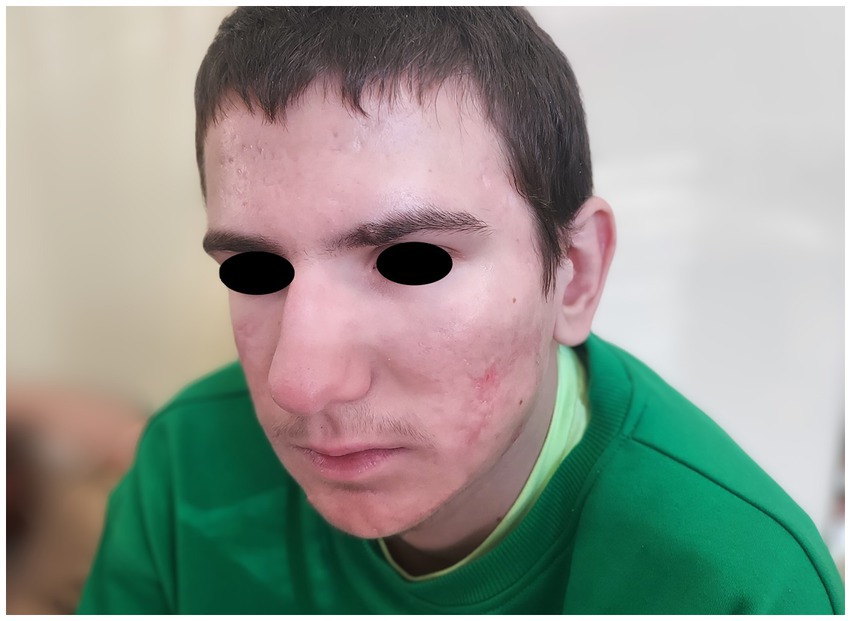
Figure 3. Patient’s face after treatment with isotretinoin and prednisone showing marked improvement.
Etiopathogenesis of AF is is not well understood (Table 1). It is a rare condition with which many general physicians are understandably unfamiliar (16). The approach to treatment is highly individualized in this condition (Table 2).
In this instance, the initial use of antibiotics alone and surgical debridement, while apparently necessary at the time, proved ineffective. While antibiotic monotherapy has shown limited efficacy, its effectiveness may improve when considering microbial colonization or anti-inflammatory effects (12, 31). Successful treatment of AF has been described with a combination of broad-spectrum systemic antibiotics (300 mg clindamycin thrice daily and 750 mg levofloxacin daily, based on microbial colonization results) (20). However, unlike our case, this treatment regimen also included 1 mg/kg/day of oral prednisolone at the beginning of treatment, gradually tapered and supplemented with low-dose oral isotretinoin. AF typically exhibits resistance to conventional acne antibiotics (16).
Data indicate that treatment protocols combining prednisolone and isotretinoin are effective in managing systemic symptoms and achieving acne clearance (19). Such treatment, though administered relatively late, was effective in this case. Earlier dermatological consultation could have potentially prevented surgical procedures. This case underscores the importance of a well-coordinated interprofessional team in managing AF. Urgent dermatological consultations are crucial to prevent delays, advocating for a combined treatment approach (16).
Systemic corticosteroids are frequently recommended as the initial treatment for AF to promptly alleviate inflammation (38). Treatment often involves starting with prednisone, then integrating low-dose isotretinoin, with the regimen adjusted based on the patient’s response and potential health screenings to mitigate risks (18). Evidence-based recommendations include initiating treatment with prednisone at a dosage of 0.5–1 mg/kg/day as a single intervention for a minimum of 4 weeks or until crusted lesions are resolved. Subsequently, low-dose isotretinoin at 0.1 mg/kg/day may be added to the regimen. Treatment with the corticosteroid should be continued alongside the low-dose isotretinoin for at least an additional 4 weeks. Post this period, the isotretinoin dosage may be incrementally increased while the corticosteroid is gradually tapered off (18).
