- 1Department of Biomedical Science, Humanitas University, Milan, Italy
- 2Department of Pathology, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy
- 3Department of Pathology, Humanitas Istituto Clinico Catanese, Catania, Italy
- 4Department of Medical Oncology, Humanitas Istituto Clinico Catanese, Catania, Italy
- 5Department of Radiology, Humanitas Istituto Clinico Catanese, Catania, Italy
- 6Department of Surgery, Humanitas Istituto Clinico Catanese, Catania, Italy
- 7Section of Oncology, Department of Medicine, University of Verona, Verona University Hospital Trust (AUOI), Verona, Italy
- 8Section of Pathology, Department of Diagnostics and Public Health, University of Verona, Verona, Italy
Neurotrophic tyrosine receptor kinase (NTRK) fusions are infrequent genetic events that can occur in various tumor types. Specifically, NTRK-rearranged sarcoma has been observed in pediatric mesenchymal tumors and, to a lesser extent, in adult mesenchymal tumors like fibrosarcoma. Recently, NTRK-rearranged uterine sarcoma (US) has been identified as a rare entity characterized by constitutive activation or overexpression of the TRK receptor, which plays a role in cell proliferation and differentiation. Since its initial description in 2018, only 46 cases of NTRK-rearranged US have been reported. In this context, herein we describe an exceptional case of an STRN3::NTRK3 fused US with histologically confirmed splenic metastasis. Notably, such localization has not been previously associated with pure uterine sarcomas in the literature. The fusion involved STRN3 (exon-3) and NTRK3 (exon-14) genes and was identified through next-generation sequencing analysis. Recognizing this specific molecular rearrangement is crucial, as it not only enables targeted therapy but also holds diagnostic significance in specific clinical scenarios.
1 Introduction
NTRK gene rearrangements are driver molecular alterations found in a wide variety of human cancers, including both common and rare tumors such as infantile fibrosarcomas, lipofibromatosis-like neural tumors, and fibrosarcoma-like uterine sarcomas (1). NTRK-rearranged uterine sarcomas are rare malignant spindle-cell neoplasms that typically arise in the uterine cervix, uterine corpus, and vagina. They primarily affect premenopausal women, with an average age of 35 years (median 30.5, ranging from 23 to 60 years) (2). Since the first report by Chiang et al. (3), only 46 cases of these rare tumors have been described. Despite their morphologically unremarkable appearance and often organ-confined nature at initial diagnosis, these neoplasms generally display aggressive clinical behavior, characterized by rapid recurrence and metastatic dissemination (4). Even though a limited number of cases has been described to date, a recent review has identified the presence of either coagulative necrosis, high mitotic count (≥8/10 HPF), lymphovascular invasion, or NTRK3 rearrangements as independent prognostic features predicting a high-risk of recurrence/metastases (5).
Tumors with NTRK gene alterations can benefit from TRK inhibitor-targeted therapy, especially in advanced or metastatic disease. Proper classification of the lesion and identification of these pathogenic mutations are clinically significant. For instance, Michal et al. (6) observed that an STRN::NTRK3 fusion in a mesenchymal tumor of the uterus was characterized by bland morphology and subsequently defined as low-grade unclassifiable. In this context, we report the first case of STRN3::NTRK3 fused high-grade sarcoma of the uterus with histologically documented splenic metastasis. This finding could expand the spectrum of these rare and fascinating entities and may be further clarified by WHO classification in the future.
2 Case description
A 58-year-old woman presented with left-sided flank abdominal pain. In her clinical history, she reported five previous pregnancies and had undergone 6 years before bilateral radical hysterectomy with bilateral uterine tube and ovary resection for a uterine polypoid neoplasm. Such a tumor was made up of a highly cellular proliferation of atypical spindle and, at times, roundish cells with a high mitotic index displacing normal uterine glands. Therefore, it had been diagnosed as an undifferentiated uterine sarcoma with “adenosarcoma-like features” due to peripheral entrapment of benign cervical glands. Abdominal ultrasound revealed a splenic cystic mass, which was subsequently confirmed by CT scan and MRI to be a 30 × 41 mm complex solid-cystic mass in the inferior pole of the spleen, showing peripheral enhancement (Figure 1A). Left para-aortic lymphadenopathy was also noted. Following a multidisciplinary consultation, the patient underwent radical splenectomy.