Although this combination appears to be effective in treating AF, various hypotheses exist regarding isotretinoin’s role in its induction. One posits that AF originates from a severe immune response (type III and IV hypersensitivity) to antigens of Propionibacterium acnes (P. acnes), possibly initiated by the weakening of the skin’s pilosebaceous ducts due to isotretinoin treatment (39). In combination therapy, isotretinoin targets the pilosebaceous unit, while prednisolone mitigates inflammation and systemic symptoms (40). Another theory proposes that a genetic predisposition to neutrophil hyperactivity leads to ineffective phagocytosis of P. acnes, causing mediator release that could explain the initial worsening of acne seen with isotretinoin treatment (41). However, findings from a 2024 multicenter study indicate no significant difference between isotretinoin-associated acute acne flares (IAF) and non-associated flares (NAF), challenging the notion of isotretinoin-induced acne flares (9). This study reassures physicians about prescribing oral isotretinoin for severe acne, noting that flares can occur with any acne treatment. More research is needed on isotretinoin’s role in AF pathogenesis. It is important to consider a cutaneous drug reaction when worsening acne occurs during therapy. Blood tests should always be performed, focusing on peripheral eosinophilia, which is a key parameter in evaluating severe cutaneous adverse drug reactions (42). However, in our case, the patient’s eosinophil count was slightly lowered upon admission and normalized by the second day, suggesting that an adverse drug reaction was unlikely.
Tetracyclines are not recommended as the primary option due to their limited efficacy (38). The effectiveness of combining tetracyclines with corticosteroids remains uncertain, and such antibiotics are advised only if patients cannot tolerate isotretinoin or corticosteroids, with specified dosages (43). The concurrent use of isotretinoin and tetracyclines may lead to an increased risk of pseudotumor cerebri syndrome (44).
AF represents a unique and aggressive form of inflammatory acne with elevated inflammatory markers like TNF-alpha levels (25). The intense inflammatory response seen in AF and the effectiveness of TNF-alpha inhibitors in treating similar chronic inflammatory skin conditions, including plaque psoriasis and hidradenitis suppurativa, have led to the off-label use of these medications by dermatologists for treatment-resistant acne (45, 46). Treating AF poses a significant challenge as conventional treatments often fall short. As mentioned, the standard approach includes a regimen of oral steroids and isotretinoin. However, for cases that do not respond to these methods, alternative treatments such as biologic medications may offer relief (18, 21). TNF-alpha inhibitors have proven to be particularly beneficial in the management of AF that does not respond to prior treatments (21, 22, 25, 26). They are also effective in cases of AF with concurrent conditions such as mentioned hidradenitis suppurativa (23), SAPHO syndrome (47), and complications arising from isotretinoin therapy, such as acute sacroiliitis (24). Additionally, TNF-alpha inhibitors can reduce the need for prolonged prednisolone use, which is particularly advantageous in pediatric patients to mitigate risks like reduced growth rates and short stature (26). Other successful treatments have been documented. They include using subcutaneous adalimumab in combination with oral doxycycline (25), adalimumab with prednisolone and minocycline (26), adalimumab alone (22, 23), and infliximab paired with methotrexate (47). Moreover, alternative biologic therapies have been explored for AF treatment, including anakinra (an IL-1 receptor antagonist) used alongside bisphosphonates and corticosteroids for AF with severe osteoarticular symptoms (27), ustekinumab (an interleukin (IL)-12/23 inhibitor) (28), and apremilast (a selective inhibitor of phosphodiesterase 4) (29).
Several cases have demonstrated success in treating AF through the use of dapsone (30, 32, 48). However, treatment with dapsone is associated with two significant adverse effects: hemolytic anemia and methemoglobinemia (49). In addition, cyclosporine (33, 34) and a combination therapy of methotrexate with isotretinoin (35) have been identified as viable alternative treatment options in instances where conventional therapies are ineffective.
Effective treatment was also described with photodynamic therapy and isotretinoin with methyl aminolevulinate (37) and 5-aminolevulinic acid (ALA-PDT) used as a photosensitizer (38).Combining ALA-PDT with oral isotretinoin has been shown to clear acne lesions significantly faster and more efficiently compared to using isotretinoin alone, while also utilizing a lower dose of isotretinoin than is typically used in treatment.
This case highlights the complexities in managing acne fulminans and the importance of a multidisciplinary approach for effective treatment. Timely diagnosis and appropriate interventions, including systemic therapy with retinoids and corticosteroids, significantly improved the patient’s condition.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
JW: Conceptualization, Data curation, Project administration, Writing – original draft. KK: Data curation, Supervision, Writing – review & editing. JS: Writing – original draft. AB: Writing – original draft. RŻ: Conceptualization, Data curation, Supervision, Writing – review & editing. RS: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Jansen MD, T, and Plewig MD, G. Acne fulminans. Int J Dermatol. (1998) 37:254–7. doi: 10.1046/j.1365-4362.1998.00443.x
2. Zaba, R, and Schwartz, RA. Acne fulminans. Medscape. (2022). Available at: (http://emedicine.medscape.com/article/1072815-overview).