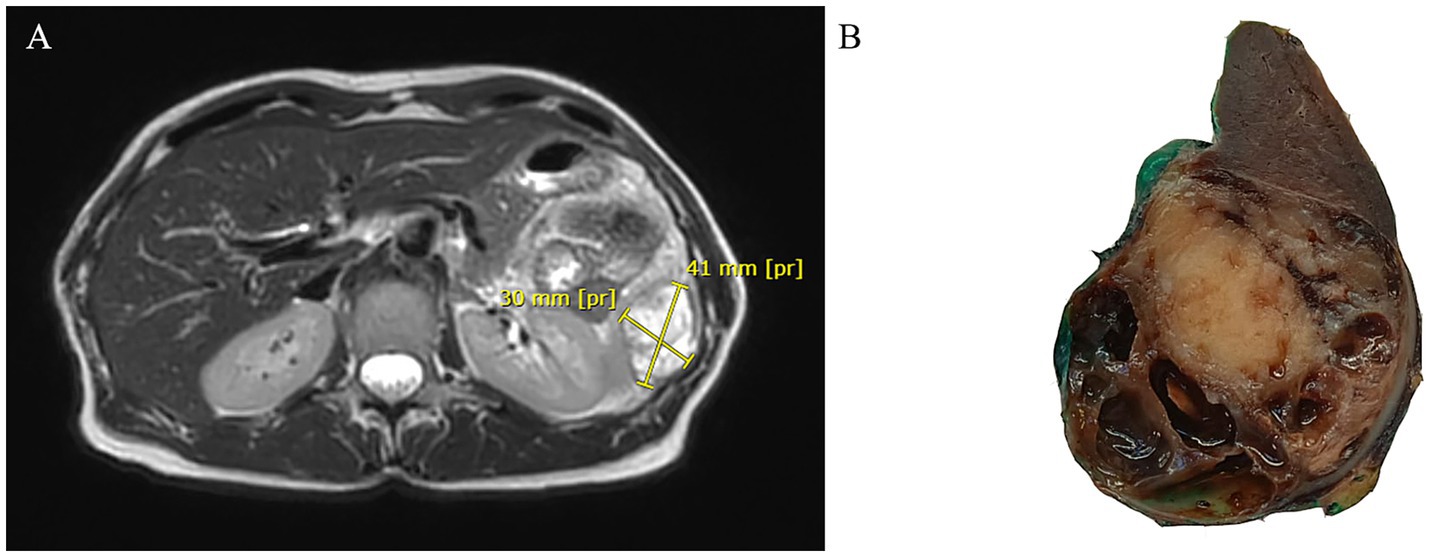
Figure 1. Abdominal MRI showing a cystic mass in the inferior pole of the spleen with peripheral enhancement in T2-weighted sequences (A). Gross section of the surgical specimen revealing a roundish mass with pushing borders at the lower pole of the spleen alternating solid and cystic areas (B).
Gross examination of the spleen revealed an irregular and multinodular appearance due to a 4.5 × 4 × 4 cm roundish intraparenchymal lesion. This lesion exhibited mucoid-myxoid, cystic-hemorrhagic, and solid gray-yellowish areas on the cut surface (Figure 1B). Paraffin-embedded formalin-fixed blocks were cut into 4 μm slides and stained with H&E. Immunohistochemistry was performed on 4 μm sections using various primary monoclonal antibodies. Notably, pan-TRK immunostaining was also conducted to analyze NTRK gene alterations. Morphologically, the neoplasm was made up of a solid-arranged proliferation of spindle and pleomorphic cells with pushing margins, along with cystic areas and myxoid stroma (Figure 2A). Geographic necrosis was observed throughout the tumor, coupled with a high mitotic rate (18/10HPF) (Figure 2B). Despite lacking characteristic architectural growth or differentiation reminiscent of a specific lineage, the histological features, combined with the patient’s previous history of a uterine polypoid neoplasm, led to the hypothesis of splenic localization by the primary uterine sarcoma (Figures 2C,D). The neoplastic cells of both the previously removed uterine tumor and the novel splenic localization exhibited the following immunophenotype: WT1+, CD10+, CD34+/− (Figure 3A), S100+ (Figure 3B), Smooth Muscle Actin+/−, ERG−, Desmin−, HMB45−, PanCK−, CK7−, p16−, ER−, PR+ (5%), AR−, AFP−, Myogenin−, and MyoD1−. The proliferation index (Ki-67) was 40%. Based on these findings, a diagnosis of high-grade spindle and pleomorphic cell sarcoma NOS (grade III FNCLCC—D3N1M2) was made. Pan-TRK immunostaining was positive in the majority of the neoplastic cells both in the splenic metastasis and primary uterine neoplasm (Figures 3C,D). To confirm pan-TRK immunopositivity, NGS-based fusion detection analysis was performed. RNA was purified, and sequencing revealed a fusion of STRN3 (exon 3)—NTRK3 (exon 14) genes (15,000 read count) in the splenic tumor, which was later also detected in the uterine neoplasm (Figure 4A). Further validation was achieved through fluorescence in situ hybridization (FISH) using a Dual Color Break Apart Probe (targeting the NTRK3 gene at 15q25.3) on tissue from the splenic metastasis, which confirmed the presence of an NTRK3 rearrangement (Figure 4B).