3. Katsambas, AD, Dessinioti, C, and Cunliffe, WJ. Clinical aspects of acne Fulminans In: Pathogenesis and treatment of acne and Rosacea. Eds. Christos C. Zouboulis, Andreas D. Katsambas, Albert M. Kligman (Berlin Heidelberg: Springer) (2014). 223–6.
4. Zaba, R, Schwartz, R, Jarmuda, S, Czarnecka Operacz, M, and Silny, W. Acne fulminans: explosive systemic form of acne. J Eur Acad Dermatol Venereol. (2011) 25:501–7. doi: 10.1111/j.1468-3083.2010.03855.x
5. Dessinioti, C, and Katsambas, A. Difficult and rare forms of acne. Clin Dermatol. (2017) 35:138–46. doi: 10.1016/j.clindermatol.2016.10.005
6. Forouzani-Haghighi, B, and Karimzadeh, I. Isotretinoin and the kidney: opportunities and threats. Clin Cosmet Investig Dermatol. (2020) 13:485–94. doi: 10.2147/CCID.S259048
7. Kurklinsky, AK, and Rooke, TW. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc. (2010) 85:552–9. doi: 10.4065/mcp.2009.0586
8. Honma, M, Murakami, M, Iinuma, S, Fujii, M, Komatsu, S, Sato, K, et al. Acne fulminans following measles infection. J Dermatol. (2009) 36:471–3. doi: 10.1111/j.1346-8138.2009.00680.x
9. Dessinioti, C, Dréno, B, Bettoli, V, Vural, S, Brzezinski, P, Nassif, A, et al. Isotretinoin-associated acne fulminans: a multicentre, retrospective study of the European academy of dermatology and venereology task force on acne, Rosacea and hidradenitis Suppurativa. J Eur Acad Dermatol Venereol. (2024) 38:197–204. doi: 10.1111/jdv.19477
10. Dall'oglio, F, Puglisi, DF, Nasca, MR, and Micali, G. Acne fulminans. G Ital Dermatol Venereol. (2020) 155:711–8. doi: 10.23736/S0392-0488.20.06711-5
11. Dréno, B, Pécastaings, S, Corvec, S, Veraldi, S, Khammari, A, and Roques, C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. J Eur Acad Dermatol Venereol. (2018) 32:5–14. doi: 10.1111/jdv.15043
12. Proença, NG . Acne fulminans. An Bras Dermatol. (2017) 92:8–10. doi: 10.1590/abd1806-4841.20176546
13. Bocquet-Trémoureux, S, Corvec, S, Khammari, A, Dagnelie, MA, Boisrobert, A, and Dreno, B. Acne fulminans and Cutibacterium acnes phylotypes. J Eur Acad Dermatol Venereol. (2020) 34:827–33. doi: 10.1111/jdv.16064
14. Divya, BL, and Rao, PN. SAPHO syndrome with acne fulminans and severe polyosteitis involving axial skeleton. Indian Dermatol Online J. (2016) 7:414–7. doi: 10.4103/2229-5178.190495
15. Li, AW, and Antaya, RJ. Isotretinoin-induced acne fulminans without systemic symptoms with concurrent exuberant granulation tissue. Pediatr Dermatol. (2018) 35:257–8. doi: 10.1111/pde.13389
16. Zito, PM, and Badri, T. Acne Fulminans. (2024). Available at: https://www.ncbi.nlm.nih.gov/books/NBK459326/ (Accessed April 22, 2024).