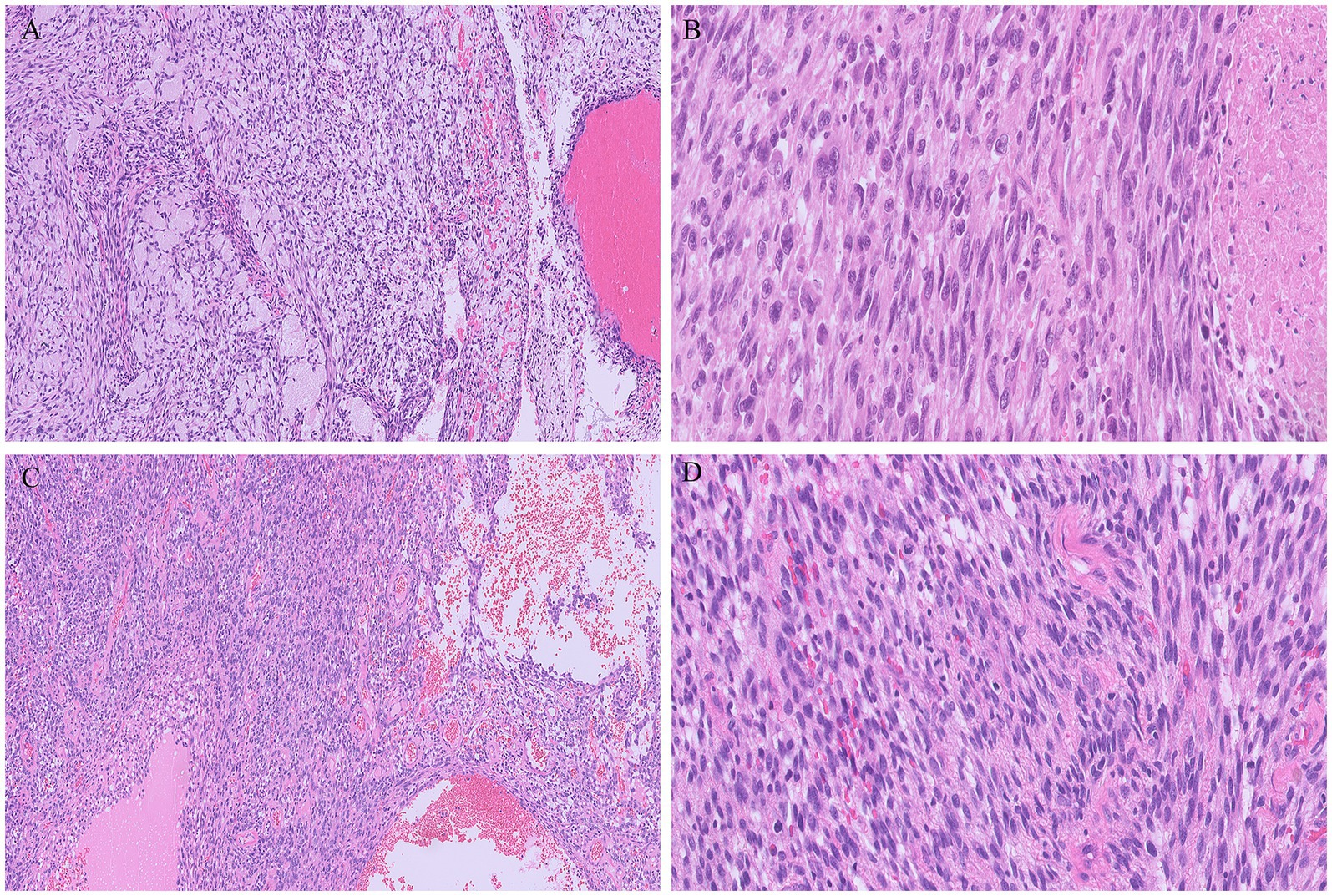
Figure 2. Metastatic tumor deposit in the spleen consisting of solid proliferation of spindle at times with a herringbone pattern of growth, intermixed with cystic areas, in a myxoid stroma (A). Multiple foci of necrosis and pleomorphic cells were evident throughout the tumor (B). The metastatic tumor morphologically resembled the uterine neoplasm (C,D), without characteristic architectural growth nor cellular differentiation reminiscent of a specific lineage.
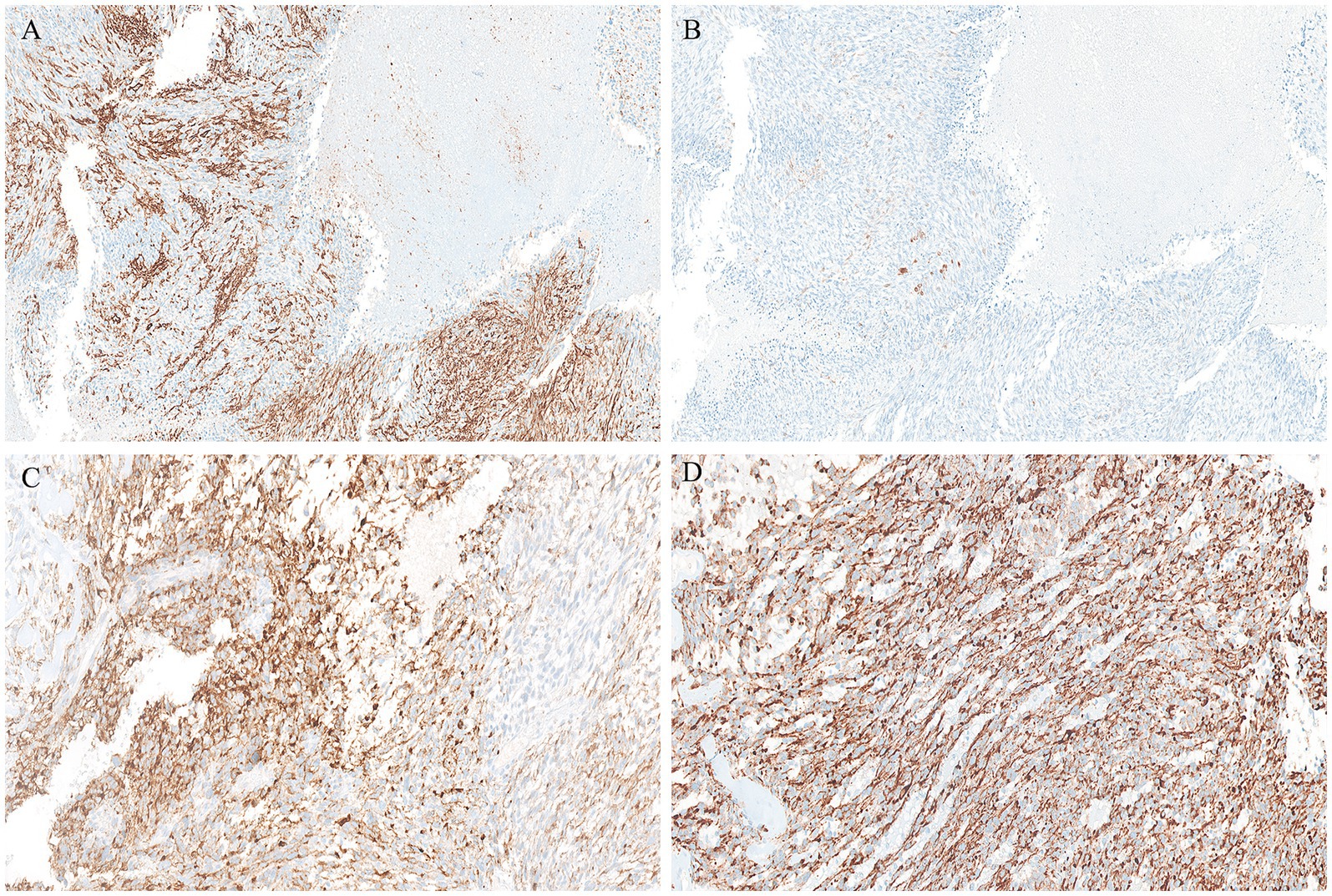
Figure 3. Positive CD34 immunohistochemical staining in the splenic metastasis (A). Conversely, S100 labelled only a few scattered cells (B). Both the splenic localization (C) and the primary uterine sarcoma (D) displayed strong PanTRK cytoplasmic expression.
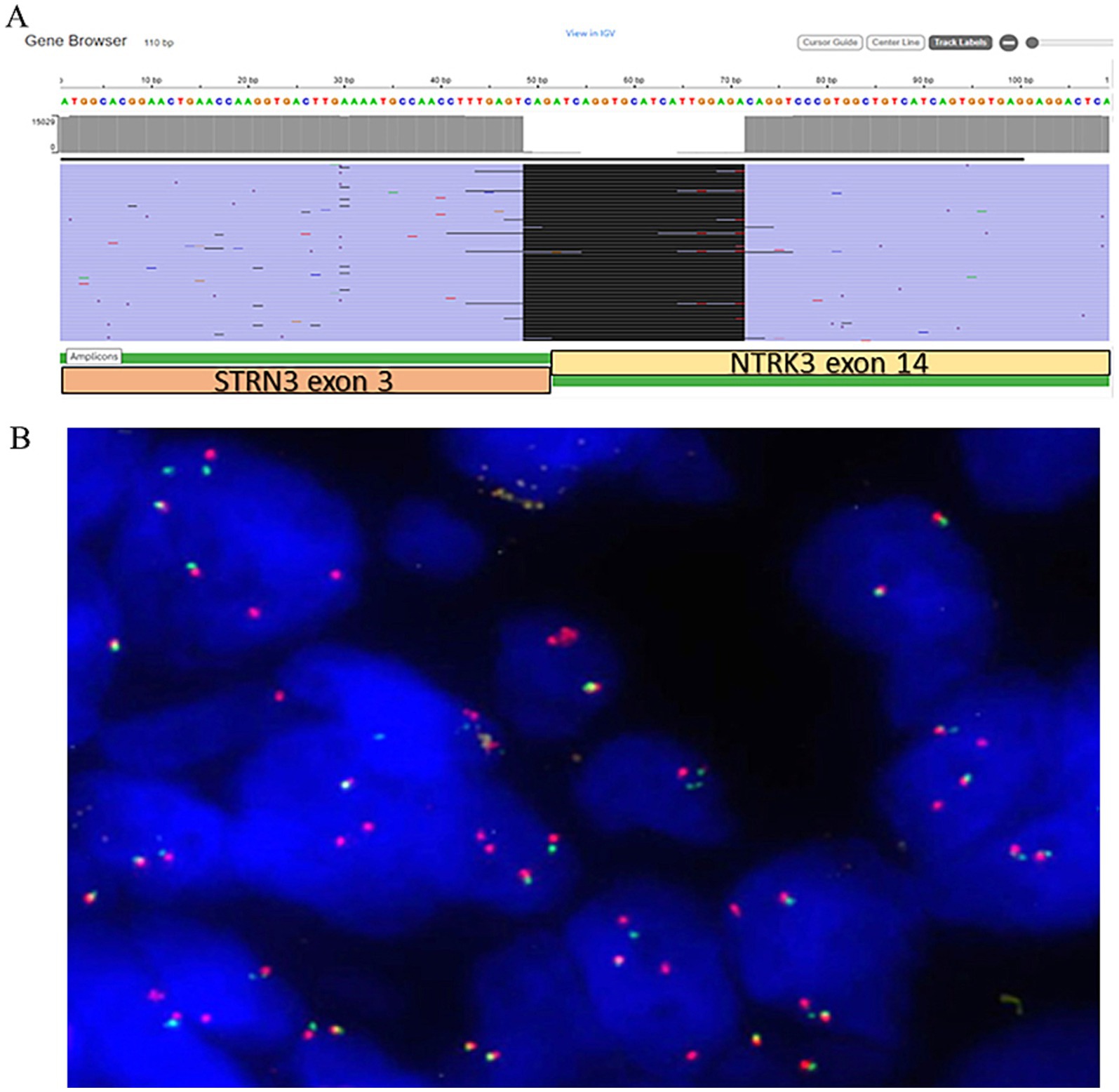
Figure 4. (A) Schematic presentation of STRN3::NTRK3 not targeted fusion in the current case (view in IGV) with the breakpoint sequence indicated. (B) NTRK3 dual color break apart FISH probes showing fused unbroken probe (yellow signals), broken 5′and 3′ probes (separated red and green signals) and multiple extra 3′ red signals in 90% of tumor cells.
Ultimately, the diagnosis of splenic metastatic TRK-rearranged sarcoma originating from a uterine primary was established. As for the present case, according to the previously mentioned prognostic score (5), it could be labeled as a high-risk tumor, due to the identification of three risk factors (high mitotic count, coagulative necrosis, NTRK3 rearrangement). Six months after surgery, two small PET-positive nodules in the Morrison peritoneal zone were noticed. Therefore, after multidisciplinary discussion, the patient was considered suitable for anti-NTRK target therapy (larotrectinib), which she is about to start in the following weeks.