17. Trave, I, Donadoni, R, Cozzani, E, D'Agostino, F, Herzum, A, and Parodi, A. Acne fulminans and its multiple associated factors: a systematic review. Eur J Dermatol. (2023) 33:624–34. doi: 10.1684/ejd.2023.4629
18. Greywal, T, Zaenglein, AL, Baldwin, HE, Bhatia, N, Chernoff, KA, Del Rosso, JQ, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. (2017) 77:109–17. doi: 10.1016/j.jaad.2016.11.028
19. Massa, AF, Burmeister, L, Bass, D, and Zouboulis, CC. Acne Fulminans: treatment experience from 26 patients. Dermatology. (2017) 233:136–40. doi: 10.1159/000473860
20. Siadat, AH, Bostakian, A, Abtahi-Naeini, B, and Shahbazi, M. Successful treatment of facial acne Fulminans: antimicrobial agents and Oral prednisolone as promising regimes. Case Rep Dermatol Med. (2017) 2017:1–3. doi: 10.1155/2017/7092910
21. Taudorf, EH, Jensen, MB, Bouazzi, D, Sand, C, Thomsen, SF, Jemec, GBE, et al. Tumor necrosis factor-α inhibitor treatment of acne fulminans – a clinical and literature review. JDDG. J Dtsch Dermatol Ges. (2024) 22:23–7. doi: 10.1111/ddg.15234
22. Marasca, C, Fabbrocini, G, Abategiovanni, L, Camela, E, Nocerino, M, Di Guida, A, et al. Adalimumab in the Management of Isotretinoin-Induced Acne Fulminans: report of a case. Skin Appendage Disord. (2021) 7:115–9. doi: 10.1159/000512032
23. Kontochristopoulos, G, Agiasofitou, E, Platsidaki, E, Kapsiocha, A, Gregoriou, S, and Rigopoulos, D. Successful treatment of coexistent acne Fulminans and severe hidradenitis Suppurativa with adalimumab. Skin Appendage Disord. (2021) 7:329–32. doi: 10.1159/000515002
24. Dawoud, N, Elnady, B, Elkhouly, T, and Yosef, A. Adalimumab as a successful treatment for acne fulminans and bilateral acute sacroiliitis with hip synovitis complicating isotretinoin therapy. Indian J Dermatol Venereol Leprol. (2018) 84:104–7. doi: 10.4103/ijdvl.IJDVL_834_16
25. Rajaii, R, Globerson, J, Arnold, N, and Mahon, M. A novel treatment of acne Fulminans with adalimumab: a case report. Spartan Med Res J. (2018) 3. doi: 10.51894/001c.7003
26. Miguel, D, Tittelbach, J, and Elsner, P. A dramatic case of acne fulminans responding to adalimumab. J Dtsch Dermatol Ges. (2019) 17:837–8. doi: 10.1111/ddg.13842
27. Oranges, T, Insalaco, A, Diociaiuti, A, Carnevale, C, Strippoli, R, Zambruno, G, et al. Severe osteoarticular involvement in isotretinoin-triggered acne fulminans: two cases successfully treated with anakinra. J Eur Acad Dermatol Venereol. (2017) 31:e277–9. doi: 10.1111/jdv.14022
28. Gier, H, Israeli, A, Cusick, A, and Merritt, D. Use of Interleukin-12/23 inhibitor for the Management of Acne Fulminans. Cureus. (2023) 15:e50352. doi: 10.7759/cureus.50352
29. Sánchez-Velázquez, A, Falkenhain-López, D, Arroyo-Andrés, J, Montero-Menárguez, J, García-Donoso, C, and Postigo-Llorente, C. Apremilast: a novel adjuvant treatment for refractory isotretinoin-induced acne fulminans. Dermatol Ther. (2022) 35:e15637. doi: 10.1111/dth.15637
30. Legal, K, and Misery, L. Isotretinoin-induced acne Fulminans without systemic symptoms treated successfully with Oral Dapsone. Acta Dermato Venereologica. (2020) 100:1–2. doi: 10.2340/00015555-3354
31. Lages, RB, Bona, SH, Silva, FVM, Gomes, AKL, and Campelo, V. Acne fulminans successfully treated with prednisone and dapsone. An Bras Dermatol. (2012) 87:612–4. doi: 10.1590/S0365-05962012000400015
32. Furukawa, F, Makino, T, Mori, S, and Shimizu, T. Successful treatment of acne fulminans with the combination of prednisolone and diaminodiphenylsulfone. J Dermatol. (2021) 48:e120–1. doi: 10.1111/1346-8138.15699
33. Giavedoni, P, Mascaró-Galy, JM, Aguilera, P, and Estrach-Panella, T. Acne fulminans successfully treated with cyclosporine and isotretinoin. J Am Acad Dermatol. (2014) 70:e38–9. doi: 10.1016/j.jaad.2013.09.043
34. Tago, O, Nagai, Y, Matsushima, Y, and Ishikawa, O. A case of acne Fulminans successfully treated with Cyclosporin a and prednisolone. Acta Dermato Venereologica. (2011) 91:337–8. doi: 10.2340/00015555-0796
35. Rodríguez-Lomba, E, Molina-López, I, Monteagudo-Sáez, I, Suárez-Fernández, R, and Campos-Domínguez, M. A case of acne fulminans with sacroiliitis successfully treated with methotrexate and isotretinoin. Dermatol Ther. (2016) 29:476–8. doi: 10.1111/dth.12382
36. Hao, J, and Wang, T. 5-Aminolevulinic acid photodynamic therapy and isotretinoin for treatment of drug-induced acne fulminans in a patient with idiopathic thrombocytopenic purpura. Photodiagn Photodyn Ther. (2020) 29:101630. doi: 10.1016/j.pdpdt.2019.101630
37. Picone, V, Potestio, L, Fabbrocini, G, Monfrecola, G, and Marasca, C. A case of acne fulminans successfully treated with photodynamic therapy. Photodermatol Photoimmunol Photomed. (2022) 38:401–3. doi: 10.1111/phpp.12761
38. Seukeran, C, and Cunliffe, WJ. The treatment of acne fulminans: a review of 25 cases. Br J Dermatol. (1999) 141:307–9. doi: 10.1046/j.1365-2133.1999.02982.x
39. Karvonen, SL, Räsänen, L, Cunliffe, WJ, Holland, KT, Karvonen, J, and Reunala, T. Delayed hypersensitivity to Propionibacterium acnes in patients with severe nodular acne and acne fulminans. Dermatology. (1994) 189:344–9. doi: 10.1159/000246876
40. Oeff, MK, Seltmann, H, Hiroi, N, Nastos, A, Makrantonaki, E, Bornstein, SR, et al. Differential regulation of toll-like receptor and CD14 pathways by Retinoids and corticosteroids in human Sebocytes. Dermatology. (2006) 213:266. doi: 10.1159/000095056
41. Perkins, W, Kv, C, Mb, H, Rm, M, and Jm, L. The effect of treatment with 13-cis-retinoic acid on the metabolic burst of peripheral blood neutrophils from patients with acne. Br J Dermatol. (1991) 124:429–32. doi: 10.1111/j.1365-2133.1991.tb00620.x
42. Drago, F, Cogorno, L, Agnoletti, AF, Ciccarese, G, and Parodi, A. A retrospective study of cutaneous drug reactions in an outpatient population. Int J Clin Pharm. (2015) 37:739–43. doi: 10.1007/s11096-015-0134-z
43. Karvonen, SL . Acne fulminans: report of clinical findings and treatment of twenty-four patients. J Am Acad Dermatol. (1993) 28:572–9. doi: 10.1016/0190-9622(93)70076-6
44. Friedman, DI . The Pseudotumor Cerebri syndrome. Neurol Clin. (2014) 32:363–96. doi: 10.1016/j.ncl.2014.01.001
45. Sand, FL, and Thomsen, SF. Adalimumab for the treatment of refractory acne Conglobata. JAMA Dermatol. (2013) 149:1306–7. doi: 10.1001/jamadermatol.2013.6678
46. Schwartz, RA, and Zaba, R. Acne conglobata. Medscape. (2022). Available at: http://emedicine.medscape.com/article/1072716-overview (Accessed April 1, 2024).
47. Iqbal, M, and Kolodney, MS. Acne fulminans with synovitis-acne-pustulosis-hyperostosis-osteitis (SAPHO) syndrome treated with infliximab. J Am Acad Dermatol. (2005) 52:S118–20. doi: 10.1016/j.jaad.2004.09.006
48. Bb, T, Jt, L, and Ag, S. Acne fulminans and erythema nodosum during isotretinoin therapy responding to dapsone. Clin Exp Dermatol. (1997) 22:26–7. doi: 10.1046/j.1365-2230.1997.1830600.x
Keywords: acne fulminans, isotretinoin, acne, acne management, anti TNF-alfa therapy, corticosteorids
Citation: Woźna J, Korecka K, Stępka J, Bałoniak A, Żaba R and Schwartz RA (2024) Acne fulminans treatment: case report and literature review. Front. Med. 11:1450666. doi: 10.3389/fmed.2024.1450666
Received: 17 June 2024; Accepted: 15 July 2024;
Published: 30 July 2024.
Edited by:
Laura Atzori, University of Cagliari, ItalyReviewed by:
Gabriele Biondi, Azienda Ospedaliero Universitaria Sassari, ItalyCopyright © 2024 Woźna, Korecka, Stępka, Bałoniak, Żaba and Schwartz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryszard Żaba, cnphYmFAdW1wLmVkdS5wbA==
†ORCID: Julia Woźna, orcid.org/0009-0006-3659-2580
Katarzyna Korecka, orcid.org/0000-0002-9473-1239
Jan Stępka, orcid.org/0009-0006-8346-6632
Andrzej Bałoniak, orcid.org/0009-0007-5517-5587
Ryszard Żaba, orcid.org/0000-0003-0756-3909
Robert A. Schwartz, orcid.org/0000-0003-3036-3825
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.