3 Discussion
The most common malignant mesenchymal neoplasm of the uterus is leiomyosarcoma, followed by low/high-grade endometrial stromal sarcoma and undifferentiated sarcoma. The WHO classification defining the different categories of uterine sarcomas is becoming increasingly complex due to the continuous discovery of cases associated with new rare mutations (7). Uterine sarcomas with NTRK gene rearrangements constitute a recently identified group of uterine spindle-shaped cell tumors, which were likely previously grouped under the spectrum of undifferentiated uterine sarcoma. These NTRK-rearranged sarcomas have the potential to respond to TRK inhibitors (2). The first case was reported by Chiang et al. (3), and to date, only 46 cases have been described in the literature (4, 6–8). Most of these tumors have been identified due to the expanding accessibility of advanced molecular methodologies, such as next-generation sequencing (NGS), which has unveiled novel diagnostic molecular alterations.
NTRK rearrangements are oncogenic events occurring in a wide variety of human cancers, including both common and rare tumor types such as infantile fibrosarcomas, lipofibromatosis-like neural tumors, and fibrosarcoma-like uterine sarcomas (1). The NTRK gene family encodes the TrkA, TrkB, and TrkC proteins, which activate downstream signaling cascades via PI3K, RAS/MAPK/ERK, and PLC-gamma pathways, ultimately leading to cellular proliferation (1).
In this report, we present the first case of STRN3::NTRK3 fused uterine sarcoma metastatic to the spleen, properly classified after the excision of the secondary distant localization. The rarity of this case lies in the significant impact that the diagnosis has on the patient’s management, with the prospect of treating potential future metastases using NTRK inhibitors. Currently, this specific variant of rearranged sarcoma is included in the WHO classification as ‘NTRK-rearranged spindle cell neoplasms’ (NTRK-SCN). This category encompasses spindle cell tumors occurring in both children and adults, forming a continuous morphological spectrum (9). The morphological features of our case align with what has been reported in the literature: the tumor consisted of poorly organized spindle and pleomorphic cells, displaying an infiltrative growth pattern within a background of myxoid and cystic stroma. Hypercellular areas, hemorrhagic foci, and geographic coagulative necrosis were also observed. As frequently noticed in other similar reports, the neoplastic cells entrapped normal uterine glands, giving the tumor an “adenosarcoma-like” appearance. However, the lack of a clear-cut peripheral cuffing of native glands by neoplastic cells is a useful morphological hint to avoid misdiagnoses (10, 11). Across the morphological spectrum of NTRK-SCN, many tumors exhibit variable immunohistochemical co-expression of CD34 and S100 protein (12, 13). Their molecular backgrounds involve various gene fusions and, less frequently, point mutations in kinase genes such as NTRK1/2/3 (1). Similarly, our case showed focal positivity for both CD34 and S100, while additional immunoreactivity may be present but is less specific for this type of neoplasm (e.g., WT1, CD10, desmin, and smooth muscle actin; not shown).
Given that these neoplasms may exhibit histological overlap with other entities, further investigation is crucial to avoid misdiagnoses. Consequently, we decided to perform pan-TRK immunohistochemical testing, which revealed expression in 50% of the cellular population. Although pan-TRK expression lacks absolute sensitivity or specificity for NTRK-rearranged sarcomas, these neoplasms typically exhibit diffuse staining of moderate to strong intensity, unlike their mimics (14). Ultimately, molecular confirmation by NGS was necessary for the diagnosis, as it outperforms other tests such as FISH or immunohistochemistry. An advantage of NGS is its ability to identify multiple oncogenic events alongside NTRK gene fusions from a single tumor sample, providing comprehensive insights into the molecular landscape of the investigated neoplasm (15). In our case, NGS results yielded interesting findings: the assay revealed an STRN3::NTRK3 fusion, with breakpoints involving exon 3 of the STRN3 gene and exon 14 of the NTRK3 gene. Regarding uterine tumors, this specific fusion has been previously reported only once by Michal et al. (6) (Table 1). Notably, although the tumor described by Michal et al. (6) was positive for S100 and CD34, with pan-TRK expression, its histological features differed from ours. Our findings align more closely with what Yamazaki et al. (16) recently described for adult fibrosarcoma, expanding the spectrum of NTRK-SCN rearrangements in the uterus. To date, STRN::NTRK translocations are rare, and the occurrence of the STRN3 variant is even rarer (Table 1) (17–20). The STRN3 gene, mapped at chromosome 14q12, encodes for the striatin 3 protein (also called SG2NA). Along with the other proteins of the striatin family, codified by the STRN and STRN4 genes, it acts as a calmodulin-binding protein physiologically playing a relevant role in estrogen signaling and neuroprotection (21). Rearrangements of the striatin family genes have been claimed as putative molecular drivers of different pathological conditions, including arrhythmogenic cardiomyopathies and cancer (21). As for this latter, apart from the above mentioned STRN::NTRK fusions in adult fibrosarcomas, STRN::ALK fusions have been identified in some thyroid and lung carcinomas, where they are thought to lead to ligand-independent activation of ALK kinase (16).
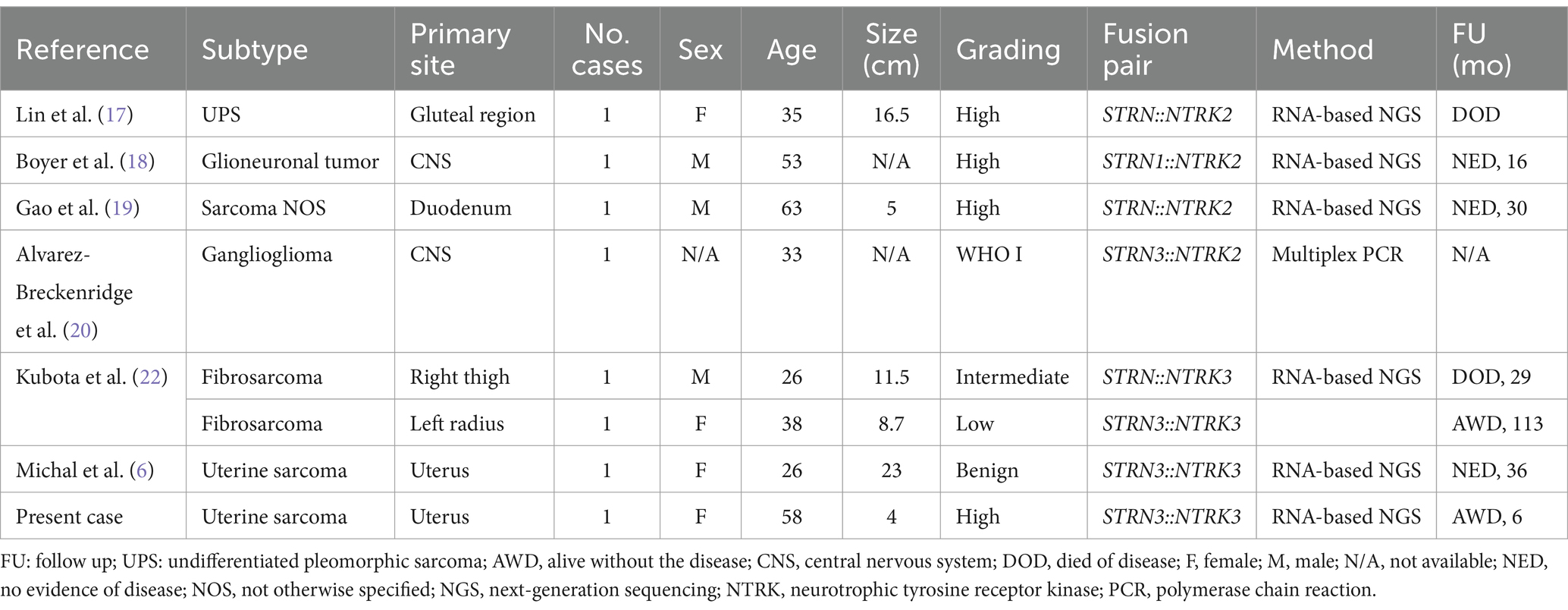
Table 1. Described STRN::NTRK fused cases stratified by subtype, organ, grading, and specific fusion pair.
The clinical significance of the exceptional genetic finding in our case lies in its impact on patient treatment. Tumors harboring NTRK rearrangements can potentially benefit from targeted therapy with TRK inhibitors (such as entrectinib and larotrectinib, approved in 2020 and 2021, respectively), which emerge as highly effective options in the event of potential metastases (22). Given the rarity of these tumors, further research is needed to better understand optimal management strategies and long-term outcomes associated with NTRK-rearranged uterine sarcomas. In localized diseases, surgical resection through hysterectomy remains the first-line treatment. Systematic genetic analysis is essential, especially for patients at high risk of relapse or in the recurrence setting, as sarcomas associated with NTRK fusion transcripts can benefit from targeted therapy (8). Current guidelines from the French Sarcoma Group and the Rare Gynecological Tumors Group emphasize integrating molecular data with accurate histopathological findings to enhance the identification of NTRK-rearranged uterine sarcomas, potentially improving clinical outcomes and enhancing patients’ quality of life (23).
This rare case underscores the importance of recognizing NTRK rearrangements, not only for treatment but also for diagnostic purposes in high-risk uterine sarcomas. Close collaboration among multidisciplinary teams remains crucial for comprehensive care in patients with NTRK-rearranged uterine sarcomas.
Data availability statement
The original contributions presented in this study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
PC: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. GB: Data curation, Methodology, Writing – original draft. AG: Data curation, Methodology, Writing – original draft. LH: Data curation, Methodology, Writing – original draft. NR: Data curation, Validation, Writing – review & editing. AR: Formal analysis, Supervision, Writing – review & editing. EC: Data curation, Investigation, Writing – review & editing. CC: Data curation, Investigation, Writing – review & editing. SG: Data curation, Investigation, Writing – review & editing. SMo: Data curation, Investigation, Writing – review & editing. SP: Data curation, Investigation, Writing – review & editing. SMa: Investigation, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Momeni-Boroujeni, A, and Chiang, S. Uterine mesenchymal tumours: recent advances. Histopathology. (2020) 76:64–75. doi: 10.1111/his.14008
2. Croce, S, Hostein, I, and McCluggage, WG. NTRK and other recently described kinase fusion positive uterine sarcomas: a review of a group of rare neoplasms. Genes Chromosomes Cancer. (2021) 60:147–59. doi: 10.1002/gcc.22910
3. Chiang, S, Cotzia, P, Hyman, DM, Drilon, A, Tap, WD, Zhang, L, et al. NTRK fusions define a novel uterine sarcoma subtype with features of fibrosarcoma. Am J Surg Pathol. (2018) 42:791–8. doi: 10.1097/PAS.0000000000001055
4. Szalai, L, Vereczkey, I, Szemes, M, Rókusz, A, Csernák, E, Tóth, E, et al. NTRK-rearranged spindle cell sarcoma of the uterine cervix with a novel NUMA1::NTRK1 fusion. Virchows Arch. (2024) 484:527–31. doi: 10.1007/s00428-023-03724-1
5. Costigan, DC, Nucci, MR, Dickson, BC, Chang, MC, Song, S, Sholl, LM, et al. NTRK -rearranged uterine sarcomas: clinicopathologic features of 15 cases, literature review, and risk stratification. Am J Surg Pathol. (2022) 46:1415–29. doi: 10.1097/PAS.0000000000001929
6. Michal, M, Hájková, V, Skálová, A, and Michal, M. STRN-NTRK3-rearranged mesenchymal tumor of the uterus: expanding the morphologic spectrum of tumors with NTRK fusions. Am J Surg Pathol. (2019) 43:1152–4. doi: 10.1097/PAS.0000000000001292
7. WHO Classification of Tumors Editorial Board. WHO classification of tumors. Female genital tumours. 5th ed. Lyon: International Agency for Research on Cancer (2020).
8. Fang, X, Huang, R, and Zhang, Z. NTRK-rearranged uterine sarcoma: a case report. Asian J Surg. (2023) 46:4764–5. doi: 10.1016/j.asjsur.2023.05.088
9. Klubíčková, N, Dermawan, JK, Mosaieby, E, Martínek, P, Vaněček, T, Hájková, V, et al. Comprehensive clinicopathological, molecular, and methylation analysis of mesenchymal tumors with NTRK and other kinase gene aberrations. J Pathol. (2024) 263:61–73. doi: 10.1002/path.6260
10. De Castro, JVA, Dos Santos, PJS, Mantoan, H, Baiocchi, G, Bovolim, G, Torrezan, G, et al. Uterine sarcoma with EML4::NTRK3 fusion: a spectrum of mesenchymal neoplasms harboring actionable gene fusions. Int J Gynecol Pathol. (2024) 43:56–60. doi: 10.1097/PGP.0000000000000957
11. Grant, L, Boyle, W, Williams, S, Pascoe, J, and Ganesan, R. Uterine neurotrophic tyrosine receptor kinase rearranged spindle cell neoplasms: three cases of an emerging entity. Int J Gynecol Pathol. (2024) 43:326–34. doi: 10.1097/PGP.0000000000000988
12. Dang, X, Xiang, T, Zhao, C, Tang, H, and Cui, P. EML4-NTRK3 fusion cervical sarcoma: a case report and literature review. Front Med. (2022) 9:832376. doi: 10.3389/fmed.2022.832376
13. Rabban, JT, Devine, WP, Sangoi, AR, Poder, L, Alvarez, E, Davis, JL, et al. NTRK fusion cervical sarcoma: a report of three cases, emphasising morphological and immunohistochemical distinction from other uterine sarcomas, including adenosarcoma. Histopathology. (2020) 77:100–11. doi: 10.1111/his.14069
14. Moura, MS, Costa, J, Velasco, V, Kommoss, F, Oliva, E, Le Loarer, F, et al. Pan-TRK immunohistochemistry in gynaecological mesenchymal tumours: diagnostic implications and pitfalls. Histopathology. (2024) 84:451–62. doi: 10.1111/his.15082
15. Penault-Llorca, F, Rudzinski, ER, and Sepulveda, AR. Testing algorithm for identification of patients with TRK fusion cancer. J Clin Pathol. (2019) 72:460–7. doi: 10.1136/jclinpath-2018-205679
16. Yamazaki, F, Nakatani, F, Asano, N, Wakai, S, Sekimizu, M, Mitani, S, et al. Novel NTRK3 fusions in fibrosarcomas of adults. Am J Surg Pathol. (2019) 43:523–30. doi: 10.1097/PAS.0000000000001194
17. Lin, R, Mallick, AB, Wang, Z-X, Brown, SA, Lu, B, and Jiang, W. Identification of dual STRN-NTRK2 rearrangements in a high grade sarcoma, with good clinical response to first-line larotrectinib therapy. Diagn Pathol. (2023) 18:116. doi: 10.1186/s13000-023-01400-1
18. Boyer, J, Birzu, C, Bielle, F, Goulas, C, Savatovsky, J, Karachi, C, et al. Dramatic response of STRN-NTRK-fused malignant glioneuronal tumor to larotrectinib in adult. Neuro Oncol. (2021) 23:1200–2. doi: 10.1093/neuonc/noab080
19. Gao, X, Xu, S, Zhu, P, Lao, IW, Yu, L, and Wang, J. Primary NTRK -rearranged spindle cell neoplasm of the gastrointestinal tract: a clinicopathological and molecular analysis of 8 cases. Am J Surg Pathol. (2024) 48:623–31. doi: 10.1097/PAS.0000000000002202
20. Alvarez-Breckenridge, C, Miller, JJ, Nayyar, N, Gill, CM, Kaneb, A, D’Andrea, M, et al. Clinical and radiographic response following targeting of BCAN-NTRK1 fusion in glioneuronal tumor. npj Precis Oncol. (2017) 1:5. doi: 10.1038/s41698-017-0009-y
21. Tanti, GK, Pandey, P, Shreya, S, and Jain, BP. Striatin family proteins: the neglected scaffolds. Biochim Biophys Acta Mol Cell Res. (2023) 1870:119430. doi: 10.1016/j.bbamcr.2023.119430
22. Kubota, Y, Kawano, M, Iwasaki, T, Itonaga, I, Tsumura, H, Kaku, N, et al. Sequential treatments with TRK inhibitors in a patient with NTRK fusion-positive sarcoma: a case report. Medicine. (2023) 102:e36232. doi: 10.1097/MD.0000000000036232
23. Croce, S, Devouassoux-Shisheboran, M, Pautier, P, Ray-Coquard, I, Treilleux, I, Neuville, A, et al. Uterine sarcomas and rare uterine mesenchymal tumors with malignant potential. Diagnostic guidelines of the French Sarcoma Group and the Rare Gynecological Tumors Group. Gynecol Oncol. (2022) 167:373–89. doi: 10.1016/j.ygyno.2022.07.031
Keywords: neurotrophic tyrosine receptor kinase (NTRK), uterine sarcoma, STRN3-NTRK3, rearranged tumors, targeted therapy
Citation: Colombo P, Buonamassa GA, Giulianini A, Hassan L, Rudini N, Rizzo A, Cavallo E, Carnaghi C, Goudarzi S, Mongiovì S, Pafumi S and Marletta S (2024) Case report: STRN3-NTRK3 fusion in uterine sarcoma with spleen metastasis: a new variant in the spectrum of NTRK-rearranged tumors. Front. Med. 11:1448491. doi: 10.3389/fmed.2024.1448491
Edited by:
Luigi Tornillo, University of Basel, SwitzerlandReviewed by:
Ranran Zhang, University of Alberta, CanadaDeyin Xing, Johns Hopkins University, United States
Copyright © 2024 Colombo, Buonamassa, Giulianini, Hassan, Rudini, Rizzo, Cavallo, Carnaghi, Goudarzi, Mongiovì, Pafumi and Marletta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Piergiuseppe Colombo, cGllcmdpdXNlcHBlLmNvbG9tYm9AaHVuaW1lZC5ldQ==
 Piergiuseppe Colombo
Piergiuseppe Colombo Giuseppina Adriana Buonamassa
Giuseppina Adriana Buonamassa Anita Giulianini1
Anita Giulianini1 Letizia Hassan
Letizia Hassan